Abstract
The Asia-Pacific Colorectal Screening (APCS) score is a risk-stratification tool that helps predict the risk for advanced colorectal neoplasia (ACN) in asymptomatic Asian populations, but has not yet been assessed for its validity of use in Mainland China.
The aim of the study was to assess the validity of APCS score in asymptomatic Chinese population, and to identify other risk factors associated with ACN.
Asymptomatic subjects (N = 1010) who underwent colonoscopy screening between 2012 and 2014 in Beijing were enrolled. APCS scores based on questionnaires were used to stratify subjects into high, moderate, and average-risk tiers. Cochran–Armitage test for trend was used to assess the association between ACN and risk tiers. Univariate and multivariate logistic regression was performed with ACN as the outcome, adjusting for APCS score, body mass index, alcohol consumption, self-reported diabetes, and use of nonsteroidal anti-inflammatory drugs as independent variables.
The average age was 53.5 (standard deviation 8.4) years. The prevalence of ACN was 4.1% overall, and in the high, moderate, and average-risk tiers, the prevalence was 8.8%, 2.83%, and 1.55%, respectively (P < 0.001). High-risk tier had 3.3 and 6.1-fold increased risk of ACN as compared with those in the moderate and average-risk tiers, respectively. In univariate analysis, high-risk tier, obesity, diabetes, and alcohol consumption were associated with ACN. In multivariate analysis, only high-risk tier was an independent predictor of ACN.
The APCS score can effectively identify a subset of asymptomatic Chinese population at high risk for ACN. Further studies are required to identify other risk factors, and the acceptability of the score to the general population will need to be further examined.
Keywords: APCS score, Chinese population, screening for colorectal cancer
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in men, and the second most common cancer in women. It is a leading cause of cancer-related mortality and morbidity worldwide.[1] CRC has traditionally been one of the commonest cancers in western populations, such as those in Europe and North America. However, there has been a rapid increase in CRC incidence and its associated mortality in Asia. China has experienced a 2 to 4-fold increase in the incidence of CRC in the past 1 decade.[2] Screening for CRC has been proven effective to reduce CRC-related mortality.[3,4] However, CRC screening is hindered due to concerns on resources, especially the limited colonoscopic capacity in many Asia-Pacific cities. Therefore, a practical screening tool to risk-stratify subjects is called for to make CRC screening more cost-effective.
The Asia-Pacific Colorectal Screening (APCS) score, developed in 2011, is a validated risk-stratification tool that helps identify individuals at risk for advanced colorectal neoplasm (ACN) amongst the asymptomatic population, which has been promulgated as an instrument to prioritize subjects for colorectal screening.[5] The score is based on elementary clinical information such as age, sex, family history, and smoking status. It is a simple, practical tool that can be used by family physicians, healthcare providers, and nurse-educators.
The validity of the APCS scoring system for predicting ACN risk in asymptomatic Asian subjects has been demonstrated.[5] However, the tool does not take into account body mass index (BMI) and other potential risk factors. A scoring system based on age, sex, smoking, family history, BMI, and self-reported diabetes[6] was recently developed for predicting the risk of colorectal neoplasm, instead of that for ACN. The latter bears a greater malignant potential, and is of greater clinical relevance. A large-scale study (N = 5220)[7] of asymptomatic Asian subjects who underwent screening colonoscopy evaluated some risk factors in addition to those incorporated in APCS system. The study identified alcohol consumption, hypertension, and BMI as being independent predictors of ACN, which could be incorporated into the APCS for prioritizing Asian asymptomatic subjects for CRC screening.
The discriminatory capability of APCS has been demonstrated in the validation cohort of the original study by Yeoh et al[5] in 2011. The study called for external validation of the APCS score in other population groups, and the validity of this tool for use in Mainland Chinese is yet to be tested. The present study aimed to evaluate the efficacy of APCS scoring system for CRC screening in a large cohort of asymptomatic subjects who underwent screening colonoscopy in Beijing, China. Furthermore, we explored other independent risk factors for ACN in Mainland China, including BMI, diabetes, alcohol intake, and use of non-steroidal anti-inflammatory drugs (NSAIDs).
2. Materials and methods
2.1. Study aims
The aim of the present study was 2-fold. Our primary aim was to assess the validity of APCS score as a risk-prediction score for ACN in asymptomatic Chinese population. Our further aim was to identify other risk factors associated with ACN.
2.2. Study participants
From September 2012 to December 2014, asymptomatic individuals aged 40 to 75 years were prospectively enrolled for CRC screening in the Peking Union Medical College Hospital (PUMCH) and the Beijing Friendship Hospital and Beijing Chaoyang Hospital. Individuals with present or past symptoms suggestive of CRC or lower gastrointestinal tract disease, such as hematochezia, anorexia, recent change in bowel habits, lower abdominal pain, or weight loss were excluded. Those with a history of CRC, colonic adenoma, diverticular disease, inflammatory bowel disease, or other contraindications to colonoscopy were also excluded. Written informed consent was obtained from all the participants before their enrollment in the study.
2.3. Study design
This study was approved by the Ethical Committee of the PUMCH. We set out to conduct a prospective, multicenter study in Chinese asymptomatic subjects based on questionnaires and colonoscopy findings, to assess the validity of APCS score, and also to identify other risk factors associated with ACN. The registration number of our study on “Chinese Clinical Trial Registry” was ChiCTR-SOD-16008774.
2.4. Questionnaire
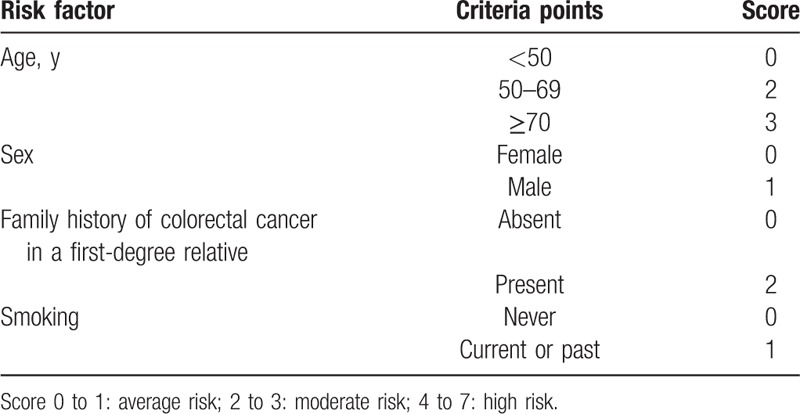
Eligible participants completed a self-administered questionnaire with the assistance of medical staff and trained volunteers. The questionnaire captured basic demographic variables, and clinical and lifestyle-related information, including age, sex, height, weight, family history of CRC in a first degree relative, smoking status (both current and past), alcohol consumption, chronic diseases including diabetes, previous medical history, and long-term medication use including NSAIDS. Alcohol consumption was defined as intake of >2 drinks per week. Use of NSAIDS was defined as continuous use of NSAIDS, including aspirin, in the past 6 months. The development process of the questionnaire was described as follows: firstly, the questionnaire should cover all the participants’ personal information such as basic demographic variables. Secondly, risk factors of ACN included in APCS score (Table 1)[5] was directly brought into our questionnaire, including age, sex, family history of CRC, and smoking. Finally, to indentify other risk factors associated with ACN, all the factors which may be associated with ACN, as far as we know, were included in the questionnaire, such as lifestyle-related information, smoking status, alcohol consumption, diabetes, and long-term medication use including NSAIDS.
Table 1.
Asia-Pacific colorectal screening score.
2.5. Colonoscopy
Colonoscopy was performed by experienced endoscopists, who were blinded to the APCS score. The Olympus CF-HQ190 colonoscope (Olympus Optical, Tokyo, Japan) was used in the study. Each individual underwent standard bowel preparation before endoscopy. In the event of poor bowel preparation, colonoscopy was performed again after repeat bowel preparation. The withdrawal observation time was ≥6 minutes to minimize the possibility of missing the colonic lesions benchmarking with the international standard of quality assurance for colonoscopy procedures. The size, location, number of lesions, and histological findings were recorded. Colorectal neoplasia includes adenoma and advanced colorectal neoplasia (ACN). ACN was defined as CRC or advanced adenoma. Advanced adenoma was defined as adenomas ≥10 mm in diameter, with villous histological features (at least 25% villous), or with high-grade dysplasia,[8] or any combination thereof.
2.6. Statistical analysis
All statistical analyses were performed with SPSS software (version 17.0). Percentages were reported as proportions and 95% confidence interval (CI). Continuous variables were expressed as mean ± standard deviation (SD) in case of parametric distribution, and median (quartile1 [Q1] − quartile3 [Q3]) in case of abnormal distribution.
Our primary aim was to assess the validity of APCS score as a risk-prediction score for ACN in asymptomatic Chinese population. Firstly, APCS scores were calculated for each subject on the basis of their age, sex, family history, and smoking status (Table 1).[5] Based on the APCS score, subjects were stratified into 3 groups (average risk [AR], score 0–1; moderate risk [MR], score 2–3; high risk [HR], score 4–7). Cochran–Armitage test for trend was used to assess the associations between the proportion of ACN in each APCS risk tier. Logistic regression analysis was performed to assess the relative risk of ACN in HR group and MR group compared with AR group. Furthermore, Pearson chi-square test was used to test the difference of the proportion of ACN between each of the 2 tiers (HR and MR, HR and AR, MR and AR). A 2-tailed P value <0.05 was considered as statistically significant.
Our secondary aim was to identify other risk factors associated with ACN. For this aim, outcome variables included the proportion of screening participants whose colonoscopy findings indicated ACN. The major covariate included the APCS score as a single variable. Additional variables tested for association with the colonoscopic outcome of ACN included BMI (underweight: <18.5; normal: 18.5–23; overweight: 23–25; obese: ≥25; the BMI cut-off points were used according to the recognized definition of obesity among Asian subjects[9]); smoking (current smokers or ex-smokers vs nonsmokers); alcohol consumption (current or past alcohol consumers vs nonconsumers); self-reported history of diabetes mellitus; and use of NSAIDs. Logistic regression analysis was performed to assess the risk factors associated with ACN. A univariate analysis was conducted between ACN and each covariate consecutively. All covariates were included into a binary logistic regression model if the initial P value was <0.1 in the univariate analysis. All variables selected in the multivariate regression analysis were detected for the presence of interactions.
3. Results
3.1. Subject characteristics
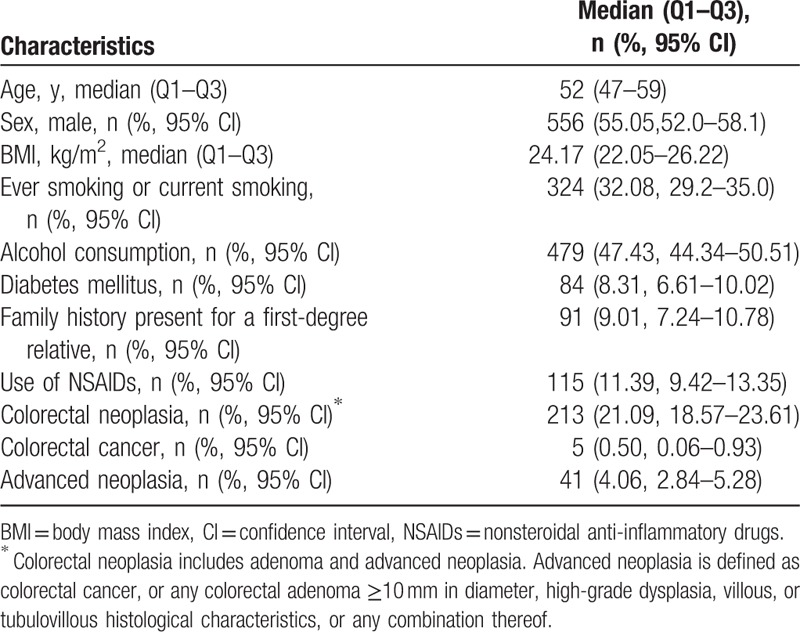
A total of 1010 participants were included, where 55.1% was men. The average age of the subjects was 52 years (Q1–Q3, 47–59 years); the average BMI was 24.17 kg/m2 (Q1–Q3, 22.05–26.22 kg/m2); 32.08% were current or past smokers; and 47.43% were alcohol drinkers. Ninety-one patients (9.0%) had a family history of at least 1 first-degree relative with CRC and 115 (11.4%) had chronic use of NSAIDs. A total of 213 (21.9%) cases of colorectal neoplasia were detected, including 5 subjects (0.5%) with colorectal cancer and 41 subjects (4.1%) with ACN (Table 2).
Table 2.
Characteristics of study participants (N = 1010).
3.2. Risk stratification by APCS score
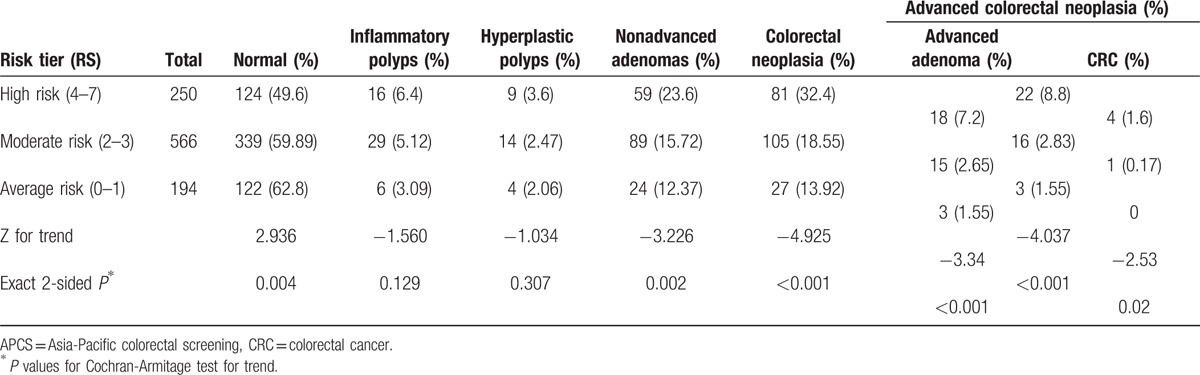
Our primary aim was to assess the validity of APCS score as a risk-prediction score for ACN in asymptomatic Chinese population. Based on the APCS score, 194 subjects (19.2%) were classified as belonging to the AR tier (score 0–1), 566 (56.0%) to the MR tier (score 2–3), and 250 (24.75%) subjects to the HR tier (score 4–7). The prevalence of ACN in the AR, MR, and HR categories was 1.6%, 2.8%, and 8.8%, respectively (P < 0.001). ACN included advanced adenoma and CRC. With the same trend as ACN, the prevalence of advanced adenoma in the AR, MR, and HR tiers was 1.6%, 2.65%, and 7.2%, respectively (P < 0.001); the prevalence of CRC in the AR, MR, and HR tiers was 0%, 0.17%, and 1.6%, respectively (P < 0.001). Similarly, the prevalence of colorectal neoplasia in the AR, MR, and HR tiers was 13.92%, 18.55%, and 32.4%, respectively (P < 0.001). Likewise, the prevalence of nonadvanced adenomas in the AR, MR, and HR tiers was 12.37%, 15.72%, and 23.6%, respectively (P < 0.05). However, there was no significant difference in the prevalence of inflammatory polyps and hyperplastic polyps in the HR, MR, and AR tiers (P = 0.307, P = 0.129, respectively) (Table 3).
Table 3.
Prevalence of colorectal neoplasia and colorectal advanced neoplasia by risk tier.
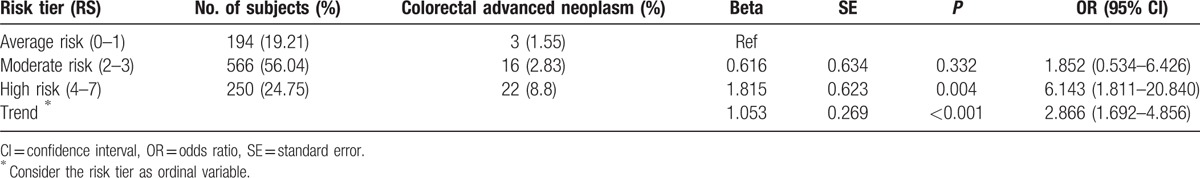
By logistic regression analysis, subjects in the HR tier and MR tier were at a 6.1-fold (95% CI 1.8–20.8, P = 0.004) and 1.9-fold (95% CI 0.5–6.4, P = 0.332) higher risk of ACN as compared with those in the AR (Table 4).
Table 4.
Prevalence of colorectal advanced neoplasia by risk tier and risk score.
To test the difference of the proportion of ACN of between each of the 2 tiers, Pearson chi-square test was used. The prevalence of ACN in HR was significantly higher than MR (P < 0.001) and AR (P = 0.001); however, there was no significant difference in the prevalence of ACN between MR and AR (P = 0.430) (Fig. 1).
Figure 1.
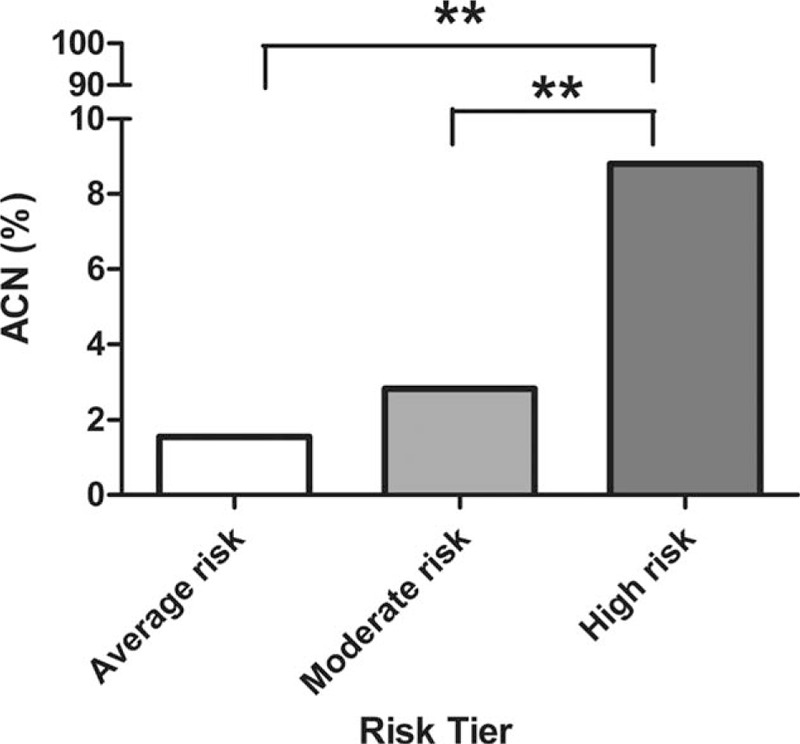
Prevalence of colorectal advanced neoplasia by risk tier. ACN = advanced colorectal neoplasia.
3.3. Factors associated with advanced colorectal neoplasia
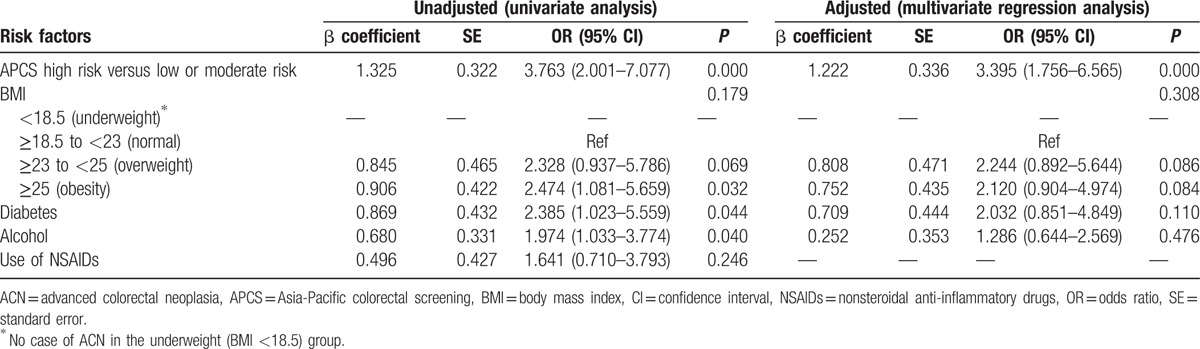
Our secondary aim was to identify other risk factors associated with ACN. From univariate analysis, APCS score (P = 0.001), obesity (P = 0.032), self-reported diabetes (P = 0.044), and alcohol consumption (P = 0.040) were significantly associated with ACN. Next, all covariates were included into a binary logistic regression model if the initial P value was <0.1 in the univariate analysis. However, in the multivariate regression model, only APCS score (adjusted odds ratio [OR] 3.395, 95% CI 1.756–6.565, P = 0.000) was found to be independently associated with ACN (Table 5).
Table 5.
Predictors of ACN on univariate and multivariate analyses.
4. Discussion
The incidence and mortality rate of CRC in Asian countries continue to increase at a rapid rate without any signs of plateau. Although robust data on the incidence and prevalence of CRC in China are lacking, according to the Chinese National Cancer Database of 2003, CRC was 1 of the 3 cancers with most rapidly increasing incidence (together with lung cancer and female breast cancer) between 1991 and 2005.[10] Lifestyle, including preference for western-style diet, physical inactivity, ethnic influences, and population migration, may have contributed to the remarkable rise in Asia.[2]
There is strong evidence that CRC screening is cost-effective[3,4] and improves survival.[11–13] Two common screening tests used for CRC screening, fecal occult blood test,[11–14] and colonoscopy[15] have been shown to be of value in significantly reducing CRC mortality, either by early detection of cancer at an early stage, or by removal of adenomatous polyps.[16] Current international guidelines from the US,[17] UK,[18] and Europe,[19,20] together with the Asia-Pacific Consensus statement,[21] recommend CRC screening in individuals >50 years of age who are at an average risk for ACN.
Nevertheless, CRC screening is a resource-intensive activity requiring equipment and necessary infrastructure, in addition to trained medical personnel,[22] which limits its wider use in deprived settings owing to the resource constraints.[23,24] Moreover, lack of awareness in the target population, inadequate advocacy by healthcare professionals, and poor compliance[25–30] are some of the other barriers that have hampered the implementation of CRC screening programs. Screening guidelines for western countries are not feasible to implement in China owing to the large population base and inadequate resource allocation for CRC screening.
The APCS score system was built based on the higher efficiency of risk-based screening in the Asia-Pacific region. Risk stratification can help improve cost-effectiveness of screening by prioritizing high-risk subjects for colonoscopy, whereas lower-risk subjects could opt for fecal tests. However, the applicability of APCS scoring system in Mainland China is yet to be established, which is now addressed by this study. Therefore, we evaluated the efficacy of APCS score as a tool to help prioritize asymptomatic Chinese population for CRC screening. Further, we also assessed other risk factors associated with ACN. Asymptomatic subjects were stratified into 3 tiers—average risk, moderate risk, and high risk—and all subjects underwent colonoscopy.
The prevalence of ACN was found to be 4.1%, which is consistent with the findings from the APCS study.[5] Moreover, the prevalence of ACN was similar to that in the validation cohort (P = 0.160) and the derivation cohort (P = 0.647). The estimated prevalence of ACN among Asian population is between 3% and 12%.[31–33] In a recent cross-sectional study conducted across multiple endoscopy units in America (N = 2993 in the derivation set), the prevalence of advanced neoplasia was 9.4%.[34] Thus, the prevalence of ACN in Chinese population seems to be comparable with that in other Asian populations, and is different from that in the western countries. This implies that the population under study could be similar to CRC screening participants in other Asian countries.
In our study, subjects in the HR tier were at a 6.14-fold increased risk of ACN as compared with those in the AR tier, which is comparable to the corresponding figures reported from Hong Kong (4.3-fold increased risk in the HR tier). This finding further reinforces the applicability of APCS scoring system to populations in Mainland China. Moreover, on univariate and multivariate logistic regression analyses, APCS score was found to be a strong predictor for ACN. Subjects in the high-risk tier had a 3.8-fold increased risk for developing ACN on univariate analysis (P < 0.000), and a 3.3-fold increased risk on multivariate analysis (P < 0.000), implying that the APCS score can independently predict the risk of ACN in the Chinese population. However, MR tier was not significantly associated with ACN risk when compared with the AR tier (P = 0.430), which is different from that reported from Hong Kong (MR tiers had 4.3-fold risk of ACN). In the MR tier, population aged between 50 and 69 years (n = 391) accounted for 69% of the subject population (n = 566), indicating that a large proportion of participants in the 50 to 69-year age group in our study belonged to the MR tier. Owing to the lack of data, we could not compare the proportion of 50 to 69-year-old subjects in the MR tier in our study with the corresponding proportion in the APCS study. It is, however, conceivable that the differences in population age structure could have influenced the observed associations in our study. Owing to this possibility, the age stratification in the APCS score system may need to be adapted to the Chinese Mainland context. Further studies are required to assess the need for refining age considerations.
In the present study, we analyzed the association between other potential risk factors for ACN. Obesity (BMI ≥25, OR 2.474, 95% CI 1.081–5.659, P = 0.032), the presence of self-reported diabetes mellitus (OR 2.385, 95% CI 1.023–5.559, P = 0.044), and alcohol consumption (OR 1.974, 95% CI 1.033–3.774, P = 0.040) were found to be associated with ACN on univariate analysis, but not with multivariate analysis.
The relationship between BMI and colorectal neoplasia has been assessed in a few studies conducted in Western subjects. Frezza et al[35] reported that obesity conferred a greater risk of colon cancer among men of all ages and in premenopausal women, than it does for postmenopausal women. Furthermore, insulin appeared to be the most consistent biochemical mediator between obesity and colon cancer.[35] In a prospective cohort study of 33,403 African-American women, those with BMI ≥35 were found to be at an increased risk of colon polyps as compared with subjects with BMI <25.[36] In Asian populations—a large cohort study of 5220 asymptomatic subjects in Hong Kong—reported a 1.55-fold increased risk of ACN among obese subjects.[7] Similar published data for Mainland China were not available. In the present study, obesity raised the risk of developing ACN by 2.474-fold in univariate analysis, but failed to predict ACN independently after multivariate analysis. The association between obesity and ACN may be explained by common risk factors such as diabetes, which, to some extent, negates the independent predictive ability of obesity for ACN.
Diabetes has been widely recognized as a risk factor for colorectal neoplasia. In a systematic review of 16 cohort studies and 8 case-control studies with more than 3.6 million subjects, diabetes was associated with a 1.26-fold increased risk for CRC.[37] In the present study, diabetes was associated with a 2.38-fold increased risk of ACN. However, the association was not observable on multivariate logistic regression analyses, which may be related to the weak association between diabetes and ACN on univariate analysis (P = 0.044) and the multicolinearity with other factors such as obesity and alcohol consumption. Apart from this, diabetes was self-reported, and different populations may either over or under-report this medical condition in varying degrees due to the absence of symptoms among the patients.
The association between alcohol consumption and ACN is not clearly understood, although it is widely recognized that alcohol consists of many carcinogenic compounds. In a pooled analysis of primary data from 8 cohort studies in North America and Europe (N > 489,000), alcohol intake correlated with a modest elevation of CRC rate, mainly at the highest levels of alcohol intake.[38] A cross-sectional study in a screening population conducted in United States found that whereas there was a more than 2-fold increased risk of screen-relevant colorectal neoplasia in people who consumed spirits and beer, people who consumed wine had a lower risk.[39] In a large cohort study conducted in Hong Kong (N = 5220), alcohol consumers were reported as having a 50% higher risk of ACN than nonconsumers.[34] The present study showed a significant relationship between alcohol consumption and development of ACN on univariate analysis (P = 0.040); however, the association did not retain its significance on multivariate analysis (P = 0.481). Many mutually shared risk factors such as diabetes and obesity may have compromised the independent predictive ability of alcohol for ACN.
A large body of epidemiological evidence shows that regular use of NSAIDs, including aspirin, over a 10 to 15-year period, reduces the relative risk of developing colorectal cancer and adenomas by 40% to 50%.[40] Recent prospective population-based studies have also corroborated the association of long-term use of NSAIDs with a lower risk for CRC.[41–43] In our study, use of NSAIDS did not correlate with the risk for developing ACN, both on univariate and multivariate analyses. The discrepancy could be attributable to the fact that the earlier studies involved long-term use of NSAIDS, and also that the protective effect of NSAIDs is dose-dependent. In our study, we did not define the duration and dose of NSAIDS intake and hence might not fulfill the criteria of long-term and high-dose use of NSAIDS. There is an absence of evidence demonstrating the effectiveness of low-dose, intermittent use of NSAIDS in the reduction of CRC risk.
Some limitations in our study need to be considered. Subjects in our study were recruited from only 3 medical centers in Beijing, and thus may not be representative of the wider Chinese population. Secondly, the age range of participants in our study was between 40 and 75 years, whereas the APCS study excluded subjects <50 years of age. Moreover, in our statistical analysis, we have not incorporated all potential confounders in the multivariate regression models. Obesity, alcohol, and diabetes were associated with risk of ACN on univariate analysis, indicating the relationship of those factors with ACN. Further studies with larger sample size are needed so that more potential covariate may be accounted for to improve the validity of the multivariate analysis. In addition, there were some gaps in collecting data on lifestyle-related variables. We did not collect detailed information on the frequency of intake and the total consumption of alcohol. Further, data on the dosage and duration of use of NSAIDs were also not included. Lastly, information related to diabetes was self-reported, which is likely to have introduced a certain degree of bias.
5. Conclusions
The APCS score system was demonstrated to be effective in prioritizing asymptomatic Chinese population for CRC screening. On univariate analysis, obesity, diabetes, and alcohol consumption were found to be associated with ACN; however, on multivariate analysis, only APCS was a significant risk factor. Further studies are required to determine alternative risk factors that may be associated with CRC in the Chinese population, and the preliminary validity of the APCS score should be studied in parallel with the acceptability and user-friendliness of using the score in screening practices.
Acknowledgments
We would like to acknowledge the contribution of Professor Joseph J.Y. Sung and his research team in the Chinese University of Hong Kong for intellectual input in this study. We also appreciate the instructive suggestions of Professor Martin C.S. Wong for this paper. We are grateful to the Asia-Pacific Working Group on Colorectal Cancer for their support for this study. We expressed our gratitude for the professional statistical guidance from Professor Li Zhang in the Department of Epidemiology and Biostatistics of Peking Union Medical College.
Footnotes
Abbreviations: ACN = advanced colorectal neoplasia, APCS score = Asia-Pacific Colorectal Screening score, AR = average risk, BMI = body mass index, CI = confidence interval, CRC = colorectal cancer, HR = high risk, MR = moderate risk, NSAIDs = non-steroidal anti-inflammatory drugs, OR = odds ratio, SD = standard deviation.
Funding: The special funding of capital health research and development, No. 2011-4001-01, Beijing Municipal Government, China, funded this study. The website was www.bjhbkj.com. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors report no conflicts of interest.
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Sung JJ, Lau JY, Goh K, et al. Increasing incidence of colorectal cancer in Asia: implications for screening. Lancet Oncol 2005; 6:871–876. [DOI] [PubMed] [Google Scholar]
- 3.Heitman SJ, Hilsden RJ, Au F, et al. Colorectal cancer screening for average-risk North Americans: an economic evaluation. PLoS Med 2010; 7:1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsoi K, Ng S, Leung M, et al. Cost-effectiveness analysis on screening for colorectal neoplasm and management of colorectal cancer in Asia. Aliment Pharmacol Therapeut 2008; 28:353–363. [DOI] [PubMed] [Google Scholar]
- 5.Yeoh K-G, Ho K-Y, Chiu H-M, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011; 2010:221168. [DOI] [PubMed] [Google Scholar]
- 6.Wong MC, Lam TY, Tsoi KK, et al. A validated tool to predict colorectal neoplasia and inform screening choice for asymptomatic subjects. Gut 2014; 63:1130–1136. [DOI] [PubMed] [Google Scholar]
- 7.Wong MC, Lam TY, Tsoi KK, et al. Predictors of advanced colorectal neoplasia for colorectal cancer screening. Am J Prevent Med 2014; 46:433–439. [DOI] [PubMed] [Google Scholar]
- 8.Imperiale TF, Wagner DR, Lin CY, et al. Results of screening colonoscopy among persons 40 to 49 years of age. N Engl J Med 2002; 346:1781–1785. [DOI] [PubMed] [Google Scholar]
- 9.WHO.I., IOTF. The Asia-Pacific Perspective. Redefining Obesity and Its Treatment. Obesity: preventing and managing the global epidemic. Geneva: WHO; 2000. [Google Scholar]
- 10.Yang L, Parkin D, Li L, et al. Estimation and projection of the national profile of cancer mortality in China: 1991–2005. Br J Cancer 2004; 90:2157–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. N Engl J Med 1993; 328:1365–1371. [DOI] [PubMed] [Google Scholar]
- 12.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348:1472–1477. [DOI] [PubMed] [Google Scholar]
- 13.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348:1467–1471. [DOI] [PubMed] [Google Scholar]
- 14.Faivre J, Dancourt V, Lejeune C, et al. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 2004; 126:1674–1680. [DOI] [PubMed] [Google Scholar]
- 15.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology∗†. CA Cancer J Clin 2008; 58:130–160. [DOI] [PubMed] [Google Scholar]
- 17.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol 2009; 104:739–750. [DOI] [PubMed] [Google Scholar]
- 18.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010; 59:666–689. [DOI] [PubMed] [Google Scholar]
- 19.Steele R, Pox C, Kuipers E, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Endoscopy 2012; 10:0032–1309802. [DOI] [PubMed] [Google Scholar]
- 20.Steele R, Rey J, Lambert R. European guidelines for quality assurance in colorectal cancer screening and diagnosis: professional requirements and training. Endoscopy 2012; 44:SE106–SE115. [DOI] [PubMed] [Google Scholar]
- 21.Sung J, Ng S, Chan F, et al. An updated Asia Pacific Consensus Recommendations on colorectal cancer screening. Gut 2015; 64:121–132. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman DA, Rex DK. Feasibility of colonoscopy screening: discussion of issues and recommendations regarding implementation. Gastrointest Endosc 2001; 54:662–667. [DOI] [PubMed] [Google Scholar]
- 23.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: data from the National Cancer Institute Survey of Colorectal Cancer Screening Practices. Am J Med 2003; 115:129–133. [DOI] [PubMed] [Google Scholar]
- 24.Levin TR. Colonoscopy capacity: can we build it? Will they come? Gastroenterology 2004; 127:1841–1844. [DOI] [PubMed] [Google Scholar]
- 25.Emmons K, Puleo E, McNeill LH, et al. Colorectal cancer screening awareness and intentions among low income, sociodemographically diverse adults under age 50. Cancer Causes Control 2008; 19:1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimeno-García AZ, Quintero E, Nicolás-Pérez D, et al. Impact of an educational video-based strategy on the behavior process associated with colorectal cancer screening: a randomized controlled study. Cancer Epidemiol 2009; 33:216–222. [DOI] [PubMed] [Google Scholar]
- 27.Kamposioras K, Mauri D, Alevizaki P, et al. Cancer screening in Greece. Guideline awareness and prescription behavior among Hellenic physicians. Eur J Intern Med 2008; 19:452–460. [DOI] [PubMed] [Google Scholar]
- 28.Domati F, Travlos E, Cirilli C, et al. Attitude of the Italian general population towards prevention and screening of the most common tumors, with special emphasis on colorectal malignancies. Intern Emerg Med 2009; 4:213–220. [DOI] [PubMed] [Google Scholar]
- 29.Inadomi JM. Taishotoyama Symposium Barriers to colorectal cancer screening: economics, capacity and adherence. J Gastroenterol Hepatol 2008; 23 (s2):S198–S204. [DOI] [PubMed] [Google Scholar]
- 30.Cai S-R, Zhang S-Z, Zhu H-H, et al. Barriers to colorectal cancer screening: a case-control study. World J Gastroenterol 2009; 15:2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sung JJ, Chan FK, Leung WK, et al. Screening for colorectal cancer in Chinese: comparison of fecal occult blood test, flexible sigmoidoscopy, and colonoscopy. Gastroenterology 2003; 124:608–614. [DOI] [PubMed] [Google Scholar]
- 32.Soon M-S, Kozarek RA, Ayub K, et al. Screening colonoscopy in Chinese and Western patients: a comparative study. Am J Gastroenterol 2005; 100:2749–2755. [DOI] [PubMed] [Google Scholar]
- 33.Chiu H-M, Wang H-P, Lee Y-C, et al. A prospective study of the frequency and the topographical distribution of colon neoplasia in asymptomatic average-risk Chinese adults as determined by colonoscopic screening. Gastrointest Endosc 2005; 61:547–553. [DOI] [PubMed] [Google Scholar]
- 34.Imperiale TF, Monahan PO, Stump TE, et al. Derivation and validation of a scoring system to stratify risk for advanced colorectal neoplasia in asymptomatic adults. Ann Intern Med 2015; 163:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut 2006; 55:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise LA, Rosenberg L, Palmer JR, et al. Anthropometric risk factors for colorectal polyps in African-American women. Obesity 2008; 16:859–868. [DOI] [PubMed] [Google Scholar]
- 37.Deng L, Gui Z, Zhao L, et al. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Digest Dis Sci 2012; 57:1576–1585. [DOI] [PubMed] [Google Scholar]
- 38.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med 2004; 140:603–613. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JC, Alpern Z, Sethi G, et al. Prevalence and risk of colorectal neoplasia in consumers of alcohol in a screening population. Am J Gastroenterol 2005; 100:2049–2055. [DOI] [PubMed] [Google Scholar]
- 40.Smalley WE, DuBois RN. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol (San Diego, CA) 1997; 39:1–20. [DOI] [PubMed] [Google Scholar]
- 41.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007; 369:1603–1613. [DOI] [PubMed] [Google Scholar]
- 42.Chan AT, Arber N, Burn J, et al. Aspirin in the chemoprevention of colorectal neoplasia: an overview. Cancer Prevent Res 2012; 5:164–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibrahim Halil S, Hassan MM, Garrett CR. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: current state-of-the science. Cancer Lett 2014; 345:249–257. [DOI] [PubMed] [Google Scholar]


