Abstract
Optic nerve sheath diameter (ONSD) seen on ocular US has been associated with increased intracranial pressure (IICP). However, most studies have analyzed normal range of ONSD and its optimal cut-off point for IICP in Caucasian populations. Considering ONSD differences according to ethnicity, previous results may not accurately reflect the association between IICP and ONSD in Koreans. Therefore, we conducted this study to investigate normal range of ONSD and its optimal threshold for detecting IICP in Korean patients.
This prospective multicenter study was performed for patients with suspected IICP. ONSD was measured 3 mm behind the globe using a 13-MHz US probe. IICP was defined as significant brain edema, midline shift, compression of ventricle or basal cistern, effacement of sulci, insufficient gray/white differentiation, and transfalcine herniation by radiologic tests. The results of the ONSD are described as the median (25th–75th percentile). The differences of ONSD according to disease entity were analyzed. A receiver operator characteristic (ROC) curve was generated to determine the optimal cut-off point for identifying IICP.
A total of 134 patients were enrolled. The patients were divided into 3 groups as follows: patients with IICP, n = 81 (60.5%); patients without IICP, n = 27 (20.1%); and control group, n = 26 (19.4%). ONSD in patients with IICP (5.9 mm [5.8–6.2]) is significantly higher than those without IICP (5.2 mm [4.8–5.4]) (P < 0.01) and normal control group (4.9 mm [4.6–5.2]) (P < 0.001). Between patients without IICP and normal control group, the difference of ONSD did not reach statistical significance (P = 0.31). ONSD >5.5 mm yielded a sensitivity of 98.77% (95% CI: 93.3%–100%) and a specificity of 85.19% (95% CI: 66.3%–95.8%).
In conclusion, the optimal cut-off point of ONSD for identifying IICP was 5.5 mm. ONSD seen on ocular US can be a feasible method for detection and serial monitoring of ICP in Korean adult patients.
Keywords: increased intracranial pressure, optic nerve sheath diameter, optimal threshold, ultrasonography
1. Introduction
Early detection of increased intracranial pressure (IICP) and prompt management is essential for patients with intracranial lesions. ICP can be accurately estimated using invasive monitoring probes, such as an intraventricular or intraparenchymal catheter. However, clinical concerns include procedure-related complications, limited technical availability due to performance by only neurosurgeon, and unsuitable patients who have coagulopathy or vital sign instability.[1]
Ultrasonography (US) has become widely used in the emergency department (ED) or intensive care unit (ICU) settings due to its noninvasive nature, real-time tracking at the bedside, and capability of repetitive examination without radiation exposure. Optic nerve sheath diameter (ONSD) seen on ocular US has been associated with IICP.[1–3] However, most studies have analyzed normal range of ONSD and its optimal cut-off point for IICP in Caucasian populations.[4] Considering ONSD differences according to ethnicity,[5] results obtained from Caucasian may not accurately reflect the association between IICP and ONSD in Koreans. Therefore, we conducted this study to investigate normal range of ONSD and its optimal threshold for detecting IICP in Korean patients.
2. Methods
This prospective multicenter study was performed in 3 institutions. Patients who visited the ED or ICU for suspected IICP from September 2013 to May 2016 were enrolled. Inclusion criteria were age >18 years; clinical signs of IICP that included headache, nausea, vomiting, or altered mentality; and radiologic tests like brain computed tomography (CT) or magnetic resonance imaging (MRI), and US within a 1-hour interval. Patients who were younger than 18 years of age, or who had an orbital trauma or mass, or cavernous sinus arachnoid cysts were excluded.[2]
Radiologic tests scans were interpreted independently by 2 neuroradiologists blinded to clinical information. IICP was defined as significant brain edema, midline shift, compression of ventricle or basal cistern, effacement of sulci, insufficient gray/white differentiation, and transfalcine herniation.[2,6,7] Ocular US was performed using a ProSound Alpha 6 13-MHz US probe (Hitachi Medical Corp., Tokyo, Japan) according to a previously reported method.[8] ONSD assessments were independently done by 2 trained investigators (JPJ and HNL). This study was approved by the Institutional Review Boards at the participating institutions (2015-131, H-1309-004-515, B-1506-304-102).
2.1. Statistical analyses
Categorical variables are presented as numbers and percentages. Continuous data are shown as the mean ± standard deviation (SD). The intraobserver and interobserver agreements were calculated using kappa statistics. The results of the ONSD are described as the median (25th–75th percentile). A comparison of ONSD was performed by Kruskal–Wallis test. Mann–Whitney U test with Bonferroni correction was conducted for all possible pairwise comparison. A receiver operator characteristic (ROC) curve was generated to determine the optimal cut-off point for identifying IICP. Sensitivity, specificity, positive predictive value, and negative predictive value were assessed. Statistics were performed using SPSS V.19 (SPSS, Chicago, IL) and MedCalc (www.medcalc.org).
3. Results
A total of 134 patients were enrolled. The 134 individuals comprised 81 patients with IICP (60.5%), 27 patients without IICP (20.1%), and 26 unaffected control group (19.4%). Detailed information on the clinical characteristics of the patients is described in Table 1. The proportion of male and the mean age of the enrolled patients according to groups were as follows: patients with IICP, 52 males (64.2%), 64.4 ± 17.6 years; patients without IICP, 20 males (74.1%), 65.2 ± 17.8 years; and control group, 9 males (34.6%), 60.8 ± 12.5 years. In patients with IICP, 42 (51.9%) presented with headache and vertigo, and 21 (25.9%) with altered mentality. In patients without IICP, 20 patients presented with headache or vertigo, and 7 (25.9%) displayed neurologic deficits including motor weakness, transient ischemic attack, and seizure. Hemorrhagic cases, such as epidural hematoma, subdural hematoma, or intracerebral hemorrhage, were found in 70 (86.4%) patients with IICP and 4 (14.8%) without IICP. For the control group, 20 (76.9%) cases of spinal stenosis or herniated nucleus pulposus and 6 (23.1%) of unruptured aneurysms were noted.
Table 1.
Baseline characteristics of the study population (n = 134).
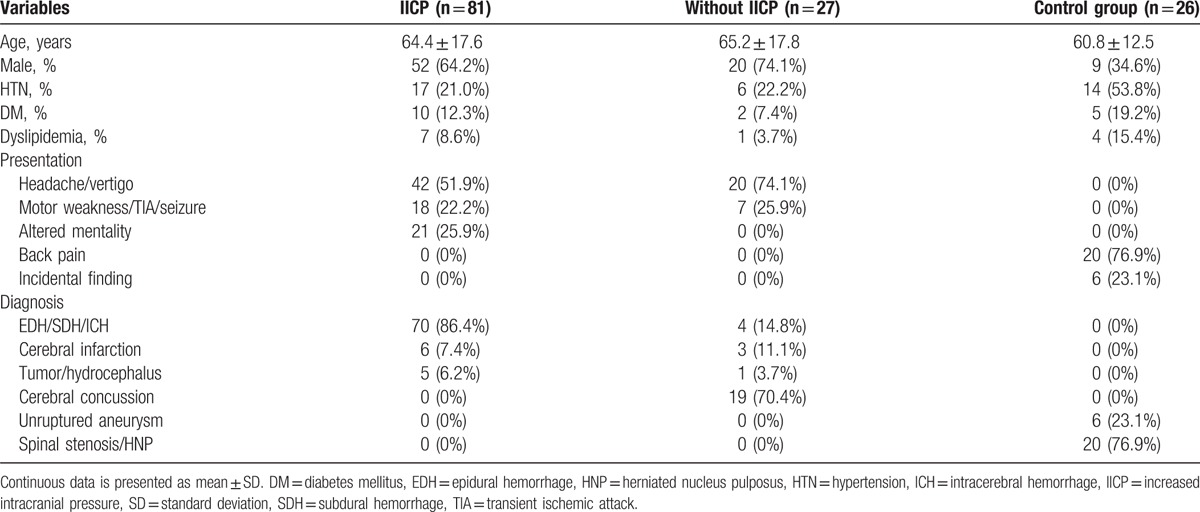
The intra- and interobserver agreements were excellent for estimating ONSD (κ = 0.91 and 0.88). ONSD in patients with IICP (5.9 mm, range 5.8–6.2 mm) was significantly higher than those without IICP (5.2 mm, range 4.8–5.4 mm) (P < 0.001) and the normal control group (4.9 mm, range 4.6–5.2 mm) (P < 0.001). More specifically, the results were further analyzed according to the diagnosis such as hemorrhage (n = 74, 83.1%) and infarction (n = 9, 10.1%), and the presence of IICP. In the hemorrhage group, IICP patients (6.0 ± 0.3 mm) showed significantly increased ONSD compared to non-IICP patients (5.4 ± 0.1 mm) (P = 0.001). In the infarction group, the mean ONSD of IICP patients was 6.1 mm (range 5.8–6.4 mm) and that of non-IICP was 5.4 mm (range 5.1–5.7 mm).
The difference of ONSD between patients without IICP and the normal control group did not reach statistical significance (P = 0.31). The area under ROC curve is 0.975. ONSD >5.5 mm yielded a sensitivity of 98.77% (95% CI: 93.3%–100%) and a specificity of 85.19% (95% CI: 66.3%–95.8%) (Fig. 1).
Figure 1.
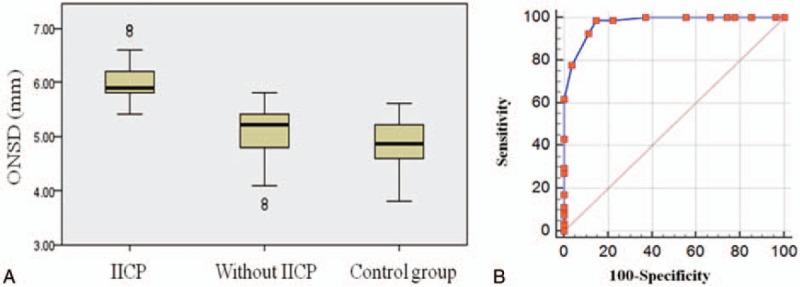
(A) ONSD in patients with IICP, without IICP, and normal control group. The bar represents the median value and 25th to 75th percentile. ONSD in patients with IICP (5.9 mm, range 5.8–6.2 mm) is significantly higher than those without IICP (5.2 mm, range 4.8–5.4 mm) (P < 0.001) and normal control group (4.9 mm, range 4.6–5.2 mm) (P < .001). (B) The area under the receiver operator characteristic curve is 0.975. ONSD >5.5 mm yielded a sensitivity of 98.77% (95% CI: 93.3%–100%) and a specificity of 85.19% (95% CI: 66.3%–95.8%). CI = confidence interval, IICP = increased intracranial pressure, ONSD = optic nerve sheath diameter.
3.1. Illustrative case
A 57-year-old man presented with a sudden onset of left hemiparesis. Diffusion MRI revealed acute cerebral infarction in the territory of the middle cerebral artery on the right side with ONSD of 5.2 mm (Fig. 2A and B). Two days later, the patient became drowsy and the right-sided hemiplegic despite antiplatelet medication and intravenous mannitolization. Brain CT scans displayed aggravation of cerebral edema. Follow-up of ocular US showed increased ONSD of 6.3 mm (Fig. 2C and D). Emergent decompressive craniectomy and wide duroplasty were performed to relieve the IICP. Postoperative CT scans demonstrated a decrease in midline shift with ONSD of 5.8 mm (Fig. 2E and F). The patient underwent further hypothermic therapy after surgical decompression. CT scans taken after 2 weeks after operation showed a substantial improvement in the extent of midline shift with ONSD of 5.4 mm (Fig. 2G and H).
Figure 2.
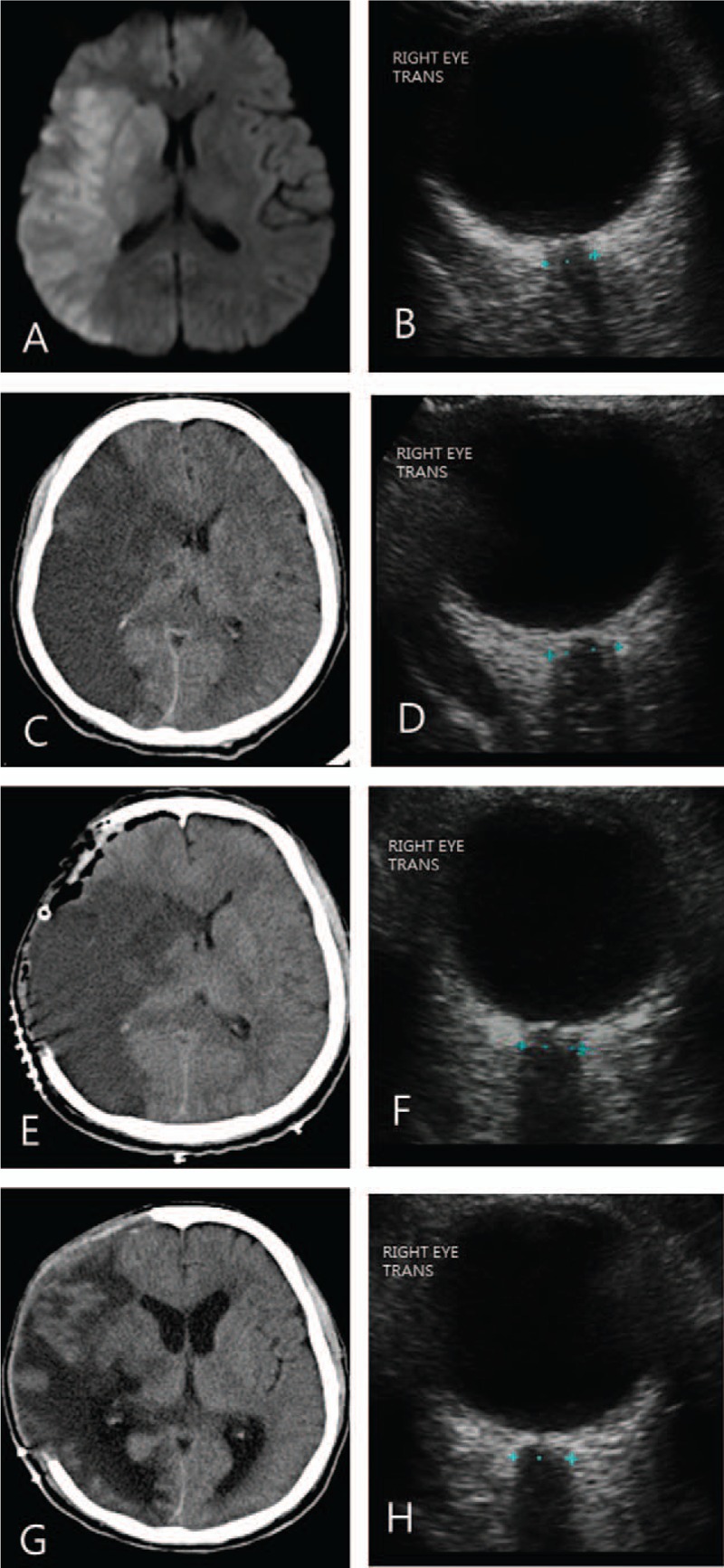
(A, B) A 57-year-old man presented with left hemiparesis because of acute middle cerebral infarction on the right side seen on diffusion magnetic resonance imaging. ONSD on the right side was measured at 5.2 mm. (C, D) The level of consciousness decreased to drowsy on the 2nd day with aggravation of cerebral edema seen on brain CT. ONSD on the right side increased to 6.3 mm. (E, F) Decompressive craniectomy decreased midline shift with ONSD of 5.8 mm. (G, H) CT scans taken after 2weeks after operation showed a substantial improvement in the extent of midline shift with ONSD of 5.4 mm. CT = computed tomography, ONSD = optic nerve sheath diameter.
4. Discussion
The present study showed that ONSD in patients with IICP had a mean size of 5.9 mm (range 5.8–6.2 mm), which was significantly higher than patients without IICP (5.2 mm, range 4.8–5.4 mm) or normal controls (4.9 mm, range 4.6–5.2 mm). The optimal ONSD cut-off point for identifying IICP was 5.5 mm which had a sensitivity of 98.77% and a specificity of 85.19%.
Invasive ICP monitoring method has been a gold standard for the evaluation of IICP. However, limitations include potential complications, such as hemorrhage or infection, and limited technical availability in institutions without a neurosurgeon.[4] Accordingly, feasibility of noninvasive methods like transcranial Doppler, tympanic membrane displacement, US, CT, and MRI have been investigated as the invasive ICP monitoring with a reference value. ONSD that defines the dural sheath diameter surrounding the optic nerve can be measured due to its cerebrospinal fluid (CSF) connection to the intracranial subarachnoid space. Liu and Kahn[9] reported that subarachnoid space pressure of ONSD showed a liner correlation with ICP change. Moretti et al[10] showed that optimal ONSD cut-off point to define IICP (>20 mm Hg) was 5.2 mm with a 93.1% sensitivity and 73.9% specificity in 63 patients with spontaneous intracerebral hemorrhage. Geeraerts et al[11] reported that adults with high ICP, defined as ICP >20 m mHg for more than 30 minutes in the 1st 48 hours after trauma, had a higher ONSD value (6.3 ± 0.6 mm) than normal ICP (5.1 ± 0.7 mm) or control group (4.9 ± 0.3 mm). Beyond the comparative tests between ONSD by ocular US and invasive ICP monitoring, ONSD correlated well with IICP seen on CT findings. Tayal et al[2] suggested that ONSD can be an alternative to CT for detecting IICP patients who visit the ED. In their study, ONSD over 5 mm had 100% sensitivity and 63% specificity in detecting IICP. Girisgin et al[1] also reported that IICP patients had higher mean ONSD than control group (6.4 mm in the IICP group vs 4.6 mm in the control group). Nevertheless, ethnic differences could be a confounding factor to set the optimal ONSD to define IICP. Ozgen and Ariyurek[12] reported a mean ONSD of 4.4 mm as seen on CT in 100 healthy Turkish volunteers. Lee et al[13] showed a mean ONSD of 4.1 mm ranging from 2.9 to 5.3 mm in Korean population using CT scan. Ko[14] also reported that the mean ONSD on MRI was 4.37 mm in patients with normal ICP. In their study, ONSD was not significantly different according to age, sex, and underlying diseases. Maude et al[15] reported a relative narrow range of ONSD (4.24–4.83 mm) in healthy volunteer in Bangladesh than that of United Kingdom (2.5–4.1 mm)[16] or Greece (2.2–4.9 mm).[17] Accordingly, we think that optimal cut-off point of ONSD for IICP could be better defined differently according to ethnicity.
Recently, Wang et al[4] assessed the mean ONSD and optimal value for defining IICP in a Chinese population. The mean ONSD in normal individuals was 3.55 ± 0.38 mm. The optimal cut-off point for IICP was 4.1 mm, which yielded a 95% sensitivity and 92% specificity. However, defining IICP based on lumbar puncture and disease severity in the study was a concern to the interpretation of the results. CSF pressure assessed by lumbar puncture tends to exhibit higher level than actual ICP in children.[18] Lenfeldt et al[19] showed that lumbar puncture opening pressure was consistently higher than ICP in adult patients with normal pressure hydrocephalus. Warden et al[20] also reported that CSF pressure by lumbar puncture overestimated the ICP within a range of 300 mmH2O. Regarding the disease severity, the authors only included patients who were managed conservatively in the general ward. Accordingly, the reported optimal cut-off of 4.1 mm in the Chinese subjects could not accurately reflect IICP patients who may require invasive treatments in ED or ICU. The aim of the present study was to investigate optimal cut-off points to indicate IICP in Korean population. The optimal ONSD cut-off point was 5.5 mm, which was higher than that of the Chinese population based on CSF pressure by lumbar puncture.[4]
Serial check-up of intracranial lesions can be challenging for ICU patients, in particular unstable patients who have multiple drains or fluid lines. Although no data concerning adverse events during transfer to radiologic tests are available in Korea, an incidence rate up to 71% has been reported.[21,22] Moreover, transfer of patients is usually done with an intern doctor or a physician assistant who may not yet have enough experience in dealing with unexpected complications during transfer.[22] In such circumstances, ONSD by ocular US can provide crucial information about the ICP at the patient’ bedside within 5 minutes. Accordingly, the need to conduct brain CT scans could be decreased while avoiding unnecessary complications during transfer.
4.1. Limitations
There are some limitations in this study. Intra- or interobserver variation are concerns. Previous studies[16,23] showed ±0.1 to 0.2 mm of intraobserver and ±0.2 to 0.3 mm of interobserver variation. Ballantyne et al[16] reported that intra- and interobserver variation reduced after the 1st 17 examinations. In this study, we also had excellent agreements for estimating ONSD (κ = 0.91 of intraobserver and 0.88 of interobserver agreements, respectively). Accordingly, we think that ONSD measured by ocular US can be an easily handled method to detect IICP with low intra- and interobserver variation, although standardization efforts are necessary. Second, some physicians may argue the possibility of the presence of idiopathic intracranial hypertension (IIH) in the enrolled patients, in particular, the control group. IIH has been reported to range from 0.9 to 2.2 per 100,000 individuals per year. Most patients with IIH present with constant daily headache and visual symptoms without intracranial abnormalities.[24–26] In this study, patients without IICP underwent radiologic imaging tests for sudden acute headache. In addition, the normal control group included patients who had had a medical check-up, with no evidence indicating IIH. Accordingly, the possibility of the presence of IIH in the enrolled patients would be low. Third, direct comparison between ONSD and invasive ICP monitoring was not performed. We do not advocate that ONSD seems to be accurate enough as an alternative to invasive ICP monitoring or radiologic tests, such as CT or MRI in all circumstances. However, we think that ONSD could be used as a screening method for the detection or serial monitoring of IICP in ED or ICU. Nevertheless, further study on the correlation between ONSD and invasive ICP monitoring is required in Koreans.
5. Conclusion
The optimal cut-off point of ONSD for identifying IICP was 5.5 mm. ONSD seen on ocular US can be a feasible method for detection and serial monitoring of ICP.
Acknowledgments
The authors thank Sung-Eun Kim for her help with the data collection and valuable contribution to the statistical analysis.
Footnotes
Abbreviations: CT = computed tomography, ED = emergency department, ICU = intensive care unit, IICP = increased intracranial pressure, IIH = idiopathic intracranial hypertension, MRI = magnetic resonance imaging, ONSD = optic nerve sheath diameter, US = ultrasonography.
The authors have no conflicts of interest to disclose.
HL and JPJ contributed equally to this work as corresponding author.
Funding/support: This study was supported by Hallym University Research Fund (HURF-2015-52) and BioGreen 21 (PJ009051) of Rural Development Administration.
The authors have no conflicts of the interest to disclose.
References
- 1.Girisgin AS, Kalkan E, Kocak S, et al. The role of optic nerve ultrasonography in the diagnosis of elevated intracranial pressure. Emerg Med J 2007; 24:251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tayal VS, Neulander M, Norton HJ, et al. Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med 2007; 49:508–514. [DOI] [PubMed] [Google Scholar]
- 3.Moretti R, Pizzi B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol Scand 2011; 55:644–652. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Feng L, Yao Y, et al. Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PloS One 2015; 10:e0117939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin G, Wang YX, Zheng ZY, et al. Ocular axial length and its associations in Chinese: the Beijing Eye Study. PloS One 2012; 7:e43172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller MT, Pasquale M, Kurek S, et al. Initial head computed tomographic scan characteristics have a linear relationship with initial intracranial pressure after trauma. J Trauma 2004; 56:967–972.discussion 972-963. [DOI] [PubMed] [Google Scholar]
- 7.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Initial management. J Neurotrauma 2000; 17:463–469. [DOI] [PubMed] [Google Scholar]
- 8.Blaivas M, Theodoro D, Sierzenski PR. Elevated intracranial pressure detected by bedside emergency ultrasonography of the optic nerve sheath. Acad Emerg Med 2003; 10:376–381. [DOI] [PubMed] [Google Scholar]
- 9.Liu D, Kahn M. Measurement and relationship of subarachnoid pressure of the optic nerve to intracranial pressures in fresh cadavers. Am J Ophthalmol 1993; 116:548–556. [DOI] [PubMed] [Google Scholar]
- 10.Moretti R, Pizzi B, Cassini F, et al. Reliability of optic nerve ultrasound for the evaluation of patients with spontaneous intracranial hemorrhage. Neurocrit Care 2009; 11:406–410. [DOI] [PubMed] [Google Scholar]
- 11.Geeraerts T, Launey Y, Martin L, et al. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med 2007; 33:1704–1711. [DOI] [PubMed] [Google Scholar]
- 12.Ozgen A, Ariyurek M. Normative measurements of orbital structures using CT. Am J Roentgenol 1998; 170:1093–1096. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Lim DW, Lee SH, et al. Normative measurements of Korean orbital structures revealed by computerized tomography. Acta Anaesthesiol Scand 2001; 79:197–200. [DOI] [PubMed] [Google Scholar]
- 14.Ko SB. Optic nerve sheath diameter on brain magnetic resonance imaging: a single center study. J Neurocrit Care 2015; 8:16–24. [Google Scholar]
- 15.Maude RR, Hossain MA, Hassan MU, et al. Transorbital sonographic evaluation of normal optic nerve sheath diameter in healthy volunteers in Bangladesh. PloS One 2013; 8:e81013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ballantyne SA, O’Neill G, Hamilton R, et al. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound 2002; 15:145–149. [DOI] [PubMed] [Google Scholar]
- 17.Soldatos T, Karakitsos D, Chatzimichail K, et al. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care 2008; 12:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cartwright C, Igbaseimokumo U. Lumbar puncture opening pressure is not a reliable measure of intracranial pressure in children. J Child Neurol 2015; 30:170–173. [DOI] [PubMed] [Google Scholar]
- 19.Lenfeldt N, Koskinen LO, Bergenheim AT, et al. CSF pressure assessed by lumbar puncture agrees with intracranial pressure. Neurology 2007; 68:155–158. [DOI] [PubMed] [Google Scholar]
- 20.Warden KF, Alizai AM, Trobe JD, et al. Short-term continuous intraparenchymal intracranial pressure monitoring in presumed idiopathic intracranial hypertension. J Neuroophthalmol 2011; 31:202–205. [DOI] [PubMed] [Google Scholar]
- 21.Smith I, Fleming S, Cernaianu A. Mishaps during transport from the intensive care unit. Crit Care Med 1990; 18:278–281. [DOI] [PubMed] [Google Scholar]
- 22.Jeon JS, Lee SH, Son YJ, et al. Mobile computed tomography: three year clinical experience in Korea. J Korean Neurosurg Soc 2013; 53:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raboel PH, Bartek J, Jr, Andresen M, et al. Intracranial pressure monitoring: invasive versus non-invasive methods – a review. Crit Care Res Pract 2012; 2012:950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 2013; 81:1159–1165. [DOI] [PubMed] [Google Scholar]
- 25.Wall M, Kupersmith MJ, Kieburtz KD, et al. The idiopathic intracranial hypertension treatment trial: clinical profile at baseline. JAMA Neurol 2014; 71:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mollan SP, Ali F, Hassan-Smith G, et al. Evolving evidence in adult idiopathic intracranial hypertension: pathophysiology and management. J Neurol Neurosurg Psychiatry 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]


