Abstract
Background:
Radiation-induced heart disease (RIHD), which affects the patients’ prognosis with both acute and late side effects, has been published extensively in the radiotherapy of breast cancer, lymphoma and other benign diseases. Studies on RIHD in lung cancer radiotherapy, however, are less extensive and clear even though the patients with lung cancer are delivered with higher doses to the heart during radiation treatment.
Methods:
In this article, after extensive literature search and analysis, we reviewed the current evidence on RIHD in lung cancer patients after their radiation treatments and investigated the potential risk factors for RIHD as compared to other types of cancers.
Result:
Cardiac toxicity has been found highly relevant in lung cancer radiotherapy. So far, the crude incidence of cardiac complications in the lung cancer patients after radiotherapy has been up to 33%.
Conclusion:
The dose to the heart, the lobar location of tumor, the treatment modality, the history of heart and pulmonary disease and smoking were considered as potential risk factors for RIHD in lung cancer radiotherapy. As treatment techniques improve over the time with better prognosis for lung cancer survivors, an improved prediction model can be established to further reduce the cardiac toxicity in lung cancer radiotherapy.
Keywords: cardiac toxicity, heart dose, lung cancer, radiation-induced heart disease, radiotherapy
1. Introduction
In the United States, lung cancer is the leading cause of cancer-related deaths and is expected to result in 158,080 deaths in 2016, accounting for about 25% of all cancer deaths.[1] The radiation toxicity to the critical structures during thoracic radiotherapy remains a potential threat to patient prognosis. Radiation-induced heart disease (RIHD) in the patients treated with mediastinal, chest, mantle, or left breast radiotherapy has been reported as the development of treatment-associated cardiac toxic effects, including ischemic heart disease, cardiomyopathy, conduction disorders, cardiac dysfunction, and heart failure or deterioration of these preexisting conditions.[2] In the case of breast cancer, Hodgkin lymphoma, and childhood cancer, radiation-induced cardiac toxicity has been studied more extensively due to a larger number of patients and better survival rates.[3,4] In these patient groups, the cardiac toxicity was regarded as late side effects after treatments.[5] The main concern following radiotherapy for lung cancer has been the toxicity to the lung and the esophagus,[6] which disinclined the studies of RIHD in lung cancer radiotherapy. In addition, the follow-up time in the lung cancer patients were usually not long enough to quantify the late effects of RIHD and to establish possible associations with dose–volume parameters, tumor laterality, and other risk factors. We therefore reviewed the current evidence on the radiation-induced cardiac toxicity and its associations with the risk factors after radiation therapy (RT) of lung cancer. As this review is a retrospective study in nature involving no human subjects, an institutional review board approval has been waived.
2. Methods
We have searched PubMed, Embase, and Cochrane Libraries on the papers that were published from January 2003 to November 2015 on radiation-induced cardiac toxicity and/or radiation dose parameters. The following terms were used in the searches: “lung cancer,” “radiation therapy,” “heart,” “cardiovascular system,” and “toxicity.” Due to the limited number of randomized controlled trials in the target population, literatures selected also included any study modalities, such as cohort designs and prospective studies. Association guidelines, protocols for the radiotherapy, and additional review articles were screened for reference.
For each published work, we reviewed the treatment modality (radiotherapy or chemo-radiotherapy), the cardiovascular morbidity and mortality, the prognosis, the survival rate, the history of heart and pulmonary disease and smoking, and the dosimetric parameters to the heart and other organs-at-risk (OARs) after radiotherapy. To compare the cardiovascular diseases (CVDs) among different carcinomas, the articles relevant to the radiation-induced cardiac toxicity in the radiotherapy of breast cancer, Hodgkin lymphoma, and childhood cancer were also reviewed.
3. Results
The literature search resulted in 10 papers related to the cardiac toxicity in lung cancer radiotherapy.[7–16] Eight of 10 references were summarized briefly in Table 1[7–14] which proved strong association of cadiac toxicity with lung cancer radiotherapy. Eight studies reported specifically on nonsmall cell lung cancer (NSCLC),[7–13] while 1 study did not clearly put forward the type or stage of lung cancer.[14] All these patients in the 8 refereed papers underwent RT alone. The follow-up time in 10 refereed papers ranged between 3 months and 7.9 years. The crude incidence of cardiac complications following lung cancer radiotherapy was reported to be as high as 33%.[8] Most of the cardiac events occurred within 2 years after treatment.
Table 1.
The reported cardiac toxicity after radiotherapy of lung cancer.
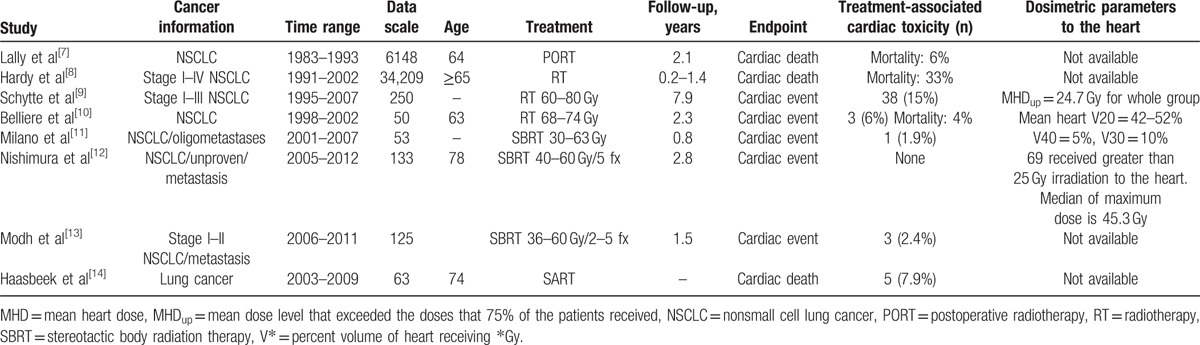
There were 2 randomized controlled trials that regarded cardiac death as the analysis endpoint.[7,8] Per International Classification of Disease-9 procedure codes, cardiac death was identified as an event where patients in the research group died of ischemic heart disease, cardiomyopathy, conduction disorders, cardiac dysfunction, or heart failure. In a multivariate analysis, Lally et al[7] included 6148 patients who underwent pneumonectomy/lobectomy, 3589 of whom received postoperative RT (PORT). They found that PORT use was associated with an increase in the hazard for heart disease mortality (hazard ratio [HR], 1.30). Similarly, Hardy et al[8] collected a cohort of 34,209 patients with stages I–IV NSCLC, 68.8% of whom were treated with chemotherapy-only, radiotherapy-only, or chemoradiation in their study. The median time to the development of toxicity ranged from 78 to 510.5 days, depending on the cardiac endpoint. An increased risk of cardiac dysfunction (HR, 1.54) was found among the patients with NSCLC treated with radiotherapy or chemoradiotherapy (CRT).
Two studies reported cardiac event was observed as the incidence of cardiac disease after the radiation treatment of lung cancer patients.[9–10] Schytte et al[9] reported 38 patients (15% of 250 patients) developed cardiac disease after RT, the majority of which was supraventricular and myocardial infarctions (IMs). Belliere et al[10] undertook a retrospective analysis of the patients with nonresectable NSCLC treated with high-dose (74 Gy) standard-fractionation 3-dimensional conformal RT. Three out of 50 patients (6%) were reported to experience radiation-induced cardiac toxicity and particularly 2 of those 3 died of cardiac failure after completion of radiotherapy.
There were 4 studies on the cardiac toxicity of lung cancer patients treated with stereotactic body radiation therapy (SBRT).[11–14] In the study of Milano et al[11] on the toxicity and outcome after SBRT for central thoracic lesions, only 1 patient developed grade 3 pericarditis (graded with Common Terminology Criteria for Adverse Events [CTCAE], version 3.0) 9 months after SBRT to a lesion near the heart, and 4 months after palliative radiation was delivered to mediastinal lymph nodes and 3 bulky lung masses. Nishimura et al[12] investigated tolerable doses to OARs in the mediastinum and pulmonary hilum following SBRT and extracted 133 patients who received greater than 25 Gy irradiation to the OARs. In this cohort of population, there were no grade 3 to 5 adverse events involving the heart (CTCAE version 4). Although it is difficult to associate cardiac events with RT in the population with frequent comorbidities, Modh et al[13] identified 3 cases of cardiac toxicity (i.e., pericardial effusion, pericarditis, and MI) in their study of SBRT for central lung tumors. Haasbeek et al[14] also reported 5 cases of cardiac failure after SBRT of centrally located lung tumors. Of 5 patients who died of cardiac failure, 1 patient who had chronic atrial fibrillation and a severe pretreatment aortic valve stenosis had a target volume overlapping the pericardium. There were 3 patients whose target volumes were proximal to their bronchial trees, and 1 patient with the lesion against the upper mediastinum.
There were 2 studies designed to monitor or prevent the cardiotoxicity in patients with lung cancer. Cao and Tan[15] divided 80 lung cancer patients into the observation group and the control group who was given L-carnitine injection. They found that the incidence rates of inflammatory heart and abnormal ECG changes, as well as the main heart symptoms like chest tightness, chest pain, and heart palpitations, were significantly lower in the observation group than in the control group (P < 0.05). They concluded that L-carnitine injection could effectively prevent the radiotherapy from inducing cardiotoxicity in lung cancer patients and contribute to the completion of radiotherapy. Gayed et al[16] evaluated 24 lung cancer patients who received concurrent or adjuvant chemotherapy with RT, or RT alone. The myocardial perfusion imaging studies performed before and after RT indicated that 29% of the patients developed cardiac complications and morbidities but none of them died from cardiac event.
4. Discussion
The effects of radiation treatments on the heart have been extensively described in breast cancer,[17–28] lymphoma,[29–31] childhood cancer,[32–34] esophageal cancer,[16,35,36] and peptic ulcer disease[37,38] patients. However, the radiological effects on the heart of lung cancer patients were not well documented, even though a large percentage of cancer patients belonged to this group, and they often received significant radiation doses to a large portion of the heart due to its close proximity to the tumors. From the limited evidence, we found that radiation-induced cardiac toxicity was associated with the radiation treatments and there were multiple relevant risk factors that could contribute to the RIHD in lung cancer patients, such as the heart dose, the tumor laterality, the treatment modality, the history of heart disease, and smoking as described below.
4.1. Radiation dose to the heart
With large prescription doses (45–60 Gy for small cell lung cancer [SCLC]; ≥66 Gy for NSCLC) in lung cancer patients, the radiation dose to a fraction of heart volume incidentally irradiated by the treatment beams could be significant, depending on the location of the tumor and involvement of the mediastinal lymph nodes.[39] Studies listed in Table 2 showed higher dose levels to the heart increased the occurrence of CVD after treatment of breast cancer, childhood cancer, and peptic ulcer disease.[17,32,33,37] And a linear relationship was found between the mean dose to the heart and the risk of cardiac mortality.[17,33,37]
Table 2.
The correlation between the heart dose and the risk of RIHD after radiotherapy.
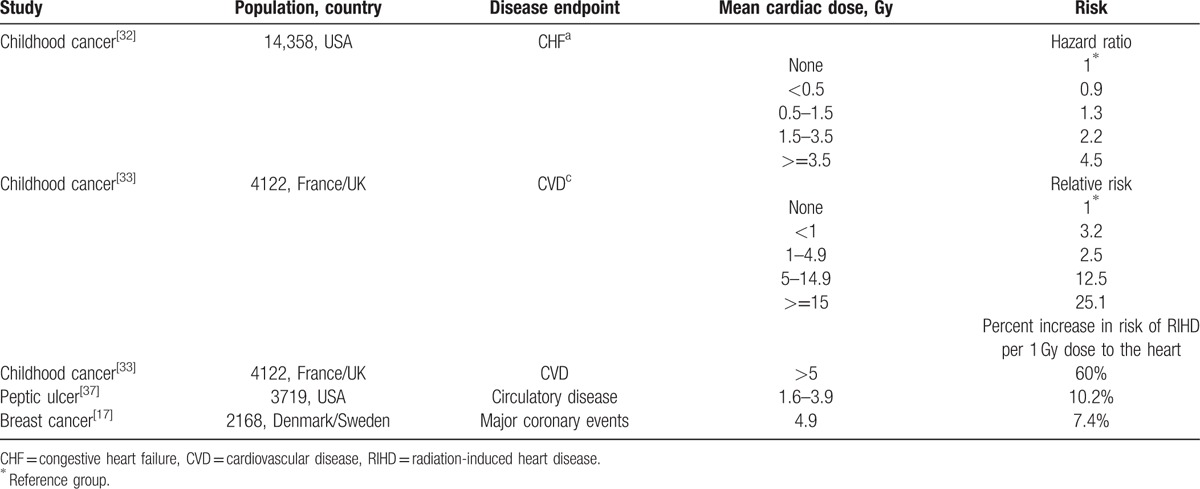
However, there were not enough direct evidence to prove the correlation between the risk of RIHD and the radiation dose to the heart in lung cancer radiotherapy.[7–14] In the study of Nishimura et al,[12] there were no records of cardiac events among the patients who received greater than 25 Gy to the heart. In radiation therapy oncology group 0617, 17 of 166 patients who were randomly assigned to receive 60 versus 74 Gy with concurrent and consolidation chemotherapy, with or without cetuximab, developed grade 3 to 5 cardiac events (CTCAE version 3.0).[40] Heart dose was significantly higher in the high-dose group (74 Gy), while the cardiac events graded 3 to 5 were more in the standard-dose group (60 Gy).
There can be multiple reasons for the less significant correlation observed between the high dose to the heart and the risk of cardiac toxicity following lung cancer radiotherapy. One reason could be that most of the reviewed literatures were not randomized controlled trials and reported the incidence of cardiac events rather than the dose to the heart. The other reason could be that the tolerable doses for the heart might be overestimated in the chosen cohort for selected studies.[12] Given that the dose to the heart in lung cancer patients is much higher than that in breast cancer patients (Table 3), the heart dose in the radiation treatment of lung cancer could not be neglected for a more rigorous study design on the incidence of RIHD. The same attention should be paid to the irradiated volume of the heart. From Table 1, the percent volumes of the heart that received 20, 30, 40, and 50 Gy on dose–volume histograms were 42–52%, 10–22%, 16%, and 9%, respectively.
Table 3.
The averaged heart dose in the whole radiation treatment courses.
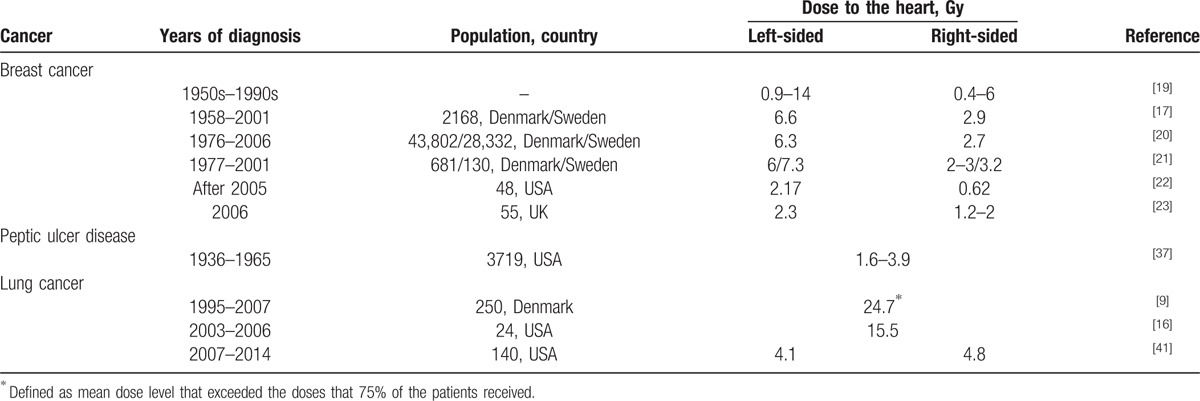
4.2. Tumor laterality
The differences in the doses to the heart between the treatments for the left- and right-sided lung cancers were not as significant as those shown in breast cancer. As shown in Table 3, there was no significant association of cardiac complications with tumor laterality in lung cancer patients (HR, 0.99 in Lally et al; 1.18 in Hardy et al). The risk of RIHD increased in breast cancer patients with left-sided tumors over the patients with right-sided tumors (HR, 1.27–1.5).[18,42–44] Due to the difference in tumor-to-heart distances, the heart normally received higher dose in the treatment of ipsilateral tumors. Remarkably, the patients with lower lobe lung tumor had a higher risk for the mortality of heart disease but PORT was not associated with the risk of cardiac death for the patients with right upper lobe tumor.[7] This could be that the cardiac dose from irradiation of lower lobe tumors was much higher than that from irradiation of upper lobe ones. Hence, the lobar location of tumor could be a risk factor for RIHD in lung cancer radiotherapy.
4.3. Radiotherapy alone versus chemoradiotherapy (CRT)
Chemotherapy or combined with radiation is the usual treatment for advanced-stage NSCLC or SCLC. Hardy et al found out that the actuarial risk of heart diseases in lung cancer patients after RT combined with chemotherapy, such as ischemic heart disease, cardiomyopathy, conduction disorders, cardiac dysfunction, and heart failure, was up to 1.53 times higher than RT alone for lung cancer patients.[8] In some other studies, chemotherapy combined with RT was shown to significantly increase the risk of developing cardiovascular toxicity in the patients with Hodgkin lymphoma[29] and esophageal cancer.[35,45,46] Hence, the treatment modality (RT alone vs CRT) would be an important risk factor for RIHD in lung cancer radiotherapy.
4.4. History of heart disease and smoking
A prior diagnosis of heart disease conferred a substantial increase in subsequent cardiac complications after the radiation treatments.[17,20] Gayed et al[16] found that the history of arrhythmia or the presence of arrhythmia prior to CRT was significantly associated with higher risk of cardiac complications after CRT in a logistic regression analysis. Particularly, 1 of 3 patients who had a recorded history of MI presented with MI after receiving a total dose of 60 Gy. Two of 3 patients with cardiac failure during radiotherapy had a history of MI in the study of Belliere et al,[10] where 66% patients had a previous history of coronary disease. One patient presented the tumor located in the left upper lobe with a suspicion of aorta invasion, receiving the dose 26 and 2.25 Gy delivered to the tumor and the heart, respectively.[10] Yet, cardiac death was not considered as a result of these escalated doses to the heart. It is therefore highly necessary to take the patient's heart disease history into consideration in assessing the risk for RIHD in the radiotherapy of lung cancers. Besides the cardiac history prior to the treatment, the patients with chronic obstructive pulmonary disease had an increased risk of sudden cardiac deaths (HR, 2.12) 5.5 years after the diagnosis of chronic obstructive pulmonary disease.[47]
Although there was no straightforward proof in the screened studies for lung cancer tumors, the habit of smoking increased the risk of MI in the patients with Hodgkin lymphoma (2-fold)[29] and breast cancer (2–2.63-fold)[25] compared to the patients who never smoked. Irradiated thoracic cancer patients should be advised to refrain from smoking to reduce their risk for CVD.
Although current evidence supports RIHD as a relevant complication following the treatment of lung cancer, the studies are not without their limitations. First, the underlying mechanism for radiation-induced cardiac damage is not clear in lung cancer patients. Irradiation can damage both cancer cells and healthy cells, and can affect blood vessels of all sizes.[3,4] Typically, complications from irradiation of the heart manifest as late effects with a range of 3 to 29 years after treatment, becoming evident in the 2nd to 3rd decade posttherapy.[3] However, in the patients with lung cancer, the follow-up durations were within 2 years after the treatment in the most of current studies,[7–14] due largely to the much poorer prognosis and survival in this group of patients as compared to other groups such as breast cancer patients. As such, it is quite challenging to investigate the underlying mechanism for radiation-induced cardiac damage. As suggested by Beukema et al,[48] the pathophysiological and clinical presentation of different cardiac events may relate to different dose–volume response relationships, leading to different underlying mechanisms. Additional evidence on the cardiac dose–volume response relationship would help to elucidate the underlying mechanism for cardiac toxicity in lung cancer radiotherapy.
Second, due to the limited number of patients and low survival rates, the endpoint in 7 of 10 studies was cardiac event.[9–13,15,16] It is not always straightforward to identify the cardiac event in lung cancer patients. Schytte et al[9] explained that if NSCLC patients developed dyspnea and chest pain, it would be recorded as a relapse of lung cancer and not as a cardiac toxicity event. Traditionally, the short-term clinical symptoms after RT (up to 6–9 months) were considered to be pulmonary toxicity effects.[49] Additionally, cardiac irradiation was proposed to be associated with the development of radiation-induced lung toxicity after high-dose RT for lung cancer.[50] The biological mechanism underpinning the short-term interplay between cardiac complications and lung toxicity is not clear-cut, potentially leading to an underestimation of cardiac toxicity after treatments for lung cancer.
Third, the reported dose–volume responses to the risk of RIHD were not conclusive from the current evidence. Two groups reported that there was no significant correlation between having high level heart dose and having a cardiac event.[9,16] However, the patient numbers involved in these studies were not comparable to those in the aforementioned studies for breast cancer, childhood cancer, and peptic ulcer disease. In 2 recent trials,[7,8] the cardiac dose distribution and the corresponding toxicity was unfortunately missing. Recently, Ming et al[41] reported that a patient with preexisting coronary artery disease and congestive heart failure developed a pericardial effusion after receiving a maximum heart dose of 67.1 Gy, which was 112% of prescription dose. They further pointed out that the radiation dose to the heart is highly linked to the RIHD in lung cancer radiotherapy, similar to the correlations observed in the childhood cancer, peptic ulcer disease, and breast cancer.[17,32,33,37]
Finally, it is not clear whether radiotherapy is an independent risk factor associated with heart disease for lung cancer patients treated with CRT. In Hardy et al work,[8] radiotherapy was only associated with the risk of cardiac dysfunction (HR, 1.54), but not significantly associated with other heart diseases. In contrast, chemoradiation treatment was associated with an increased risk of cardiac dysfunction (HR, 2.36), ischemic heart disease (HR, 1.10), heart failure (HR, 1.20), and conduction disorders (HR, 1.37). Further clarification would be needed as to the mechanism for radiation-induced and chemoradiation-induced heart disease.
The studies of cardiac toxicity in lung cancer radiotherapy are promising but challenging. Given that the heart has long been considered as a relatively dose resistant organ, it is surprizing that current evidence presented in this review showed that the crude incidence of RIHD after lung cancer radiotherapy was up to 33%.[7–14] It should be noted that half of the reports listed in Table 1 often involved 2D radiotherapy where the doses to the heart were much higher as compared to the modern radiotherapy techniques.[9,16] Modern radiotherapy of lung cancer has been largely driven technologically, from the simpler 3-dimensional conformal RT to the more complicated intensity-modulated radiotherapy, dynamic conformal arc therapy, and volumetric modulated arc therapy.[51–54] As pointed out by Lee et al,[55] modern radiotherapy techniques are capable of providing improved tumor irradiation and sparing of the adjacent organs to an extent that was not possible before. Intensity-modulated radiotherapy with noncoplanar fields[56] and adaptive radiotherapy[57] have been shown to preserve the heart in irradiation of lung tumors. On the other hand, the mean heart doses for lung cancer patients were comparable to those for breast cancer patients (Table 3). As demonstrated by Darby et al,[17] for every gray of mean heart dose increase, the major coronary events increased by 7.4%. Particularly for the high dose delivered with SBRT techniques, a large percent of the heart volume was irradiated with high dose and the median maximum dose was as much as 45.3 Gy.[11,12] As the survival rate increases and prognosis improves due to the use of modern techniques in radiotherapy, the benefits of RT for lung cancer may be offset by the risk of radiation-induced cardiac toxicity. Hence, RIHD may still be a big concern for lung cancer patients treated with modern radiotherapy techniques.
Although this review indicated that RIHD is a clinically relevant issue in lung cancer radiotherapy, additional data with long-term follow-up and accurate heart dose distribution would be needed to make an accurate multivariable prediction model on radiation-induced cardiac toxicity. This model should include multiple risk factors, such as the dosimetric parameters of the heart, the lobar location of tumor, the past medical history of cardiac, and pulmonary disease and smoking. These multiple risk factors could also be used to design effective preventing strategies for lung cancer patients receiving radiation treatments. In order to avoid underestimation of cardiac toxicity, cardiac screening could be used to help identify cardiac morbidity in those lung cancer patients. For example, myocardial perfusion imaging with different tracer agent has been used to demonstrate myocardial perfusion defects in the RT fields of some patients who had been treated for esophageal cancer and lung cancer.[16,58] L-carnitine injection before RT was also proven to be efficient to prevent cardiotoxicity in lung cancer patients.[15]
5. Conclusion
This review concludes that RIHD is a potential threat for lung cancer survivors after treatment, though more evidence is needed. The crude incidence of cardiac complications following lung cancer radiotherapy was found to be up to 33% in current studies. The dose to the heart, the lobar location of tumor, the treatment modality, the history of heart and pulmonary disease, and smoking were important risk factors for RIHD in lung cancer radiotherapy. As treatment techniques improve over the time with better prognosis for lung cancer survivors, an improved prediction model can be established to further reduce the cardiac toxicity in lung cancer radiotherapy.
Acknowledgments
The authors thank the valuable discussion and comments provided by Dr Lujun Zhao for this manuscript. The authors also thank William Hinchcliffe and Qian Zeng for their kind help with the proofreading.
Footnotes
Abbreviations: CRT = chemoradiotherapy, CTCAE = Common Terminology Criteria for Adverse Events, CVD = cardiovascular disease, HR = hazard ratio, MI = myocardial infarction, NSCLC = nonsmall cell lung cancer, OAR = organ-at-risk, PORT = postoperative radiation therapy, RIHD = radiation-induced heart disease, RT = radiation therapy, SBRT = stereotactic body radiation therapy.
The authors have no funding and conflicts of interest to disclose.
References
- 1.American Cancer Society. Cancer Facts & Figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 2.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol 2007; 25:3991–4008. [DOI] [PubMed] [Google Scholar]
- 3.Jaworski C, Mariani JA, Wheeler G, et al. Cardiac complications of thoracic irradiation. J Am Coll Cardiol 2013; 61:2319–2328. [DOI] [PubMed] [Google Scholar]
- 4.Filopei J, Frishman W. Radiation-induced heart disease. Cardiol Rev 2012; 20:184–188. [DOI] [PubMed] [Google Scholar]
- 5.Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003; 13:346–356. [DOI] [PubMed] [Google Scholar]
- 6.Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol 2010; 28:2181–2190. [DOI] [PubMed] [Google Scholar]
- 7.Lally BE, Detterbeck FC, Geiger AM, et al. The risk of death from heart disease in patients with nonsmall cell lung cancer who receive postoperative radiotherapy: analysis of the Surveillance, Epidemiology, and End Results database. Cancer 2007; 110:911–917. [DOI] [PubMed] [Google Scholar]
- 8.Hardy D, Liu CC, Cormier JN, et al. Cardiac toxicity in association with chemotherapy and radiation therapy in a large cohort of older patients with non-small-cell lung cancer. Ann Oncol 2010; 21:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schytte T, Hansen O, Stolberg-Rohr T, et al. Cardiac toxicity and radiation dose to the heart in definitive treated non-small cell lung cancer. Acta Oncol 2010; 49:1058–1060. [DOI] [PubMed] [Google Scholar]
- 10.Belliere A, Girard N, Chapet O, et al. Feasibility of high-dose three-dimensional radiation therapy in the treatment of localised non-small-cell lung cancer. Cancer Radiother 2009; 13:298–304. [DOI] [PubMed] [Google Scholar]
- 11.Milano MT, Chen Y, Katz AW, et al. Central thoracic lesions treated with hypofractionated stereotactic body radiotherapy. Radiother Oncol 2009; 91:301–306. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura S, Takeda A, Sanuki N, et al. Toxicities of organs at risk in the mediastinal and hilar regions following stereotactic body radiotherapy for centrally located lung tumors. J Thorac Oncol 2014; 9:1370–1376. [DOI] [PubMed] [Google Scholar]
- 13.Modh A, Rimner A, Williams E, et al. Local control and toxicity in a large cohort of central lung tumors treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2014; 90:1168–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haasbeek CJ, Lagerwaard FJ, Slotman BJ, et al. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. J Thorac Oncol 2011; 6:2036–2043. [DOI] [PubMed] [Google Scholar]
- 15.Cao J, Tan H. Observation on the effects of L-carnitine in the prevention of radiotherapy induced cardiotoxicity of patients with lung cancer. Anti-Tumor Pharmacy 2013; 3:290. [Google Scholar]
- 16.Gayed I, Gohar S, Liao Z, et al. The clinical implications of myocardial perfusion abnormalities in patients with esophageal or lung cancer after chemoradiation therapy. Int J Cardiovasc Imaging 2009; 25:487–495. [DOI] [PubMed] [Google Scholar]
- 17.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368:987–998. [DOI] [PubMed] [Google Scholar]
- 18.Darby S, McGale P, Peto R, et al. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ 2003; 326:256–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor CW, Nisbet A, McGale P, et al. Cardiac exposures in breast cancer radiotherapy: 1950s–1990s. Int J Radiat Oncol Biol Phys 2007; 69:1484–1495. [DOI] [PubMed] [Google Scholar]
- 20.McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011; 100:167–175. [DOI] [PubMed] [Google Scholar]
- 21.Taylor CW, Bronnum D, Darby SC, et al. Cardiac dose estimates from Danish and Swedish breast cancer radiotherapy during 1977–2001. Radiother Oncol 2011; 100:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner DJ, Shuryak I, Jozsef G, et al. Risk and risk reduction of major coronary events associated with contemporary breast radiotherapy. JAMA Intern Med 2014; 174:158–160. [DOI] [PubMed] [Google Scholar]
- 23.Taylor CW, Povall JM, McGale P, et al. Cardiac dose from tangential breast cancer radiotherapy in the year 2006. Int J Radiat Oncol Biol Phys 2008; 72:501–507. [DOI] [PubMed] [Google Scholar]
- 24.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300 000 women in US SEER cancer registries. Lancet Oncol 2005; 6:557–565. [DOI] [PubMed] [Google Scholar]
- 25.Hooning MJ, Botma A, Aleman BMP, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007; 99:365–375. [DOI] [PubMed] [Google Scholar]
- 26.Demirci S, Nam J, Hubbs JL, et al. Radiation-induced cardiac toxicity after therapy for breast cancer: interaction between treatment era and follow-up duration. Int J Radiat Oncol Biol Phys 2009; 73:980–987. [DOI] [PubMed] [Google Scholar]
- 27.Borger JH, Hooning MJ, Boersma LJ, et al. Cardiotoxic effects of tangential breast irradiation in early breast cancer patients: the role of irradiated heart volume. Int J Radiat Oncol Biol Phys 2007; 69:1131–1138. [DOI] [PubMed] [Google Scholar]
- 28.Taylor CW, McGale P, Povall JM, et al. Estimating cardiac exposure from breast cancer radiotherapy in clinical practice. Int J Radiat Oncol Biol Phys 2009; 73:1061–1068. [DOI] [PubMed] [Google Scholar]
- 29.Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007; 109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst 2007; 99:206–214. [DOI] [PubMed] [Google Scholar]
- 31.Castellino SM, Geiger AM, Mertens AC, et al. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood 2011; 117:1806–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ 2009; 339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol 2010; 28:1308–1315. [DOI] [PubMed] [Google Scholar]
- 34.Kucharska W, Negrusz-Kawecka M, Gromkowska M. Cardiotoxicity of oncological treatment in Children. Adv Clin Exp Med 2012; 21:281–288. [PubMed] [Google Scholar]
- 35.Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys 2008; 70:707–714. [DOI] [PubMed] [Google Scholar]
- 36.Martel MK, Sahijdak WM, Ten Haken RK, et al. Fraction size and dose parameters related to the incidence of pericardial effusions. Int J Radiat Oncol Biol Phys 1998; 40:155–161. [DOI] [PubMed] [Google Scholar]
- 37.Little MP, Kleinerman RA, Stovall M, et al. Analysis of dose response for circulatory disease after radiotherapy for benign disease. Int J Radiat Oncol Biol Phys 2012; 84:1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carr ZA, Land CE, Kleinerman RA, et al. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys 2005; 61:842–850. [DOI] [PubMed] [Google Scholar]
- 39.Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010; 76 (3 Suppl):S77–S85. [DOI] [PubMed] [Google Scholar]
- 40.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ming X, Feng Y, Liu H, et al. Cardiac exposure in the dynamic conformal arc therapy, intensity-modulated radiotherapy and volumetric modulated arc therapy of lung cancer. PLoS One 2015; 10:e0144211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 2007; 25:3031–3037. [DOI] [PubMed] [Google Scholar]
- 43.Roychoudhuri R, Robinson D, Putcha V, et al. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population-based study. BMC Cancer 2007; 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giordano SH, Kuo YF, Freeman JL, et al. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst 2005; 97:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tait LM, Meyer JE, McSpadden E, et al. Women at increased risk for cardiac toxicity following chemoradiation therapy for esophageal carcinoma. Pract Radiat Oncol 2013; 3:e149–e155. [DOI] [PubMed] [Google Scholar]
- 46.Shirai K, Tamaki Y, Kitamoto Y, et al. Dose-volume histogram parameters and clinical factors associated with pleural effusion after chemoradiotherapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys 2011; 80:1002–1007. [DOI] [PubMed] [Google Scholar]
- 47.Lahousse L, Niemeijer MN, van den Berg ME, et al. Chronic obstructive pulmonary disease and sudden cardiac death: the Rotterdam study. Eur Heart J 2015; 36:1754–1761. [DOI] [PubMed] [Google Scholar]
- 48.Beukema JC, Van Luijk P, Widder J, et al. Is cardiac toxicity a relevant issue in the radiation treatment of esophageal cancer? Radiother Oncol 2015; 114:85–90. [DOI] [PubMed] [Google Scholar]
- 49.Nalbantov G, Kietselaer B, Vandecasteele K, et al. Cardiac comorbidity is an independent risk factor for radiation-induced lung toxicity in lung cancer patients. Radiother Oncol 2013; 109:100–106. [DOI] [PubMed] [Google Scholar]
- 50.Huang EX, Hope AJ, Lindsay PE, et al. Heart irradiation as a risk factor for radiation pneumonitis. Acta Oncol 2011; 50:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeda A, Kunieda E, Sanuki N, et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Int J Radiat Oncol Biol Phys 2009; 74:363–369. [DOI] [PubMed] [Google Scholar]
- 52.Grills IS, Yan D, Martinez AA, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003; 57:875–890. [DOI] [PubMed] [Google Scholar]
- 53.Holt A, van Vliet-Vroegindeweij C, Mans A, et al. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2011; 81:1560–1567. [DOI] [PubMed] [Google Scholar]
- 54.Merrow CE, Wang IZ, Podgorsak MB. A dosimetric evaluation of VMAT for the treatment of non-small cell lung cancer. J Appl Clin Med Phys 2013; 14:4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee TF, Yang J, Huang EY, et al. Technical advancement of radiation therapy. Biomed Res Int 2013; 2014:549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chapet O, Khodri M, Jalade P, et al. Potential benefits of using non coplanar field and intensity modulated radiation therapy to preserve the heart in irradiation of lung tumors in the middle and lower lobes. Radiother Oncol 2006; 80:333–340. [DOI] [PubMed] [Google Scholar]
- 57.Kataria T, Gupta D, Bisht SS, et al. Adaptive radiotherapy in lung cancer: dosimetric benefits and clinical outcome. Br J Radiol 2014; 87:20130643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Umezawa R, Takase K, Jingu K, et al. Evaluation of radiation-induced myocardial damage using iodine-123 beta-methyl-iodophenyl pentadecanoic acid scintigraphy. J Radiat Res 2013; 54:880–889. [DOI] [PMC free article] [PubMed] [Google Scholar]


