Abstract
Objective
The primary focus of this study was to investigate the effects of local profound hypothermia and to explore the possible mechanism in adult rats with spinal cord injury.
Study Design and Methods
Spinal cord injury models were established by placing aneurysm clips on T10. An epidural perfusion device was applied to maintain a steady temperature (18 °C) for 120 min with gradual rewarming to 37 °C Total hypothermic duration lasted up to about 170 min. The expression of axon regeneration inhibitors was tested by Western blot and real-time PCR. Luxol Fast Blue (LFB) stain and Bielschowsky silver stain were used to observe spinal cord morphology. Motor function of the hind limbs (BBB score) was monitored for 21 days.
Results
The expressions of RhoA, ROCK-II, NG2, Neurocan, Brevican, and Nogo-A were downregulated by regional hypothermia (RH) after spinal cord injury. Subsequent observation showed that rats that had received RH had an alleviated demyelinating condition and a greater number of nerve fibers. Furthermore, the RH group achieved higher BBB scores than the spinal cord injury (SCI) group.
Conclusions
Recovery of hind limb function in rats can be promoted by local profound hypothermia; this may be caused by the suppression of axon regeneration inhibitors.
Keywords: Profound hypothermia, Spinal cord injury, RhoA, ROCK
Introduction
Spinal cord injury (SCI) is a catastrophic neurological event that can seriously affect quality of life; however, there are no treatments with relatively superior curative effects. After years of exploration several therapeutic methods, such as stem cell transplantation1 and hyperbaric oxygen therapy,2 have been used to treat patients suffering from SCI. Of all the methods, hypothermia has been used to protect the central nervous system (CNS) after cardiac arrest hypoxic ischemic encephalopathy,3 aortic aneurysm surgery,4–6 and spinal cord injury, in many experimental and clinical studies.7–9 However, some controversial issues need to be resolved, such as the temperature range, detrimental consequences, the method of introduction, and the duration of hypothermia.
The pathophysiology of SCI is complex and involves both primary and secondary mechanisms. During the period after SCI a host of biochemical changes occur, some of which are immune activation, apoptosis, intracellular Ca2 + overload, impaired spinal cord blood flow, excess release of excitatory amino acids, and altered gene expression.10–14 In addition to the changes mentioned above, the failure of axon regeneration is another important detrimental consequence of SCI.15 Chondroitin sulfate proteoglycans (CSPGs) are a family of inhibitory extracellular matrix molecules highly expressed at CNS injury sites, where they can restrict anatomical plasticity by inhibiting sprouting and reorganization.16–18 In animal SCI models the expression levels of some well-characterized CSPGs, such as NG2 and neurocan, increase rapidly within 24 h, and then reach a peak around 8–14 days after injury. The levels may remain high for at least several weeks.19,20 Apart from the CSPGs, Nogo-A also plays an important role in inhibiting axon regeneration by causing growth cone collapse.21,22 Unlike CSPGs, Nogo transcripts are downregulated in the central parts of lesion sites but upregulated at the borders.23,24 Together with CSPGs, Nogo-A actives RhoA (a substance belonging to the Rho subfamily of GTPases within the Ras superfamily) and can regulate organization of the actin cytoskeleton, gene expression, and cell proliferation. Through regulating growth cone formation and elongating neuritis, both RhoA and its main downstream protein Rho kinase II (ROCK-II) play a significant role in the regenerative process. The regeneration of axons is restrained by the upregulation and activation of the RhoA-ROCK pathway after spinal cord injury.25,26 In rat SCI models, RhoA mRNA as well as protein expression are enhanced significantly 7 d after injury.27 Recently, a large number of studies have reported the beneficial effects of Rhoa-ROCK inhibitors (C3-exoenzmye, fasudil, Y-27632, ibuprofen, siRhoA, and p21),28–32 Nogo-A antibody,33 and the catabolic enzyme of CSPGs (chondroitinase ABC)34 on the regeneration of inhibited axons, plasticity of uninjured pathways, and neuroprotection of injured projection neurons.
In the current study we explored the neuroprotective effects of local profound hypothermia and the possible mechanism of action after experimental spinal cord injury in rats.
Methods
Animals
Seventy-nine adult male Sprague–Dawley rats weighing 240–260 g were used in this study. Experimental protocols were approved by the Animal Care and Use Committee of Nanjing University and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. All rats were housed in standard conditions and given ad libitum access to food and water.
Spinal cord injury
Firstly, the rats were anesthetized with 10% chloral hydrate (0.35 mL/100 g) through intraperitoneal injection. Half the quantity (0.17 mL/100 g) was then injected into the rats every hour during the entire experiment to achieve continuous anesthesia. Rats were placed in the prone position on a warming pad. They were shaved, aseptically prepared, and a midline longitudinal incision was created to expose the region of interest (T8–T12). Subsequently, a laminectomy was performed at the T10 level using an operating microscope (M500-N, LEICA, Heerbrugg, Switzerland) to expose the surface of the spinal dura mater. Finally, a 10 g aneurysm clip (Kent Scientific, Torrington, CT, USA) was used to induce a 2-min compressive spinal cord injury.
Application of local profound hypothermia
An epidural hypothermia device was applied at the injured site according to our previous study.35 Hypothermia was initiated by infusing 4 °C saline into the epidural area through the inflow catheter, which was connected to an infusion pump. A catheter on the other side ensured sufficient outflow. By adjusting the speed of perfusion, the temperature was allowed to stabilize at target and the temperature of the surface of the spinal cord was kept at 18 °C for about 120 min. The hypothermia device was then removed to allow gradual whole body rewarming. Because of the low speed of temperature variation, the total hypothermia time was longer than 160 min (Figure 1).
Figure 1.

Rats with spinal cord injury (A). Rats with the hypothermic device (B).
Western blot analysis
The 20 rats for the Western blot analysis were divided into four groups (SCI 2 d n = 5, SCI 8 days, n = 5, SCI + hypothermia 2 days, n = 5, SCI + hypothermia 8 days, n = 5). Protein concentrations were determined by the Bradford method. Equal amounts of protein per lane were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene-difluoride (PVDF) membranes. The membranes were blocked for 2 hours in blocking buffer [Tris-buffered saline/0.05% Tween 20 (TBST) containing 5% skim milk] and then incubated overnight at 4 °C with primary antibodies against RhoA (1:1200; Abcam, Cambridge, MA, USA), ROCK-II (1:1000; Abcam, USA) and β-actin (1:5000; Bioworld Technology, St. Louis Park, MN, USA) in blocking buffer. After being washed with TBST (3 × 10 min), the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated IgG (1:5000; Bioworld Technology, USA) for 2 hours at room temperature. The protein bands were visualized by enhanced chemiluminescence (ECL) Western blot detection reagents (Millipore, Billerica, MA, USA). Band density was quantified using Un-Scan-It 6.1 software (Silk Scientific Inc., Orem, UT, USA).
Real-time quantitative polymerase chain reaction (PCR)
Thirty-five rats were divided into three groups [sham n = 5, SCI n = 15 (2, 8, and 14 days, n = 5), SCI + hypothermia n = 15 (2, 8, and 14 days, n = 5)] Total RNA was extracted from spinal cord samples (in the test of Nogo-A, we used the samples around contusion injury sites) with RNAiso Plus (TaKaRa Bio, Japan). The concentration and purity of total RNA were determined by a spectrophotometer (OD260/280 1.8–2.0) and 1% agarose gel electrophoresis. To avoid RNA degradation, some of the RNA was immediately reverse transcribed to cDNA with the PrimeScript RT reagent kit (TaKaRa Bio, Kusatsu, Japan), and the surplus RNA was kept at −80 °C. The primers were designed according to PubMed GenBank and synthesized by Invitrogen Life Technologies (Shanghai, China). The primer sequences are given in Table 1. The quantitative real-time PCR analysis was performed by using the Mx3000P System (Stratagene, CA, USA) with real-time SYBR Green PCR technology. The PCR amplification program consisted of an initial denaturation step of 95 °C for 30 seconds, followed by 40 cycles of 95 °C for 5 seconds, and a 30 seconds annealing and elongation step at 60 °C. All samples were analyzed in triplicate. β-Actin was used as an endogenous reference “housekeeping” gene. Relative change in mRNA expression was determined by the equation:
Table 1.
Primer sequences of RhoA, ROCK-II, NG2, Neurocan , Brevican and Nogo-A
| Primer Sequences (5′–3′) | Name |
|---|---|
| GTAAGACATGCTTGCTCATA | Rhoa forward |
| CTCCGTCTTTGGTCTTTGCT | Rhoa reverse |
| CATACACCACATGTCGCTCG | ROCK-II for |
| AGCCCAGACAAACCTCTCCA | ROCK-II rev |
| GAG ACC CTT TTT GCT CTT CCT G | Nogo-A for |
| AAT GAT GGG CAA AGC TGT GCT G | Nogo-A rev |
| CTG TGT ACC GCT TCG CCA AC | Neurocan for |
| TGG GAC CCC CTG GAG TAG AA | Neurocan rev |
| CAG GAG GAC CTG TGG GTG TG | Brevican for |
| CAG GGG CTG GGG ATA CAG TC | Brevican rev |
| TTG CTC CAG CTC CAC TCA GG | NG-2(cspg 4) for |
| CAG GCC CAC TTC ATC ACC AG | NG-2(cspg 4) rev |
| CCCATCTATGAGGGTTACGC | actin for |
| TTTAATGTCACGCACGATTTC | actin rev |
Tissue processing and histochemistry
Following animal perfusion with 4% paraformaldehyde, the spinal cords containing the lesion area were dissected out. Spinal cord blocks (sham n = 4, SCI n = 4, SCI + hypothermia n = 4) were transversely cut (8 μm) at the indicated levels rostral or caudal to the lesion. Myelination of the spinal cord was evaluated via Luxol Fast Blue (LFB) staining. Briefly, spinal cord sections were dehydrated in a gradient of ethanol, and stained in 0.1% solvent blue 38 (Sigma-Aldrich Co., LLC, St. Louis, MO, USA) in acidified 95% ethanol overnight at 60 °C. After rinsing with 95% ethanol and distilled water, sections were then differentiated with 0.05% Li2CO3 and 35% ethanol several times until the contrast between gray matter and white matter was clearly detected. The pictures were analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Silver Spring, MD, USA).
Other spinal cord blocks (sham n = 4, SCI n = 4, SCI + hypothermia n = 4) were cut longitudinally (8 μm). Nerve fibers were observed via modified Bielschowsky's silver staining. Spinal cord sections were dehydrated in a gradient of ethanol, rinsed in distilled water three times, and stained in 3% silver nitrate solution at 37 °C for 30 min away from light. After being rinsed three times, sections were reduced for 5 min with 10% formaldehyde until they turned a light brown color and then rinsed a further three times. Ammonium silver alcohol solution was then added to sections (200 μl/section) for 5 min. Finally, sections were reduced again in 8% formaldehyde until the color became dark brown. All sections were visualized using an Olympus IX71 inverted microscope system.
Behavioral testing
BBB scoring
Animals were evaluated using the BBB open field locomotor test36 immediately after the introduction of injury, and on postoperative days 1, 3, 5, 7, 14, and 21. BBB scores reflect a 21-point open field locomotor scale, where 0 indicates no locomotion and 21 normal motor functions. Rats’ hind limb movements, trunk position and stability, stepping, coordination, paw placement, toe clearance, and tail position were analyzed during the evaluation period. Two blinded observers evaluated the scores individually, and the mean value of the two observers’ scores was used.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics, version 19 (IBM Corp., Armonk, NY, USA). The data are expressed as mean ± S.E.M. Paired t-tests, 1-way ANOVAs with Tamhane's T2 and repeated measures ANOVAs were used in data analysis. Values of ***P < 0.001, **P < 0.01, and *P < 0.05 were considered statistically significant.
Results
Temperature management and duration
The temperature level reached 18 °C within 10 min after the hypothermic device was applied. The regional temperature was maintained at the required level within a small fluctuation range (within 0.5 °C) for 120 min, and the rewarming period lasted 38.21 ± 2.35 min. Using a warming pad, rectal temperature was maintained at 37.5 ± 0.3 °C during the experiment.
Expression of RhoA and ROCK-II proteins
Western blot testing demonstrated that RhoA and ROCK-II proteins were expressed at relatively low levels at 2 days after SCI but were elevated at 8 days in both the SCI group and the SCI + hypothermia group. RhoA and ROCK-II protein expression was significantly higher in the SCI group than in the SCI + hypothermia group at both 2 days (the relative expression of RhoA P < 0.001, ROCK-II P < 0.05) and 8 days (RhoA P < 0.01, ROCK-II P < 0.01; Figure 2).
Figure 2.
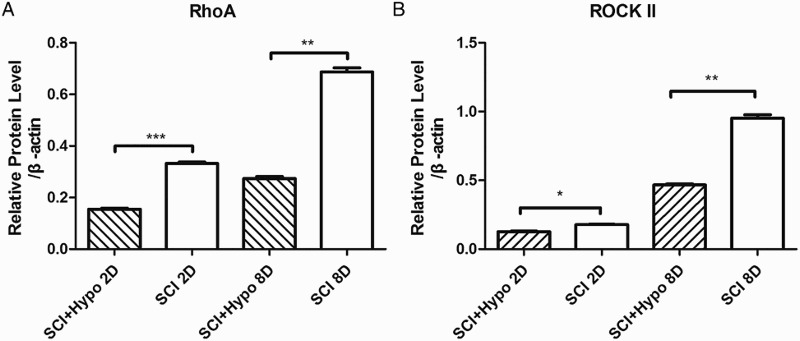
Time course of RhoA and ROCK-II protein expression after SCI. Expression levels of proteins were normalized to those of β-actin. The expression levels of RhoA and ROCK-II proteins were significantly decreased at 2 d and 8 d in the SCI + hypothermia group compared with those in the SCI group (the relative expression of RhoA at 2 d: SCI + hypothermia 0.155 ± 0.007 times; SCI 0.332 ± 0.012 times, P < 0.001, ROCK-II: SCI + hypothermia 0.128 ± 0.005 times; SCI 0.178 ± 0.006 times, P < 0.05. RhoA at 8 d: SCI + hypothermia 0.273 ± 0.014 times; SCI 0.087 ± 0.03 times, P < 0.01, ROCK-II: SCI + hypothermia 0.467 ± 0.01 times; SCI 0.9521 ± 0.04 times, P < 0.01). (A) A typical Western blot result. (B) Quantification of data.
Expressions of RhoA, ROCK-II, NG2, Neurocan, Brevican, and Nogo-A mRNAs
Except for Brevican, other mRNA expression began to increase as early as 2 d after SCI and peaked at 8 days. Similarly, the expression levels of mRNA were higher in the SCI group than in the SCI + hypothermia group at 2 days, 8 days, and 14 days. Brevican mRNA expression was downregulated at 2 days and upregulated at 8 days after SCI, even though the expression level was still higher in the SCI group than in the SCI + hypothermia group at 2 days, 8 days, and 14 days (Figure 3).
Figure 3.
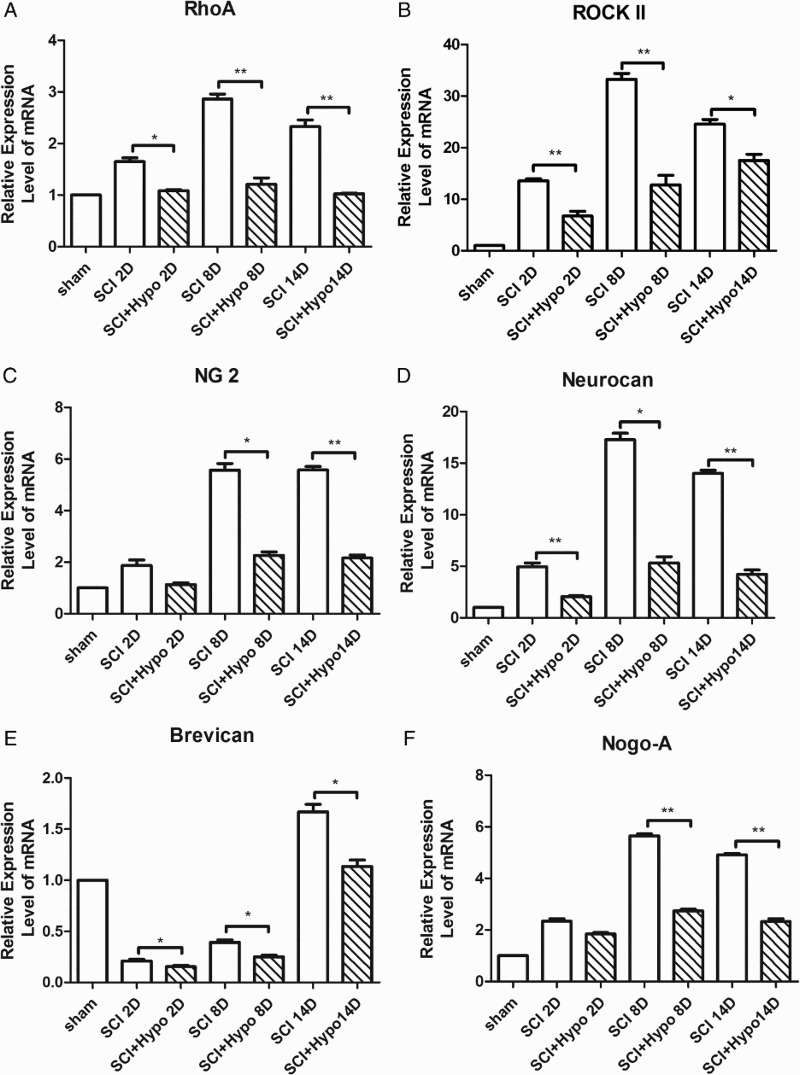
Time course of RhoA (A), ROCK-II (B), NG2(C), Neurocan (D), Brevican (E), and Nogo-A (F) mRNAs expressions after SCI. The data were normalized to β-actin and were expressed as fold increase over the sham group. The expression levels of mRNAs significantly decreased at 8 days and 14 days in the SCI + hypothermia group compared with those in the SCI group.
LFB and modified Bielschowsky's silver staining
Six representative images of LFB staining are shown in Figure 4. The LFB staining results indicated that axonal myelination at the SCI site increased significantly at D 21 in the SCI + hypothermia group compared with that in the SCI group. The integrated optical density (IOD) of the SCI + hypothermia group (442.67 ± 50.36) was much higher than that of the SCI group (picture B 186.35 ± 35.69, P < 0.01). More characteristic vacuolation was observed in the SCI group than in the SCI + hypothermia group.
Figure 4.
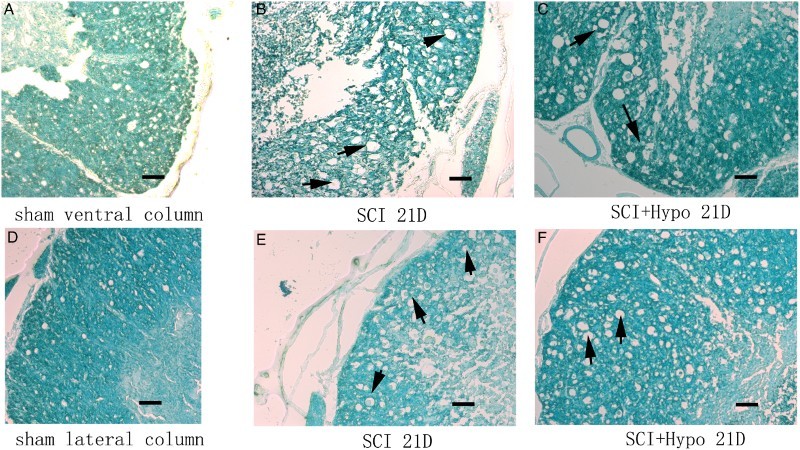
Representative LFB staining of spinal cord (A-C ventral column, D-E: lateral column) on D 21 from the sham, SCI and SCI + hypothermia groups. All black arrows indicate characteristic vacuolation. Scale bar = 200 μm.
Representative images of modified Bielschowsky's silver staining are shown in Figure 5. Compared with sections in the SCI group, there were more nerve fibers observed at the lesion site in SCI rats at D 21 in the SCI + hypothermia group.
Figure 5.

Bielschowsky's silver staining of the spinal cord. The spinal cord tissues on D 21 from the Sham group (A), the SCI + hypothermia group (B), and the SCI group (C). All black arrows indicate the nerve fibers. Scale bar = 200 μm.
Locomotor function recovery
The preoperative motor function of all rats was normal (BBB Scale = 21). At 1 day after SCI, all animals in the SCI and SCI + hypothermia groups were completely paraplegic. In the following days, a slow recovery of hind limb locomotion, characterized by slight movement in one or two joints, was observed in both groups. The RH group achieved a higher BBB score than the SCI group at D 7 (SCI + hypothermia 9.15 ± 1.02; SCI 6.33 ± 0.58, P = 0.016), D 14 (SCI + hypothermia 13.25 ± 1.26; SCI 9.75 ± 0.96, P = 0.004) and D 21 (SCI + hypothermia 15.25 ± 1.32; SCI 12.25 ± 0.86, P = 0.018; Figure 6).
Figure 6.
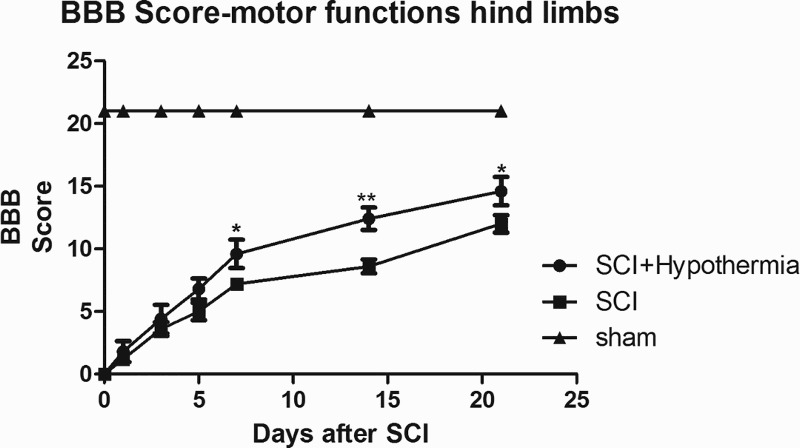
The BBB scores of rats after SCI in the Sham, SCI and SCI + hypothermia groups. The BBB scores of rats in the SCI + hypothermia group were higher than those of the SCI group at D 7 (SCI + hypothermia 9.15 ± 1.02; SCI 6.33 ± 0.58, P = 0.016), D 14 (SCI + hypothermia 13.25 ± 1.26; SCI 9.75 ± 0.96, P = 0.004) and D 21 SCI + hypothermia 15.25 ± 1.32; SCI 12.25 ± 0.86, P = 0.018).
Discussion
Axon regeneration is much more problematic in the central nervous system than in the peripheral nervous system. CNS injury, especially of the spinal cord, often causes serious damage to patients and can lead to lifelong disabilities. When the neuroprotective effects of hypothermia were identified in the 1940s, it was proven to reduce oxygen consumption and metabolic rate, steady the blood flow, and inhibit the release of excitatory neurotransmitters, apoptosis, and inflammation.10–14 As a result, hypothermia has been widely used in the treatment of cardiac arrest hypoxic ischemic encephalopathy, aortic aneurysm surgery, strokes, and spinal cord injury.3–9
The beneficial effect of regional hypothermia and its possible mechanism
The effect of hypothermia on axons has rarely been studied, unlike its effect on metabolism, hemodynamics, apoptosis, and inflammation.10–14 In the present study, the LFB staining results showed that axonal myelination at the lesion site in SCI rats was significantly increased at 21 days in the SCI + hypothermia group compared with the SCI group. The modified Bielschowsky's silver staining results also showed that there were more nerve fibers observed at the lesion site in SCI rats at D 21 in the SCI + hypothermia group. All of these findings may explain the reason why the BBB scores were markedly increased by the regional/local profound hypothermia at D 7, D 14 D, and D 21 after SCI.
In our study, the mRNA levels of NG2, Neurocan, Brevican, and Nogo-A were downregulated significantly in the SCI + hypothermia group. NG2, Neurocan, and Brevican [three important types of CSPG mainly expressed at CNS injury sites] were rapidly upregulated at the lesion site by reactive astrocytes in the glial scar tissues. Spinal cord trauma usually induces over-expression of CSPGs in the lesion penumbra, with higher levels in the epicenter of scar tissue.37 In addition to the physical barrier of scar tissue including reactive astrocytes, meningeal cells, fibroblasts, and microglia, the increased levels of CSPGs form a potent chemical barrier for axon regeneration by preventing elongation.16,19,20 Nogo-A was discovered as a myelin-associated inhibitor of axon growth and its mRNA was upregulated around the lesion. Nogo-A protein was strongly expressed in injured dorsal column fibers and their sprouts that entered the lesion site. Binding to its receptors, Nogo-A could obviously inhibit axon regeneration.21–24 Thus, surmounting strong suppression of CSPG inhibitors and Nogo-A is a major target for therapeutic intervention following CNS injuries, including SCI.33,34 Our study found that hypothermia can downregulate the gene expressions of NG2, Neurocan, Brevican, and Nogo-A. This may be one of the important mechanisms for explaining why more axons remained in the SCI + hypothermia group. Hypothermia could inhibit the activation of astrocytes and microglia.38,39 The regeneration inhibitors were expressed in this sense. Therefore, we assumed that the effect of local profound hypothermia on expressions of NG2, Neurocan, Brevican, and Nogo-A may be caused by the inhibition of astrocytes and microglia.
The intracellular effects of most axon growth-suppressing proteins, including CSPGs and Nogo-A, are mediated by the activation of the small GTP-binding protein RhoA, a signaling molecule that regulates neuronal morphogenesis via interaction with a number of other molecules, including serine/threonine kinases, tyrosine kinases, lipid kinases, lipases, oxidases, and scaffold proteins. In particular, the activated (GTP-bound) form of Rho can bind and directly activate ROCK. This activation leads to phosphorylation of several target proteins, including myosin light chain, and mediating cytoskeletal rearrangements, and disassembly in neurons and collapse of growth cones.25,26,40 In the current study, the protein and mRNA expression levels of RhoA and ROCK-II were downregulated in the SCI + hypothermia group; we believe that regional profound hypothermia could suppress this pathway, including RhoA and ROCK-II. As a result, more axons and improved behavioral recovery were observed after SCI. Since hypothermia could downregulate the expression of CSPGs and Nogo-A, which could in turn active RhoA by binding to their respective receptors, activation of the RhoA and ROCK-II pathway may be inhibited by hypothermia.
Local profound hypothermia
The efficacy of hypothermia is associated with three important factors: the methods of hypothermia induction, the duration, and the temperature range. According to existing small clinical trials, systematic hypothermia both increases the incidence of cardiac arrhythmias and pneumonia and causes serious consequences such as chills, immunosuppression, electrolyte disorders, and mild coagulopathy.41–43 RH can largely avoid the complications mentioned above and achieve a lower temperature range than systematic hypothermia. While there are numerous advantages of RH,5,9 its use is restricted because of the technical complexity of laminectomy procedures44 and the relatively shorter duration of hypothermia.
In this study we used a mixture of ice and saline for perfusion, as the temperature could approach the ideal level more quickly (10 min) and remain more stable during the perfusion than with pure cold saline. Although the duration of hypothermia in our study (120 min) was shorter than those of other studies (duration could be as long as three or more days),45,46 a neuroprotective effect was found. This may be because we used profound hypothermia with a lower temperature level of 18C instead of mild hypothermia, since profound hypothermia has been proved to be more efficient in reducing oxygen consumption and metabolic rate, and inhibiting inflammation. As a result, we observed a significant recovery of locomotor function despite the shorter duration of hypothermia.
In some clinical trials, ice blankets, medicine, and endovascular devices have been used to induce hypothermia.41–43 These methods could maintain the level of temperature for a long time, but failed to achieve the lowest temperature the patients could tolerate. A recent clinical study9 found that the use of regional/local profound hypothermia during fixation surgery could improve the prognosis of patients with severe SCI. The majority of patients may need emergency surgery because of the severity of SCI, and this may make intraoperative regional/local profound hypothermia a potential adjunct in treating SCI in the future.
Conclusion
The present experimental study demonstrated the beneficial effects of regional profound hypothermia on the recovery of locomotor function. Moreover, our results strongly suggest that regional profound hypothermia could improve locomotor function by inhibiting the pathways of CSPGs, Nogo-A, RhoA, and ROCK-II. We hope these results will be an inspiration for further studies and will promote the potential clinical utilization of regional profound hypothermia in the future.
Acknowledgments
This work was supported by grants from National Science Foundation of China(81371356). The authors are grateful for support from Mengliang Zhou, Lili Wen, Jing He and their colleagues in the Department of Neurosurgery, Jinling Hospital.
Disclaimer statements
Contributors NL, LZ and YZ helped the author in westernblot and PCR; HC as the corresponding author helped in the design of study and supervised the whole process. The authors are grateful for support from Mengliang Zhou, Lili Wen, Jing He and their colleagues in the Department of Neurosurgery, Jinling Hospital.
Funding National Science Foundation.
Conflicts of interest There are no conflicts-of-interest.
Ethics approval This study was performed on rats, the paper has received approval from Research Ethical Commitee of Jinling hospital.
References
- 1.John WM, Xiaozhong L, Yun Q, Su L, Shannon KM, Dorothy T, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 1999;12(1):1410–2. [DOI] [PubMed] [Google Scholar]
- 2.Asamoto S1, Sugiyama H, Doi H, Iida M, Nagao T, Matsumoto K. Hyperbaric oxygen (HBO) therapy for acute traumatic cervical spinal cord injury. Spinal Cord 2000;38(3):538–40. doi: 10.1038/sj.sc.3101023 [DOI] [PubMed] [Google Scholar]
- 3.Nikolov NM, Cunningham AJ. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346(8):549–56. doi: 10.1056/NEJMoa012689 [DOI] [PubMed] [Google Scholar]
- 4.Conrad MF, Crawford RS, Davison JK, Cambria RP. Thoracoabdominal aneurysm repair: a 20-year perspective. Ann Thorac Surg 2007;83(2):856–61, 890–2. doi: 10.1016/j.athoracsur.2006.10.096 [DOI] [PubMed] [Google Scholar]
- 5.Tabayashi K, Saiki Y, Kokubo H, Takahashi G, Akasaka J, Yoshida S, et al. Protection from postischemic spinal cord injury by perfusion cooling of the epidural space during most or all of a descending thoracic or thoracoabdominal aneurysm repair. Gen Thorac Cardiovasc Surg 2010;58(5):228–34. doi: 10.1007/s11748-009-0495-0 [DOI] [PubMed] [Google Scholar]
- 6.Hsu CC, Kwan GN, van Driel ML, Rophael JA. Distal aortic perfusion during thoracoabdominal aneurysm repair for prevention of paraplegia. Cochrane Database Syst Rev 2012;3:CD8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bricolo A, Ore GD, Da PR, Faccioli F. Local cooling in spinal cord injury. Surg Neurol 1976;6(2):101–6. [PubMed] [Google Scholar]
- 8.Ha KY, Kim YH. Neuroprotective effect of moderate epidural hypothermia after spinal cord injury in rats. Spine (Phila Pa 1976) 2008;33(19):2059–65. doi: 10.1097/BRS.0b013e31818018f6 [DOI] [PubMed] [Google Scholar]
- 9.Hansebout RR, Hansebout CR. Local cooling for traumatic spinal cord injury: outcomes in 20 patients and review of the literature. J Neurosurg Spine 2014;20(5):550–61. doi: 10.3171/2014.2.SPINE13318 [DOI] [PubMed] [Google Scholar]
- 10.Schumacher PA, Siman RG, Fehlings MG. Pretreatment with calpain inhibitor CEP-4143 inhibits calpain I activation and cytoskeletal degradation, improves neurological function, and enhances axonal survival after traumatic spinal cord injury. J Neurochem 2000;74(4):1646–55. doi: 10.1046/j.1471-4159.2000.0741646.x [DOI] [PubMed] [Google Scholar]
- 11.Eldadah BA, Faden AI. Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma 2000;17(10):811–29. doi: 10.1089/neu.2000.17.811 [DOI] [PubMed] [Google Scholar]
- 12.Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785 [DOI] [PubMed] [Google Scholar]
- 13.Charles HT. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol 1995;5(4):407–13. doi: 10.1111/j.1750-3639.1995.tb00619.x [DOI] [PubMed] [Google Scholar]
- 14.Craenen G, Jeftinija S, Grants I, Lucas JH. The role of excitatory amino acids in hypothermic injury to mammalian spinal cord neurons. J Neurotrauma 1996;13(12):809–18. doi: 10.1089/neu.1996.13.809 [DOI] [PubMed] [Google Scholar]
- 15.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev 1996;76(2):319–70. [DOI] [PubMed] [Google Scholar]
- 16.Fidler PS, Schuette K, Asher RA, Dobbertin A, Thornton SR, Calle-Patino Y, et al. Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2. J Neurosci 1999;19(20):8778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedlander DR, Milev P, Karthikeyan L, Margolis RK, Margolis RU, Grumet M. The neuronal chondroitin sulfate proteoglycan neurocan binds to the neural cell adhesion molecules Ng-CAM/L1/NILE and N-CAM, and inhibits neuronal adhesion and neurite outgrowth. J Cell Biol 1994;125(3):669–80. doi: 10.1083/jcb.125.3.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quaglia X, Beggah AT, Seidenbecher C, Zurn AD. Delayed priming promotes CNS regeneration post-rhizotomy in Neurocan and Brevican-deficient mice. Brain 2008;131(1):240–49. doi: 10.1093/brain/awm279 [DOI] [PubMed] [Google Scholar]
- 19.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan and versican are differentially regulated following spinal cord injury. Exp Neurol 2003;182(2):399–411. doi: 10.1016/S0014-4886(03)00087-6 [DOI] [PubMed] [Google Scholar]
- 20.Xiufeng T, Jeannette ED, Stephen JD. Changes in distribution, cell associations, and protein expression levels of NG2, Neurocan, Phosphacan, Brevican, Versican V2, and Tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res 2003;71(3):427–44. doi: 10.1002/jnr.10523 [DOI] [PubMed] [Google Scholar]
- 21.Cafferty WB, Strittmatter SM. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J Neurosci 2006;26(47):12242–50. doi: 10.1523/JNEUROSCI.3827-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maio SC, Andrea BH, Marjan EV, Marcus F, Lisa S, Adrian AS, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 2000;403(6768):434–9. doi: 10.1038/35000219 [DOI] [PubMed] [Google Scholar]
- 23.Hunt D, Coffin RS, Prinjha RK, Campbell G, Anderson PN. Nogo-A expression in the intact and injured nervous system. Mol Cell Neurosci 2003;24(4):1083–102. doi: 10.1016/j.mcn.2003.09.002 [DOI] [PubMed] [Google Scholar]
- 24.Xingxing W, Soo-Jin C, Helen T, Timothy V, Charles AG, Stephen MS. Localization of Nogo-A and Nogo-66 receptor proteins at sites of axon-myelin and synaptic contact. J Neurosci. 2002;22(13):5505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 2003;22(3):319–30. doi: 10.1016/S1044-7431(02)00035-0 [DOI] [PubMed] [Google Scholar]
- 26.Barbara N, Thomas O, Jens F, R.Anne M, Christine EB. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 2002;22(23):10368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joo-Kyung Sung, Liyan Miao, John WC, Lixin H. A possible role of RhoA/Rho-kinase in experimental spinal cord injury in rat. Brain Research 2003;959(1):29–38. doi: 10.1016/S0006-8993(02)03717-4 [DOI] [PubMed] [Google Scholar]
- 28.Masahito H , Masakazu T, Kazuhiko W, Atsushi N, Teruhide T, Yoshio S, et al. Protein kinase inhibition by fasudil hydrochloride promotes neurological recovery after spinal cord injury in rats. J Neurosurg 2003;93(1 Suppl):94–101. [DOI] [PubMed] [Google Scholar]
- 29.John D, Ankur RP, Xiao-Li Y, Robert B, Craig MP, Shuxin Li. A molecular mechanism for ibuprofen mediated RhoA inhibition in neurons. J Neurosci 2010;30(3):963–72. doi: 10.1523/JNEUROSCI.5045-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anja H, Fred H, Ingo J, Seija L, Uwe-Karsten H, Wolfgang B, et al. Inhibition of Rho-dependent pathways by Clostridium botulinum C3 protein induces a proinflammatory profile in microglia. Glia 2008;56(11):1162–75. doi: 10.1002/glia.20687 [DOI] [PubMed] [Google Scholar]
- 31.Alyson EF, Bayan TT, Stephen MS. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 2003;23(4):1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watzlawick R, Sena ES, Dirnagl U, Brommer B, Kopp MA, Macleod MR, et al. Effect and reporting bias of RhoA/ROCK-blockade intervention on locomotor recovery after spinal cord injury: a systematic review and meta-analysis. JAMA Neurol 2014;71(1):91–9. doi: 10.1001/jamaneurol.2013.4684 [DOI] [PubMed] [Google Scholar]
- 33.Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci 2001;21(10):3665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elizabeth JB, Lawrence DM, Reena JP, Von RK, Gavin SB, Preena NP, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002;416(6881):636–40. doi: 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- 35.Ning L, Lei T, Wei W, Huchen L, Yuan Z, Xiaoyu X, et al. Regional hypothermia inhibits spinal cord somatosensory-evoked potentials without neural damage in uninjured rats. J Neurotrauma 2013;30(15):1325–33. doi: 10.1089/neu.2012.2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1 [DOI] [PubMed] [Google Scholar]
- 37.Stephen JAD, Michael TFitch, Stacey PM, Alison KH, Geoffrey R, Jerry S. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 1997;390(6661):680–3. [DOI] [PubMed] [Google Scholar]
- 38.Takeshi S, Shino S, Hiroshi Y, Masanori T. Neuroprotection following mild hypothermia after spinal cord ischemia in rats. J Vasc Surg 2013;57(1):173–81. doi: 10.1016/j.jvs.2012.05.101 [DOI] [PubMed] [Google Scholar]
- 39.Morino T, Ogata T, Takeba J, Yamamoto H. Microglia inhibition is a target of mild hypothermic treatment after the spinal cord injury. Spinal Cord 2008;46(6):425–31. doi: 10.1038/sj.sc.3102163 [DOI] [PubMed] [Google Scholar]
- 40.Liqun L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci 2000;1(3):173–80. doi: 10.1038/35044547 [DOI] [PubMed] [Google Scholar]
- 41.Soliman HM, Mercan D, Lobo SS, Mélot C, Vincent JL. Development of ionized hypomagnesemia is associated with higher mortality rates. Crit Care Med 2003;31(4):1082–87. doi: 10.1097/01.CCM.0000060867.17556.A0 [DOI] [PubMed] [Google Scholar]
- 42.Melhuish T. Linking hypothermia and hyperglycemia. Nurs Manage 2009;40(12):42–5. doi: 10.1097/01.NUMA.0000365472.26379.be [DOI] [PubMed] [Google Scholar]
- 43.Lenhardt R. The effect of anesthesia on body temperature control. Front Biosci (Schol Ed) 2010;2:1145–54. doi: 10.2741/S123 [DOI] [PubMed] [Google Scholar]
- 44.Amar AP, Levy ML. Surgical controversies in the management of spinal cord injury. J Am Coll Surg 1999;188(5):550–66. doi: 10.1016/S1072-7515(99)00013-7 [DOI] [PubMed] [Google Scholar]
- 45.Yu WR, Westergren H, Farooque M, Holtz A, Olsson Y. Systemic hypothermia following compression injury of rat spinal cord: reduction of plasma protein extravasation demonstrated by immunohistochemistry. Acta Neuropathol 1999;98(1):15–21. doi: 10.1007/s004010051046 [DOI] [PubMed] [Google Scholar]
- 46.Shibuya S, Miyamoto O, Janjua NA, Itano T, Mori S, Norimatsu H. Post-traumatic moderate systemic hypothermia reduces TUNEL positive cells following spinal cord injury in rat. Spinal Cord 2004;42(1):29–34. doi: 10.1038/sj.sc.3101516 [DOI] [PubMed] [Google Scholar]


