Abstract
Anti-tumor necrosis factor-α (TNFα) therapy has improved the prognosis of many chronic inflammatory diseases. It appears to be well-tolerated by liver-transplant patients. However, their use and their safety in kidney-transplant patients have yet to be determined.
In this retrospective study, we identified 16 adult kidney-transplant patients aged 46.5 years (34–51.8) who received anti-TNFα therapy from 7 kidney transplantation centers. The indications for this treatment included: chronic inflammatory bowel disease (n = 8), inflammatory arthritis (n = 5), AA amyloidosis (n = 1), psoriasis (n = 1), and microscopic polyangiitis (n = 1).
Anti-TNFα therapies resulted in a clinical response in 13/16 patients (81%). Estimated glomerular filtration rates (MDRD-4) were similar on day 0 and at 24 months (M24) after anti-TNFα treatment had been initiated (41 [12–55] and 40 [21–53] mL/min/1.73 m2, respectively). Two allograft losses were observed. The 1st case was due to antibody-mediated rejection (M18), while the 2nd was the result of AA amyloidosis recurrence (M20). There were several complications: 8 patients (50%) developed 23 serious infections (18 bacterial, 4 viral, and 1 fungal) and 4 developed cancer. Five patients died (infection n = 2, cardiac AA amyloidosis n = 1, intraalveolar hemorrhage following microscopic polyangiitis n = 1, and acute respiratory distress syndrome n = 1). On univariate analysis, recipient age associated with death (P = 0.009) and infection development (P = 0.06).
Using anti-TNFα therapies, remission can be achieved in chronic inflammatory diseases in kidney-transplant patients. However, concommitant anti-TNFα and immunosuppresive therapies must be used with caution due to the high risk of infection, particularly after the age of 50.
Keywords: anti-TNFα therapy, chronic inflammatory disease, inflammatory arthritis, inflammatory bowel disease, kidney transplantation
1. Introduction
The advent of tumor necrosis factor-α (TNFα) inhibitors (anti-TNFα therapies) within the past decade has resulted in a revolution in the management of severe chronic rheumatoid (psoriatic arthritis, ankylosing spondylitis, or rheumatoid arthritis), gastrointestinal (Crohn disease and ulcerative colitis), and dermatologic (psoriasis) inflammatory diseases.
TNFα is a pleiotropic cytokine produced by immune cells (macrophages, dendritic cells, and T lymphocytes).[1] TNFα binds to 2 surface receptors, namely, TNFR1 and TNFR2, and activates different signaling pathways associated with cell proliferation; inflammation induction, immune modulation, and proinflammatory cytokine production; and cell apoptosis.[1,2]
Very few studies regarding the use of anti-TNFα therapy in kidney transplant patients have been conducted. Organ transplant patients have been excluded from anti-TNFα drug safety studies due to the increased risks of infection[3] and cancer specific to this population.[4,5] Anti-TNFα treatment is associated with an increased incidence of severe bacterial,[6,7] viral (i.e., herpesvirus),[8] and opportunistic (i.e., tuberculosis)[9,10] infections and may cause certain cancers.[11] Anti-TNFα drugs also cause hypersensitivity reactions, which may contribute to autoimmune disease development, particularly drug-induced lupus.[4] To date, the literature includes only case series regarding organ transplant patients treated with anti-TNFα drugs.[12–18]
We retrospectively evaluated 16 kidney transplant patients treated with anti-TNFα drugs for chronic inflammatory diseases. Our study objectives were to describe the indications for anti-TNFα treatment, the responses to treatment, and the safety of these drugs in this population of immunocompromised patients.
2. Patients and methods
2.1. Methods
This multicenter retrospective observational study was conducted in 7French kidney transplant centers. We identified 16 patients (11 male) aged 46.5 years (34–51.8) from the ASTRE prospective database of the Spiesser Kidney Transplantation Group (Angers, Caen, Clermont-Ferrand, Reims, and Strasbourg University Hospitals) and the DIVAT prospective database (Necker and Toulouse University Hospitals). Patients aged at least 18 years who had undergone kidney transplantation and received anti-TNFα treatment were included in this study. The ethical committee of the institution approved the extraction of data from the patient charts.
Patient medical charts and demographics were retrieved from the hospital registries, and the following data were recorded: age, gender, nephropathy, past history of infection or malignancy, date of transplantation, and postoperative immunosuppressive regimen. We examined the kidney transplantation outcomes of these patients, including patient and graft survival, occurrence of acute rejection episodes, infections, cancer, adverse event with TNF therapy, causes of graft loss, and patient death. The glomerular filtration rate (GFR) was estimated (eGFR) using the Modification of Diet in Renal Disease 4 (MDRD4) formula. Failing data were retrospectively found in the patient record.
2.2. Statistics
All analyses were performed using Stata software (version 13, StataCorp, College Station, TX) and were performed for a 2-sided type I error of α5%. Baseline characteristics are presented as the mean ± standard deviation (SD) or the median (interquartile range) for continuous data (assumption of normality assessed via the Shapiro–Wilk test) and as numbers and percentages for categorical data. Quantitative variables were compared between independent groups (infection yes/no and death yes/no) by Student t test or the Mann–Whitney U test if the conditions of the t test were not met (normality and homoscedasticity were analyzed using the Fisher–Snedecor test). When appropriate, comparisons between independent groups were analyzed using a Chi-squared test or Fischer exact test for categorical variables. The relationships between quantitative outcomes were analyzed using correlation coefficients (Pearson or Spearman, according to the statistical distribution). Regarding the evolution of the eGFR (longitudinal repeated data), random-effects models were used to account for between- and within-patient variability. Finally, due to the design of this study (meta-analysis of individual data), these analyses were completed using generalized linear mixed models (logistic regression for dichotomous dependent variables: infection yes/no and death yes/no) to study the fixed effects described previously, and the study was considered a random effect (to measure between- and within-study variability). Given the sample size, no meta-regression analysis was performed based on the results of the multivariate analysis. Last, sensitivity analysis was performed to measure the impact of missing data.
3. Results
3.1. Patient characteristics
Patient characteristics are summarized in Table 1. Prior to kidney transplantation, 2 patients developed neoplasms (breast adenocarcinoma and basal-cell carcinoma), and 3 patients developed serious infections requiring hospitalization. The 1st patient (P#14) developed 3 bacterial infections (urinary tract, gastrointestinal, and cutaneous) during his 1st kidney transplantation, the 2nd patient (P#7) developed eye shingles, and the 3rd patient (P#10) developed dialysis catheter-related staphylococcal septicemia.
Table 1.
Characteristics of kidney transplant recipients treated with anti-TNFα therapy.
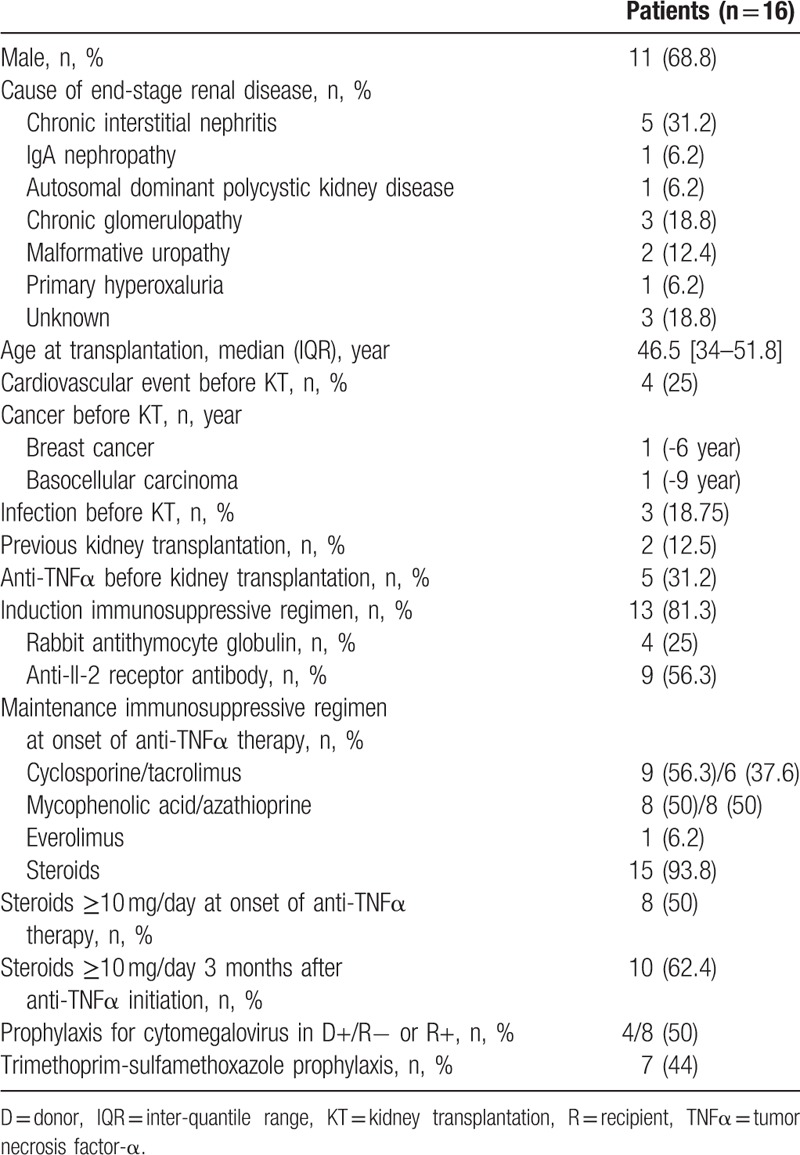
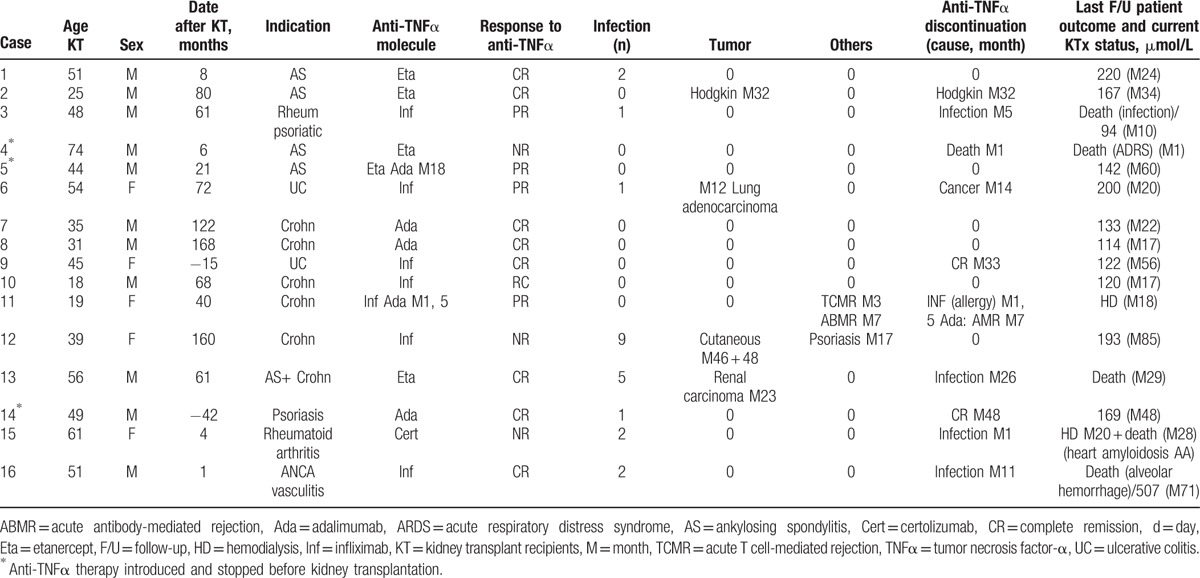
Five patients began anti-TNFα treatment before kidney transplantation (Table 1). Three patients (P#4, P#5, and P#15) discontinued treatment following transplantation before resuming it at 4, 6, and 22 months, respectively, after transplantation (Table 2). Patients P#9 and P#14 continued their treatments, which they had initiated at 15 and 42 months, respectively, prior to transplantation (Table 2).
Table 2.
Anti-TNFα treatment indications, tolerance and outcomes in 16 kidney transplant recipients.
Cytomegalovirus prophylaxis was administered to 4/8 patients (50%) at risk for viral reactivation or primary graft infection upon anti-TNFα treatment initiation. Seven patients (44%) received Pneumocystis pneumonia prophylaxis (trimethoprim/sulfamethoxazole) upon anti-TNFα treatment initiation.
The indications for anti-TNFα treatment were rheumatoid (n = 5, 31%), gastrointestinal (n = 8, 50%), and dermatologic (n = 1, 6%) disease, as well as microscopic polyangiitis (n = 1, 6%) and familial Mediterranean fever (n = 1, 6%) (Table 2).
3.2. Clinical response to anti-TNFα treatment
Overall, clinical responses were observed by clinicians in 13/16 cases. A complete response was observed in 9 cases (56%), and a partial response was observed in 4 cases (25%) (Table 2). Two complete responses (40%) and 2 partial responses (40%) were noted in patients with chronic rheumatoid disease (n = 5); 5 complete responses (62.5%) and 2 partial responses (25%) were noted in patients with chronic inflammatory bowel disease (n = 8); 1 complete response was noted in a patient with pustular psoriasis, resulting in adalimumab discontinuation at M48; and 1 complete response was noted in a patient being treated for microscopic polyangiitis. One patient (P#15) with AA amyloidosis discontinued treatment at M1 due to a urinary tract infection; thus, anti-TNFα treatment effectiveness could not be determined in this patient.
3.3. Infectious complications
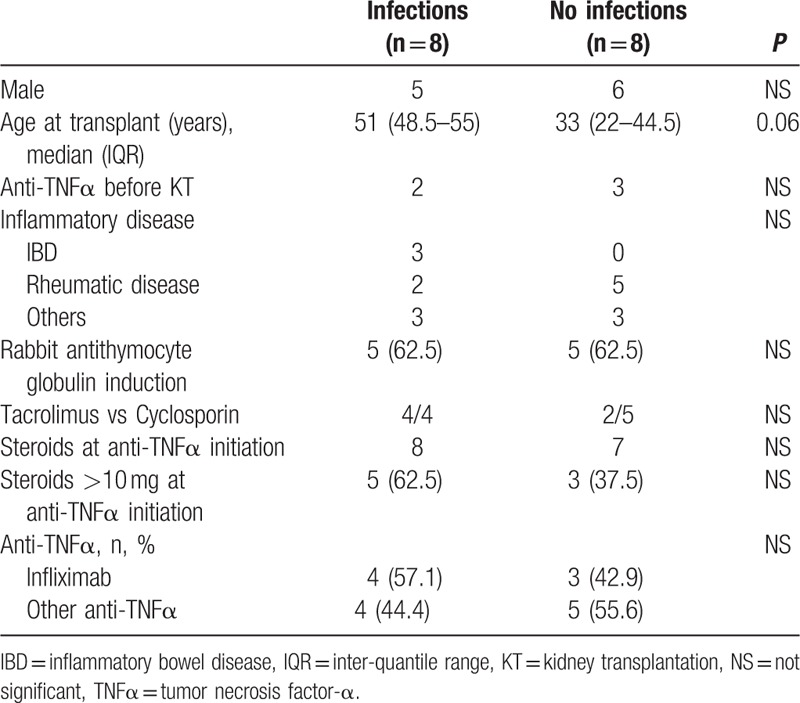
Eight patients (50%) developed 23 serious infections while receiving anti-TNFα treatment (1.43 infections per patient) (Tables 3 and 4). These patients mainly developed bacterial infections (n = 16) (primarily upper urinary tract infections and acute cholecystitis), although viral infections (n = 4) (varicella-zoster virus [VZV], cytomegalovirus, and BK viremia) and a fungal infection were also noted. On univariate analysis, the mean ages of the patients who did and did not develop infection were 51.1 ± 6.4 and 36.8 ± 18.3 years, respectively, P = 0.06 (Table 4). Induction therapy, corticosteroid doses ≥ 10 mg, and pretransplant anti-TNFα treatment were not associated with infection risk (Table 4).
Table 3.
Description of severe infectious complications that occurred during anti-TNFα therapy.
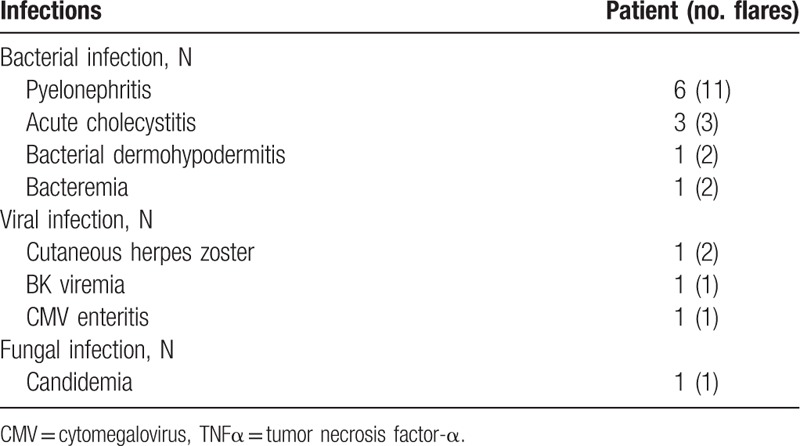
Table 4.
Infectious complications in our 16 kidney transplant recipients treated with anti-TNFα therapy: univariate analysis.
3.4. Cancer
Four patients (25%) developed cancer while receiving anti-TNFα treatment (Table 2). Three patients with chronic inflammatory bowel disease developed a solid tumor or multiple solid tumors after biological therapy initiation. These included 1 case of lung adenocarcinoma at M12, which resulted in infliximab discontinuation at M14 (P#6 was still alive at the last follow-up at M20); 2 basal-cell carcinomas at M46 and M48 (P#12); and a papillary adenoma of the kidney at M23 (P#13 died of septicemia at M26). P#2 developed Hodgkin lymphoma at M32, which resulted in anti-TNFα treatment discontinuation.
3.5. Changes in the glomerular filtration rate and graft survival
The estimated GFR remained stable over time during anti-TNFα treatment as follows: 41 (12–55) mL/min/1.73 m2 at D0, 44.5 (33–56) mL/min/1.73 m2 at M3, 41 (34–57) mL/min/1.73 m2 at M12, and 40 (21–53) mL/min/1.73 m2 at M24 (Fig. 1, Table 2).
Figure 1.
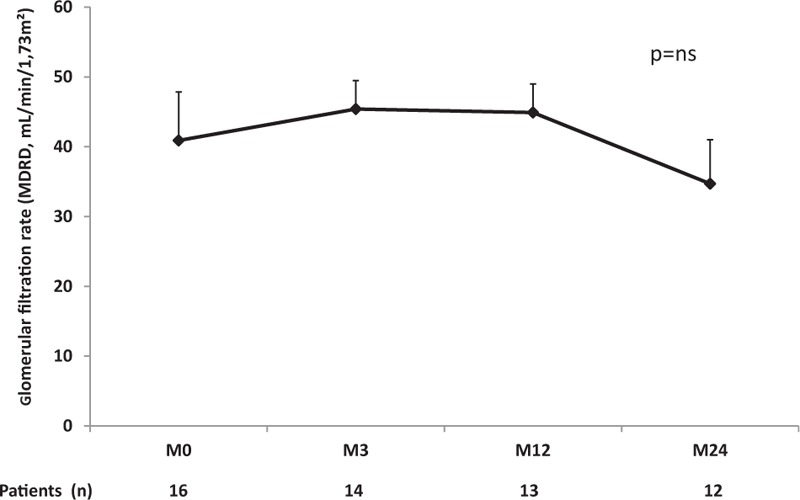
Evolution of estimated glomerular filtration rates (MDRD, mL/min/1.73 m2) of kidney transplant recipients treated with anti-TNFα therapy. MDRD = Modification of Diet in Renal Disease 4, TNFα = tumor necrosis factor-α
Two patients (12.5%) lost their grafts. The 1st, P#15, developed AA amyloidosis recurrence in the graft at M29 of anti-TNFα treatment despite certolizumab treatment. The 2nd, P#11, who exhibited donor-specific antibodies before anti-TNFα treatment, lost the graft (Table 2) at M18 (58 months after transplantation) due to antibody-mediated rejection. The patient had been receiving adalimumab for Crohn disease and developed a cutaneous rash while receiving infliximab treatment (M1.5), which resulted in the initiation of adalimumab treatment. A graft biopsy performed at M3 due to increased blood creatinine levels revealed an inflammatory infiltrate occupying 25% to 30% of the biopsy and signs of t1 tubulitis consistent with either borderline rejection (Banff 2007)[19] or acute tubulointerstitial nephritis. High-dose corticosteroid therapy and anti-TNFα treatment discontinuation improved graft function. Adalimumab was subsequently resumed at M6. A biopsy conducted at M8 due to further graft function deterioration and increased Luminex preexisting donor-specific antihuman leukocyte antigen antibody (DQ7) mean fluorescence intensity showed acute antibody-mediated rejection (g1 + ptc2).[19] No treatment reduction was observed (cyclosporin A, azathioprine, and steroids) after anti-TNFα treatment initiation, nor was treatment reduction observed during the preceding 3 months.
3.6. Patient survival
Overall, 5 patients died a median of 28 months (1–71) after anti-TNFα therapy initiation (Table 2). Two patients died of bacterial infections. One of them (P#3) died of septic shock of gastrointestinal origin at M10 of anti-TNFα treatment despite treatment discontinuation at M5 due to gangrenous cholecystitis accompanied by biliary peritonitis. The 2nd developed multiple infections, including 2 bacterial infections, 1 BK viral infection, and 1 candidal infection. He died of a bacterial infection at M29. Two patients died after anti-TNFα treatment was discontinued. P#15 lost the renal graft at M20 due to AA amyloidosis recurrence and died at M28 of cardiac AA amyloidosis. Patient P#16, who had microscopic polyangiitis, died of intraalveolar hemorrhage at M71 despite treatment discontinuation at M11 following 2 VZV infections, the 1st of which involved the thorax, and the 2nd of which involved the eyes. Another patient (P#4) died of acute respiratory distress syndrome of unknown cause at M1 after anti-TNFα treatment (10 months after transplantation). On univariate analysis, the patients who died were significantly older (58.0 ± 10.2 vs 37.3 ± 12.7 years; P = 0.009) than their surviving counterparts.
4. Discussion
To our knowledge, our study reports the largest case series of kidney transplant patients treated with anti-TNFα drugs. The clinical response rate noted in our study (13/16; 81.3%) is similar to that noted among all solid-organ transplant patients in the literature who received anti-TNFα treatment (29/36; 80.6%) (Tables 5 and 6).
Table 5.
Anti-TNFα treatment for inflammatory bowel disease in solid organ recipients, a review of the literature.
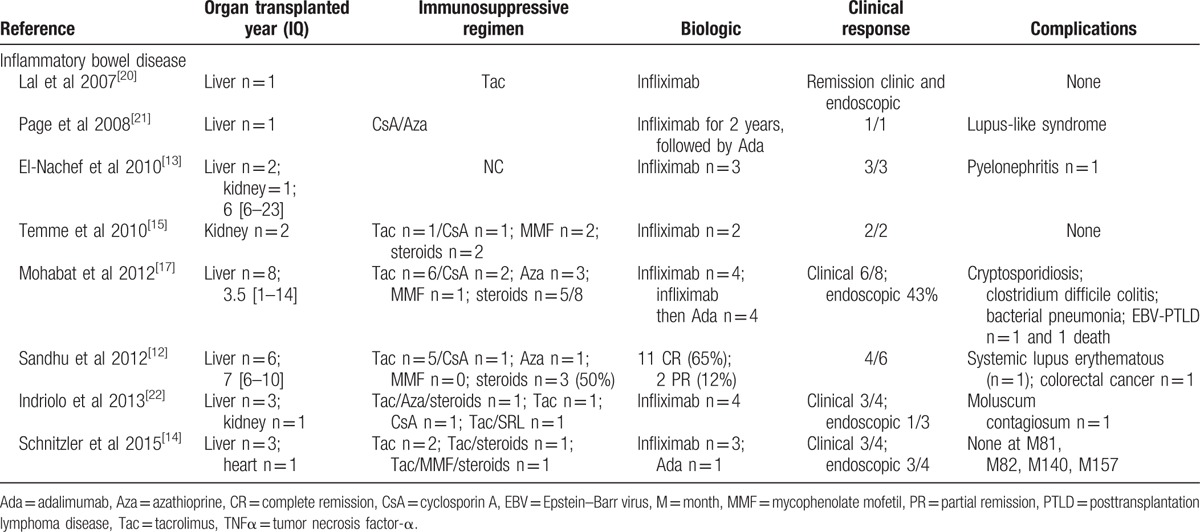
Table 6.
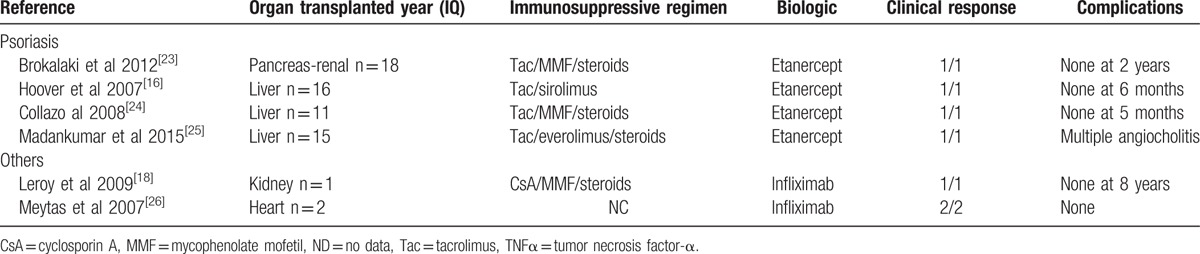
Anti-TNFα treatment for psoriasis or other indications in solid organ recipients, a review of the literature.
However, in our series, we observed a particularly high rate of severe infections (8/16, 50% of patients). Most of these infections were bacterial and resulted in permanent treatment discontinuation in 4 patients (Table 2) and death in 2 patients. These outcomes have rarely been reported in previous series. Only 1 study, which evaluated liver transplant patients who received anti-TNFα treatment for inflammatory bowel disease, observed a similar infection rate (3/8, 37.5%).[17] Combining the results of our series with those of previously reported series (Table 5) showed that 12/52 patients (23.2%) developed infections while receiving anti-TNFα treatment. Anti-TNFα therapy administration in patients who have not undergone organ transplantation is associated with an increased risk of bacterial infection,[6,7] viral reactivation (particularly herpesviruses),[8] and opportunistic infections, especially tuberculosis.[8] This risk seems to be heightened: during the first 3 months after anti-TNFα therapy initiation,[27] is also increased by the presence of underlying disease, and the combination of immunosuppressive treatment and corticosteroids >10 mg/day.[10] This was not observed in our series (Table 4). In addition to being more frequent among immunocompromised patients receiving anti-TNFα therapy, infections seem to be particularly severe in these patients. We noted a relationship between infection occurrence in patients receiving anti-TNFα therapy and patient death (4 of 5 patients died, P = 0.04). International guidelines[28] underscore the importance of detecting and treating tuberculosis. It may be necessary: to place kidney transplant patients on antiinfection prophylaxis upon anti-TNFα therapy initiation, in addition to surveilling these patients closely; and to decrease immusoppressive regimen dose (i.e., steroids).
In our study, 4 patients (25%) developed either solid tumors (n = 3) or hematologic malignancies (n = 1) (Table 2). No patients died as a direct result of these cancers following a median follow-up of 7 months (2–47). Two other cases of neoplasms following anti-TNFα therapy (6/52 organ transplant patients overall, 11.5%) have been reported in liver transplant patients, 1 case of Epstein–Barr-virus-associated lymphoma and 1 case of colorectal adenocarcinoma (Table 4). The risk of lymphoma and solid tumors (particularly tumors of the skin) is heightened after kidney transplantation, as well as in the setting of inflammatory bowel disease[11,29–33] and rheumatoid arthritis.[34–36] However, patients with spondyloarthritis do not appear to have an increased cancer risk.[37] The relationship between anti-TNFα therapy and cancer is unclear.[38] In patients with inflammatory bowel disease, anti-TNFα therapy may increase the risk of nonmelanoma skin cancer, although this risk is mainly related to thiopurine use,[39] as well as the risk of melanoma,[29] and reduce the incidence of colorectal adenocarcinoma by controlling bowel inflammation.[40] Patients with rheumatoid arthritis receiving anti-TNFα therapy seem to be at greater risk for nonmelanoma skin cancer[41] and melanoma,[42] whereas patients being treated for spondyloarthritis do not seem to have an increased cancer risk.[37] In our study, 2 patients developed breast cancer (−6 years) and basal-cell carcinoma (−9 years), respectively, before receiving anti-TNFα therapy. Neither of these patients relapsed after transplantation. Following kidney transplantation, the risk of cancer recurrence may reach 20% depending on the type of cancer.[43] Therefore, based on the cancer history of the patient, a waiting period may be necessary before kidney transplantation is authorized.[5] Initiating anti-TNFα therapy does not seem to contribute to subsequent cancer recurrence.[44,45]
We observed 1 case of a kidney graft loss (P#11, Table 2) at M18 secondary to antibody-mediated rejection in a patient treated with adalimumab for Crohn disease. No cases of organ rejection have been reported in the literature (Tables 5 and 6). TNFα and its receptors, TNFR1 and TNFR2, play important roles in kidney transplant rejection.[1] In animals, anti-TNFα therapy may prolong kidney graft survival in the event of rejection.[46] Thus, the abovementioned antibody-mediated rejection was probably unrelated to the use of this drug; thus, we cannot rule out autoimmune adalimumab-induced acute interstitial nephritis at M3.[47,48] Other autoimmune phenomena with renal manifestations have been reported in the setting of anti-TNFα therapy, namely, lupus[49–51] and glomerulonephritis.[52] In the literature, two cases of cutaneous lupus have been reported in liver transplant patients who received infliximab and adalimumab, respectively (Table 5), although neither patient presented with kidney involvement.
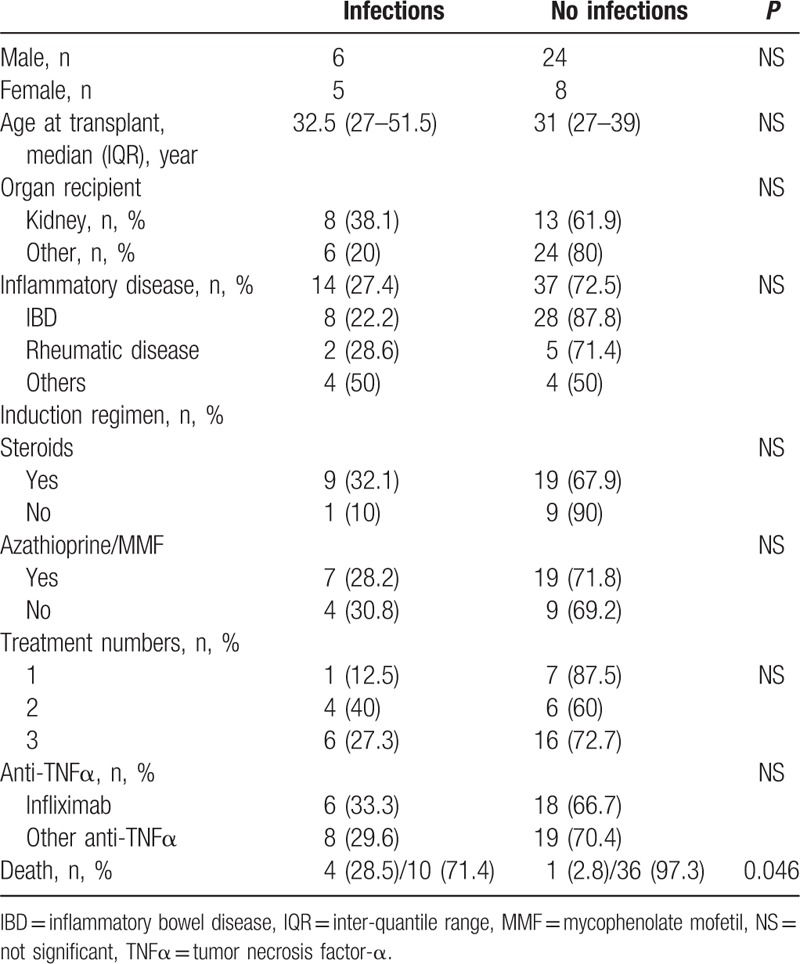
Our study had several limitations. First, the retrospective nature of our study may have resulted in an underestimation of the number of kidney transplant patients who received anti-TNFα therapy. Second, because of the small size of our series, the number of indications (chronic inflammatory bowel disease, chronic rheumatoid diseases, or others) for anti-TNFα therapy and the variety of drugs used, only patient age was identified as a risk factor for infection and death (Table 4). In the literature, age is an independent risk factor for infection and death in patients with chronic inflammatory diseases, regardless of whether or not these patients have been treated with biological therapy,[53] as well as in patients who have undergone kidney transplantation.[54] On univariate analysis, no other risk factors for infection (i.e., organs transplanted, indications, numbers of concomitant immunosuppressive treatments, or corticosteroid doses) were identified after reviewing all solid-organ transplant patients reported in the literature (Table 7).
Table 7.
Univariate analysis of infections in solid organ recipients treated with anti-TNFα therapy in the literature and in our study.
In conclusion, anti-TNFα therapies are effective for treating chronic inflammatory diseases in kidney transplant patients and do not lead to graft function deterioration. However, infection and cancer rates are particularly high among these immunocompromised patients. Regular screening for infection and cancer may thus be recommended for this at-risk population, in addition to antiinfection prophylaxis. Tailoring concomitant immunosuppressive therapy must be investigated in further studies to ensure that anti-TNFα therapy is safe in kidney transplant patients.
Footnotes
Abbreviations: GFR = glomerular filtration rate, TNFα = tumor necrosis factor-α.
The authors have no conflicts of interest to disclose.
References
- 1.Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int 2015; 87:281–296. [DOI] [PubMed] [Google Scholar]
- 2.Palladino MA, Bahjat FR, Theodorakis EA, et al. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov 2003; 2:736–746. [DOI] [PubMed] [Google Scholar]
- 3.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med 2007; 357:2601–2614. [DOI] [PubMed] [Google Scholar]
- 4.Nanau RM, Neuman MG. Safety of anti-tumor necrosis factor therapies in arthritis patients. J Pharm Pharm Sci 2014; 17:324–361. [DOI] [PubMed] [Google Scholar]
- 5.AlBugami M, Kiberd B. Malignancies: pre and post transplantation strategies. Transplant Rev (Orlando) 2014; 28:76–83. [DOI] [PubMed] [Google Scholar]
- 6.Pena-Sagredo JL, Hernandez MV, Fernandez-Llanio N, et al. Listeria monocytogenes infection in patients with rheumatic diseases on TNF-alpha antagonist therapy: the Spanish Study Group experience. Clin Exp Rheumatol 2008; 26:854–859. [PubMed] [Google Scholar]
- 7.Lanternier F, Tubach F, Ravaud P, et al. Incidence and risk factors of Legionella pneumophila pneumonia during anti-tumor necrosis factor therapy: a prospective French study. Chest 2013; 144:990–998. [DOI] [PubMed] [Google Scholar]
- 8.Winthrop KL, Baddley JW, Chen L, et al. Association between the initiation of anti-tumor necrosis factor therapy and the risk of herpes zoster. JAMA 2013; 309:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winthrop KL, Baxter R, Liu L, et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis 2013; 72:37–42. [DOI] [PubMed] [Google Scholar]
- 10.Salmon-Ceron D, Tubach F, Lortholary O, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis 2011; 70:616–623. [DOI] [PubMed] [Google Scholar]
- 11.Lebrec H, Ponce R, Preston BD, et al. Tumor necrosis factor, tumor necrosis factor inhibition, and cancer risk. Curr Med Res Opin 2015; 31:557–574. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu A, Alameel T, Dale CH, et al. The safety and efficacy of antitumour necrosis factor-alpha therapy for inflammatory bowel disease in patients post liver transplantation: a case series. Aliment Pharmacol Ther 2012; 36:159–165. [DOI] [PubMed] [Google Scholar]
- 13.El-Nachef N, Terdiman J, Mahadevan U. Anti-tumor necrosis factor therapy for inflammatory bowel disease in the setting of immunosuppression for solid organ transplantation. Am J Gastroenterol 2010; 105:1210–1211. [DOI] [PubMed] [Google Scholar]
- 14.Schnitzler F, Friedrich M, Stallhofer J, et al. Solid organ transplantation in patients with inflammatory bowel diseases (IBD): analysis of transplantation outcome and IBD activity in a large single center cohort. PLoS One 2015; 10:e0135807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Temme J, Koziolek M, Bramlage C, et al. Infliximab as therapeutic option in steroid-refractory ulcerative colitis after kidney transplantation: case report. Transplant Proc 2010; 42:3880–3882. [DOI] [PubMed] [Google Scholar]
- 16.Hoover WD. Etanercept therapy for severe plaque psoriasis in a patient who underwent a liver transplant. Cutis 2007; 80:211–214. [PubMed] [Google Scholar]
- 17.Mohabbat AB, Sandborn WJ, Loftus EV, Jr, et al. Anti-tumour necrosis factor treatment of inflammatory bowel disease in liver transplant recipients. Aliment Pharmacol Ther 2012; 36:569–574. [DOI] [PubMed] [Google Scholar]
- 18.Leroy S, Guigonis V, Bruckner D, et al. Successful anti-TNFalpha treatment in a child with posttransplant recurrent focal segmental glomerulosclerosis. Am J Transplant 2009; 9:858–861. [DOI] [PubMed] [Google Scholar]
- 19.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant 2008; 8:753–760. [DOI] [PubMed] [Google Scholar]
- 20.Lal S, Steinhart AH. Infliximab for ulcerative colitis following liver transplantation. Eur J Gastroenterol Hepatol 2007; 19:277–280. [DOI] [PubMed] [Google Scholar]
- 21.Page AV, Liles WC. Tumor necrosis factor-alpha inhibitor-induced lupus-like syndrome presenting as fever of unknown origin in a liver transplant recipient: case report and concise review of the literature. Transplant Proc 2008; 40:1768–1770. [DOI] [PubMed] [Google Scholar]
- 22.Indriolo A, Fagiuoli S, Pasulo L, et al. Letter: infliximab therapy in inflammatory bowel disease patients after liver transplantation. Aliment Pharmacol Ther 2013; 37:840–842. [DOI] [PubMed] [Google Scholar]
- 23.Brokalaki EI, Voshege N, Witzke O, et al. Treatment of severe psoriasis with etanercept in a pancreas-kidney transplant recipient. Transplant Proc 2012; 44:2776–2777. [DOI] [PubMed] [Google Scholar]
- 24.Collazo MH, Gonzalez JR, Torres EA. Etanercept therapy for psoriasis in a patient with concomitant hepatitis C and liver transplant. P R Health Sci J 2008; 27:346–347. [PubMed] [Google Scholar]
- 25.Madankumar R, Teperman LW, Stein JA. Use of etanercept for psoriasis in a liver transplant recipient. JAAD Case Rep 2015; 1:S36–S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metyas S, La D, Arkfeld DG. The use of the tumour necrosis factor antagonist infliximab in heart transplant recipients: two case reports. Ann Rheum Dis 2007; 66:1544–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnott ID, Watts D, Satsangi J. Azathioprine and anti-TNF alpha therapies in Crohn's disease: a review of pharmacology, clinical efficacy and safety. Pharmacol Res 2003; 47:1–10. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber S, Campieri M, Colombel JF, et al. Use of anti-tumour necrosis factor agents in inflammatory bowel disease. European guidelines for 2001–2003. Int J Colorectal Dis 2001; 16:1–11.discussion 2–3. [DOI] [PubMed] [Google Scholar]
- 29.Long MD, Martin CF, Pipkin CA, et al. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology 2012; 143:390.e1–399.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009; 374:1617–1625. [DOI] [PubMed] [Google Scholar]
- 31.Magro F, Peyrin-Biroulet L, Sokol H, et al. Extra-intestinal malignancies in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (III). J Crohns Colitis 2014; 8:31–44. [DOI] [PubMed] [Google Scholar]
- 32.Soderlund S, Brandt L, Lapidus A, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology 2009; 136:1561–1567.quiz 818-9. [DOI] [PubMed] [Google Scholar]
- 33.Castano-Milla C, Chaparro M, Gisbert JP. Systematic review with meta-analysis: the declining risk of colorectal cancer in ulcerative colitis. Aliment Pharmacol Ther 2014; 39:645–659. [DOI] [PubMed] [Google Scholar]
- 34.Baecklund E, Iliadou A, Askling J, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum 2006; 54:692–701. [DOI] [PubMed] [Google Scholar]
- 35.Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006; 295:2275–2285. [DOI] [PubMed] [Google Scholar]
- 36.Amari W, Zeringue AL, McDonald JR, et al. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford) 2011; 50:1431–1439. [DOI] [PubMed] [Google Scholar]
- 37.Hellgren K, Dreyer L, Arkema EV, et al. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis 2016; May 4. pii: annrheumdis-2016-209270. doi: 10.1136/annrheumdis-2016-209270. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-alpha therapy in inflammatory bowel disease. Aliment Pharmacol Ther 2014; 39:447–458. [DOI] [PubMed] [Google Scholar]
- 39.Peyrin-Biroulet L, Khosrotehrani K, Carrat F, et al. Increased risk for nonmelanoma skin cancers in patients who receive thiopurines for inflammatory bowel disease. Gastroenterology 2011; 141:1621.e1-5–1628.e1-5. [DOI] [PubMed] [Google Scholar]
- 40.Baars JE, Looman CW, Steyerberg EW, et al. The risk of inflammatory bowel disease-related colorectal carcinoma is limited: results from a nationwide nested case-control study. Am J Gastroenterol 2011; 106:319–328. [DOI] [PubMed] [Google Scholar]
- 41.Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016; 152:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raaschou P, Simard JF, Holmqvist M, et al. Rheumatoid arthritis, anti-tumour necrosis factor therapy, and risk of malignant melanoma: nationwide population based prospective cohort study from Sweden. BMJ 2013; 346:f1939. [DOI] [PubMed] [Google Scholar]
- 43.Penn I. The effect of immunosuppression on pre-existing cancers. Transplantation 1993; 55:742–747. [DOI] [PubMed] [Google Scholar]
- 44.Bernheim O, Axelrad J, Itzkowitz SH, et al. Previous cancer/lymphoma and refractory inflammatory bowel disease. Dig Dis 2015; 33 (Suppl 1):44–49. [DOI] [PubMed] [Google Scholar]
- 45.Axelrad J, Bernheim O, Colombel JF, et al. Risk of new or recurrent cancer in patients with inflammatory bowel disease and previous cancer exposed to immunosuppressive and anti-tumor necrosis factor agents. Clin Gastroenterol Hepatol 2016; 14:58–64. [DOI] [PubMed] [Google Scholar]
- 46.Imagawa DK, Millis JM, Seu P, et al. The role of tumor necrosis factor in allograft rejection. III. Evidence that anti-TNF antibody therapy prolongs allograft survival in rats with acute rejection. Transplantation 1991; 51:57–62. [DOI] [PubMed] [Google Scholar]
- 47.Korsten P, Sweiss NJ, Nagorsnik U, et al. Drug-induced granulomatous interstitial nephritis in a patient with ankylosing spondylitis during therapy with adalimumab. Am J Kidney Dis 2010; 56:e17–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshioka T, Yamakawa T, Yamaguchi M, et al. [Granulomatous interstitial nephritis in a patient with Behcet's disease treated with infliximab]. Nihon Jinzo Gakkai Shi 2013; 55:1412–1417. [PubMed] [Google Scholar]
- 49.Williams VL, Cohen PR. TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists. Int J Dermatol 2011; 50:619–625. [DOI] [PubMed] [Google Scholar]
- 50.Shakoor N, Michalska M, Harris CA, et al. Drug-induced systemic lupus erythematosus associated with etanercept therapy. Lancet 2002; 359:579–580. [DOI] [PubMed] [Google Scholar]
- 51.Williams EL, Gadola S, Edwards CJ. Anti-TNF-induced lupus. Rheumatology (Oxford) 2009; 48:716–720. [DOI] [PubMed] [Google Scholar]
- 52.Stokes MB, Foster K, Markowitz GS, et al. Development of glomerulonephritis during anti-TNF-alpha therapy for rheumatoid arthritis. Nephrol Dial Transplant 2005; 20:1400–1406. [DOI] [PubMed] [Google Scholar]
- 53.Lahiri M, Dixon WG. Risk of infection with biologic antirheumatic therapies in patients with rheumatoid arthritis. Best Pract Res Clin Rheumatol 2015; 29:290–305. [DOI] [PubMed] [Google Scholar]
- 54.Knoll GA. Kidney transplantation in the older adult. Am J Kidney Dis 2013; 61:790–797. [DOI] [PubMed] [Google Scholar]


