Abstract
Amebiasis is caused by Entamoeba histolytica infection and can produce a broad range of clinical signs, from asymptomatic cases to patients with obvious symptoms. The current epidemiological and clinical statuses of amebiasis make it a serious public health problem worldwide. The Entamoeba life cycle consists of the trophozoite, the causative agent for amebiasis, and the cyst, the form responsible for transmission. These two stages are connected by “encystation” and “excystation.” Hence, developing novel strategies to control encystation and excystation will potentially lead to new measures to block the transmission of amebiasis by interrupting the life cycle of the causative agent. Here, we highlight studies investigating encystation using inhibitory chemicals and categorize them based on the molecules inhibited. We also present a perspective on new strategies to prevent the transmission of amebiasis.
Entamoeba histolytica Infection and Amebiasis: Behavior of the Causative Agent and the Parasite Life Cycle
Amebiasis is caused by Entamoeba histolytica infection, a protozoan parasite belonging to the phylum Amoebozoa. Infected individuals show a wide range of clinical signs and can be asymptomatic or have obvious symptoms, such as diarrhea, dysentery, fever, and abdominal pains owing to invasive infection. As a consequence of the invasive infection, various extra-intestinal manifestations may also arise: for example, amebic liver, lung, or brain abscesses. Worldwide, 35–50 million symptomatic cases occur annually, leading to approximately 55,000 deaths [1]. However, only a few drugs are available, and no effective vaccines exist [2,3]. Furthermore, because of the high occurrence of asymptomatic infections, amebiasis morbidity is thought to be much higher than estimates that are only based on the number of reported symptomatic cases. These clinical and epidemiological statuses make amebiasis a serious public health problem worldwide [4].
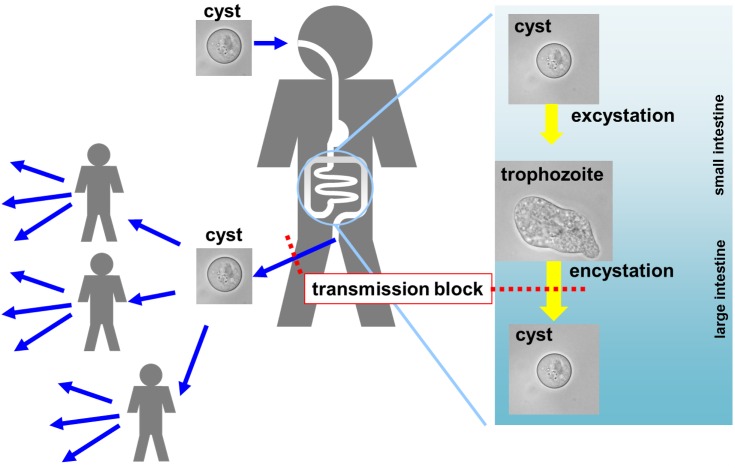
E. histolytica infection usually occurs by oral ingestion of cysts, the dormant form of the parasite (Fig 1) [5]. The cysts, particularly in endemic regions, can be found in various materials or on surfaces (including human hands) that have been contaminated with feces. This can directly or indirectly lead to oral intake. Because the cysts are resistant to severe environments (e.g., temperature, osmotic pressure, pH, and nutrient deprivation), they can pass through the stringent acidic conditions of the stomach and duodenum and reach the small intestine, where they hatch and become proliferative trophozoites. They then passively move to and colonize the large intestine and proliferate there. The proliferating trophozoites sometimes undergo progression to invasive steps that are closely associated with the clinical manifestations and pathogenesis of severe amebiasis [6,7]. Meanwhile, some proliferating trophozoites differentiate into cysts. The newly formed cysts, together with trophozoites, are then excreted during bowel movements. Only the excreted cysts are a source of infection because the trophozoites are labile to environmental assault. Hence, cysts are solely responsible for transmitting amebiasis, and blocking cyst formation halts the spread of this infectious disease to other individuals.
Fig 1. E. histolytica infection and spread of amebiasis; behavior of the causative agent and the parasite life cycle.
Schematic of E. histolytica infection and spread of amebiasis. The Entamoeba life cycle is essentially composed of the proliferating trophozoite and dormant cyst stages. Encystation and excystation are transition steps from trophozoites to cysts and vice versa. Transmission of amebiasis is solely mediated by cysts, and thus blocking encystation halts the spread of this infectious disease.
In this review, we focus on the transition step in the differentiation from the proliferative trophozoite into the dormant cyst: “encystation.” The life cycle of E. histolytica is essentially composed of the trophozoite and cyst stages, which are connected by encystation and excystation (see Fig 1). Encystation and excystation are fundamental cell differentiation processes and are, therefore, important from a biological as well as a medical perspective. Understanding the underlying molecular and cellular mechanisms will not only provide new insights into cellular differentiation but will also provide rationales and potential targets for the development of new preventive measures against amebiasis, such as drugs for blocking transmission (see Fig 1) [8,9]. Here, we take a medical perspective and discuss studies of Entamoeba encystation using inhibitory chemicals, and we categorize these chemicals based on the molecules they inhibit. We also present a perspective for developing transmission-blocking and prophylactic strategies against amebiasis.
Molecules and Processes Involved in Entamoeba Encystation
All the studies described in this review have investigated E. invadens, a reptilian parasite, and not E. histolytica. This is because the in vitro culture of E. invadens has been adopted as a model system for encystation studies (Box 1) [8,10,11].
Box 1. The In Vitro Culture of E. invadens: A Model System for the Study of Encystation
The in vitro culture of E. invadens: The strains of E. histolytica available in the laboratory do not encyst after adaptation to culture conditions; however, E. invadens strains are able to undergo in vitro encystation. The E. invadens life cycle is the same as that of E. histolytica, and the symptoms caused by E. invadens infection are similar to those of E. histolytica [11,59].
In this system, encystation is induced by exposing E. invadens trophozoites to environmental changes that trigger encystation: carbon source deprivation [60], hypoosmotic shock [61], or both [11,62]. This usually involves the routine maintenance of trophozoites in Bisate-Iron-Serum-33 (BI-S-33) medium (a standard culture medium [26,63,64]) and transfer to encystation medium [11,22,52], which provides a decreased carbon source and a reduced osmotic level.
This model system, together with ever-expanding databases such as AmoebaDB, The National Center for Biotechnology Information (NCBI), and Kyoto Encyclopedia of Genes and Genomes (KEGG), has boosted our understanding of encystation.
Gal-Terminated Ligands and Their Receptors
Distinct lines of evidence demonstrate that galactose (Gal)-terminated ligands and their receptors are involved in encystation:
Excess Gal, but not N-acetyl-galactosamine (GalNAc), impaired cyst formation when added in the in vitro encystation system [12,13]. Furthermore, excess Gal treatment made E. invadens form an aberrant cyst, termed a “wall-less cyst” [14].
Coculture of E. invadens trophozoites with the flagellate Crithidia fasciculata induced encystation. This induction was abolished by the addition of Gal to the culture medium or pretreatment of Crithidia with β-galactosidase, indicating the importance of the interaction between Crithidia and Entamoeba cells via Gal residues on the Crithidia surface [12].
Type III mucin or asialofetuin, both of which have a Gal residue as a terminal sugar, can substitute for adult bovine serum (ABS)—a critical component in the in vitro encystation system [9]—when used within an optimal concentration range (20 and 0.1–10 μg/mL, respectively). Their action is abolished by β-galactosidase pretreatment [13].
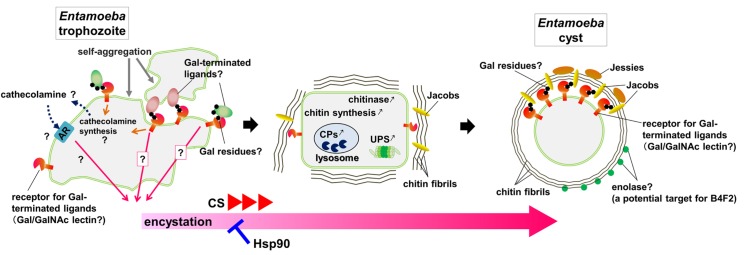
These findings indicate that Gal-terminated ligands are important for Entamoeba cell aggregation, a prerequisite for encystation [12,15,16]. They also suggest the presence of a cell surface molecule that mediates cell–cell interaction via binding to Gal-terminated ligands. Both the Gal-terminated ligands and their receptors await identification. However, Entamoeba Gal/GalNAc lectin is a candidate for the receptor of Gal-terminated ligands because it is among the best-characterized cell surface proteins of Entamoeba [1,17], it plays important roles in various processes of E. histolytica pathogenicity—including adherence, cytolysis, invasion, and resistance to lysis by complement—and it mediates binding to bacteria, red blood cells, epithelial cells, or mucin-coated tissues [16]. Hence, it is plausible that Entamoeba Gal/GalNAc lectin also participates in encystation [16]. Nevertheless, the following important issue needs to be confirmed: how do Entamoeba Gal-terminated ligands and their receptors commit a cell to encystation? (see Fig 2).
Fig 2. Schematic of proposed Entamoeba cyst formation.
Depiction of the processes involved in Entamoeba cyst formation and their potential causal links. Entamoeba trophozoite self-aggregation via cell–cell interaction is an essential prerequisite process for encystation and is mediated by the binding of receptors to Gal-terminated ligands (left). Diverse pathways are implicated in the regulation of encystation (left and center). Numerous components are involved in forming intact cysts (right); some may be exclusive components in the cyst wall, and others may play versatile roles throughout the processes of cyst formation. Abbreviations used: AR, adrenergic receptor; B4F2, a monoclonal antibody; CP, cysteine protease; CS, cholesteryl sulfate; Gal, galactose; GalNAc, N-acetyl-galactosamine; Hsp90, heat shock protein 90; and UPS, ubiquitin proteasome system.
The importance of interactions of Gal-terminated ligands and their receptors has also been demonstrated for the formation of the Entamoeba cyst wall structure. Biochemical analyses identified Jacob as the most abundant glycoprotein in the cyst wall. Jacob can bind to the cyst wall component, chitin (see the “Chitin metabolism” section), and also to purified E. histolytica Gal/GalNAc lectin. Moreover, immunoelectron microscopy revealed that Jacob is released from multiple loci to the surface of encysting E. invadens cells, while low levels of Jacob are seen on the surface of “wall-less cysts” [14]. Another biochemical analysis showed that the protein Jessie can bind to chitin via its chitin-binding domain and also self-aggregate via its unique C-terminal domain [18]. Collectively, these data indicate that a cell surface Gal-lectin, Jacob, and Jessie are important protein components in the formation of an intact cyst (see Fig 2) [8,9,14,18]. However, several questions still remain to be answered (see Fig 2); for example, do Jacob and its family members indeed have a Gal residue as their terminal sugar? Are there any Gal-terminated ligands other than the Jacob family? Is Entamoeba Gal/GalNAc lectin indeed a receptor for Jacob and/or other putative Gal-terminated ligands that is needed to form the cyst wall structure?
Ultimately, elucidation of the molecular and cellular mechanisms to which Gal-terminated ligands and Gal-lectin are committed will provide potential targets and help develop strategies to control encystation and also inform new mechanistic insight in encystation.
Autocrine Catecholamine System
Catecholamine is a vertebrate hormone synthesized from tyrosine and exerts crucial roles via adrenergic receptor (AR)-mediated signal transduction [19,20]. Coppi and colleagues demonstrated several lines of evidence that an autocrine catecholamine system is involved in Entamoeba encystation [21].
Catecholamines can be a substitute for ABS, a critical component in the in vitro encystation system [11], when used within an optimum concentration range; omitting ABS from the standard encystation medium decreased encystation efficiency to ~30%, while the addition of catecholamines to serum-free medium dose-dependently restored the efficiency to over 90%, which can be routinely obtained in the in vitro encystation system. A biphasic effect could be observed for epinephrine and norepinephrine; for example, epinephrine showed maximal activity at 1–10 μM and 1–10 mM.
Furthermore, both β-AR and β1-AR agonists could dose-dependently restore encystation efficiency when added to serum-free medium with a maximal level at 0.1 and 1 μM, respectively. In contrast, an α-AR agonist showed no significant effect in a concentration range of 0.01–10 μM. Consistently, only β-AR and β1-AR antagonists, but not α-AR and β2-AR antagonists, showed negative effects on encystation efficiency when added to standard encystation medium or to serum-free medium supplemented with epinephrine, a catecholamine. These results suggest the participation of the β1-AR–mediated catecholamine signaling pathway in encystation.
Results obtained by quantitative and qualitative biochemical analyses also suggest that Entamoeba trophozoite cells express an AR-like molecule on the cell surface and that they can produce catecholamines that are released into the medium during encystation [21]. Together, these results indicate that the Entamoeba autocrine catecholamine system uses β1-type receptors for encystation.
Nevertheless, the intriguing finding that autocrine regulation of catecholamine release via AR-mediated signal transduction plays a role in the cellular differentiation of a unicellular parasitic organism, Entamoeba, was inconsistent in a number of ways with the reported genome sequence (AmoebaDB). No counterparts of vertebrate ARs are encoded in the Entamoeba genome. Neither are any counterparts of vertebrate enzymes involved in the catecholamine synthetic pathway. These inconsistencies raise two critical questions; why do AR ligands, agonists, and antagonists all show significant, consistent (positive or negative) effects on encystation? How does Entamoeba produce catecholamines?
Cholesteryl Sulfate Synthesis in Sulfur Metabolism
Cholesteryl sulfate (CS) is a common sulfate metabolite in mammals. Recently, CS was shown to play an important role in Entamoeba encystation [22].
Sulfur metabolism provides a variety of sulfur-containing biomolecules, such as methionine, cysteine, Fe-S clusters, sulfolipids, and sulfoproteins [23–25]. In Entamoeba, six sulfolipids (including CS) are synthesized as the terminal products of this metabolism [22]. Furthermore, CS enhanced encystation efficiency in the in vitro culture system within an optimum concentration range (50–200 μM). Conversely, inhibition of CS synthesis by chlorate—an inhibitor targeting ATP sulfurylase in sulfolipid synthesis—dose-dependently reduced encystation efficiency at >100 mM; this concentration range is sublethal for E. invadens trophozoite growth (IC50, 69.3 ± 6.6 mM). These lines of evidence imply that CS participates in the regulation of encystation and that factors involved in CS synthesis are potential targets for developing means to control encystation.
It is worth mentioning that the idea of CS playing an important role in the regulation of encystation provides an answer to the long-standing enigma of the role of Entamoeba mitosomes [22]. Entamoeba mitosomes are derived from canonical mitochondria and have largely lost typical mitochondrial functions during the course of eukaryote evolution; thus, their raison d'être and function have been a long-standing enigma (see Box 2) [22,26–29]. Furthermore, this unique mitosome-linked feature contributes to the adaptation of Entamoeba to its parasitic lifestyle (Box 2) [22].
Box 2. Entamoeba Mitosomes Contribute to Parasitic Lifestyle Adaptation
A role of mitosomes: Recently, Entamoeba mitosomes were shown to atypically compartmentalize sulfate activation and to be necessary for CS synthesis [26]. The regulation of encystation via CS synthesis is, therefore, an important role of Entamoeba mitosomes [22].
Furthermore, interestingly, this feature is not conserved in Mastigamoeba, a nonparasitic close relative of Entamoeba; Mastigamoeba likewise compartmentalizes sulfate activation into mitochondrion-related organelles, which have diversified from canonical mitochondria, but cannot synthesize CS because the cholesteryl sulfotransferase gene is absent in the genome [22,65]. These findings suggest that a unique feature of Entamoeba mitosomes contributes to its adaptation to its parasitic lifestyle.
Hence, elucidation of the underlying molecular and cellular mechanisms modulated by CS will provide not only a new mechanistic insight into encystation but also a new paradigm for linking organellology and evolutionary adaptation to parasitism.
Hsp90
In the process of encystation, negative as well as positive factors are involved. Heat shock protein 90 (Hsp90) is such a negative regulator of encystation [30]. Hsp90 is a chaperone that is highly conserved from bacteria to mammals and plays crucial roles via interaction with co-chaperones [31–34]. Protozoan parasites are, however, an exception; they maintain the conserved Hsp90 but lack some co-chaperones. Indeed, E. histolytica has a highly conserved Hsp90 but lacks the co-chaperones p23, cyclophilin 40 (Cyp40), cell division cycle 37 (Cdc37), and full-length Activator of the Hsp90 ATPase-1 (Aha1), although it does possess a novel truncated Aha1 [32,35]. Singh et al. demonstrated that the ATPase activity of purified recombinant E. histolytica Hsp90 (EhHsp90) was inhibited by 17-allylamino-17-demethoxygeldanamycin (17-AAG), a well-known inhibitor of Hsp90 with an IC50 value of 30.9 μM [35,36]. Furthermore, 17-AAG inhibited the growth of E. histolytica trophozoites with an IC50 value of 546 nM. These results suggest that 17-AAG targets native EhHsp90 in vivo and that EhHsp90 plays an indispensable role in E. histolytica trophozoites [35].
Hsp90 has recently been shown to play a role in encystation [30]. Encystation efficiency was enhanced by almost two-fold by pretreatment of E. invadens with 600 nM 17-AAG for 24 hours prior to inducing encystation; the concentration used is a sublethal dose for E. invadens trophozoite growth (IC50, 711 nM). Consistently, mRNA levels of Hsp90 and co-chaperones are dramatically down-regulated during encystation, suggesting that Hsp90 acts as a negative regulator of encystation. Hsp90, therefore, appears to play versatile roles in the parasitic life cycle of Entamoeba, and elucidation of the underlying molecular and cellular mechanisms in which Hsp90 and co-chaperones participate will inform numerous topics on parasitism as well as differentiation.
Chitin Metabolism
Chitin metabolism may provide potential targets for developing strategies to block the transmission of amebiasis because chitin is present in the cyst wall [5,8,9,15,18] and is not a component of mammalian cells [37,38]. Two independent groups published inconsistent results; one study showed that polyoxin D and Nikkomycin (structural analogs of uridine diphosphate-N-acetylglucosamine, a substrate for chitin synthase) inhibited the cyst formation in a dose-dependent manner when added to the in vitro culture (2–500 and 10–50 μg/mL, respectively) [39]. The other study showed that chitin synthase activity in E. invadens cyst lysates was not inhibited by polyoxin D or Nikkomycin even at 100 μg/mL [40]. This inconsistency raises two questions: Is the Entamoeba chitin synthase a target of polyoxin D and Nikkomycin? Is the Entamoeba chitin synthase a redundant enzyme? The Entamoeba chitin synthase may indeed be redundant because two chitin synthases are encoded in the genome (AmoebaDB); however, the critical question of what is the target of polyoxin D and Nikkomycin remains unanswered.
The importance of the chitin anabolism during encystation is underscored by a study exploiting the Entamoeba database (AmoebaDB) and a gene knockdown approach [41]. All of the transcripts for the putative genes encoding the enzymes in this pathway are up-regulated during encystation. Moreover, the addition of a double-stranded RNA encoding part of an enzyme in this pathway, glucosamine-6-phosphate isomerase, to the in vitro culture reduces its mRNA level, resulting in significant impairment of chitin synthesis. Furthermore, the addition of glycogen phosphorylase inhibitor to the encystation-inducing in vitro culture reduced the chitin level of cysts; glycogen phosphorylase supplies glucose to chitin synthesis by the breakdown of glycogen. Nevertheless, a causal link between the level of chitin synthesis and encystation efficacy is not currently substantiated.
As well as chitin anabolism, the importance of its catabolism has been demonstrated [42]. The addition of allosamidin, an inhibitor of chitinase, to the culture delayed the progression of the early phase of encystation but could not abolish cyst formation in the in vitro culture. In addition, chitinase activity in E. invadens cell extracts prepared from the encystation-inducing in vitro culture was inhibited by allosamidin with a Ki value of 65 nM. The effect on the ability of cells to form cysts was observed within a concentration range of 80–320 μM, more than three orders of magnitude higher than its Ki value on the enzyme. It is, therefore, necessary to determine whether the target of allosamidin is chitinase. Ultimately, unraveling the involvement of chitin metabolism in encystation is needed.
Proteolytic Systems: Lysosomes and Ubiquitin Proteasome System
In eukaryotes, two proteolytic systems—i.e., the lysosome system and the ubiquitin (Ub) proteasome system (UPS)—control intracellular protein levels within optimal ranges [43,44]. In Entamoeba, these two systems play important roles in encystation as well as in proliferation of trophozoites and pathogenicity [1,45].
(1) Cysteine proteases in lysosomes
Involvement of lysosomes in encystation was shown by using specific irreversible cysteine protease (CP) inhibitors, Z-Phe-Arg-CH2F (benzyloxycarbonyl phenylalanyl arginyl fluoromethyl ketone) and E64; CP is known as an important virulence factor in Entamoeba [46–49]. Sharma et al. described the significant ability of Z-Phe-Arg-CH2F to reduce cyst formation efficiency in the in vitro encystation system [46].
Gonzalez et al. demonstrated that the addition of 20 μM E64 significantly delayed the progression of the early phase of encystation but could not abolish cyst formation in the in vitro culture [47,50]. This indicates that CPs only partially contribute to encystation. Alternatively, it implies that the E64 inhibitory effect was not fully exerted in the reported experimental conditions; during incubation, inadequately supplemented E64 was titrated by the turnover of E64-bound CPs and/or by the presence of different types of E64-sensitive proteolytic enzymes, resulting in abrogation of the inhibitory effect. In the studies using CP inhibitors, indirect effects on encystation caused by lysosome dysfunction in trophozoites also needs to be considered [51].
Among 64 CP genes encoded in the E. invadens genome (AmoebaDB), 11 CP mRNAs were up-regulated upon induction of encystation in vitro [52]. In the cysts isolated from amebiasis patients, 8 E. histolytica CP mRNAs were also up-regulated, 2 of which were counterparts of the CP genes detected in the above-mentioned 11 E. invadens genes [52,53]. Collectively, these results indicate that CP plays an important role in encystation, although the nature of this involvement requires elucidation.
(2) Ubiquitin proteasome system
The UPS, another essential intracellular proteolytic system, is typically composed of sequential chain reactions, comprising Ub activation, conjugation, ligation, and elongation, as well as protein degradation in the proteasome, a protein complex containing various proteases [44,54]. This proteolytic system also has an important influence on encystation.
Eichinger and colleagues substantiated the involvement of the UPS in encystation [47]. Lactacystin, a specific proteasome inhibitor, blocked cyst formation in a dose-dependent manner with an IC50 value of 1.25–2.5 μM when added in the in vitro culture. Enrichment of the lactacystin-inhibitable, chymotrypsin-like activity was achieved by column chromatography of the lactacystin-treated E. invadens cell lysate. The enriched fractions show trypsin-like and peptidyl-glutamyl peptide-hydrolyzing (caspase-like) activities. All three of these activities were inhibited by lactacystin at ≤50 μM, which is characteristic of the proteasome. Furthermore, the fractions display typical features of eukaryotic 20S proteasome core subunits—i.e., molecular masses and isoelectric points of major UPS proteins and typical structures as visualized by negatively stained electron microscopy. These studies confirm that, similarly to other organisms, the proteasome in Entamoeba is the target of lactacystin and that the proteasome plays an important role in encystation.
The transcriptional up-regulation of the Ub gene occurred coincidently with other encystation-specific genes (e.g., gene 122 and chitinase 1) 24 hours after the initiation of encystation in vitro [47]. Moreover, β-lactone and MG-132, specific proteasome inhibitors, also dose-dependently inhibited cyst formation in the in vitro culture, with IC50 values of ~1.1 and ~60 μM, respectively [45]. These results further indicate that the UPS plays an important role in encystation. Nevertheless, it cannot be ruled out that overall trophozoite health is affected by proteasome dysfunction that indirectly causes decreased efficiency of cyst formation.
A gene previously identified to encode the E. invadens Ub (AF016643) [47] does not exist in AmoebaDB. Furthermore, a gene annotated as the Ub gene in the AmoebaDB (EIN_063840) does not show high amino acid sequence conservation with other Ubs from different organisms. Therefore, identifying bona fide Entamoeba Ub genes is necessary to address the important issue of how the UPS is involved in encystation.
Enolase: A Potential Target for a Monoclonal Antibody (B4F2)
Segovia-Gamboa et al. reported the isolation of a monoclonal antibody, B4F2, by screening monoclonal antibodies raised against E. invadens intact cysts that could inhibit encystation in the in vitro system [55]. The movement of B4F2’s target molecule during encystation was also shown. Moreover, enolase was demonstrated to be a potential target for B4F2. These findings indicate a role of enolase, a glycolytic enzyme, in encystation; however, further study is needed to confirm whether the B4F2 antigen is indeed enolase.
Conclusions and Future Perspectives
Amebiasis is a serious public health problem; therefore, the development of novel strategies to manipulate E. histolytica encystation is important for realizing new preventive measures. Impairing the ability to form cysts can block the spread of the disease because dormancy of the E. histolytica cyst is crucial for disease transmission. This type of approach cannot directly eliminate the causative agent, the trophozoite, from infected patients, so it would not cure amebiasis. However, it could be an effective approach to mitigate the disease because high numbers of asymptomatic patients who do not require clinical treatment are unconsciously spreading the disease (see Fig 1). A combination approach of eliminating the causative agent itself and interrupting its life cycle would be effective against infectious diseases such as amebiasis and would accelerate a reduction in endemicity.
Most of the molecules described and discussed in this review are potential targets for the development of transmission-blocking strategies. Ideal targets are molecules that exist exclusively in the parasite and not in the host. However, enzymes that have human and E. histolytica counterparts can also be considered as targets if they have distinct features. These features include differences of primary structure, which can be useful for candidate selection (Table 1), and enzyme characteristics, such as substrate specificity, catalytic mechanism, and three-dimensional structures, which are relevant to drug design. Another important factor is the organs and tissues in which the human enzymes are expressed because this has a large bearing on the most efficient drug delivery system. Thus, it will be necessary to differentiate various characteristics of E. histolytica enzymes from their human counterparts.
Table 1. Relevant information on proteins described in this review.
| Described in | Protein | Specific inhibitor | Entamoeba histolytica | Human counterpart | Percentage amino acid sequence identity |
|---|---|---|---|---|---|
| Gal-terminated ligands and their receptors | Gal/GalNAc lectin | + | - | ||
| Autocrine catecholamine system | catecholamine synthetic pathway | -? | + | ||
| adrenergic receptor | -? | + | |||
| Cholesteryl sulfate synthesis in sulfur metabolism | cholesteryl sulfotransferase | XP_649714 | NP_814444 | no homology | |
| ATP sulfurylase | chlorate | XP_653570 | NP_005434 | 26.5% | |
| Hsp90 | Hsp90 | 17-AAG | XP_653132 | NP_005339 | 64.1% |
| Chitin metabolism | chitin synthesis pathway | + | - | ||
| chitinase | allosamidin | XP_652205 | AAI05681 | 39.7% | |
| Proteolytic systems | proteasome subunit beta type 5 | lactacystin | XP_653800 | NP_002788 | 44.8% |
| ubiquitin | XP_650163 | EAX04503 | 89.5% | ||
| A potential target for a monoclonal antibody (B4F2) | enolase? | XP_649161 | NP_001966 | 62.4% | |
The studies reviewed also describe the important molecular processes involved in encystation; however, the underlying mechanisms are not completely understood. The processes described appear to function separately, and it is necessary to determine causal connections to produce an orchestrated network for the regulation of cyst formation (Fig 2). As described in this review, chemicals that can target specific molecules participating in cyst formation can be effective; nevertheless, a major concern is whether their effects result directly from inhibition of target molecules that specifically function in encystation or indirectly because the target molecule also plays important roles in trophozoite proliferation. For example, some target molecules may be involved in fundamental processes of cell maintenance throughout the Entamoeba life cycle (see the “Proteolytic systems” section; [56–58]). This concern can be addressed using a combination approach of gene knockdown (gene knockout has not been achieved in Entamoeba) and supplementation with the metabolite produced by a target enzyme.
Further molecular characterization and elucidation of the underlying mechanism of encystation, both in vitro and in vivo, are essential to provide a solid scientific basis to enable translation of research knowledge into clinically useful materials (see Fig 2). As the ultimate goal, prophylactic drugs as well as transmission-blocking drugs and vaccines against amebiasis will hopefully be developed in the future.
Funding Statement
We thank the support from the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (24117517, 26117719, 16K19117, 16H01365) to FM, (25460594) to HY, and (23406009, 26305013) to SH, and the Cooperative Research Grants of NEKKEN, 2011, 2013, 2014, 2015 to FM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ralston KS, Petri WA. The ways of a killer: how does Entamoeba histolytica elicit host cell death? Essays Biochem. 2011;51:193–210. 10.1042/bse0510193 [DOI] [PubMed] [Google Scholar]
- 2. Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr. Amebiasis. N Engl J Med. 2003;348(16):1565–73. . [DOI] [PubMed] [Google Scholar]
- 3. Quach J, St-Pierre J, Chadee K. The future for vaccine development against Entamoeba histolytica . Hum Vaccin Immunother. 2014;10(6):1514–21. 10.4161/hv.27796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watanabe K, Petri WA Jr. Molecular biology research to benefit patients with Entamoeba histolytica infection. Mol Microbiol. 2015;98(2):208–17. 10.1111/mmi.13131 [DOI] [PubMed] [Google Scholar]
- 5. Samuelson J, Bushkin GG, Chatterjee A, Robbins PW. Strategies to discover the structural components of cyst and oocyst walls. Eukaryot Cell. 2013;12(12):1578–87. 10.1128/EC.00213-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moonah SN, Jiang NM, Petri WA Jr. Host immune response to intestinal amebiasis. PLoS Pathog. 2013;9(8):e1003489 10.1371/journal.ppat.1003489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Begum S, Quach J, Chadee K. Immune Evasion Mechanisms of Entamoeba histolytica: Progression to Disease. Front Microbiol. 2015;6:1394 10.3389/fmicb.2015.01394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aguilar-Diaz H, Carrero JC, Arguello-Garcia R, Laclette JP, Morales-Montor J. Cyst and encystment in protozoan parasites: optimal targets for new life-cycle interrupting strategies? Trends Parasitol. 2011;27(10):450–8. 10.1016/j.pt.2011.06.003 [DOI] [PubMed] [Google Scholar]
- 9. Samuelson J, Robbins P. A simple fibril and lectin model for cyst walls of Entamoeba and perhaps Giardia . Trends Parasitol. 2011;27(1):17–22. 10.1016/j.pt.2010.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eichinger D. Encystation in parasitic protozoa. Curr Opin Microbiol. 2001;4(4):421–6. 10.1016/s1369-5274(00)00229-0 . [DOI] [PubMed] [Google Scholar]
- 11. Sanchez L, Enea V, Eichinger D. Identification of a developmentally regulated transcript expressed during encystation of Entamoeba invadens . Mol Biochem Parasitol. 1994;67(1):125–35. 10.1016/0166-6851(94)90102-3 . [DOI] [PubMed] [Google Scholar]
- 12. Cho J, Eichinger D. Crithidia fasciculata induces encystation of Entamoeba invadens in a galactose-dependent manner. J Parasitol. 1998;84(4):705–10. 10.2307/3284574 . [DOI] [PubMed] [Google Scholar]
- 13. Coppi A, Eichinger D. Regulation of Entamoeba invadens encystation and gene expression with galactose and N-acetylglucosamine. Mol Biochem Parasitol. 1999;102(1):67–77. 10.1016/s0166-6851(99)00085-7 . [DOI] [PubMed] [Google Scholar]
- 14. Frisardi M, Ghosh SK, Field J, Van Dellen K, Rogers R, Robbins P, et al. The most abundant glycoprotein of amebic cyst walls (Jacob) is a lectin with five Cys-rich, chitin-binding domains. Infect Immun. 2000;68(7):4217–24. 10.1128/iai.68.7.4217-4224.2000 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eichinger D. Encystation of entamoeba parasites. Bioessays. 1997;19(7):633–9. 10.1002/bies.950190714 . [DOI] [PubMed] [Google Scholar]
- 16. Eichinger D. A role for a galactose lectin and its ligands during encystment of Entamoeba . J Eukaryot Microbiol. 2001;48(1):17–21. 10.1111/j.1550-7408.2001.tb00411.x . [DOI] [PubMed] [Google Scholar]
- 17. Aguirre Garcia M, Gutierrez-Kobeh L, Lopez Vancell R. Entamoeba histolytica: adhesins and lectins in the trophozoite surface. Molecules. 2015;20(2):2802–15. 10.3390/molecules20022802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chatterjee A, Ghosh SK, Jang K, Bullitt E, Moore L, Robbins PW, et al. Evidence for a "wattle and daub" model of the cyst wall of entamoeba. PLoS Pathog. 2009;5(7):e1000498 10.1371/journal.ppat.1000498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caron MG, Lefkowitz RJ. Catecholamine receptors: structure, function, and regulation. Recent Prog Horm Res. 1993;48:277–90. . [DOI] [PubMed] [Google Scholar]
- 20. Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;361(1):1–15. . [DOI] [PubMed] [Google Scholar]
- 21. Coppi A, Merali S, Eichinger D. The enteric parasite Entamoeba uses an autocrine catecholamine system during differentiation into the infectious cyst stage. J Biol Chem. 2002;277(10):8083–90. 10.1074/jbc.M111895200 . [DOI] [PubMed] [Google Scholar]
- 22. Mi-ichi F, Miyamoto T, Takao S, Jeelani G, Hashimoto T, Hara H, et al. Entamoeba mitosomes play an important role in encystation by association with cholesteryl sulfate synthesis. Proc Natl Acad Sci U S A. 2015;112(22):E2884–90. 10.1073/pnas.1423718112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yoshinari K, Petrotchenko EV, Pedersen LC, Negishi M. Crystal structure-based studies of cytosolic sulfotransferase. J Biochem Mol Toxicol. 2001;15(2):67–75. . [DOI] [PubMed] [Google Scholar]
- 24. Rath VL, Verdugo D, Hemmerich S. Sulfotransferase structural biology and inhibitor discovery. Drug Discov Today. 2004;9(23):1003–11. . [DOI] [PubMed] [Google Scholar]
- 25. Koprivova A, Kopriva S. Molecular mechanisms of regulation of sulfate assimilation: first steps on a long road. Front Plant Sci. 2014;5:589 10.3389/fpls.2014.00589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mi-ichi F, Abu Yousuf M, Nakada-Tsukui K, Nozaki T. Mitosomes in Entamoeba histolytica contain a sulfate activation pathway. Proc Natl Acad Sci U S A. 2009;106(51):21731–6. 10.1073/pnas.0907106106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mi-ichi F, Nozawa A, Yoshida H, Tozawa Y, Nozaki T. Evidence that the Entamoeba histolytica Mitochondrial Carrier Family Links Mitosomal and Cytosolic Pathways through Exchange of 3'-Phosphoadenosine 5'-Phosphosulfate and ATP. Eukaryot Cell. 2015;14(11):1144–50. 10.1128/EC.00130-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aguilera P, Barry T, Tovar J. Entamoeba histolytica mitosomes: organelles in search of a function. Exp Parasitol. 2008;118(1):10–6. . [DOI] [PubMed] [Google Scholar]
- 29. van der Giezen M. Hydrogenosomes and mitosomes: conservation and evolution of functions. J Eukaryot Microbiol. 2009;56(3):221–31. 10.1111/j.1550-7408.2009.00407.x [DOI] [PubMed] [Google Scholar]
- 30. Singh M, Sharma S, Bhattacharya A, Tatu U. Heat Shock Protein 90 regulates encystation in Entamoeba . Front Microbiol. 2015;6:1125 10.3389/fmicb.2015.01125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sidera K, Patsavoudi E. HSP90 inhibitors: current development and potential in cancer therapy. Recent Pat Anticancer Drug Discov. 2014;9(1):1–20. . [PubMed] [Google Scholar]
- 32. Singh C, Atri N. Chemo-informatic design of antibiotic geldenamycin analogs to target stress proteins HSP90 of pathogenic protozoan parasites. Bioinformation. 2013;9(7):329–33. 10.6026/97320630009329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Didenko T, Duarte AM, Karagoz GE, Rudiger SG. Hsp90 structure and function studied by NMR spectroscopy. Biochim Biophys Acta. 2012;1823(3):636–47. 10.1016/j.bbamcr.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 34. Johnson JL, Brown C. Plasticity of the Hsp90 chaperone machine in divergent eukaryotic organisms. Cell Stress Chaperones. 2009;14(1):83–94. 10.1007/s12192-008-0058-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Singh M, Shah V, Tatu U. A novel C-terminal homologue of Aha1 co-chaperone binds to heat shock protein 90 and stimulates its ATPase activity in Entamoeba histolytica . J Mol Biol. 2014;426(8):1786–98. 10.1016/j.jmb.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 36. Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3(3):213–7. . [DOI] [PubMed] [Google Scholar]
- 37. Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. 10.1146/annurev-physiol-012110-142250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanneganti M, Kamba A, Mizoguchi E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. J Epithel Biol Pharmacol. 2012;5:1–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Avron B, Deutsch RM, Mirelman D. Chitin synthesis inhibitors prevent cyst formation by Entamoeba trophozoites. Biochem Biophys Res Commun. 1982;108(2):815–21. . [DOI] [PubMed] [Google Scholar]
- 40. Das S, Gillin FD. Chitin synthase in encysting Entamoeba invadens . Biochem J. 1991;280 (Pt 3):641–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samanta SK, Ghosh SK. The chitin biosynthesis pathway in Entamoeba and the role of glucosamine-6-P isomerase by RNA interference. Mol Biochem Parasitol. 2012;186(1):60–8. 10.1016/j.molbiopara.2012.09.011 [DOI] [PubMed] [Google Scholar]
- 42. Villagomez-Castro JC, Calvo-Mendez C, Lopez-Romero E. Chitinase activity in encysting Entamoeba invadens and its inhibition by allosamidin. Mol Biochem Parasitol. 1992;52(1):53–62. . [DOI] [PubMed] [Google Scholar]
- 43. Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584(7):1393–8. 10.1016/j.febslet.2009.12.047 [DOI] [PubMed] [Google Scholar]
- 44. Pickart CM. Back to the future with ubiquitin. Cell. 2004;116(2):181–90. . [DOI] [PubMed] [Google Scholar]
- 45. Makioka A, Kumagai M, Ohtomo H, Kobayashi S, Takeuchi T. Effect of proteasome inhibitors on the growth, encystation, and excystation of Entamoeba histolytica and Entamoeba invadens . Parasitol Res. 2002;88(5):454–9. . [DOI] [PubMed] [Google Scholar]
- 46. Sharma M, Hirata K, Herdman S, Reed S. Entamoeba invadens: characterization of cysteine proteinases. Exp Parasitol. 1996;84(1):84–91. . [DOI] [PubMed] [Google Scholar]
- 47. Gonzalez J, Bai G, Frevert U, Corey EJ, Eichinger D. Proteasome-dependent cyst formation and stage-specific ubiquitin mRNA accumulation in Entamoeba invadens . Eur J Biochem. 1999;264(3):897–904. . [DOI] [PubMed] [Google Scholar]
- 48. Garcia-Calvo M, Peterson EP, Leiting B, Ruel R, Nicholson DW, Thornberry NA. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J Biol Chem. 1998;273(49):32608–13. . [DOI] [PubMed] [Google Scholar]
- 49. Que X, Reed SL. Cysteine proteinases and the pathogenesis of amebiasis. Clin Microbiol Rev. 2000;13(2):196–206. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gonzalez J, Frevert U, Corey EJ, Nussenzweig V, Eichinger D. Proteasome function is required for encystation of Entamoeba invadens . Arch Med Res. 1997;28 Spec No:139–40. . [PubMed] [Google Scholar]
- 51. de Meester F, Shaw E, Scholze H, Stolarsky T, Mirelman D. Specific labeling of cysteine proteinases in pathogenic and nonpathogenic Entamoeba histolytica . Infect Immun. 1990;58(5):1396–401. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. De Cadiz AE, Jeelani G, Nakada-Tsukui K, Caler E, Nozaki T. Transcriptome analysis of encystation in Entamoeba invadens . PLoS ONE. 2013;8(9):e74840 10.1371/journal.pone.0074840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ehrenkaufer GM, Haque R, Hackney JA, Eichinger DJ, Singh U. Identification of developmentally regulated genes in Entamoeba histolytica: insights into mechanisms of stage conversion in a protozoan parasite. Cell Microbiol. 2007;9(6):1426–44. 10.1111/j.1462-5822.2006.00882.x . [DOI] [PubMed] [Google Scholar]
- 54. Baumeister W, Walz J, Zuhl F, Seemuller E. The proteasome: paradigm of a self-compartmentalizing protease. Cell. 1998;92(3):367–80. . [DOI] [PubMed] [Google Scholar]
- 55. Segovia-Gamboa NC, Chavez-Munguia B, Medina-Flores Y, Cazares-Raga FE, Hernandez-Ramirez VI, Martinez-Palomo A, et al. Entamoeba invadens, encystation process and enolase. Exp Parasitol. 2010;125(2):63–9. 10.1016/j.exppara.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 56. Makioka A, Kumagai M, Kobayashi S, Takeuchi T. Involvement of signaling through protein kinase C and phosphatidylinositol 3-kinase in the excystation and metacystic development of Entamoeba invadens . Parasitol Res. 2003;91(3):204–8. 10.1007/s00436-003-0955-x . [DOI] [PubMed] [Google Scholar]
- 57. Makioka A, Kumagai M, Kobayashi S, Takeuchi T. Different effects of cytochalasins on the growth and differentiation of Entamoeba invadens . Parasitol Res. 2004;93(1):68–71. 10.1007/s00436-004-1106-8 . [DOI] [PubMed] [Google Scholar]
- 58. Ehrenkaufer GM, Weedall GD, Williams D, Lorenzi HA, Caler E, Hall N, et al. The genome and transcriptome of the enteric parasite Entamoeba invadens, a model for encystation. Genome Biol. 2013;14(7):R77 10.1186/gb-2013-14-7-r77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kojimoto A, Uchida K, Horii Y, Okumura S, Yamaguch R, Tateyama S. Amebiasis in four ball pythons, Python reginus . J Vet Med Sci. 2001;63(12):1365–8. . [DOI] [PubMed] [Google Scholar]
- 60. Vazquezdelara-Cisneros LG, Arroyo-Begovich A. Induction of encystation of Entamoeba invadens by removal of glucose from the culture medium. J Parasitol. 1984;70(5):629–33. . [PubMed] [Google Scholar]
- 61. Bailey GB, Rengypian S. Osmotic stress as a factor controlling encystation of Entamoeba invadens . Arch Invest Med (Mex). 1980;11(1 Suppl):11–6. . [PubMed] [Google Scholar]
- 62. Avron B, Stolarsky T, Chayen A, Mirelman D. Encystation of Entamoeba invadens IP-1 is induced by lowering the osmotic pressure and depletion of nutrients from the medium. J Protozool. 1986;33(4):522–5. . [DOI] [PubMed] [Google Scholar]
- 63. Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba . Trans R Soc Trop Med Hyg. 1978;72(4):431–2. . [DOI] [PubMed] [Google Scholar]
- 64. Deloer S, Nakamura R, Mi-ichi F, Adachi K, Kobayashi S, Hamano S. Mouse models of amoebiasis and culture methods of amoeba. Parasitol Int. 2016;65(5):520–5. 10.1016/j.parint.2016.03.012 [DOI] [PubMed] [Google Scholar]
- 65. Nyvltova E, Stairs CW, Hrdy I, Ridl J, Mach J, Paces J, et al. Lateral gene transfer and gene duplication played a key role in the evolution of Mastigamoeba balamuthi hydrogenosomes. Mol Biol Evol. 2015;32(4):1039–55. 10.1093/molbev/msu408 [DOI] [PMC free article] [PubMed] [Google Scholar]