Abstract
Remifentanil and nitrous oxide (N2O) are 2 commonly used anesthetic agents. Both these agents are known risk factors for postoperative nausea and vomiting (PONV). However, remifentanil and N2O have not been directly compared in a published study. Remifentanil can induce acute tolerance or hyperalgesia, thus affecting postoperative pain. The objective of this retrospective study is to compare the effects of remifentanil and N2O on PONV and pain in patients receiving intravenous patient-controlled analgesia (IV-PCA) after thyroidectomy.
We analyzed the electronic medical records of 992 patients receiving fentanyl-based IV-PCA after thyroidectomy at Chung-Ang University Hospital from January 1, 2010 to April 30, 2016. We categorized the patients according to anesthetic agents used: group N2O (n = 745) and group remifentanil (n = 247). The propensity score matching method was used to match patients in the 2 groups based on their covariates. Finally, 128 matched subjects were selected from each group.
There were no differences between groups for all covariates after propensity score matching. The numeric rating scale for nausea (0.55 ± 0.88 vs 0.27 ± 0.76, P = 0.01) was higher and complete response (88 [68.8%] vs 106 [82.8%], P = 0.001) was lower in group N2O compared with group remifentanil on postoperative day 0. However, the visual analog scale score for pain (3.47 ± 2.02 vs 3.97 ± 1.48, P = 0.025) was higher in group remifentanil than group N2O on postoperative day 0.
In patients receiving IV-PCA after thyroidectomy, postoperative nausea was lower but postoperative pain was higher in group remifentanil.
Keywords: nitrous oxide, pain, postoperative, postoperative nausea and vomiting, remifentanil
1. Introduction
A characteristic complication of general anesthesia is postoperative nausea and vomiting (PONV). PONV can induce both postoperative pain and surgical wound dehiscence.[1] Moreover, PONV can reduce overall patient satisfaction and increase both the length of hospital stay and the overall medical cost by increasing the risk of pulmonary aspiration, electrolyte abnormality, and dehydration.[2,3] The risk factors for PONV include patient-related factors (female, nonsmoker, and history of PONV or motion sickness), perioperative opioid usage, anesthetic methods, and surgical type.[4]
Thyroidectomy patients constitute a high-risk group for PONV. Indeed, the incidence of PONV is higher (63–84% of thyroidectomy patients) than that in patients who underwent other surgeries.[5] Thus, many studies have investigated the effects of various pharmacologic agents, combinations of pharmacologic agents, and anesthetic or surgical methods for reducing PONV in these patients.[6–11]
Remifentanil and nitrous oxide (N2O) are commonly used anesthetic agents. Remifentanil is an ultra-short acting opioid used in anesthetic practice for relieving pain and improving hemodynamic stability during anesthetic and surgical management.[12] However, remifentanil is known to significantly increase the risk of PONV, although reports concerning the effects of this agent are inconsistent.[13,14] In addition, continuous infusion of remifentanil may induce acute opioid tolerance or hyperalgesia.[15]
N2O has been widely used as an adjuvant to clinical anesthetic practice. The analgesic properties and general properties of this gas that render it suitable for this purpose include its low solubility, rapid onset and short half-life, and low cost. Like remifentanil, N2O is also a well-known risk factor for PONV, especially in cases where patients were exposed to N2O for a prolonged time.[16,17] However, no comparative study evaluating PONV and postoperative pain in remifentanil- and N2O-based anesthetic methods has been reported. In the present retrospective study, we aim to compare the effects of remifentanil and N2O on PONV in patients undergoing thyroidectomy and using opioids for postoperative pain management.
2. Methods
2.1. Study design
This retrospective study was approved and informed consent was exempted by the Institutional Review Board of Chung-Ang University Hospital. All data were anonymized and de-identified at the start of the study. We analyzed the electronic medical records of 992 patients receiving fentanyl-based intravenous patient-controlled analgesia (IV-PCA) after thyroidectomy at Chung-Ang University Hospital from January 1, 2010 to April 30, 2016. The STROBE (Strengthening the Reporting of Observational Studies in epidemiology) checklist was used for constructing this manuscript.[18] We excluded cases where the patient was under 19 years old, cases with missing data for variables or outcome measures, cases in which the patient did not receive fentanyl-based IV-PCA or prior participation into other randomized controlled trials. We categorized the patients according to anesthetic agents used: group N2O (n = 745) and group remifentanil (n = 247).
2.2. Data collection
We collected and sorted data regarding risk factors for PONV related to patient characteristics (age, gender, height, weight, and history of smoking, PONV or motion sickness), risk factors related to anesthetic use (operation time, use of glycopyrrolate as premedication, and type of anesthetic agents used [desflurane vs sevoflurane vs propofol]), and risk factors related to PCA (dosage of fentanyl, use of nefopam, palonosetron, and ramosetron in IV-PCA). Postoperative variables included severity of nausea and pain, number of incidences of vomiting, incidence of headache, usage of rescue antiemetics, usage of rescue analgesics, and incidence of complete response (CR). These variables were measured on postoperative days (POD) 0 and 1. CR was defined as no nausea, no vomiting, and no requirement of antiemetic during the postoperative period at POD 0 or 1. The severity of pain was recorded on a 10-point visual analog scale (VAS) while the severity of nausea was recorded on a numerical rating scale (none = 0/mild = 1/moderate = 2/severe = 3/worst imaginable = 4).
2.3. Statistics
Because this was a retrospective cohort study, patients were not randomized prior to intervention. For this reason, we used the propensity score matching method to reduce the bias due to confounding factors. The propensity score was calculated using logistic regression analysis, and accounting for covariates such as age, gender, height, weight, history of smoking, PONV or motion sickness, operation time, use of glycopyrrolate as premedication, type of anesthetic agents used, dosage of fentanyl, and use of nefopam, palonosetron, and ramosetron in IV-PCA. We adopted the nearest available match between the 2 groups according to propensity score similarities (caliper radius of 0.001). We subsequently calculated standardized differences (STDs) for covariates to evaluate the balance between each matched group. STD is defined as the difference in means between 2 groups in units of standard deviation (SD). When the value of STD is lower than 20%, a good comparison between the groups is considered to have been achieved.[19]
For continuous variables, the data distribution was first evaluated for normality using the Shapiro–Wilk test. Normally distributed data were then compared using parametric methods and non-normally distributed data were analyzed using nonparametric methods. Prior to matching, an unpaired t test or Mann–Whitney U test was used for comparing continuous variables, and a chi-squared analysis or Fisher exact test was used for comparing descriptive variables. After matching, statistical differences between the remifentanil group and N2O group were evaluated using a paired t test, Wilcoxon signed-rank test, and McNamara test. Continuous variables were expressed as mean ± SD and descriptive variables were expressed as absolute number (%).
P value <0.05 was considered statistically significant. All analyses of data were conducted using the Statistical Package for the Social Sciences software suite (version 23; IBM Corp., Armonk, NY).
3. Results
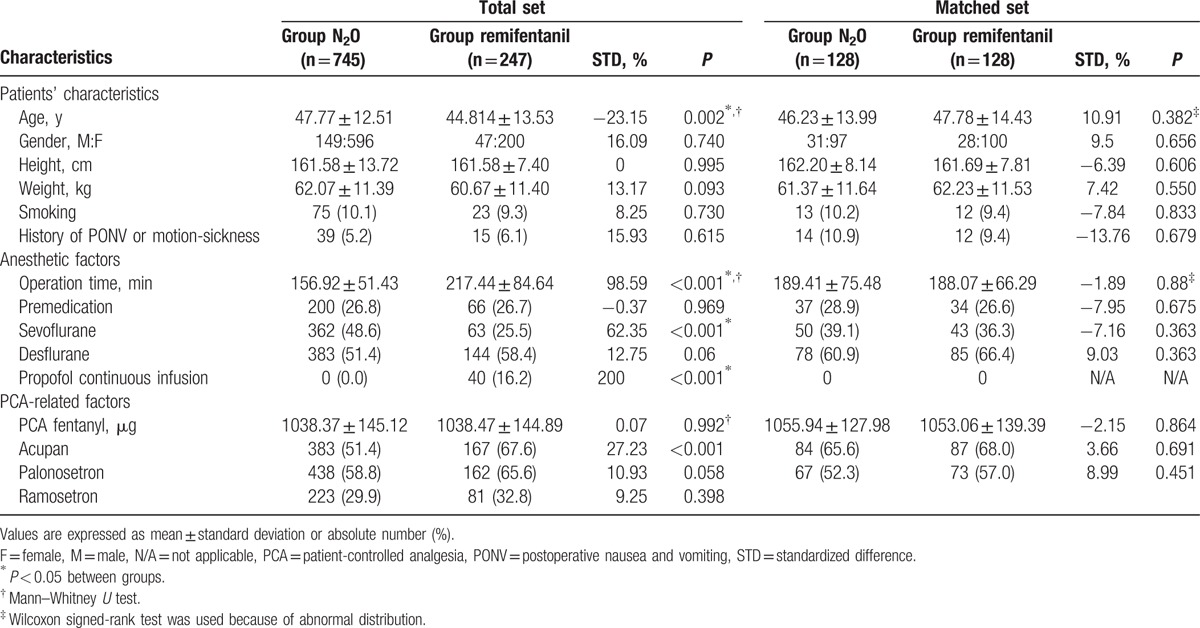
The records of 992 patients receiving fentanyl-based IV-PCA after thyroidectomy at Chung-Ang University Hospital from January 1, 2010 to April 3, 2016 were collected and categorized into group N2O (n = 745) or group remifentanil (n = 247). Table 1 shows the overall demographic characteristics of all patients (and also the characteristics of the matched cohorts).
Table 1.
Patients’ characteristics in total and matched cohorts.
3.1. Group N2O versus group remifentanil in the overall series
Before propensity score matching, 5 variables among 15 confounding variables showed poor STD scores. These variables were as follows: age, operation time, use of sevoflurane, desflurane, or propofol continuous infusion, and use of nefopam in IV-PCA (Table 1).
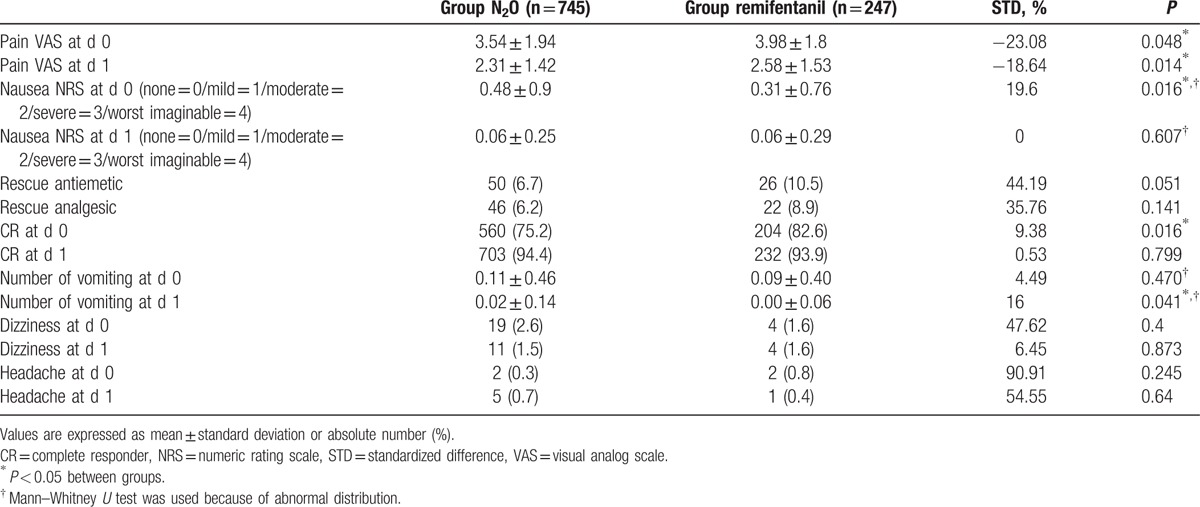
Except for VAS of pain on POD 0 and 1, the numeric rating scale (NRS) of nausea on POD 0, the number of vomiting episodes on POD 1, and complete responder on POD 0, there was no significant difference in variables between the 2 groups (Table 2).
Table 2.
Postoperative variables before matching.
3.2. Group N2O versus group remifentanil according to propensity score analysis
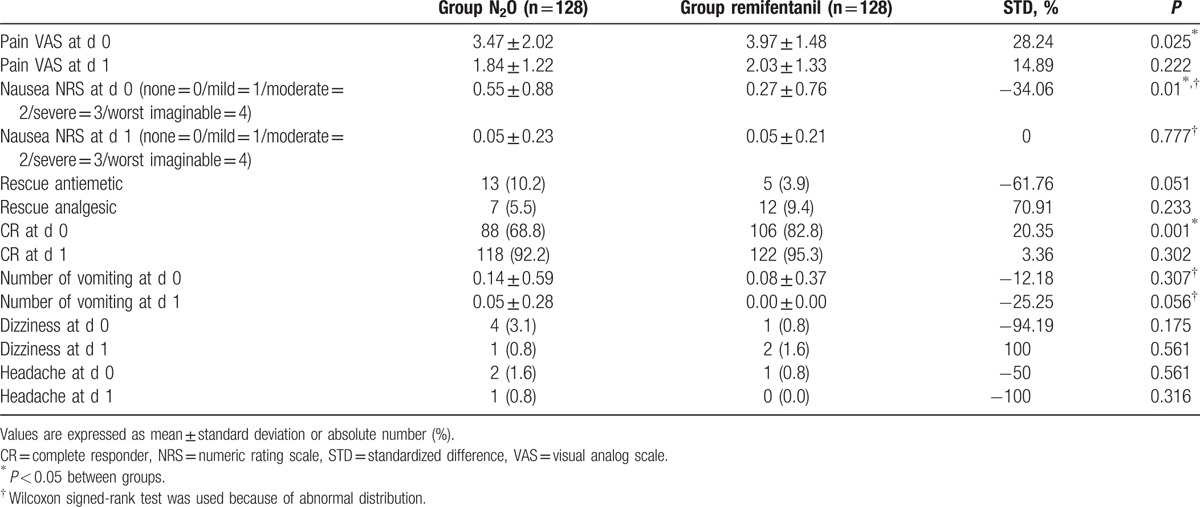
After matching, there were 128 patients in each group. All 15 confounding variables showed acceptable STDs (<20%), confirming that the matching procedure was efficient in creating a balance between the 2 groups (Table 1). The VAS score of pain (3.47 ± 2.02 vs 3.97 ± 1.48, P < 0.025) and CR (88 [68.8%] vs 106 [82.8%], P = 0.001) on POD 0 were both higher in the remifentanil group. The NRS of nausea (0.55 ± 0.88 vs 0.27 ± 0.76, P = 0.01) was higher in the N2O group than in the remifentanil group (Table 3).
Table 3.
Postoperative variables after matching.
4. Discussion
According to our study, the VAS score of pain and CR on POD 0 were both higher in patients receiving IV-PCA using general anesthesia with remifentanil than in patients receiving IV-PCA using general anesthesia with N2O after thyroidectomy. The NRS of nausea was lower using remifentanil than when using N2O.
A common postoperative complaint of patients after thyroidectomy is PONV, which has a remarkably higher incidence following this procedure than following other surgeries.[5] There are various risk factors for PONV, including age, sex, and use of perioperative opioid. In particular, an important factor explaining the higher incidence of PONV in patients receiving thyroidectomy is surgical manipulation (especially manipulation involving vagus nerve stimulation).[4] Further, vomiting after thyroidectomy may cause serious complications, including postoperative bleeding and neck hematoma, which can lead to surgical wound dehiscence and potentially bleeding-induced upper airway obstruction.[20] Therefore, proper prophylaxis or treatment for PONV in these patients is very important. Although various pharmacological prophylactic methods have been studied extensively in patients undergoing thyroidectomy,[5,9–11] the effect of these methods is still controversial. Thus, it may be easier and more efficacious to identify and reduce modifiable risk factors, including anesthetic agents, for PONV.
Remifentanil continues to be extensively used in anesthetic practice, since it is rapidly degraded by tissue esterase and has a short half-life. However, there is some controversy concerning the effect of remifentanil on PONV. Several previous studies[12,13] reported that remifentanil use increased the incidence of PONV, while other studies (remifentanil vs other opioids[21] and sevoflurane with remifentanil vs sevoflurane)[22] reported that remifentanil did not increase incidence of PONV. Since remifentanil can lead to acute tolerance and hyperalgesia,[15,23,24] remifentanil use during thyroidectomy may increase postoperative pain in the immediate period after surgery, and in consequence result in higher postoperative rescue analgesic requirements after infusion of remifentanil has stopped.[25] These features of remifentanil may potentially cause a vicious cycle of PONV by increasing the need for use of postoperative narcotic analgesics.
N2O shares similar features with remifentanil. It has a rapid onset of action and a short half-life. It is known to increase the incidence of PONV via several different mechanisms, including stimulation of the central nervous system through catecholamine release,[26] induction of changes in middle ear pressure which subsequently influence the vestibular system,[27] and by an increase in abdominal bowel distension.[28] The association of N2O with severe PONV is especially strong in patients of Asian ethnicity.[16]
Although patients in our study have a high-risk factor for PONV (opioid was used in PCA, thyroidectomy is a high-risk surgery for PONV, the proportion of female gender [75.8% in N2O group vs 78.1% in remifentanil group, P = 0.656] was high, and all patients were of Asian ethnicity), the percentage of patients who were not CR was 31.2% in the N2O group and 17.2% in the remifentanil group. Thus, incidence of PONV was lower in both groups in comparison with a previous study.[5] The prophylactic use of antiemetic before the end of surgery and also mixed in with PCA, routine use of more effective antiemetics (2nd generation antiemetics such as palonosetron and ramosetron were used), and reduced stimulation of the vagus nerve by the surgical team, might all have contributed to the lower incidence of PONV in our study compared with the earlier study.
In our comparison between the 2 groups, remifentanil, N2O, and volatile anesthetics are all known risk factors of PONV. However, remifentanil use or N2O use effectively reduced the total usage of the intraoperative volatile anesthetics, and both remifentanil and N2O are eliminated very rapidly. Therefore, their effect on PONV may be expected to wear off promptly. However, while N2O was generally kept at a constant concentration during the maintenance of general anesthesia, the infusion rate of remifentanil was titrated in real-time according to the hemodynamic status and the somatic signs of the patient. In consequence, there was a further reduction in the usage of volatile agents in the remifentanil group relative to the N2O group. Since the total usage of intraoperative volatile anesthetic was more effectively titrated and consequently reduced in the remifentanil group in comparison with the N2O group, the risk factor for volatile anesthetic is higher in the N2O group. Indeed, this aspect may account for our result that NRS of nausea was lower and that there was a higher number of CR on POD 0 in the remifentanil group.
Conversely, the VAS of postoperative pain was higher with remifentanil than with N2O, although this was observed only on POD 0. There was, however, no difference in rescue analgesic requirement between the 2 groups. Thus, remifentanil use has distinct disadvantages as well as advantages. The many advantages of remifentanil use include the short-acting nature and rapid recovery, while the major disadvantage is acute tolerance and hyperalgesia. Acute tolerance is induced rapidly after total dose of intraoperative opioid infusion is increased or when short acting opioids like remifentanil are used.[25] Opioid-induced hyperalgesia is increased when the total dose of opioid during surgery is increased, and the intensity of pain due to surgery is high.[15] The clinical effects of these characteristics are still controversial. Several previous studies[24,29] have reported that remifentanil-induced hyperalgesia increased the requirement of postoperative analgesia. However, in agreement with results reported in the present study, another study[30] reported no difference in rescue analgesic consumption. These discrepancies may be due to differences in the doses of intraoperative remifentanil infusion. However, additional confounding factors and bias that affect the results of these studies cannot be ruled out. Therefore, we assume that although remifentanil-induced hyperalgesia influences the degree of pain in patients, additional administration of rescue narcotic analgesic is not needed.
This study had several limitations. First, as in other retrospective studies, our data were imperfect, including missing or incomplete data. Since patients were asked to recall the severity or events of their nausea and vomiting at POD 0 or 1, recall bias may be a problem, and the incidence of PONV may be underestimated because less severe PONV may be ignored. Second, our study used data from a single-medical center. Hence, caution is advised in generalizing from the results presented in our study, and further randomized controlled trials should be performed.
In conclusion, the severity and incidence of PONV in patients receiving fentanyl-based IV-PCA after thyroidectomy under general anesthesia was lower in the remifentanil group. Although the postoperative pain was higher in the remifentanil group than that in the N2O group, there was no difference in the use of rescue narcotic analgesic between the 2 groups.
Footnotes
Abbreviations: CR = complete responder, IV-PCA = intravenous patient-controlled analgesia, N2O = nitrous oxide, NRS = numeric rating scale, POD = postoperative days, PONV = postoperative nausea and vomiting, SD = standard deviation, STD = standardized difference, VAS = visual analog scale.
The authors have no funding and conflicts of interest to disclose.
References
- 1.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth 2002; 89:409–423. [PubMed] [Google Scholar]
- 2.Kovac AL. Update on the management of postoperative nausea and vomiting. Drugs 2013; 73:1525–1547. [DOI] [PubMed] [Google Scholar]
- 3.Golembiewski J, Chernin E, Chopra T. Prevention and treatment of postoperative nausea and vomiting. Am J Health Syst Pharm 2005; 62:1247–1260. [DOI] [PubMed] [Google Scholar]
- 4.Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999; 91:693–700. [DOI] [PubMed] [Google Scholar]
- 5.Sonner JM, Hynson JM, Clark O, et al. Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth 1997; 9:398–402. [DOI] [PubMed] [Google Scholar]
- 6.Wang JJ, Ho ST, Lee SC, et al. The use of dexamethasone for preventing postoperative nausea and vomiting in females undergoing thyroidectomy: a dose-ranging study. Anesth Analg 2000; 91:1404–1407. [DOI] [PubMed] [Google Scholar]
- 7.Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000; 90:186–194. [DOI] [PubMed] [Google Scholar]
- 8.Park JW, Jun JW, Lim YH, et al. The comparative study to evaluate the effect of palonosetron monotherapy versus palonosetron with dexamethasone combination therapy for prevention of postoperative nausea and vomiting. Korean J Anesthesiol 2012; 63:334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauger PG, Shanks A, Morris M, et al. Propofol decreases early postoperative nausea and vomiting in patients undergoing thyroid and parathyroid operations. World J Surg 2008; 32:1525–1534. [DOI] [PubMed] [Google Scholar]
- 10.Fujii Y. The benefits and risks of different therapies in preventing postoperative nausea and vomiting in patients undergoing thyroid surgery. Curr Drug Safety 2008; 3:27–34. [DOI] [PubMed] [Google Scholar]
- 11.Lee MJ, Lee KC, Kim HY, et al. Comparison of ramosetron plus dexamethasone with ramosetron alone on postoperative nausea, vomiting, shivering and pain after thyroid surgery. Korean J Pain 2015; 28:39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosow CE. An overview of remifentanil. Anesth Analg 1999; 89 (suppl):S1–S3. [DOI] [PubMed] [Google Scholar]
- 13.Apfel CC, Bacher A, Biedler A, et al. A factorial trial of six interventions for the prevention of postoperative nausea and vomiting. Der Anaesthesist 2005; 54:201–209. [DOI] [PubMed] [Google Scholar]
- 14.Casati A, Albertin A, Danelli G, et al. Implementing sevoflurane anesthesia with small doses opioid for upper abdominal surgery. Postoperative respiratory function after either remifentanil or fentanyl. Minerva Anestesiol 2001; 67:621–628. [PubMed] [Google Scholar]
- 15.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology 2006; 104:570–587. [DOI] [PubMed] [Google Scholar]
- 16.Myles PS, Chan MT, Kasza J, et al. Severe nausea and vomiting in the evaluation of nitrous oxide in the gas mixture for anesthesia II trial. Anesthesiology 2016; 124:1032–1040. [DOI] [PubMed] [Google Scholar]
- 17.Leslie K, Myles PS, Chan MT, et al. Risk factors for severe postoperative nausea and vomiting in a randomized trial of nitrous oxide-based vs nitrous oxide-free anaesthesia. Br J Anaesth 2008; 101:498–505. [DOI] [PubMed] [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (Lond, Engl) 2007; 370:1453–1457. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003. Stat Med 2008; 27:2037–2049. [DOI] [PubMed] [Google Scholar]
- 20.Thompson DP, Ashley FL. Face-lift complications: a study of 922 cases performed in a 6-year period. Plast Reconstr Surg 1978; 61:40–49. [DOI] [PubMed] [Google Scholar]
- 21.Jabalameli M, Rouholamin S, Gourtanian F. A comparison of the effects of fentanyl and remifentanil on nausea, vomiting, and pain after cesarean section. Iran J Med Sci 2011; 36:183–187. [PMC free article] [PubMed] [Google Scholar]
- 22.Oh AY, Kim JH, Hwang JW, et al. Incidence of postoperative nausea and vomiting after paediatric strabismus surgery with sevoflurane or remifentanil-sevoflurane. Br J Anaesth 2010; 104:756–760. [DOI] [PubMed] [Google Scholar]
- 23.Stricker PA, Kraemer FW, Ganesh A. Severe remifentanil-induced acute opioid tolerance following awake craniotomy in an adolescent. J Clin Anesth 2009; 21:124–126. [DOI] [PubMed] [Google Scholar]
- 24.de Hoogd S, Ahlers SJ, van Dongen EP, et al. Is intraoperative remifentanil associated with acute or chronic postoperative pain after prolonged surgery? An update of the literature. Clin J Pain 2016; 32:726–735. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu R, Turan AM, Orhan-Sungur M, et al. Remifentanil for general anaesthesia: a systematic review. Anaesthesia 2007; 62:1266–1280. [DOI] [PubMed] [Google Scholar]
- 26.Jenkins LC, Lahay D. Central mechanisms of vomiting related to catecholamine response: anaesthetic implication. Can Anaesth Soc J 1971; 18:434–441. [DOI] [PubMed] [Google Scholar]
- 27.Perreault L, Normandin N, Plamondon L, et al. Middle ear pressure variations during nitrous oxide and oxygen anaesthesia. Can Anaesth Soc J 1982; 29:428–434. [DOI] [PubMed] [Google Scholar]
- 28.Sun R, Jia WQ, Zhang P, et al. Nitrous oxide-based techniques versus nitrous oxide-free techniques for general anaesthesia. Cochrane Database Syst Rev 2015; 11:CD008984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hood DD, Curry R, Eisenach JC. Intravenous remifentanil produces withdrawal hyperalgesia in volunteers with capsaicin-induced hyperalgesia. Anesth Analg 2003; 97:810–815. [DOI] [PubMed] [Google Scholar]
- 30.Cortinez LI, Brandes V, Munoz HR, et al. No clinical evidence of acute opioid tolerance after remifentanil-based anaesthesia. Br J Anaesth 2001; 87:866–869. [DOI] [PubMed] [Google Scholar]


