Abstract
Durable local control of irradiated cancer and distant abscopal effects are presumably immune mediated. To evaluate the role of radiotherapy (RT) for limited progression after anti-CTLA4 checkpoint inhibition, medical records of all patients with surgically incurable stage III or IV melanoma from a single institution who received ipilimumab as first-line immunotherapy and subsequent RT were reviewed. Sixteen patients who received RT to all sites of limited melanoma progression were analyzed. Eight patients with an incomplete initial response to ipilimumab received RT to new or progressive disease, whereas the remaining 8 patients with a complete initial response to ipilimumab received RT to sites of subsequent recurrence. The median interval from ipilimumab initiation to start of RT was 30 weeks (range, 15–130 wk), a timeframe where delayed response to ipilimumab is rare. The RT dose was predominantly 30 Gy in 5 fractions (41%) or 36 Gy in 6 fractions (26%). Brain radiation was limited to stereotactic radiosurgery in a single patient. The median local control with RT was 31.4 months. The median disease control was 18.7 months, defined as the interval from completion of RT to the start of additional systemic therapy known to impact survival (anti-programmed death-1 or targeted BRAF therapy), hospice enrollment, or death. The overall survival at 1 and 2 years was 87% and 61%, respectively. Seven patients (44%) had no evidence of melanoma at median follow-up of 29.5 months since completion of RT with no additional therapy. This series supports use of RT to limited sites of progression following ipilimumab as an alternative to other systemic treatments such as anti-programmed death-1 antibodies.
Key Words: melanoma, ipilimumab, radiation therapy, immune response, clinical
Despite marked progress in recent years with BRAF-targeted therapy and immune checkpoint inhibition, stage IV melanoma remains a largely incurable disease. Approximately half of melanomas harbor BRAF codon 600 mutations targetable with combined BRAF and MEK kinase inhibitors.1 Although initial response rates are impressive, median progression-free survival is less than a year, and approximately 20% remain progression free at 3 years consisting predominantly of those with relatively low tumor burden at baseline.1–3 Ipilimumab, an anti-cytotoxic T-lymphocyte antigen (CTLA)-4 monoclonal antibody, was the first immune checkpoint inhibitor approved for advanced melanoma on the basis of improved overall survival in randomized phase III trials.4,5 In the initial phase III trial, single-agent ipilimumab produced an overall response rate of 11% with an additional 18% achieving stable disease 12 weeks from therapy initiation.4 Mean time to response was 12 weeks. None with progression at 24 weeks later responded, and only 6% of patients initially achieving stable disease experienced delayed objective response beyond week 24. Thus, the majority of ipilimumab responses occur in <3 months. Delayed responses to ipilimumab beyond 6 months are rare, and those progressing at 6 months virtually never subsequently respond. Among those achieving objective response following ipilimumab, 66% progressed within 2 years.4 Recent pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in 1861 patients with advanced melanoma demonstrated median overall survival of 11.4 months with a plateau at 22% in the survival curve beginning around 3 years.6 Therefore, the vast majority of patients treated with ipilimumab will require subsequent anticancer therapy. We reasoned that radiotherapy (RT) to sites of limited melanoma progression following ipilimumab could treat sites of immune escape and potentially enhance systemic immune response. This retrospective report reviews our experience with surgically incurable stage III or stage IV melanoma treated with RT to limited sites of progression in patients with otherwise good response to ipilimumab.
MATERIALS AND METHODS
Ipilimumab for surgically incurable stage III or IV melanoma was the predominant first-line therapy used at the University of Alabama at Birmingham (UAB) after Food and Drug Administration approval of ipilimumab in March 2011 for this patient population. The records of all melanoma patients over 18 years of age who received immunotherapy at UAB from August 2011 to August 2015 were reviewed. Patients were excluded if anti-programmed death (PD)-1 monoclonal antibodies were administered before or currently with ipilimumab or if whole-brain RT was necessary for palliation of extensive brain metastases. Patients who received RT to all sites of limited progressive disease after treatment with ipilimumab with no other intervening treatment were analyzed. To prevent confounding from delayed responses to ipilimumab, patients were excluded if the first fraction of RT was delivered <15 weeks from the start of ipilimumab. In those who had a complete clinical and radiographic response to ipilimumab, RT was delivered to all sites of detectable relapse. In patients with detectable, residual melanoma following ipilimumab, RT was delivered to all new or progressive sites with observation of stable and/or responding sites. Irradiation of a single site was defined as treatment that encompassed a single planning target volume. Follow-up was measured from the first day of ipilimumab infusion until the date of last clinical follow-up or death. Local control was defined as radiographic and clinical stability or regression of all lesions targeted with RT. Disease control was defined as the time from the last day of RT to the first of one of the following events: start of additional systemic therapy known to impact survival (anti-PD-1 or targeted BRAF therapy), hospice enrollment, or death. The overall survival was measured from the completion of RT. Statistical analysis was performed using SAS 9.4 software (SAS Institute, Cary, NC). Estimates of overall survival, local control, and disease control were measured using the Kaplan-Meier product limit method.7 Cox regression analysis was used to determine hazard ratios (HRs) between subgroups.8 A total of 16 patients were analyzed. Complete blood counts obtained within 4 weeks of RT initiation were analyzed for any correlation between total white blood count, absolute neutrophil count, absolute lymphocyte count, or eosinophilia and clinical outcomes. This study was conducted with approval from the UAB institutional review board.
RESULTS
Patient and Treatment Characteristics
Of the 16 patients analyzed in this study, the majority was male (63%) and the median age at diagnosis of metastatic or surgically incurable disease was 61 years (range, 46–85 y). Most patients (81%) had a documented cutaneous primary melanoma and the remainder had melanoma of unknown primary site. All patients with tissue available after 2013 were tested for BRAF codon 600 mutations (either at initial diagnosis or at time of metastatic progression) and 2 patients were identified as harboring a mutation. One BRAF-mutated patient received ipilimumab due to progression of disease while on BRAF-targeted therapy. No patient had known central nervous system metastases before ipilimumab.
Ipilimumab was administered intravenously at 3 mg/kg and the majority completed 4 doses (94%) with 1 patient discontinuing therapy after 3 doses due to autoimmune colitis requiring steroids. The median time from the first ipilimumab dose to the start of RT was 30 weeks (range, 15–130 wk). Half of the patients had a complete clinical and radiographic response to ipilimumab and were irradiated to the only site(s) of relapse. The other half had detectable, residual melanoma following ipilimumab, and RT was delivered to new or progressive sites with observation of stable and/or responding sites. Characteristics of the 27 irradiated lesions in our cohort of 16 patients are described in Table 1. The most common RT dose and fractionation regimens were 30 Gy in 5 fractions (41%) or 36 Gy in 6 fractions (26%), which were delivered every other day, Monday through Friday. One patient was treated with stereotactic radiosurgery (SRS) to a dose of 20 Gy in a single fraction to 3 sites of isolated intracranial progression. Biological effective dose (BED) calculations were performed to estimate the effective total dose if delivered in 2 Gy fractions and assumed a tumor α/β ratio of 10. BED calculations were not performed for single fraction regimens due to the uncertainty of extrapolating the linear-quadratic model for nonfractionated treatments. Half of the patients were irradiated to a single site of disease. If patients were treated at >1 site of disease, it was common practice to use the same dose and fractionation regimen, although this decision was made on an individual basis. As our demographic consisted of situations where all sites of active disease were targeted with RT, it was not possible to consistently evaluate for abscopal responses at active untreated metastatic sites.
TABLE 1.
Treatment Characteristics
Clinical Outcomes
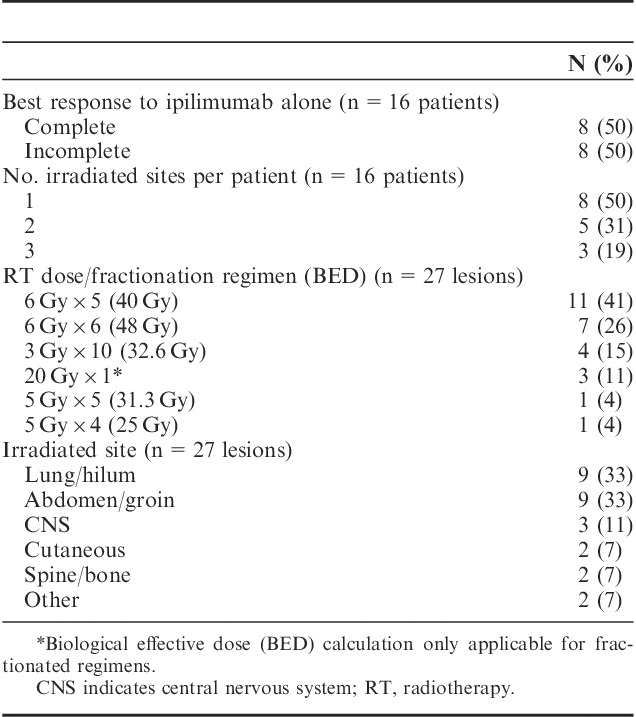
Most patients (n=8) had an initial complete response to ipilimumab. Of the remaining 8 patients, 3 achieved an initial partial response and 5 had stable disease following ipilimumab. The median follow-up from initiation of ipilimumab for the entire cohort and for living patients was 25.5 months (range, 12–49 mo) and 34 months (range, 23–49 mo), respectively. Seven patients (44%) experienced a local failure at one or more irradiated lesions. The median duration of local control was 31.4 months for all patients (Fig. 1A). Local control at 1 and 2 years was 83% and 63%, respectively, for the 8 patients where RT was delivered to all sites of progression after a complete response to ipilimumab. Corresponding local control of the treated lesion(s) was 50% at both 1 and 2 years for those with detectable, residual melanoma following ipilimumab (HR, 2.53; 95% confidence interval, 0.46–13.99) (Fig. 1B). The median disease control was 18.7 months for the entire cohort (Fig. 2). The overall survival at 1 and 2 years was 87% and 61%, respectively (Fig. 1C). The overall survival at 1 and 2 years was 100% and 80%, respectively, for the 8 patients where RT was delivered to all sites of progression after a complete response to ipilimumab. For those with an incomplete response or stable disease as best response to ipilimumab the corresponding overall survival at 1 and 2 years was 75% and 47%, respectively (HR, 3.29; 95% confidence interval, 0.37–29.51) (Fig. 1D). Seven patients (44%) had no clinical or radiographic evidence of active disease at a median follow-up of 29.5 months since completion of RT. Six of these patients received 30–36 Gy in 5–6 fractions and 1 patient with 3 brain metastases received single fraction SRS of 20 Gy to all 3 lesions. The RT schedules used in these patients are analogous to regimens selected for modern melanoma trials to enhance immune response. There has been no evidence of melanoma progression in the central nervous system or elsewhere after over 2 years of surveillance in the patient who received SRS. There was no apparent correlation between interval from ipilimumab to RT, number of irradiated sites, tissues irradiated or pre-RT complete blood counts, and clinical outcomes. Three additional patients were treated with salvage anti-PD-1 monoclonal antibodies at the time of disease progression after RT, and all have experienced continuous melanoma control for up to 22+ months. Thus, 10 of 16 patients have excellent melanoma control at this writing. There were no documented grade≥3 radiation related acute toxicities according to the Common Terminology Criteria for Adverse Events. Imaging from representative patients treated with RT at the time of limited melanoma progression after ipilimumab as first-line immunotherapy are shown in Figure 3.
FIGURE 1.
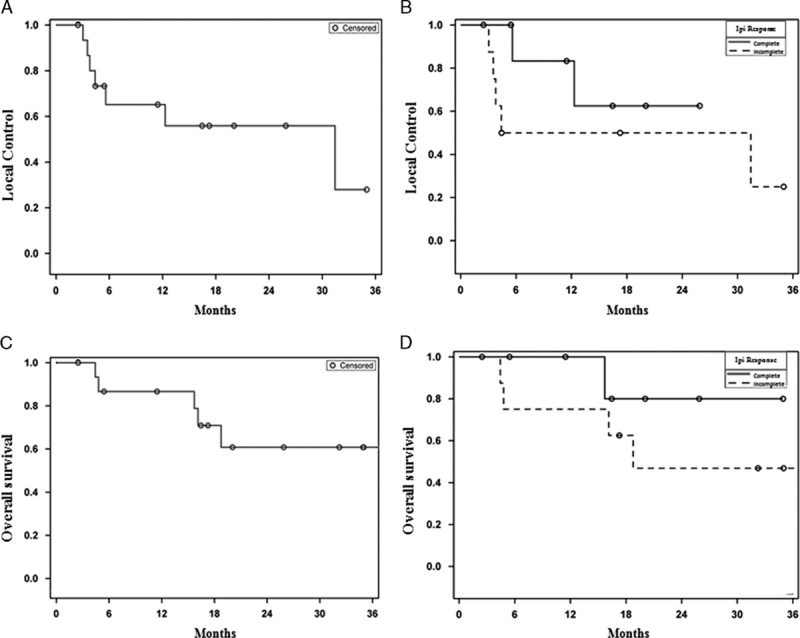
A and C, Kaplan-Meier estimates of local control of the irradiated disease and overall survival for the entire cohort. B and D, Kaplan-Meier estimates of local control and overall survival for patients with complete best response to ipilimumab (solid) compared with those with detectable, residual melanoma following ipilimumab (dashed).
FIGURE 2.
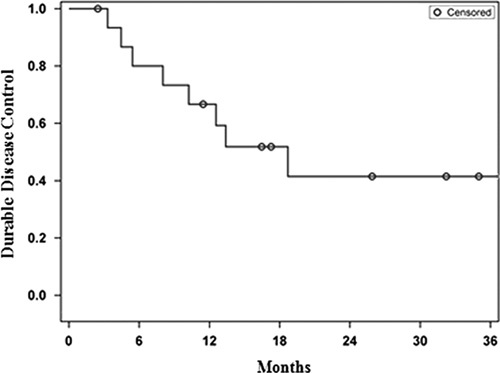
Kaplan-Meier estimate of disease control.
FIGURE 3.
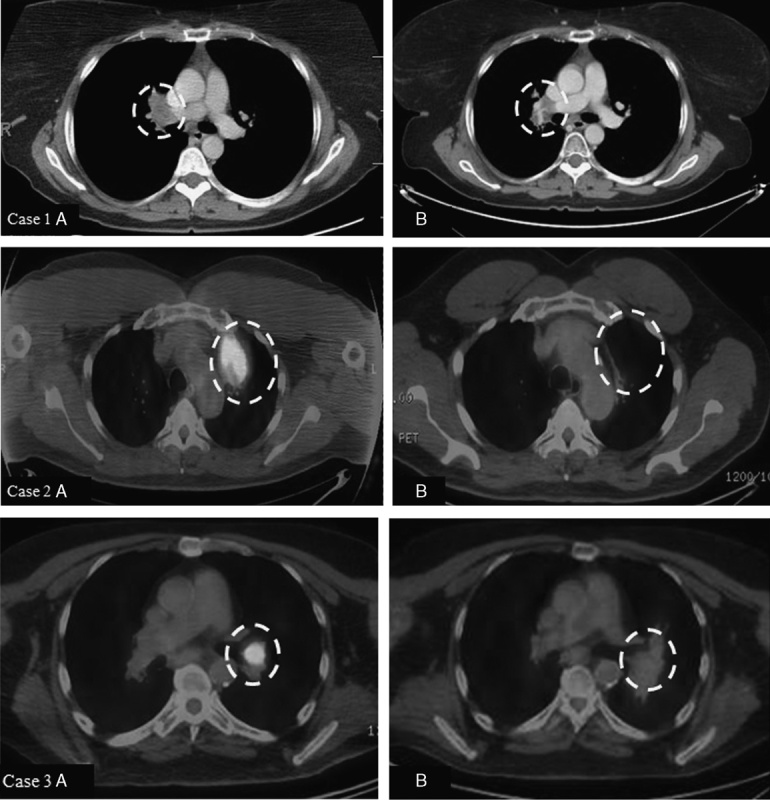
Case 1. A 48-year-old woman with stage IIIC melanoma of the right arm underwent wide local excision and axillary lymph node dissection in 2010. Computed tomography (CT) of the chest demonstrated multiple bilateral pulmonary nodules, and wedge resection of a left lower lobe lesion in July 2012 demonstrated metastatic disease. She completed ipilimumab in December 2012, and CT scan in February 2015 showed isolated progression in the right hilum (1A). She completed 36 Gy in 6 fractions to the right hilum in March 2015 followed by radiographic response. Her most recent CT chest in February 2016 showed stable findings consistent with posttreatment change and no new areas of melanoma involvement (1B). Case 2. A 55-year-old man underwent resection of primary cutaneous melanoma from the left postauricular region and was subsequently diagnosed with metastases to the left parotid and left lung in 2011. He completed ipilimumab in January 2012 with stable left parotid disease and progression at 2 sites in the left upper lobe 1 year later (2A). He completed 36 Gy in 6 fractions to 2 left upper lobe lesions in February 2013 with observation of the parotid disease. He required resection of the parotid nodule in January 2014 that confirmed melanoma. His most recent imaging from January 2016 shows no evidence of active melanoma (2B). Case 3. A 66-year-old man with stage IIIA melanoma of the left cheek diagnosed in 2012 who later developed metastatic disease to the lung in January 2014 was treated with ipilimumab. His lung lesions showed improvement; however, he developed a new and progressive left hilar lesion (3A) which was treated with 30 Gy in 5 fractions in August 2014. His most recent imaging from December 2015 shows no evidence of active melanoma (3B). The white dashed circles highlight the areas of interest described for each image.
DISCUSSION
In this small series of 16 patients treated with RT to limited sites of melanoma progression following ipilimumab, our findings of 31.4 months median local control of the irradiated disease and 18.7 months median disease control were encouraging. Furthermore, overall survival of 61% at 2 years from the start of RT for ipilimumab-refractory melanoma dramatically exceeded expectations considering pooled analysis demonstrating median overall survival of 11 months for advanced melanoma patients from the start of ipilimumab. All 16 patients in our cohort were managed in the era preceding Food and Drug Administration approval of first-line systemic therapy with anti-PD-1 monoclonal antibodies, pembrolizumab and nivolumab.9,10 However, phase II trials of these agents in patients with metastatic melanoma who have progressed after ipilimumab showed response rates of 21%–30% and median progression-free survival of 5 months.11,12 Thus, a brief course of RT using a hypofractionated schedule provides an attractive complementary or alternative approach to patients with limited sites of melanoma progression with otherwise good response to ipilimumab. In fact, 44% of our cohort was free of detectable melanoma at a median follow-up of 29.5 months following RT with no additional therapy. It should be noted that our cohort had a relatively good prognosis before RT with immunogenic tumors benefiting to varying degrees from prior ipilimumab. No grade 3 or 4 acute adverse events were observed with RT and patients were able to defer more time-consuming, costly, and toxic treatment options.
Ionizing radiation affects the inflammatory tumor microenvironment, increases antigen presentation by myeloid cells within the tumor stroma, and enhances recruitment of tumor-reactive effector T cells.13–15 In murine models as little as a single 2 Gy fraction of gamma irradiation resulted in polarization of tumor-associated macrophages from M2 toward M1 phenotype favoring effectual cytolytic T-cell function and tumor rejection.16 RT also expands the T-cell receptor repertoire through increased peptide pool diversity resulting from epigenetic modifications within irradiated tumor cells enhancing transcription of quiescent genes capable of priming at the treated site and recognition of much lower level antigen expression at distant tumor sites.17 Recent dramatic advances in melanoma immunotherapy have heightened interest in the ability of RT to enhance immune response as investigators seek to define the role of radiation in modern melanoma treatment.
It is also becoming increasingly apparent that durable local control following RT for most malignancies involves induction of innate and adaptive immune responses to eliminate tumor cells surviving the direct effects of ionizing radiation.14,18,19 Prior experience with RT for macroscopic melanoma metastases has shown median local control of approximately 6 months.20,21 Although our cohort has the favorable characteristics of prior response or disease stabilization following ipilimumab, the median local control of 31 months observed in our series was unexpected durable. Although immune enhancement by RT is an attractive hypothesis to explain the favorable outcomes reported herein, immune response analysis was not part of this study.
The abscopal effect is a phenomenon where local RT is associated with regression of metastatic cancer remote from the irradiated site.22,23 It has long been observed in melanoma patients and in murine models has been demonstrated to depend upon a functional immune system.24,25 Recent studies have examined synergy between immune checkpoint inhibitors and RT.26 In murine models of mammary and colon cancer, a single fraction of 12 or 20 Gy, respectively, and anti-PD-L1 synergistically reduced local accumulation of tumor infiltrating myeloid-derived suppressor cells and enhanced local and distant tumor rejection.27 Two retrospective reviews reported in the same journal have examined the effects of palliative radiation after ipilimumab in advanced melanoma patients.28,29 The first reported partial abscopal response defined simply as dimensional reduction of metastases outside the irradiated area in 9 of 21 patients (43%). The second paper reported that RT was associated with an improved frequency (11% vs. 25%) and rate of index lesion response outside the radiation field. Although useful for hypothesis generation, these studies shared 2 key limitations: (1) the most commonly irradiated site was the brain, a sanctuary organ from robust immune surveillance and (2) the majority of “abscopal” responses occurred within 3–6 months of ipilimumab initiation such that a portion may have represented delayed response to checkpoint inhibition. In our series, 81% of patients began RT for melanoma progression >24 weeks after ipilimumab initiation, a timeframe in which delayed response to ipilimumab alone is very rare.4 Our 16 patient cohort could not be directly analyzed for abscopal responses as all new or progressive lesions at the time of RT were treated. However, hypofractionated RT regimens of 6 Gy×5 (BED, 40 Gy) or 6 Gy×6 (BED, 48 Gy) predominating in our series are similar to those used in cases of abscopal responses in humans.26,30–32 The median disease control of 18.7 months and 44% of our cohort NED without further therapy at median follow-up of 29.5 months were encouraging. Limitations of this study include its retrospective nature, small cohort size, and lack of uniformity in RT dose and fractionation. Although enhancement of systemic immunity by RT following at least partially successful treatment with ipilimumab is an attractive hypothesis supported by other preclinical observations, this study does not provide direct evidence of this mechanism. Selection of patients with favorable characteristics including immunogenic tumors transiently controlled by ipilimumab may have contributed to better than expected outcomes.
Use of RT to regress limited sites of immune escape following ipilimumab could logically be extrapolated to follow other immune checkpoint inhibitors such as anti-PD-1 monoclonal antibodies, which have recently received a first-line indication in advanced melanoma. In a recent letter to Nature, investigators at the University of Pennsylvania demonstrated that RT combined with anti-CTLA4 and anti-PD-1 activate nonredundant immune mechanisms in a murine melanoma model.31 It is interesting to note that all 3 patients in our series receiving anti-PD-1 therapy after ipilimumab and RT have experienced continuous melanoma control. Although surgery would sometimes be feasible in such patients, it would often result in greater morbidity and would not be expected to enhance immunologic control of melanoma elsewhere. Clearly, therapy for widespread melanoma progression following checkpoint inhibition needs to focus on other systemic agents. However, RT should be considered for limited sites of progression in patients with otherwise good response to checkpoint inhibition as an alternative to potentially more toxic systemic treatments. The only parameter identified to correlate with improved clinical outcomes following RT was prior complete rather than incomplete best response to ipilimumab. Future prospective trials to investigate this approach and the optimal RT dose, timing of checkpoint inhibitor and RT delivery, and fractionation regimen are warranted. Such trials should include correlative laboratory studies to identify tumor and host immune parameters predictive of local and distant melanoma control.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
All authors have declared that there are no financial conflicts of interest with regard to this work.
REFERENCES
- 1.Long GV, Stroyakovskiy D, Gogas H, et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: a multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444–451. [DOI] [PubMed] [Google Scholar]
- 2.Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867–1876. [DOI] [PubMed] [Google Scholar]
- 3.Long GV, Weber JS, Infante JR, et al. Overall survival and durable responses in patients with BRAF V600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clini Oncol. 2016;34:871–878. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. [DOI] [PubMed] [Google Scholar]
- 6.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–229. [Google Scholar]
- 9.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 10.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16:908–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber J, Gibney G, Kudchadkar RR, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. 2016;4:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res. 2011;71:2488–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204:49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klug F, Prakash H, Huber PE, et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24:589–602. [DOI] [PubMed] [Google Scholar]
- 17.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. [DOI] [PubMed] [Google Scholar]
- 19.Deng L, Liang H, Fu S, et al. From DNA damage to nucleic acid sensing: a strategy to enhance radiation therapy. Clin Cancer Res. 2016;22:20–25. [DOI] [PubMed] [Google Scholar]
- 20.Olivier KR, Schild SE, Morris CG, et al. A higher radiotherapy dose is associated with more durable palliation and longer survival in patients with metastatic melanoma. Cancer. 2007;110:1791–1795. [DOI] [PubMed] [Google Scholar]
- 21.Gimbel MI, Delman KA, Zager JS. Therapy for unresectable recurrent and in-transit extremity melanoma. Cancer Control. 2008;15:225–232. [DOI] [PubMed] [Google Scholar]
- 22.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol. 1975;48:863–866. [DOI] [PubMed] [Google Scholar]
- 25.Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. [DOI] [PubMed] [Google Scholar]
- 26.Vanpouille-Box C, Pilones KA, Wennerberg E, et al. In situ vaccination by radiotherapy to improve responses to anti-CTLA-4 treatment. Vaccine. 2015;33:7415–7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. 2015;4:e1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1:1325–1332. [DOI] [PubMed] [Google Scholar]
- 31.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vatner RE, Cooper BT, Vanpouille-Box C, et al. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325 doi: 10.3389/fonc.2014.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]


