Abstract
Introduction
Impaired wound healing has been widely reported in diabetes. Linoleic acid (LA) accelerates the skin wound healing process in non-diabetic rats. However, LA has not been tested in diabetic animals.
Objectives
We investigated whether oral administration of pure LA improves wound healing in streptozotocin-induced diabetic rats.
Methods
Dorsal wounds were induced in streptozotocin-induced type-1 diabetic rats treated or not with LA (0.22 g/kg b.w.) for 10 days. Wound closure was daily assessed for two weeks. Wound tissues were collected at specific time-points and used to measure fatty acid composition, and contents of cytokines, growth factors and eicosanoids. Histological and qPCR analyses were employed to examine the dynamics of cell migration during the healing process.
Results
LA reduced the wound area 14 days after wound induction. LA also increased the concentrations of cytokine-induced neutrophil chemotaxis (CINC-2αβ), tumor necrosis factor-α (TNF-α) and leukotriene B4 (LTB4), and reduced the expression of macrophage chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1 (MIP-1). These results together with the histological analysis, which showed accumulation of leukocytes in the wound early in the healing process, indicate that LA brought forward the inflammatory phase and improved wound healing in diabetic rats. Angiogenesis was induced by LA through elevation in tissue content of key mediators of this process: vascular-endothelial growth factor (VEGF) and angiopoietin-2 (ANGPT-2).
Conclusions
Oral administration of LA hastened wound closure in diabetic rats by improving the inflammatory phase and angiogenesis.
Introduction
Wound healing is a physiological and essential process that must initiate as soon as tissue damage occurs. It is divided into 4 phases: 1) the formation of a clot, to stop the bleeding; 2) the inflammatory phase, with the recruitment of immune cells and release of inflammatory mediators; 3) the proliferative phase, with formation of granulation tissue, that plays an important role in new vessel formation; 4) the remodeling phase, when the spatial reorganization of collagen fibers and re-epithelization occur. Various cell types including neutrophils, macrophages, fibroblasts, endothelial cells and keratinocytes, and a great number of mediators (e.g. cytokines, lipid derived molecules, growth factors) orchestrate the wound healing phases. Alterations in duration or intensity of the inflammatory phase modify the onset of the next phase and hence impair the wound healing process [1, 2].
Types 1 and 2 diabetes exhibit different etiologies, however, both are associated with hyperglycemia and impairment in wound healing through mechanisms involving exacerbation and chronification of the inflammatory response [2–4]. Hard-to-heal wounds are a well-known diabetic complication [5]; 25% of diabetic patients had experienced a non-healing ulcer and 28% of them underwent amputation related to poor wound healing [5]. Chronic wounds have an imbalanced production of pro- and anti-inflammatory mediators such as TNF-α, IL-1β, VEGF and IL-10 [6–8], hindering proper healing. The sustained expression of pro-inflammatory cytokines and chemokines are associated with increased numbers of neutrophils in late wound tissues and impairment in tissue repair in db/db mice [4]. The recruitment of macrophages is also impaired and there is a predominance of M1 pro-inflammatory macrophage subtype in the harmed area. The permanence of M1 macrophages in wound tissue increases the production of inflammatory mediators and blocks inflammation resolution. As a consequence, the progression to angiogenesis not occurs [3, 9].
Angiogenesis is defined as the formation of new vessels from preexisting vessels [10]. It plays a critical role in wound healing, since it reestablishes the supply of oxygen and nutrients to damaged area as well as promotes the migration of cells that will build up the tissue. Angiogenesis is up regulated by growth factors such as VEGF and ANGPT-2, that will promote the genesis of new vessels by acting on endothelial cells [11]. On the other hand, it is down regulated by angiostatin and TGF-β (tumor growth factor-β) that, not only, reduce the synthesis of pro-angiogenic factors but also antagonize some of their effects [12]. Then, both inflammation and angiogenesis play pivotal roles in injured tissue repair. These two processes are impaired in diabetes, resulting in delayed wound healing. Compounds that reestablish inflammation and angiogenesis and then normalize the wound healing process are of great importance for diabetic patients.
Skin wounds are popularly treated with natural compounds such as nut oils in developping countries. Although this provides the basis for the pharmaceutical formulations of healing ointments, little is known about how these products act on the wound healing process. We previously reported that oral administration of pure linoleic acid (LA), an abundant fatty acid of nut oils, improves the wound healing process in non-diabetic animals [13]. LA (18:2, ω-6) is an essential fatty acid widely present in the western diet. LA constitutes 40% of the fatty acids in the human skin and plays an important role for its function. However, there is no consense about the effects of LA on inflammatory response yet. We reported that oral administration of LA has pro- and anti-inflammatory effects in non-diabetic rats. LA increased the influx of inflammatory cells into the injured tissue, changed neutrophil [14] and macrophage [15] fatty acid composition, and reduced the production of cytokines and reactive oxygen species (ROS).
The information above led us to investigate the effects of oral administration of LA on skin repair in diabetic rats. The key steps of wound healing, inflammation and angiogenesis, were assessed. We hypothesized that LA may hasten wound healing by acting on inflammatory response and angiogenesis. To test this hypothesis the experiments were performed in vivo in streptozotocin-induced diabetic rats orally suplemented with pure LA.
Materials and methods
Animals
Male Wistar rats (from the Institute of Biomedical Sciences, Sao Paulo University, Brazil) were maintained at 23°C under a light: dark cycle of 12:12 h and received food (Nuvital, Curitiba, Brazil, containing 22% of protein, 4,5% of fat, 40,8% of carbohydrate and 8% of fiber) and water ad libitum. Linoleic acid constitutes 40% of the fatty acids in the chow. The complete fatty acid composition of chow was previously published [14]. The Animal Care Committee of the Institute of Biomedical Sciences approved the experimental procedure of this study (Protocol number: 86).
Induction of diabetes mellitus
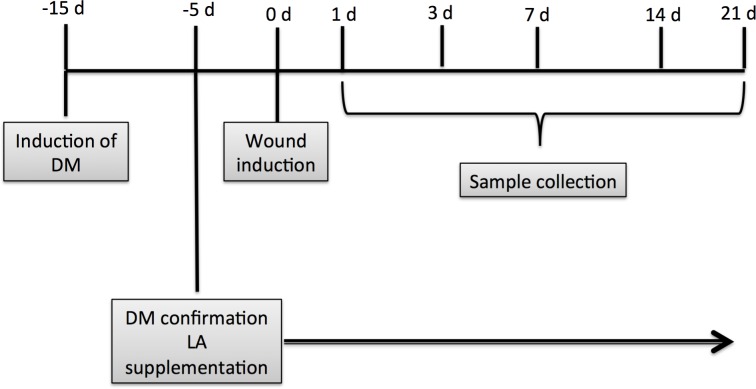
Type I diabetes mellitus was induced by streptozotocin injection as previously reported [16]. This drug destroys pancreatic beta cells resulting in a marked reduction in insulin release and consequently hyperglycemia. Diabetes was confirmed three days after induction by blood glucose concentrations above 250 mg/dL as determined by the Accu-Check Active glucometer (Roche, Mannheim, Germany). After ten days, diabetic animals were divided into two groups: untreated diabetic (D) and diabetic that received oral LA supplementation (DLA) (Fig 1).
Fig 1. Experimental protocol.
Administration of LA
Oral administration of pure LA (Sigma-Aldrich Co, St Louis, MO, USA) was initiated ten days after diabetes induction and maintained daily during the experimental period. Unesterified LA, at a dose of 0.22 g/kg b.w, was administered by gavage. The total calories associated with LA dose are low (1.98 cal/day) and so, we used water administration as control [14]. The D group received water (same LA volume). Considering the chow FA composition [14] and the food intake (g/day) of the animals (data not shown), the LA dose used in the present study represents an increase of 7% in the total ingestion of LA compared with the chow diet.
Skin wound induction
After five days of LA administration, the animals were anesthetized with xilazine (7 mg/kg b.w.) and ketamine (14 mg/kg b.w.) and an area of 10 mm2 in dorsal region skin was shaved and removed by surgery. Animals were killed by overdose of the anesthetics xilazine (21 mg/kg b.w.) and ketamine (42 mg/kg b.w.), 1, 3, 7 or 14 days after the surgery.
Determination of wound tissue fatty acid composition
The fatty acid composition of the wounds was determined by gas chromatography (GC) as previously described [17]. Results of individual fatty acids are expressed as percentage of total fatty acids.
Skin wound closure assessment
Animals were anesthetized with isoflurane. The wounds were daily photographed using a Sony cyber shot camera (model DSC-S950S 10 mP; 4 x Optical zoom) by the same examiner, as previously described [13]. Wound closure was defined as a reduction of wound area and results are expressed as percentage of the original wound area.
Eicosanoid measurements in wound tissue
The concentrations of leukotriene B4 (LTB4) and 15 (S) hydroxyeicosatetraenoic acid (15(S)-HETE) were measured in scar tissue homogenates using ELISA kits according to manufacturer's instructions (Cayman Chemical, Ann Arbor, MI, USA).
Histological examination of the wound tissue
Wound lesions with adjacent normal skin were removed, fixed in Bouin for 24 h at room temperature, processed and embedded in Paraplast®. Seven μm sections were stained with hematoxilin/eosin to evaluate the general morphology of the wound.
Morphometric analysis of blood vessels
Digital photomicrographs were obtained using a Leitz Aristoplan optical microscope (Leica) with a 20x objective and a Nikon (DS-Ril) camera. The NIS-Elements software was employed for image capturing. Only the dermal wound region, just below the crust, was photographed (2–5 pictures per animal, 3–4 animals per group). The Image J public software (NIH, Bethesda, US) was used for morphometric analysis using the grid plugin. A grid of 130 points was used in each photograph and the number of points observed in the interior of small blood vessels was counted and expressed as percentage of the total points, representing the area occupied by vessels.
Cytokine contents in wound tissue
Wound lesions removed at 1, 3 and 7 days after lesion induction were wrapped up in aluminium paper, dropped into dry ice and kept frozen (-80°C). CINC-2αβ, IL-1β, TNF-α, IL-6 and VEGF were assessed by ELISA as previously described [13] using the Duo Set kit (R&D System, Minneapolis, MN, USA) and normalized by protein concentration as measured by the Bradford method [18].
Real-time polymerase chain reaction
Total RNA was extracted (RNAeasy Mini Kit, Qiagen, Venlo, Netherlands) from wound tissue and reverse-transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Reactions were performed using SYBR-Green PCR master mix (Invitrogen, Carlsbad, CA, USA) in a Rotor Gene Q (Qiagen, Germantown, Maryland, MD, USA). mRNA expression was normalized by the D values in unwounded skin. The sequences of the primers used are described in the S1 Table.
Measurement of NF-KB and AP-1 activation in wound tissue
Wound tissue removed at 1 and 24h after lesion was processed as previously described [13, 19]. The blots were analyzed by scanner densitometry (Image Master 1D, Amersham Biosciences) and results expressed as arbitrary units in relation to diabetic animals.
Statistical Analysis
Comparisons between groups were performed using Student’s t test. In some experiments (cytokines, skin fatty acid composition and mRNA expression), two-way analysis of variance (ANOVA) and Bonferroni post-test were used. The significance was set at p<0.05.
Results
All streptozotocin-induced diabetic animals used in this study had blood glucose levels close to 400 mg/dL. None other plasma measurement (e.g. ketone bodies) was considered for this purpose as also reported by others [16, 20, 21]. The diabetes protocol used was established considering the animals lost around 10% of their body weight and they would not survive for longer period without insulin administration. The diabetic rats were not treated with insulin due to its direct effects on the wound healing process [20]. The combination of high glycemia, intense weight loss and general catabolic state could compromise the interpretation of results obtained in a condition of prolonged diabetes state. Ten days after streptozotocin-induced diabetes, a full-thickness biopsy was performed and wound closure was assessed over time. The diabetic condition protocol used did delay wound healing as indicated by the analysis of wound closure in control (non-diabetic) and diabetic (non-treated) rats.
Pure LA was orally administered to diabetic rats daily for 5 days prior to the full-thickness biopsy and then until wound closure (Fig 1). The dose (0.22 g/kg/day) of LA and the duration of the administration did not induce any change in the nutritional status of the animals (data not shown). The amount of LA given is unlikely to have increased plasma ketone body levels. In fact, the dose of LA given represents an increase of 7% in the total ingestion of LA compared with the chow diet.
Oral administration of LA changed skin fatty acid composition and modulated eicosanoid production in wound tissue
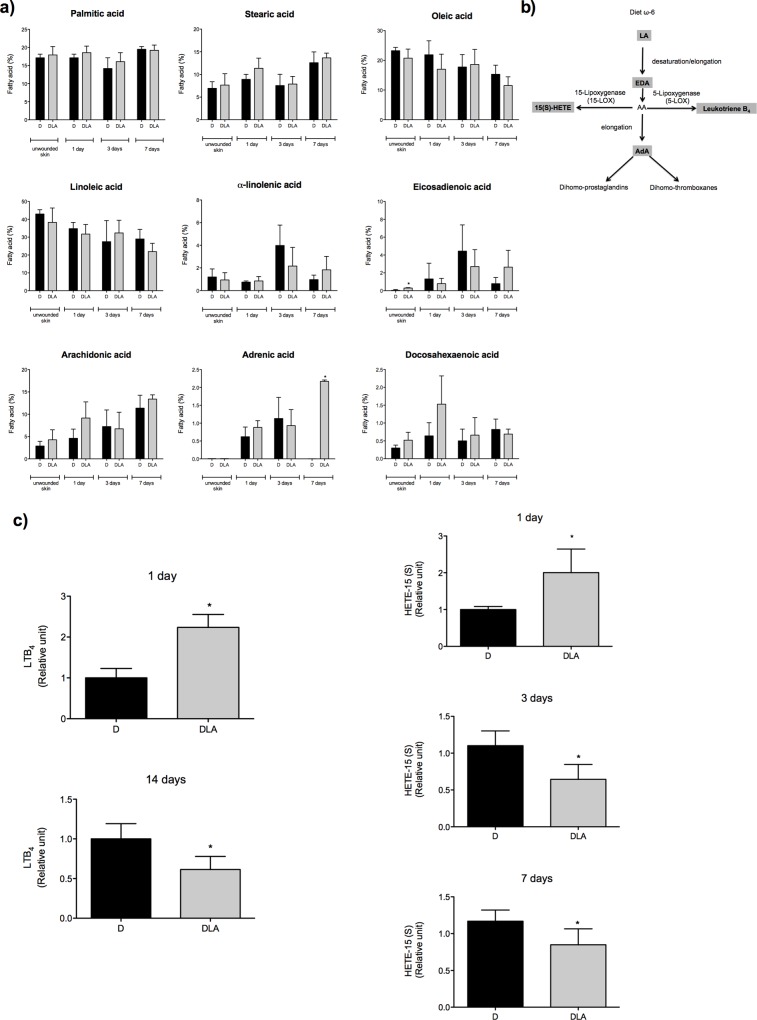
We previously reported that the same treatment protocol increases the proportion of LA in neutrophils [14] and macrophages [15]. LA increased eicosadienoic (EDA) percentages on unwounded skin. On the 7th day, LA elevated the adrenic acid (AdA) percentages in wound tissue (Fig 2A).
Fig 2. Fatty acid composition and eicosanoids production during wound healing.
(a) Fatty acid composition in wound tissue from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented as mean ± SD. D (3 rats) and DLA (7 rats). (*) Indicates significant differences between D and DLA rats (p<0.001). (b) Scheme showing LA metabolism and generation of eicosanoids. (c) LTB4 and HETE-15 (S) concentrations in wound tissues from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented as mean ± SD. D (3 rats) and DLA (5 rats). (*) Indicates significant differences between D and DLA rats (LTB4 1d –p = 0.002; LTB4 14d –p = 0.02; HETE-15 (S) 1d –p = 0.03; HETE-15 (S) 3d –p = 0.001; HETE-15 (S) 7d –p = 0.04).
The concentrations of LTB4 and 15(S)-HETE, two eicosanoids derived from AA, which can be generated from LA (Fig 2B) were measured. Concentrations of both eicosanoids were increased in the wound tissue one-day post-wounding and were reduced after 3 and 7 days (15(S)-HETE) or 14 days (LTB4) in the DLA group (Fig 2C).
LA improved skin repair in diabetic rats
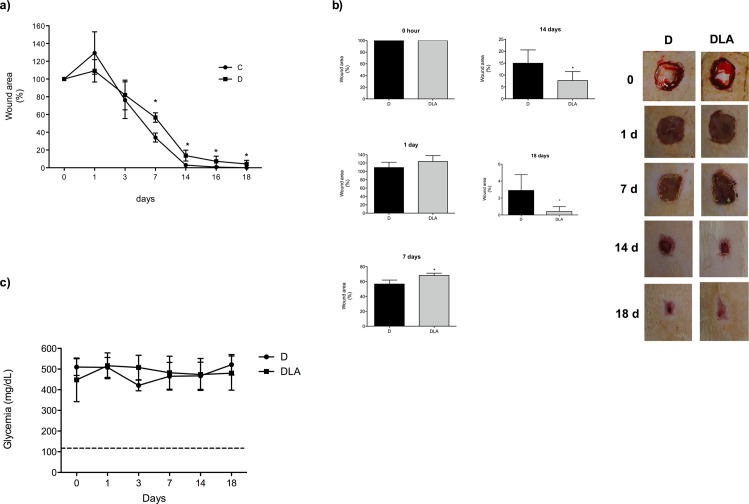
Fourteen days post-wounding, the original wound area was reduced by over 95% in the control group being fully closed by day 18 (Fig 3A). In comparison, wound closure was much slower in diabetic animals. At the 7th day after wound induction, diabetic animals had a larger wound area (p = 0.002) than the control group (D: 56 ± 2% vs. C: 34 ± 3%, mean ± SEM of 5–9 animals per group) (Fig 3A). The delay in wound healing remained in diabetic animals and wounds were not fully healed up to 18 days after induction.
Fig 3. Time course of wound healing and glycemia.
(a) Macroscopic and time course of wound closure in control (C) and diabetic rats (D). (*) Indicates significant differences among C versus D (p = 0.006) (b) Macroscopic and time course of wound closure in diabetic (D) and diabetic rats treated with LA (DLA) (*) Indicates significant differences between D and DLA (p = 0.02). Representative photos of the wound tissue obtained during the time-course of 18 days. Results are presented as mean ± SD. D (5 rats) and DLA (9 rats). (c) Glycemia of rats during the wound healing process: (D) diabetic; (DLA) diabetic rats treated with LA. Dashed line indicates the mean of glycemia in control rats.
Administration of LA hastened wound closure in diabetic rats (Fig 3B), an effect that was independent of any change in glycemia (Fig 3C). The wound area was reduced in the DLA group from the 14th to the 18th day post-wounding in relation to D animals (Fig 3B). In order to verify if the effect on wound closure was specific for LA, we performed the same analysis in diabetic rats treated with pure oleic acid (OA), a monounsaturated 18-carbon chain fatty acid (S1 Fig). In contrast to the effect of LA, OA caused a delay in the wound closure of diabetic rats (DOA group) when compared to D animals but did not modify glycemia (S1 Fig). Taking together, these results suggest that the improvement in wound healing is specific for LA treatment.
LA induced inflammatory cell migration and increased formation of new vessels in wound tissue
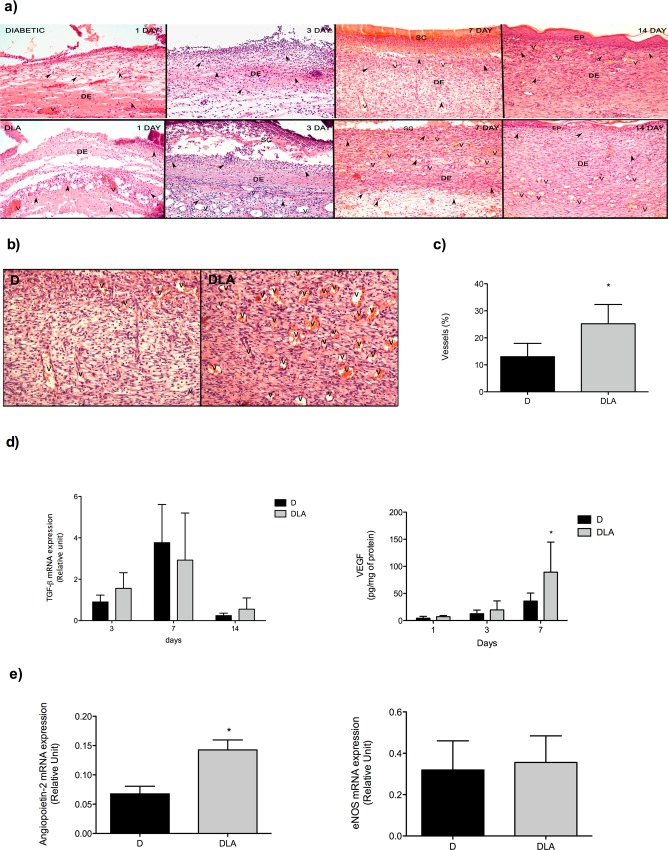
Histological analysis of wounds from diabetic rats exhibited inflammation in the dermis on the first day and intense neutrophil influx into the tissue from the 3rd until to the 14th day post-wounding (Fig 4A). A few vessels were observed in wounds of diabetic rats (Fig 4A).
Fig 4. Histological analysis and angiogenic growth factors expression in wound tissue.
(a) Samples were isolated from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA) at the 1st, 3rd, 7th and 14th days after wounding. (b) Representative new vessel formation in wound tissue from the D and DLA groups. Samples were collected on the 7th day after wounding. (c) Vessels quantification. Results are presented as mean ± SD. D (4 rats) and DLA (5 rats). (*) Indicates significant difference between D and DLA (p = 0.0001). (d) TGF-β mRNA expression and VEGF concentration in wound tissues from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented as mean ± SD. D (9 rats) and DLA (4 rats). (*) Indicates significant difference between D and DLA (VEGF–p<0.01). (e) eNOS and ANGPT-2 mRNA expression in wound tissues from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented as mean ± SD. D (9 rats) and DLA (4 rats). (*) Indicates significant difference between D and DLA (ANGPT-2 –p = 0.01). V: vessel. DE: derm. Objective 10X.
Wounds were more inflamed in DLA group than in D animals on the first day after wounding. Significant edema and high number of neutrophils were found in the crust (Fig 4A). On the 3rd day, neutrophils were abundant at the surface of the wound but in lower number than in the D group. There were more newly formed vessels from the 3rd day until the 14th day after wounding in the DLA group in relation to D animals (Fig 4B and 4C).
To explain the increase in vessel number observed in the DLA group, we measured mRNA expression of tissue factors that regulate angiogenesis. Although there was no difference in TGF-β expression, the concentration of VEGF was elevated in DLA rats (Fig 4D), 7 days after wound induction. Considering this effect on VEGF, we analysed the expression of other pro-angiogênic factors at the 7th day after tissue injury and observed that DLA increased ANGPT-2 mRNA expression but did not alter eNOS (endothelial nitric oxide synthase) expression (Fig 4E). These effects of LA were in agreement with the presence of new vessels observed in the histological analysis (Fig 4A, 4B and 4C). Thus, LA induced migration of inflammatory cells and increased the formation of new vessels in wound tissue.
LA affected both the early and the late cell recruitment
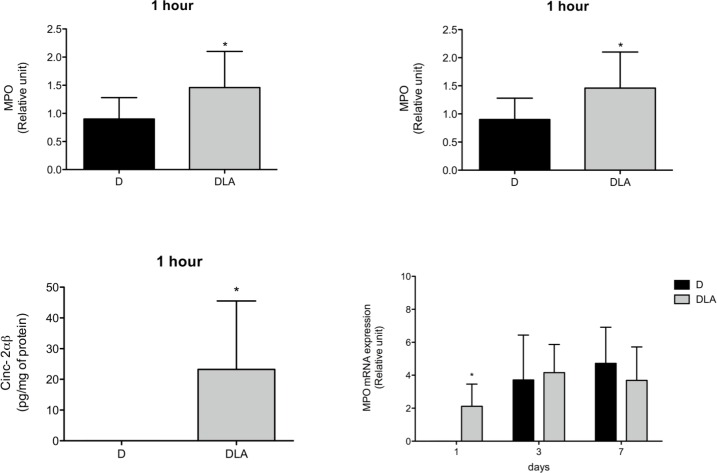
In order to evaluate the kinetics of inflammatory cell migration into wound tissues, mRNA expression of neutrophil (myeloperoxidase—MPO) and macrophage (F4/80) markers were measured during the wound healing process. MPO activity and chemokine concentrations were also measured at different time points in the wound tissue. LA increased MPO mRNA expression and activity one hour after wound induction. This was followed by elevation in CINC-2αβ, an important neutrophil chemoattractant agent (Fig 5). The increase in MPO mRNA expression persisted until the 1st day after wound induction.
Fig 5. Myeloperoxidase and CINC-2αβ contents.
Myeloperoxidase (MPO) activity (1 hour), mRNA expression (1 h, 1, 3 and 7 days) and CINC-2αβ concentration (1 h) in wound tissue. Results are presented as mean ± SD. D (6 rats) and DLA (6 rats). (*) Indicates significant differences between D and DLA rats (MPO activity–p = 0.02; mRNA expression 1h 0.006; mRNA 1 day–p = 0.03; CINC-2αβ –p = 0.04).
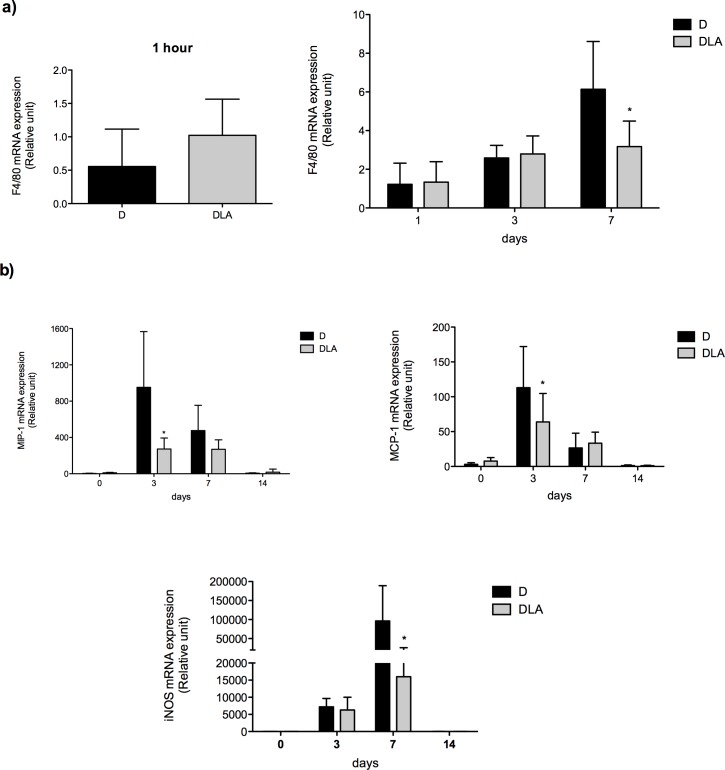
After neutrophils, the next cell population that migrates into an injured area is macrophage. LA did not change F4/80 (macrophage marker) expression during the inflammatory phase of wound healing (Fig 6A). However, LA diminished it at 7th day. This result was followed by reduction in the contents of chemoattractant cytokines (MIP-1 and MCP-1) and of iNOS expression (Fig 6B) that is increased in activated macrophage [22]. So, LA treatment accelerated the early migration of neutrophils through, at least in part, an increase in production of chemoattractants or neutrophil responsiveness to them. LA also modified migration of macrophages (later) and production of macrophage-related chemoattractants.
Fig 6. Macrophages cell markers expression.
(a) mRNA expression of F4/80 in wound tissue 1 hour and 1–7 days after wounding. Results are presented as mean ± SD. D (5 rats) and DLA (10 rats). (b) mRNA expression of MIP-1, MCP-1 and iNOS in wound tissue from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented mean ± SD. D (5 animals) and DLA (9 animals). (*) Indicates significant differences between D and DLA rats (p<0.001)
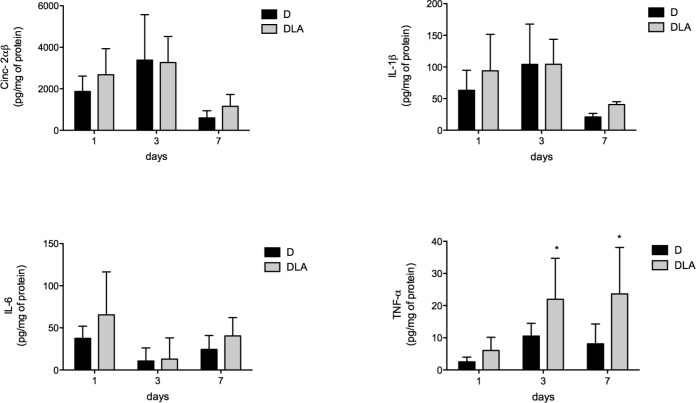
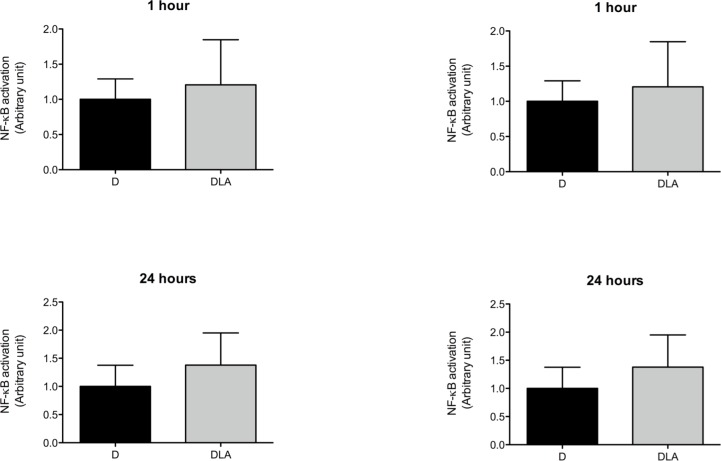
LA hastened the inflammatory phase
TNF-α concentration was raised in wounds on days 3 and 7 after lesion in the DLA group in comparison to D rats (Fig 7). We did not observe any change in IL-6 or IL-1β levels between the experimental groups (Fig 7). We also evaluated activation of NF-κB and AP-1 in the wound tissue. No alteration was observed in NF-κB activation. However, LA inhibited AP-1 activation 1 and 24 hours after wound induction in diabetic animals (Fig 8).
Fig 7. Cytokines production during wound healing.
CINC-2α, IL-6, IL-1β and TNF-α concentrations in wound tissue from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented mean ± SD. D (5 animals) and DLA (6 animals). (*) Indicates significant differences between D and DLA rats (TNF-α 3d –p<0,05; TNF-α 7d –p<0.01)
Fig 8. Transcription factors activation.
NF-KB and AP-1 activation in wound tissues from diabetic rats (D) and diabetic rats treated with linoleic acid (DLA). Results are presented as mean ± SD. D (5 animals) and DLA (8 animals). (*) Indicates significant difference between D and DLA rats (AP-1 1 h–p = 0.02; AP-1 24hs–p = 0.001)
Oral administration of LA hastened wound healing inflammation and angiogenesis steps in diabetic rats by: 1) increasing inflammatory cell influx through chemoattractant agent (CINC-2αβ) production and LTB4 generation; 2) regulation in gene expression (MIP, MCP and iNOS), through AP-1 modulation; 3) induction of vessel formation via production of pro-angiogenic factors (ANGPT-2 and VEGF).
Discussion
The animals herein used had a glycemia around 400 mg/dL (not affected by the treatment with LA or wound process). Despite the short period of diabetes impaired in wound healing was reported, in comparison to non-diabetic animals, which resembles the human condition. Oral administration of LA to diabetic rats hastened the influx of neutrophils (early), reduced macrophage (late) abundance, and modulated the production/release of cytokines (CINC-2αβ and TNF-α), growth factors (VEGF) and eicosanoids (LTB4 and 15(S)-HETE) that drive the healing process. These modifications in LA treated rats were associated with new vessel formation and improvement of the wound healing process.
In order to examine if the effects of LA on wound healing process were due to LA incorporation in the skin, we evaluated skin fatty acid composition by gas chromatography (GC). Although no differences were observed in LA or AA incorporation, oral administration of LA increased eicosadienoic (EDA– 20:2 ω-6) and adrenic acid (AdA—22:4ω-6) incorporation (Fig 2A). EDA is a product of LA elongation that also modifies the inflammatory response, however, in a less intense manner when compared to LA or AA [23]. AdA is an AA elongation product, which can be metabolized to dihomo-eicosanoids or docosanoids [24, 25]. A reduction in AdA formation has been described in type 1-diabetes [26]. Importantly, AdA reduces AA metabolism and inhibits AA-derived eicosanoid formations [27]. In the present study, LA increased AdA incorporation and reduced 15(S)-HETE (Fig 2C).
15-HETE plays a key role in the early phase of wound healing since it controls clot formation through platelet aggregation and thrombin production [28]. Long standing release of 15-HETE is positively associated with wound tissue infiltration of neutrophils and macrophages [29]. The presence of 15-HETE in the latter phase of wound healing reflects a persistent influx of inflammatory cells into the tissue and consequently wound chronification.
Fatty acids can generate a wide range of bioactive molecules named oxylipins [30]. Oxylipins are products formed by PUFA oxidation and the most well known class is the AA-derived eicosanoids [30]. However, they can also be derived from LA such as 13 hydroxyoctadecadienoic acid (13-HODE) and 9,10-cis epoxide of linoleic acid (9,10 EpOME). Considering that HODE and EpOME oxilipins can modulate inflammatory responses, a limitation of the present study is the fact we did not measure these molecules during the wound healing process.
Diabetes mellitus is associated with chronic inflammation and poor wound healing [31]. The inflammatory phase of wound healing in diabetes exhibits accumulation and persistence of primed inflammatory cells in the lesion area [32], resulting in exacerbated production of pro-inflammatory mediators that cause surrounding tissue damage and impairs wound resolution [3, 31, 33].
During inflammation, leukocyte recruitment cascade, a sequential adhesive interaction between leukocytes and endothelial cells, takes place [34]. LA administration induced neutrophil infiltration in the first hours after wounding that returned to basal values 3 days latter. The possible mechanisms involved in LA-induced cell migration are: increased adhesion molecule expression in leukocytes [14] and endothelium [35] and release of chemoattractants such as MCP, LTB4 and CINC-2αβ [36]. The earlier expression of CINC-2αβ induced by LA, also reported in the present study (Fig 5), is associated with an increase in neutrophil influx into damaged tissue and with acceleration of colonic wound healing [37]. Once in the injured area, neutrophils phagocyte dead cells and microorganisms and produce cytokines that attract macrophages to wounded site.
Macrophages modify their phenotype in response to the wound environment. Due to their plasticity, different states of polarization were described for these cells, in which M1 (pro-inflammatory) and M2 (pro-resolution) are the extremes [38]. In a short time wound healing, the switch of M1 to M2 macrophages hastens the resolution of inflammation enabling the progression to the proliferative phase [39]. On the other hand, in chronic wounds, the persistence of M1 macrophages in the tissue exacerbates the inflammatory response and blocks the progression to wound resolution [3].
Considering the importance of macrophages on wound healing, we investigated if LA could influence their recruitment to the wound area. Although we did not analyze M1/M2 markers, we found that LA diminished the expression of a global macrophage marker (F4/80) and reduced the production macrophage derived chemokines (MCP-1 and MIP-1) in the late inflammatory phase (7 days). MCP-1 is a chemokine produced by several cell types including keratinocytes, endothelial cells and resident macrophages, which induces migration of inflammatory cells to injured tissue. Maximum expression of MCP-1 occurs 1–2 days after wounding and declines progressively until to the 7th day of the wound healing process in control conditions [40].
We have previously demonstrated that LA induces transient AP-1 activation in skin of non-diabetic rat [13], favoring the recruitment and activation of inflammatory cells. The effect was not found herein in diabetic rat. LA reduced AP-1 activation at 1 and 24 hours after wounding. Neub et al. [41] stated that reduction in AP-1 activity is needed to restore normal wound healing and prolonged AP-1 activation is described in chronic wounds [42]. The modulation of AP-1 activation in skin is shared by other fatty acids such as docosahexaenoic acid (DHA) and by eicosanoids such as 13-hydroxyoctadecadienoic acid (13-HODE) and 15-hydroxyeicosatrienoic acid (15-HETrE) [43]. These reports together indicate that LA modulates recruitment of cells through inhibition of AP-1 activity and consequent reduction on chemokine production.
After recruitment, leukocytes produce a range of inflammatory mediators such as cytokines, ROS, and growth factors to resolve inflammation. IL-6, IL-1β and TNF-α play a very important role in this process. IL-6 promotes the migratory response of epithelial cells [44] and wound remodeling [45]. IL-6 deficient mice exhibit impaired angiogenesis, macrophage infiltration and re-epithelization, resulting in delayed wound healing [46]. IL-1β inhibits type I collagen production and upregulates metalloproteinase-1, which degrades collagen fibers [47]. Collagen is the main component of the extracellular matrix and plays an important role in the wound healing remodeling [48]. The reduction in type I collagen induces premature collagen synthesis and poor healing.
TNF-α is an important regulator of cell migration. Naaldijk et al. [49] reported that the presence of TNF-α in the medium increases migration of mesenchymal stem cells in a transwell assay. The migratory cell response plays a critical role in the proliferative phase of wound healing. TNF-α also induces angiogenesis in vivo [50] and in vitro [51] through increased VEGF production. Increased TNF-α and VEGF production in diabetic animals treated with LA explains the augmented number of new vessels and improved healing. Increased VEGF levels and number of new vessels formed support the proposition that LA induces angiogenesis.
Angiogenesis is necessary to deliver immune cells, nutrients and oxygen and to remove debris from the damaged tissue. Impairment in formation of new blood vessels retards the healing process and induces ulceration [52]. There is a wide range of growth factors that regulate angiogenesis [52–54]. Diabetes per se leads to increased TGF-β expression during tissue repair. High levels of TGF-β increase extracellular matrix deposition that impairs the vascularization process [55, 56]. Geng et al. [55] reported that there is an inverse correlation between TGF-β expression and VEGF concentration in colon tumors. TGF-β reduces VEGF stability by inducing ubiquitination and degradation of this growth factor, with no effect on VEGF mRNA levels [55].
Growth factors and cytokines released during inflammation are involved in the abluminal sprouting and formation of new vessels from an existing vessel [57–59]. Nishioka et al. [60] described that in vivo administration of LA induces angiogenesis through angiostatin suppression. Angiostatin is a proteolytic fragment of plasminogen and suppresses angiogenesis by inhibiting endothelial cell proliferation and migration and by inducing endothelial cell apoptosis [61]. In the present study, we did not detect angiostatin mRNA expression seven days after the wound in any group (data not shown). However, LA increased VEGF production and expression of ANGPT-2. This latter protein is induced by growth factors such as VEGF after endothelial cell [11] and/or fibroblast/myofibroblast [62] activation. The presence of ANGPT-2 primes endothelial cells to respond to inflammatory cytokines, resulting in expression of adhesion molecules and transmigration of inflammatory cells [11]. LA increased the production of pro-angiogenic factors, which might be associated with elevation in vascularization. These effects of LA are in agreement with the presence of new vessels observed in the histological analysis (Fig 4A, 4B and 4C).
In summary, oral administration of LA to diabetic animals brought forward the inflammatory response and induced angiogenesis. The pro-healing effects of LA hasted the healing process in diabetic rats.
Supporting Information
Results are presented as mean ± SD of 7 animals in each group. (#) Indicates differences in relation to D (10d –p = 0.04; 16d –p = 0.03; 18d –p = 0.03). Glycemia of rats during the wound healing process: (D) diabetic; (DOA) diabetic rats treated with OA. Dashed line indicates the mean of glycemia in control rats.
(TIF)
(DOCX)
Acknowledgments
The authors are indebted to the technical support of José R. Mendonça, Dr.Tatiana C. A. Loureiro, and Dr. Gilson M. Murata.
Data Availability
All the relevant data are within the paper.
Funding Statement
This research was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) grants 2012/10653-9 and 2013/06810-4, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq - 446562/2014-9), and Guggenheim Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6 Epub 2014/12/05. 10.1126/scitranslmed.3009337 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–25. Epub 2007/02/15. 10.1038/sj.jid.5700701 . [DOI] [PubMed] [Google Scholar]
- 3.Mirza RE, Fang MM, Weinheimer-Haus EM, Ennis WJ, Koh TJ. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 2014;63(3):1103–14. Epub 2013/11/07. 10.2337/db13-0927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115(2):245–53. Epub 2000/08/22. 10.1046/j.1523-1747.2000.00029.x . [DOI] [PubMed] [Google Scholar]
- 5.Bakker K, Schaper NC. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28 Suppl 1:116–8. Epub 2012/03/01. 10.1002/dmrr.2254 . [DOI] [PubMed] [Google Scholar]
- 6.Eming SA, Koch M, Krieger A, Brachvogel B, Kreft S, Bruckner-Tuderman L, et al. Differential proteomic analysis distinguishes tissue repair biomarker signatures in wound exudates obtained from normal healing and chronic wounds. J Proteome Res. 2010;9(9):4758–66. Epub 2010/07/30. 10.1021/pr100456d . [DOI] [PubMed] [Google Scholar]
- 7.Beidler SK, Douillet CD, Berndt DF, Keagy BA, Rich PB, Marston WA. Inflammatory cytokine levels in chronic venous insufficiency ulcer tissue before and after compression therapy. J Vasc Surg. 2009;49(4):1013–20. Epub 2009/04/04. 10.1016/j.jvs.2008.11.049 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kubo H, Hayashi T, Ago K, Ago M, Kanekura T, Ogata M. Temporal expression of wound healing-related genes in skin burn injury. Leg Med (Tokyo). 2014;16(1):8–13. Epub 2013/11/26. 10.1016/j.legalmed.2013.10.002 . [DOI] [PubMed] [Google Scholar]
- 9.Altavilla D, Saitta A, Cucinotta D, Galeano M, Deodato B, Colonna M, et al. Inhibition of lipid peroxidation restores impaired vascular endothelial growth factor expression and stimulates wound healing and angiogenesis in the genetically diabetic mouse. Diabetes. 2001;50(3):667–74. Epub 2001/03/15. . [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–95. Epub 2000/03/31. 10.1038/74651 . [DOI] [PubMed] [Google Scholar]
- 11.Fiedler U, Augustin HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol. 2006;27(12):552–8. Epub 2006/10/19. 10.1016/j.it.2006.10.004 . [DOI] [PubMed] [Google Scholar]
- 12.Radziwon-Balicka A, Ramer C, Moncada de la Rosa C, Zielnik-Drabik B, Jurasz P. Angiostatin inhibits endothelial MMP-2 and MMP-14 expression: a hypoxia specific mechanism of action. Vascul Pharmacol. 2013;58(4):280–91. Epub 2012/12/12. 10.1016/j.vph.2012.11.003 . [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues HG, Vinolo MA, Magdalon J, Vitzel K, Nachbar RT, Pessoa AF, et al. Oral administration of oleic or linoleic acid accelerates the inflammatory phase of wound healing. J Invest Dermatol. 2012;132(1):208–15. Epub 2011/09/02. 10.1038/jid.2011.265 . [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues HG, Vinolo MA, Magdalon J, Fujiwara H, Cavalcanti DM, Farsky SH, et al. Dietary free oleic and linoleic acid enhances neutrophil function and modulates the inflammatory response in rats. Lipids. 2010;45(9):809–19. Epub 2010/08/24. 10.1007/s11745-010-3461-9 . [DOI] [PubMed] [Google Scholar]
- 15.Magdalon J, Vinolo MA, Rodrigues HG, Paschoal VA, Torres RP, Mancini-Filho J, et al. Oral administration of oleic or linoleic acids modulates the production of inflammatory mediators by rat macrophages. Lipids. 2012;47(8):803–12. Epub 2012/06/15. 10.1007/s11745-012-3687-9 . [DOI] [PubMed] [Google Scholar]
- 16.Kato N, Hou Y, Lu Z, Lu C, Nagano H, Suzuma K, et al. Kallidinogenase normalizes retinal vasopermeability in streptozotocin-induced diabetic rats: potential roles of vascular endothelial growth factor and nitric oxide. Eur J Pharmacol. 2009;606(1–3):187–90. Epub 2009/04/21. 10.1016/j.ejphar.2009.01.027 . [DOI] [PubMed] [Google Scholar]
- 17.Sain J, Gonzalez MA, Lasa A, Scalerandi MV, Bernal CA, Portillo MP. Effects of trans-fatty acids on liver lipid metabolism in mice fed on diets showing different fatty acid composition. Ann Nutr Metab. 2013;62(3):242–9. Epub 2013/04/19. 10.1159/000339453 . [DOI] [PubMed] [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical biochemistry. 1976;72:248–54. . [DOI] [PubMed] [Google Scholar]
- 19.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22(9):849–55. Epub 2010/12/21. 10.1016/j.jnutbio.2010.07.009 . [DOI] [PubMed] [Google Scholar]
- 20.Lima MH, Caricilli AM, de Abreu LL, Araujo EP, Pelegrinelli FF, Thirone AC, et al. Topical insulin accelerates wound healing in diabetes by enhancing the AKT and ERK pathways: a double-blind placebo-controlled clinical trial. PLoS One. 2012;7(5):e36974 Epub 2012/06/05. 10.1371/journal.pone.0036974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YJ, Lian ZY, Liu G, Zhou HY, Yang HJ. RNA sequencing reveals retinal transcriptome changes in STZ-induced diabetic rats. Mol Med Rep. 2016;13(3):2101–9. Epub 2016/01/20. 10.3892/mmr.2016.4793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattila JT, Thomas AC. Nitric oxide synthase: non-canonical expression patterns. Front Immunol. 2014;5:478 Epub 2014/10/28. 10.3389/fimmu.2014.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YS, Huang WC, Li CW, Chuang LT. Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Mol Cell Biochem. 2011;358(1–2):85–94. Epub 2011/06/21. 10.1007/s11010-011-0924-0 . [DOI] [PubMed] [Google Scholar]
- 24.Sprecher H, VanRollins M, Sun F, Wyche A, Needleman P. Dihomo-prostaglandins and -thromboxane. A prostaglandin family from adrenic acid that may be preferentially synthesized in the kidney. J Biol Chem. 1982;257(7):3912–8. Epub 1982/04/10. . [PubMed] [Google Scholar]
- 25.Harkewicz R, Fahy E, Andreyev A, Dennis EA. Arachidonate-derived dihomoprostaglandin production observed in endotoxin-stimulated macrophage-like cells. J Biol Chem. 2007;282(5):2899–910. Epub 2006/12/01. 10.1074/jbc.M610067200 . [DOI] [PubMed] [Google Scholar]
- 26.Ghebremeskel K, Thomas B, Lowy C, Min Y, Crawford MA. Type 1 diabetes compromises plasma arachidonic and docosahexaenoic acids in newborn babies. Lipids. 2004;39(4):335–42. Epub 2004/09/11. . [DOI] [PubMed] [Google Scholar]
- 27.Campbell WB, Falck JR, Okita JR, Johnson AR, Callahan KS. Synthesis of dihomoprostaglandins from adrenic acid (7,10,13,16-docosatetraenoic acid) by human endothelial cells. Biochim Biophys Acta. 1985;837(1):67–76. Epub 1985/10/23. . [DOI] [PubMed] [Google Scholar]
- 28.Vijil C, Hermansson C, Jeppsson A, Bergstrom G, Hulten LM. Arachidonate 15-lipoxygenase enzyme products increase platelet aggregation and thrombin generation. PLoS One. 2014;9(2):e88546 Epub 2014/02/18. 10.1371/journal.pone.0088546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conrad DJ, Kuhn H, Mulkins M, Highland E, Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992;89(1):217–21. Epub 1992/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabbs M, Leng S, Devassy JG, Monirujjaman M, Aukema HM. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv Nutr. 2015;6(5):513–40. Epub 2015/09/17. 10.3945/an.114.007732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wicks K, Torbica T, Mace KA. Myeloid cell dysfunction and the pathogenesis of the diabetic chronic wound. Semin Immunol. 2014;26(4):341–53. Epub 2014/06/24. 10.1016/j.smim.2014.04.006 . [DOI] [PubMed] [Google Scholar]
- 32.Bannon P, Wood S, Restivo T, Campbell L, Hardman MJ, Mace KA. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech. 2013;6(6):1434–47. Epub 2013/09/24. 10.1242/dmm.012237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acosta JB, del Barco DG, Vera DC, Savigne W, Lopez-Saura P, Guillen Nieto G, et al. The pro-inflammatory environment in recalcitrant diabetic foot wounds. Int Wound J. 2008;5(4):530–9. Epub 2008/11/14. 10.1111/j.1742-481X.2008.00457.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noda K, Nakao S, Ishida S, Ishibashi T. Leukocyte adhesion molecules in diabetic retinopathy. J Ophthalmol. 2012;2012:279037 Epub 2011/12/02. 10.1155/2012/279037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matesanz N, Jewhurst V, Trimble ER, McGinty A, Owens D, Tomkin GH, et al. Linoleic acid increases monocyte chemotaxis and adhesion to human aortic endothelial cells through protein kinase C- and cyclooxygenase-2-dependent mechanisms. J Nutr Biochem. 2012;23(6):685–90. Epub 2011/08/16. 10.1016/j.jnutbio.2011.03.020 . [DOI] [PubMed] [Google Scholar]
- 36.Fang IM, Yang CH, Yang CM. Docosahexaenoic acid reduces linoleic acid induced monocyte chemoattractant protein-1 expression via PPARgamma and nuclear factor-kappaB pathway in retinal pigment epithelial cells. Mol Nutr Food Res. 2014;58(10):2053–65. Epub 2014/07/22. 10.1002/mnfr.201400196 . [DOI] [PubMed] [Google Scholar]
- 37.Brasken P. Healing of experimental colon anastomosis. Eur J Surg Suppl. 1991;(566):1–51. Epub 1991/01/01. . [PubMed] [Google Scholar]
- 38.Schumann T, Adhikary T, Wortmann A, Finkernagel F, Lieber S, Schnitzer E, et al. Deregulation of PPARbeta/delta target genes in tumor-associated macrophages by fatty acid ligands in the ovarian cancer microenvironment. Oncotarget. 2015;6(15):13416–33. Epub 2015/05/15. 10.18632/oncotarget.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shook B, Xiao E, Kumamoto Y, Iwasaki A, Horsley V. CD301b+ macrophages are essential for effective skin wound healing. J Invest Dermatol. 2016. Epub 2016/06/12. 10.1016/j.jid.2016.05.107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alzoghaibi MA, Zubaidi AM. Upregulation of the proinflammatory cytokine-induced neutrophil chemoattractant-1 and monocyte chemoattractant protein-1 in rats' intestinal anastomotic wound healing—does it matter? Asian J Surg. 2014;37(2):86–92. Epub 2013/09/26. 10.1016/j.asjsur.2013.07.016 . [DOI] [PubMed] [Google Scholar]
- 41.Neub A, Houdek P, Ohnemus U, Moll I, Brandner JM. Biphasic regulation of AP-1 subunits during human epidermal wound healing. J Invest Dermatol. 2007;127(10):2453–62. Epub 2007/05/15. 10.1038/sj.jid.5700864 . [DOI] [PubMed] [Google Scholar]
- 42.Ouahes N, Phillips TJ, Park HY. Expression of c-fos and c-Ha-ras proto-oncogenes is induced in human chronic wounds. Dermatol Surg. 1998;24(12):1354–7; discussion 8. Epub 1998/12/29. . [DOI] [PubMed] [Google Scholar]
- 43.Xi S, Pham H, Ziboh VA. Suppression of proto-oncogene (AP-1) in a model of skin epidermal hyperproliferation is reversed by topical application of 13-hydroxyoctadecadienoic acid and 15-hydroxyeicosatrienoic acid. Prostaglandins Leukot Essent Fatty Acids. 2000;62(1):13–9. Epub 2000/04/15. . [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Zeng X, Ma G, Li N, Li Y, Miao Q, et al. [Effects of interleukin-6 in epithelial-mesenchymal transition of Barrett's esophagus cells]. Zhonghua Yi Xue Za Zhi. 2014;94(4):296–300. Epub 2014/04/16. . [PubMed] [Google Scholar]
- 45.Tang J, Liu H., Gao C., Mu L., Yang S., Rong M., Zhang Z., Liu J., Ding Q., Lai R.. A Small Peptide with Potential Ability to Promote Wound Healing. PLoS One. 2014;9(3):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. Faseb J. 2000;14(15):2525–31. Epub 2000/12/02. 10.1096/fj.00-0073com . [DOI] [PubMed] [Google Scholar]
- 47.Qin Z, Okubo T, Voorhees JJ, Fisher GJ, Quan T. Elevated cysteine-rich protein 61 (CCN1) promotes skin aging via upregulation of IL-1beta in chronically sun-exposed human skin. Age (Dordr). 2014;36(1):353–64. Epub 2013/07/25. 10.1007/s11357-013-9565-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paraguassú GM, Xavier F.C.A, Cangussu M.C.T, Ramalho M.J.P, Cury P.R, dos Santos J.N, Pinheiro A.L.B, Ramalho L.M.P. Effect of Laser Phototherapy (k660 nm) on Type I and III Collagen Expression During Wound Healing in Hypothyroid Rats: An Immunohistochemical Study in a Rodent Model. Photomedicine and Laser Therapie. 2014;32(5):1–8. [DOI] [PubMed] [Google Scholar]
- 49.Naaldijk Y, Johnson AA, Ishak S, Meisel HJ, Hohaus C, Stolzing A. Migrational changes of mesenchymal stem cells in response to cytokines, growth factors, hypoxia, and aging. Exp Cell Res. 2015;338(1):97–104. Epub 2015/09/04. 10.1016/j.yexcr.2015.08.019 . [DOI] [PubMed] [Google Scholar]
- 50.Hutton DL, Kondragunta R, Moore EM, Hung BP, Jia X, Grayson WL. Tumor necrosis factor improves vascularization in osteogenic grafts engineered with human adipose-derived stem/stromal cells. PLoS One. 2014;9(9):e107199 Epub 2014/09/24. 10.1371/journal.pone.0107199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111(10):4997–5007. Epub 2008/03/14. 10.1182/blood-2007-08-108597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodnar RJ. Chemokine Regulation of Angiogenesis During Wound Healing. Adv Wound Care (New Rochelle). 2015;4(11):641–50. Epub 2015/11/07. 10.1089/wound.2014.0594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12(9):551–64. Epub 2011/08/24. 10.1038/nrm3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chin LC, Kumar P, Palmer JA, Rophael JA, Dolderer JH, Thomas GP, et al. The influence of nitric oxide synthase 2 on cutaneous wound angiogenesis. Br J Dermatol. 2011;165(6):1223–35. Epub 2011/09/08. 10.1111/j.1365-2133.2011.10599.x . [DOI] [PubMed] [Google Scholar]
- 55.Geng L, Chaudhuri A, Talmon G, Wisecarver JL, Wang J. TGF-Beta suppresses VEGFA-mediated angiogenesis in colon cancer metastasis. PLoS One. 2013;8(3):e59918 Epub 2013/03/29. 10.1371/journal.pone.0059918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci. 2013;92(22):1037–45. Epub 2013/04/23. 10.1016/j.lfs.2013.04.001 . [DOI] [PubMed] [Google Scholar]
- 57.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, et al. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45(12):2077–86. Epub 2009/06/06. 10.1016/j.ejca.2009.04.026 . [DOI] [PubMed] [Google Scholar]
- 58.Luo X, Jia R, Yao Q, Xu Y, Luo Z, Wang N. Docosahexaenoic acid attenuates adipose tissue angiogenesis and insulin resistance in high fat diet-fed mid-aged mice via a sirt1-dependent mechanism. Mol Nutr Food Res. 2016. Epub 2016/01/12. 10.1002/mnfr.201500714 . [DOI] [PubMed] [Google Scholar]
- 59.Fang Y, Shen J, Yao M, Beagley KW, Hambly BD, Bao S. Granulocyte-macrophage colony-stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines. Br J Dermatol. 2010;162(3):478–86. Epub 2009/10/06. 10.1111/j.1365-2133.2009.09528.x . [DOI] [PubMed] [Google Scholar]
- 60.Nishioka N, Matsuoka T, Yashiro M, Hirakawa K, Olden K, Roberts JD. Linoleic acid enhances angiogenesis through suppression of angiostatin induced by plasminogen activator inhibitor 1. Br J Cancer. 2011;105(11):1750–8. Epub 2011/10/22. 10.1038/bjc.2011.434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Radziwon-Balicka A, Moncada de la Rosa C, Zielnik B, Doroszko A, Jurasz P. Temporal and pharmacological characterization of angiostatin release and generation by human platelets: implications for endothelial cell migration. PLoS One. 2013;8(3):e59281 Epub 2013/04/05. 10.1371/journal.pone.0059281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staton CA, Valluru M, Hoh L, Reed MW, Brown NJ. Angiopoietin-1, angiopoietin-2 and Tie-2 receptor expression in human dermal wound repair and scarring. Br J Dermatol. 2010;163(5):920–7. Epub 2010/07/17. 10.1111/j.1365-2133.2010.09940.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results are presented as mean ± SD of 7 animals in each group. (#) Indicates differences in relation to D (10d –p = 0.04; 16d –p = 0.03; 18d –p = 0.03). Glycemia of rats during the wound healing process: (D) diabetic; (DOA) diabetic rats treated with OA. Dashed line indicates the mean of glycemia in control rats.
(TIF)
(DOCX)
Data Availability Statement
All the relevant data are within the paper.