Abstract
Membrane vesicles (MVs) are spherical particles naturally released from the membrane of Gram-negative bacteria. Bacterial MV production is associated with a range of phenotypes including biofilm formation, horizontal gene transfer, toxin delivery, modulation of host immune responses and virulence. This study reports comparative profiling of MVs from bacterial strains isolated from three widely disperse geographical areas. Mass spectrometry identified 119, 159 and 142 proteins in MVs from three different strains of Piscirickettsia salmonis isolated from salmonids in Chile (LF-89), Norway (NVI 5692) and Canada (NVI 5892), respectively. MV comparison revealed several strain-specific differences related to higher virulence capability for LF-89 MVs, both in vivo and in vitro, and stronger similarities between the NVI 5692 and NVI 5892 MV proteome. The MVs were similar in size and appearance as analyzed by electron microscopy and dynamic light scattering. The MVs from all three strains were internalized by both commercial and primary immune cell cultures, which suggest a potential role of the MVs in the bacterium’s utilization of leukocytes. When MVs were injected into an adult zebrafish infection model, an upregulation of several pro-inflammatory genes were observed in spleen and kidney, indicating a modulating effect on the immune system. The present study is the first comparative analysis of P. salmonis derived MVs, highlighting strain-specific vesicle characteristics. The results further illustrate that the MV proteome from one bacterial strain is not representative of all bacterial strains within one species.
Introduction
Membrane vesicles (MVs) are 50 to 250 nm spherical structures, enclosed by a single or double membrane, secreted from the surface of many Gram-negative bacteria during all stages of growth [1–3]. Proteomic and biochemical characterization has revealed that the vesicles contain a variety of bacterial components, including periplasmic and outer membrane proteins as well as lipopolysaccharides (LPS), DNA, RNA and cytoplasmic proteins [4–7]. Together they represent several aspects of the bacteria, but in a non-replicative form. MVs have also been reported to contain several important immunogenic factors, such as toxins [8], chaperons [9], and active enzymes [5]. The mechanisms of the MV formation and their biological role have yet to be clearly defined. However, bacterial MV secretion has been associated with several phenotypes including biofilm formation [10], bacterial survival [11], toxin delivery [12], cell-to-cell communication [13], and host-pathogen interactions [14]. MVs from infectious agents have also been found in both tissue and fluid samples from patients [15–17], indicating that the vesicle secretion plays an important role in the bacterial pathogenesis. The MV secretion is shown to be upregulated during stress and environmental changes [11]. These include treatment with membrane active antibiotics, nutrient depletion, temperature alteration and chemical exposure [18–21], alterations which the bacterium may encounter both in its natural environment and within a host. Alterations in MV production upon environmental changes can be exemplified by the human opportunistic pathogen Pseudomonas aeruginosa, which increases its secretion of MVs during treatment with gentamycin [18]. Similar observations have been done for Shigella dysenteriae serotype 1, which displayed a significantly higher concentration of the shiga toxin inside the MVs when treated with mitomycin C [19]. Pathogenic bacteria, in general, have the tendency to produce more MVs compared to their non-pathogenic counterparts, and for marine bacteria the vesicle production is reported to be important for survival [22–24]. Marine isolates of Alteromonas have been shown not only to persist, but also to grow in seawater media when supplemented with purified MVs from Prochlorococcus. In contrast, the control group displayed a reduced viability when grown in non-supplemented seawater media [24–25]. Furthermore, comparative MV protein profiling of two clinical isolates of Acinetobacter baumannii has revealed potential strain-specific links between vesicle content and virulence factors [26]. Taken together, it illustrates that the release of MVs may play an important role for bacterial survival and pathogenesis within a host.
In a host, isolated MVs have been shown to induce an immune response by activating the production of various cytokines, and have therefore been investigated and used as vaccines [4, 27]. However, modulating the immune response could also be beneficial for bacterial pathogens [28]. For a pathogen to successfully establish an infection, it needs to overcome the host’s initial immune defense [29]. The human pathogen Moraxella catarrhalis has been shown to utilize MV secretion in order to modify the B-cell response, to avoid direct contact with the host’s immune cells [30]. Furthermore, MVs form both Helicobacter pylori, Pseudomonas aeruginosa and Neisseria gonorrhea has been shown to upregulate the expression of nuclear factor NF-κB and the intracellular pattern recognition receptor NOD1 in vitro, promoting inflammation and pathology in infected hosts [31]. NF-κB is shown to be important for regulating the expression of several inflammatory and immune genes [32], while NOD1 has been described as a key pathogen recognition molecule (PRM) for the innate immune response [33]. Thus, the release of MVs interacting with the immune response could be beneficial for the pathogen in order to fight of the host’s defense system. Nonetheless, MV-based vaccines have successfully been used for epidemic control in Cuba, Norway, Brazil, and New Zealand against serogroup B meningococcal disease [34–37]. MVs used in vaccination of fish have also been reported to give good protection against Edwardsiella tarda in olive flounder (Paralichthys olivaceus) [38], Flavobacterium psychrophilum in rainbow trout (Oncorhynchus mykiss) [39], and Francisella noatunensis in zebrafish (Danio rerio) [40].
The Gram-negative intracellular bacterium Piscirickettsia salmonis is the etiologic agent of salmonid rickettsial septicaemia (SRS), a chronic and often fatal disease in salmonid and a variety of marine fish species [41–42]. P. salmonis was and characterized from Coho salmon (Oncorhyncus kisutch) in 1989 after a devastating epizootic in the Chilean aquaculture industry [41]. The bacteria has since then been recognized as an emerging problem as outbreaks of SRS has been reported across the world [43–45]. Strains of P. salmonis has been identified in salmon net-pens in Norway, Canada, Ireland and Scotland, but with a reduced virulence compared to the Chilean strains [46]. P. salmonis has been shown to infect, replicate and survive within macrophages as a part of its infection strategy. The infection process includes the formation of vacuoles within the host cells, enabling the bacterium to avoid the fish’s primary immune defense [44, 47–49]. The mechanisms behind P. salmonis ability to utilize macrophages are still poorly understood, but a Dot/Icm Type IV Secretion System homolog, has been identified within the genome of P. salmonis, and might be involved in the inhibition of phagosome-lysosome fusion during infection [50]. Furthermore, the heat shock protein ClpB and virulence factor BipA, proteins known to modulate the host cells defense mechanisms, has been reported to be expressed by the bacterium [51]. Nonetheless, the specific function of the Dot/Icm system, ClpB and BipA during SRS are still unknown. Thus, the mechanisms behind P. salmonis pathogenesis are poorly understood and further research is needed to characterize the bacterium.
MVs are of interest as they are considered to be important virulence factor and the secretion of MVs from P. salmonis was recently described for LF-89 [52]. As a geographic difference in virulence of SRS outbreaks have been reported [46], the present study focused on evaluating potential strain-specific differences in MV properties using three geographically disperse isolates of the bacterium including P. salmonis isolated from Norway (NVI 5692), Canada (NVI 5892) and Chile (LF-89). The identification and comparison of the proteins packed into MVs were analyzed to give new insight to the adaptation and virulence of P. salmonis. We show that intact MVs can be isolated from the three P. salmonis strains. Comparative MV profiling revealed several strain-specific factors, and in depth-analysis revealed that the vesicles contain a variety of proteins and that MVs may have a biological function both in vivo and in vitro.
Material and Methods
Bacterial Strains and growth conditions
Three isolates of P. salmonis were used for the characterization of MVs: LF-89 (type-strain ATCC VR 1361) isolated from Coho salmon (Oncorhyncus kisutch) in Chile [41], and NVI 5692 and NVI 5892 isolated from Atlantic salmon (Salmon salar) in Norway and Canada, respectively [53] (Kindly donated by Duncan J. Colquhoun, Norwegian University of Life Science). All three isolates were routinely grown at 20°C on Eugon Chocolate Agar (ECA), containing 30.4 g/L BD Bacto TM Eugon Broth (Becton, Dickinson and Company, Franklin lakes, NJ, USA), 15 g/L Agar Bacteriological (Thermo Fisher Scientific, Hudson, NH, USA) and 5% bovine blood (Håtunalab AB) [54] or in EBFC containing BD Bacto TM Eugon Broth supplemented with 2 mM FeCl3 (Sigma-Aldrich Co., St. Louis, MO, USA) and 1% Casamino Acids (BD) with agitation (100 rpm) for 7–10 days, depending on the isolate. The bacterial stocks used were frozen in autoclaved 10% skimmed milk (BD Difco) or in BD Bacto TM Eugon Broth supplemented with 20% glycerol (Sigma-Aldrich) and stored at—80°C.
Purification and fluorescent labeling of MVs from Piscirickettsia salmonis
10 mL of exponential-growth phase cultures of each P. salmonis isolate was used to inoculate 200 mL of EBFC. The cells were grown at 20°C with agitation, and growth curves were measured by using optical density reading at 600 nm until the isolates reached late exponential-phase. MVs were isolated as described [40]. In short, the bacterial cells were removed by centrifugation (10 minutes, 15 000 g, 4°C), and the supernatant filtered sequentially through a 0.45- and 0.22 μm/pore filter in order to remove the remaining bacterial cells. The filtrate was then ultra-centrifuged sequentially at 125 000 g at 4°C for 2 hours and 125 000 g at 4°C for 30 minutes, to eliminate cell debris and aggregates. The MVs were resuspended in 100 μL 1x phosphate buffered saline (PBS) pH 7.2, and protein concentration determined by a Picodrop spectrophotometer (Picodrop Limited, UK). MV aliquots (10 μL) were spread onto ECA plates to check for sterility, and the remaining sample was stored at -80°C until use. A Zetasizer Nano ZS (Malvern instruments Ltd., UK) was used to conduct dynamic light scattering measurements, to determine the MVs size [55]. A velocity gradient centrifugation was preformed to evaluate the purity of the MV isolation, and each layer of the gradient investigated by transmission electron microscopy for quality control [7]. The labeling of MVs with fluorescein isothiocyanate (FITC; Sigma-Aldrich, USA) was done according to the method described [56], with some minor modifications. Vesicles were incubated for 1 hour at 25°C with 1 mg/mL isothiocyanate and pelleted at 25 900 rpm for 30 minutes. The FITC-labeled MVs were then washed three timed with 50 mM HEPES, resuspended in PBS and monitored for sterility and protein concentration as described above.
Isolation of outer- and inner membranes using water lysis
10 mL of exponential-growth phase cultures of each P. salmonis isolate was used to inoculate 200 mL of EBFC. The cells were grown at 20°C with agitation until the isolates reached late exponential-phase. For the preparation of mixed membrane fractions the cell cultures were split into four sterile 50 mL Falcon tube and a water lysis protocol was used [57]. The mixed membranes were separated by a linear sucrose gradient of: 55%, 50%, 45%, 40%, 35% and 30% (w/w) sucrose in Tris-EDTA buffer. The mixed membrane samples were placed on top of each gradient and the samples ultra-centrifuged at 38 000 rpm at 4°C for 17 hours. The sucrose layers were carefully removed and the membrane fractions harvested, the inner membranes were at the 35–40% interface and the outer membranes at the 50–55% interface. The protein concentration was determined by a Picodrop spectrophotometer, and membrane aliquots (10 μL) were spread onto ECA plates to check for sterility. The remaining samples were stored at -80°C until use.
SDS-PAGE
A standard SDS-PAGE procedure was used [58]. Briefly, 20 μg of membrane fractions and MVs isolated from LF-89, NVI 5692 and NVI 5892 was loaded onto a 12% (w/v) SDS polyacrylamide gel. The proteins separated through SDS-PAGE were stained with Coomassie Blue, and the image was acquired and evaluated using Gel doc™ XR+ with Image Lab™ software (Bio-Rad, Munich, Germany). Protein molecular weight standards were obtained from Bio-Rad.
Electron Microscopy
For transmission electron microscopy carbon coated Formvar copper grids were placed on a drop of MV suspension for 5 minutes. The grids were then washed three times with PBS and the samples were fixed in 1% glutaraldehyde (Sigma-Aldrich) for 4 minutes. The samples were washed three times with PBS, two times with Milli-Q (MQ) water, stained for 20 seconds with 4% uranyl acetate (Sigma-Aldrich) in MQ water, washed once with MQ water and finally left on a solution of (9:1) methyl-cellulose (Sigma-Aldrich) with 4% uranyl acetate for 10 minutes on ice. The grids were then dried and viewed in a Philips CM200 transmission electron microscope and the images were acquired using the iTEM software (Olympus, PA, USA). For scanning electron microscopy a drop of bacterial suspension was placed on pre-coated poly-L-lysine (Sigma-Aldrich) coverslips (Thermo Scientific) and fixed overnight at 4°C with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer pH 7.4. The coverslips were then washed twice in 0.1M sodium cacodylate buffer pH 7.4 for 10 minutes, and the samples dehydrated in a graded ethanol series for 10 minutes at 70%, 90%, 96% and 100% and for 15 minutes 4 times in 100% ethanol. Dehydrated samples were subsequently critical-point dried using carbon dioxide in a CPD 030 critical-point dryer (Bal-Tec, CA, USA), then mounted on stub with carbon-circles colloidal silver and sputter coated with a Cressington coating system 308R. The samples were viewed in a Hitachi S-4800 scanning electron microscopy, and images acquired using Scandium software (Olympus)
Liquid chromatography-mass spectrometry
Three biological replicates of MVs harvested from P. salmonis LF-89, NVI 5692 and NVI 5892 were diluted to 40 μg of total protein in PBS and the samples were centrifuged at 16,000 g for 20 minutes at 4°C (Centrifuge 5415R, Eppendorf, Hamburg, Germany) and the supernatant discarded. Proteins were re-dissolved in 50 μL 6 M urea and 100 mM ammonium bicarbonate, pH 7.8. For reduction and alkylation of cysteines, 2.5 μL of 200 mM DTT in 100 mM Tris-HCl, pH 8 was added and the samples were incubated at 37°C for 1 hour followed by addition of 7.5 μL 200 mM iodoacetamide for 1 hour at room temperature in the dark. The alkylation reaction was quenched by adding 10 μL 200 mM DTT at 37°C for 1 hour. Subsequently, the proteins were digested with 10 μg trypsin (Promega, sequencing grade) overnight at 37°C. The digestion was stopped by adding 5 μL 50% formic acid and the generated peptides were purified using a ZipTip C18 (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions, and dried using a Speed Vac concentrator (Concentrator Plus, Eppendorf, Hamburg, Germany). The tryptic peptides were analyzed using an Ultimate 3000 nano-UHPLC system connected to a Q Exactive mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with a nano electrospray ion source (S1 File). The MVs from NVI 5692 and NVI 5892 were analyzed as routinely performed by the Australian Proteome Analysis Facility (APAF) and MVs from LF-89 by the Proteomic unit at the University of Oslo.
Proteomic data analysis
Raw spectra files were converted into mgf format and processed using the global proteome machine (GPM) software with version 2.2.1 of X!Tandem algorithm [59] and a nonredundant output file was generated for protein identifications with log (e) values less than -1. Peptide identification was determined using a 0.8 Da fragment ion tolerance. Protein sequences extracted from the genome of LF-89 = ATCC VR-1361 [60] were used as the search database. A database of reversed sequences was searched to determine the false discovery rate at protein level. The three protein identification output files from each biological replicate of peptide samples were combined together to produce a single merged output file for each strains MV fraction. To ensure data quality, identified proteins were filtered based on two criteria: reproducible identification across three replicates and a total spectral count of >6, making the minimum number of peptides used to identify each protein an average value of 2 per replicate [61]. The subcellular location and functions for each of the identified MV proteins was predicted using PSORTb 3.0.2 [62] and their gene otology (GO) molecular function derived from The UniProt database [63]. The proteins were also subject to in silico analysis using VirulentPred, which predicts bacterial virulence proteins based on their sequences information [64].
Flow analysis of MVs in fish cells
Zebrafish were anesthetized in tricaine methanesulfonate (MS-222, Sigma-Aldrich). Kidney and spleen were isolated as described [65]. Ten whole kidneys or spleens were pooled in 1 mL media; Leibovitz L-15 medium (Gibco) supplemented with 2% fetal bovine serum (FBS), penicillin (10 μg/mL) and streptomycin (10 μg/mL). Single-cell suspensions were generated by gentle teasing of the tissue on a 40 μm cell strainer with a plunger from a 1 mL syringe, collected in a 50 mL tube and rinsed twice with 1 mL media. The cells were cultivated in a concentration of 1x106 cells/mL in 24 well plates. The SHK-1 Salmo salar macrophage-like cell line (passage 58) was maintained at 20°C in L-15 medium supplemented with 15% FBS in 25 cm2 flasks. For the microscopy imaging to observe cytopathic effect at different time points, 3 x105 cells/mL were cultivated in μ-slide IV (Ibidi) with 20 μg/mL of MVs. Cells were analyzed with a Nikon inverted Microscope ECLIPSE TE300. The ability of kidney, spleen or SHK-1 cells to endocytose MVs in vitro was measured following methods described previously [66]. Briefly, 1 mL of cells (1x106 cells) per sample was incubated for 1 hour at 20°C with 10 μg, 20 μg or 40 μg of FITC-MVs. After the incubation with MVs, the cells were washed three times with ice-cold phosphate buffered saline (PBS) and analyzed by flow cytometry using a Beckman Coulter (GaLLios). At least 10.000 events were collected for each sample. Data were analyzed using Kaluza software v.1.2 (Beckman Coulter) and macrophage/lymphocytes gated using Side scatter (SSC) (granularity) and Forward scatter (FSC) (size) parameters. Discrimination of aggregates from singlets was preformed using side scatter-W (SSC-W) versus side scatter (SSC) and Hoechst stains were used for the separation of dead and live cells. The fluorescence of the FITC conjugated MVs was measured before and after the addition of trypan blue (0,025% final concentration), to quench extracellular fluorescence. Incorporation of MVs-FITC was measured at 520 nm (FL1). The significant differences in percentage of MV uptake for each cell type was calculated using a Two-way ANOVA, Tukey`s multiple comparison test.
Intraperitoneal injection of Piscirickettsia salmonis derived MVs in adult zebrafish
The biological effect of MVs in vivo were assessed by using 10–11 months old male and female Zebrafish Danio rerio wild type strain AB obtained from the model fish unit at the Norwegian University of Life Science. The fish were acclimatized to room temperature (20 ± 2°C) two weeks prior to the experimental setup. The fish were fed every morning with brine shrimp (Scanbur AS, Nittedal, Norway) and SDS 400 Scientific Fish Food (Scanbur AS) in the afternoon. Experimental groups of 20 fish were anesthetized by immersion in water containing 100 mg/mL tricaine methanesulfonate (MS-222, Sigma Aldrich) buffered with bicarbonate to pH 7–7.5. The fishwere injected intraperitoneally (i.p.) with 20 μL of PBS, 1x108 colony forming units (CFU) of LF-89, NVI 5692, NVI 5892 or a total of 40 μg MVs in PBS isolated from LF-89, NVI 5692 and NVI 5892 respectively, by using a 27 g needle [40, 67]. After injection, the fish were immediately returned to recovery tanks and kept in separate 6-liters polycarbonate tanks (Pentair, USA), in which 50% of the water was manually changed daily. Fish that did not resume normal behavior after the injections were removed from the experiment and euthanized with an overdose of 250 mg/mL tricaine methanesulfonate. The water was provided by the model fish unit at the Norwegian University of Life Science and was supplemented with 0.55 g/L Instant Ocean sea salt, 0.053 g/L Sodium Bicarbonate and 0.015 g/L Calcium Chloride. The tanks were housed in a water-system with a controlled temperature (20°C) and with a cycle consisting of 14 hours of light and 10 hours of darkness. The fish were closely monitored, and the animal’s health recorded twice a day. Moribund or fish that clearly showed deviant behavior and clinical symptoms not consistent with good animal welfare (greatly reduced level of activity, response to environment and appetite), were euthanized as previously described. Water parameters were monitored every third day using commercial test kits (TetraTest kit): pH, NO2-, NO32-, NH3/NH4+ and water hardness. All zebrafish experiment was approved by NARA (The Norwegian Animal Research Authority) and waste water decontaminated by chlorination and tested for sterility before disposal.
RNA isolation and quantitative real-time PCR
For RNA isolation, three randomly chosen fish from each experimental group were sacrificed by an overdose of tricaine methanesulfonate (250 mg/mL) after 14 days, and kidney and spleen harvested. The organs were kept in RNAlater (Ambion) and stored at 4°C until further processing. The tissue was homogenized in 600 μL with buffer RLT (supplemented in RNeasy Mini Kit, QIAGEN) using a mortar and pestle (Sigma-Aldrich), followed by passing the lysate through a blunt 20 gauge needle fitted to a small 1 mL syringe (BD). Total RNA was extracted using the QIAGEN RNeasy kit according to the manufactures instructions, including a 15 minute on-column DNase treatment using an RNase-free DNase set (QIAGEN). The RNA was diluted in 30 μL RNase-free H2O (QIAGEN). RNA quantity and quality was measured with a Picodrop spectrophotometer. Reverse transcription reaction was performed by using High Capacity RNA to cDNA kit (Applied Biosystems). Quantitative real-time PCR (RT-qPCR) was carried out for each of the sampling points for a defined set of genes. These included major histocompatibility complex II (MHC II), cluster of differentiation 40 (cd40), interferon gamma (ifnγ), tumor necrosis factor alpha (tnfα), suppressors of cytokine signaling 3a and 3b (socs3a and socs3b), macrophage expressed gene 1 (mpeg1), nucleotide binding and oligomerization domain 1 and 2 (nod1 and nod2), and the six interleukins: il-1β, il-6, il-8, il-10 and il-12a. QuantiTec bioinformatically validated primers were obtained from QIAGEN (Hilden, Germany) for most of the genes used; the remaining primers were obtained from Life Technologies Inc. (Carlsbad, CA, USA). Primers are listed in S1 Table. RT-qPCR was performed in triplicates using a Lightcycler® 480 (Roche, Basel, Switzerland) as previously described [54]. 18S ribosomal RNA (18S) and Elongation factor-1 alpha (ef-1α) were used as reference genes for the normalization of the relative transcription levels of each gene, and the normalized immune response data of MV injected fish was standardized against the transcription levels of PBS injected fish for each time point. The significance of difference in relative gene expression levels between MV or bacterial challenges fish and PBS injected fish was calculated by a Student t test assuming unequal variance.
Results and Discussion
Isolation and phenotypic characterization of Piscirickettsia salmonis MVs
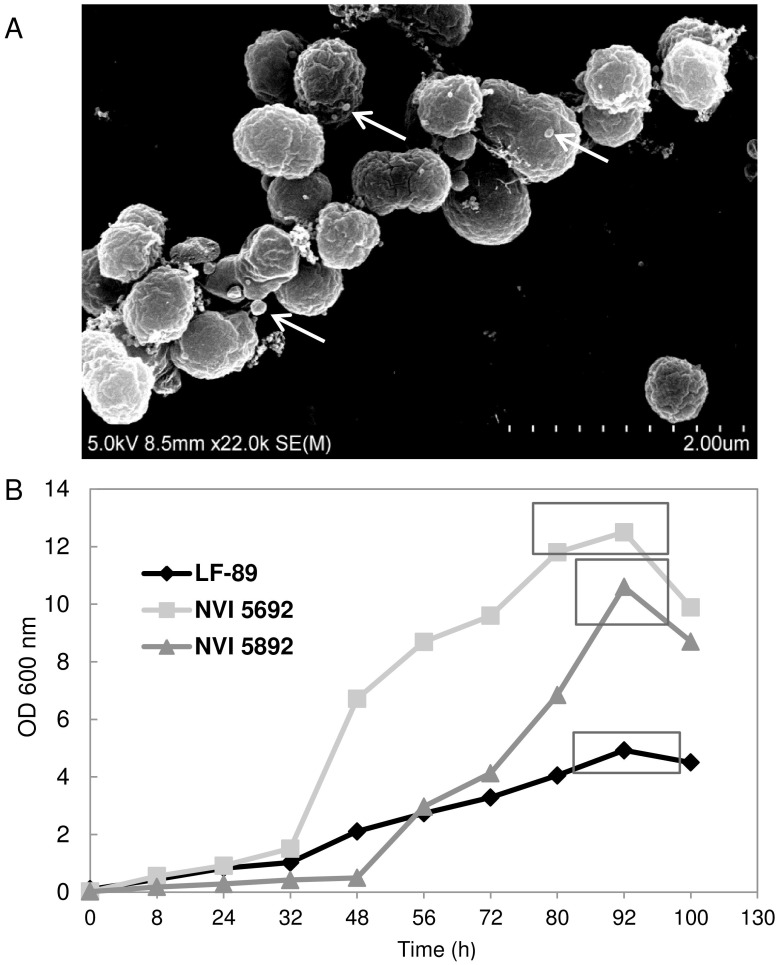
The P. salmonis derived MVs were observed on the surface of bacterial cells when exanimated by scanning electron microscopy, demonstrating that vesicles may bud off from the bacterial membrane during growth in liquid medium (Fig 1A). All three strains of P. salmonis, LF-89, NVI 5692 and NVI 5892 produced small spherical MVs when grown to late exponential-phase in EBFC medium. In vitro growth has previously been obtained for P. salmonis up to an optical density of OD620 = 1.8 in AUSTRAL-SRS medium [68] and OD600 = 2 in BM1 [69]. For NVI 5692 and NVI 5892 the late exponential to stationary growth-phase is reached at an optical density of OD60 0 = 10–12 with a measured CFU ~5x109 in EBFC (Fig 1B). LF-89 expressed a reduced growth pattern in comparison to the other strains, reaching its late exponential to stationary growth-phase at an optical density of OD600 = 4–5 with CFU measured to ~2x109 (Fig 1B). Thus, the EBFC medium enhances the optimal growth of the three P. salmonis isolates up to six fold from previously published media. The three strains do, however express a divergent growth pattern in EBFC reaching different optical densities within the same period, which could potentially affect the MV production, lipid content and protein composition. As NVI 5692 and NVI 5892 reach their exponential-phase between OD600 = 10–12, the two strains will have a higher cell density prior to the isolation compared to LF-89, thus the bacterial cultures were diluted to an equal cell number before harvesting the vesicles [70]. Alternatively, MVs could be harvested from NVI 5692 and NVI 5892 at an optical density of OD600 = 4–5, but the cultures would then not have been in late exponential-phase in contrast to LF-89. MVs isolated from P. aeruginosa grown in both cultures has been reported to display differences in both lipid and protein composition when harvested from exponential and stationary phase. These data indicate that the MV properties could be growth phase dependent [71]. Furthermore, as the different stages of bacterial growth has been reported to affect protein expression in general [72–73], harvesting MVs from cultures in an equal growth phase is preferable over a variation in cell density.
Fig 1. Identification and isolation of membrane vesicles isolated from Piscirickettsia salmonis.
(A) Bacterial cultures of P. salmonis NVI 5692 grown in EBFC is viewed by scanning election microscopy. Arrows indicate MVs secreted from the bacterial cells. (B) Growth curves of P. salmonis in EBFC medium, square show time point for isolation of MV (n = 3).
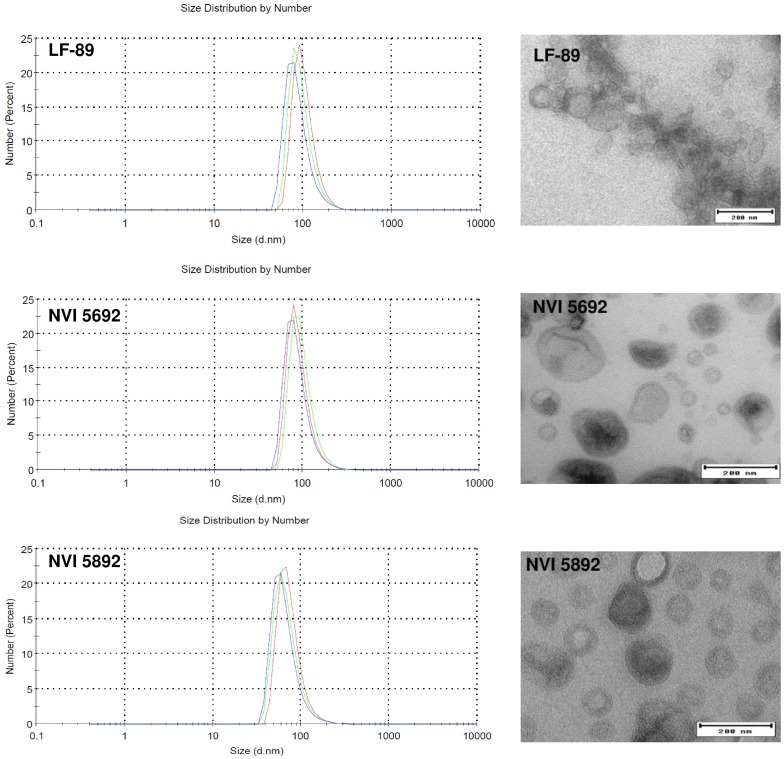
Examination of the MVs isolated from EBFC medium by transmission electron microscopy and dynamic light scattering revealed a phenotypical similarity between the three strains of P. salmonis, analogues in both size and distribution (Fig 2). MVs from all three strains were also dominated by double membrane vesicles (S1 Fig), indicating that the MVs contain both an plasma and outer membrane, similar to what has been described for other Gram-negative bacteria [3]. Vesicles isolated from LF-89 had a higher tendency to form clusters of MVs compared to NVI 5692 and NVI 5892, however, some collections of vesicles were observed for all strains. Both image analysis using the iTEM software and Dynamic light scattering was used to determine the size distribution for the MVs between the three stains of P. salmonis. All strains were shown to have MVs ranging from 10–220 nm in size, with an average between 80–100 nm (Fig 2), similar to what has been reported for other bacterial species [5, 74–75]. The isolated vesicles from all three strains of P. salmonis were also compared to their outer membrane fractions by SDS-PAGE and coomassie blue staining (S2 Fig). Although not directly quantitative, coomassie blue staining is useful to examine differences in protein composition between samples. For all three strains of P. salmonis the vesicles resembled, but were not identical to the membrane fractions. This indicates that the vesicles are purified fractions and not membranes from lysed bacterium; however, the presence of non-MV associated material cannot be completely excluded.
Fig 2. Size distribution and imaging analysis of Piscirickettsia salmonis membrane vesicles.
Vesicle size and range analyzed by dynamic light scattering (left panels) (n = 3) and electron transmission microscopy imaging (right panels) of MVs isolated from LF-89, NVI 5692 and NVI 5892. Bar size, 200 nm.
Identification of MV proteins from Piscirickettsia salmonis and their predicted subcellular distribution
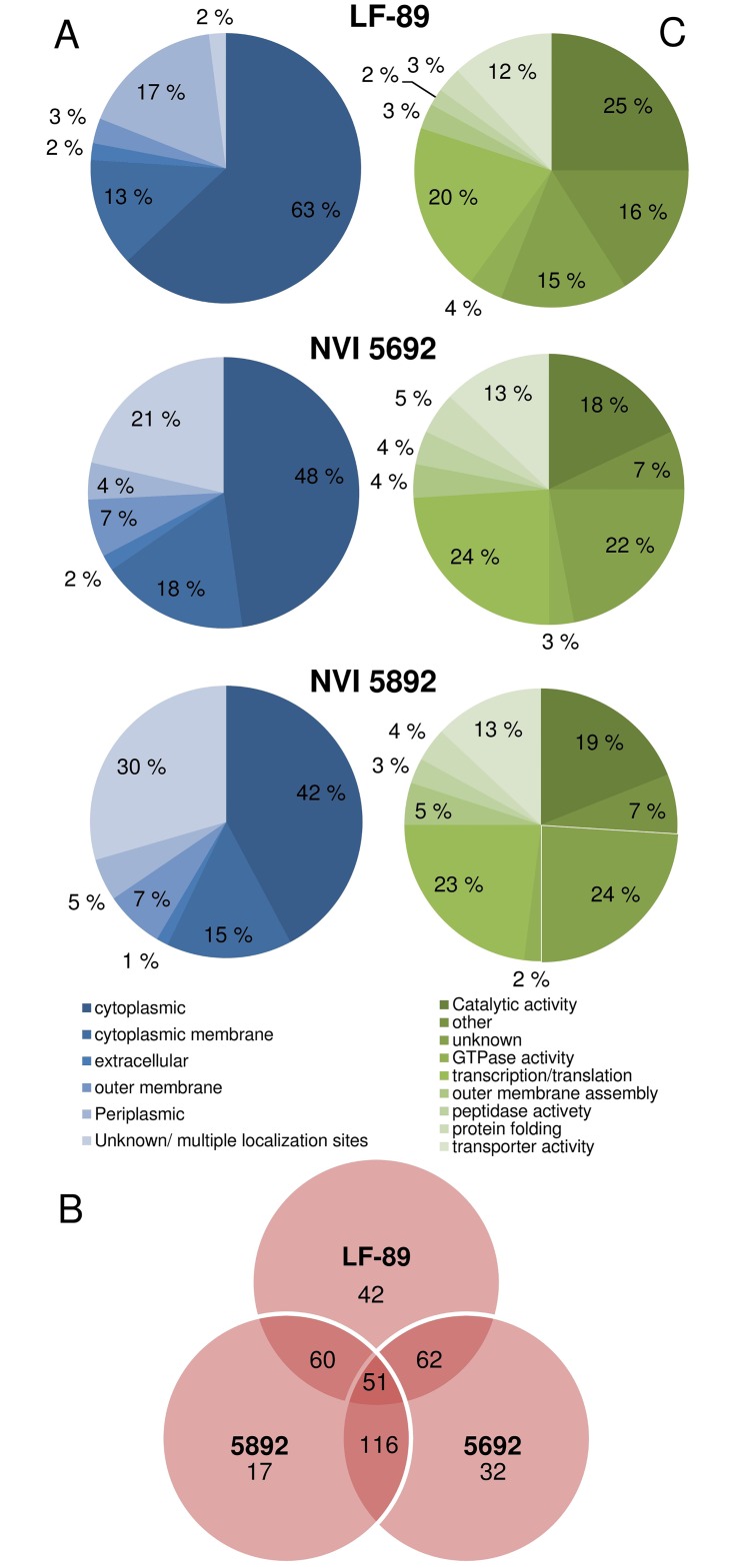
The total number of proteins identified from liquid chromatography-mass spectrometry (MS) of P. salmonis derived MVs identified 119 (FDR 0.18%) different proteins in vesicles isolated from LF-89, 159 (FDR 0.16%) from NVI 5692 and 142 (FDR 0.16%) from NVI 5892 (S2–S4 Tables). However, as the genomes of NVI 5692 and NVI 5892 have not been sequenced, the identified proteins by MS are based on the genome of LF-89 for all three strains, limiting the protein identification. The PSORTb 3.0.2 identified the potential subcellular localization of 98%, 79% and 70% of the P. salmonis MV proteins identified for LF-89, NVI 5692 and NVI 5892, respectively. The majority of the identified proteins (~ 60%) in MVs isolated from P. salmonis were predicted to be cytoplasmic proteins (Fig 3A). The high number of cytoplasmic proteins is most likely do to the presences of a double membrane in the majority of the MVs (S1 Fig). As the formation of double membrane vesicles are characterized by a disruption of both the plasma and outer membrane, high amounts of cytoplasmic proteins have been shown to be packed into the MVs [3]. We cannot, however, fully exclude the presence of cytoplasmic contaminants in the samples, which could contribute to a higher number of proteins been identified as cytoplasmic. The presence of several cytoplasmic proteins have, nonetheless, been identified in proteomic studies of several bacterial derived MVs [5, 9, 76–77], and it has been suggested that some of these proteins could be sorted into the vesicles during the MV formation [2, 78–79]. This may implicate that the MV production is specific and not a random event, allowing for selective incorporation of proteins into the vesicles. Compared to the hypothetical proteome of P. salmonis LF-89 (ATCC VR 1361) (S3 Fig), the cytoplasmic proteins were downregulated in the vesicles, while outer membrane proteins were enriched. An enrichment of outer membrane proteins in bacterial derived vesicles has been described for several other Gram-negative bacteria including Vibrio cholerae [80], Neisseria meningitides [81] and Mycobacterium tuberculosis [77]. An escalation of outer membrane proteins in the P. salmonis derived MVs, may therefore play an important role in the biological function of the vesicles, as membrane proteins often function as an interface between the pathogen and its host. An in-depth analysis of the MV proteins revealed a strain-specific difference, were the highest similarity were observed between the vesicles from NVI 5692 and NVI 5892 (Fig 3B). Strain-specific variations of MV content has been reported for Haemophilus influenza, revealing that certain outer membrane proteins were enriched or excluded in MVs from different isolates [82]. A variation between the three isolates is therefore not unique for P. salmonis, however, why the LF-89 derived MVs differentiates from the Norwegian and Canadian strain has yet to be revealed.
Fig 3. Proteomic characterizations of Piscirickettsia salmonis membrane vesicles.
The identified proteins in the MVs were grouped into families according to their (A) predicted subcellular localization and (C) putative function. (B) Venn diagram comparing MV proteins from three different strains of P. salmonis.
Functional classification of proteins identified in Piscirickettsia salmonis MVs
The identified MV proteins were further categorized based on their predicted functions, where limited differences were identified between the MVs from the three stains of P. salmonis. Both proteins involved in translation/transcription and catalytic activity were abundant in all the samples (Fig 3C). Proteomic profiling during different stages of bacterial growth has shown that proteins involved in DNA and RNA synthesis are upregulated during the log-phase [72–73, 83]. A study of the Acinetobacter baumannii proteome has shown that several translation-related proteins are upregulated during both exponential and early stationary phase. These include 50S ribosomal protein L3, L5, L6, 30S ribosomal protein S2, S8 and elongation factor G and Tu [84]. Therefore, proteins involved in transcription and translation may naturally be packed into the vesicles, as these are upregulated by the bacterium during early growth stages. A differentiation in protein levels has been observed for MVs harvested from Salmonella enterica grown in different medium. MVs isolated from S. enterica grown in LB medium were reported to have higher number of translation/transcription proteins compared to S. enterica grown in acidic MgM media. The MgM cultures on the other hand, had a higher abundance of proteins involved in transporter activity [85]. Thus, the bacterial growth conditions could have an impact on the vesicles protein composition. However, growth phase dependent packing of P. salmonis MVs was not performed in the present work and will be interesting to investigate in future studies.
In addition to a high abundance of translation/transcription proteins in the P. salmonis MVs, approximately one fourth of all the proteins were assigned a catalytic activity. The identification of catalytic proteins in MVs is reported in several species [4, 14, 86] and the packing of active enzymes into MVs is proposed to have an important role in virulence. This can be exemplified by studies of Pseudomonas aeruginosa derived MVs, which, has been shown to contain active chromosomally encoded β-lactamase [87]. β-lactamases are enzymes that deactivate β-lactam antibiotics like penicillin and cephamycins by interruption of the β-lactam ring and thus its activity, providing bacterial resistance against antibiotic treatment [88]. Furthermore, P. aeruginosa has been reported to upregulate its production of MVs in the presence of antibiotics [86]. Therefore, the presence of enzymatic proteins within MVs may provide increased survival and resistance during infections. Proteins displaying catalytic activity have also been reported in non-virulent bacteria, including Bacteroides fragilis and Bacteroides thetaiotaomicron, members of the human microbiota. Both B. fragilis and B. theaiotaomicron derived MVs were reported to display sugar-hydrolyzing activity [89]. The catalytic activities of the P. salmonis derived MVs were not determined in this study, but based on the bioinformatics analysis the vesicles harbor a variety of enzymes.
Identification of strain-specific MV proteins and their relation to virulence and adaptation
The full protein content of all three strains of P. salmonis derived MVs is listed in the S2–S4 Tables, while the 20 most abundant proteins identified by mass spectrometry analysis are shown in Table 1. As the proteomic analysis of the MVs was performed on a collection of vesicles, rather than a single MV, the 20 most abundant proteins are most likely presents in multiple MVs, due to the high number of total mass spectra assigned to the individual proteins. Of the 20 most abundant proteins, six proteins are common for all the strains, while ten proteins overlap between NVI 5692 and NVI 5892. These data illustrate a higher similarity between the Norwegian and Canadian strains compared to the Chilean. When taking the total proteomic profile of the strains-specific vesicles into account, similar findings were identified. 42 proteins were identified only in the vesicles derived from LF-89, in contrast to 32 in NVI 5692 and 17 in NVI 5892 (Fig 3B). To which degree these individual differences in protein content affects the vesicles biological role is not known, but they might contribute to a differentiation in virulence and adaptation for the three strains of P. salmonis. NVI 5692 and NVI 5892 are both isolated from Atlantic salmon from the northern Atlantic Ocean in Norway and Canada respectively, while LF-89 isolated from Coho salmon form the South Pacific Ocean in the south of Chile. Strains of P. salmonis found in the south of Chile, including LF-89, are reported to cause a high mortality rate in salmonids, with an accumulating mortality reaching almost 90% [90–91]. Outbreaks of P. salmonis in Norway and Canada have been, in contrast to Chile, less severe. Out of 14 fish farms in Norway affected during 1988–1992, only 35% of the fish displayed multiple symptoms of SRS. The mortality rate has also been, as in Canada, subsequently lower, ranging from 2–30% [92–93]. The reason for the higher severity of P. salmonis outbreaks in Chile compared to other graphical areas is yet to be revealed. Different strains of P. salmonis have been shown to be closely related independently of their geographical distribution, as 16S and intergenic spacer ITS-1 sequencing of different isolates has shown that strains from both Chile, Norway and Canada has a high phylogenetic similarity [94–96]. Other factors, including environmental and geographical variations, might therefore contribute to a variation in virulence among the different strains of P. salmonis.
Table 1. Top 20 proteins most commonly identified by label-free shotgun proteomics in Piscirickettsia salmonis membrane vesicles.
| LF-89 | NVI 5692 | NVI 592 | |||
|---|---|---|---|---|---|
| Identified protein | Total number of spectra | Identified protein | Total number of spectra | Identified protein | Total number of spectra |
| Outer membrane family protein | 81 | Putative uncharacterized protein* | 144 | Putative uncharacterized protein* | 171 |
| DNA-directed RNA polymerase subunit beta | 79 | Putative uncharacterized protein** | 136 | Peptidyl-prolyl cis-trans isomerase** | 140 |
| Bacterial DNA-binding family protein* | 72 | Prolyl oligopeptidase family protein** | 133 | Outer membrane beta-barrel domain protein** | 104 |
| Chaperone protein DnaK* | 63 | Type I secretion outer membrane TolC family protein** | 89 | Outer membrane protein assembly factor BamA** | 89 |
| 60kDa chaperonin GroEL | 58 | SH3 domain of the SH3b1 type family protein* | 86 | Prolyl oligopeptidase family protein** | 83 |
| 30s ribosomal protein S1* | 44 | Conjugal transfer/type IV secretion DotA/TraY family protein** | 81 | Type I secretion outer membrane, TolC family protein** | 82 |
| SH3 domain of the SH3b1 type family protein* | 44 | Outer membrane beta-barrel domain protein** | 81 | Outer membrane family protein | 79 |
| ATP synthase subunit beta | 40 | Outer membrane protein assembly factor BamA** | 72 | Chaperone protein DnaK* | 74 |
| Succinyl-CoA synthetase subunit beta | 37 | 30s ribosomal protein S1* | 69 | SH3 domain of the SH3b1 type family protein* | 72 |
| Adenylosuccinate synthetase | 35 | Peptidyl-prolyl cis-trans isomerase** | 65 | Conjugal transfer family protein** | 71 |
| 50S ribosomal protein L2* | 35 | Bacterial DNA-binding family protein* | 65 | OmpA family protein | 68 |
| Pyruvate dehydrogenase E1 component | 35 | 50S ribosomal protein L2* | 64 | Outer membrane protein assembly factor BamD** | 64 |
| Translation elongation factor Tu | 32 | Outer membrane protein assembly factor BamD** | 63 | Bacterial DNA-binding family protein* | 62 |
| ATP synthase subunit alpha | 31 | Chaperone protein HtpG | 60 | Conjugal transfer/type IV secretion DotA/TraY family protein** | 61 |
| 30S ribosomal protein S10 | 31 | Conjugal transfer family protein** | 60 | Glycerophosphoryl diester phosphodiesterase family protein** | 57 |
| Acetyl-CoA carboxylase, biotin carboxylase subunit | 30 | NAD-specific glutamate dehydrogenase | 59 | 50S ribosomal protein L2* | 57 |
| GTP-binding protein TypA/BipA | 30 | ATP synthase subunit alpha | 57 | 30s ribosomal protein S1* | 55 |
| Adenylosuccinate lyase | 30 | Chaperone protein DnaK * | 57 | Putative uncharacterized protein | 52 |
| Putative uncharacterized protein* | 29 | Glycerophosphoryl diester phosphodiesterase family protein** | 56 | ostA-like family protein | 48 |
| Glutamine synthetase | 28 | Succinyl-CoA synthetase subunit beta | 50 | DSBA-like thioredoxin domain protein | 46 |
*Proteins identified in MVs from all three strains of P. salmonis
**Proteins identified in MVs from P. salmonis strains NVI 5692 and NVI 5892
Nonetheless, the three strains have several proteins in common, and many of these proteins are highly represented in the vesicles of all three isolates. This indicates that MVs can have similar functions although isolated from three geographically disperse strains of P. salmonis. To evaluate the potential virulence of the P. salmonis derived MVs, the vesicles were subjected to in silico analysis using VirulentPred, to predict putative virulence factors [64]. Based on the VirulentPred analysis, almost 50% of the MV proteins were predicted to be associated with virulence in all three strains (results not shown). Some of these proteins were in addition among the most commonly identified protein by the proteomic analysis, which includes TolC, GroEL and DnaK (Table 1). TolC is involved in multidrug resistance and has previously been described as an virulence factor in the human pathogen Francisella tularensis [97]. Deletion of the TolC orthologue in F. tularensis did exhibit a significant reduction of virulence in mice, suggesting that TolC is involved in the bacterial pathogenesis of F. tularensis [97]. TolC has also been reported to be important for environmental adaptation, protein secretion and drug resistance in several Gram-negative bacteria [98]. The identification of TolC in P. salmonis derived MVs may reflect their presence in the bacterial membrane, although it is not known if the proteins play an active role in the bacterial vesicles. While TolC is identified as the top six most abundant proteins in NVI 5692 and NVI 5892, it does not reach the top 20 list in the LF-89 strain (Table 1).
Certain proteins do not need to be active to have a biological role. This can be exemplified for chaperone proteins, including GroEL and DnaK, which initially prevents protein aggregation by either refolding or degrading misfolded proteins [99]. Both of these proteins has been shown to be highly immunogenic independently of their function [100], and reported to induce the expression and release of the pro-inflammatory cytokines IL-6 and tumor necrosis factor alpha (TNFα) in human monocytes, both individually and in combination [101–102]. Treatment of HUVEC cells with Escherichia coli derived GroEL and DnaK has further been shown to upregulate the release of intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), important for the recruiting of leucocytes, in addition to IL-6 in a dose dependent manner [101]. Thus, the high abundance of GroEL and DnaK in P. salmonis derived MVs might contribute to an increased immunogenic effect of the vesicles.
Dose-dependent internalization of MVs by in vitro cell cultures
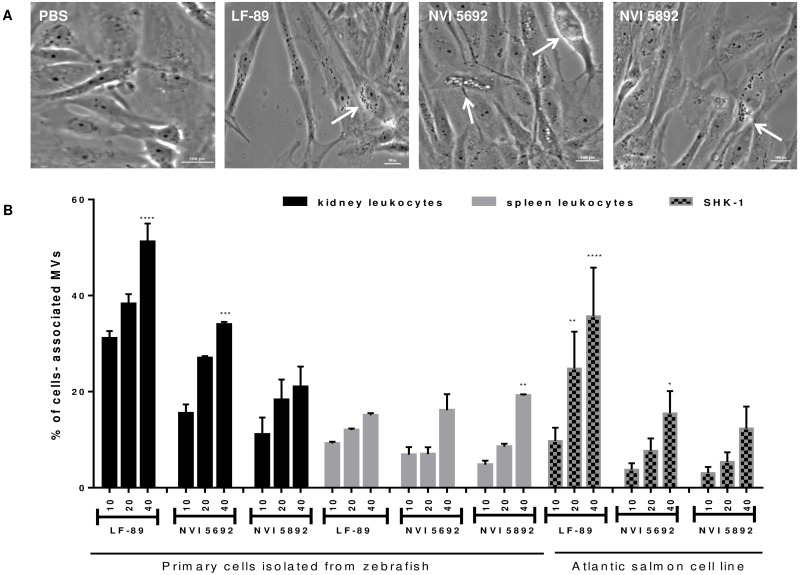
To investigate the biological role of the P. salmonis derived MVs, the vesicles interactions with both commercial and primary cell cultures were assessed by microscopic examination and flow cytometry. As the appearance of a cytopathic effect (CPE) has previously been used to evaluate the susceptibility of cell lines to P. salmonis [42, 103], the CPE after exposure to MVs was evaluated using a salmon head-kidney cell line (SHK-1) (Fig 4A). A 20 μg/mL concentration of P. salmonis derived vesicles was added to SHK-1 cultures, and the CPE was observed after 48 hours by the formation of round vacuoles within the cell (Fig 4A). P. salmonis have previously been reported to infect SHK-1 cells [104], and the CPE induced by vesicles isolated from P. salmonis might therefore indicate a virulent effect of the MVs. Cellular damage caused by bacterial vesicles has been reported in a variety of cell lines as exemplified by RAW264.7, THP-1, and HL60 cells treated with MVs from Acinetobacter baumanii, Aggregatibacter actinomycetemcomitans and Actinobacillus actinomycetem- comitans, respectively [26, 76, 78]. The secretion of MVs has also been observed from the fish pathogen Francisella noatunensis subsp noatunensis during infections in primary cod leukocytes and within zebrafish embryos [40, 105].
Fig 4. Internalization and effect of membrane vesicles isolated from Piscirickettsia salmonis in fish cells.
(A) Cytopathic effect of 20 μg/mL MVs in SHK-1 cells. The cytopathic effect is characterized by the production of rounded vacuoles (arrow). Bar size, 100 μm. (B) The effect of three different MV concentrations (10, 20 and 40 μg/mL) on internalization in SHK-1 cells and kidney and spleen primary leukocytes isolated from adult zebrafish assessed by flow cytometry (n = 3). Results are presented as mean ± SD. Asterisks indicate statistical significances between the different concentrations of MVs within each cell type (Two-way ANOVA, Tukey`s multiple comparison test). P value: **** < 0.0001; *** < 0.001; ** < 0.01; * < 0.1.
The interaction between P. salmonis derived MVs and cultured cells were further evaluated using flow cytometric analysis in combination with FITC-labeled vesicles. A concentration of 10 μg/mL of FITC conjugated MVs from all three strains of P. salmonis were shown to be internalized by both kidney and spleen primary leukocytes isolated from adult zebrafish (S4 Fig). To investigate the dose-response in the incorporation of MVs two additional doses of FITC conjugated MVs (20 and 40 μg/mL) from all three bacterium were incubated with primary culture or SHK-1, and the incorporation analyzed by flow cytometry. Increasing concentrations of vesicles resulted in a linear enrichment of MVs association with zebrafish primary cells and in SHK-1 cells (Fig 4B). Vesicles isolated from LF-89 were shown to display a significantly higher degree of association with primary zebrafish kidney leukocytes compared to SHK-1 cells and primary zebrafish spleen leukocytes (p<0.001). NVI 5692 and NVI 5892 derived MVs displayed a similar internalization in all three cell types (Fig 4B).
A dose-dependent uptake of MVs has previously been described for Brucella abortus [56]. Moreover, B. abortus derived vesicles has been reported to modulate the innate immune response in human epithelial and monocytes cells, as well as increasing the adherence and internalization of B. abortus in vitro [56]. In general, the cellular process for internalization of MVs is not known, but studies with B. abortus and H. pylori has shown a potential uptake of MVs by the clathrin-mediated endocytosis, the main pathway for receptor-mediated endocytosis in most eukaryotic cells [56, 106–107]. A similar infection strategy has been suggested for P. salmonis, indicating that the utilization of macrophages is dependent upon the interaction with host-cell clathrin and actin [108]. The capability to exploit host cells for survival by manipulating cellular processes by protein secretion and specific effectors has been described for a range of pathogens [109]. E.g. membrane vesicles isolated from Legionella pneumophila inhibit the fusion of phagosomes with lysosomes in primary mouse macrophages [110]. Therefore, P. salmonis could potentially utilize the MV secretion as a survival strategy to replicate within macrophages. Both the primary and commercial cell lines revealed a higher association with LF-89 derived MVs in contrast to NVI 5692 and NVI 5892 in this study. This suggests that MVs might promote bacterial survival within macrophages, which could explain the higher virulence reported for the Chilean strain. Thus, bacterial release of MVs within the host may contribute to the utilization of host cells and modulations of the immune system during an infection. To which degree MVs are released during SRS outbreaks has yet to be investigated but was recently shown in CHSE-cells [52].
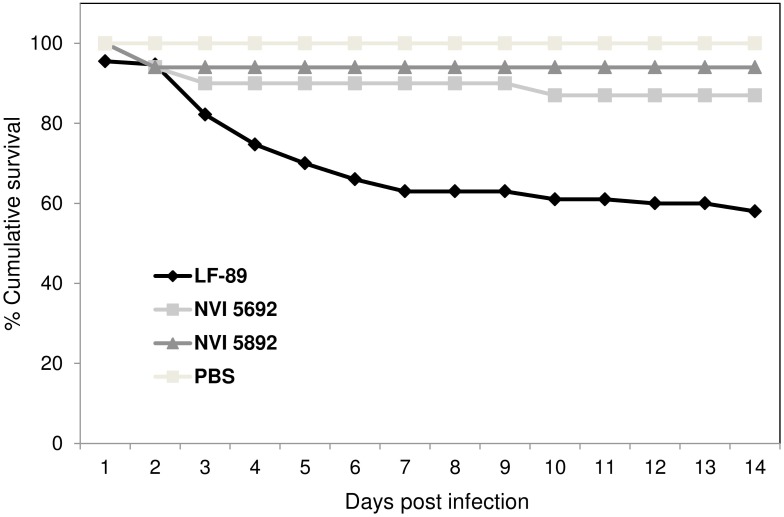
Studies of Piscirickettsia salmonis derived vesicles in adult zebrafish
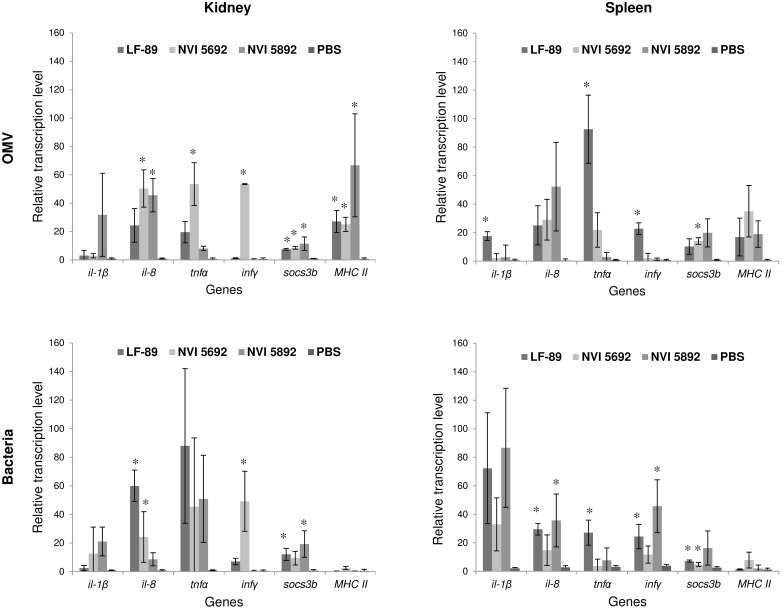
To study the potential effect of MVs in vivo, adult zebrafish were injected with MVs from the three strains of P. salmonis. In recent years, zebrafish has proven to be a unique model for the study of leukocytes subset, immune cell migration and host-pathogen interaction [111]. The effects of MVs have previously only been described for F. noatunensis in a zebrafish model [40], with no observed cytotoxic effects. For zebrafish injected with 40 μg MVs from NVI 5692 and NVI 5892, no behavioral alterations were observed over two weeks compared to the PBS control group (Fig 5). Interestingly, fish injected with MVs from LF-89 presented a reduction in activity and approximately three days post injection mortalities were detected. A rapid decrease in accumulative survival up to 50% were registered for the LF-89 MV group during the first seven days, indicating an initial acute phase, which stabilized by day 9 (Fig 5). In the NVI 5692 and NVI 5892 MV group, less than 10% mortalities were registered, most likely due to complications from the injections, as they occurred shortly after the procedure. Kidney and spleen samples were harvested two weeks post-injection to evaluate the MVs immunogenic effect in the fish. Of the genes analyzed no significant up or down regulation were detected for il-6, il-10, il-12a, socs3a, mpeg1, cd40, nod1 and nod2. This could mean that the MVs do not affect the selected genes, or that it occurs at an earlier time point. An increased expression of immune genes was, however observed for il-1β, il-8, tnfα, infγ, socs3b and MHC II in all three groups injected with P. salmonis derived MVs (Fig 6). The gene expression profile for the fish challenged with MVs were in most cases similar to the ones challenged with live bacteria, indicating that the vesicles mimic their mother cells. Several pathogens, including the intracellular ones, modify the suppressor of cytokine signaling (Socs) to inhibit the host’s ability to clear an infection [112]. Thus, the effect of MVs on the socs3b gene expression was investigated, showing a significant increased expression. Such alterations of the cytokine secretion is a common modification initiated by several intracellular pathogens, enabling prolonged utilization of macrophages, by downregulating the host’s defense mechanisms [113]. To what degree the P. salmonis derived MVs can modulate the cytokine expression has not previously been explored. However, as socs3b in combination with several other immune related genes were upregulate after exposure to MVs, the P. salmonis derived vesicles might display immunogenic abilities. However, further studies including sampling at earlier time points are needed to fully evaluate the potential immunogenic effect of the vesicles.
Fig 5. Adult zebrafish challenged with membrane vesicles isolated from Piscirickettsia salmonis.
Cumulative survival of adult zebrafish injected with 40 μg of MVs isolated from the three different strains of P. salmonis (LF-89, NVI 5692 and NVI 5892) or PBS (n = 20).
Fig 6. Immune gene transcription of adult zebrafish challenged with P. salmonis and isolated membrane vesicles analyzed by RT-qPCR.
Immune gene expression of kidney and spleen, isolated 14 days post injection with either 40 μg MVs isolated from three different strains of P. salmonis or 1x107 CFU of the same bacteria strains (LF-89, NVI 5692 and NVI 5892). Results are presented as mean +/- SD. Asterisk indicate significantly upregulated genes compared to the PBS control p<0.05, two tailed unpaired Student’s t-test (n = 3).
These immunogenic abilities are, nonetheless, partly the reason why MVs over the last decades have been explored and successfully used as vaccine components [1, 36]. Thus, several pro-inflammatory genes were investigated and shown to be significantly upregulated in zebrafish injected with the P. salmonis derived vesicles, including il-1β, il-8, tnfα and infγ. The inflammatory cascade of an infection begins with receptors involved in the binding and uptake of infectious agents and their products by cells of the innate immune system. This is then followed by the production of pro-inflammatory cytokines, such as TNFα, IL-1, IL-8 and IFNγ [114]. In the present work, we show that P. salmonis derived MVs are able to upregulated genes involved in an inflammatory response, as well as genes related to antigen representing cells, (MHC II) in an adult zebrafish model. Interestingly, the MHC II genes were significantly upregulated in fish injected with MVs compared to fish injected with live bacteria (p<0.05). However, as P. salmonis is an intracellular bacteria utilizing macrophages as a part of its infection strategy, a low MHC II expression might be expected [115]. On the other hand, the MVs do not replicate within a host, and might therefore be taken up and degraded by antigen representing cells, increasing the MHC II expression, which also makes them interesting as potential vaccine components. Several of the genes investigated have been reported to be upregulated at early time points post vaccination in salmon [116–117]. Olive flounder injected with E. tarda derived vesicles has been reported to display immunogenic alterations due to MV exposure, including upregulation of il-1β and il-6 detected in kidney samples at 3 hours post challenge and maintained up to 5 days [38]. Similar results are found for Japanese flounder immunized with vesicles isolated from Vibrio anguillarum, where they observed, an increased production of pro-inflammatory cytokines, including tnfα, il-1β and il-6, during the first 48 hours [118]. Furthermore, when MVs isolated from E. tarda was tested as a vaccine candidate towards edwardsiellosis, it provided an equal level of protection as Formalin-killed E. tarda cells [38]. An activation of the innate immune system, including upregulation of pro-inflammatory cytokines, plays an important role in the adaptive immune response by attracting the antigen presenting cells [119]. Thus, the up-regulation of immune-related genes detected in this study, might indicate a potential activation of the host’s immune system initiated by the MVs. Whether this activation is mediated by i.e. toll-like receptors (TLRs) or not, is not known. Considering the composition of MVs, containing several molecules and proteins identified as pathogen associated molecular pattern (PAMPS) including LPS, carbohydrates, heat-shock proteins (HSPs) and nuclei sequence motifs suggest the participation of TLRs as a bridge between innate and adaptive immunity, making P. salmonis MVs interesting as a vaccine candidate. As mortalities were observed for fish immunized with vesicles isolated from LF-89, further dose-response studies are needed to evaluate the vesicles long-term effect. However, as an effect was observed both in vivo as well as in vitro by the P. salmonis derived MVs, the vesicles could be an integral part of the bacterium’s pathogenesis as suggested by others [52]. It could be argued that mortalities observed in the zebrafish when exposed LF-89 derived MVs compared to the two other strains are caused by differences in the LPS. LPS isolated from E. coli has been shown to have a limited effect in adult zebrafish [120], but an immunogenic response has been observed in zebrafish larva exposed to E. coli and P. aeruginosa LPS [121–122]. There is, however, still a lack of knowledge regarding the immunogenic effect of LPS from fish pathogens, and studies of P. salmonis derived LPS would be interesting to follow up in future studies.
Conclusion
The present study is the first investigation of MV proteomes from bacterial species isolated from a wide geographical area. It is also the first in-depth analysis of MVs from multiple P. salmonis isolates. We show that MVs derived from LF-89, a high-virulent strain isolated from Chile, differs compared to the MVs of a Norwegian and Canadian strain, isolated from low infection-associated areas. According to the number of shared proteins in the MVs, we can identify two cluster, one that include NVI 5692 and NVI 5892 derived vesicles and a second one including LF-89 MVs. In general, these results illustrate that the MVs proteome analyzed from one bacterial strain is not representative of all bacterial strains within the same species. Furthermore, our findings also demonstrate that P. salmonis derived MVs are able to associate within both primary and commercial fish cells suggesting a potential role with fish immune cells. The use of zebrafish as a model for studies of MVs allowed us to investigate toxicity and the immunogenic effects upon injection with the bacterial derived vesicles indicating that the P. salmonis derived MVs are able to modulate the host’s immune response. These data indicates that the P. salmonis derived MVs could be important for the bacterium’s virulence, which also make them relevant as potential vaccine candidate against SRS.
Ethics Statement
All animal experiments were approved by the Norwegian Animal Research Authority, approval no. 2014/3409, FOTS ID 5959/ and 2014/239007 FOTS ID 7028 and treated according to institutional guidelines.
Supporting Information
Electron microscopy image analysis of MVs, exemplified by NVI 5692, showing the presences of both single membrane vesicles (OMV) consisting of an outer membrane (OM), and double membrane vesicles (I-OMV) containing a periplasmic membrane (PM) and an outer membrane.
(PDF)
Membrane fractions (M) and membrane vesicles (MVs) (20μg) isolated from three strains of P. salmonis, NVI 5692, NVI 5892 and LF-89 were applied to 12% SDS-PAGE and stained with coomassie blue.
(PDF)
Pie chart showing the cellular localization for the theoretical proteome of P. salmonis LF-89 (ATCC VR 1361).
(PDF)
Percentage of SHK-1, Atlantic salmon cell line, spleen and kidney primary cells isolated from adult zebrafish able to incorporate 10μg/mL of FITC conjugated membrane vesicles isolated from P. salmonis strains LF-89, NVI 5692 and NVI 5892 as assessed by flow cytometry (n = 3).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank Dylan Xavier at the Australian Proteome Analysis Facility for experimental advice and help running the LC-MS/MS and Karl Hassan (Macquarie University, Australia) for all the helpful assistance. We thank Bernd Thiede for running the LC-MS/MS at the proteomics unit at the University of Oslo, Atnje Hofgaard for assisting with the electron scanning microscopy, Erik Ropstad for advice regarding the zebrafish experiments, Ana C.S. Tevara for help with zebrafish supply and housing, Ruth Halsne for proofreading the manuscript and Duncan J. Colquhoun for providing the bacterial strains used in the experiments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was financially supported by the University of Oslo www.uio.no (JIT, LXL, EKB, PL and HWL), The Research Council of Norway http://www.forskningsradet.no/ (Biotek2021 Program Grant no# 233849) (JIT, LXL and HWL), Biostruct travel grant http://site.uit.no/biostruct/ (JIT) and EU 7FP Marie Curie IRSES staff exchange program grant (FP7-PEOPLE-2009-IRSES) (JIT and ITP) to whom we express our gratitude.
References
- 1.Ellis TN, Kuehn MJ. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiology and Molecular Biology Reviews. 2010;74(1):81–94. 10.1128/MMBR.00031-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–84. 10.1146/annurev.micro.091208.073413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perez-Cruz C, Delgado L, Lopez-Iglesias C, Mercade E. Outer-inner membrane vesicles naturally secreted by Gram-negative pathogenic bacteria. PLoS One. 2015;10(1):e0116896 10.1371/journal.pone.0116896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee E-Y, Choi D-S, Kim K-P, Gho YS. Proteomics in Gram-negative bacterial outer membrane vesicles. Mass Spectrometry Reviews. 2008;27(6):535–55. 10.1002/mas.20175 [DOI] [PubMed] [Google Scholar]
- 5.Pierson T, Matrakas D, Taylor YU, Manyam G, Morozov VN, Zhou W, et al. Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. Journal of Proteome Research. 2011;10(3):954–67. 10.1021/pr1009756 [DOI] [PubMed] [Google Scholar]
- 6.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–87. 10.1038/nri3837 [DOI] [PubMed] [Google Scholar]
- 7.Horstman AL, Kuehn MJ. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. Journal of Biological Chemistry. 2000;275(17):12489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chatterjee D, Chaudhuri K. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS letters. 2011;585(9):1357–62. 10.1016/j.febslet.2011.04.017 [DOI] [PubMed] [Google Scholar]
- 9.Alzahrani H, Winter J, Boocock D, De Girolamo L, Forsythe SJ. Characterization of outer membrane vesicles from a neonatal meningitic strain of Cronobacter sakazakii. FEMS Microbiol Lett. 2015;362(12):fnv085 10.1093/femsle/fnv085 [DOI] [PubMed] [Google Scholar]
- 10.Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. Journal of Bacteriology. 2006;188(16):5945–57. 10.1128/JB.00257-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63(2):545–58. 10.1111/j.1365-2958.2006.05522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J. 2004;23(23):4538–49. 10.1038/sj.emboj.7600471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mashburn-Warren L, Howe J, Garidel P, Richter W, Steiniger F, Roessle M, et al. Interaction of quorum signals with outer membrane lipids: insights into prokaryotic membrane vesicle formation. Molecular Microbiology. 2008;69(2):491–502. 10.1111/j.1365-2958.2008.06302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host–pathogen interaction. Genes & Development. 2005;19(22):2645–55. [DOI] [PubMed] [Google Scholar]
- 15.Keenan J, Day T, Neal S, Cook B, Perez-Perez G, Allardyce R, et al. A role for the bacterial outer membrane in the pathogenesis of Helicobacter pylori infection 2000. 2000-01-01 00:00:00. 259–64 p. [DOI] [PubMed] [Google Scholar]
- 16.Namork E, Brandtzaeg P. Fatal meningococcal septicaemia with “blebbing” meningococcus. The Lancet. 2002;360(9347):1741. [DOI] [PubMed] [Google Scholar]
- 17.Stephens DS, Edwards KM, Morris F, McGee ZA. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. Journal of Infectious Diseases. 1982;146(4):568 [DOI] [PubMed] [Google Scholar]
- 18.Kadurugamuwa JL, Beveridge TJ. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. Journal of Antimicrobial Chemotherapy. 1997;40(5):615–21. [DOI] [PubMed] [Google Scholar]
- 19.Dutta S, Iida K, Takade A, Meno Y, Nair GB, Yoshida S. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol Immunol. 2004;48(12):965–9. [DOI] [PubMed] [Google Scholar]
- 20.MacDonald IA, Kuehn MJ. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. Journal of Bacteriology. 2013;195(13):2971–81. 10.1128/JB.02267-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Applied and Environmental Microbiology. 2014;80(11):3469–83. 10.1128/AEM.04248-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai CH, Listgarten MA, Hammond BF. Comparative ultrastructure of leukotoxic and non-leukotoxic strains of Actinobacillus actinomycetemcomitans. J Periodontal Res. 1981;16(4):379–89. [DOI] [PubMed] [Google Scholar]
- 23.Wai SN, Takade A, Amako K. The release of outer-membrane vesicles from the strains of enterotocigenic echerichia-coli. Microbiol Immunol. 1995;39(7):451–6. [DOI] [PubMed] [Google Scholar]
- 24.Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. Bacterial vesicles in marine ecosystems. Science. 2014;343(6167):183–6. 10.1126/science.1243457 [DOI] [PubMed] [Google Scholar]
- 25.Tanoue E, Nishiyama S, Kamo M, Tsugita A. Bacterial membranes: possible source of a major dissolved protein in seawater. Geochimica et Cosmochimica Acta. 1995;59(12):2643–8. [Google Scholar]
- 26.Li Z-T, Zhang R-L, Bi X-G, Xu L, Fan M, Xie D, et al. Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microbial pathogenesis. 2015;81:46–52. 10.1016/j.micpath.2015.03.009 [DOI] [PubMed] [Google Scholar]
- 27.Collins BS. Gram-negative outer membrane vesicles in vaccine development. Discovery medicine. 2011;12(62):7–15. [PubMed] [Google Scholar]
- 28.Amano A, Takeuchi H, Furuta N. Outer membrane vesicles function as offensive weapons in host–parasite interactions. Microbes and Infection. 2010;12(11):791–8. 10.1016/j.micinf.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 29.Hornef MW, Wick MJ, Rhen M, Normark S. Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol. 2002;3(11):1033–40. 10.1038/ni1102-1033 [DOI] [PubMed] [Google Scholar]
- 30.Perez Vidakovics MLA, Jendholm J, Mörgelin M, Månsson A, Larsson C, Cardell L-O, et al. B cell activation by outer membrane vesicles—A novel virulence mechanism. PLoS Pathogens. 2010;6(1):e1000724 10.1371/journal.ppat.1000724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaparakis M, Turnbull L, Carneiro L, Firth S, Coleman HA, Parkington HC, et al. Bacterial membrane vesicles deliver peptidoglycan to NOD1 in epithelial cells. Cellular Microbiology. 2010;12(3):372–85. 10.1111/j.1462-5822.2009.01404.x [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ. Nuclear factor-κB. The International Journal of Biochemistry & Cell Biology. 1997;29(6):867–70. [DOI] [PubMed] [Google Scholar]
- 33.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7(12):1250–7. 10.1038/ni1412 [DOI] [PubMed] [Google Scholar]
- 34.Danzig L. Meningococcal vaccines. The Pediatric Infectious Disease Journal. 2004;23(12):S285–S92. [PubMed] [Google Scholar]
- 35.Granoff DM. Review of Meningococcal group B vaccines. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;50(S2):S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holst J, Oster P, Arnold R, Tatley MV, Naess LM, Aaberge IS, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Human vaccines & immunotherapeutics. 2013;9(6):1241–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collins BS. Gram-negative outer membrane vesicles in vaccine development. Discovery medicine. 2011;12(62):7–15. [PubMed] [Google Scholar]
- 38.Park SB, Jang HB, Nho SW, Cha IS, Hikima J, Ohtani M, et al. Outer membrane vesicles as a candidate vaccine against edwardsiellosis. PLoS One. 2011;6(3):e17629 10.1371/journal.pone.0017629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki M, Kondo M, Nakatsuka Y, Kawai K, Oshima S-I. Stationary phase culture supernatant containing membrane vesicles induced immunity to rainbow trout Oncorhynchus mykiss fry syndrome. Vaccine. 2007;25(3):561–9. 10.1016/j.vaccine.2006.07.047 [DOI] [PubMed] [Google Scholar]
- 40.Brudal E, Lampe EO, Reubsaet L, Roos N, Hegna IK, Thrane IM, et al. Vaccination with outer membrane vesicles from Francisella noatunensis reduces development of francisellosis in a zebrafish model. Fish Shellfish Immunol. 2015;42(1):50–7. 10.1016/j.fsi.2014.10.025 [DOI] [PubMed] [Google Scholar]
- 41.Fryer JL, Lannan CN, Giovannoni SJ, Wood ND. Piscirickettsia salmonis gen. nov., sp. nov., the causative agent of an epizootic disease in salmonid fishes. Int J Syst Bacteriol. 1992;42(1):120–6. 10.1099/00207713-42-1-120 [DOI] [PubMed] [Google Scholar]
- 42.Fryer JL, Hedrick RP. Piscirickettsia salmonis: a Gram-negative intracellular bacterial pathogen of fish. Journal of fish diseases. 2003;26(5):251–62. [DOI] [PubMed] [Google Scholar]
- 43.Mauel MJ, Miller DL. Piscirickettsiosis and piscirickettsiosis-like infections in fish: a review. Vet Microbiol. 2002;87(4):279–89. [DOI] [PubMed] [Google Scholar]
- 44.McCarthy UM, Bron JE, Brown L, Pourahmad F, Bricknell IR, Thompson KD, et al. Survival and replication of Piscirickettsia salmonis in rainbow trout head kidney macrophages. Fish Shellfish Immunol. 2008;25(5):477–84. 10.1016/j.fsi.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 45.Fryer JL, Lannan CN. Rickettsial infections of fish. Annual Review of Fish Diseases. 1996;6:3–13. [Google Scholar]
- 46.Rozas M, Enríquez R. Piscirickettsiosis and Piscirickettsia salmonis in fish: a review. Journal of fish diseases. 2014;37(3):163–88. 10.1111/jfd.12211 [DOI] [PubMed] [Google Scholar]
- 47.Ramirez R, Gomez FA, Marshall SH. The infection process of Piscirickettsia salmonis in fish macrophages is dependent upon interaction with host-cell clathrin and actin. FEMS Microbiol Lett. 2015;362(1):1–8. [DOI] [PubMed] [Google Scholar]
- 48.Rojas V, Galanti N, Bols NC, Marshall SH. Productive infection of Piscirickettsia salmonis in macrophages and monocyte-like cells from rainbow trout, a possible survival strategy. Journal of cellular biochemistry. 2009;108(3):631–7. 10.1002/jcb.22295 [DOI] [PubMed] [Google Scholar]
- 49.Rojas V, Galanti N, Bols NC, Jimenez V, Paredes R, Marshall SH. Piscirickettsia salmonis induces apoptosis in macrophages and monocyte-like cells from rainbow trout. Journal of cellular biochemistry. 2010;110(2):468–76. 10.1002/jcb.22560 [DOI] [PubMed] [Google Scholar]
- 50.Gomez FA, Tobar JA, Henriquez V, Sola M, Altamirano C, Marshall SH. Evidence of the presence of a functional Dot/Icm type IV-B secretion system in the fish bacterial pathogen Piscirickettsia salmonis. PLoS One. 2013;8(1):e54934 10.1371/journal.pone.0054934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isla A, Haussmann D, Vera T, Kausel G, Figueroa J. Identification of the clpB and bipA genes and an evaluation of their expression as related to intracellular survival for the bacterial pathogen Piscirickettsia salmonis. Veterinary microbiology. 2014;173(3–4):390–4. 10.1016/j.vetmic.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 52.Oliver C, Valenzuela K, Hernández M, Sandoval R, Haro RE, Avendaño-Herrera R, et al. Characterization and pathogenic role of outer membrane vesicles produced by the fish pathogen Piscirickettsia salmonis under in vitro conditions. Veterinary microbiology. 2016;184:94–101. 10.1016/j.vetmic.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 53.Mikalsen J, Skjærvik O, Wiik-Nielsen J, Wasmuth MA, Colquhoun DJ. Agar culture of Piscirickettsia salmonis, a serious pathogen of farmed salmonid and marine fish. FEMS microbiology letters. 2008;278(1):43–7. 10.1111/j.1574-6968.2007.00977.x [DOI] [PubMed] [Google Scholar]
- 54.Brudal E, Ulanova LS, E OL, Rishovd AL, Griffiths G, Winther-Larsen HC. Establishment of three Francisella infections in zebrafish embryos at different temperatures. Infect Immun. 2014;82(6):2180–94. 10.1128/IAI.00077-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pistone S, Qoragllu D, Smistad G, Hiorth M. Multivariate analysis for the optimization of polysaccharide-based nanoparticles prepared by self-assembly. Colloids and Surfaces B: Biointerfaces. 2016;146:136–43. 10.1016/j.colsurfb.2016.05.055 [DOI] [PubMed] [Google Scholar]
- 56.Pollak CN, Delpino MV, Fossati CA, Baldi PC. Outer membrane vesicles from Brucella abortus promote bacterial internalization by human monocytes and modulate their innate immune response. PLoS ONE. 2012;7(11):e50214 10.1371/journal.pone.0050214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.A. W, Sanderson NM, O'Reilly J, Rutherford NG, Poolman B, Henderson PJF. The amplified expression, identification, purification, assay, and properties of hexahistidine-tagged bacterial membrane transport proteins In Membrane transport—A practical approach. Baldwin SA, editor. Oxford University Press: Oxford, UK; 2000. [Google Scholar]
- 58.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–5. [DOI] [PubMed] [Google Scholar]
- 59.Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004;20(9):1466–7. 10.1093/bioinformatics/bth092 [DOI] [PubMed] [Google Scholar]
- 60.Pulgar R, Travisany D, Zuniga A, Maass A, Cambiazo V. Complete genome sequence of Piscirickettsia salmonis LF-89 (ATCC VR-1361) a major pathogen of farmed salmonid fish. Journal of biotechnology. 2015;212:30–1. 10.1016/j.jbiotec.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 61.Mirzaei M, Pascovici D, Keighley T, George I, Voelckel C, Heenan PB, et al. Shotgun proteomic profiling of five species of New Zealand Pachycladon. Proteomics. 2011;11(1):166–71. 10.1002/pmic.200900816 [DOI] [PubMed] [Google Scholar]
- 62.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, et al. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26(13):1608–15. 10.1093/bioinformatics/btq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Consortium TU. UniProt: a hub for protein information. Nucleic Acids Research. 2015;43(D1):D204–D12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Garg A, Gupta D. VirulentPred: a SVM based prediction method for virulent proteins in bacterial pathogens. BMC Bioinformatics. 2008;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Palic D, Andreasen CB, Ostojic J, Tell RM, Roth JA. Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. Journal of immunological methods. 2007;319(1–2):87–97. 10.1016/j.jim.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 66.Hohn C, Lee SR, Pinchuk LM, Petrie-Hanson L. Zebrafish kidney phagocytes utilize macropinocytosis and Ca+-dependent endocytic mechanisms. PloS one. 2009;4(2):e4314 10.1371/journal.pone.0004314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cosma CL, Swaim LE, Volkman H, Ramakrishnan L, Davis JM. Zebrafish and frog models of Mycobacterium marinum infection. Current protocols in microbiology. 2006;Chapter 10:Unit 10B.2 10.1002/0471729256.mc10b02s3 [DOI] [PubMed] [Google Scholar]
- 68.Yanez AJ, Valenzuela K, Silva H, Retamales J, Romero A, Enriquez R, et al. Broth medium for the successful culture of the fish pathogen Piscirickettsia salmonis. Diseases of aquatic organisms. 2012;97(3):197–205. 10.3354/dao02403 [DOI] [PubMed] [Google Scholar]
- 69.Henriquez M, Gonzalez E, Marshall SH, Henriquez V, Gomez FA, Martinez I, et al. A novel liquid medium for the efficient growth of the salmonid pathogen Piscirickettsia salmonis and optimization of culture conditions. PLoS One. 2013;8(9):e71830 10.1371/journal.pone.0071830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klimentová J, Stulík J. Methods of isolation and purification of outer membrane vesicles from Gram-negative bacteria. Microbiological Research. 2015;170:1–9. 10.1016/j.micres.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 71.Tashiro Y, Ichikawa S, Shimizu M, Toyofuku M, Takaya N, Nakajima-Kambe T, et al. Variation of physiochemical properties and cell association activity of membrane vesicles with growth phase in Pseudomonas aeruginosa. Applied and Environmental Microbiology. 2010;76(11):3732–9. 10.1128/AEM.02794-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee KJ, Bae SM, Lee MR, Yeon SM, Lee YH, Kim KS. Proteomic analysis of growth phase-dependent proteins of Streptococcus pneumoniae. Proteomics. 2006;6(4):1274–82. 10.1002/pmic.200500415 [DOI] [PubMed] [Google Scholar]
- 73.Cohen DP, Renes J, Bouwman FG, Zoetendal EG, Mariman E, de Vos WM, et al. Proteomic analysis of log to stationary growth phase Lactobacillus plantarum cells and a 2-DE database. Proteomics. 2006;6(24):6485–93. 10.1002/pmic.200600361 [DOI] [PubMed] [Google Scholar]
- 74.Bauman SJ, Kuehn MJ. Purification of outer membrane vesicles from Pseudomonas aeruginosa and their activation of an IL-8 response. Microbes and Infection. 2006;8(9–10):2400–8. 10.1016/j.micinf.2006.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kwon S-O, Gho YS, Lee JC, Kim SI. Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii isolate. FEMS microbiology letters. 2009;297(2):150–6. 10.1111/j.1574-6968.2009.01669.x [DOI] [PubMed] [Google Scholar]
- 76.Kieselbach T, Zijnge V, Granstrom E, Oscarsson J. Proteomics of Aggregatibacter actinomycetemcomitans Outer Membrane Vesicles. PLoS One. 2015;10(9):e0138591 10.1371/journal.pone.0138591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee J, Kim SH, Choi DS, Lee JS, Kim DK, Go G, et al. Proteomic analysis of extracellular vesicles derived from Mycobacterium tuberculosis. Proteomics. 2015. [DOI] [PubMed] [Google Scholar]
- 78.Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microbial pathogenesis. 2002;32(1):1–13. 10.1006/mpat.2001.0474 [DOI] [PubMed] [Google Scholar]
- 79.Siljamäki P, Varmanen P, Kankainen M, Sukura A, Savijoki K, Nyman TA. Comparative exoprotein profiling of different Staphylococcus epidermidis strains reveals potential link between nonclassical protein export and virulence. Journal of Proteome Research. 2014;13(7):3249–61. 10.1021/pr500075j [DOI] [PubMed] [Google Scholar]
- 80.Altindis E, Fu Y, Mekalanos JJ. Proteomic analysis of Vibrio cholerae outer membrane vesicles. Proceedings of the National Academy of Sciences. 2014;111(15):E1548–E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lappann M, Otto A, Becher D, Vogel U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J Bacteriol. 2013;195(19):4425–35. 10.1128/JB.00625-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roier S, Blume T, Klug L, Wagner GE, Elhenawy W, Zangger K, et al. A basis for vaccine development: Comparative characterization of Haemophilus influenzae outer membrane vesicles. International Journal of Medical Microbiology. 2015;305(3):298–309. 10.1016/j.ijmm.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 83.Folio P, Chavant P, Chafsey I, Belkorchia A, Chambon C, Hébraud M. Two-dimensional electrophoresis database of Listeria monocytogenes EGDe proteome and proteomic analysis of mid-log and stationary growth phase cells. Proteomics. 2004;4(10):3187–201. 10.1002/pmic.200300841 [DOI] [PubMed] [Google Scholar]
- 84.Soares NC, Cabral MP, Gayoso C, Mallo S, Rodriguez-Velo P, Fernández-Moreira E, et al. Associating growth-phase-related changes in the proteome of Acinetobacter baumannii with increased resistance to oxidative stress. Journal of Proteome Research. 2010;9(4):1951–64. 10.1021/pr901116r [DOI] [PubMed] [Google Scholar]
- 85.Bai J, Kim SI, Ryu S, Yoon H. Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar Typhimurium. Infect Immun. 2014;82(10):4001–10. 10.1128/IAI.01416-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kadurugamuwa JL, Beveridge TJ. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. Journal of Bacteriology. 1995;177(14):3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ciofu O, Beveridge TJ, Kadurugamuwa J, Walther-Rasmussen J, Høiby N. Chromosomal β-lactamase is packaged into membrane vesicles and secreted from Pseudomonas aeruginosa. Journal of Antimicrobial Chemotherapy. 2000;45(1):9–13. [DOI] [PubMed] [Google Scholar]
- 88.Bush K, Jacoby GA. Updated functional classification of β-Lactamases. Antimicrobial Agents and Chemotherapy. 2010;54(3):969–76. 10.1128/AAC.01009-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elhenawy W, Debelyy MO, Feldman MF. Preferential packing of acidic glycosidases and proteases into bacteroides outer membrane vesicles. mBio. 2014;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lannan CN, Fryer JL. Piscirickettsia salmonis, a major pathogen of salmonid fish in Chile. Fisheries Research. 1993;17(1–2):115–21. [Google Scholar]
- 91.Branson EJ, Diaz-Munoz DN. Description of a new disease condition occurring in farmed coho salmon, Oncorhynchus kisutch (Walbaum), in South America. Journal of fish diseases. 1991;14(2):147–56. [Google Scholar]
- 92.Olsen A, Melby H, Speilberg L, Evensen Ø, Håstein T. Piscirickettsia salmonis infection in Atlantic salmon Salmo salar in Norway-epidemiological, pathological and microbiological findings. Diseases of aquatic organisms. 1997;31(1):35–48. [Google Scholar]
- 93.Cusack RR, Groman DB, Jones SRM. Rickettsial infection in farmed Atlantic salmon in eastern Canada. The Canadian Veterinary Journal. 2002;43(6):435–40. [PMC free article] [PubMed] [Google Scholar]
- 94.Fryer JL, Mauel MJ. The rickettsia: an emerging group of pathogens in fish. Emerging Infectious Diseases. 1997;3(2):137–44. 10.3201/eid0302.970206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mauel MJ, Giovannoni SJ, Fryer JL. Phylogenetic analysis of Piscirickettsia salmonis by 16S, internal transcribed spacer (ITS) and 23S ribosomal DNA sequencing. Diseases of aquatic organisms. 1999;35(2):115–23. 10.3354/dao035115 [DOI] [PubMed] [Google Scholar]
- 96.Contreras-Lynch S, Olmos P, Vargas A, Figueroa J, Gonzalez-Stegmaier R, Enriquez R, et al. Identification and genetic characterization of Piscirickettsia salmonis in native fish from southern Chile. Diseases of aquatic organisms. 2015;115(3):233–44. 10.3354/dao02892 [DOI] [PubMed] [Google Scholar]
- 97.Gil H, Platz GJ, Forestal CA, Monfett M, Bakshi CS, Sellati TJ, et al. Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(34):12897–902. 10.1073/pnas.0602582103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koronakis V, Eswaran J, Hughes C. Structure and function of TolC: the bacterial exit duct for proteins and drugs. Annual review of biochemistry. 2004;73:467–89. 10.1146/annurev.biochem.73.011303.074104 [DOI] [PubMed] [Google Scholar]
- 99.Susin MF, Baldini RL, Gueiros-Filho F, Gomes SL. GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. Journal of Bacteriology. 2006;188(23):8044–53. 10.1128/JB.00824-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wallin RPA, Lundqvist A, Moré SH, von Bonin A, Kiessling R, Ljunggren H-G. Heat-shock proteins as activators of the innate immune system. Trends in Immunology. 2002;23(3):130–5. [DOI] [PubMed] [Google Scholar]
- 101.Galdiero M, de l'Ero GC, Marcatili A. Cytokine and adhesion molecule expression in human monocytes and endothelial cells stimulated with bacterial heat shock proteins. Infection and Immunity. 1997;65(2):699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xolalpa W, Vallecillo AJ, Lara M, Mendoza-Hernandez G, Comini M, Spallek R, et al. Identification of novel bacterial plasminogen-binding proteins in the human pathogen Mycobacterium tuberculosis. Proteomics. 2007;7(18):3332–41. 10.1002/pmic.200600876 [DOI] [PubMed] [Google Scholar]
- 103.Almendras FE, Jones SR, Fuentealba C, Wright GM. In vitro infection of a cell line from Ictalurus nebulosus with Piscirickettsia salmonis. Canadian Journal of Veterinary Research. 1997;61(1):66–8. [PMC free article] [PubMed] [Google Scholar]
- 104.Vera T, Isla A, Cuevas A, Figueroa J. Un nuevo medio de cultivo líquido para el patógeno Piscirickettsia salmonis. Archivos de medicina veterinaria. 2012;44:273–7. [Google Scholar]
- 105.Bakkemo KR, Mikkelsen H, Bordevik M, Torgersen J, Winther-Larsen HC, Vanberg C, et al. Intracellular localisation and innate immune responses following Francisella noatunensis infection of Atlantic cod (Gadus morhua) macrophages. Fish Shellfish Immunol. 2011;31(6):993–1004. 10.1016/j.fsi.2011.08.020 [DOI] [PubMed] [Google Scholar]
- 106.Parker H, Chitcholtan K, Hampton MB, Keenan JI. Uptake of Helicobacter pylori outer membrane vesicles by gastric epithelial cells. Infection and Immunity. 2010;78(12):5054–61. 10.1128/IAI.00299-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2006;7(6):404–14. 10.1038/nrm1940 [DOI] [PubMed] [Google Scholar]
- 108.Ramírez R, Gómez FA, Marshall SH. The infection process of Piscirickettsia salmonis in fish macrophages is dependent upon interaction with host-cell clathrin and actin. FEMS Microbiology Letters. 2014. [DOI] [PubMed] [Google Scholar]
- 109.Winchell CG, Steele S, Kawula T, Voth DE. Dining in: intracellular bacterial pathogen interplay with autophagy. Current Opinion in Microbiology. 2016;29:9–14. 10.1016/j.mib.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez-Moreira E, Helbig JH, Swanson MS. Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes. Infection and Immunity. 2006;74(6):3285–95. 10.1128/IAI.01382-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van der Sar AM, Appelmelk BJ, Vandenbroucke-Grauls CMJE, Bitter W. A star with stripes: zebrafish as an infection model. Trends in Microbiology. 2004;12(10):451–7. 10.1016/j.tim.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 112.Dalpke A, Heeg K, Bartz H, Baetz A. Regulation of innate immunity by suppressor of cytokine signaling (SOCS) proteins. Immunobiology. 2008;213(3–4):225–35. 10.1016/j.imbio.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 113.Thi EP, Lambertz U, Reiner NE. Sleeping with the Enemy: How Intracellular Pathogens Cope with a Macrophage Lifestyle. PLoS Pathogens. 2012;8(3):e1002551 10.1371/journal.ppat.1002551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Medzhitov R, Janeway CA Jr. Innate immunity: impact on the adaptive immune response. Current opinion in immunology. 1997;9(1):4–9. [DOI] [PubMed] [Google Scholar]
- 115.Robinson JH, Delvig AA. Diversity in MHC class II antigen presentation. Immunology. 2002;105(3):252–62. 10.1046/j.0019-2805.2001.01358.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Haugland Ø, Torgersen J, Syed M, Evensen Ø. Expression profiles of inflammatory and immune-related genes in Atlantic salmon (Salmo salar L.) at early time post vaccination. Vaccine. 2005;23(48–49):5488–99. 10.1016/j.vaccine.2005.07.034 [DOI] [PubMed] [Google Scholar]
- 117.Fast MD, Johnson SC, Jones SRM. Differential expression of the pro-inflammatory cytokines IL-1β-1, TNFα-1 and IL-8 in vaccinated pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon juveniles. Fish & Shellfish Immunology. 2007;22(4):403–7. [DOI] [PubMed] [Google Scholar]
- 118.Hong G-E, Kim D-G, Park E-M, Nam B-H, Kim Y-O, Kong I-S. Identification of Vibrio anguillarum outer membrane vesicles related to immunostimulation in the Japanese Flounder, Paralichthys olivaceus. Bioscience, Biotechnology, and Biochemistry. 2009;73(2):437–9. 10.1271/bbb.80580 [DOI] [PubMed] [Google Scholar]
- 119.Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296(5566):298–300. 10.1126/science.1068883 [DOI] [PubMed] [Google Scholar]
- 120.Sepulcre MP, Alcaraz-Pérez F, López-Muñoz A, Roca FJ, Meseguer J, Cayuela ML, et al. Evolution of lipopolysaccharide (LPS) recognition and signaling: Fish TLR4 does not recognize LPS and negatively regulates NF-κB activation. The Journal of Immunology. 2009;182(4):1836–45. 10.4049/jimmunol.0801755 [DOI] [PubMed] [Google Scholar]
- 121.Yang LL, Wang GQ, Yang LM, Huang ZB, Zhang WQ, Yu LZ. Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: a novel screening method for anti-inflammatory drugs. Molecules (Basel, Switzerland). 2014;19(2):2390–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Novoa B, Bowman TV, Zon L, Figueras A. LPS response and tolerance in the zebrafish (Danio rerio). Fish & shellfish immunology. 2009;26(2):326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electron microscopy image analysis of MVs, exemplified by NVI 5692, showing the presences of both single membrane vesicles (OMV) consisting of an outer membrane (OM), and double membrane vesicles (I-OMV) containing a periplasmic membrane (PM) and an outer membrane.
(PDF)
Membrane fractions (M) and membrane vesicles (MVs) (20μg) isolated from three strains of P. salmonis, NVI 5692, NVI 5892 and LF-89 were applied to 12% SDS-PAGE and stained with coomassie blue.
(PDF)
Pie chart showing the cellular localization for the theoretical proteome of P. salmonis LF-89 (ATCC VR 1361).
(PDF)
Percentage of SHK-1, Atlantic salmon cell line, spleen and kidney primary cells isolated from adult zebrafish able to incorporate 10μg/mL of FITC conjugated membrane vesicles isolated from P. salmonis strains LF-89, NVI 5692 and NVI 5892 as assessed by flow cytometry (n = 3).
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.