Abstract
Respiratory syncytial virus (RSV) is one of the most common etiological agents of childhood respiratory infections globally. Information on seasonality of different antigenic groups is scarce. We aimed to describe the frequency, seasonality, and age of children infected by RSV antigenic groups A (RSVA) and B (RSVB) among children with ARI in a 4-year period.
Children (6–23 months old) with respiratory infection for ≤7 days were enrolled in a prospective cross-sectional study, from September, 2009 to October, 2013, in Salvador, in a tropical region of Brazil. Upon recruitment, demographic, clinical data, and nasopharyngeal aspirates (NPA) were collected. A multiplex quantitative real-time polymerase chain reaction (RT-PCR) with a group-specific primer and probeset for RSVA and RSVB was used. Seasonal distribution of infection by RSV different antigenic groups was evaluated by Prais-Wisten regression.
Of 560 cases, the mean age was 11.4 ± 4.5 months and there were 287 (51.3%) girls. Overall, RSV was detected in 139 (24.8%; 95% CI: 21.4%–28.5%) cases, RSVA in 74 (13.2%; 95% CI: 10.6%–16.2%) cases, and RSVB in 67 (12.0%; 95% CI: 9.5%–14.9%) cases. Two (0.4%; 95% CI: 0.06%–1.2%) cases had coinfection. RSVA frequency was 9.6%, 18.4%, 21.6%, and 3.1% in 2010, 2011, 2012, and 2013, respectively. RSVB frequency was 19.2%, 0.7%, 1.4%, and 35.4% in the same years. RSVA was more frequently found from August to January than February to July (18.2% vs. 6.4%, P < 0.001). RSVB was more frequently found (P < 0.001) between March and June (36.0%) than July to October (1.0%) or November to February (1.6%). RSVB infection showed seasonal distribution and positive association with humidity (P = 0.02) whereas RSVA did not. RSVA was more common among children ≥1-year-old (17.8% vs. 1.8%; P = 0.02), as opposed to RSVB (11.5% vs. 12.2%; P = 0.8).
One quarter of patients had RSV infection. RSVA compromised more frequently children aged ≥1 year. RSVA predominated in 2011 and 2012 whereas RSVB predominated in 2010 and 2013. In regard to months, RSVA was more frequent from August to January whereas RSVB was more often detected between March and June. Markedly different monthly as well as yearly patterns for RSVA and RSVB reveal independent RSV antigenic groups’ epidemics.
Keywords: acute respiratory infection, immunoprophylaxis, palivizumab, RSV, RSVA, RSVB, seasonality
1. Introduction
Acute respiratory infections (ARI) are the leading cause of mortality in children less than 5 years old worldwide.[1] Viruses are major contributors to the morbidity and mortality of ARI, and respiratory syncytial virus (RSV) is one of the most common etiological agents of respiratory infections such as bronchiolitis and pneumonia.[2,3] It is estimated that nearly all children have been infected by RSV by their second birthday.[4] Surveillance to track RSV circulation is essential to establish effective preventive measures to control RSV-associated severe ARI episodes.[5] RSV incidence can be highly variable within countries and between regions within a country depending on the level of seasonal constraining.[6] Temperature, rainfall, and humidity have been postulated to significantly impact RSV seasonality[7] and information on seasonality of different antigenic groups is scarce.
In this study, we aimed to describe the frequency, seasonality, and age of children infected by RSV antigenic groups A (RSVA) and B (RSVB) among children with ARI in a 4-year period.
2. Methods
2.1. Study design
This prospective cross-sectional study evaluated children with ARI seen at the Pediatric Emergency Department of the Federal University of Bahia Hospital, in Salvador, Northeastern Brazil, between September, 2009 and October, 2013. This city is located in a tropical region, the latitude being 12°58′16″S, the longitude 38°30′39″W, and the altitude 8 m. Inclusion criteria comprised children aged from 6 to 23 months with fever, sneeze, running nose, nasal blockage, or cough for up to 7 days. Children transferred from other hospitals or reporting a previous episode of wheeze were excluded. Clinical and demographic data, as well as findings on physical examination, were collected using a standardized questionnaire.
2.2. Clinical definitions
Fever was defined as axillary temperature >37.4 °C,[8] and tachypnea as respiratory rate (RR) ≥50 breaths/min among children aged 6 to 11 months and RR ≥40 breaths/min in children from 12 months of age upwards.[9] Lower respiratory tract infection (LRTI) was considered when the child had tachypnea and/or any respiratory distress or crackles or wheezing.[10] Age strata were defined as: under 1 year when children were ≤364 days old, and 1 year and above when children were ≥365 days old.
2.3. Laboratory procedures
Nasopharyngeal aspirate (NPA) samples were collected at study enrollment, placed in Nuclisens Lysis Buffer (Biomeriux, Boxtel, The Netherlands) and frozen at −70 °C. Total ribonucleic acid was extracted using RNEasy (Qiagen's, Hilden, Germany) following manufacturer's instructions. A multiplex quantitative RT-PCR with a group-specific primer and probeset for RSVA and RSVB was used to identify the different antigenic groups of the virus.[11] The carboxyterminal region of the G protein was amplified using the One-Step RT-PCR (Qiagen, Westburg, The Netherlands). The RSVA forward and reverse primers, G267FW and F164RV, respectively, and the RSVB forward and reverse primers, BGF and BGR, respectively, were employed.[11]
2.4. Meteorological data
Data about rainfall, relative humidity, air temperature, and hours of sunshine were provided by the Institute of Environment and Water Resources (INMET) in the State of Bahia with no missing data over the months studied. The meteorological station that provided the data is located approximately 2 km away from the Emergency Department where the study was carried out. Rainfall was measured as daily precipitation, in millimeters, and was analyzed as the total quantity of the month. Relative humidity (%) and air temperature (°C) were measured three times per day, and these were averaged monthly. Hours of sunshine were measured as daily duration of sunshine, in hours, and were calculated as monthly totals.
2.5. Statistical analysis
Sample size was estimated on an expected frequency of RSV infection of 30%, total width of confidence interval equal to 0.10, and 99% confidence level. Thus, the sample size was estimated at 558 cases. Categorical variables were presented as absolute numbers (percentage) and continuous variables as the median (interquartile range [IQR]). 95% Confidence Interval (95% CI) was calculated for the outcome variables. Categorical variables were compared using the corrected chi-square or Fisher's exact test, as appropriate. The seasons and their corresponding months were: summer (January, February, March), fall (April, May, June), winter (July, August, September), and spring (October, November, December). Time series analysis using Prais-Winsten generalized linear regression (Y(i) = b0 + b1∗X(i) + b2∗sin[2πX(i)/12] + b3∗cos[2πX(i)/12]—in which “Y(i)" is the time series measure for each moment “i”; “b0” is a constant; “b1” is the trend indicator; “b2” and “b3” are seasonality indicators) was used for seasonality detection of logarithmic transformed monthly RSVA or RSVB number of cases. This regression model detects monthly fluctuations in the number of cases that are consistent between years when the coefficient “b2” or “b3” is statistically different from zero. The association between logarithmically transformed monthly RSVA or RSVB number of cases with monthly values of meteorological factors was evaluated by multiple regression using Prais-Winsten generalized linear regression. Statistical tests were two-tailed, with a significance level of 0.05. The software Stata version 12 (StataCorp, USA) was used for these analyses.
2.6. Ethical approval
The study was approved by the Ethics Committee at the Federal University of Bahia. Parents or legal guardians signed written informed consent forms before enrollment of each study participant.
3. Results
Among 1154 evaluated children, 504 (43.7%) reported at least 1 previous episode of wheezing, 16 (1.4%) did not have NPA collected successfully, 11 (1.0%) came from other hospitals and 63 (5.4%) did not give consent. Thus, the study group comprised 560 cases. The mean age was 11.4 ± 4.5 months and there were 287 (51.3%) girls. The most frequent complaints were cough (86.8%), running nose (83.9%), fever (82.5%), and sneeze (76.4%). On physical examination, the most frequent findings were fever (40.3%), ronchi (37.0%), and tachypnea (23.6%). Overall, 230 (41.1%) patients had lower respiratory tract involvement, out of which 130 (57%) had tachypnea, 92 (40%) presented wheezing, 51 (22.2%) presented crackles, and 32 (13.9%) presented thoracic recession. Forty-six (8.2%) children attended day care centers.
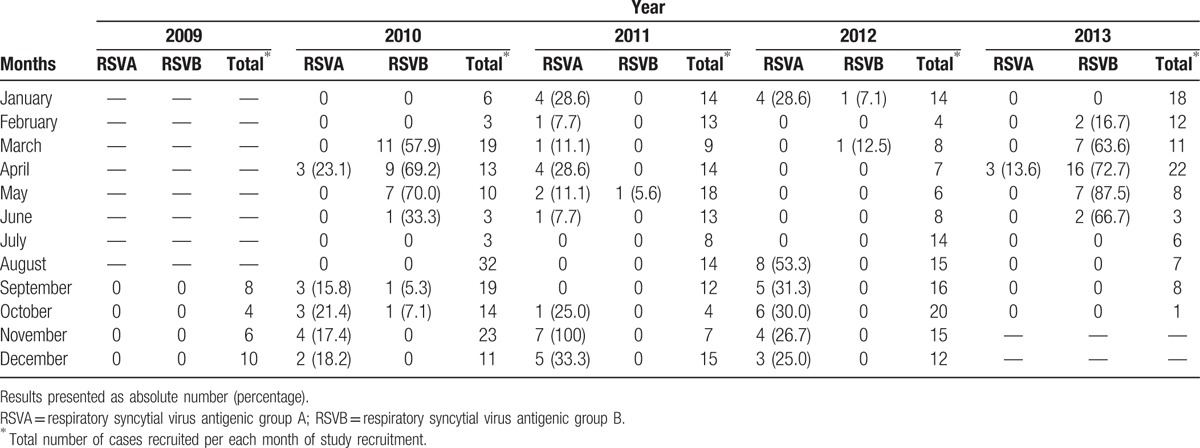
Overall, RSV was detected in 139 (24.8%; 95% CI: 21.4%–28.5%) cases, RSVA in 74 (13.2%; 95% CI: 10.6%–16.2%) cases, and RSVB in 67 (12.0%; 95% CI: 9.5%–14.9%) cases. Two (0.4%; 95% CI: 0.06%–1.2%) were coinfected. Table 1 shows the distribution of RSVA or B cases per year of study. RSVA occurred more frequently in 2011 and 2012 whereas RSVB occurred more often in 2010 and 2013. RSVB frequency demonstrated seasonal distribution (b2 = −0.44 and b3 = −0.24; P = 0.01) and was more frequent (P < 0.001) in March to June (36.0%) compared with July to October (1.0%) or November to February (1.6%). Conversely, RSVA was more frequent from August to January compared with February to July (18.2% vs. 6.4%, P < 0.001). RSVA cases were distributed in several distinct months in different years. The RSVA seasonal pattern was not statistically significant on a monthly basis. Table 2 shows the monthly distribution of RSVA and RSVB during the study period. It is possible to verify that there was an alternating pattern between RSVA and RSVB cases.
Table 1.
Distribution of respiratory syncytial virus antigenic group A and respiratory syncytial virus antigenic group B cases per year of study among children with acute respiratory infection aged 6 to 23 months in a University Hospital in Salvador, Northeast Brazil, between September, 2009 and October, 2013.
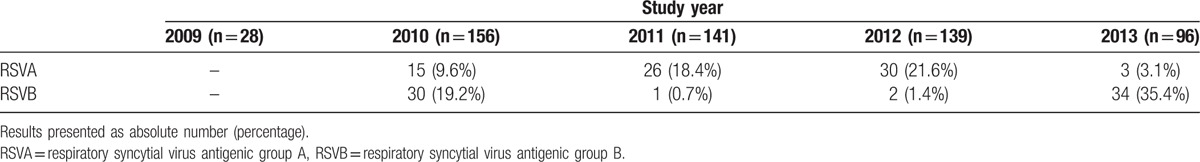
Table 2.
Monthly distribution of respiratory syncytial virus antigenic group A and respiratory syncytial virus antigenic group B cases among children with acute respiratory infection aged 6 to 23 months in a University Hospital in Salvador, Northeast Brazil, between September, 2009 and October, 2013.
There was a positive association between monthly RSVB number of cases and humidity (multiple regression coefficient for humidity = 0.10; P = 0.02). There was no association between RSVA frequency and meteorological factors. Figure 1 shows the monthly distribution of mean relative humidity and of RSVA and RSVB number of cases during the study period. All meteorological factors showed seasonal distribution (Fig. 2).
Figure 1.
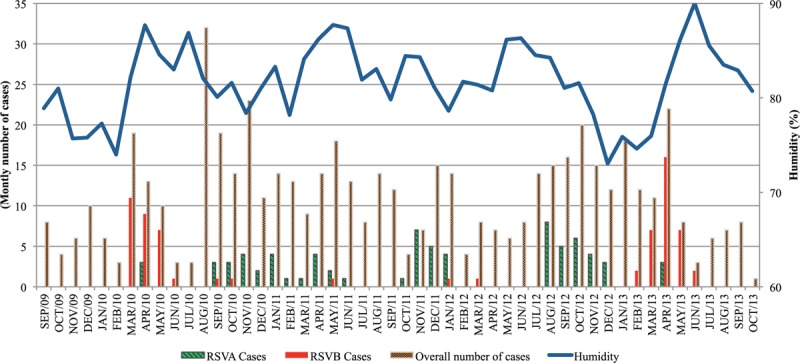
Monthly distribution of respiratory syncytial virus antigenic group A and respiratory syncytial virus antigenic group B cases among total number of children with acute respiratory infection aged 6 to 23 months and of mean relative humidity (%) in Salvador, Northeastern Brazil, between September, 2009 and October, 2013.
Figure 2.
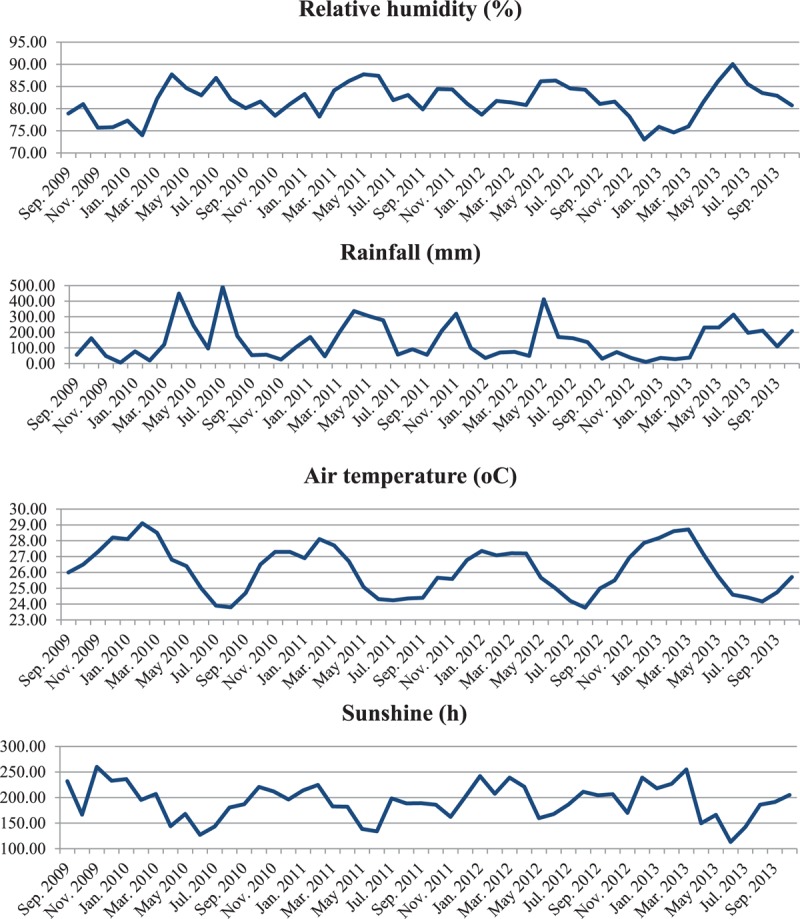
Seasonal distribution of meteorological factors during the study period in Salvador, Northeastern Brazil.
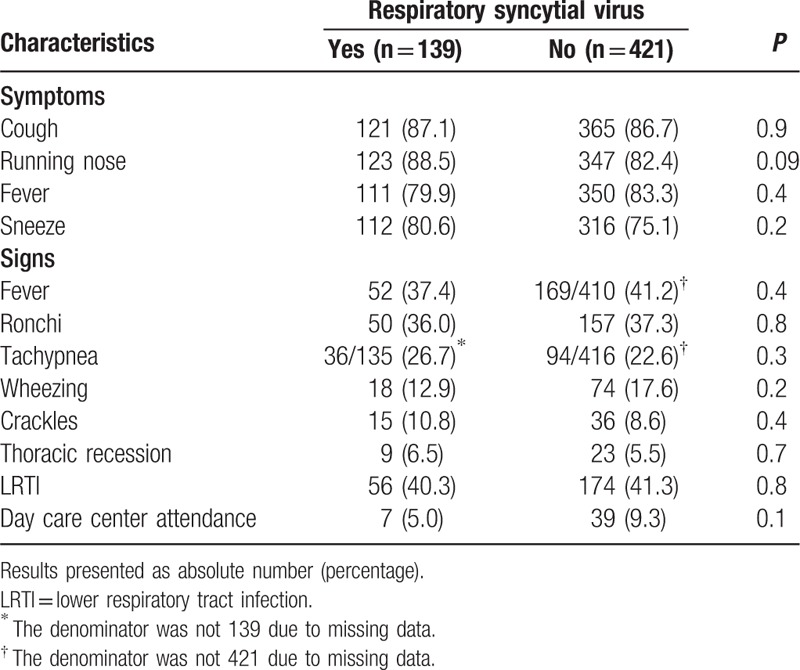
Table 3 shows that RSVA was more frequent among children aged 1 year and above compared with younger children (17.8% vs. 10.8%; P = 0.02). No difference was found in the distribution of RSVB in regard to age strata (11.5% vs. 12.2%; P = 0.8). RSVA was similarly found among patients with or without their lower respiratory tract being compromised (12.6% vs. 13.6%; P = 0.7). Likewise, RSVB was similarly distributed among these subgroups (12.2% vs. 11.8%; P = 0.9). Table 4 compares the frequency of symptoms, signs, and day care center attendance between cases with or without RSV detected. No difference was found.
Table 3.
Comparison of different respiratory syncytial virus antigenic groups infection frequency among children with acute respiratory infection aged 6 to 11 months or 12 to 23 months.
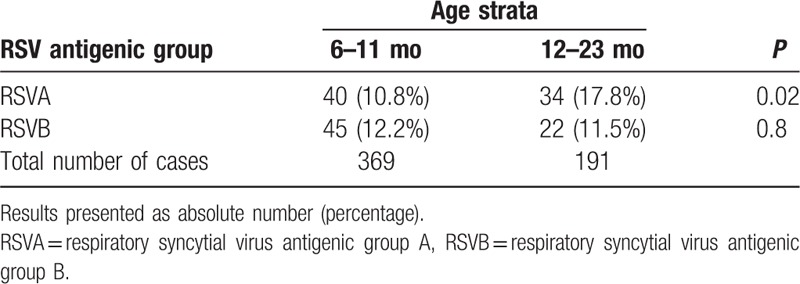
Table 4.
Comparison of the frequency of symptoms, signs, and day care center attendance between children with acute respiratory infection with or without respiratory syncytial virus detected in nasopharyngeal aspirates.
4. Discussion
RSVB infection showed seasonal distribution with higher frequency in late summer and fall and had positive correlation with humidity. In contrast, RSVA infection did not show a consistent seasonal pattern throughout the 4 years study. That is, RSVA and RSVB infection occurred in different months in this tropical city. To the best of our knowledge, this study is the first to demonstrate that infection by the different antigenic groups RSVA or RSVB occurs in different months during the year.
Our study has limitations. It was conducted in a single center. Similar studies enrolling children at multiple centers throughout the full 12 calendar months and for at least 4 years are necessary in order to reach definitive conclusions about the different seasonal characteristics of RSVA and RSVB. Moreover, genotype data on the different circulating subtypes are not available. Additionally, children aged under 6 months were not included. However, the option to study children aged ≥6 months was due to the fact that these children usually go out and are in contact with people living in the community. Breastfeeding is very frequent in our country during the first 6 months of life as maternity leave lasts for 6 months after delivery. Therefore, children aged <6 months are less exposed than those aged ≥6 months.
RSV infection distribution has been recently addressed between April 2012 and March 2013 among children younger than 2 years hospitalized with LRTI in 4 cities in Northeastern Brazil; however, the infection distribution of the different antigenic groups A and B was not assessed.[12] It has been shown that the activity of RSVA and B usually occurs in association with the rainy season and there is evidence that temperature and associated factors, primarily humidity, are important factors in the seasonality.[7] These factors can influence the fusion of viruses with the cellular membranes allowing for cell entry and replication. Humidity strongly influences virus stability and transmissibility.[13] Herein, infection by RSVB was positively associated with humidity. Different studies conducted in Belgium, Finland, Pakistan, the UK, and Uruguay have presented the cocirculation of RSVA and B in the same period of the year.[11,14–16] However, in our study, which lasted 4 years and was conducted in the tropical city of Salvador, Brazil, the monthly circulation of RSVA and B was markedly different. There were different periods of high humidity during the 4 years studied (Fig. 1). Herein, RSVA and B circulated not only in different months but also in different years with alternating yearly patterns (Table 1). That is why RSVA and RSVB coinfection rate was very low (0.4%). The pattern of 2 to 4 consecutive years of group A predominance followed by a single intervening year in which group B is dominant was reported in countries like Uruguay and Finland.[14,15] Competitive relationship and cross-immunity between these antigenic groups have been reported in the population. Following infection, individuals gain transient immunity which average duration is 2 years.[15]
Among our cases, overall, RSV was found in 24.8%. A study conducted in Southeastern Brazil reported that 27.4% of children aged less than 2 years with ARI had RSV.[2] Other studies conducted in the Northern and Southeastern Brazil presented similar frequencies in children aged less than 2 to 3 years with ARI.[17,18] One study carried out in another city in Northeastern Brazil showed that 48% of the children had RSV. This finding may be attributed to the study period, as the study was carried out only in the rainy season.[19] It has been shown that RSV frequencies depend on local climate and can vary between 27.8% and 37.9% in temperate, subtropical, and tropical countries, in the same age population.[6,20–23] We showed that there was no difference between the overall RSV groups frequency (13.2% vs.12.0%). However, RSVA is usually predominant in the majority of the studies involving children with ARI.[12,14,20,24] RSVA predominance has been attributed to the higher variability among these group strains and normally the dominant strain has a mechanism for frequent reinfections by evasion of immunity induced by previous strains.[25] This absence of RSVA predominance in our study may be explained by the unique total length of the study (4 years), of which 2 years were dominated by RSVA and 2 years were dominated by RSVB.
We found that RSVA was more common among children aged 1 year and above. A study conducted in 7 different countries presented that, across all countries, RSVA was more frequent in children aged 36 to 59 months whereas the lowest frequency was detected in the 6 to 11 months old group. In that study, children between 6 months and 10 years of age were included.[26] In general, it has been described that RSV is frequent in children under 1 year of age. However, the distribution of different antigenic groups (A and B) is not commonly reported.
Almost half of the children presented lower respiratory tract involvement (41.1%) and the frequency of RSVA and B was not different among children with or without such involvement. It has been reported that children with RSV infection present frequent involvement of the lower respiratory tract.[27,28] Our samples were collected from children in the emergency department irrespective of severity. Moreover, the etiological diagnoses were performed with very sensitive methods. That is, this study included primarily children with ARI (not only LRTI) and RSV was searched for by very sensitive tests. This strategy allowed us to detect RSV among children with ARI, without predefined selection in favor of cases with more severe presentation (with lower respiratory tract involvement).
The monthly and yearly distribution of infection by RSV is usually addressed during fall and winter months, all over the world. As such, the distribution of the cases infected by RSV is known to occur during the RSV season. Based on these findings, recommendations for immunoprophylaxis for high risk groups of severe disease have been made as the RSV distribution along the year impacts on the immunoprophylaxis calendar with monoclonal antibody. In Brazil, palivizumab applications are recommended between February and July in the Northeastern region.[29] This practice is based on the assumption that RSV circulates between March and July in these regions. This routine is influenced by guidelines developed and implemented in countries in temperate regions.[30] However, we demonstrated that RSV infection occurs not only during the expected RSV season. Indeed, RSVB infection occurs during the RSV season but this is not the case for RSVA. Taking into account that our results were provided in a 4-year period of data collection, guidelines for specific immunoprophylaxis against RSV should be reviewed in the tropical region. In the current form, prophylaxis is appropriate for infection by RSVB but not for infection by RSVA. Furthermore, our findings highlight the necessity to implement RSV vaccine, which should be effective against both antigenic groups A and B, as vaccination confers protection for longer periods of time than the current available passive immunization (palivizumab). Further studies conducted in tropical regions with data collection during the whole year calendar for at least 4 years would be highly appreciated. The duration of our study was fundamental in order to find our results.
5. Conclusion
We conclude that seasonality was markedly different between distinct antigenic groups, when RSVA presented a non-seasonal pattern. A positive association between RSVB and humidity was found. One quarter of the patients had RSV detected, similar to other studies in the same region. RSVA compromised more frequently children aged 1 year and above. Guidelines for specific immunoprophyl axis against RSV should be reviewed in the tropical region as the circulation of RSV is supposedly at the same time among otherwise healthy children as among high risk groups of severe RSV infection.
Acknowledgments
5.1. The acute respiratory infection and wheeze study group phase I and II
Elaine N. Pacheco,1 Eliana Silva,2 Eveline Xavier,1 Karen S. Miranda,2 Laíse R. Neri,2 Patrícia F. Silva,2 Ramom S. Amoedo,1 Rômulo B. Menezes,2 Sylvia Patrícia T. Ledo2 and Thamirys Marinho.1
Affiliations:1Federal University of Bahia School of Medicine, Salvador, CEP 40020-210, Brazil.
2Bahiana School of Medicine, Bahiana Foundation for Science Development, Salvador, CEP 40290-000, Bahia, Brazil.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, ARI = acute respiratory infections, IQR = interquartile range, LRTI = lower respiratory tract infection, NPA = nasopharyngeal aspirates, RR = respiratory rate, RSV = respiratory syncytial virus, RSVA = respiratory syncytial virus antigenic group A, RSVB = respiratory syncytial virus antigenic group B, RT-PCR = real-time polymerase chain reaction.
JVW and CMNC are senior authors and have contributed equally to this work.
AB, CIO, and CMNC are senior investigators at Brazilian Council for Scientific and Technological Development (CNPq), Brazil.
Members of the group are listed at the end of this article.
This work was supported by the Bahia State Agency for Research Funding (FAPESB) [grant numbers APP0045/2009 and PNX0019/2009]. FAPESB had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
There was no writing assistance.
The authors have no conflicts of interest to disclose.
References
- 1.Nair H, Simões EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013; 381:1380–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Durigon GS, Oliveira DB, Felicio MC, et al. Poor outcome of acute respiratory infection in young children with underlying health condition in Brazil. Int J Infect Dis 2015; 34:e3–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nascimento-Carvalho CM, Cardoso MRA, Barral A, et al. Seasonal patterns of viral and bacterial infections among children hospitalized with community-acquired pneumonia in a tropical region. Scan J Infect Dis 2010; 42:839–844. [DOI] [PubMed] [Google Scholar]
- 4.Borchers AT, Chang C, Gershwin ME, et al. Respiratory syncytial virus - a comprehensive review. Clin Rev Allerg Immnunol 2013; 45:331–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimberlin DW, Brady MT, Jackson MA, Long SS. American Academy of Pediatrics. Respiratory syncytial virus: initiation and termination of immunoprophylaxis. Red Bood: 2015 Report of the Committee on Infectious Diseases 30th ed.Elk Grove Village, IL: American Academy of Pediatrics; 2015. 672. [Google Scholar]
- 6.Moore HC, Jacoby P, Hogan AB, et al. Modelling the seasonal epidemics of respiratory syncytial virus in young children. PLoS One 2014; 9:e100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes AK, Manangan AP, Iwane MK, et al. Respiratory syncytial virus circulation in seven countries with global disease detection regional centers. J Infect Dis 2013; 208 (Suppl. 3):S246–S254. [DOI] [PubMed] [Google Scholar]
- 8.El-Radhi AS, Barry W. Thermometry in paediatric practice. Arch Dis Child 2006; 91:351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Integrated Management of Childhood Illness Chart Booklet. (WC 503.2). Geneva: WHO, 2008. Available at: http://whqlibdoc.who.int/publications/2008/9789241597289_eng.pdf Accessed 15 January 2009. [Google Scholar]
- 10.Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011; 66 (Suppl. 2):ii1–ii23. [DOI] [PubMed] [Google Scholar]
- 11.Houspie L, Lemey P, Keyaerts E, et al. Circulation of HRSV in Belgium: from multiple genotype circulation to prolonged circulation of predominant genotypes. PLoS One 2013; 8:e60416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurgel RQ, Bezerra PG, Duarte MC, et al. Relative frequency, possible risk factors, viral codetection rates, and seasonality of respiratory syncytial virus among children with lower respiratory tract infection in Northeastern Brazil. Medicine 2016; 95:e3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sloan C, Moore ML, Hartert T. Impact of pollution, climate, and socio-demographic factors on spatiotemporal dynamics of seasonal respiratory viruses. Clin Transl Sci 2011; 4:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbiza J, Delfraro A, Frabasile S. Molecular epidemiology of human respiratory syncytial virus in Uruguai: 1985-2001—a review. Mem Inst Oswaldo Cruz 2005; 100:221–230. [DOI] [PubMed] [Google Scholar]
- 15.White LJ, Waris M, Cane PA, et al. The transmission dynamics of groups A and B human respiratory syncytial virus (hRSV) in England & Wales and Finland: seasonality and cross-protection. Epidemiol Infect 2005; 133:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aamir UB, Alam MM, Sadia H, et al. Molecular characterization of circulating respiratory syncytial virus (RSV) genotypes in Gilgit Baltistan Province of Pakistan during 2011–2012 winter season. PLoS One 2013; 8:e74018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamarão LM, Ramos FL, Mello WA, et al. Prevalence and clinical features of respiratory syncytial virus in children hospitalized for community-acquired pneumonia in northern Brazil. BMC Infect Dis 2012; 12:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferone EA, Berezin EN, Durigon GS, et al. Clinical and epidemiological aspects related to the detection of adenovirus or respiratory syncytial virus in infants hospitalized for acute lower respiratory tract infection. J Pediatr (Rio J) 2014; 90:42–49. [DOI] [PubMed] [Google Scholar]
- 19.Cuevas LE, Nasser AM, Dove W, et al. Human metapneumovirus and respiratory syncytial virus, Brazil. Emerg Infect Dis 2003; 9:1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang ZY, Du LN, Chen X, et al. Genetic variability of respiratory syncytial viruses (RSV) prevalent in southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J Clin Microbiol 2010; 48:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hara M, Takao S, Shimazu Y, et al. Three-year study of viral etiology and features of febrile respiratory tract infections in Japanese pediatric outpatients. Pediatr Infect Dis J 2014; 33:687–692. [DOI] [PubMed] [Google Scholar]
- 22.McCraken JP, Prill MM, Arvelo W, et al. Respiratory syncytial virus infection in Guatemala, 2007-2012. J Infect Dis 2013; 208:S197–S206. [DOI] [PubMed] [Google Scholar]
- 23.Hacimustafaoglu M, Celebi S, Bozdemir SE, et al. RSV frequency in children below 2 years hospitalized for lower respiratory tract infections. Turk J Pediatr 2013; 55:130–139. [PubMed] [Google Scholar]
- 24.Oliveira TF, Freitas GR, Ribeiro LZ, et al. Prevalence and clinical aspects of respiratory syncytial vírus A and B groups in children seen at Hospital de Clínicas of Uberlândia, MG, Brazil. Mem Inst Oswaldo Cruz 2008; 103:417–422. [DOI] [PubMed] [Google Scholar]
- 25.Gardinassi LG, Simas PV, Gomes DE, et al. Diversity and adaptation of human syncytial virus genotypes circulating in two distinct communities: public hospital and day care center. Viruses 2012; 4:2432–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nolan T, Borja-Tabora C, Lopez P, et al. Prevalence and incidence of respiratory syncytial virus and other respiratory viral infections in children aged 6 months to 10 years with influenza-like illness enrolled in a randomized trial. Clin Infect Dis 2015; 60:e80–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calegari T, Queiroz DA, Yokosawa J, et al. Clinical-epidemiological evaluation of respiratory syncytial virus infection in children attended in a public hospital in Midwestern Brazil. Braz J Infect Dis 2005; 9:156–161. [DOI] [PubMed] [Google Scholar]
- 28.Panayiotou C, Richter J, Koliou M, et al. Epidemiology of respiratory syncytial virus in children in Cyprus during three consecutive winter seasons (2010-2013): age distribution, seasonality and association between prevalent genotypes and disease severity. Epidemiol Infect 2014; 142:2406–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brazilian Ministry of Health. Technical note no. 05/2015. To establish the seasonality of respiratory syncytial virus in Brazil and to offer information in regard to the recommended use of palivizumab [in Portuguese].[Brasil. Ministério da Saúde. Nota técnica conjunta n° 05/2015. 2015 Estabelecer a sazonalidade do vírus sincicial respiratório no Brasil e oferecer esclarecimentos referentes ao protocolo de uso do Palivizumabe.]. [Google Scholar]
- 30.American Academy of Pediatrics. Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014; 134:415–420. [DOI] [PubMed] [Google Scholar]


