Abstract
Background and objectives:
Vasospasm-related injury such as delayed ischemic neurological defect (DIND) or cerebral infarction is an important prognostic factor for aneurismal subarachnoid hemorrhage (SAH). Whether cerebrospinal fluid (CSF) drainage can achieve a better outcome in aneurismal SAH patients after coiling or clipping remains the subject of debate. Here, we report a meta-analysis of the related available literature to assess the effect of continuous CSF drainage on clinical outcomes in patients with aneurismal SAH.
Methods:
Case-control studies regarding the association between aneurismal SAH and CSF drainage were systematically identified through online databases (PubMed, Web of Science, Elsevier Science Direct, and Springer Link). Inclusion and exclusion criteria were defined for the eligible studies. The fixed-effects model was performed when homogeneity was indicated. Alternatively, the random-effects model was utilized.
Results:
This meta-analysis included 11 studies. Continuous CSF drainage obviously improved patients’ long-term outcome (odds ratio [OR] of 2.86, 95% confidence interval [CI], 1.37–5.98, P < 0.01). CSF drainage also reduced angiographic vasospasm (OR of 0.35, 95% CI, 0.23–0.51, P < 0.01), symptomatic vasospasm (OR of 0.32, 95% CI, 0.32–0.43, P < 0.01), and DIND (OR of 0.48, 95% CI, 0.25–0.91, P = 0.03), but there was no significant difference between the CSF drainage group and the no CSF drainage group on shunt-dependent hydrocephalus (SDHC) prevention (OR of 1.04, 95% CI, 0.52–2.07, P = 0.91). Further analysis on lumbar drainage (LD) and external ventricular drainage (EVD) indicated that LD had a better outcome (OR of 3.11, 95% CI, 1.18–8.23, P = 0.02), whereas no significant difference in vasospasm-related injury was detected between the groups (OR of 1.13, 95% CI, 0.54–2.37, P = 0.75).
Conclusion:
Continuous CSF drainage is an effective treatment for aneurismal SAH patients; lumbar drainage showed lower complications, but more well-designed studies are required to verify and consolidate this conclusion.
Keywords: aneurismal subarachnoid hemorrhage, complication, CSF drainage, DIND, hydrocephalus, lumbar drainage, meta-analysis, outcome, vasospasm
1. Introduction
Spontaneous subarachnoid hemorrhage (SAH) is a major cause of stroke, accounting for approximately 5% to 7% of cases [1,2]; ruptured aneurysm represents 80% of SAH cases.[3] The incidence of aneurismal SAH is reported to range from 2 to 16 per 100,000.[4] When aneurismal SAH occurs, the following problems should be considered as soon as possible: (1) re-bleeding, (2) increased intracranial pressure and acute hydrocephalus, (3) delayed ischemic neurological deficit (DIND) or cerebral infarction due to vasospasm.[5] Early aneurysm clipping or endovascular coiling can prevent re-bleeding, but vasospasm-related injury remains a threat to patient outcomes.
The management of vasospasm-related injury is difficult. DIND may occur in nearly 50% of patients with vasospasm and can lead to cerebral infarction or death.[6,7] Among all of the pathogenic mechanisms, a blood clot in the subarachnoid space is believed to be an important cause of DIND.[8,9] Treatment by removal of blood clots combined with irrigation of the cisterns with or without thrombolytic or fibrinolytic agents has been studied,[10–14] but the conclusions of these studies were inconsistent. Some studies suggested that cerebrospinal fluid (CSF) drainage reduced the vasospasm-related DIND and improved outcomes,[7,15] whereas others found that CSF drainage had no effect on outcomes.[11,16]
We performed a meta-analysis to determine the effect of CSF drainage on the Glasgow Outcome Scale (GOS) or the modified Rankin Scale (mRS), death rate, vasospasm-related DIND or cerebral infarction, and shunt-dependent hydrocephalus after more than 6 months and to analyze the complications associated with different CSF drainage methods, such as lumbar drainage (LD) and external ventricular drainage (EVD).
2. Method
2.1. Ethical review
The clinical ethics committee of the Second Affiliated Hospital of Zhejiang University School of Medicine approved the study.
2.2. Literature search
We comprehensively searched several electronic databases, including PubMed, Elsevier Science Direct, Web of Science, and Springer Link. The key search terms were “subarachnoid hemorrhage,” “cerebrospinal fluid drainage,” “aneurysm,” “vasospasm,” “delayed ischemic neurological deficit (DIND),” “hydrocephalus,” “cerebral infarction,” “complication,” and “outcome.” All papers published until February, 2016, were included. Additionally, reference lists in the identified publications and the main electronic sources of ongoing trials were also examined. Three authors (CG, JYC, LW) independently evaluated the search results by reading the titles, whereas 2 other reviewing authors (CQ, XBY) independently reviewed the abstracts of the initially screened papers; disagreements were settled by the senior authors (GC and YYD).
Our inclusion criteria for studies were: (1) patients with a digital subtraction angiography (DSA) or computed tomography angiography (CTA) confirmation of aneurismal SAH; (2) randomized controlled trials (RCTs), prospective cohort studies, and retrospective case-control studies; and (3) quality score >5 on the Newcastle–Ottawa Scale (NOS)[17] and quality score >3 on the 7-point modified Jadad scoring system.[18] The exclusion criteria were: (1) a system review or case report; (2) published in a language other than English; (3) only the abstract of a study was available; and (4) studies in which participants presented with coagulopathy (glioma, cirrhosis, etc.).
2.3. Data abstraction
Two review authors (CQ, XBY) independently extracted data using a uniform standardized form until an agreement was reached. The primary outcomes were the Glasgow Outcome Scale (GOS), the modified Rankin Scale (mRS), and death rate, all of which were collected >6 months after the intervention during the follow-up period. The secondary outcomes were vasospasm, DIND or cerebral infarction, shunt-dependent hydrocephalus (SDHC). Other related factors, including population characteristics and CSF drainage complications, such as ischemic or hemorrhagic events, seizure, meningitis, and spinal nerve root injury, were also extracted.
2.4. Statistical analysis
Data were processed in Review Manager Version 5.3 from the Cochrane Collaboration and STATA 13.0. Dichotomous variables are presented as an odds ratio (OR) with a 95% confidence interval (CI). If the I2 value, which indicated heterogeneity, was <50%, a fixed effect model was used; otherwise, a random effect model was adopted. A P < 0.05 was considered significant for all analyses. In addition, Begg's test,[19] Egger's test,[20] and funnel plots were used to detect potential publication bias.
3. Results
3.1. Literature selection and characteristics
The detailed search process is illustrated in the flow chart of Fig. 1. We retrieved 2307 records after the initial search strategy; 15 records were kept for further analysis after scanning the title and abstract. Four records were excluded because they had no data. Finally, 11 articles were included [7,11,14–16,21–27]; among these, 6 articles reported outcomes in 808 patients (GOS >3 or mRS <3 is considered as good outcome), 5 studies reported death rates in 768 patients, 4 studies assessed angiographic vasospasm in 519 patients, 7 studies evaluated symptomatic vasospasm in 886 patients, 5 studies evaluated cerebral infarction in 730 patients, 9 papers focused on shunt-dependent hydrocephalus in 1075 patients, and 2 studies compared lumbar drainage and external ventricular drainage. All of the included studies demonstrated high methodological quality (Table 1).
Figure 1.
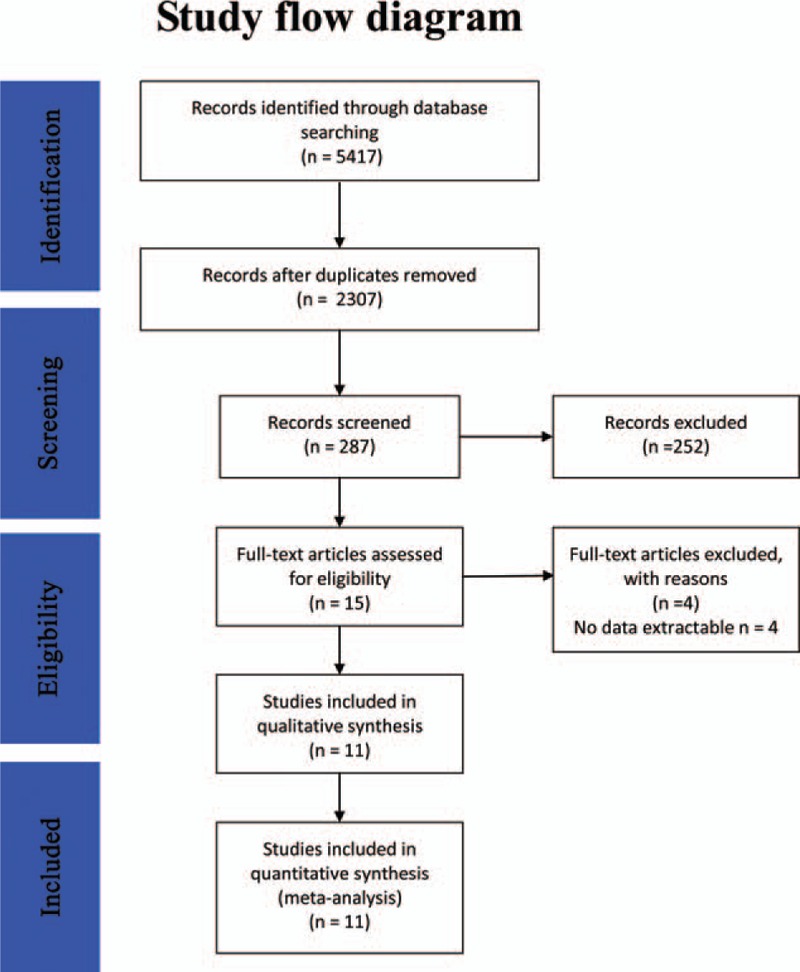
A flow diagram of the selection process for CSF drainage treatment on aneurismal SAH. CSF = cerebrospinal fluid, SAH = subarachnoid hemorrhage.
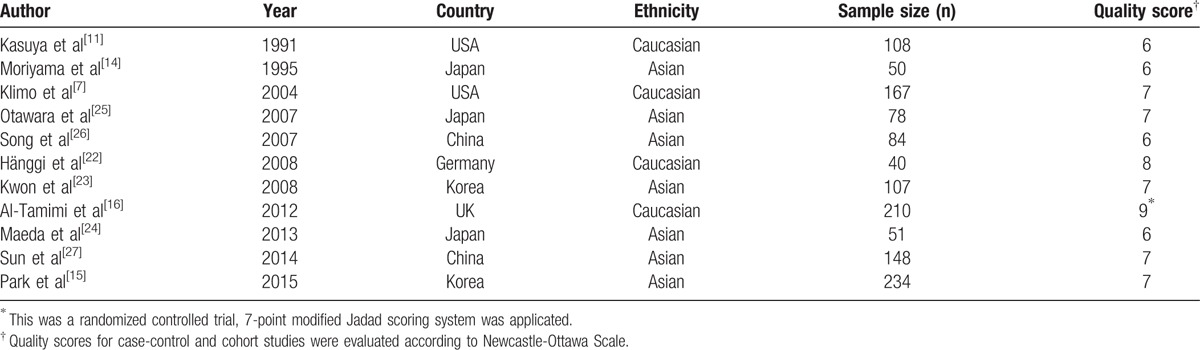
Table 1.
Main characteristics of studies included in this meta-analysis.
3.2. The effect of CSF drainage on outcome improvement in aneurismal SAH patients
A total of 808 patients from 5 studies were included (413 patients underwent CSF drainage and 395 patients received conventional treatment). Based on GOS or mRS, patients with CSF drainage experienced better outcomes (OR of 2.86, 95% CI, 1.37–5.98, P < 0.01, I2 = 75%) (Fig. 2A). No publication bias was found (Begg's test, z = 1.50, P = 0.133; Egger's test, t = 1.32, P = 0.257) (Fig. 3A). In the death analysis, 768 patients from 5 studies were included (393 patients underwent CSF drainage and 375 patients had conventional treatment); CSF drainage resulted in a significant reduction in the death rate (OR of 0.39, 95% CI, 0.20–0.75, P < 0.01, I2 = 0%) (Fig. 2B). No publication bias was detected (Begg's test, z = 0.34, P = 0.734; Egger's test, t = −0.83, P = 0.493) (Fig. 3B).
Figure 2.
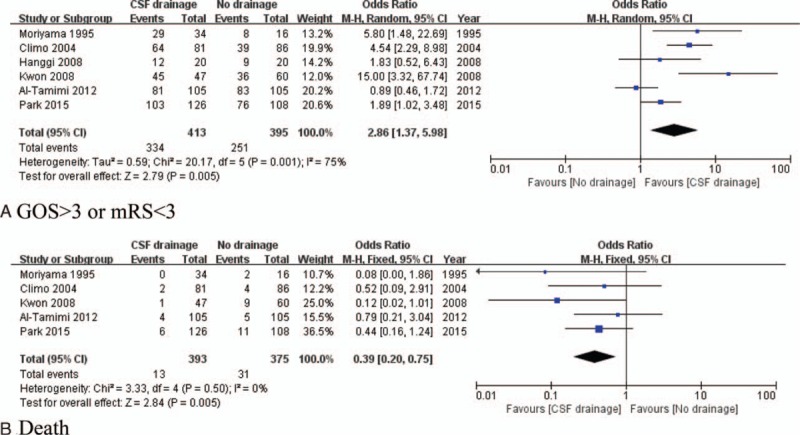
Forest plots for outcomes between CSF drainage and no CSF drainage treatment groups: (A) CSF drainage group had better long-term recovery than no CSF drainage group; (B) CSF drainage group had lower death rate than no CSF drainage group. CSF = cerebrospinal fluid.
Figure 3.
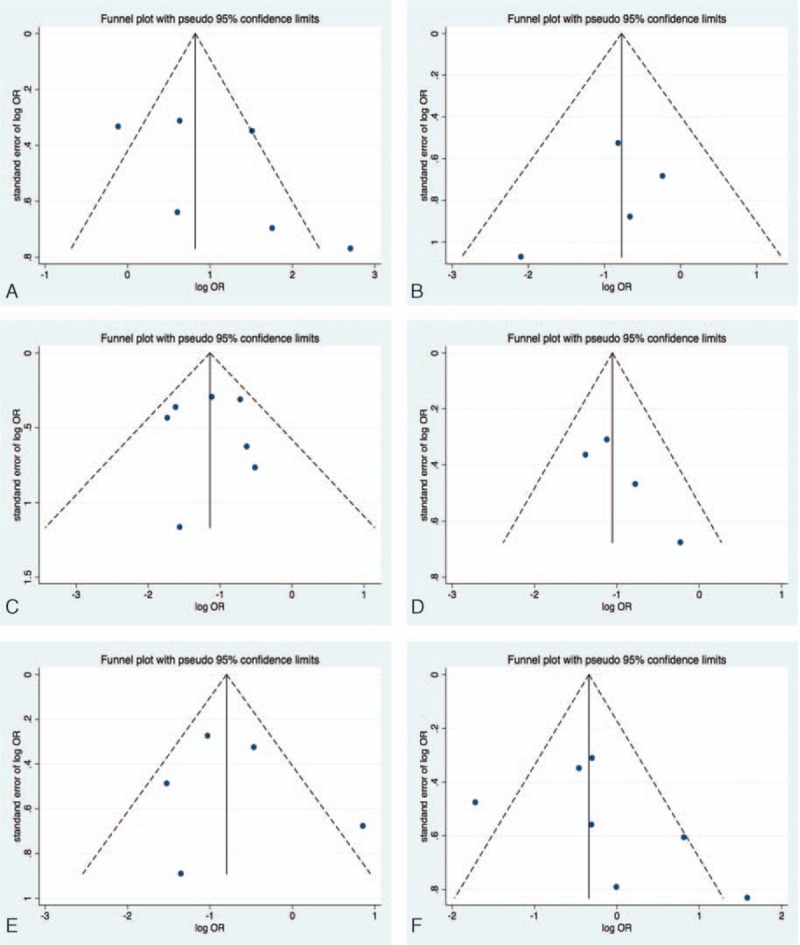
Funnel plots of publication bias: (A) studies comparing GOS or mRS between CSF drainage and non-CSF drainage groups; (B) studies comparing the death rate between CSF drainage and non-CSF drainage groups; (C) studies comparing angiographic vasospasm prevention between CSF drainage and non-CSF drainage groups; (D) studies comparing symptomatic vasospasm prevention between CSF drainage and non-CSF drainage groups; (e) studies comparing cerebral infarction between CSF drainage and non-CSF drainage groups; (f) studies comparing shunt-dependent hydrocephalus between CSF drainage and non-CSF drainage groups. CSF = cerebrospinal fluid, GOS = Glasgow outcome scale.
3.3. The effect of CSF drainage on prevention of vasospasm, vasospasm-related DIND, or cerebral infarction in aneurismal SAH patients
For the analysis of angiographic vasospasm prevention, 519 patients from 4 studies were analyzed (265 patients underwent CSF drainage surgery and 254 patients had no CSF drainage). CSF drainage demonstrated significant efficacy (OR of 0.35, 95% CI, 0.23–0.51, P < 0.01, I2 = 0%) (Fig. 4A). No publication bias was found (Begg's test, z = 0.30, P = 0.764; Egger's test, t = 0.06, P = 0.951) (Fig. 3C). For symptomatic vasospasm prevention analysis, 886 patients from 7 studies were included (451 patients with CSF drainage and 435 patients without CSF drainage), CSF drainage was found to reduce the occurrence of symptomatic vasospasm (OR of 0.32, 95% CI, 0.32–0.43, P < 0.01, I2 = 12%) (Fig. 4B). Publication bias was not detected (Begg's test, z = 1.02, P = 0.308; Egger's test, t = 2.26, P = 0.152) (Fig. 3D). For the analysis of cerebral infarction, 730 patients from 5 studies were included (409 patients underwent CSF drainage and 321 patients had no drainage surgery). CSF drainage reduced the occurrence of vasospasm-related cerebral infarction (OR of 0.48, 95% CI, 0.25–0.91, P = 0.03, I2 = 61%) (Fig. 4C). No publication bias was observed in these studies (Begg's test, z = 0.24, P = 0.806; Egger's test, t = 0.35, P = 0.752) (Fig. 3E).
Figure 4.
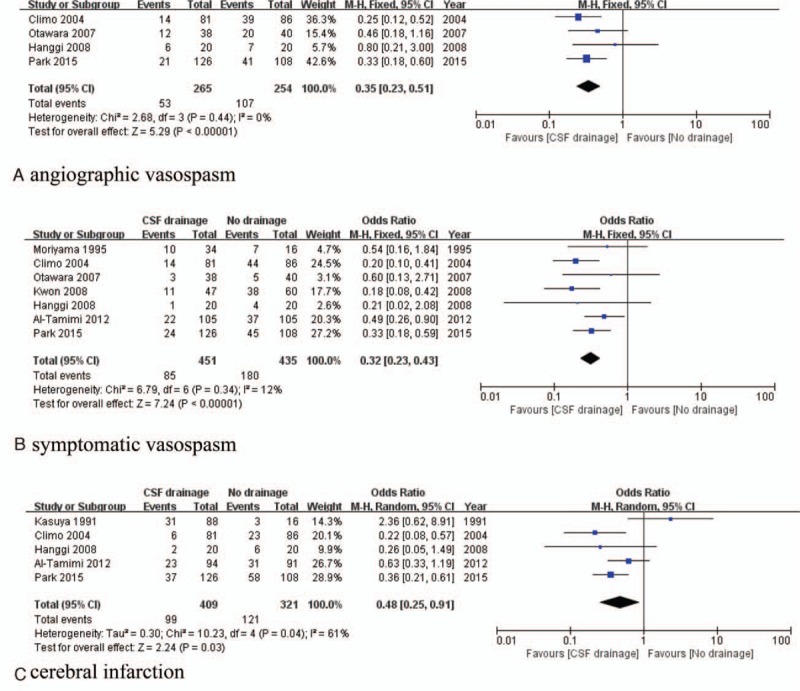
Forest plots for vasospasm-related injury prevention between CSF drainage and no CSF drainage treatment: (A) CSF drainage reduced angiographic vasospasm risk compared with no CSF drainage; (B) CSF drainage reduced sympatomatic vasospasm risk compared with no CSF drainage; (C) CSF drainage reduced DIND or cerebral infarction risk compared with no CSF drainage. CSF = cerebrospinal fluid, DIND = delayed ischemic neurological deficit.
3.4. The effect of CSF drainage on SDHC in aneurismal SAH patients
Nine studies with 1075 patients were included. No difference was observed in terms of SDHC (OR of 1.04, 95% CI, 0.52–2.07, P = 0.91, I2 = 68%) (Fig. 5A). No publication bias was detected (Begg's test, z = 1.50, P = 0.133; Egger's test, t = 1.08, P = 0.329) (Fig. 3F).
Figure 5.
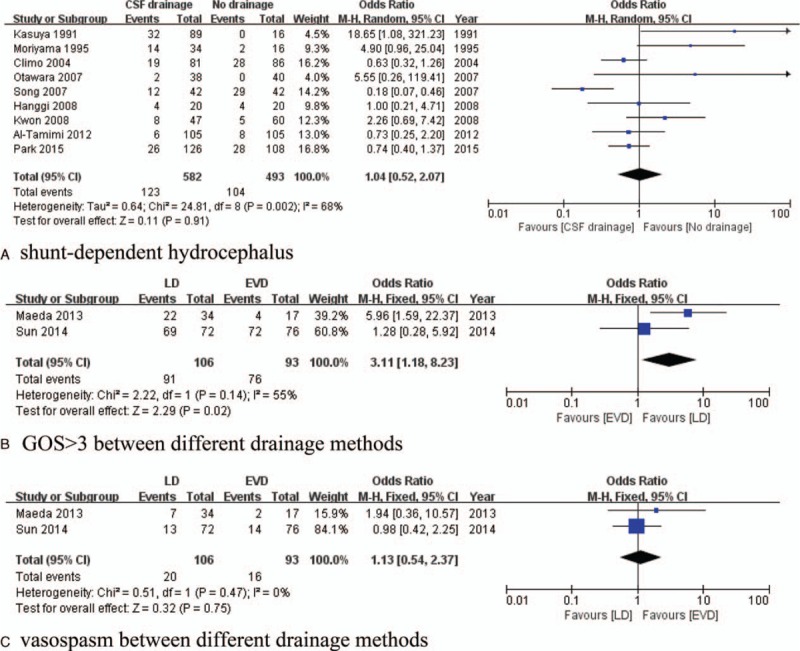
Forest plots for CSF drainage on SDHC prevention and different drainage method comparison: (A) No difference was found in SDHC prevention between CSF drainage group and no CSF drainage group; (B) LD had better long-term recovery than EVD; (C) no difference was found in vasospasm prevention between LD and EVD. CSF = cerebrospinal fluid, EVD = external ventricular drainage, LD = lumbar drainage, SDHC = shunt-dependent hydrocephalus.
3.5. The effect of different methods of CSF drainage and complications of CSF drainage requiring intervention
Two studies were included in this analysis (106 patients underwent LD and 93 patients had EVD). The LD group demonstrated good outcomes (OR of 3.11, 95% CI, 1.18–8.23, P = 0.02, I2 = 55%) (Fig. 5B), but no differences were observed in terms of vasospasm prevention (OR of 1.13, 95% CI, 0.54–2.37, P = 0.75, I2 = 0%) (Fig. 5C). Six studies mentioned CSF drainage complications; 22 cases of complications requiring intervention were reported among 417 patients who underwent CSF drainage (22/417, 5.3%) (10 meningitis or ventriculitis, 2 intracerebral hemorrhage, 2 catheter breakage, 3 neurological worsening, 3 low-pressure headaches, and 2 pseudomeningoceles).
4. Discussion
The prognosis of aneurismal SAH patients is closely related with re-bleeding events which can be caused by including preoperative, intraoperative, or postoperative aneurysm rupture. Various risk factors, such as aneurysm size, location, Hunt & Hess grade, can contribute to the re-bleeding.[28,29] Early clipping or coiling of aneurysms have obviously reduced re-bleeding rates among SAH patients; however, DIND or cerebral infarction due to vasospasm still threatens life and is a major cause of disability.[30] Previous research suggested that blood clots in the subarachnoid space and their breakdown products were closely related to vasospasm.[8] Based on this, many researchers have attempted to solve the problem in various ways.[14,21] Among all of the accepted methods, post-operative continuous CSF drainage has been the focus of most recent attention.[15,24] However, most of the studies were retrospective and the reported results have been inconsistent. Here, we present the first meta-analysis evaluating the effects of post-operative continuous CSF drainage on DIND prevention and outcome improvement in SAH patients.
Our meta-analysis found that CSF drainage reduced the occurrence of both angiographic (20.0% [53/265] vs 42.1% [107/254], OR of 0.35, P < 0.01) and symptomatic (18.8% [85/451] vs 41.4% [180/435], OR of 0.32, P < 0.01) vasospasm compared to no CSF drainage. We also found that CSF drainage significantly reduced the occurrence of DIND or cerebral infarction compared to no CSF drainage (24.2% [99/409] vs 37.4% [121/321], OR of 0.48, P = 0.03). Beyond the acute phase, we also found that the CSF drainage group had better long-term recovery (80.9% [334/413] vs 63.5% [251/395], OR of 2.86, P < 0.01), defined as GOS >3 or mRS <3, and lower death rates (3.3% [13/393] vs 8.3% [31/375], OR of 0.39, P < 0.01). These findings suggest that CSF drainage is an effective treatment for aneurismal SAH after clipping or coiling the aneurysm. On the other hand, CSF drainage might result in drainage-related complications such as ventriculitis or meningitis, neurological function decline, low-pressure headache, and even intracerebral hemorrhage.[7,15,16,31] In this meta-analysis, we found the total incidence of complications to be 5.3% (22/417); all patients recovered after appropriate intervention. Among these complications, neurological function decline was the result of overly rapid drainage of CSF[7]; therefore, close supervision and tight regulation of the drainage speed are necessary in patients undergoing CSF drainage. CSF drainage-related infection, which was closely related to site leakage and the duration of catheterization,[31] showed favorable outcomes with early aggressive treatment such as re-catheterization and antibiotics.
Bae et al[32] reported that SDHC was related to the initial presence of intraventricular hemorrhage (IVH). It seemed that reduction of the blood clot in the subarachnoid space might prevent SDHC, but recently Sugawara et al[34] and Tso et al[33] reported that EVD and increased daily CSF output were predictive of SDHC. In our meta-analysis, the incidence rate of SDHC was not reduced by CSF drainage compared to no drainage (21.1% [123/582] vs 21.1% [104/493], OR of 1.04, P = 0.91). Experimental research has shown that SDHC due to aneurismal SAH was closely related to TGF-β levels, which could accelerate the proliferation of meningeal cells; this finding will require confirmation through appropriate clinical trials.[35]
Both LD and EVD are commonly used for CSF drainage because of their safety and efficacy. Our meta-analysis showed that between these 2 methods, there was no difference in vasospasm prevention (18.9% [20/106] vs17.2% [16/93], OR of 1.13, P = 0.75), but the LD group had better long-term outcomes (85.8% [91/106] vs 81.7% [76/93], OR of 3.11, P = 0.02). Only the study of Sun et al[27] reported that the EVD group had a higher rate of intracerebral hemorrhage. Because only 2 studies compared LD and EVD in terms of CSF drainage outcomes, the results are not convincing and more well-designed studies will be required.
Despite these meaningful findings, there were several limitations in our study. First, only 1 RCT was available and most of the included studies were retrospective. Second, only a few studies reported clear inclusion and exclusion criteria, the latent difference of patients’ baseline conditions such as the location of aneurysms and Hunt & Hess grades may reduce the persuasion of research results, and the differences in the diagnostic criteria and techniques between different hospitals can also introduce bias. Third, the experience in aneurysm clipping or coiling is a crucial factor associated with prognosis, the inconsistent quality of treatment was a shortcoming of this study. Finally, detailed data on CSF drainage complications were insufficient and therefore an advanced analysis could not be conducted.
In conclusion, postoperative continuous CSF drainage reduced vasospasm-related injury and improved outcomes in patients with aneurismal SAH; however, no effect of CSF drainage on SDHC prevention was observed. In addition, LD and EVD had similar effects on vasospasm prevention. Based on these results, we recommend continuous CSF drainage for aneurismal SAH treatment. Although lower complication was found in the LD group, the choice between LD and EVD is still inconclusive due to too few researches; more well-designed RCTs need to be performed.
Footnotes
Abbreviations: CI = confidence interval, CSF = cerebrospinal fluid, CTA = computed tomography angiography, DIND = delayed ischemic neurological deficit, DSA = digital subtraction angiography, EVD = external ventricular drainage, GOS = Glasgow Outcome Scale, H-H = Hunt & Hess, IVH = intraventricular hemorrhage, LD = lumbar drainage, mRS = modified Rankin Scale, NOS = Newcastle–Ottawa Scale, OR = odds ratio, RCTs = randomized controlled trials, SAH = subarachnoid hemorrhage, SDHC = shunt-dependent hydrocephalus.
CQ and XY contributed equally to this study.
The authors have no funding or conflicts of interest to disclose.
References
- 1.Becker KJ. Epidemiology and clinical presentation of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am 1998; 9:435–444. [PubMed] [Google Scholar]
- 2.Kaptain GJ, Lanzino G, Kassell NF. Subarachnoid haemorrhage: epidemiology, risk factors, and treatment options. Drugs Aging 2000; 17:183–199. [DOI] [PubMed] [Google Scholar]
- 3.van Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001; 124 (pt 2):249–278. [DOI] [PubMed] [Google Scholar]
- 4.Feigin VL, Lawes CM, Bennett DA, et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 2009; 8:355–369. [DOI] [PubMed] [Google Scholar]
- 5.Murayama Y, Malisch T, Guglielmi G, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: report on 69 cases. J Neurosurg 1997; 87:830–835. [DOI] [PubMed] [Google Scholar]
- 6.Kassell NF, Torner JC, Haley EC, Jr, et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: overall management results. J Neurosurg 1990; 73:18–36. [DOI] [PubMed] [Google Scholar]
- 7.Klimo P, Jr, Kestle JR, MacDonald JD, et al. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg 2004; 100:215–224. [DOI] [PubMed] [Google Scholar]
- 8.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery 1980; 6:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke 2001; 32:2012–2020. [DOI] [PubMed] [Google Scholar]
- 10.Inagawa T, Kamiya K, Matsuda Y. Effect of continuous cisternal drainage on cerebral vasospasm. Acta Neurochir (Wien) 1991; 112:28–36. [DOI] [PubMed] [Google Scholar]
- 11.Kasuya H, Shimizu T, Kagawa M. The effect of continuous drainage of cerebrospinal fluid in patients with subarachnoid hemorrhage: a retrospective analysis of 108 patients. Neurosurgery 1991; 28:56–59. [DOI] [PubMed] [Google Scholar]
- 12.Hoekema D, Schmidt RH, Ross I. Lumbar drainage for subarachnoid hemorrhage: technical considerations and safety analysis. Neurocrit Care 2007; 7:3–9. [DOI] [PubMed] [Google Scholar]
- 13.Mizoi K, Yoshimoto T, Takahashi A, et al. Prospective study on the prevention of cerebral vasospasm by intrathecal fibrinolytic therapy with tissue-type plasminogen activator. J Neurosurg 1993; 78:430–437. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama E, Matsumoto Y, Meguro T, et al. Combined cisternal drainage and intrathecal urokinase injection therapy for prevention of vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 1995; 35:732–736. [DOI] [PubMed] [Google Scholar]
- 15.Park S, Yang N, Seo E. The effectiveness of lumbar cerebrospinal fluid drainage to reduce the cerebral vasospasm after surgical clipping for aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 2015; 57:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Tamimi YZ, Bhargava D, Feltbower RG, et al. Lumbar drainage of cerebrospinal fluid after aneurysmal subarachnoid hemorrhage: a prospective, randomized, controlled trial (LUMAS). Stroke 2012; 43:677–682. [DOI] [PubMed] [Google Scholar]
- 17.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 18.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Findlay JM, Kassell NF, Weir BK, et al. A randomized trial of intraoperative, intracisternal tissue plasminogen activator for the prevention of vasospasm. Neurosurgery 1995; 37:168–176.discussion 177-168. [PubMed] [Google Scholar]
- 22.Hanggi D, Liersch J, Turowski B, et al. The effect of lumboventricular lavage and simultaneous low-frequency head-motion therapy after severe subarachnoid hemorrhage: results of a single center prospective Phase II trial. J Neurosurg 2008; 108:1192–1199. [DOI] [PubMed] [Google Scholar]
- 23.Kwon OY, Kim YJ, Kim YJ, et al. The utility and benefits of external lumbar CSF drainage after endovascular coiling on aneurysmal subarachnoid hemorrhage. J Korean Neurosurg Soc 2008; 43:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda Y, Shirao S, Yoneda H, et al. Comparison of lumbar drainage and external ventricular drainage for clearance of subarachnoid clots after Guglielmi detachable coil embolization for aneurysmal subarachnoid hemorrhage. Clin Neurol Neurosurg 2013; 115:965–970. [DOI] [PubMed] [Google Scholar]
- 25.Otawara Y, Ogasawara K, Kubo Y, et al. Effect of continuous cisternal cerebrospinal fluid drainage for patients with thin subarachnoid hemorrhage. Vasc Health Risk Manag 2007; 3:401–404. [PMC free article] [PubMed] [Google Scholar]
- 26.Song JN, Liu SX, Bao G, et al. Effect of drainage of the cerebrospinal fluid at the acute period of aneurysmal subarachnoid hemorrhage on the formation of hydrocephalus. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2007; 19:329–331. [PubMed] [Google Scholar]
- 27.Sun C, Du H, Yin L, et al. Choice for the removal of bloody cerebrospinal fluid in postcoiling aneurysmal subarachnoid hemorrhage: external ventricular drainage or lumbar drainage? Turk Neurosurg 2014; 24:737–744. [DOI] [PubMed] [Google Scholar]
- 28.Chowdhury T, Petropolis A, Wilkinson M, et al. Controversies in the anesthetic management of intraoperative rupture of intracranial aneurysm. Anesthesiol Res Pract 2014; 2014:595837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury T, Cappellani RB, Sandu N, et al. Perioperative variables contributing to the rupture of intracranial aneurysm: an update. Scient World J 2013; 2013:396404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim GM, Macdonald RL. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. Neurocrit Care 2013; 19:140–149. [DOI] [PubMed] [Google Scholar]
- 31.Liang H, Zhang L, Gao A, et al. Risk factors for infections related to lumbar drainage in spontaneous subarachnoid hemorrhage. Neurocrit Care 2016; 25:243–249. [DOI] [PubMed] [Google Scholar]
- 32.Bae IS, Yi HJ, Choi KS, et al. Comparison of incidence and risk factors for shunt-dependent hydrocephalus in aneurysmal subarachnoid hemorrhage patients. J Cerebrovasc Endovasc Neurosurg 2014; 16:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tso MK, Ibrahim GM, Macdonald RL. Predictors of shunt-dependent hydrocephalus following aneurysmal subarachnoid hemorrhage. World Neurosurg 2016; 86:226–232. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara T, Maehara T, Nariai T, et al. Independent predictors of shunt-dependent normal pressure hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg Sci 2016; 60:154–158. [PubMed] [Google Scholar]
- 35.Li T, Zhang P, Yuan B, et al. Thrombin-induced TGF-beta1 pathway: a cause of communicating hydrocephalus post subarachnoid hemorrhage. Int J Mol Med 2013; 31:660–666. [DOI] [PubMed] [Google Scholar]


