Abstract
The objective of the study is to assess the TNF-α levels in PCOS patients and healthy controls. A comprehensive electronic search in Medline, Embase, and the Cochrane Library database was conducted up to July 2016. Random-effects model was used to estimate the standardized mean differences (SMDs) with 95% confidence intervals (CIs). Twenty-nine studies with a total of 1960 participants (1046 PCOS patients and 914 controls) were included in this meta-analysis. The TNF-α levels in PCOS patients were significantly higher than those in controls (random-effects, SMD = 0.60, 95% CI = 0.28–0.92, P<0.001). With regard to the subgroup analyses stratified by ethnicity, study quality, methods, and BMI, significantly high TNF-α levels were found in patients with PCOS in almost all of these subgroups. In the subgroup stratified by HOMA-IR ratio and T ratio, significant differences were only observed in the subgroups with HOMA-IR ratio of >1.72(SMD = 0.967, 95% CI = 0.103–1.831, P = 0.028, I2 = 93.5%) and T ratio>2.10 (SMD = 1.420, 95% CI = 0.429–2.411, P = 0.005, I2 = 96.1%). By meta-regression it was suggested that ethnicity might contribute little to the heterogeneity between the included studies. Through cumulative meta-analysis and sensitivity analysis it was supposed that the higher TNF-α levels of PCOS patients compared to healthy controls was stable and reliable. This meta-analysis suggests that the circulating TNF-α levels in women with PCOS are significantly higher than those in healthy controls. It may be involved in promoting insulin resistance and androgen excess of PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common heterogeneous endocrine disorders, which affects 5%–10% of women in reproductive age and is considered as one of the leading causes of female infertility[1]. It is characterized by biochemical or clinical hyperandrogenism, polycystic ovaries on ultrasonography and oligo-ovulation or anovulation. Besides, PCOS frequently accompanies with metabolic abnormality such as insulin resistance and obesity which predisposes women with PCOS to type 2 diabetes mellitus(T2DM)[2] and cardiovascular disease[3].
PCOS is also a proinflammatory state. Low-grade chronic inflammation in women with PCOS is involved in the pathogenesis of T2DM and cardiovascular disease[4]. Tumor necrosis factor-alpha (TNF-α) is a major proinflammatory cytokine and expressed mainly in monocytes, macrophages and adipose tissue. Serum levels of TNF-α were elevated in both obesity and T2DM [5]. TNF-α played a role in the pathogenesis of insulin resistance [5]. It inhibited tyrosine phosphorylation of insulin receptor and insulin receptor substrate-1 in muscle and fat cells [5]. Besides, it down-regulated the expression of the glucose transporter type 4 which was necessary for cellular transport of glucose [6]. TNF-α might also play a key role in the development of cardiovascular disease. Elevated levels of TNF-α were reported to be associated with an increased risk of future myocardial infarction[7]. Therefore, TNF-α may be a key mediator which is linked to T2DM and cardiovascular diseases in women with PCOS. Therefore, TNF-α may be a useful biomarker for the diagnosis of PCOS and the treatment of T2DM and cardiovascular diseases in women with PCOS.
Until recently, a number of studies have investigated the changes of TNF-α levels in PCOS patients. However, the results of these studies were contradictory rather than conclusive. Some studies reported significant elevation of TNF-α levels in PCOS women compared with healthy controls[8–15], but these were not confirmed in similar studies[16–28], with some studies even reporting decreased TNF-α levels[29]. Two meta-analysis of comparing circulating TNF-α levels in women with PCOS and healthy controls were reported in 2011[30, 31]. The analysis by toulis et al[31] revealed that TNF-α levels were higher in women with PCOS than in controls, but the other meta-analysis found no significant difference in TNF-α levels of PCOS women and controls[30]. During the last 5 years, many more relevant studies have been published and presented inconsistent results [11–15, 23–28]. Moreover, those two meta-analysis did not report the relations between TNF-α levels and the characteristics of PCOS, such as body mass index (BMI), insulin resistance, and androgen status. Thus, a updated meta-analysis of the literature was conducted to investigate the circulating TNF-α levels in PCOS women compared to healthy controls and the relations between TNF-α levels and the characteristics of PCOS.
Material and Methods
Search Strategy
A comprehensive electronic search in Medline, Embase, and the Cochrane Library database was conducted from inception to July 2016, using both free words and index terms specific to each search platform (Emtree in Embase.com and MeSH in Cochrane Library). The search strategies were based on combinations of the keywords: ‘tumor necrosis factor-alpha’, ‘TNF-alpha’, ‘tumor necrosis factor-α’, ‘TNF-α’ coupled with ‘polycystic ovary syndrome’, or ‘PCOS’. The detailed search strategies are listed in supplementary data (S1 File). The electronic investigation was supplemented with a manual search of references of all articles retrieved. The latest searches were undertaken to 1 July 2016. There were no language restrictions. Two reviewers independently searched the electronic databases, and screened the titles, abstracts, and full-text after excluding duplicated studies. Any discrepancy in the screening process was resolved by consultation of the group.
Study selection
Studies were included in the analysis if: 1. the study reported circulating TNF-α levels in women with PCOS and healthy women controls. 2. the study provided TNF-α means (M) and standard deviation (SD) or sufficient information to calculate them. Letters, case reports, editorials, and conference abstracts were excluded. Additionally, studies in which patients with diseases more than PCOS, as well as studies involving pregnant patients were also excluded.
Quality Score Assessment
Quality evaluation of included studies was conducted according to the Newcastle-Ottawa Scale[32] with minor modification. The quality assessment criteria used in the study were: 1. whether the diagnosis of the PCOS was with independent validation; 2. whether the involved cases were representative of population; 3. whether the controls enrolled were from the same community; 4. whether the controls were described to have no history of disease; 5. whether the cases and controls were matched for age or BMI; 6. Whether the cases and controls were matched for additional factor, such as drinking or smoking status; 7. whether the sample size was >50. For each criterion a score of 0 or 1 was assigned according to whether the criterion was satisfactorily fulfilled. According to the quality score assessment, the distribution of the scores was between 0 and 6. Studies with score of 5 or above are classified as high quality studies. Others are categorized as low quality studies.
Data Extraction
Two of the authors independently extracted the data from the included studies. The general characteristics of the study were extracted using a standardized data extraction form. The general characteristics (name of the first author, year of publication, country, diagnostic criteria, matched factors, number of the PCOS group and control group) were listed. Further, the following data of the PCOS and control groups were extracted and double-checked: methods used to measure TNF-α levels, BMI, age, TNF-α levels, insulin sensitivity status, and total testosterone levels. If the two investigators could not reach an agreement, the dispute was resolved by a third reviewer.
Statistical Analysis
TNF-α levels were extracted as M±SDs in each study. Missing SDs were calculated by reported standard error. Where appropriate, the data was completed through communication with the authors. Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) values were used to measure the degree of insulin resistance. When not reported, missing HOMA-IR values were calculated on the basis of mean fasting glucose and insulin values, using the Oxford Diabetes Trials Unit calculator (www.dtu.ox.ac.uk). The relative between-group difference in insulin resistance was expressed as the ratio of mean HOMA-IR value in the PCOS group to that of the controls. Similarly, the relative difference in androgen status was expressed by the ratio of mean total testosterone (T ratio) in PCOS women to controls. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) was used to estimate the effect size.
Heterogeneity across studies was quantified using the Q-statistic and inconsistency index (I2). If P < 0.10, the heterogeneity was considered as statistically significant. If I2> 50%, it was considered as large heterogeneity; if I2 = 25–50%, as moderate heterogeneity and if I2 < 25%, as absent of heterogeneity. In case of large heterogeneity (I2>50%), random-effects model was applied since it was usually more conservative. Otherwise, a fixed-effects model was used. When the results revealed statistically significant heterogeneity, possible explanations were investigated by subgroup analysis according to the following factors: ethnicity, quality score, methods used to measure TNF-α levels, BMI, HOMA-IR ratio, and T ratio. The BMI was categorized into two groups: a lean group (BMI <25 kg/m2) and an obese group (BMI ≥25 kg/m2). The HOMA-IR ratio and T ratio were categorized according to their quartile intervals. A meta-regression analysis was performed to investigate the sources of heterogeneity. The SMD was used as the dependent variable, and the year, ethnicity, sample Size, quality score, BMI, age, HOMA-IR ratio, T ratio, and sample size were used as explanatory covariates. A multivariable analysis was further performed if more than one variable were significant at the 0.1 level. A sensitivity analysis, with the studies omitted one by one, was performed to examine the influence of individual studies. A cumulative sequential meta-analysis of the studies was performed according to their year of publication. To assess the publication bias bias, begg’s test, egger’s test and trim-and-fill was applied. All statistical analyses were carried out with STATA software (version 10.0; Stata Corporation, College Station, TX).
Results
Literature Selection
A flow chart showing the study selection is presented in Fig 1. Using the search strategy predefined 522 potentially relevant studies were identified. After removing duplicated records and reviewing the titles and abstracts, 52 potential eligible studies that evaluate the TNF-α levels in PCOS and controls were identified for full-text assessment. Of these, 28 articles that did not fulfill the selection criteria were excluded(S2 File). Ten studies were excluded because the data was asymmetric distributed and presented by median and range[33–42]. Three studies were excluded because the data was presented by bar graph and unable to be obtained from the authors[43–45]. The control group of eight excluded studies was inappropriate[46–53]. In three excluded studies the levels of TNF-α were not tested in blood sample[54–56] and in another three studies the mRNA level of TNF-α in adipose tissue was tested[57–59].The subjects of two studies were overlapped[14, 60], so one of them was omitted[60]. Since several studies reported the data separately according to different categories of BMI (<25 kg/m2 or ≥25 kg/m2)[25, 27, 28, 61, 62], these five articles were separated as ten studies. Ultimately, 24 articles (29 studies) were included in this meta-analysis[8–29, 61, 62].
Fig 1. Flow chart showing the study selection for this meta-analysis.
Systematic review
The involved studies enrolled 1960 participants (1046 PCOS patients and 914 controls). The main characteristics of the included studies are demonstrated in Table 1. These studies were published between 1999 and 2015. The majority of the studies were conducted in Asia, whereas six were done in America, five in Europe, and one in Australia. Nineteen of the reports were conducted in Caucasian population, and ten study were conducted in Asian population (S2 Table). For the diagnosis of PCOS, 16 of the studies used Rotterdam criteria, 11 used National Institutes of Health (NIH) criteria, and another 2 used Androgen Excess Society (AES) criteria. The major matched factors for the PCOS and control group were BMI, age, waist circumference, and smoking. Ten of the studies found higher TNF-α levels in the PCOS group compared with the controls, and 18 studies found no statistically significant difference. Only one study reported decreased TNF-α levels in the PCOS group.
Table 1. Characteristics of studies included in the meta-analysis.
| Study | Year | Country | Study Design | Diagnosticcriteria | Sample size | Matched factors | TNF-α Level | Methods | PCOS | Control | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCOS | control | ||||||||||
| Gonzalez[61] | 1999 | USA | Case-control | NIH | 12 | 20 | Age,BMI | ↑ | ELISA | 3.31±0.87 | 0.91±0.54 |
| Gonzalez[61] | 1999 | USA | Case-control | NIH | 22 | 20 | Age,BMI | NS | ELISA | 4.11±0.89 | 4.65±1.47 |
| Escobar-Morreale[16] | 2001 | Spain | Case-control | NIH | 21 | 27 | BMI | NS | CL | 7.3±3.7 | 5.6±4.0 |
| Araya[8] | 2002 | Chile | Case-control | NIH | 16 | 11 | Age | ↑ | CL | 6.73±2.34 | 4.82±1.15 |
| Escobar-Morreale[17] | 2003 | Spain | Case-control | NIH | 35 | 28 | BMI,Smoking | NS | CL | 3.74±1.18 | 3.53±1.32 |
| Sayin[9] | 2003 | Turkey | Case-control | NIH | 21 | 14 | Age,BMI | ↑ | CL | 23.67±25.57 | 7.58±6.66 |
| Tarkun[10] | 2006 | Turkey | Case-control | Rotterdam | 32 | 25 | Age,BMI,WHR | ↑ | CL | 5.27±1.59 | 4.48±0.81 |
| Vgontzas[18] | 2006 | USA | Case-control | NIH | 42 | 17 | BMI | NS | ELISA | 4.05±1.94 | 3.79±0.82 |
| Moran[19] | 2007 | Australia | Case-control | Rotterdam | 15 | 17 | BMI,Smoking | NS | ELISA | 6.0±5.1 | 5.8±3.5 |
| Olszanecka[20] | 2007 | Poland | Case-control | NIH | 39 | 34 | Age,BMI,Smoking | NS | ELISA | 5.5±2.0 | 6.8±3.5 |
| Jakubowska[29] | 2008 | Poland | Case-control | Rotterdam | 29 | 29 | Age,BMI,WHR | ↓ | RIA | 10.69±13.14 | 14.95±13.3 |
| Arika[21] | 2009 | Turkey | Case-control | Rotterdam | 39 | 30 | Age,BMI,WC | NS | CL | 11.52±5.68 | 13.84±10.72 |
| Samy[62] | 2009 | Egypt | Case-control | Rotterdam | 56 | 35 | Age,BMI | NS | ELISA | 3.72±1.26 | 3.66±1.02 |
| Samy[62] | 2009 | Egypt | Case-control | Rotterdam | 52 | 40 | Age,BMI | ↑ | ELISA | 6.87±1.12 | 3.76±1.04 |
| Soares[22] | 2009 | Brazil | Case-control | Rotterdam | 40 | 50 | Age,BMI,Waist | NS | CL | 10.08±7.38 | 12.25±6.54 |
| Ilie[23] | 2011 | Romania | Case-control | AES | 45 | 32 | Age,BMI | NS | ELISA | 8.2±5.49 | 7.26±2.54 |
| Victor[11] | 2011 | Spain | Case-control | Rotterdam | 39 | 43 | Age,BMI,WC | ↑ | MBIA | 5.1±0.9 | 3.2±1.8 |
| Xiong[12] | 2011 | China | Case-control | Rotterdam | 86 | 50 | Age | ↑ | CL | 2.312±1.762 | 1.751±1.725 |
| Choi[13] | 2012 | Korea | Case-control | Rotterdam | 37 | 33 | BMI,smoking | ↑ | ELISA | 3.19±1.19 | 1.79±0.78 |
| Wang[24] | 2012 | China | Case-control | Rotterdam | 35 | 35 | Age,BMI,WC | NS | ELISA | 4.1±0.3 | 3.1±0.2 |
| Lee[25] | 2013 | Korea | Case-control | NIH | 20 | 20 | Age,BMI | NS | RIA | 1.06±0.47 | 1.19±0.88 |
| Lee[25] | 2013 | Korea | Case-control | NIH | 20 | 20 | Age,BMI | NS | RIA | 0.98±0.52 | 1.05±0.59 |
| Li[14] | 2013 | China | Case-control | NIH | 16 | 18 | Age,BMI,WC | ↑ | ELISA | 68.66±35.92 | 30.69±16.67 |
| Pawelczak[26] | 2014 | USA | Case-control | Rotterdam | 23 | 12 | Age,BMI | NS | MBIA | 7.4±4.08 | 4.8±3.16 |
| Thathapudi[15] | 2014 | India | Case-control | AES | 204 | 204 | Age | ↑ | ELISA | 13.24±10 | 5.5±3.8 |
| Agacayak[27] | 2015 | Turkey | Case-control | Rotterdam | 15 | 15 | Age,BMI | NS | ELISA | 313±248 | 294±292.2 |
| Agacayak[27] | 2015 | Turkey | Case-control | Rotterdam | 15 | 15 | Age,BMI | NS | ELISA | 212±242.1 | 214.5±233 |
| souza[28] | 2015 | Turkey | Case-control | Rotterdam | 8 | 10 | Age,BMI | NS | CL | 7.65±4.83 | 6.37±1.38 |
| souza[28] | 2015 | Turkey | Case-control | Rotterdam | 12 | 10 | Age,BMI | NS | CL | 10.47±6.92 | 8.11±3 |
NIH, National Institutes of Health; AES, Androgen Excess Society; BMI, body mass index; WC, Waist circumference; WHR, Waist-to-Hip Ratio, NS, no significant difference; ELISA, enzyme-linked immunosorbent assay; CL, chemiluminescence; RIA, radioimmunoassay; MBIA, multiplexing bead immunoassay
Furthermore, the detailed quality score for each study was listed in S2 Table. Thirteen studies scored 7 or above were categorized as high quality studies, and the other 16 studies were classified as low quality ones. The BMI in 13 of the studies was <25 kg/m2, and 14 of the studies ≥25 kg/m2 (S2 Table). Twenty-one studies reported the HOMA-IR ratio and 22 studies reported the T-ratio between PCOS women and controls to account for a difference in TNF-α levels and a percentage of the potential variability of across-study results (S2 Table).
Meta-Analysis
Pooled analysis
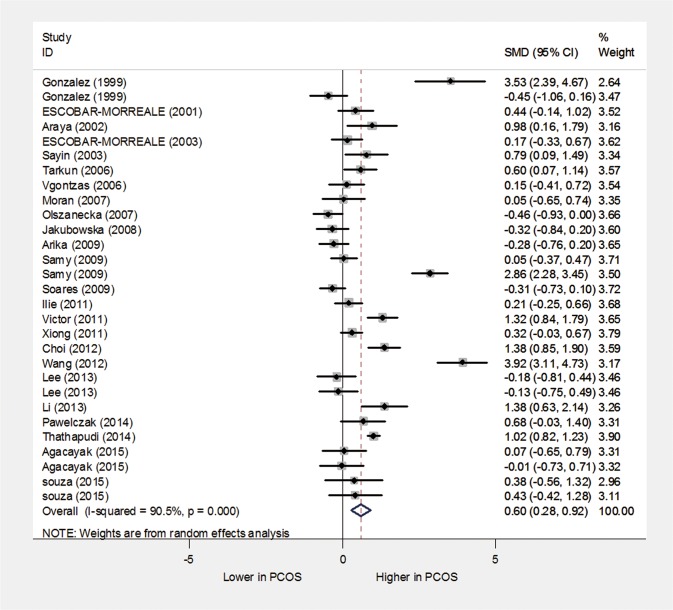
The overall effect of the pooled analysis indicated that the TNF-α levels in the PCOS patients were significantly higher than in healthy controls (random-effects, SMD = 0.60, 95% CI = 0.28–0.92, P<0.001; Fig 2). However, significant heterogeneity was found across the included studies (I2 = 90.5% and P<0.001).
Fig 2. The quantitative synthesis for TNF-α levels in PCOS patients compared with controls.
Subgroup analyses
Subgroup analyses were carried out according to the different categories of ethnicity, quality score, methods used to measure TNF-α levels, BMI, HOMA-IR ratio, and T ratio. The quartile intervals for the HOMA2-IR ratio were ≤1.39, 1.40–1.53, 1.54–1.89, and >1.89. The quartile intervals for the T ratio were ≤1.64, 1.65–1.81, 1.82–2.10, and >2.10. Studies with unavailable data of HOMA2-IR and total testosterone were categorized as the unknown group. The results of the subgroup analyses were shown in Table 2.
Table 2. Subgroup meta-analysis of TNF-α levels and polycystic ovary syndrome.
| Characteristic | Studies | SMD (95% CI) | P | Heterogeneity | |
|---|---|---|---|---|---|
| I2 | p | ||||
| Ethnicity | |||||
| Caucasian | 19 | 0.308(0.005,0.612) | 0.047 | 79.8% | 0.000 |
| Asia | 10 | 1.103(0.489,1.718) | 0.000 | 94.0% | 0.000 |
| Study quality | |||||
| Low score | 16 | 0.360(0.070,0.650) | 0.015 | 69.1% | 0.000 |
| High score | 13 | 0.840(0.285,1.395) | 0.003 | 94.8% | 0.000 |
| Methods | |||||
| ELISA | 14 | 0.932(0.361,1.502) | 0.001 | 94.1% | 0.000 |
| CL | 10 | 0.284(0.020,0.548) | 0.035 | 54.0% | 0.021 |
| RIA | 3 | -0.255(-0.560,0.110) | 0.188 | 0.0% | 0.883 |
| MBIA | 2 | 1.059(0.451,1.666) | 0.001 | 51.4% | 0.152 |
| BMI | |||||
| <25 | 13 | 0.829(0.280,1.379) | 0.003 | 92.2% | 0.000 |
| ≥25 | 16 | 0.431(0.024,0.838) | 0.038 | 89.3% | 0.000 |
| HOMA-IR ratio | |||||
| Unknown | 8 | 0.511(-0.022,1.044) | 0.060 | 82.7% | 0.000 |
| ≤1.39 | 5 | 0.614(-0.370,1.599) | 0.221 | 94.9% | 0.000 |
| 1.40–1.53 | 5 | 0.626(-0.020,1.272) | 0.057 | 85.8% | 0.000 |
| 1.54–1.89 | 6 | 0.335(-0.354,1.023) | 0.341 | 85.2% | 0.000 |
| >1.89 | 5 | 0.967(0.103,1.831) | 0.028 | 93.5% | 0.000 |
| T ratio | |||||
| Unknown | 7 | 0.695(-0.017,1.406) | 0.056 | 84.5% | 0.000 |
| ≤1.64 | 6 | 0.217(-0.104,0.537) | 0.186 | 53.5% | 0.056 |
| 1.65–1.81 | 5 | 0.258(-0.302,0.818) | 0.366 | 86.4% | 0.000 |
| 1.82–2.10 | 5 | 0.295(-0.290,0.881) | 0.323 | 75.7% | 0.002 |
| >2.10 | 6 | 1.420(0.429,2.411) | 0.005 | 96.1% | 0.000 |
With regard to the subgroup analysis by ethnicity, significantly higher TNF-α levels were found in patients with PCOS in both Caucasian (SMD = 0.308, 95%CI = 0.005–0.612, P = 0.047, I2 = 79.8%) and Asia ethnicity (SMD = 1.103, 95%CI = 0.489–1.718, P = 0.000, I2 = 94.0%) (S1 Fig, Table 2). There were also significant differences in TNF-α levels of low-score studies (SMD = 0.360, 95%CI = 0.070–0.650, P = 0.015, I2 = 69.1%) and high-score studies (SMD = 0.840, 95%CI = 0.070–0.650, P = 0.003, I2 = 94.8%) (S2 Fig, Table 2).
The methods used to measure TNF-α levels included enzyme-linked immunosorbent assay(ELISA,), chemiluminescence(CL), radioimmunoassay(RIA), and multiplexing bead immunoassay(MBIA). The TNF-α levels were also significant higher in the ELISA group(SMD = 0.932, 95%CI = 0.361–1.502, P = 0.001, I2 = 94.1%), CL group(SMD = 0.284, 95%CI = 0.020–0.548, P = 0.035, I2 = 54.0%), and MBIA group(SMD = 1.059, 95%CI = 0.451–1.666, P = 0.001, I2 = 51.4%), but not in the RIA group (SMD = -0.255, 95%CI = -0.560–0.110, P = 0.188, I2 = 0.0%) (S3 Fig, Table 2).
Subgroup analyses also demonstrated higher TNF-α levels in subgroups with BMI of <25 kg/m2 and ≥25 kg/m2 (SMD = 0.829, 95% CI = 0.280–1.379, P = 0.003, I2 = 92.2% and SMD = 0.431, 95%CI = 0.024–0.838, P = 0.038, I2 = 89.3%, respectively; S4 Fig, Table 2).
In the subgroup analysis, significant difference in the TNF-α levels of PCOS patients versus the controls were observed in the subgroup with HOMA-IR ratio >1.72(SMD = 0.967, 95% CI = 0.103–1.831, P = 0.028, I2 = 93.5%)(S5 Fig, Table 2). However, there was no significant difference in the subgroups with unknown HOMA2-IR ratio, HOMA2-IR ratios ≤1.39, 1.40–1.53, and 1.54–1.89 (S5 Fig, Table 2). There was also a significant difference in the TNF-α levels in the subgroup with T ratio>2.10 (SMD = 1.420, 95% CI = 0.429–2.411, P = 0.005, I2 = 96.1%) but not in unknown T ratio group and low T ratio groups (T ratio ≤1.64, 1.65–1.81, and 1.82–2.10) (S6 Fig, Table 2).
Meta-regression analysis
To further investigate the sources of heterogeneity, meta-regression analysis was conducted. SMD was used as the dependent variable, and year, ethnicity, sample Size, quality score, BMI, Age, HOMA-IR ratio, and T ratio were entered as explanatory covariates. Results of the univariate analysis are presented in Table 3. If the regression coefficient of the covariates were significant at the level of 0.1, then the covariates were entered into the multivariate meta-regression. Based on the univariate meta-regression analysis, the regression coefficient of the ethnicity (Asia and Australia) was significant at the level of 0.1 (P = 0.069). Therefore, subsequent multivariate meta-regression could not be done. The results of meta-regression suggested that ethnicity might contribute little to the heterogeneity between included studies, and other covariates failed to account for the heterogeneity.
Table 3. Univariate meta-regression analysis for the potential variables between studies.
| Studies | Coefficient | SE | t | P | 95%CI | |
|---|---|---|---|---|---|---|
| Year | 29 | -0.008 | 0.043 | -0.18 | 0.859 | (0.096,0.080) |
| Ethnicity | 29 | -0.760 | 0.402 | -1.89 | 0.069 | (1.585,0.065) |
| Sample Size | 29 | 0.001 | 0.003 | 0.45 | 0.653 | (0.004,0.007) |
| Quality score | 29 | 0.132 | 0.194 | 0.68 | 0.501 | (0.266,0.532) |
| BMI | 26 | -0.049 | 0.039 | -1.25 | 0.224 | (0.130,0.032) |
| Age | 25 | -0.016 | 0.065 | -0.24 | 0.812 | (0.150,0.119) |
| HOMA-IR ratio | 21 | 0.555 | 0.400 | 1.39 | 0.181 | (0.282,1.392) |
| T ratio | 22 | 0.472 | 0.304 | 1.55 | 0.136 | (0.162,1.106) |
Cumulative meta-analysis
To explore the changes in the TNF-α levels of PCOS patients over time, a cumulative meta-analysis was carried out (Fig 3). The random-effects pooled SMD was insignificantly larger or smaller than zero from the first study in 1999 to the study in 2009 by Soares et al., representing no statistically significant difference in TNF-α level between PCOS patients and healthy controls. A statistically significant difference was consistently observed since added the study by Ilie et al. (SMD = 0.44, 95% CI = 0.01–0.87), and the tendency of SMD became stable after that study, which indicating the stability of differences in the TNF-α levels of the PCOS patients over time.
Fig 3. Cumulative meta-analysis for TNF-α levels in PCOS patients compared with controls.
Sensitivity analysis
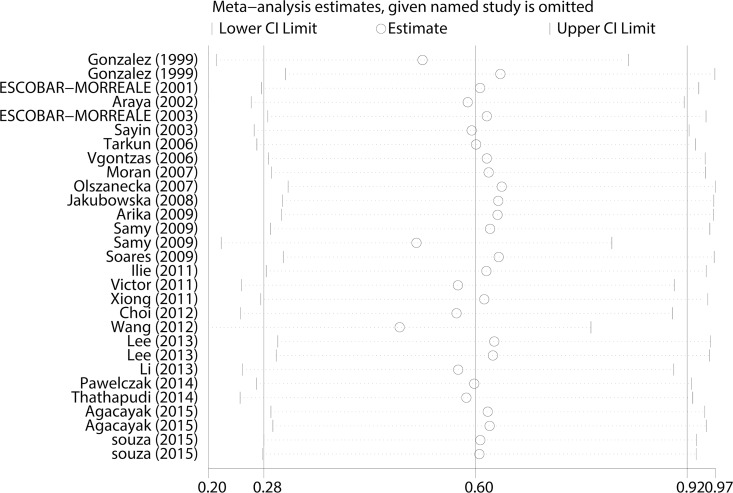
A sensitivity analysis was performed by omitting one study at a time and calculating the pooled SMDs and 95% CIs for the remaining studies. There was no change in the effect when any one of the studies was excluded (Fig 4), which indicated that the results of the meta-analysis was reliable and stable. In three of included studies the BMI of the PCOS group and control group was unmatched. Therefore, the effect was recalculated after discarded these data in that three studies and there was also significant difference in the TNF-α levels of the PCOS patients compared with that of the controls (SMD = 0.589, 95% CI = 0.213–0.962, P = 0.002).
Fig 4. Sensitivity analysis for TNF-α levels in PCOS patients compared with controls.
Studies that might contributed to the heterogeneity[11, 13, 15, 20–22, 24, 29, 61, 62], as confirmed by a Galbraith plot analysis (S7 Fig), were then excluded. The TNF-α levels remained significantly higher in the PCOS patients compared to the controls (SMD = 0.335, 95% CI = 0.162–0.508, P<0.001), but the heterogeneity decreased significantly (I2 = 25.7% and P = 0.159).
Publication bias
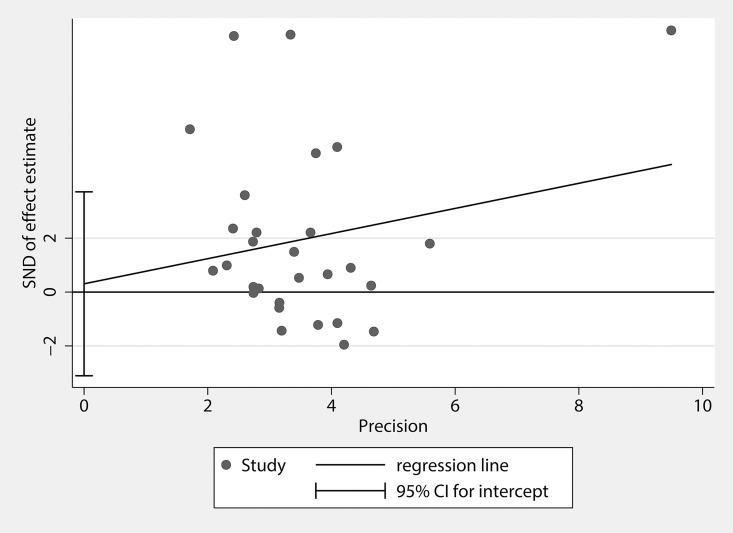
Publication bias of the studies was evaluated by Begg’s funnel plots and Egger’s tests. The Begg’s funnel plot was slightly asymmetrical in distribution (P = 0.028), which indicated the possibility of publication bias. But the Egger’s test suggested no statistically significant asymmetry of the funnel plot (P = 0.855, Fig 5). We further undertook analysis using the trim-and-fill method[63]. The results from the trim-and-fill analysis did not change the summary estimate of effect, suggesting that publication bias is unlikely to affect the result of the meta-analysis.
Fig 5. Graph of Egger’s test for publication bias in the studies of the meta-analysis of TNF-α levels in PCOS patients compared with controls.
Discussion
In this meta-analysis with 29 included studies it was demonstrated that the TNF-α levels in PCOS patients were significantly higher than in controls, which were consistent with the results of toulis’s [31]. With regard to the subgroup analyses stratified by ethnicity and study quality, significantly high TNF-α levels were found in patients with PCOS in all of these subgroups. Furthermore, raised TNF-α levels were found both in lean and in obese PCOS patients. In the subgroup stratified by HOMA ratio and T ratio, significant difference was only observed in the quartiles with HOMA-IR ratio >1.72 and T ratio >2.10. So elevated TNF-α levels are related to the insulin resistance and androgen status in women with PCOS. By meta-regression it was suggested that ethnicity might contribute little to the heterogeneity between included studies. Through cumulative meta-analysis and sensitivity analysis it was concluded the difference in the TNF-α levels of the PCOS patients compared to the controls was stable and reliable.
Two meta-analyses of comparing circulating TNF-α levels in women with PCOS and healthy controls were conducted in 2011[30, 31]. The analysis by toulis et al[31] with 13 studies revealed that TNF-α levels were higher in women with PCOS than in controls, but the other meta-analysis with 9 studies conducted by Escobar-Morreale et al found there was no significant difference in TNF-α levels of PCOS women and controls[30]. In Escobar-Morreale’s meta-analysis four studies with fewer than 25 PCOS women were excluded, which resulted in the inconsistent effect with toulis’s study. So we conducted the analysis after discarding the seven studies with fewer than 25 PCOS patients[8, 9, 14, 16, 19, 26, 28], the effect direction (SMD = 0.594, 95% CI = 0.192–0.996, P = 0.004) was the same as the analysis with 29 studies. This study included 29 studies and the sample size was twice more than that of the previous studies. With the expansion of sample size, the corresponding statistical power can be increased. The BMI of the cases and controls was unmatched in three of included studies. Considering that the levels of proinflammatory cytokines are usually elevated in obesity [64], potential selection bias could influence the results in BMI-unmatched studies. So the effect was recalculated after excluding these data and the result was also significant different in the TNF-α levels.
In our study TNF-α levels of PCOS patients were higher than those of the controls in the subgroups with high HOMA-IR ratio and T ratio, but no significant difference in the low HOMA2-IR ratio and T ratio subgroups. This indicates that the elevated TNF-α levels are directly related to the insulin resistance and androgen excess of PCOS. TNF-α levels were elevated in both obesity and metabolic syndrome[65]. It decreased the cellular response to insulin in adipocytes, hepatocytes and human muscle cells [64].In Gonzalez’s study women with PCOS failed to suppress monocyte-derived TNF-α and IL-6 release in response to glucose ingestion, and this response is independent of excess adiposity[56]. Monocyte-derived cytokine release was directly related to insulin resistance and androgen excess[56]. Combined with our study it was supposed that the raised TNF-α levels are not an intrinsic characteristic of PCOS, but it may be involved in promoting insulin resistance and androgen excess of PCOS. The mechanism of TNF-α to participate in insulin resistance and androgen excess could be of potential research interest in PCOS.
To our knowledge, this is the most comprehensive meta-analysis uptodate to evaluate the association between TNF-α levels and PCOS. Substantial number of cases and controls were pooled from publications concerned with circulating TNF-α levels and PCOS, which greatly increased statistical power of the analysis and provided enough evidence to draw a safe conclusion. Sensitivity analysis suggested that no single study influenced the pooled SMD qualitatively. And cumulative meta-analysis showed that no significant change occurred in pooled SMD since 2009, indicating the stability of the association between high TNF-α levels and PCOS. In addition, no obvious publication bias was detected in this meta-analysis, which indicated that the pooled results of our study should be reliable. Taken together, these data further confirm the reliability and stability of the meta-analysis results.
The main limitation of the present study is the significant heterogeneity across the included studies. After stratified by methods to measure TNF-α levels, it is found that the heterogeneity was decreased in the CL, RIA, and MBIA group, but also high in the ELISA group. So the heterogeneity in this meta-analysis might be partly attributed to the differences on the sensitivity and accuracy of different methods used to measure TNF-α levels. In the subgroup of T ratio ≤1.64 this heterogeneity decreased to 53.5%. So the differences in T levels may also be related to the heterogeneity. By meta-regression only ethnicity was found to contribute little to the heterogeneity. According to Galbraith plot analysis ten studies that might contributed to the heterogeneity were found. After excluding these studies the TNF-α levels remained significantly high in the PCOS patients, but the heterogeneity decreased to 25.7%. Although we conducted subgroup analysis, meta-regression analysis, and sensitivity analysis, the sources of heterogeneity were not satisfactorily explained. It might reflect clinical heterogeneity related to geographical differences, smoking status, concomitant subclinical inflammatory diseases, and variability in PCOS diagnostic criteria. But it was not possible to take into account different PCOS phenotypes.
Although the publication bias was denied by egger’s test and trim-and-fill method, the possibility of undetected bias cannot be absolutely excluded. In addition, missing data on the HOMA-IR ratio and T ratio in some studies may produce a certain degree of systemic bias. The results were based on unadjusted estimates, whereas a more precise evaluation should consider the confounding factors such as smoking status, alcoholic consumption, environmental factors, and other diet lifestyle. There was an increase of TNF-α in patients with dyslipidemia [64], but because of data limitation we were not able to conduct further analysis stratified by the lipid metabolism of included women. These limitations must be considered when interpreting the results of this meta-analysis.
Conclusions
This meta-analysis suggested that circulating TNF-α levels in women with PCOS were significantly higher than those in healthy controls and that a high serum TNF-α concentration was related to insulin resistance and androgen excess but not to the BMI. Therefore, a high TNF-α level is not an intrinsic characteristic of PCOS, but it may be involved in promoting insulin resistance and hyperandrogenism of PCOS.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
This research was supported by National Natural Science Foundation of China (81401173), and Natural Science Foundation from Yangzhou (YZ2014021).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by National Natural Science Foundation of China (81401173), and Natural Science Foundation from Yangzhou (YZ2014021).
References
- 1.Franks S. Polycystic ovary syndrome. The New England journal of medicine. 1995;333(13):853–61. 10.1056/NEJM199509283331307 . [DOI] [PubMed] [Google Scholar]
- 2.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. The Journal of clinical endocrinology and metabolism. 1999;84(1):165–9. 10.1210/jcem.84.1.5393 . [DOI] [PubMed] [Google Scholar]
- 3.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocrine reviews. 2003;24(3):302–12. 10.1210/er.2003-0004 . [DOI] [PubMed] [Google Scholar]
- 4.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. Journal of internal medicine. 2003;254(1):32–44. . [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. The Journal of clinical investigation. 1994;94(4):1543–9. 10.1172/JCI117495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephens JM, Pekala PH. Transcriptional repression of the GLUT4 and C/EBP genes in 3T3-L1 adipocytes by tumor necrosis factor-alpha. The Journal of biological chemistry. 1991;266(32):21839–45. . [PubMed] [Google Scholar]
- 7.Halawa B, Salomon P, Jolda-Mydlowska B, Zysko D. [Levels of tumor necrosis factor (TNF-alpha) and interleukin 6 (IL-6) in serum of patients with acute myocardial infarction]. Polskie Archiwum Medycyny Wewnetrznej. 1999;101(3):197–203. . [PubMed] [Google Scholar]
- 8.Araya AV, Aguirre A, Romero C, Miranda C, Molina MC, Ferreira A. Evaluation of tumor necrosis factor alpha production in ex vivo short term cultured whole blood from women with polycystic ovary syndrome. Eur Cytokine Netw. 2002;13(4):419–24. . [PubMed] [Google Scholar]
- 9.Sayin NC, Gucer F, Balkanli-Kaplan P, Yuce MA, Ciftci S, Kucuk M, et al. Elevated serum TNF-alpha levels in normal-weight women with polycystic ovaries or the polycystic ovary syndrome. J Reprod Med. 2003;48(3):165–70. . [PubMed] [Google Scholar]
- 10.Tarkun I, Cetinarslan B, Turemen E, Canturk Z, Biyikli M. Association between circulating tumor necrosis factor-alpha, interleukin-6, and insulin resistance in normal-weight women with polycystic ovary syndrome. Metabolic Syndrome and Related Disorders. 2006;4(2):122–8. 10.1089/met.2006.4.122 . [DOI] [PubMed] [Google Scholar]
- 11.Victor VM, Rocha M, Banuls C, Alvarez A, de Pablo C, Sanchez-Serrano M, et al. Induction of Oxidative Stress and Human Leukocyte/Endothelial Cell Interactions in Polycystic Ovary Syndrome Patients with Insulin Resistance. Journal of Clinical Endocrinology & Metabolism. 2011;96(10):3115–22. 10.1210/jc.2011-0651 . [DOI] [PubMed] [Google Scholar]
- 12.Xiong YL, Liang XY, Yang X, Li Y, Wei LN. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2011;159(1):148–50. 10.1016/j.ejogrb.2011.07.012 . [DOI] [PubMed] [Google Scholar]
- 13.Choi YS, Yang HI, Cho S, Jung JA, Jeon YE, Kim HY, et al. Serum asymmetric dimethylarginine, apelin, and tumor necrosis factor-alpha levels in non-obese women with polycystic ovary syndrome. Steroids. 2012;77(13):1352–8. 10.1016/j.steroids.2012.08.005 . [DOI] [PubMed] [Google Scholar]
- 14.Li S, Tao T, Wang L, Mao X, Zheng J, Zhao A, et al. The Expression of 11 beta-HSDs, GR, and H6PDH in Subcutaneous Adipose Tissue from Polycystic Ovary Syndrome Subjects. Hormone and Metabolic Research. 2013;45(11):802–7. 10.1055/s-0033-1345186 . [DOI] [PubMed] [Google Scholar]
- 15.Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q. Tumor Necrosis Factor-Alpha and Polycystic Ovarian Syndrome: A Clinical, Biochemical, and Molecular Genetic Study. Genetic Testing and Molecular Biomarkers. 2014;18(9):605–9. 10.1089/gtmb.2014.0151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Escobar-Morreale HF, Calvo RM, Sancho J, San Millan JL. TNF-alpha and hyperandrogenism: A clinical, biochemical, and molecular genetic study. Journal of Clinical Endocrinology & Metabolism. 2001;86(8):3761–7. 10.1210/jc.86.8.3761 . [DOI] [PubMed] [Google Scholar]
- 17.Escobar-Morreale HF, Villuendas G, Botella-Carretero JI, Sancho J, San Millan JL. Obesity, and not insulin resistance, is the major determinant of serum inflammatory cardiovascular risk markers in pre-menopausal women. Diabetologia. 2003;46(5):625–33. 10.1007/s00125-003-1090-z . [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Trakada G, Bixler EO, Lin HM, Pejovic S, Zoumakis E, et al. Plasma interleukin 6 levels are elevated in polycystic ovary syndrome independently of obesity or sleep apnea. Metab-Clin Exp. 2006;55(8):1076–82. 10.1016/j.metabol.2006.04.002 . [DOI] [PubMed] [Google Scholar]
- 19.Moran LJ, Noakes M, Clifton PM, Wittert GA, Belobrajdic DP, Norman RJ. C-reactive protein before and after weight loss in overweight women with and without polycystic ovary syndrome. Journal of Clinical Endocrinology & Metabolism. 2007;92(8):2944–51. 10.1210/jc.2006-2336 . [DOI] [PubMed] [Google Scholar]
- 20.Olszanecka-Glinianowicz M, Banag M, Zahorska-MarkiewiCz B, Janowska J, Kocelak P, Madej P, et al. Is the polycystic ovary syndrome associated with chronic inflammation per se? European Journal of Obstetrics & Gynecology and Reproductive Biology. 2007;133(2):197–202. 10.1016/j.ejogrb.2006.10.037 . [DOI] [PubMed] [Google Scholar]
- 21.Arikan S, Akay H, Bahceci M, Tuzcu A, Gokalp D. The evaluation of endothelial function with flow-mediated dilatation and carotid intima media thickness in young nonobese polycystic ovary syndrom patients; existence of insulin resistance alone may not represent an adequate condition for deterioration of endothelial function. Fertility and Sterility. 2009;91(2):450–5. 10.1016/j.fertnstert.2007.11.057 . [DOI] [PubMed] [Google Scholar]
- 22.Soares GM, Vieira CS, Martins WP, Franceschini SA, dos Reis RM, de Sa MFS, et al. Increased arterial stiffness in nonobese women with polycystic ovary syndrome (PCOS) without comorbidities: one more characteristic inherent to the syndrome? Clinical Endocrinology. 2009;71(3):406–11. 10.1111/j.1365-2265.2008.03506.x . [DOI] [PubMed] [Google Scholar]
- 23.Ilie IR, Pepene CE, Marian I, Mocan T, Hazi G, Dragotoiu G, et al. The polycystic ovary syndrome (pcos) status and cardiovascular risk in young women. Central European Journal of Medicine. 2011;6(1):64–75. 10.2478/s11536-010-0054-1 . [DOI] [Google Scholar]
- 24.Wang YX, Zhu WJ. Evaluation of Adiponectin, Resistin, IL-6, TNF-α in Obese and Non-obese Women with Polycystic Ovary Syndrome. Journal of Reproduction and Contraception. 2012;23(4):237–44. [Google Scholar]
- 25.Lee H, Oh J-Y, Sung Y-A. Adipokines, insulin-like growth factor binding protein-3 levels, and insulin sensitivity in women with polycystic ovary syndrome. Korean Journal of Internal Medicine. 2013;28(4):456–63. 10.3904/kjim.2013.28.4.456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pawelczak M, Rosenthal J, Milla S, Liu Y-H, Shah B. Evaluation of the Pro-inflammatory Cytokine Tumor Necrosis Factor-alpha in Adolescents with Polycystic Ovary Syndrome. Journal of Pediatric and Adolescent Gynecology. 2014;27(6):356–9. 10.1016/j.jpag.2014.01.104 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agacayak E, Tunc SY, Sak S, Basaranoglu S, Yuksel H, Turgut A, et al. Levels of Neopterin and other Inflammatory Markers in Obese and Non-Obese Patients with Polycystic Ovary Syndrome. Medical Science Monitor. 2015;21:2446–55. 10.12659/MSM.894368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza dos Santos AC, Soares NP, Costa EC, Ferrezini de Sa JC, Azevedo GD, Araujo Moura Lemos TM. The impact of body mass on inflammatory markers and insulin resistance in polycystic ovary syndrome. Gynecological Endocrinology. 2015;31(3):225–8. 10.3109/09513590.2014.976546 . [DOI] [PubMed] [Google Scholar]
- 29.Jakubowska J, Bohdanowicz-Pawlak A, Milewicz A, Szymczak J, Bednarek-Tupikowska G, Demissie M. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecological Endocrinology. 2008;24(7):378–84. 10.1080/09513590802128968 . [DOI] [PubMed] [Google Scholar]
- 30.Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–58 e1-2. 10.1016/j.fertnstert.2010.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, et al. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Human reproduction update. 2011;17(6):741–60. 10.1093/humupd/dmr025 . [DOI] [PubMed] [Google Scholar]
- 32.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 33.Amato G, Conte M, Mazziotti G, Lalli E, Vitolo G, Tucker AT, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101(6):1177–82. 10.1016/s0029-7844(03)00233-3 . [DOI] [PubMed] [Google Scholar]
- 34.Puder JJ, Varga S, Nusbaumer CPG, Zulewski H, Bilz S, Muller B, et al. Women with polycystic ovary syndrome are sensitive to the TNF-alpha-lowering effect of glucose-induced hyperinsulinaemia. European Journal of Clinical Investigation. 2006;36(12):883–9. 10.1111/j.1365-2362.2006.01734.x . [DOI] [PubMed] [Google Scholar]
- 35.Shroff R, Kerchner A, Maifeld M, Van Beek EJR, Jagasia D, Dokras A. Young obese women with polycystic ovary syndrome have evidence of early coronary atherosclerosis. Journal of Clinical Endocrinology & Metabolism. 2007;92(12):4609–14. 10.1210/jc.2007-1343 . [DOI] [PubMed] [Google Scholar]
- 36.Ozcaka O, Ceyhan BO, Akcali A, Bicakci N, Lappin DF, Buduneli N. Is There an Interaction Between Polycystic Ovary Syndrome and Gingival Inflammation? Journal of Periodontology. 2012;83(12):1529–37. 10.1902/jop.2012.110588 . [DOI] [PubMed] [Google Scholar]
- 37.Svendsen PF, Christiansen M, Hedley PL, Nilas L, Pedersen SB, Madsbad S. Adipose expression of adipocytokines in women with polycystic ovary syndrome. Fertility and Sterility. 2012;98(1). 10.1016/j.fertnstert.2012.03.056 . [DOI] [PubMed] [Google Scholar]
- 38.Barcellos CRG, Rocha MP, Hayashida SAY, Dantas WS, Yance VRV, Marcondes JAM. Obesity, but not polycystic ovary syndrome, affects circulating markers of low-grade inflammation in young women without major cardiovascular risk factors. Hormones. 2015;14(2):251–7. 10.14310/horm.2002.1584 [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi Barcellos CR, Rocha MP, Yamashita Hayashida SA, Dantas WS, Vieira Yance VdR, Miguel Marcondes JA. Obesity, but not polycystic ovary syndrome, affects circulating markers of low-grade inflammation in young women without major cardiovascular risk factors. Hormones-International Journal of Endocrinology and Metabolism. 2015;14(2):251–7. . [DOI] [PubMed] [Google Scholar]
- 40.Puder JJ, Varga S, Kraenzlin M, De Geyter C, Keller U, Muller B. Central fat excess in polycystic ovary syndrome: Relation to low-grade inflammation and insulin resistance. Journal of Clinical Endocrinology & Metabolism. 2005;90(11):6014–21. 10.1210/jc.2005-1002 . [DOI] [PubMed] [Google Scholar]
- 41.Gao H, Meng J, Xu M, Zhang S, Ghose B, Liu J, et al. Serum Heat Shock Protein 70 Concentration in Relation to Polycystic Ovary Syndrome in a Non-Obese Chinese Population. Plos One. 2013;8(6). 10.1371/journal.pone.0067727 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomann R, Rossinelli N, Keller U, Tirri BF, De Geyter C, Ruiz J, et al. Differences in low-grade chronic inflammation and insulin resistance in women with previous gestational diabetes mellitus and women with polycystic ovary syndrome. Gynecological endocrinology: the official journal of the International Society of Gynecological Endocrinology. 2008;24(4):199–206. 10.1080/09513590801893398 . [DOI] [PubMed] [Google Scholar]
- 43.Knebel B, Janssen OE, Hahn S, Jacob S, Gleich J, Kotzka J, et al. Increased low grade inflammatory serum markers in patients with polycystic ovary syndrome (PCOS) and their relationship to PPAR gamma gene variants. Experimental and Clinical Endocrinology & Diabetes. 2008;116(8):481–6. 10.1055/s-2008-1058085 . [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez F, Sia CL, Shepard MK, Rote NS, Minium J. Inflammation in Response to Glucose Ingestion Is Independent of Excess Abdominal Adiposity in Normal-Weight Women with Polycystic Ovary Syndrome. Journal of Clinical Endocrinology & Metabolism. 2012;97(11):4071–9. 10.1210/jc.2012-2131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Victor VM, Rovira-Llopis S, Bañuls C, Diaz-Morales N, Lopez-Domenech S, Escribano-López I, et al. Metformin modulates human leukocyte/endothelial cell interactions and proinflammatory cytokines in polycystic ovary syndrome patients. Atherosclerosis. 2015;242(1):167–73. 10.1016/j.atherosclerosis.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 46.Kawamura S, Maesawa C, Nakamura K, Nakayama K, Morita M, Hiruma Y, et al. Predisposition for borderline personality disorder with comorbid major depression is associated with that for polycystic ovary syndrome in female Japanese population. Neuropsychiatr Dis Treat. 2011;7:655–62. 10.2147/ndt.s25504 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oner G, Muderris II. Clinical, endocrine and metabolic effects of metformin vs N-acetyl-cysteine in women with polycystic ovary syndrome. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2011;159(1):127–31. 10.1016/j.ejogrb.2011.07.005 . [DOI] [PubMed] [Google Scholar]
- 48.Oner G, Muderris II. Efficacy of omega-3 in the treatment of polycystic ovary syndrome. Journal of Obstetrics and Gynaecology. 2013;33(3):289–91. 10.3109/01443615.2012.751365 [DOI] [PubMed] [Google Scholar]
- 49.Almario RU, Karakas SE. Roles of Circulating WNT-Signaling Proteins and WNT-Inhibitors in Human Adiposity, Insulin Resistance, Insulin Secretion, and Inflammation. Hormone and Metabolic Research. 2015;47(2):152–7. 10.1055/s-0034-1384521 . [DOI] [PubMed] [Google Scholar]
- 50.Gower BA, Goss AM. A Lower-Carbohydrate, Higher-Fat Diet Reduces Abdominal and Intermuscular Fat and Increases Insulin Sensitivity in Adults at Risk of Type 2 Diabetes. Journal of Nutrition. 2015;145(1):177–83. 10.3945/jn.114.195065 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Covington JD, Tam CS, Pasarica M, Redman LM. Higher circulating leukocytes in women with PCOS is reversed by aerobic exercise. Biochimie. 2016;124:27–33. 10.1016/j.biochi.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Kocelak P, Janowska J, Semik-Grabarczyk E. The effect of weight loss on inflammation in obese women with polycystic ovary syndrome. Endokrynologia Polska. 2008;59(1):13–7. . [PubMed] [Google Scholar]
- 53.Omu AE, Al-Azemi MK, Makhseed M, Al-Oattan F, Ismail AA, Al-Tahir S, et al. Differential expression of T-helper cytokines in the peritoneal fluid of women with normal ovarian cycle compared with women with chronic anovulation. Acta obstetricia et gynecologica Scandinavica. 2003;82(7):603–9. . [DOI] [PubMed] [Google Scholar]
- 54.Victor VM, Rocha M, Banuls C, Sanchez-Serrano M, Sola E, Gomez M, et al. Mitochondrial Complex I Impairment in Leukocytes from Polycystic Ovary Syndrome Patients with Insulin Resistance. Journal of Clinical Endocrinology & Metabolism. 2009;94(9):3505–12. 10.1210/jc.2009-0466 . [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez F, Kirwan JP, Rote NS, Minium J. Evidence of mononuclear cell preactivation in the fasting state in polycystic ovary syndrome. American Journal of Obstetrics and Gynecology. 2014;211(6). 10.1016/j.ajog.2014.06.044 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez F, Sia CL, Shepard MK, Rote NS, Minium J. The Altered Mononuclear Cell-Derived Cytokine Response to Glucose Ingestion Is Not Regulated by Excess Adiposity in Polycystic Ovary Syndrome. Journal of Clinical Endocrinology & Metabolism. 2014;99(11):E2244–E51. 10.1210/jc.2014-2046 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindholm A, Blomquist C, Bixo M, Dahlbom I, Hansson T, Poromaa IS, et al. No difference in markers of adipose tissue inflammation between overweight women with polycystic ovary syndrome and weight-matched controls. Human Reproduction. 2011;26(6):1478–85. 10.1093/humrep/der096 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seow K-M, Lin Y-H, Hwang J-L, Wang P-H, Ho L-T, Lin Y-H, et al. Expression levels of haem oxygenase-I in the omental adipose tissue and peripheral blood mononuclear cells of women with polycystic ovary syndrome. Human Reproduction. 2011;26(2):431–7. 10.1093/humrep/deq351 . [DOI] [PubMed] [Google Scholar]
- 59.Huang ZH, Manickam B, Ryvkin V, Zhou XJ, Fantuzzi G, Mazzone T, et al. PCOS Is Associated with Increased CD11c Expression and Crown-Like Structures in Adipose Tissue and Increased Central Abdominal Fat Depots Independent of Obesity. Journal of Clinical Endocrinology & Metabolism. 2013;98(1):E17–E24. 10.1210/jc.2012-2697 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tao T, Li S, Zhao A, Zhang Y, Liu W. Expression of the CD11c gene in subcutaneous adipose tissue is associated with cytokine level and insulin resistance in women with polycystic ovary syndrome. European Journal of Endocrinology. 2012;167(5):705–13. 10.1530/eje-12-0340 . [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metab-Clin Exp. 1999;48(4):437–41. 10.1016/s0026-0495(99)90100-2 . [DOI] [PubMed] [Google Scholar]
- 62.Samy N, Hashim M, Sayed M, Said M. Clinical significance of inflammatory markers in polycystic ovary syndrome: Their relationship to insulin resistance and Body Mass Index. Dis Markers. 2009;26(4):163–70. 10.3233/dma-2009-0627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. . [DOI] [PubMed] [Google Scholar]
- 64.Ramirez Alvarado MM, Sanchez Roitz C. [Tumor necrosis factor-alpha, insulin resistance, the lipoprotein metabolism and obesity in humans]. Nutricion hospitalaria. 2012;27(6):1751–7. 10.3305/nh.2012.27.6.6004 . [DOI] [PubMed] [Google Scholar]
- 65.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–45. 10.1146/annurev-immunol-031210-101322 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(DOC)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.