Abstract
Background:
The diagnostic accuracy of interleukin-6 (IL-6) in predicting bacterial infection in cirrhotic patients remains unclear. The aim of this meta-analysis is to explore the potential diagnostic value of IL-6 in cirrhotic patients.
Methods:
We systematically searched PubMed, Embase (via OvidSP), Web of Science, the Cochrane Library, and Scopus for studies published from inception to October 2015. Studies were enrolled if they included assessment of the accuracy of IL-6 in the diagnosis of bacterial infection in cirrhotic patients and provided sufficient data to construct a 2 × 2 contingency table.
Results:
Totally, 535 studies were searched in the initial database and finally 6 studies involving 741 patients were included for the final analysis. The pooled sensitivity, specificity and diagnostic odds ratio were 0.85 (95% confidence interval [CI], 0.64–0.94), 0.91 (95% CI, 0.80–0.96) and 52.89 (95% CI, 15.21–183.86), respectively. The pooled positive likelihood ratio was 8.99 (95% CI, 4.13–19.55) and the pooled negative likelihood ratio was 0.17 (95% CI, 0.07–0.43). The area under the receiver operating characteristic curve was 0.94 (95% CI, 0.92–0.96).
Conclusion:
This meta-analysis suggests IL-6 has a high diagnostic value for the differentiation of bacterial infection in patients with cirrhosis.
Keywords: bacterial infection, cirrhosis, interleukin-6, meta-analysis
1. Introduction
Bacterial infection is very common in patients with liver cirrhosis, the prevalence of bacterial infection on admission or during hospitalization is about 30% in cirrhotic population.[1–3] Infection has been considered to be one of the triggers of complications in cirrhosis.[4,5] Severe infection may cause increasing mortality in those patients.[6,7] Data show that any delay of appropriate antibiotics therapy is associated with an increase in death rate.[8,9] Therefore, a timely detection of bacterial infection is crucial for improving the prognosis.
However, it is not easy to make accurate diagnosis of infection in cirrhotic population, because the clinical manifestations of bacterial infection in those patients could be subtle and less specific.[5] Routine blood test, C-reactive protein (CRP), and procalcitonin (PCT) are the currently available and widely used diagnostic methods. Those indicators have been demonstrated to have some limitations for identification bacterial infection in cirrhotic patients.[5]
Recently, several studies revealed that interleukin-6 (IL-6) could serve as a promising diagnostic tool in cirrhotic population.[10–12] IL-6 is a proinflammatory cytokine. It increases earlier after exposure to bacterial components than PCT and CRP do.[12,13] The IL-6 has higher sensitivity and specificity than the other biomarkers in early diagnosis of sepsis.[12] However, the sample sizes in most studies were relatively small. In present study, we performed a meta-analysis to explore the potential diagnostic value of serum IL-6 in predicting bacterial infection in cirrhotic patients.
2. Methods
2.1. Search strategy and selection criteria
We systematically searched the following databases for studies focusing on the accuracy of IL-6 for the diagnosis of bacterial infection in patients with liver cirrhosis: PubMed, Embase (via OvidSP), Web of Science, the Cochrane Library, and Scopus.
The electronic databases published up to October 2015 with the following MeSH terms and free text were searched: “(interleukin-6 OR IL-6) AND (“liver cirrhosis” OR cirrhotic) AND (bacterial infection).” We also hand-searched the reference lists of each primary study and reviews for additional citations. No language restrictions were applied on initial searching of published studies. Two investigators (YLW, SL) performed the reference selection independently. Discrepancies were resolved by a consensus meeting. If agreements still could not be reached, they were resolved by a 3rd investigator (YYZ).
After removing the duplicate references, the title and abstract were screened in the 1st round. Full-text copies of potentially relevant articles were retrieved for review in the 2nd round. Only those articles published in English or Chinese were finally included for analysis, although there were no language restrictions on primary searching. The included studies should fulfill the following criteria: study populations are patients with liver cirrhosis; include the results of an IL-6 test; one of the endpoints should be infection; and include calculations for sensitivity, specificity, or have sufficient data to construct a 2 × 2 contingency table. Case reports, case series, editorials, review articles, or clinical guidelines were excluded.
2.2. Data extraction and quality assessment
Data extraction and quality evaluation were carried out independently by 2 investigators (YLW, SL). Disagreements were resolved by discussion between the 2 researchers. Information from each included study was extracted on: the demographic characteristics of study; measurement methods of IL-6; and the true positive value, false positive value, false negative value, true negative value, and cut-off value of each study. The methodological quality of the studies was evaluated using the Quality Assessment of Diagnostic Accuracy Studies-2 tool,[14] as recommended by the Cochrane Handbook of Diagnostic Test Accuracy Reviews.[15] This tool consists of 4 domains: patient selection, index test, reference standard, and flow and timing. Each domain was assessed in terms of risk of bias, and the 1st 3 domains were also considered in terms of applicability.
2.3. Statistical analysis and data synthesis
The 2 × 2 tables (numbers of true positives, false positives, true negatives, and false negatives) of each included study were constructed according to the data of the articles. If multiple cut-off points for IL-6 analysis were reported in a same article, only the cut-off value with the largest overall accuracy was used for final analysis. The study results were demonstrated in paired forest plots as sensitivity and specificity with 95% confidence intervals (95% CIs). A bivariate binomial mixed model was used to compute the pooled estimates of sensitivity and specificity, positive likelihood ratio, negative likelihood ratio (NLR), and diagnostic odds ratio (DOR). A summary receiver operating characteristic curve was constructed.[16,17] Heterogeneity was quantitatively assessed using the I2 statistic with significance set at P < 0.10 and I2 > 50%.[18] Effective sample size funnel plots versus the log DOR, as well as Deeks test, a regression test of asymmetry were used to examine possible publication bias.[19] All analyses were conducted using Stata, Version 12.0 (Stata Corp, College Station, TX), MetaDisc (version1.4), and Revman Manger 5.3.
Ethical approval and patient consent were not necessary, as this study was a meta-analysis, which was based on previous published data.
3. Results
A total of 535 articles were retrieved in the initial database search. After removing the duplicates, 272 articles were identified. We excluded 255 articles after reviewing the titles and abstracts. After a full-text review, further 11 articles were excluded, leaving 6 for final meta-analysis (Fig. 1). No more relevant articles were further identified after searching the reference lists of the identified articles or relevant review articles.
Figure 1.
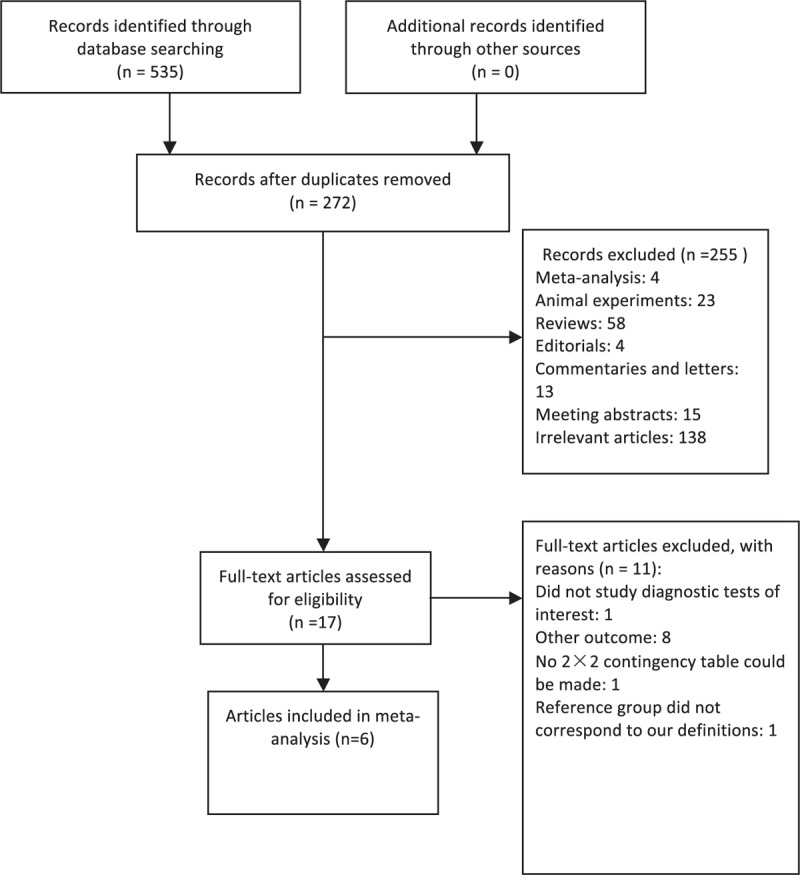
Flow diagram of study identification and inclusion.
Publication years of the 6 included studies[10,20–24] spanned from 1993 to 2015. A total of 741 subjects (32–425 for each study) were included. Table 1 showed the demographic characteristics of the 6 included studies. The diagnosis of cirrhosis was mostly based on clinical presentations and laboratory examinations and/or histopathological diagnosis. Etiologies of cirrhosis included alcoholic-related, viral-related, nonalcoholic steatohepatitis-related, and cryptogenic. There was no significant difference between infectious and noninfectious patients in cirrhotic etiologies (Table 1). Spontaneous bacterial peritonitis (SBP) was defined as an infection of the ascitic fluid without any intraabdominal source of infection. The ascitic fluid neutrophil count should be higher than 250 cells/mm3 and/or a positive culture. Urinary tract infection was considered if polymorphnuclear cell count >105 mm3 and/or a positive culture. Pneumonia was diagnosed based on the symptoms and a positive sputum culture and/or a typical chest X-ray. Systemic infection was diagnosed when 2 positive blood cultures were found. We used 4 quality categories as described in Quality Assessment of Diagnostic Accuracy Studies-2 to evaluate the risk of bias and applicability of each study and designated an overall high or low risk of bias for each category. The results of the methodological quality of the 6 included studies were shown in Fig. 2.
Table 1.
Demographic characteristics of the included studies.
Figure 2.
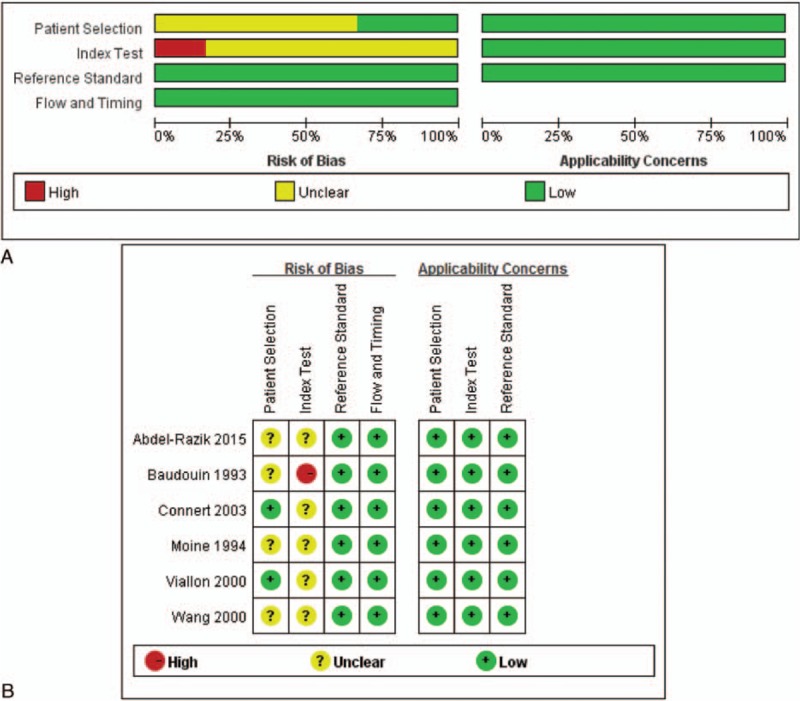
(A) Risk of bias and applicability concerns graph; (B) risk of bias and applicability concerns summary.
The pooled sensitivity, specificity, and DOR were 0.85 (95% CI, 0.64–0.94), 0.91 (95% CI, 0.80–0.96), and 52.89 (95% CI, 15.21–183.86), respectively (Fig. 3), with the quantity I2 of 85.72%, 70.23%, and 99.84%, indicating significant heterogeneity. The pooled positive likelihood ratio and NLR were 8.99 (95% CI, 4.13–19.55) and 0.17 (95% CI, 0.07–0.43), respectively (Fig. 4). The area under the ROC curve (AUROC) was 0.94 (95%CI, 0.92–0.96) (Fig. 5), indicating high diagnostic accuracy. Taken together, these results suggested that IL-6 had a favorable accuracy for diagnosis.
Figure 3.
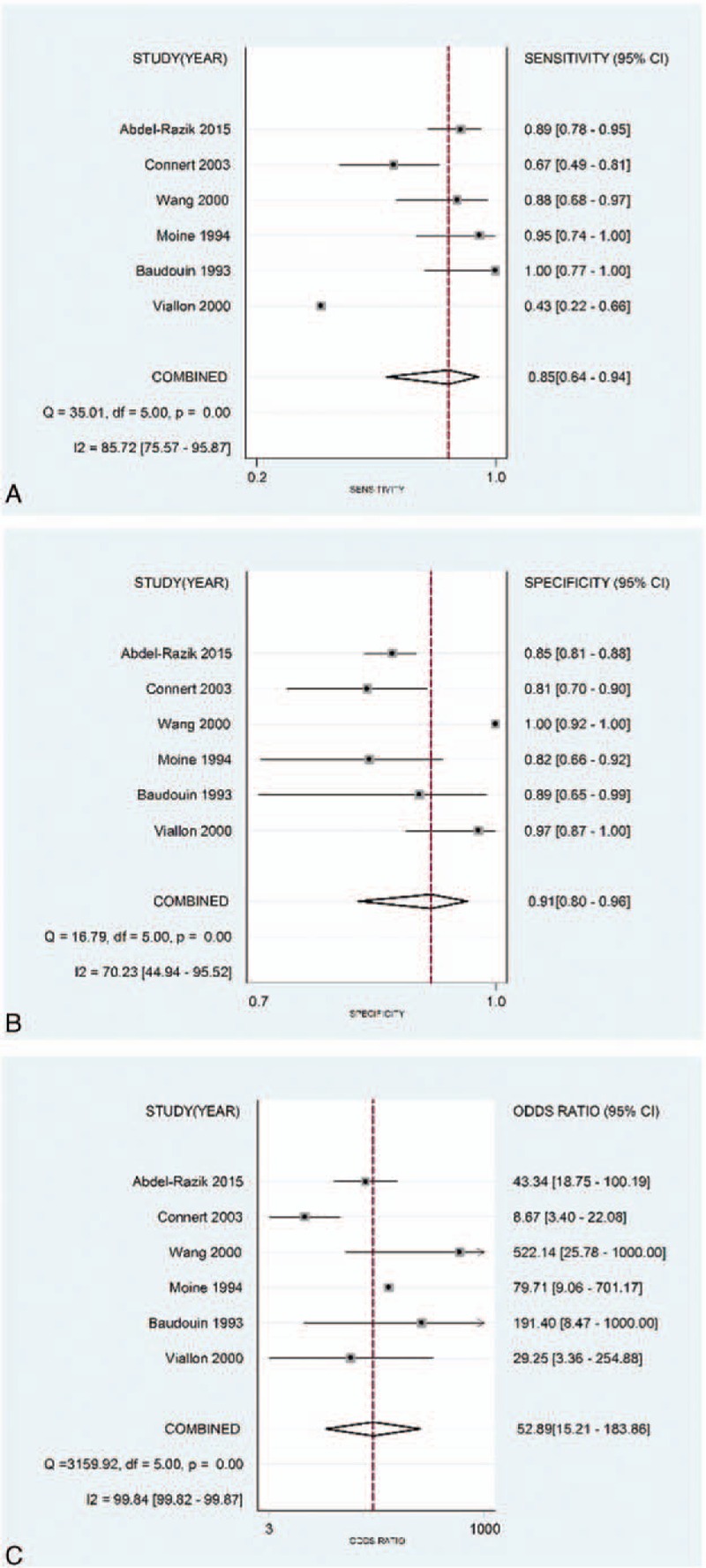
(A) Forest plots of the sensitivity of interleukin-6 (IL-6) for the diagnosis of bacterial infection in cirrhotic patients; (B) forest plots of the specificity of IL-6 for the diagnosis of bacterial infection in cirrhotic patients; and (C) forest plots of the diagnostic odds ratios of IL-6 for the diagnosis of bacterial infection in cirrhotic patients.
Figure 4.
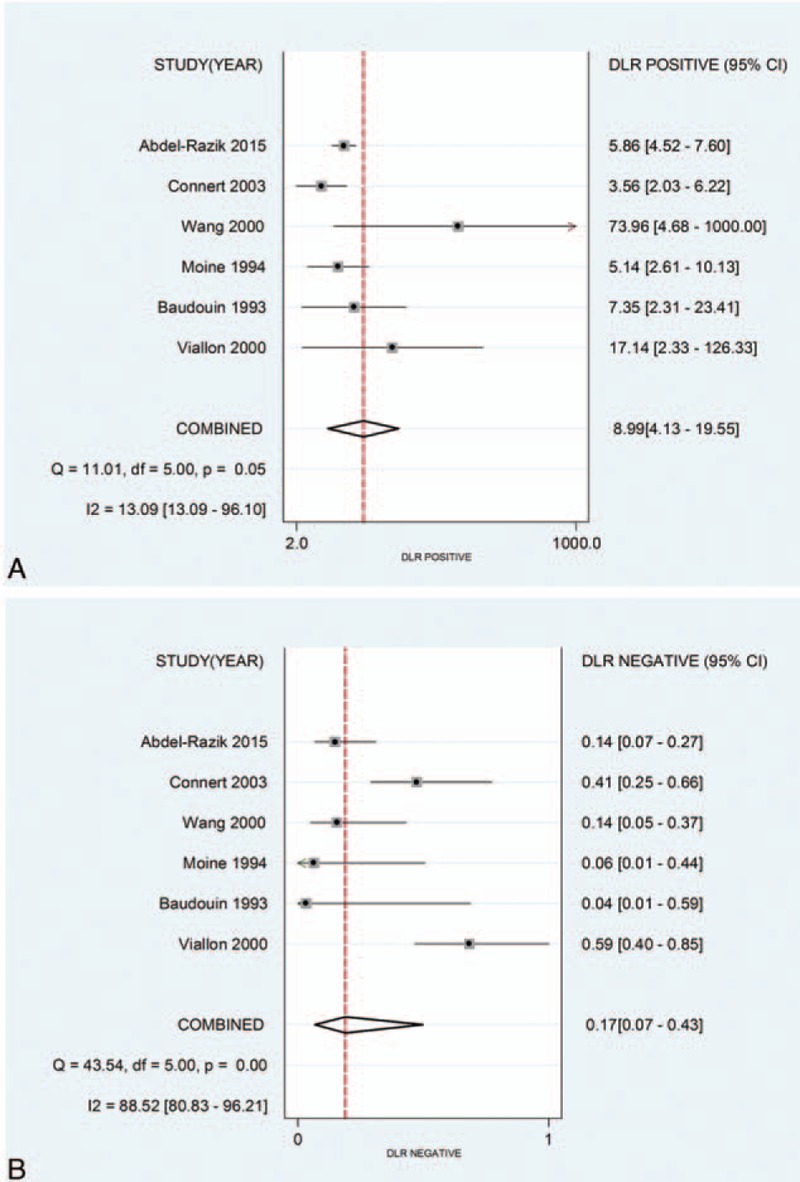
(A) Forest plots of the positive likelihood of interleukin-6 (IL-6) for the diagnosis of bacterial infection in cirrhotic patients; (B) forest plots of the negative likelihood of IL-6 for the diagnosis of bacterial infection in cirrhotic patients.
Figure 5.
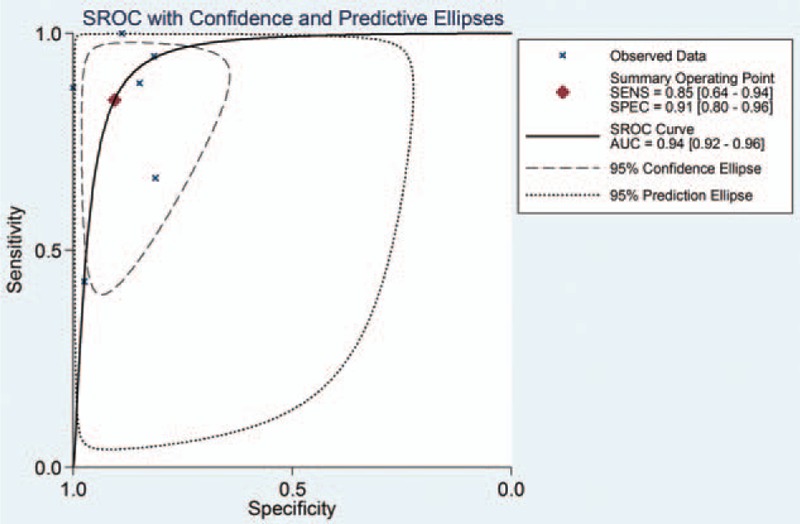
SROC curve of IL-6 for the diagnosis of bacterial infection in cirrhotic patients. IL-6 = interleukin-6, SROC = summary receiver operating characteristic.
Statistical heterogeneity was tested using I2 statistic. Significant heterogeneities were found for the pooled sensitivity, specificity, NLR, and DOR. A threshold analysis was used to explore the effect (Spearman correlation coefficient = 0.143, P = 0.787), and the results were with no statistically significant difference.
Deeks funnel plot asymmetry and the results of the Deeks test (P = 0.481) did not suggest potential publication bias.
4. Discussion
A timely and accurate diagnosis of bacterial infection is crucial for patients with cirrhosis, as any missed diagnosis may delay the initiation of antibiotic therapy and then result in high mortality. On the other hand, doctors tend to prescribe antibiotics to lower the infective risk. However, excessive use of antibiotics may lead to drug resistance. Therefore, a rapid bedside detection of bacterial infection with high sensitivity and specificity is important.
In present study, meta-analysis was employed to investigate the accuracy of IL-6 for the differentiation of bacterial infection in cirrhotic patients. The results suggested that IL-6 had high sensitivity and specificity in diagnosis of bacterial infection. Moreover, the AUROC and pooled DOR also indicated a high degree of diagnostic accuracy. All these results suggested that IL-6 had a favorable accuracy for diagnosis and could serve as a good biomarker in cirrhotic patients with bacterial infection. Besides cirrhotic patients, meta-analyses of IL-6 for the diagnosis of sepsis or bacterial infection in neonatal also draw same conclusions.[25,26]
IL-6 is a proinflammatory cytokine. It is involved in the initiation of acute-phase response in humans during bacterial infection. Serum IL-6 level is very low in population without infection but increases rapidly and sharply during the early stage of bacterial infection.[27] The assay method of IL-6 is accurate, fast, and simple. The dramatic change of IL-6 during bacterial infection and the stable testing method of IL-6 facilitate decision making in clinical practice.[12,13,28]
There were some studies including assessments of the diagnostic accuracy of PCT, CRP in cirrhotic patients.[29,30] Those results indicated that the pooled sensitivity and specificity were only 0.79 and 0.89 for PCT, and 0.77 and 0.85 for CRP.[29] It was difficult to evaluate which biomarker was better merely based on meta-analysis results. However, previously studies showed that CRP has less diagnostic capacity in the cirrhotic population.[31] A recent study also suggested that elevated PCT might indicate liver injury rather than infection in patients with liver failure.[32] The diagnostic accuracy of CRP and PCT in cirrhosis patients still remains controversial.[31]
But as infection is complex and dynamic and the pathophysiological status of cirrhosis is special, it is hard to find out an ideal marker with satisfying sensitivity and specificity for detecting bacterial infection in cirrhotic patients. Nevertheless, IL-6 had been shown to be one of the most promising parameters. If IL-6 becomes a routine test in clinical setting, it would improve antibiotic management in cirrhosis, reduce mortality and antibiotic resistance.
This meta-analysis had several limitations. First, though we did our best to search eligible studies, this study only included 6 studies. The reason of this may be that we only included publications in English and Chinese and the usage of IL-6 in cirrhotic patients had not been well studied. Second, there was significant heterogeneity between studies. Generally, the threshold effect was a very common source of heterogeneity in a diagnostic study, but we did not find significant differences in threshold analysis. And besides that, the type of infection, different criteria to diagnose infection, age, gender, or IL-6 assay of included studies were also different. All these differences could contribute to the heterogeneity. A meta-regression would be helpful to explore the source of the heterogeneities; however, the limited numbers of studies included did not allow to carry out meta-regression. These results should be interpreted cautiously, for the heterogeneity seriously affected the accuracy of our results. Because of relatively small sample size of included studies and heterogeneity, further intensive researches with big sample size should be conducted. Third, we could not determine the ideal cutoff point for the IL-6 test, because there were several assay methods for IL-6 test and we did not have enough data. Fourth, the validity of meta-analysis depends on the quality of the included studies. Two of the included studies used a case–control design, which is prone to overestimate the sensitivity of the test by excluding patients with undetermined diagnosis.[33] Finally, several studies also analyzed the correlation between IL-6 level and the severity or the prognosis of bacterial infection.[11,12] The results confirmed the values of IL-6 as a prognostic biomarker. However, this issue was not considered in this study due to insufficient data.
5. Conclusion
This meta-analysis suggests IL-6 has high diagnostic value for the differentiation of bacterial infection in patients with cirrhosis. Further larger, multicenter studies are needed to confirm its predictive value before the IL-6 test is widely used in the clinical setting.
Acknowledgments
The authors thank the Pilot Project of Fujian Science and Technology Department (2015Y0057) and the Pilot Project of Fujian Science and Technology Department (2016Y0040) for the support.
Footnotes
Abbreviations: CI = confidence interval, CRP = C-reactive protein, DOR = diagnostic odds ratio, IL-6 = interleukin-6, NLR = negative likelihood ratio, PCT = procalcitonin.
Authorship: YW and SL designed the research study and wrote the paper, MW helped to perform the statistic analysis, and YZ contributed to the design of the study.
Funding/support: This study was financially supported by the Pilot Project of Fujian Science and Technology Department (2015Y0057) and the Pilot Project of Fujian Science and Technology Department (2016Y0040).
The authors have no conflicts of interest to disclose.
References
- 1.Fernandez J, Navasa M, Gomez J, et al. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology 2002; 35:140–148. [DOI] [PubMed] [Google Scholar]
- 2.Fernandez J, Acevedo J, Castro M, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology 2012; 55:1551–1561. [DOI] [PubMed] [Google Scholar]
- 3.Pant C, Olyaee M, Gilroy R, et al. Emergency department visits related to cirrhosis: a retrospective study of the nationwide emergency department sample 2006 to 2011. Medicine 2015; 94:e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gustot T, Felleiter P, Pickkers P, et al. Impact of infection on the prognosis of critically ill cirrhotic patients: results from a large worldwide study. Liver Int 2014; 34:1496–1503. [DOI] [PubMed] [Google Scholar]
- 5.Jalan R, Fernandez J, Wiest R, et al. Bacterial infections in cirrhosis: a position statement based on the EASL Special Conference 2013. J Hepatol 2014; 60:1310–1324. [DOI] [PubMed] [Google Scholar]
- 6.Foreman MG, Mannino DM, Moss M. Cirrhosis as a risk factor for sepsis and death: analysis of the National Hospital Discharge Survey. Chest 2003; 124:1016–1020. [DOI] [PubMed] [Google Scholar]
- 7.Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 2010; 139:1246–1256.1256.e1-5. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–1596. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh CC, Lee CC, Chan TY, et al. Clinical features and impact of empirical therapy in cirrhotic adults with community-onset bacteremia. Am J Emerg Med 2015; 33:222–228. [DOI] [PubMed] [Google Scholar]
- 10.Wang SS, Lee FY, Chan CC, et al. Sequential changes in plasma cytokine and endotoxin levels in cirrhotic patients with bacterial infection. Clin Sci 2000; 98:419–425. [PubMed] [Google Scholar]
- 11.Suliman MA, Khalil FM, Alkindi SS, et al. Tumor necrosis factor-alpha and interleukin-6 in cirrhotic patients with spontaneous bacterial peritonitis. World J Gastrointest Pathophysiol 2012; 3:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin S, Huang Z, Wang M, et al. Interleukin-6 as an early diagnostic marker for bacterial sepsis in patients with liver cirrhosis. J Crit Care 2015; 30:732–738. [DOI] [PubMed] [Google Scholar]
- 13.Dahaba AA, Metzler H. Procalcitonin's role in the sepsis cascade. Is procalcitonin a sepsis marker or mediator? Minerva Anestesiol 2009; 75:447–452. [PubMed] [Google Scholar]
- 14.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155:529–536. [DOI] [PubMed] [Google Scholar]
- 15.Deeks JJ, Bossuyt PM, Gatsonis C. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2009. Available from: http://srdta.cochrane.org/ (Accessed December 31, 2015). [Google Scholar]
- 16.Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol 2006; 59:1331–1332. [DOI] [PubMed] [Google Scholar]
- 17.Reitsma JB, Glas AS, Rutjes AW, et al. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005; 58:982–990. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 19.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005; 58:882–893. [DOI] [PubMed] [Google Scholar]
- 20.Byl B, Roucloux I, Crusiaux A, et al. Tumor necrosis factor alpha and interleukin 6 plasma levels in infected cirrhotic patients. Gastroenterology 1993; 104:1492–1497. [DOI] [PubMed] [Google Scholar]
- 21.Moine OL, Devière J, Devaster JM, et al. Interleukin-6: an early marker of bacterial infection in decompensated cirrhosis. J Hepatol 1994; 20:819–824. [DOI] [PubMed] [Google Scholar]
- 22.Viallon A, Zeni F, Pouzet V, et al. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med 2000; 26:1082–1088. [DOI] [PubMed] [Google Scholar]
- 23.Connert S, Stremmel W, Elsing C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z Gastroenterol 2003; 41:165–170. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Razik A, Mousa N, Elbaz S, et al. Diagnostic utility of interferon gamma-induced protein 10 kDa in spontaneous bacterial peritonitis: Single-center study. Eur J Gastroenterol Hepatol 2015; 27:1087–1093. [DOI] [PubMed] [Google Scholar]
- 25.Hou T, Huang D, Zeng R, et al. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: a systematic review and meta-analysis. Int J Clin Exp Med 2015; 8:15238–15245. [PMC free article] [PubMed] [Google Scholar]
- 26.Shahkar L, Keshtkar A, Mirfazeli A, et al. The role of IL-6 for predicting neonatal sepsis: a systematic review and meta-analysis. Iran J Pediatr 2011; 21:411–417. [PMC free article] [PubMed] [Google Scholar]
- 27.Panacek EA, Kaul M. IL-6 as a marker of excessive TNF-α activity in sepsis. Sepsis 1999; 3:65–73. [Google Scholar]
- 28.Buck C, Bundschu J, Gallati H, et al. Interleukin-6: a sensitive parameter for the early diagnosis of neonatal bacterial infection. Pediatrics 1994; 93:54–58. [PubMed] [Google Scholar]
- 29.Lin KH, Wang FL, Wu MS, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection in patients with liver cirrhosis: a systematic review and meta-analysis. Diagn Microbiol Infect Dis 2014; 80:72–78. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Li L, Qu C, et al. Diagnostic accuracy of serum procalcitonin for spontaneous bacterial peritonitis due to end-stage liver disease: a meta-analysis. Medicine 2015; 94:e2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol 2012; 56 (Suppl 1):S1–S12. [DOI] [PubMed] [Google Scholar]
- 32.Rule JA, Hynan LS, Attar N, et al. Procalcitonin identifies cell injury, not bacterial infection, in acute liver failure. PLoS One 2015; 10:e0138566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999; 282:1061–1066. [DOI] [PubMed] [Google Scholar]


