Abstract
Auditory-Targeted Cognitive Training (ATCT), which aims to improve auditory information processing efficiency, has shown great promise for remediating cognitive deficits in schizophrenia (SZ). However, there is substantial heterogeneity in the degree of cognitive gains made during ATCT, and some patients show negligible benefit after completing therapeutic doses of training. Identifying individual differences that can be measured early in the course of ATCT and that predict subsequent cognitive benefits from the intervention is therefore important. The present study calculated a variety of performance metrics during the initial hour of exposure to ATCT Sound Sweeps, a frequency discrimination time-order judgment task, and investigated the relationships of these metrics to demographic, clinical, and cognitive characteristics of SZ patients.
Thirty-seven SZ outpatients completed measures of auditory attention, working memory, verbal memory, and executive functioning, followed by one hour of Sound Sweeps training. Performance metrics, calculated after the first training level, the first training stage (Levels 1–4), and the entire hour of training included baseline and best auditory processing speed (APS) scores, as well as percent improvement in APS after training. The number of training levels completed by each participant was also calculated.
Baseline and best APS correlated with performance in all cognitive domains, whereas APS improvements only correlated with verbal memory. Number of training levels completed was marginally associated with auditory attention only.
Conclusions
Sound Sweeps performance correlates with a range of neurocognitive abilities. APS improvement may provide a particularly sensitive index of “plasticity potential” within the neural network underlying verbal learning and memory.
Keywords: schizophrenia, psychosis, cognition, cognitive remediation, auditory processing, training
1.1 Introduction
Neurocognitive deficits represent a core feature of schizophrenia (SZ) that impinge upon daily psychosocial functioning (Green, 1996; Green et al., 2000), and efforts at remediating cognitive deficits have generally shown a modest degree of efficacy at the group level (McGurk et al., 2007; Wykes et al., 2011). As is often the case for psychiatric interventions, cognitive remediation modalities are typically developed for the average patient and implemented in the absence of knowledge about individual variation in genes, brain function, pathophysiology, and environment that might influence treatment outcomes. This one-size-fits-all approach to cognitive remediation is particularly problematic given data that suggest that up to 45% of people with SZ demonstrate virtually no cognitive enhancement after undergoing a therapeutic dose (≥32 hours) of computerized cognitive training (Murthy et al., 2012). For patients and clinicians, the costs associated with these time- and resource-intensive interventions can be prohibitive. Thus, cognitive training is an excellent example of a treatment that may benefit from a recently announced “precision medicine” initiative by NIH, which aims to promote the systematic investigation of individual differences that play a role in illness and health. Ultimately, the initiative aims to facilitate data-driven prediction of benefit for individual patients from specific treatments at any point during the course of illness. Put simply, precision medicine strives to provide the “right treatment” to the “right person” at the “right time.”
Despite the substantial advances made in cognitive remediation over recent years, the ability to characterize the SZ patients for whom any form of cognitive remediation is the “right” intervention continues to elude practitioners. Accordingly, the present study aims to investigate several auditory processing measures for their ability to reflect early neural target engagement during initial exposure to Auditory Targeted Cognitive Training (ATCT), a computerized intervention that has shown particular promise for enhancing cognition in SZ (Fisher et al., 2009; Fisher et al., 2015; Popov et al., 2011).
While conventional cognitive remediation techniques typically target cognition from the “top-down” (e.g. teaching memory encoding strategies, problem solving approaches, etc.), ATCT focuses on “bottom-up” or feed-forward training of auditory processing fidelity and efficiency, while simultaneously harnessing attention and working memory operations. ATCT explicitly employs known mechanisms to maximize cortical neuroplasticity, for example, by delivering exercises with specifically-defined learning targets delivered at high intensity (greater than 1,000 trials throughout a full course of training), and by maintaining difficulty levels that are carefully titrated in accordance with individual patient performance (Merzenich et al., 2013). Moreover, correct responses are reinforced with sounds and visual animations, consistent with literature suggesting a neuromodulatory effect of subcortical reward processing centers on cortical representations of selectively attended sensory inputs (Merzenich et al., 2014; Vinogradov et al., 2012). ATCT thus aims to efficiently modify the frontotemporal cortical dynamics subserving both basic perceptual processes and higher-order cognitive operations (Vinogradov et al., 2012).
Studies examining the efficacy of ATCT in SZ patients have shown large improvements (d=0.86–0.89) in verbal learning and memory, verbal working memory, and global cognition after 40–50 hours of training; moderate improvements have also been detected in non-trained visual problems solving skills (Fisher et al., 2009; 2015). Furthermore, in a recent multi-site study, significant gains in MATRICS Consensus Cognitive Battery (MCCB) composite and verbal learning scores were observed after 20 hours of training. While these gains no longer achieved statistical significance after 40h of training, perhaps due in part to subject attrition, the effect sizes (d≈0.39) remained non-trivial (Keefe et al., 2012).
Despite its apparent efficacy at the group level, individual response to ATCT is highly variable (Murthy et al., 2012). Although some patient characteristics, such as self-reported anticipatory pleasure, appear to correlate with ATCT response (Fisher et al., 2015), there are currently no established methods for identifying early in treatment (e.g. within the first hour) the individuals most likely to benefit from ATCT. Auditory perceptual improvements (i.e. auditory “tuning”) gained during treatment may be a key predictor of ATCT response, as they have been shown to correspond to overall degree of cognitive enhancement (Murthy et al., 2012; Popov et al., 2011; 2012; 2015). Notably, Fisher et al. (2015) found that auditory processing speed improvements after 20 hours of training correlated with degree of cognitive enhancement after up to 40 hours of training, suggesting that these early auditory processing improvements may reflect the “plasticity potential” of the frontotemporal network and thus index the likelihood of cognitive benefit from ATCT. Since the most dramatic improvements in auditory processing have been shown to occur very early in the course of training, with maximal gains evident after the first training session and incremental gains following subsequent training sessions (Menning et al, 2000), detailed examination of the auditory perceptual dynamics occurring within the initial exposure to ATCT might thus account for some variation in individual training response and subsequently inform future predictive algorithms for guiding treatment of SZ.
Challenges exist, however, in quantifying the perceptual gains made within or across training sessions or patients, given the individualized and continually adaptive nature of ATCT, an intervention that was primarily designed for clinical rather than academic purposes. As such, the aim of the present study was to examine several measures of auditory perceptual improvement during the initial hour of ATCT for their relationships to critical demographic, clinical, and cognitive characteristics of SZ patients. Several such performance metrics have been utilized in previous studies, including percentage of auditory frequency discrimination exercises completed (i.e. the percentage of training trials completed out of the total number of trials available; Fisher et al., 2009), and auditory processing speed (APS; i.e. the length of auditory stimuli for which participants are able to make accurate time-order judgments; Fisher et al., 2015; Keefe et al., 2012; Mahncke et al., 2006; Murthy et al., 2012; Smith et al., 2009). While relationships between these auditory processing measures and ATCT-related cognitive gains have been detected across therapeutic training intervals (e.g., 20–40h; Fisher et al, 2009; 2015), no previous studies have examined whether auditory processing improvements during the initial exposure to ATCT are related to demographic, clinical, and cognitive characteristics of patients at baseline. We hypothesized that better auditory processing at baseline, as well as larger improvements in auditory processing after one hour of ATCT, would be associated with 1) younger patient age, 2) later age of illness onset, 3) less severe clinical symptoms, and 4) better cognitive performance.
2.1 Methods
2.1.1 Participants
Participants included 37 ATCT-naïve SZ outpatients recruited from community treatment programs and via physician referral following our well-established procedures (e.g. Takahashi et al, 2013). All participants were evaluated for their capacity to provide informed consent and gave written consent via methods approved by the UCSD IRB prior to participation (UCSD Protocol #:130453). SZ diagnoses were confirmed via the Structured Clinical Interview for DSM-IV (First et al., 1996). Participants were excluded on the basis of Axis I psychiatric and neurological disorders other than SZ, head injury with loss of consciousness longer than 15 minutes, and stroke. Urine toxicology screenings were conducted to rule out recent drug use (except tobacco and caffeine). Scales for the Assessment of Positive and Negative Symptoms (SAPS; SANS, Andreasen, 1984a; b) were used to assess clinical symptoms. Demographic, clinical, and cognitive data are presented in Table 1. Mean participant age was 44.7 (range: 23 to 63), and 76% of participants were male. On average, participants obtained 12.6 years of education and performed ½ to 1 standard deviation below age- and education- matched normative control groups in all cognitive domains (Table 1).
Table 1.
Demographic, Clinical, and Cognitive Characteristics of Sample
| Max. Score | Mean (SD) | T-score | |
|---|---|---|---|
| Age | – | 44.7 (9.7) | – |
| Sex (% Male) | – | 75.7 | – |
| Education (Years) | – | 12.6 (2.4) | – |
| Age of Illness Onset | – | 21.1 (7.2) | – |
| No. of Hospitalizations | – | 1.3 (3.8) | – |
| Global Positive Symptom Rating | 15.0 | 4.8 (3.4) | – |
| Global Negative Symptom Rating | 25.0 | 16.2 (5.1) | – |
| Auditory Attention | 21 | 11.2 (2.3) | 44.0* |
| Auditory Working Memory | 21 | 8.2 (2.0) | 42.0* |
| Verbal Memory | 75.0 | 39.4 (9.0) | 41.0** |
| Executive Functioning | – | 16.1 (13.2) | 41.2** |
Calculated from archival age-matched nonpsychiatric control sample
Calculated from published age- and education-matched nonpsychiatric norms (Kongs et al., 2000; Wechsler, 1997)
2.1.2 Cognitive Measures
Auditory attention and working memory were assessed with Letter-Number Sequencing Forward and Reorder conditions, respectively (LNS-F/LNS-R; Lee et al., 2015; Wechsler, 1997). Total recall score from the California Verbal Learning Test, 2nd Ed. (CVLT-II, Delis et al., 2000) indexed verbal memory, and executive functions were measured with the number of perseverative responses obtained on the Wisconsin Card Sorting Test-64 (WCST; Heaton et al., 1993), with higher score indicating worse performance.
2.1.3 Targeted Cognitive Training
ATCT (PositScience; brainhq.com) is a computerized cognitive training program that targets both low-level auditory perceptual processes and higher order attention and working memory operations. The program includes a variety of exercises aimed at training a range of auditory-dependent abilities, including auditory tone discrimination, speech syllable discrimination, and memory for verbal instructional sequences. The present study utilized one of the most basic training exercises, “Sound Sweeps”, an auditory frequency discrimination time-order judgment task. In this exercise, participants were presented with pairs of frequency-modulated sound “sweeps” and indicated whether they perceived each sweep as becoming higher or lower in pitch. The training is continuously adaptive (Adcock et al., 2009) – sweep duration, frequency range, and interstimulus interval (ISI) become shorter after correct responses, but longer after incorrect responses. Correct responses are rewarded with reinforcing visual and auditory stimuli. Training is divided into stages, with each stage comprised of levels that differ by stimulus frequency and ISI (Figure 1). Within each level, participants are presented with 20 trials in which to discriminate the shortest/fastest sweep pairs possible. Baseline auditory processing speed (APS) is calculated for each level based on the shortest duration of stimuli that participants are able to correctly discriminate upon initial exposure to that level. To progress to the next training level, participants must either match their baseline APS score (i.e. discriminate stimulus pairs of equal duration) or surpass their baseline APS score (i.e. discriminate stimulus pairs that are shorter in duration) and maintain that level of performance throughout the remainder of 20 trials. If, however, participants reach a predetermined “goal” threshold by discriminating sweeps substantially shorter than their baseline score, they automatically progress to the next training level without completing the remainder of the 20 trials. Baseline and best APS scores are calculated for each level, with possible scores ranging from 13–1,000ms and lower scores indicating better APS.
Figure 1.
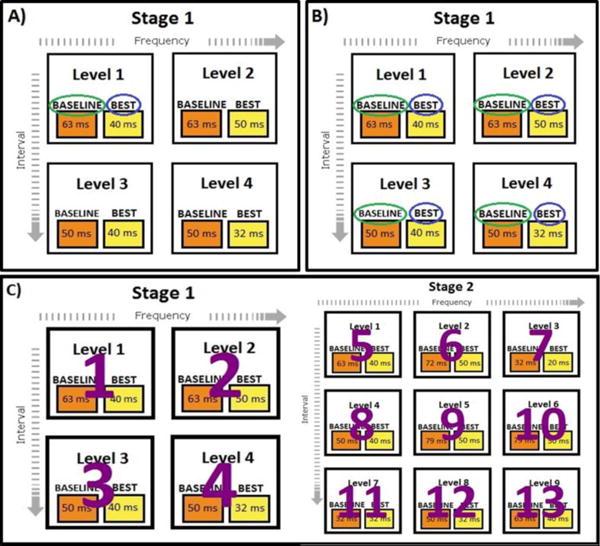
ATCT performance metrics examined in the present study. Scores represent the duration of sounds in ms that participants were able to discriminate direction of frequency modulation. Panel A represents the “initial” improvement metric, in which baseline (green circle) and best (blue circle) scores from the first level of the first training stage were compared. Panel B illustrates the “composite” improvement metrics, for which baseline and best scores were average across levels that differed by stimulus frequency and ISI and then compared. Two composite metrics were calculated – one from the first stage of training only, and one from all levels completed by participants. Panel C represents the “levels completed” metric, which consisted of the number of levels participants were able to complete during one hour of ATCT.
A practice block of Sound Sweep exercises was administered prior to training to ensure familiarity with computers and comprehension of task instructions. All participants successfully completed the practice block and demonstrated an understanding of the task before beginning the training. Thus, the practice block served to minimize early variability in performance due to factors other than APS. A research assistant monitored the session, which lasted one hour.
Ten investigational ATCT performance metrics were calculated to characterize baseline APS, best APS, and within-session APS improvements accrued throughout the one hour of training. APS improvement was calculated as the percentage of change in stimulus duration that participants were able to discriminate within a given level or group of levels. As described below, APS and APS improvement were calculated over varying time frames corresponding to the first level, stage, and hour of ATCT.
Level 1 metrics
Baseline APS, best APS, and percent improvement in APS were calculated for the first level within the first stage of training. Level 1 baseline APS score was thought to provide a measure of auditory perception prior to engaging in any training, whereas Level 1 best APS score was thought to provide a measure of capacity for improvement in APS pursuant to the initial training exposure.
Stage 1 metrics
The first “stage” of training is comprised of the first four training levels, Levels 1–4. A range of stimulus frequencies, durations, and ISIs are trained across these four levels; averaging performance across these levels is thought to yield “composite” indicators of baseline APS, best APS, and APS improvement.
Hour 1 metrics
Similar to Stage 1, the Hour 1 performance metrics consisted of the average baseline APS, best APS, and APS improvement across all levels completed by participants during the entire hour of training. These metrics were expected to provide a more comprehensive composite measure of auditory processing speed than the Stage 1 metrics, as most participants progressed beyond the first stage of training during the hour, and the one-hour scores were therefore derived from a broader range of auditory stimuli than were the Stage 1 metrics.
Number of levels completed
Given that participants with a greater propensity for neuroplasticity may be better able to surpass their baseline scores and progress through the training more quickly, the total number of levels completed by each participant within the hour of training was calculated and considered a proxy for auditory learning.
2.1.4 Statistical Analyses
Means and standard deviations were calculated for demographic, clinical, and cognitive variables (Table 1). Pearson’s bivariate correlations assessed relationships between the ten ATCT performance metrics and demographic variables, clinical characteristics, and baseline cognitive performance, in order to both validate the metrics and identify predictors of early auditory processing speed improvement. A significance threshold of a=0.05 was used for all analyses.
3.1 Results
Due to the individually adaptive nature of the training, all participants exhibited some APS improvements, as shown in Figure 2. In Level 1, average baseline APS was 204ms (range=32–750ms), and average best APS was 132ms (range=25–398ms), resulting in an average of 33% improvement in APS score across Level 1 (range=0–58%). In Stage 1, average baseline APS was 211ms (range=39–673ms), average best APS was 144ms (range=30–538ms), and average APS improvement was 30% (range=13–63%). Finally, across Hour 1, average baseline APS was 192ms (range=41–602ms), and average best APS was 133ms (range=33–480), resulting in an average of 30% improvement (range=16–53%). Participants completed an average of 12 training levels (range=2–23).
Figure 2.
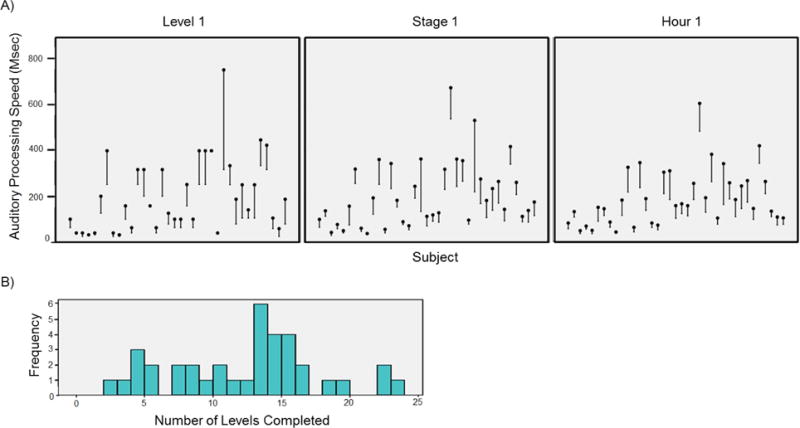
Data from each investigational ATCT performance metric. A) Performance improvement trajectories for each participant during Level 1, Stage 1, and Hour 1. Black dots represent baseline auditory processing speed (APS) and lines represent APS improvement trajectories. Lower score indicates better performance. B) Histogram of the number of training levels completed by participants during the one-hour training session.
Relationships between ATCT metrics and demographic, clinical, and cognitive variables are shown in Table 2. In contrast to our hypotheses, no significant relationships were detected between any of the ATCT performance metrics and demographic or clinical variables (r’s≤0.31, p’s≥0.06). There were, however, several medium or large correlations between ATCT performance metrics and baseline cognitive performance. Level 1 baseline and best APS scores were significantly and negatively correlated with auditory attention and working memory (i.e. ability to discriminate shorter sounds was associated with better auditory attention and working memory; r’s≤−0.36, p’s<0.03). In addition to attention and working memory, Stage 1 average baseline and best APS scores were also significantly associated with executive functioning (r’s≥0.48, p’s≤0.02), whereas Hour 1 average baseline and best APS scores were significantly correlated with performance across all cognitive domains (r’s≥0.37, p’s≤0.03). Percentage improvement in APS at Level 1 and Hour 1 was significantly and positively associated with baseline verbal memory (r’s>0.34, p’s<0.05); however, Stage 1 percentage improvement in APS was not significantly associated with baseline performance in any cognitive domain. Number of levels completed was marginally associated with auditory attention (r=0.33, p=0.051) only.
4.1 Discussion
The present study aimed to evaluate a variety of potential ATCT performance metrics for their ability to index early auditory “target engagement” as well as their possible utility in future ATCT studies. In so doing, we faced the challenges of extracting information from a cognitive training paradigm that was designed for therapeutic rather than experimental purposes. Consistent with previous meta-analytic findings (McGurk et al., 2007; Wykes et al., 2011), none of the ATCT performance metrics examined in the present study were associated with any demographic or clinical variables. Nevertheless, several noteworthy relationships between these metrics and baseline cognitive performance were observed.
Both baseline and best APS scores derived from Level 1 of the training demonstrated moderate-to-strong correlations with auditory attention and working memory. Stage 1 APS scores that were averaged across a range of auditory stimuli were further associated with executive functioning, whereas APS scores averaged across the entire hour of training (Hour 1 metrics) were also moderately associated with baseline verbal memory. These findings are consistent with previous research demonstrating relationships between auditory perception and performance in complex cognitive domains (Javitt, 2009; Kawakubo et al., 2006; Leitman et al., 2005; Light, Swerdlow, & Braff, 2007; Rissling et al., 2014). As evidenced by their moderate-to-strong associations with all cognitive domains, the composite metrics calculated after a full hour of training appear to provide a more robust indicator of auditory perceptual efficiency than do metrics derived from Level 1 or Stage 1.
Interestingly, a different pattern emerged for the APS improvement metrics, which were only associated with verbal memory performance at baseline. Although the small and nonsignificant correlations between APS improvement and attention, working memory, and executive functioning partially contradicted our predictions, our findings nevertheless suggest that the Sound Sweeps auditory frequency discrimination exercise may indeed engage components of the frontotemporal verbal learning network targeted by the broader ATCT intervention (Fisher et al., 2009; 2010; 2015; Vinogradov et al., 2012). Specifically, the degree of APS improvement occurring after one hour of exposure to auditory frequency discrimination training may indicate the degree to which adaptive tuning has taken place within temporal cortex; the positive association we observed between baseline verbal memory scores and APS improvement suggests that this tuning may be greater (or may occur more rapidly) in patients with relatively strong verbal memory abilities prior to training. Taken in context with previous research showing a positive relationship between APS improvements and cognitive enhancement (Fisher et al., 2015; Murthy et al., 2012; Popov et al., 2011; 2012; 2015), our results further suggest that patients with relatively intact verbal memory performance at baseline may ultimately be shown to respond better to ATCT than might patients with relative verbal memory impairments. This assertion makes intuitive sense, given that the learning and memory capabilities indexed by measures like the CVLT-II presumably engage similar neuroplastic mechanisms to those engaged by ATCT (e.g. brain-derived neurotrophic factor signaling; Gorski et al., 2003; Huang et al., 1999; Vinogradov et al., 2009). Conversely, successful performance on behavioral measures of attention, working memory, and executive functioning does not rely heavily on short-term learning-induced neuroplasticity and these measures at baseline thus may not provide a sensitive index of “plasticity potential.” Consistent with our proposed use of “biomarkers of health” to guide personalized intervention strategies (Light & Swerdlow, 2014), this evidence of spared, rather than deficient, frontotemporal plasticity on measures of learning and memory may ultimately prove predictive of ATCT benefit.
In contrast to the APS improvement metrics, the number of levels completed metric was marginally associated with baseline auditory attention only, suggesting that this metric is less sensitive than the APS improvement metrics to frontotemporal plasticity, and instead may reflect participants’ capacity for task engagement and ability to selectively attend to training exercises. Given that matching or surpassing one’s baseline score is the only requirement for progressing through the training levels, the total number of levels completed does not account for the extent to which auditory learning has occurred – it merely indicates that it has occurred. In addition, the number of levels completed may be susceptible to influence from various non-cognitive factors, such as participant motivation, fatigue, need for breaks, etc., and it therefore appears to lack the richness of the APS improvement metrics for quantifying auditory learning.
With regard to identifying the SZ patients for whom ATCT is the “right” intervention, data from the present study hold some interesting implications that should be explored further in future research. Importantly, the lack of relationships between APS improvement, patient demographics, and clinical characteristics suggests that even older and more symptomatic patients may indeed benefit from the intervention. Although relatively strong verbal memory at baseline may signal the presence of frontotemporal plasticity necessary for cognitive enhancement, it is imperative to note that our preliminary findings do not suggest that patients with verbal memory impairments will not benefit from ATCT. In fact, patients in our sample who performed below the median score on the CVLT-II still demonstrated APS improvements that differed significantly from zero (t(16)=16.73, p<.001). It simply remains unclear whether, after a prolonged course of ATCT, these comparatively small APS improvements would translate into the same degree of cognitive enhancement as might be expected in less impaired subjects. A longer course of treatment may ultimately be required for patients with lower baseline “plasticity potential.” Alternatively, pharmacologic augmentation of ATCT (e.g. Light & Swerdlow, 2014; Swerdlow, 2011) may be beneficial for maximizing neuroplastic improvements in these patients. Furthermore, the notion that patients with relatively intact verbal memory may respond best to ATCT introduces the possible issue of cognitive ceiling effects. That is, if patients already perform relatively well on verbal memory measures at baseline, is there a need for training? Data from our sample show that a majority of patients (62%) performed at least one standard deviation below age- and education-matched nonpsychiatric controls in the domain of verbal memory (range: z=−3.12 to 0.71); thus, even patients with relatively high memory performance at baseline would still likely have “room” for improvement.
Despite the interesting implications of the present findings, it should be kept in mind that the relationship between APS improvement and verbal memory was only a medium-sized effect, and as previously noted, our study included the administration of only one hour of ATCT and one ATCT task (Sound Sweeps). Future research should examine these early indicators of auditory learning in the context of a therapeutic “dose” of ATCT in a larger sample to determine whether they can truly distinguish individuals who vary by degree of cognitive enhancement. Other limitations of the present study include the restricted range of perceptual improvements permitted within the Sound Sweeps exercise (i.e. participants automatically progressed to the next training level after reaching a predetermined perceptual threshold beyond their baseline score – the best scores obtained by participants may therefore underestimate their “true” auditory learning capacity). The present study also lacked experimental control of medications and nicotine use. Medication-related exclusion criteria were omitted in order to improve generalizability of findings to real-world community-dwelling patients. Patients in the study were prescribed heterogeneous medication regimens, including agents that may enhance or blunt ATCT’s effects (e.g. anticholinergic medications; Vinogradov et al., 2009). Randomized controlled trials are needed to experimentally evaluate the potential effects of medication or other pharmacological agents on ATCT response. Finally, multiple analyses of highly intercorrelated performance metrics were conducted in the present study. Given the small sample size, these analyses are considered exploratory, and it is possible that – in a larger sample – more conservative tests controlling for multiple comparisons might yield different results. Again, additional research is needed to verify the preliminary findings presented here. Nevertheless, as shown in Figure 3, the strong associations among the Hour 1 baseline and best APS metrics, attention, and working memory can be interpreted with a high degree of confidence and, at minimum, our findings suggest that the ATCT exercises target functionally-relevant cognitive domains in SZ.
Figure 3.
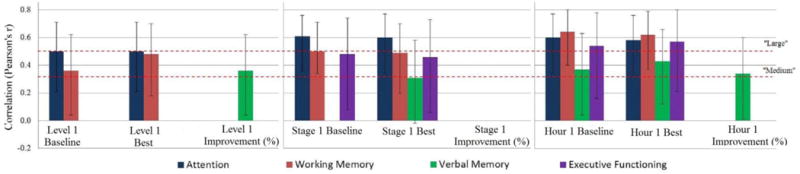
“Medium” and “Large” Correlations Among ATCT Performance Metrics and Cognitive Domains
This study is the first to demonstrate that the auditory system plasticity requisite for cognitive enhancement from ATCT in SZ may be evident as early as the initial training session. Our data provide tentative support for use of the APS improvement metrics, especially those averaged across Hour 1 of training, for their ability to “probe” the plasticity potential of the frontotemporal verbal learning network. Our data also suggest that relatively high verbal memory performance at baseline may serve as an additional indicator of plasticity potential and could thus predict the likelihood of eventual cognitive benefit from ATCT. Auditory ERP biomarkers that provide a direct “assay” of neural processes underlying auditory perception (e.g. Light & Makeig, 2015; Light & Swerdlow, 2014; Perez et al., 2014; Tarasenko et al., 2014) may prove more even sensitive than these behavioral measures in detecting individual differences in auditory learning during the initial stages of treatment; they therefore also hold great promise for predicting and monitoring response to ATCT and other forms of cognitive remediation. Studies are currently underway that examine the predictive utility of these biomarkers in the context of a randomized ATCT trial. Although we cannot yet determine the “right” conditions under which to prescribe ATCT, the present findings offer hope that ATCT or similar forms of cognitive remediation may one day be delivered as “precision” therapies for SZ.
Acknowledgments
The authors thank Ms. Marlena Pela for her assistance with data collection. This research is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, the Medical Research Service of the Veterans Affairs San Diego Health Care System, the Department of Veterans Affairs VISN-22 Mental Illness Research, Education, and Clinical Center (MIRECC), the Brain and Behavior Research Foundation (NARSAD), and by the National Institutes of Mental Health (UL1TR000100, MH42228, MH065571, MH094151, MH093453, MH094320, MH081944, MH59803).
Role of the Funding Source
Study sponsors played no role in study design; data collection, analysis, or interpretation; manuscript preparation; or the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Dr. Tarasenko designed the study, conducted the analyses, and prepared the manuscript. Dr. Perez assisted with data collection and interpretation and manuscript preparation. Mr. Pianka assisted with data collection and manuscript preparation. Dr. Vinogradov consulted on study methods and assisted with data interpretation and manuscript preparation. Dr. Braff provided resources to assist with study completion and assisted with manuscript preparation. Dr. Swerdlow assisted with data interpretation and manuscript preparation. Dr. Light provided resources for study completion and assisted with study conceptualization, design, data interpretation, and manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflicts of Interest
Dr. Vinogradov is a paid consultant to Brain Plasticity Inc., a company with a commercial interest in cognitive training software. Dr. Swerdlow is a consultant for Genco Sciences, Inc. Dr. Light has served as a consultant for Astellas, Forum, and NeuroVerse for matters unrelated to this study. Drs. Tarasenko, Perez, Braff and Mr. Pianka report no biomedical financial interests or potential conflicts of interest.
References
- Adcock RA, Dale C, Fisher M, Aldebot S, Genevsky A, Simpson GV, Nagarajan S, Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35(6):1132–41. doi: 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) University of Iowa; Iowa City: 1984a. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) University of Iowa; Iowa City: 1984b. [Google Scholar]
- Delis DC, Kramer JK, Kaplan E, Ober BA. California Verbal Learning Test. 2nd. Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J, Benjamin L. Structured clinical interview for DSM-IV Axis II disorders (SCID-II, Version 2.0) New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166(7):805–11. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: An interim report on the effects 6 months later. Schizophr Bull. 2010;36(4):869–79. doi: 10.1093/schbul/sbn170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Loewy R, Carter C, Lee A, Ragland JD, Niendam T, et al. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophr Bull. 2015;41(1):250–8. doi: 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Balogh SA, Wehner JM, Jones KR. Learning deficits in forebrain-restricted brain-derived neurotrophic factor mutant mice. Neuroscience. 2003;121:341–354. doi: 10.1016/S0306-4522(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153(3):321–30. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff?”. Schizophr Bull. 2000;26(1):119–36. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtis G. Manual, revised and expanded. Odessa: Psychological Assessment Resources Inc; 1993. Wisconsin Card Sorting Test (WCST) [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98(6):739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–75. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo Y, Kasai K, Kudo N, Rogers MA, Nakagome K, Itoh K, Kato N. Phonetic mismatch negativity predicts verbal memory deficits in schizophrenia. NeuroReport. 2006;17(10):1043–6. doi: 10.1097/01.wnr.0000221828.10846.ba. [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Vinogradov S, Medalia A, Buckley PF, Caroff SN, D’Souza DC, et al. Feasibility and pilot efficacy results from the multisite Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) randomized controlled trial. J Clin Psychiatry. 2012;73(7):1016–1022. doi: 10.4088/JCP.11m07100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. Professional Manual. Psychological Assessment Resources, Inc; Lutz, FL: 2000. Wisconsin Card Sorting Test – 64 Card Version. [Google Scholar]
- Lee J, Green MF, Calkins ME, Greenwood TA, Gur RE, Gur RC, et al. Verbal working memory in schizophrenia from the Consortium on the Genetics of Schizophrenia (COGS) Study: The moderating role of smoking status and antipsychotic medications. Schizophr Res. 2015;163(1–3):24–31. doi: 10.1016/j.schres.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Light GA, Makeig S. Electroencephalographic biomarkers of psychosis: Present and future. Biol Psychiatry. 2015;77(2):87–9. doi: 10.1016/j.biopsych.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR. Neurophysiological biomarkers informing the clinical neuroscience of schizophrenia: Mismatch negativity and prepulse inhibition of startle. Curr Top Behav Neurosci. 2014;21:293–314. doi: 10.1007/7854_2014_316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19(10):1624–32. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahncke HW, Bronstone A, Merzenich MM. Brain plasticity and functional losses in the aged: Scientific bases for a novel intervention. In: Møller AR, editor. Progress in Brain Research. Elsevier B.V; 2006. pp. 81–109. [DOI] [PubMed] [Google Scholar]
- Menning H, Roberts LE, Pantev C. Plastic changes in the auditory cortex induced by intensive frequency discrimination training. Neuroreport. 2000;11(4):817–22. doi: 10.1097/00001756-200003200-00032. [DOI] [PubMed] [Google Scholar]
- Merzenich M, Nahum M, Van Vleet T. Changing Brains: Applying Brain Plasticity to Advance and Recover Human Ability. Elsevier; 2013. [Google Scholar]
- Merzenich MM, Van Vleet TM, Nahum M. Brain plasticity-based therapeutics. Front Hum Neurosci. 2014;8:1–16. doi: 10.3389/fnhum.2014.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia. Am J Psychiatry. 2007;164(12):1791–802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy NV, Mahncke H, Wexler BE, Maruff P, Inamdar A, Zucchetto M, et al. Computerized cognitive remediation training for schizophrenia: An open label, multi-site, multinational methodology study. Schizophr Res. 2012;139:87–91. doi: 10.1016/j.schres.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Perez VB, Swerdlow NR, Braff DL, Näätänen R, Light GA. Using biomarkers to inform diagnosis, guide treatments and track response to interventions in psychotic illnesses. Biomark Med. 2014;8(1):9–14. doi: 10.2217/bmm.13.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov TG, Carolus A, Schubring D, Popova P, Miller GA, Rockstroh BS. Targeted training modifies oscillatory brain activity in schizophrenia patients. NeuroImage: Clinical. 2015;7:807–814. doi: 10.1016/j.nicl.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA. Specific cognitive training normalizes auditory sensory gating in schizophrenia: A randomized trial. Biol Psychiatry. 2011;69:465–471. doi: 10.1016/j.biopsych.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Popov T, Rockstroh B, Weisz N, Elbert T, Miller GA. Adjusting brain dynamics in schizophrenia by means of perceptual and cognitive training. PLoS ONE. 2012;7(7):e39051. doi: 10.1371/journal.pone.0039051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissling AJ, Miyakoshi M, Sugar CA, Braff DL, Makeig S, Light GA. Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. Neuroimage Clin. 2014;6:424–37. doi: 10.1016/j.nicl.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: Results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR. Are we studying and treating schizophrenia correctly? Schizophr Res. 2011;130:1–10. doi: 10.1016/j.schres.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Rissling AJ, Pascual-Marqui R, Kirihara K, Pela M, Sprock J, et al. Neural substrates of normal and impaired preattentive sensory discrimination in large cohorts of nonpsychiatric subjects and schizophrenia patients as indexed by MMN and P3a change detection responses. Neuroimage. 2013;66:594–603. doi: 10.1016/j.neuroimage.2012.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko MA, Swerdlow NR, Makeig S, Braff DL, Light GA. The auditory brainstem response to complex sounds: a potential biomarker for guiding treatment of psychosis. Front Psychiatry. 2014;5:142. doi: 10.3389/fpsyt.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology Reviews. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–62. doi: 10.1176/appi.ajp.2009.09010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168(5):472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]


