Abstract
In spite of hyporesponsivity to Mycobacterium leprae, borderline lepromatous (BL) patients show clinical and immunological instability, and undergo frequent acute inflammatory episodes such as type 1 reaction (T1R), which may cause nerve damages. This work focused on the participation of T cell subsets from blood and skin at T1R onset. We observed a significantly increased ex vivo frequency of both effector and memory CD4+ and CD8+ T cells in T1R group. Besides, ex vivo frequency of T cell homing receptor, the Cutaneous Leukocyte-associated Antigen (CLA) was significantly increased in T cells from T1R patients. M. leprae induced a higher frequency of CD4+ TEM and CD8+ TEF cells, as well as of CD8+/TEMRA (terminally differentiated effector T cells) subset, which expressed high CD69+. The presence of IFN-γ‒producing-CD4+ TEF and naïve and effector CD8+ T lymphocytes was significant in T1R. TBX21 expression was significantly higher in T1R, while BL showed increased GATA3 and FOXP3 expression. In T1R, TBX21 expression was strongly correlated with CD8+/IFN-γ‒ T cells frequency. The number of double positive CD8+/CLA+ and CD45RA+/CLA+ cells was significantly higher in skin lesions from T1R, in comparison with non-reactional BL group. The observed increase of ex vivo T cells at T1R onset suggests intravascular activation at the beginning of reactional episodes. The antigen-specific response in T1R group confirmed the higher number of CD8+/CLA+ and CD45RA+/CLA+ cells in T1R lesions suggests possible migration of these cells activated by M. leprae components inside the vascular compartment to skin and participation in T1R physiopathology.
Introduction
Leprosy is a chronic infectious disease caused by the obligate intracellular pathogen Mycobacterium leprae, which affects about 200,000 new individuals worldwide [1]. M. leprae preferably infects skin macrophages and Schwann cells from peripheral nerves, and the variety of clinical and pathological features of the disease according to the host immune response gives rise to a spectrum of polar forms. At the lepromatous pole, patients showing anergy or hyporesponsivity to M. leprae antigens and present disseminated lesions with high bacillary load, as opposed to tuberculoid ones, who exhibit a preserved specific cellular immune response, with limited lesions and a restricted growth of the pathogen. The so-called borderline forms (BL, BB and BT) are intermediary and range between the two poles [2].
The major cause of deformities and neural disabilities in leprosy relates to immune reactions that affect 30–50% of patients during the clinical course of the disease. Reactional episodes are characterized by a sudden, intense and unregulated inflammatory response, being subdivided into Reversal Reaction (T1R or RR) and Erythema Nodosum Leprosum (T2R or ENL) [3, 4].
Although the triggering mechanisms of such reactions still require a better clarification, some studies describe risk factors that would be related to the development thereof, such as the bacillary load and the clinical forms. However, literature also suggests other factors, such as age, gender and the presence of co-infections, and several combinations between them may be related to the type of reaction under examination [4, 5].
T1R presents a gradual development, and its natural course may last several weeks. It primarily affects borderline patients, being rarely detected in polar lepromatous patients. As to its clinical aspects, T1R is characterized by an increased inflammatory process in pre-existing skin lesions, as well as by the appearance of new granulomatous lesions and localized set of symptoms [5]. In T1R patients, cell-mediated immune response is the predominant cause of neuritis, and, if not suitably treated with corticosteroids, it provokes disabilities and deformities. Indeed, T1R is the leading cause of physical impairment in leprosy [6].
Among borderline patients, immunopathology of T1R is still poorly understood and most studies do not discriminate borderline forms [7], [8] BL patients are clinically unstable and should be studied on a separate basis. While BT skin lesions show granuloma formation with a predominance of epithelioid and giant cells without M. leprae, BL patients present a diffuse inflammatory infiltrate, with a large number of T lymphocytes in the dermis and abundant bacilli. At the onset of T1R clinical symptoms, BL patients also develop granulomas in the skin lesions and produce mediators of the inflammatory response [9, 10], suggesting that T cells can be activated and play a critical role in the immunopathogenesis of these episodes.
The most recent studies on T-cell participation in leprosy only encompass the characterization of responses to different M. leprae antigens, almost always combined with sorologic tests, aiming at finding a biomarker of exposure to the pathogen and to the early diagnosis of the infection [11, 12]. Originally described by Sallusto et al., T-cell subsets are differentiated according to the expression of surface molecules [13]. Among them, one should particularly refer to CCR7 and CD45RA. Thus, TNAÏVE cells present CCR7+/CD45RA+ phenotype, central memory (TCM) are CCR7+/CD45RA-, effector memory (TEM) are CCR7-/CD45RA-, and effector cells (TEF) are CCR7-/CD45RA+ [14, 15]. Several subsets of T-cells have been showing a relevant participation in the immunopathology of infectious diseases, including memory T-cells, which used to be well-known only by virtue of the protective role played by them [16, 17]. However, there is still a few number of studies on the effective participation of different T-cells subsets in the pathogenesis of leprosy per se, as well as on the reactional episodes thereof.
In this context, the main purpose of our work was to determine the participation of ex vivo and in vitro T-cell response to M. leprae in blood and skin lesions from BL patients at the onset of T1R. Indeed, evaluations of the T-cell phenotype with special attention to activation/homing, cytokine production and memory profile were performed as a possible contribution to understand the pathogenesis of T1R in this form of leprosy.
Material and Methods
Ethical considerations
The study was approved by the Institutional Ethics Committee of the Oswaldo Cruz Foundation/FIOCRUZ (permit protocol number 518/09) and an informed written consent was obtained from all individuals prior to specimen collection.
Studied population
This study included 32 individuals, among whom 12 were BL patients with T1R (immediately after diagnosis of the reactional episode and without use of immunosuppressant drugs), 10 were non-reactional BL patients immediately after diagnosis and before the beginning of multidrugtherapy (MDT). All patients were diagnosed according to Ridley and Jopling [18] criteria and accompanied at Leprosy Outpatient Unit–FIOCRUZ. We also used blood samples from 10 healthy volunteers (HV) with the same social-economic background as the patients and living in Rio de Janeiro city, which is known to be endemic for leprosy. Neither leprosy patients below 15 years-old nor any other affected by acute or chronic infectious comorbidities were included in this study. For the sake of privacy and well-being of the studied individuals, we refrained from disclosing their identity.
PBMC collection and culture and in vitro stimulation assays
Peripheral blood mononuclear cells (PBMC) were obtained under endotoxin free conditions from heparinized venous blood of patients and healthy donors in Ficoll-Hypaque (GE Healthcare, Uppsala, AB, Sweden) density centrifugation. After a separation, part of freshly isolated PBMC were resuspended at 1x106/mL in PBS for ex vivo analysis and the remaining were cultured in AIM V (Gibco BRL, Gaithersburg, MD, USA) at 5 x 105 of cultured PBMC/well for 6 hours in 96-well U bottom culture plates (Costar, Cambridge, MA, USA) in the presence of 1μg/mL anti-CD28 and anti-CD49d co-stimulatory molecules (BD Bioscience, San José, CA, USA). Then, the cells were stimulated with 1μg/mL of enteroxin B from Staphylococcus aureus (SEB, Sigma, St. Louis, MO, USA) or 20μg/mL of irradiated and sonicated armadillo-derived M. leprae (ML; supplied under the agreement NIH/NIAID contract N01 AI-25469 with Colorado State University, CO, USA). For the assays of intracellular cytokines detection, the cultures were kept at 37°C with 5% CO2 and 70% humidity, and, during the last two hours, 10 μg/mL of the protein transport inhibitor brefeldin A was added (GolgiPlug, BD Bioscience). The kinetics of responses to M. leprae and SEB were previously determined in healthy volunteers, reaching a peak at 6 hour-cultures.
Analysis of surface molecules and intracellular cytokines on CD4+ and CD8+ T subsets by flow cytometry
Newly obtained PBMC (ex vivo) together with the 6-hour cultures were resuspended in PBS (Gibco) 0,02% ethylenediamine tetraacetic acid (EDTA; Sigma) and stained with DAPI (Live/Dead Kit, Invitrogen, Grand Island, NY, USA) for separation of dead cells, according to the manufacturer’s instructions. Briefly, PBMC were incubated with DAPI (Invitrogen) for 30 minutes, washed by centrifugation and once more incubated in PBS containing Fc-receptor blocking solution (Biolegend Inc., San Diego, CA, USA). After a new wash step, PBMC were resuspended in flow cytometry buffer (PBS with 1% FCS and 0.01% sodium azide, all from Gibco) and incubated for 30 minutes at 4°C, with surface monoclonal antibodies anti-CD3 V500, anti-CCR7 PerCp, anti-CD4 or anti-CD8 APC, anti-CD69 APC-Cy7, anti-CD45RA Alexa Fluor 488 and anti-CLA FITC (all 1:50 dilution; Biolegend). Appropriate isotype controls (Biolegend) were used in all analysis. Then, PBMC were resuspended in 1% paraformaldehyde (PA; Sigma) and incubated for 30 minutes at 4°C. 6-hour cell cultures were resuspended in 1:10 permeabilization buffer (PERM-2; BD Biosciences), homogenized and incubated for 10 minutes at room temperature. After this period, PBMC were washed by centrifugation and resuspended in flow cytometry buffer (as previously described). Then, PBMC were stained with monoclonal antibodies for intracellular cytokines anti-IFN-γ PE-Cy7, anti-TNF Alexa700, anti-IL-10 PE and their respective isotype controls (Biolegend) for 30 minutes at 4°C. After other washes by centrifugation, PBMC were resuspended in 1% PA (Sigma). The cells were acquired on a FACSAria (with DIVA Software, BD Biosciences); 20,000 events/sample were acquired within the lymphocyte region for the ex vivo, and 50,000 events/sample for the 6-hour cultures. In flow cytometric analyzes, results are reported as % of median ± standard error of the median (SEM) for ex vivo data and range and quartiles (25th and 75th percentile) for data referring to cytokines-producing T cells.
PAXgene whole blood RNA extraction and quantitative real time PCR (qRT-PCR)
Whole blood was obtained by venous puncture and stored in PAXgene tubes (BD Biosciences) at -80°C, for a period below six months. Whole RNA was prepared using PAXgene Blood RNA Kit (Qiagen) according to the manufacturer’s instructions. RNA was quantified on a Nanodrop ND-1000 spectrophotometer (Nanodrop, Wilmington, DE, USA). cDNA synthesis carried out using the Superscript III RT-PCR kit (Applied Biosystems, Branchbug, NJ, USA). Taqman real time PCRs were performed via the universal PCR Master Mix (x2) and specific primers and probes (Applied Biosystems). Briefly, PCR was performed in the ABI Prism 7000 sequence detection system (Applied Biosystems) at 50°C for 2 min, 95°C for 10 min, 45 cycles of 95°C for 15 s, and 60°C for 1 min. The studied genes were TBX21 (Hs00203436), GATA3 (Hs00203436), RORC (Hs01076122), STAT3 (Hs00374280), STAT4 (Hs00374280), STAT6 (Hs00598625) and FOXP3 (Hs01085834). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 5’-CCGCATCTTCTTGTGCAGTG-3’) was used as an endogenous control and mRNA were quantified using the ΔCt method [ΔCt = Ct (target gene)—Ct (endogenous gene)]. qPCR conditions were the same as described above for the gene expression analysis (Applied Biosystems).
Immunofluorescence studies
Punch skin biopsy (6 mm diameter) was obtained from BL patients at the onset of disease and/or before treatment of T1R leprosy. Skin specimens were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetechnical, Tokyo, Japan), and snap frozen in liquid nitrogen. Longitudinal sections were cut at 16 μm on a cryostat (Leica CM 1850, Germany), mounted on gelatin-coated slides, and fixed in cold acetone for 15 minutes. Sections were washed 3 times with 10 mM PBS, pH 7.4, and then blocked by incubation with 10% normal goat serum (NGS, Invitrogen, USA) and 1% BSA (Bovine Serum Albumin, Sigma) for 1 h at room temperature. Incubation with the primary antibodies was performed overnight at 4°C in a humidity chamber, as such anti-CD4 (clone RPA-T4, isotype mouse IgG1), anti-CD8 (clone SK1, isotype mouse IgG1), anti-CD45RA (clone UCHL1, isotype mouse IgG2b) and anti-CLA (clone HECA-452, isotype rat IgM), all of them BD Pharmingen, were used in a 1:25 dilution. After wash with 10 mM PBS pH 7.4 (3 x 5 min each), sections were incubated at 90 min with rabbit anti-rat Alexa Fluor 488-conjugated and rabbit anti-mouse Alexa Fluor 633-conjugated, all obtained from Invitrogen and all at 1:200 dilution, in a humidity chamber, at room temperature. Secondary antibodies alone were used as negative controls. After a final series of washes in 10 mM PBS pH 7.4 (3 x 5 min each), cell nuclei were counterstained with DAPI (4’, 6’-diamidino-2-phenylindole, Sigma-Aldrich), and each coverslip was placed upside down on a slide containing a drop of SlowFade Antifade solution (Molecular Probes, OR, USA). The immunofluorescence analysis was performed with an Axio Observer Z1 Colibri microscope (Zeiss, Göttingen, Germany). For quantitative analysis of CD4+, CD8+ and CD45+ cells either expressing or not CLA, 10 microscopic fields were imaged and the number of positive cells was counted in each field. The results were obtained through a mean of fields’ counts, as determined by two independent observers. Images were processed via AxioVision software (Zeiss).
Statistical and graphic analysis
The data were analyzed using GraphPad Prism version 6.0 (GraphPad, San Diego, CA, USA). The nonparametric Kruskal-Wallis test with post-test Dunns were employed to determine differences between stimulated (ML or SEB) and unstimulated cells (UNS). Mann–Whitney test was used to group comparisons, and Pearson’s test for a correlation analysis. We used a statistical significance level of p<0.05.
Results
Patients’ demographic and clinical features
According to Ridley and Jopling criteria [18], all leprosy patients examined in this study were classified by two experienced pathologists as borderline lepromatous (BL) or T1R. The mean age did not present a significant variation, as well as the gender of the studied individuals. T1R group showed a mean age of 41.25 years-old (ranging from 15 to 69 years-old), while BL group presented a mean age of 44.3 years-old (ranging from 18 to 63 years-old). Healthy volunteers (HV) showed a mean age of 38.3 (ranging from 20 to 59 years-old). As to T1R, 41.7% (n = 5) already presented the episode at the diagnosis of the disease and not treated until then, while 58.3% (n = 7) presented T1R during MDT. In T1R group, the mean of baciloscopic index (BI) corresponded to 2.27 (ranging from 1.25 to 3.75) while among non-reactional BL patients this mean corresponded to 3.28 (ranging from 2.5 to 4). In relation to the lepromin skin test (LST), all BL patients showed negative results, while only 25% (n = 3) of T1R patients were positive (≥ 5.0mm). As regards disability grade (DG), 83.3% (n = 10) of T1R patients already showed a loss of sensitivity or disabilities resulting from the disease, while among BL 60% (n = 6) already presented some kind of impairment (Table 1).
Table 1. Identification of study populations.
| IDa | Form of leprosy | Gender | Age (ys.) | BIb | LSTc | DGd | MDTe (number of doses) |
|---|---|---|---|---|---|---|---|
| HR001 | T1R | F | 69 | 1.75 | NEG | 1 | 10° |
| HR002 | T1R | F | 25 | 2.5 | NEG | 1 | 8° |
| HR003 | T1R | M | 28 | 1.75 | NEG | 2 | 8° |
| HR004 | T1R | M | 22 | 2.5 | POS | 0 | 9° |
| HR005 | T1R | F | 29 | 1.5 | NEG | 1 | NTf |
| HR006 | T1R | M | 54 | 3.75 | NEG | 3 | NT |
| HR007 | T1R | F | 68 | 2.5 | POS | 2 | NT |
| HR008 | T1R | M | 59 | 2.5 | NEG | 2 | 7° |
| HR009 | T1R | F | 42 | 1.5 | NEG | 1 | NT |
| HR010 | T1R | F | 28 | 2.25 | NEG | 0 | 7° |
| HR011 | T1R | F | 56 | 3.5 | NEG | 3 | NT |
| HR012 | T1R | M | 15 | 1.25 | POS | 1 | 9° |
| BS001 | BL | M | 29 | 3.75 | NEG | 2 | NT |
| BS002 | BL | M | 61 | 4.0 | NEG | 2 | NT |
| BS003 | BL | F | 63 | 3.75 | NEG | 1 | NT |
| BS004 | BL | F | 56 | 2.75 | NEG | 1 | NT |
| BS005 | BL | M | 31 | 2.5 | NEG | 0 | NT |
| BS006 | BL | M | 33 | 3.0 | NEG | 0 | NT |
| BS007 | BL | F | 42 | 2.55 | NEG | 1 | NT |
| BS008 | BL | F | 53 | 3.75 | NEG | 0 | NT |
| BS009 | BL | M | 57 | 4.0 | NEG | 1 | NT |
| BS010 | BL | F | 18 | 2.75 | NEG | 0 | NT |
| SD001 | HV | F | 25 | - | - | - | - |
| SD002 | HV | F | 52 | - | - | - | - |
| SD003 | HV | F | 29 | - | - | - | - |
| SD004 | HV | F | 39 | - | - | - | - |
| SD005 | HV | F | 42 | - | - | - | - |
| SD006 | HV | M | 20 | - | - | - | - |
| SD007 | HV | M | 32 | - | - | - | - |
| SD008 | HV | M | 39 | - | - | - | - |
| SD009 | HV | M | 59 | - | - | - | - |
| SD010 | HV | M | 46 | - | - | - | - |
aID: randomized code for each patient or heathy subject in order to safeguard their identity.
bBI: bacteriological index.
cLST: lepromin skin test [NEG = negative (5.0 mm) and POS = positive (≥5.0 mm)].
dDG: disability grade.
eMDT: multidrug therapy currently recognized by WHO (12 doses).
fNT: not treated.
Determination of ex vivo T lymphocytes subsets
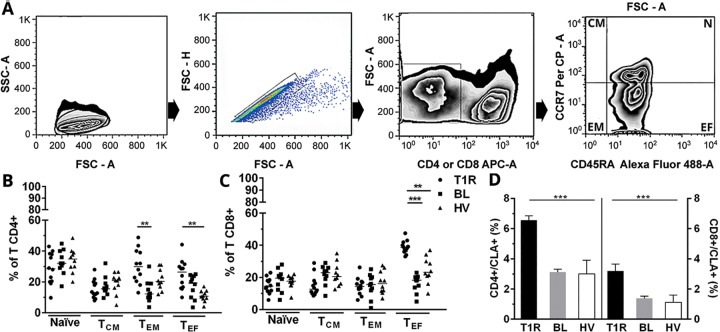
CD3+/CD4+ and CD3+/CD8+ blood T cells of BL patients with and without T1R were analyzed immediately after PBMC isolation, as well as of healthy volunteers, to characterize the predominant ex vivo T cell populations in the two groups, following to the analysis methodology shown in Fig 1A. According to our results, CD4+ T cell subsets presented significant alterations in TEM and TEF cells from the studied groups (p<0.01; Fig 1B). As to CD8+ T cells, except for the TEF cells (T1R versus BL p<0.001 and T1R versus HV p<0.01), other subsets showed no significant difference between groups (Fig 1C). Effector CD8+ T cell subsets from BL patients appear to be significantly increased at the onset of T1R, suggesting the participation of these cells at this reactional episode. To determine whether T1R alters the expression of Cutaneous Leucocyte-associated Antigen (CLA) molecules in circulating lymphocytes, CLA was evaluated ex vivo in CD4+ and CD8+ T cell subpopulations. In T1R patients, the CLA+ phenotype was significantly higher among T CD4+ than CD8+ T cells, but both showed significant differences when compared to BL and healthy volunteers (T1R versus BL and HV p<0.001; Fig 1D).
Fig 1. Increase of ex vivo CD4+ and CD8+ T subsets from BL patients at T1R.
(A) Methodology for analysis of lymphocytic subsets isolated from newly obtained peripheral blood (time zero, T0) in FACSAria flow cytometer. Exclusion of dead cells (kit Live/Dead) and determination of lymphocyte region (FSC-A versus SSC-A; first dot plot at left). Exclusion of cell clumps by FSC-H X FSC-A (second dot plot at left). Determination of CD3+/CD4+ or CD3+/CD8+ T cells regions, by using specific monoclonal antibodies and isotype control (third dot plot) and subset analysis by marking with anti-CD45RA Alexa Fluor 488 versus anti-CCR7 PerCP antibodies (first dot plot at right). Ex vivo frequencies of CD4+ (B) and CD8+ T subsets (C). Significantly increased ex vivo frequency of double positive CD4+/CLA+ and CD8+/CLA+ T cells in T1R patients (D). Results represent a median ± SEM of isolated experiments from T1R (n = 12), BL (n = 10) and HV (n = 10). Significance levels are shown by the graphs, being **p<0.01 and ***p<0.001. Mann–Whitney test; comparison between groups.
In vitro analysis of T-cell memory subsets in response to M. leprae
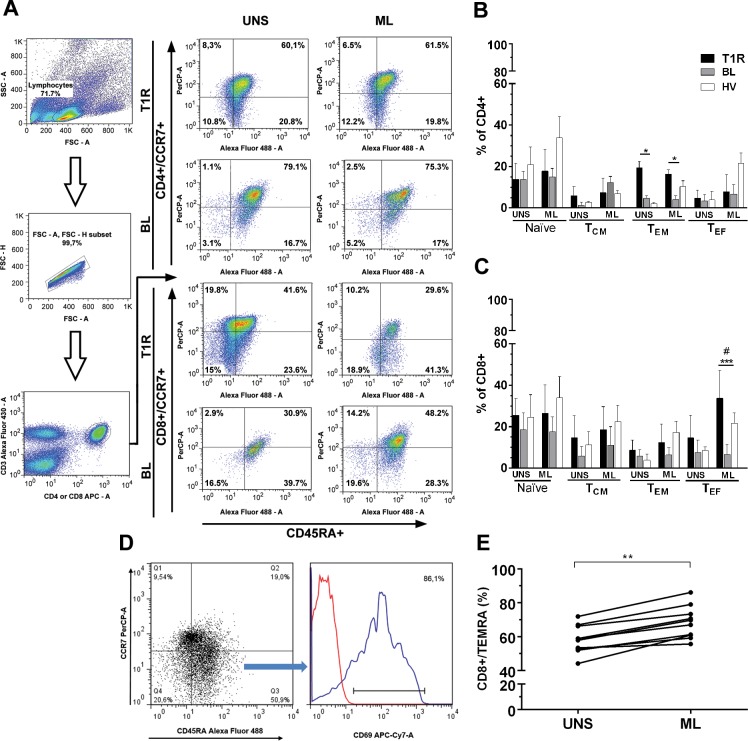
Then, in order to characterize the main subsets involved in the M. leprae-specific response shown by BL patients at the onset of T1R, we followed the cell marking technique mentioned in our methodology, as well as the standard analysis (Fig 2A). Upon comparison between the groups, the activation of CD4+ T cells by M. leprae did not undergo significant changes, except in TEM subsets. In this subset, the difference was significant among T1R and BL groups and also in T1R patients regardless of the culture conditions (UNS and ML, p<0.05; Fig 2B). Among CD8+ T cells, all subsets presented an increased activation threshold, even in unstimulated cultures. However, such values did not show significant differences in comparison with the two other groups evaluated under the same conditions. M. leprae induced a significant increase of CD8+ TEF in T1R group when compared to BL patients, (p<0.001), as well as to control group (p<0.05; Fig 2C). During the experiments, cultures were also stimulated with SEB (as positive control) and, as expected, all the results were positive (data not shown). The predominance of previously activated CD8+ T lymphocytes in T1R group, as well as after M. leprae stimulation, allows us to suggest that these subsets may contribute to the appearance of T1R. In addition to this finding, M. leprae also promoted an increase of CD8+/CD45RA+/CCR7-/CD69+high cells (Fig 2D), as known as terminally differentiated effector T cells (TEMRA) in T1R patients (p<0.05; Fig 2E). Finally, we note a negative correlation between M. leprae-specific CD8+ TEF cells, particularly between IFN-γ producing one, and the positivity to LST at the onset of T1R (r = -0.187, p = 0.47). These results are shown in S1 Fig.
Fig 2. M. leprae increases the percentage of activated CD4+ (TEM) and CD8+ (TEF and TEMRA) phenotypes in T1R patients.
(A) Analysis methodology, as described by Fig 1 legend, in addition to subsets distribution upon unstimulated 6h-culture (UNS) and with 20μg/mL M. leprae (ML). The bars represent medians of CD4+ (B) and CD8+ (C) T activated cells. The lines above the bars represent SEM of isolated experiments from R1T (n = 12), BL (n = 10) and HV (n = 10). Kruskal-Wallis test with post-test Dunns were employed to determine differences between stimulated with ML or SEB (data not shown) and unstimulated cells (UNS). Mann–Whitney test was used for comparison between groups. Significance levels are shown by the graphs, being *p<0.05, **p<0.01 and ***p<0.001. # indicates CD69+hi expressing TEF cells (%), as shown by figure D. (E) Among T1R patients, CD8+/TEMRA cells were increased by M. leprae (ML) in comparison with UNS.
Frequency of IFN-γ-, TNF- and IL-10-producing T cell subsets in response to M. leprae
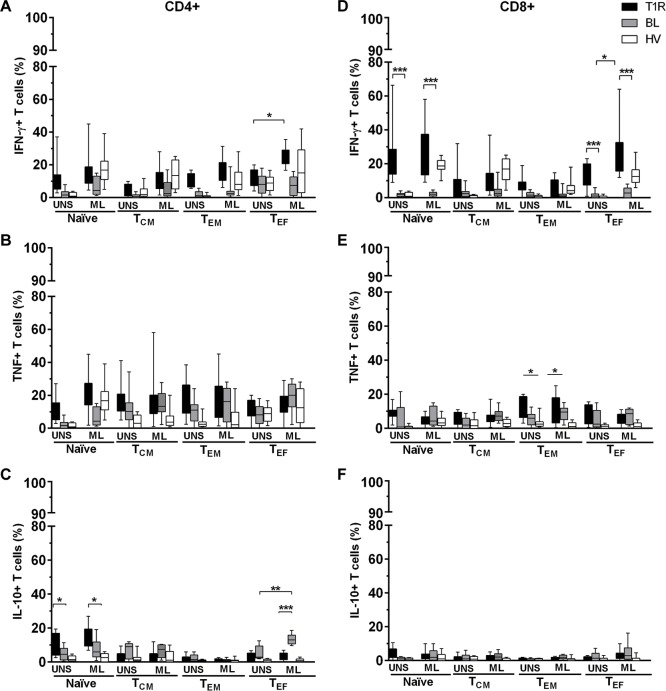
IFN-γ, TNF and IL-10 are mediators traditionally associated with leprosy pathogenesis per se. In order to evaluate the participation of these cytokine-producing T lymphocytes in T1R, both M. leprae -stimulated and unstimulated PBMC cultures were permeabilized, stained and analyzed by flow cytometry. We observed a general increase in the frequency of all IFN-γ-producing CD4+ T subsets from T1R group, regardless of culture conditions. However, CD4+/IFN-γ+ TEF in T1R group presented a significant difference between UNS- and ML-stimulated cells (p<0.05; Fig 3A). TNF-producing CD4+ T cells also presented a higher frequency in T1R group, both in unstimulated cultures and in response to M. leprae. However, there was no significant difference in relation to BL patients or to healthy volunteers. IL-10 producing CD4+ TNAÏVE were significantly increased in T1R group, in comparison with BL patients and with healthy volunteers. This finding was observed both in UNS- (p<0.05) and in ML-stimulated cultures (p<0.05; Fig 3C). IL-10+/CD4+ TCM and TEM did not changed under any culture conditions. M. leprae induced an increased frequency of CD4+/IL-10+ TEF in BL group (p<0.05; Fig 3C). As observed among CD4+ T cells, the expression of IFN-γ-producing CD8+ TNAÏVE also appeared to be significantly increased in T1R group in comparison with BL and HV subjects, under both culture conditions (p<0.05; Fig 3D). CD8+/IFN-γ+ TCM and TEM frequencies were also increased in T1R group in relation to BL patients and HV individuals. However, only IFN-γ-producing CD8+ TEF cells presented a significant difference between culture conditions and among the studied groups (p<0.05; Fig 3D). The frequency of TNF-producing CD4+ T subsets did not differ between studied groups. However, we observed a significant increase in TNF+/CD8+ TEM cells (p<0.05; Fig 3E). Finally, CD8+/IL-10+ T subsets also did not differ. As expected, SEB increased the frequency of all the studied cytokine-producing T cell subsets (Fig 3A–3F). As combined with prior data, these results suggest that, at the onset of the reactional episode, IFN-γ-producing T cells may have influenced the increased TEMRA phenotype expression found in T1R group. Among BL patients, the increased frequency of IL-10+ CD4+ T subsets may have exert a suppressive role by inducing an inhibitory effect on cell-mediated immune responses towards M. leprae.
Fig 3. IFN-γ- and TNF-producing CD4+ and CD8+ T cells are predominant in T1R group, while IL-10-producing CD4+ T cells are increased in non-reactional BL patients.
IFN-γ-, TNF- and IL-10-producing CD4+ (A-C) and CD8+ (D-F) T subsets in 6h-PBMC culture were: unstimulated (UNS), 20μg/mL M. leprae (ML) and 1μg/mL SEB (data not shown). Results are reported as % of median and interquartiles (25th and 75th percentile) in T1R (n = 12), BL (n = 10) and HV (n = 10) groups. Kruskal-Wallis with post-test Dunns were employed to determine differences between stimulated (ML or SEB) and unstimulated cells (UNS). Mann–Whitney test was used to group comparisons; *p<0.05, **p<0.01 and ***p<0,001.
Gene expression of transcription factors involved in T lymphocytes differentiation
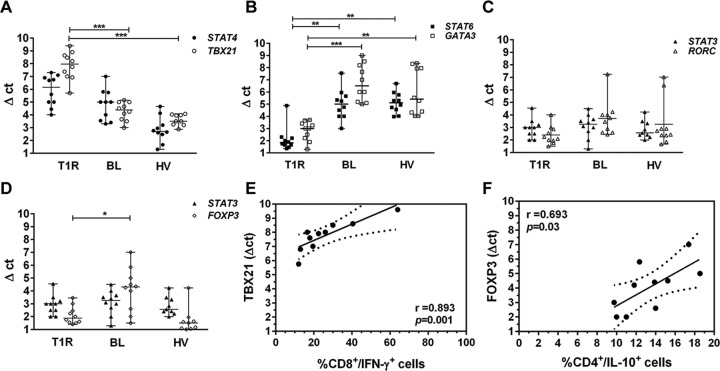
In order to evaluate the gene expression of transcription factors responsible for T lymphocytes differentiation, we carried out a real time qPCR with cDNA collected from peripheral blood obtained by PAXGene tube. We used STAT4/TBX21 gene pair to identify Th1, STAT6/GATA3 for Th2, STAT3/RORC for Th17 and STAT3/FOXP3 for Treg profile. mRNA expression of STAT4/TBX21 genes was increased in T1R group, in comparison with the same number of BL patients, as well as healthy volunteers (HV group). In fact, TBX21 per se was significantly higher among T1R patients (p<0.001; Fig 4A). Then, we evaluated STAT6/GATA3 expression, which was higher among BL patients (p<0.001) and HV group (p<0.05) than in T1R (Fig 4B), thus suggesting a tendency to the development of Th2 profile in BL and HV. As we observed the genes inducing a differentiation for Th17 profile, we noted a slight increase of RORC and STAT3 among non-reactional BL patients in comparison with T1R group, without significant difference (Fig 4C). The last transcription factor evaluated was FOXP3 expression, that presented a difference between the groups, as the values were significantly higher in BL patients than in T1R group (p<0.05; Fig 4D). As described above, STAT3 expression did not allow a discrimination between BL patients either affected or not by T1R. Likewise, it was not observed among healthy volunteers. Our gene expression analysis suggests T lymphocytes differentiation in T1R refers to Th1 phenotype, with an increased expression of TBX21, while, in non-reactional BL form, it relates to Th2 through an increase of STAT6 and GATA3. One should not rule out the participation of Treg among non-reactional BL patients, as FOXP3 expression was significantly increased in this group, in comparison with T1R patients (p<0.05; Fig 4D). As IFN-γ producing CD8+ TEF cells and TBX21 were significantly higher in T1R group, we considered a possible correlation between them. Fig 4E discloses a strong correlation obtained from such analysis (r = 0.8936, p<0.001). Likewise, we observed a significant positive correlation between IL-10-producing CD4+ TEF and FOXP3 in BL group (r = 0.693, p<0.05; Fig 4F). We still carried out a correlation analysis between gene expression of all the evaluated transcription factors and all studied cytokine-producing T lymphocytes subsets in T1R and Bl groups, but reached no significant results (data not shown).
Fig 4. Increased TBX21 in T1R group is associated with IFN-γ+-producing CD8+ T cells, while FOXP3 is associated with IL-10+-producing CD4+ T cells in BL patients.
Scattergram showing the expression of gene pairs STAT4/TBX21 (A), STAT6/GATA3 (B) STAT3/RORC (C) and STAT3/FOXP3 (D). Quantitative RT-PCR analysis in peripheral blood of T1R patients (open symbols) and BL (closed symbols) and HV (open triangles). The y-axis of each graph represents the relative expression of the respective genes calculated using the Δct method and normalized against GAPDH mRNA. Results are reported by Δct mean ± range of 10 independent samples of each group. *p<0.05, **p<0.01 and **p<0.001 (Mann–Whitney test). Spearman rank correlations of TBX21 expression with IFN-γ-producing CD8+ T cells from T1R group (E), and FOXP3 expression with IL-10-producing CD4+ T cells from BL patients (F) are shown.
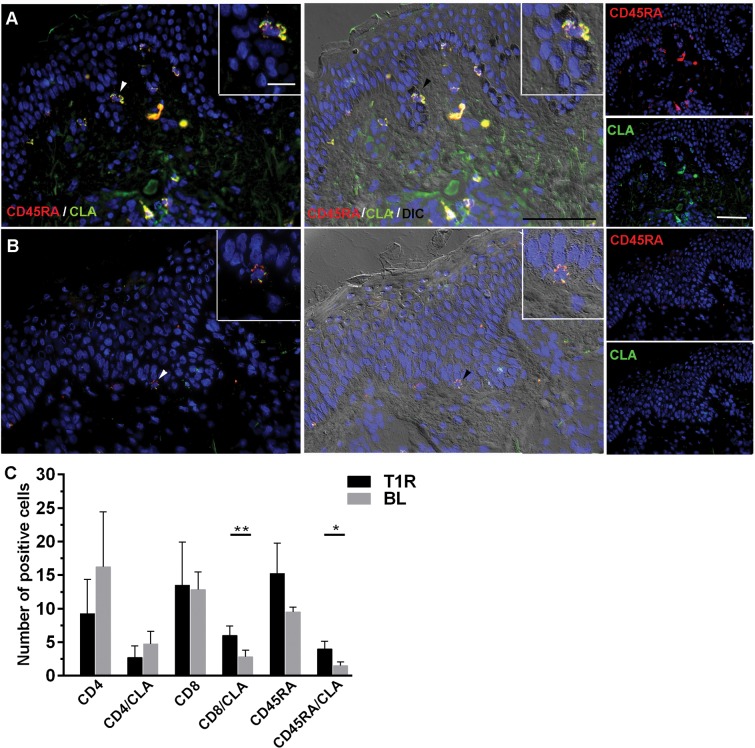
Measurement of T cell subsets and homing regulation marker in skin lesions by immunofluorescense
CLA (Cutaneous Leucocyte-associated Antigen), member of the selectin family, is expressed in T cells that can be driven to inflamed tissues through endothelial cells. In order to examine any possible regulation of CLA on T cell subsets in the context of T1R, we used immunofluorescence to analyze skin lesions in biopsies obtained from 6 studied patients (3 T1R and 3 BL). We observed a preferential distribution of colocalizated CD45RA+/CLA+ cells through the intraepithelial region of T1R lesions. Fig 5A shows a representative experiment of T1R studied patient. However, in non-reactional BL patients these cells were rare and CLA+ staining intensity was smaller (Fig 5B). With respect to the number of T cells in the lesions, as observed in Fig 5C, CD4+ T cells either expressing or not CLA were found in a higher number in lesions from BL patients compared with T1R group, although there was no significant difference. However, CD8+ cells were detected in a higher number in reactional lesions, as well as CD8+/CLA+ T cells, which appeared to be more significantly present in these inflammatory infiltrates (p<0.01). Likewise, in terms of maturation profile, CD45RA+ cells were found in a higher number in lesions from T1R patients. Finally, in relation to CD45RA+/CLA+ cells, the difference between the groups was significant (p<0.05). According to the cell distribution in the lesions, it is possible that double-positive cells (CD45RA+/CLA+) detected in a higher number in T1R group are CD8+ T cells, being thus compatible with TEMRA phenotype as observed in the blood from such patients. A relevant limitation for the demonstration is this hypothesis lies on the impossibility of evaluating CCR7 marker in skin lesions.
Fig 5. CD8+/CLA+ and CD45RA+/CLA+ T lymphocytes are predominantly expressed in T1R lesions.
Immunofluorescence assays were performed to determine the number of T cell markers (CD4, CD8 and CD45RA) expressing or not CLA. Representative images presented CD45RA+ (red, Alexa Fluor 633; upper right panels) and CLA+ (green, Alexa Fluor 488; lower right panels), colocalization (yellow, white arrow head and insert; left panels). The nuclei (blue) were stained using DAPI. Scale bar = 50 mm. Data are representative of 3 specimens of each group, being one T1R (A) and one BL patient (B). Images were visualized and obtained by a Zeiss Colibri fluorescent microscope. The graph shows single and double-positive CD4+/CLA+, CD8+/CLA+ and CD45RA+/CLA+ cells in T1R and BL skin infiltrates (C). *p<0.05 and **p<0.01 (Mann-Whitney test; group comparisons).
Discussion
In spite of the significant reduction of leprosy per se worldwide since MDT implementation, reactional states still remain a major public health concern. A reactivation of cellular immune response in borderline leprosy patients during T1R was previously described, although most studies do not discriminate borderline forms [7, 12, 13]. M. leprae-specific hyporesponsivity in blood leukocytes from classical BL patients is well known [10]. Nevertheless, as T1R patients show granulomatous skin lesions, one should not rule out the participation of T lymphocytes in the immunopathogenesis of such episodes [14]. Thus, considering that these individuals present different clinical and immunological features, this work only focused on BL patients who developed Type 1 Reaction (T1R), by comparing them with newly diagnosed patients (without reactional signs and symptoms) before MDT, as well as with healthy volunteers (HV).
In our study, T1R group was mostly composed by female individuals, showed a mean age below the non-reactional group. These data are in perfect accordance with works from other groups on risk factors to the onset of reactions [4, 5]. Of note, our T1R group also presents a high rate (83.3%) of patients with some constant level of disability, such as nerve impairment and physical disabilities. In relation to lepromin (LST), all non-reactional BL patients showed negative skin test, thus indicating hyporesponsiveness or anergy, while 25% of T1R patients were responsive, suggesting that both T1R and some BL individuals were more responsive to M. leprae antigens in the skin. Indeed, leprosy reactions may induce a strong release of antigens from M. leprae fragments deriving from the use of antimicrobial drugs. Nevertheless, we did not observe significant clinical and/laboratorial differences between patients who came to present T1R, in comparison with patients in treatment.
We analyzed PBMC cells phenotype just upon T1R diagnosis. The significant ex vivo increased frequency of CD4+ TEM/TEF and CD8+ TEF cells found in T1R group suggested an intravascular leukocyte activation at the onset of reactions. When compared to naïve or central memory T cells, these subsets, which are great IFN-γproducers, were already shown to be able to mount faster responses [19]. As still refers to findings from ex vivo PBMC, the significant increase of CLA+ cells both in CD4+ and CD8+ T cells in T1R group, in contrast to non-reactional BL group, corroborates the hypothesis that, in T1R, part of intravascular T cells may migrate to skin and develop local inflammatory response. Austin et al. showed that T cells directed to the skin via CLA may also mediate inflammatory responses and CLA+ T cells have previously been shown to be enriched in the inflammatory lesions of psoriasis where Th1 cytokine producing cells are thought to have a pathological role [20].
Therefore, we also aimed at the identification of M. leprae-specific T subsets in T1R patients. According to in vitro analysis, M. leprae induced a significantly increased frequency of activated CD4+ TEM and TEF, as well as of CD8+ TEF cells in this group. The venous blood collection from T1R individuals just upon appearance of early reactional signs and symptoms corroborates our findings concerning the activation of T cells still in blood. Prior studies indicate a participation of effector and effector memory T cells in the immunopathology of severe cases of both human pulmonary tuberculosis and cutaneous leishmaniosis, which are also caused by intracellular pathogens [21, 22]. Moreover, we observed that M. leprae induced a significantly increased CD69 expression in CD8+/CD45RA+/CCR7- T cells, and indicated terminally differentiated effector T cells (TEMRA) in T1R group. The high frequency of M. leprae-specific CD8+ TEMRA cells may be associated with T1R severity, as the two patients showing the highest CD69 expression (>85% of positive cells) also presented the most intense inflammatory response (HR006 and HR011 patients; data not shown). Similar findings were disclosed by Oliveira et al., who also demonstrated an increase in both CD4+ and CD8+ TEMRA cells in peripheral blood leucocytes in co-infected HIV/leprosy patients showing T1R. Authors also suggested that CD8+ TEM cells triggered T1R in HIV/leprosy patients [23].
To understand further the functional activity of T lymphocytes subsets, we characterized the frequency of IFN-γ, TNF and IL-10-producing T cells. The first two cytokines are known to be important in leprosy immune reactivation [8, 24], [25], while IL-10 is correlated with the pathogenesis of multibacillary forms [26, 27]. In T1R group, even without stimuli, we observed a higher frequency of IFN-γ-producing CD4+ TEF in comparison with BL and HV groups. M. leprae induced a significantly increased frequency of IFN-γ-producing CD4+ TEF. Likewise, the frequency of unstimulated and antigen-specific naïve and effector IFN-γ-producing CD8+ T cells was higher in T1R group. Such increased frequency of IFN-γ-producing T cells among T1R patients was consistent with prior studies, although such works were performed with skin lesions or assessed cytokine production in serum or in PBMC culture supernatants [7, 28].
Although more frequent in ENL, previous studies indicated that TNF mediates immune-pathologic effects, such as fever and tissue damage, in both type 1 and type 2 leprosy reactions [29, 30]. Our work did not find a significant increase of TNF-producing CD4+ T subsets, upon comparison of both culture conditions and studied groups. However, the frequency of TNF-producing CD8+ TEM cells was significantly increased in T1R patients in comparison with BL and HV groups. This finding occurred both in unstimulated cultures and in response to M. leprae. At the onset of reactions, T1R CD8+ TEM cells are possibly activated by M. leprae components, and may produce TNF and play a cytotoxic role. In another possibility not investigated in this study, such TNF-producing cells may be unconventional double-positive CD8/γδ T cells. A previous work from our group demonstrated an increased expression ofγδ+ T cells in lepromatous patients with severe type 2 reaction and increased TNF level in blood circulation [31].
Moreover, T1R group presented a significantly increased frequency of M. leprae-specific CD4+/IL-10+ TNAÏVE cells. Considering the marked IL-10 action on downregulation of inflammatory responses, through inhibition of Th1 responses, such result was surprising. IL-10 is produced by several T lymphocytes subsets, and is not subject to an epigenetic regulation, as is the case with IFN-γ [32]. Possibly, these IL-10-producing T cells could counterbalance the activation of IFN-γ- and TNF-producing-T cells in T1R. As to reactional group, the significantly increased frequency of M. leprae–induced CD4+/IL-10+ TEF is compatible with findings from other works that showed more abundant IL-10 levels produced by type 2 macrophages during mycobacterial infections, thus resulting in a decreased nitric oxide production and in enhanced intracellular bacterial growth [33, 34]. A recent family-based meta-analysis study actually confirmed an association between IL-10 promoter polymorphisms and leprosy. However, the authors did not study patients presenting reactional episodes [35].
As to cytokine participation in the immunoinflammatory response observed in T1R group, the increased frequency of IFN-γ-producing T cells may have influenced the higher expression of both effector and memory phenotypes found in the blood from these patients. Within this context, Obar et al., demonstrated that higher inflammatory levels and increased antigen-specific responses favored the generation of short-term CD8+ TEF cells [36]. On their turn, the maintenance of TEM requires ongoing antigen stimuli, and it is possible that, in T1R, intense fragmentation/death of M. leprae leads to the release of new epitopes resulting in enhanced antigen presentation, which activate such cells. Thus, our data shed light on how varying the context of T cell priming alters downstream effector and TEMRA CD8+ T cell differentiation in leprosy type 1 reaction.
Gene expression analysis of transcription factors for T lymphocytes differentiation showed a significant increase of TBX21 expression in T1R patients, in comparison with BL group. As we compared the two groups, STAT4 was also slightly increased, although this difference was not significant. TBX21, also designed T-bet, was proposed to be the master switch for Th1 development based on its IFN-γ induction and its direct activation of IFN-γ reporter activity [37, 38]. In leprosy, Quiroga et al. demonstrated by western blot that TBX21 is expressed in PBMC from BT patients, and correlated this finding with IFN-γ production in supernatants from M. leprae-stimulated cultures [38]. Our data suggest that the pro-inflammatory microenvironment found in T1R may favor an increased TBX21 expression. In contrast, BL patients displayed a significant increase of transcriptional factors pair of genes that had driven a Th2 differentiation (STAT6 and GATA3). It was already suggested that TBX21:GATA3 ratio may reflect Th1/Th2 cytokine balance, and that the lower is such ratio, the higher will be the differentiation for Th2 profile [39]. As expected, our study showed that this ratio was considerably reduced in BL group. Among these patients, we noted a significant increase of FOXP3, which is compatible with Th1 pattern inhibition. Both natural and induced Treg cells were observed in skin lesions and peripheral blood in lepromatous leprosy and the immune suppression observed during the course of the disease was linked to an increased FOXP3 expression [40]. In our work, we noted a strong correlation between TBX21 expression and the frequency of IFN-γ-producing CD8+ T cells, which corroborates our hypothesis as to the T cell activation in T1R group. The strong negative correlation between FOXP3 expression and IL-10-producing CD4+ T cells at the onset of T1R in our patients indicates a possible inhibition of Treg cells in T1R. Consistent with our observation, Geluk et al. showed a decrease in Treg subsets in a recent case report focusing a BL patient at the onset of T1R [41]. More recently, a work showed increased Th17 and reduced Treg cells among T1R patients. The authors associated such findings with an increased IL-17A gene expression, IL-17 and IL-6 levels, and with reduced gene expression of FOXP3 and accompanied by decrease of TGF-βproduction. Nevertheless, the studied T1R patients presented a BT form, which is known to differ from BL patients under immunological aspects [42]. Among non-reactional BL patients, we found a positive correlation between FOXP3 expression and IL-10-producing CD4+ T cells. In parallel, other authors demonstrated an increased CD4+/IL10+ Treg cell number in PBMC of lepromatous (BL/LL) patients and increased IL-10 levels in culture supernatants [43]. So far, our results allow us to hypothesize that T1R in BL patients is triggered by an early M. leprae activation of circulating effector and memory T cells. These cells may rapidly migrate towards skin lesions and nerve trunks, thus leading to the appearance of reactional symptoms.
A prior study from our group detected increased macrophages, epithelioid cells and dendritic cells in skin lesions from BL patients at T1R. Authors attributed such fact to an abrupt release of immune inflammatory mediators disrupting the predominantly immunosuppressive milieu in multibacillary leprosy [44]. In our work we found a significantly higher number of both double-positive CD8+/CLA+ T cells and CD45RA+/CLA+ in T1R skin biopsies. It is well known that CLA is expressed in T cells that can be driven to inflamed tissues, and that M. leprae specifically up-regulated CLA [45]. Of note, we observed a preferential distribution of co-localized cells through the intraepithelial sites of T1R lesions, bordering basal layer. These cells may have been possibly attracted to the skin by chemokines secreted by macrophages and/or keratinocytes. IFN-γ inducible protein CXCL10/IP-10 was demonstrated to be increased in T1R lesions [46]. In other study, immunohistochemistry analysis showed increased CCR5 (RANTES receptor) and CXCR2 levels in biopsy specimens from T1R patients [47]. Another possibility that still requires investigation from our group lies on the double-positive cells (CD45RA+/CLA+) detected in a higher number in T1R group, that may also be CD8+ T cells. Consistent with such a possibility, a recent work in cutaneous leishmaniosis demonstrated that both effector and memory T cells co-expressing CLA might potentially influence the cell composition of inflammatory infiltrate, thus contributing to the severity of the disease [48]. As this study was primarily focused on T1R patients, we understand that further deeper studies of skin lesions from BL patients before, during and after the reaction may clarify the nature of in situ interactions between potentially migrating and/or resident immune cells. In fact, skin lesions share similar features with peripheral blood mononuclear cells, and provide additional information on local immune responses that may be collaborating with T1R pathogenesis in BL leprosy.
Conclusions
Our work provides evidences of a potential role played by circulating CD4+ TEM and CD8+ TEF and TEMRA lymphocytes and pro-inflammatory cytokines at the onset of T1R in BL patients. In these patients, immune cell activation inside the lesion sites, such as skin and peripheral nerves, appears to be directly influenced by circulation. However, neither correlation nor significant difference between reactivity to cutaneous test, treatment status, disability grade and ex vivo or in vivo results were observed. Given the complex interaction between effector/memory T cells and cytokines/chemokines, longitudinal follow-up studies may be required to the clarification of such mechanisms.
Supporting Information
Negative correlation between cutaneous test for leprosy prognostic and CD8+/IFN-γ+ TEF cells frequency from T1R patients. Data from LST are shown in millimeters (mm) of cutaneous induration for a better visualization of results. Spearman correlation test.
(TIF)
Acknowledgments
Our recognition to the Program for Technological Development in Tools for Health—PDTIS/FIOCRUZ for use of its Flow cytometry (FACSAria) facility. To Katia Magalhaes for editing the text, Bernardo Pascarelli for the efficient help in formatting the references and Dayse Goes for the graphic work.
Data Availability
All relevant data are within the paper.
Funding Statement
LNS is a postgraduate student sponsored by FIOCRUZ/CAPES (process number 14.06.38.047). This investigation received financial support from the National Counsel of Technological and Scientific Development - CNPq (PAPES VI/Fiocruz, process number 407838/2012-0). ENS is fellow sponsored by CNPq (process number 305885/2014-6). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Global leprosy update, 2015: time for action, accountability and inclusion. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2015;91(35):405–20. Epub 2016/09/07. . [PubMed] [Google Scholar]
- 2.Talhari C, Talhari S, Penna GO. Clinical aspects of leprosy. Clinics in dermatology. 2015;33(1):26–37. Epub 2014/11/30. 10.1016/j.clindermatol.2014.07.002 . [DOI] [PubMed] [Google Scholar]
- 3.Nery JA, Vieira LM, de Matos HJ, Gallo ME, Sarno EN. Reactional states in multibacillary Hansen disease patients during multidrug therapy. Revista do Instituto de Medicina Tropical de Sao Paulo. 1998;40(6):363–70. Epub 1999/08/07. . [DOI] [PubMed] [Google Scholar]
- 4.Scollard DM, Smith T, Bhoopat L, Theetranont C, Rangdaeng S, Morens DM. Epidemiologic characteristics of leprosy reactions. International journal of leprosy and other mycobacterial diseases: official organ of the International Leprosy Association. 1994;62(4):559–67. Epub 1994/12/01. . [PubMed] [Google Scholar]
- 5.Nery JA, Bernardes Filho F, Quintanilha J, Machado AM, Oliveira Sde S, Sales AM. Understanding the type 1 reactional state for early diagnosis and treatment: a way to avoid disability in leprosy. Anais brasileiros de dermatologia. 2013;88(5):787–92. Epub 2013/11/01. 10.1590/abd1806-4841.20132004 ; PubMed Central PMCID: PMCPmc3798356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker SL, Lockwood DN. Leprosy type 1 (reversal) reactions and their management. Leprosy review. 2008;79(4):372–86. Epub 2009/03/12. . [PubMed] [Google Scholar]
- 7.Verhagen CE, Wierenga EA, Buffing AA, Chand MA, Faber WR, Das PK. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin: a follow-up study. Journal of immunology (Baltimore, Md: 1950). 1997;159(9):4474–83. Epub 1997/10/31. . [PubMed] [Google Scholar]
- 8.Stefani MM, Guerra JG, Sousa AL, Costa MB, Oliveira ML, Martelli CT, et al. Potential plasma markers of Type 1 and Type 2 leprosy reactions: a preliminary report. BMC infectious diseases. 2009;9:75 Epub 2009/05/29. 10.1186/1471-2334-9-75 ; PubMed Central PMCID: PMCPmc2696458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayanan RB, Laal S, Sharma AK, Bhutani LK, Nath I. Differences in predominant T cell phenotypes and distribution pattern in reactional lesions of tuberculoid and lepromatous leprosy. Clinical and experimental immunology. 1984;55(3):623–8. Epub 1984/03/01. ; PubMed Central PMCID: PMCPmc1535912. [PMC free article] [PubMed] [Google Scholar]
- 10.Sreenivasan P, Misra RS, Wilfred D, Nath I. Lepromatous leprosy patients show T helper 1-like cytokine profile with differential expression of interleukin-10 during type 1 and 2 reactions. Immunology. 1998;95(4):529–36. Epub 1999/01/20. ; PubMed Central PMCID: PMCPmc1364348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duthie MS, Orcullo FM, Abbelana J, Maghanoy A, Balagon MF. Comparative evaluation of antibody detection tests to facilitate the diagnosis of multibacillary leprosy. Applied microbiology and biotechnology. 2016;100(7):3267–75. Epub 2016/01/29. 10.1007/s00253-016-7328-8 . [DOI] [PubMed] [Google Scholar]
- 12.Martins MV, Guimaraes MM, Spencer JS, Hacker MA, Costa LS, Carvalho FM, et al. Pathogen-specific epitopes as epidemiological tools for defining the magnitude of Mycobacterium leprae transmission in areas endemic for leprosy. PLoS neglected tropical diseases. 2012;6(4):e1616 Epub 2012/05/01. 10.1371/journal.pntd.0001616 ; PubMed Central PMCID: PMCPmc3335884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. Epub 1999/10/28. 10.1038/44385 . [DOI] [PubMed] [Google Scholar]
- 14.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nature immunology. 2010;11(8):674–80. Epub 2010/07/21. 10.1038/ni.1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annual review of immunology. 2013;31:137–61. Epub 2012/12/12. 10.1146/annurev-immunol-032712-095954 . [DOI] [PubMed] [Google Scholar]
- 16.Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang AT, et al. Progression of clinical tuberculosis is associated with a Th2 immune response signature in combination with elevated levels of SOCS3. Clinical immunology (Orlando, Fla). 2014;151(2):84–99. Epub 2014/03/04. 10.1016/j.clim.2014.01.010 . [DOI] [PubMed] [Google Scholar]
- 17.Novais FO, Carvalho LP, Graff JW, Beiting DP, Ruthel G, Roos DS, et al. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS pathogens. 2013;9(7):e1003504 Epub 2013/07/23. 10.1371/journal.ppat.1003504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. International journal of leprosy and other mycobacterial diseases: official organ of the International Leprosy Association. 1966;34(3):255–73. Epub 1966/07/01. . [PubMed] [Google Scholar]
- 19.Moraes MO, Sarno EN, Almeida AS, Saraiva BC, Nery JA, Martins RC, et al. Cytokine mRNA expression in leprosy: a possible role for interferon-gamma and interleukin-12 in reactions (RR and ENL). Scandinavian journal of immunology. 1999;50(5):541–9. Epub 1999/11/17. . [DOI] [PubMed] [Google Scholar]
- 20.Austin LM, Ozawa M, Kikuchi T, Walters IB, Krueger JG. The majority of epidermal T cells in Psoriasis vulgaris lesions can produce type 1 cytokines, interferon-gamma, interleukin-2, and tumor necrosis factor-alpha, defining TC1 (cytotoxic T lymphocyte) and TH1 effector populations: a type 1 differentiation bias is also measured in circulating blood T cells in psoriatic patients. The Journal of investigative dermatology. 1999;113(5):752–9. Epub 1999/11/26. 10.1046/j.1523-1747.1999.00749.x . [DOI] [PubMed] [Google Scholar]
- 21.Keshavarz Valian H, Nateghi Rostami M, Tasbihi M, Miramin Mohammadi A, Eskandari SE, Sarrafnejad A, et al. CCR7+ central and CCR7- effector memory CD4+ T cells in human cutaneous leishmaniasis. Journal of clinical immunology. 2013;33(1):220–34. Epub 2012/09/20. 10.1007/s10875-012-9788-7 . [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Cao Z, Jiang J, Niu H, Dong M, Tong A, et al. Association of mycobacterial antigen-specific CD4(+) memory T cell subsets with outcome of pulmonary tuberculosis. The Journal of infection. 2010;60(2):133–9. Epub 2009/11/03. 10.1016/j.jinf.2009.10.048 . [DOI] [PubMed] [Google Scholar]
- 23.de Oliveira AL, Amadeu TP, de Franca Gomes AC, Menezes VM, da Costa Nery JA, Pinheiro RO, et al. Role of CD8(+) T cells in triggering reversal reaction in HIV/leprosy patients. Immunology. 2013;140(1):47–60. Epub 2013/04/10. 10.1111/imm.12108 ; PubMed Central PMCID: PMCPmc3809705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarno EN, Grau GE, Vieira LM, Nery JA. Serum levels of tumour necrosis factor-alpha and interleukin-1 beta during leprosy reactional states. Clinical and experimental immunology. 1991;84(1):103–8. Epub 1991/04/01. [PMC free article] [PubMed] [Google Scholar]
- 25.Sampaio EP, Sarno EN. Expression and cytokine secretion in the states of immune reactivation in leprosy. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica [et al]. 1998;31(1):69–76. Epub 1998/08/01. . [DOI] [PubMed] [Google Scholar]
- 26.Sieling PA, Abrams JS, Yamamura M, Salgame P, Bloom BR, Rea TH, et al. Immunosuppressive roles for IL-10 and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. Journal of immunology (Baltimore, Md: 1950). 1993;150(12):5501–10. Epub 1993/06/15. . [PubMed] [Google Scholar]
- 27.Lima MC, Pereira GM, Rumjanek FD, Gomes HM, Duppre N, Sampaio EP, et al. Immunological cytokine correlates of protective immunity and pathogenesis in leprosy. Scandinavian journal of immunology. 2000;51(4):419–28. Epub 2000/03/29. . [DOI] [PubMed] [Google Scholar]
- 28.Andrade PR, Pinheiro RO, Sales AM, Illarramendi X, de Mattos Barbosa MG, Moraes MO, et al. Type 1 reaction in leprosy: a model for a better understanding of tissue immunity under an immunopathological condition. Expert review of clinical immunology. 2015;11(3):391–407. Epub 2015/02/11. 10.1586/1744666x.2015.1012501 . [DOI] [PubMed] [Google Scholar]
- 29.Barnes PF, Chatterjee D, Brennan PJ, Rea TH, Modlin RL. Tumor necrosis factor production in patients with leprosy. Infection and immunity. 1992;60(4):1441–6. Epub 1992/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockwood DN, Suneetha L, Sagili KD, Chaduvula MV, Mohammed I, van Brakel W, et al. Cytokine and protein markers of leprosy reactions in skin and nerves: baseline results for the North Indian INFIR cohort. PLoS neglected tropical diseases. 2011;5(12):e1327 Epub 2011/12/20. 10.1371/journal.pntd.0001327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esquenazi D, Moreira AL, Miranda A, Nery JA, Alvarenga FF, Sarno EN, et al. Clinical, immunological and histological aspects of an uncommon type II reaction in patients with lepromatous leprosy. Clinical and experimental dermatology. 2008;33(3):294–7. Epub 2008/02/12. 10.1111/j.1365-2230.2007.02654.x . [DOI] [PubMed] [Google Scholar]
- 32.Dong J, Ivascu C, Chang HD, Wu P, Angeli R, Maggi L, et al. IL-10 is excluded from the functional cytokine memory of human CD4+ memory T lymphocytes. Journal of immunology (Baltimore, Md: 1950). 2007;179(4):2389–96. Epub 2007/08/07. . [DOI] [PubMed] [Google Scholar]
- 33.Moura DF, de Mattos KA, Amadeu TP, Andrade PR, Sales JS, Schmitz V, et al. CD163 favors Mycobacterium leprae survival and persistence by promoting anti-inflammatory pathways in lepromatous macrophages. European journal of immunology. 2012;42(11):2925–36. Epub 2012/08/02. 10.1002/eji.201142198 . [DOI] [PubMed] [Google Scholar]
- 34.Redford PS, Murray PJ, O'Garra A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal immunology. 2011;4(3):261–70. Epub 2011/04/01. 10.1038/mi.2011.7 . [DOI] [PubMed] [Google Scholar]
- 35.Alvarado-Arnez LE, Amaral EP, Sales-Marques C, Duraes SM, Cardoso CC, Nunes Sarno E, et al. Association of IL10 Polymorphisms and Leprosy: A Meta-Analysis. PloS one. 2015;10(9):e0136282 Epub 2015/09/05. 10.1371/journal.pone.0136282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. Journal of immunology (Baltimore, Md: 1950). 2011;187(10):4967–78. Epub 2011/10/12. 10.4049/jimmunol.1102335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. Pillars article: A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000. 100: 655–669. Journal of immunology (Baltimore, Md: 1950). 2015;194(7):2961–75. Epub 2015/03/22. . [PubMed] [Google Scholar]
- 38.Quiroga MF, Martinez GJ, Pasquinelli V, Costas MA, Bracco MM, Malbran A, et al. Activation of signaling lymphocytic activation molecule triggers a signaling cascade that enhances Th1 responses in human intracellular infection. Journal of immunology (Baltimore, Md: 1950). 2004;173(6):4120–9. Epub 2004/09/10. . [DOI] [PubMed] [Google Scholar]
- 39.Chakir H, Wang H, Lefebvre DE, Webb J, Scott FW. T-bet/GATA-3 ratio as a measure of the Th1/Th2 cytokine profile in mixed cell populations: predominant role of GATA-3. Journal of immunological methods. 2003;278(1–2):157–69. Epub 2003/09/06. . [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Naqvi RA, Ali R, Rani R, Khanna N, Rao DN. FoxP3 provides competitive fitness to CD4(+)CD25(+) T cells in leprosy patients via transcriptional regulation. European journal of immunology. 2014;44(2):431–9. Epub 2013/11/12. 10.1002/eji.201343649 . [DOI] [PubMed] [Google Scholar]
- 41.Geluk A, van Meijgaarden KE, Wilson L, Bobosha K, van der Ploeg-van Schip JJ, van den Eeden SJ, et al. Longitudinal immune responses and gene expression profiles in type 1 leprosy reactions. Journal of clinical immunology. 2014;34(2):245–55. Epub 2013/12/29. 10.1007/s10875-013-9979-x . [DOI] [PubMed] [Google Scholar]
- 42.Saini C, Siddiqui A, Ramesh V, Nath I. Leprosy Reactions Show Increased Th17 Cell Activity and Reduced FOXP3+ Tregs with Concomitant Decrease in TGF-beta and Increase in IL-6. PLoS neglected tropical diseases. 2016;10(4):e0004592 Epub 2016/04/02. 10.1371/journal.pntd.0004592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palermo ML, Pagliari C, Trindade MA, Yamashitafuji TM, Duarte AJ, Cacere CR, et al. Increased expression of regulatory T cells and down-regulatory molecules in lepromatous leprosy. The American journal of tropical medicine and hygiene. 2012;86(5):878–83. Epub 2012/05/05. 10.4269/ajtmh.2012.12-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andrade PR, Amadeu TP, Nery JA, Pinheiro RO, Sarno EN. CD123, the plasmacytoid dendritic cell phenotypic marker, is abundant in leprosy type 1 reaction. The British journal of dermatology. 2015;172(1):268–71. Epub 2014/09/27. 10.1111/bjd.13430 . [DOI] [PubMed] [Google Scholar]
- 45.Sieling PA, Legaspi A, Ochoa MT, Rea TH, Modlin RL. Regulation of human T-cell homing receptor expression in cutaneous bacterial infection. Immunology. 2007;120(4):518–25. Epub 2007/03/09. 10.1111/j.1365-2567.2006.02528.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scollard DM, Chaduvula MV, Martinez A, Fowlkes N, Nath I, Stryjewska BM, et al. Increased CXC ligand 10 levels and gene expression in type 1 leprosy reactions. Clinical and vaccine immunology: CVI. 2011;18(6):947–53. Epub 2011/04/22. 10.1128/cvi.00042-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkaldy AA, Musonda AC, Khanolkhar-Young S, Suneetha S, Lockwood DN. Expression of CC and CXC chemokines and chemokine receptors in human leprosy skin lesions. Clinical and experimental immunology. 2003;134(3):447–53. Epub 2003/11/25. 10.1111/j.1365-2249.2003.02306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendes-Aguiar Cde O, Gomes-Silva A, Nunes E Jr., Pereira-Carvalho R, Nogueira RS, Oliveira-Neto Mde P, et al. The skin homing receptor cutaneous leucocyte-associated antigen (CLA) is up-regulated by Leishmania antigens in T lymphocytes during active cutaneous leishmaniasis. Clinical and experimental immunology. 2009;157(3):377–84. Epub 2009/08/12. 10.1111/j.1365-2249.2009.03970.x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Negative correlation between cutaneous test for leprosy prognostic and CD8+/IFN-γ+ TEF cells frequency from T1R patients. Data from LST are shown in millimeters (mm) of cutaneous induration for a better visualization of results. Spearman correlation test.
(TIF)
Data Availability Statement
All relevant data are within the paper.