Supplemental Digital Content is available in the text
Keywords: HIV-1, nucleotide reverse transcriptase inhibitors, tenofovir alafenamide, tenofovir disoproxil fumarate
Abstract
Background:
To date, a definite conclusion about efficiency and safety of tenofovir alafenamide for patients with HIV-1 is not available. The aim of the study was to investigate the efficacy and safety of TAF versus TDF in antiretroviral regimens for patients with HIV-1.
Methods:
PUBMED, MEDLINE, and EMBASE database were searched in March 2016, with no language restriction, for randomized controlled trials (RCTs).
Results:
Six RCTs (n = 5888) met entry criteria. At week 48, viral suppression rates were similar between TAF and TDF group (90.2% vs 89.5%) for the naive patients. Interestingly, the rate was higher in patients who switched to TAF regimens compared with patients who continued previous TDF regimens (96.4% vs 93.1%). Both groups were generally well tolerated with high barrier to resistance. As compared to TDF, TAF had significantly smaller reductions in eGFR-CG, smaller changes in RBP/Cr and urineβ-2 M/Cr ratio, and less reduction in spine and hip BMD for the treatment-naive patients. Moreover, the switched group had significant efficacy advantages of improving renal function and BMD, including significant decreases in urine albumin/Cr, urine protein/Cr, urine RBP/Cr, and urine β-2 M/Cr ratios, and increases in hip and spine BMD by 1.47% and 1.56%,respectively, as compared with continued TDF regimens.
Conclusions:
TAF has a similar tolerability, safety, and effectiveness to TDF and probably less adverse events related to renal and bone density outcomes in the treatment of naive and experienced patients with HIV-1.
1. Introduction
Nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs) have played an indispensable role in the treatment and prevention of HIV-1 infection, and led to marked declines in the morbidity and mortality of patients with HIV-1.[1–4] As non-AIDS comorbidities are increasingly common, current guidelines recommend that begin antiretroviral therapy (ART) earlier for all HIV-positive individuals regardless of CD4 count.[5] Therefore, antiretroviral drug regimens with optimal tolerability, long-term safety, and durable efficacy are increasingly important.
Tenofovir disoproxil fumarate (TDF), the first approved oral prodrug of tenofovir (TFV), has been used in combination ART for the treatment of HIV-1 infection since 2001. Despite its potent and generally well tolerated, TDF can cause clinically significant renal toxic effects[6,7] and a greater decline in bone mineral density (BMD) relative to some other NRTIs.[8–10] After oral administration, TDF is metabolized to TFV which, in turn, is phosphorylated intracellularly to the active moiety TFV diphosphate (TFV-DP). However, higher circulating plasma levels of TFV has been associated with both renal and bone adverse effects of TDF.[11] Tenofovir alafenamide (TAF), a novel oral prodrug of TFV, is metabolized to TFV intracellularly, rather than in the plasma, which results in substantially higher intracellular concentrations of the active metabolite TFV-DP and lower plasma levels of TFV compared with TDF.[12,13] As a result, the dose of TAF is less than one-tenth of the dose of TDF, which is believed to reduce the risk of renal and bone toxicity.[11]
To date, some phase 2 and 3 randomized-controlled trials have shown that TAF has similar antiviral activity, with a significantly ability of reducing the risk of renal and bone toxicity, as compared with TDF. However, recently 1 trial reported that the drug-related adverse events were more common in the TAF group.[18] In addition, due to the limited sample sizes of these studies and subsequent different index for comparing outcomes, a definite conclusion about efficiency and safety of TAF in treatment of HIV-1 infection is not available. Herein, we conducted this meta-analysis by integrating published data to compare the efficacy and safety of TAF versus TDF in antiretroviral regimens for patients with HIV-1 and ultimately provide evidence for clinical application of TAF.
2. Materials and methods
2.1. Search strategy
We searched PubMed, MEDLINE, and EMBASE up to March, 2016. The following keywords were used for the search: HIV-1, tenofovir disoproxil fumarate, and tenofovir alafenamide were used to find relevant citations. In addition, reference lists from retrieved documents were reviewed, and a manual search was conducted to supplement the computer search. The search results were downloaded to a reference database and were further screened by 2 authors (WHL and YXD).
2.2. Inclusion and exclusion criteria
The following inclusion criteria were used for this meta-analysis: (1) randomized trials, (2) eligible patients were HIV-1-infected adults (aged ≥18 years), and treatment with tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens. The following types of studies were excluded: (1) patients with positive hepatitis B surface antigen or hepatitis C antibody or a new AIDS-defining illness within 30 days of screening, (2) studies not reporting any efficacy measures or not conveying sufficient statistical information, (3) studies are not comparative trials of TDF versus TAF.
2.3. Outcome measures
Efficacy and safety outcomes at 48 weeks after starting treatment were evaluated. Efficacy measures were considered as follows: HIV-1 RNA level (<50 c/mL), virologic failure with resistance. Safety for each drug was evaluated with the following outcomes at 48 weeks: adverse events, grade 3 or 4 laboratory abnormalities, renal outcomes and bone outcomes.
2.4. Data extraction
Data extraction was assessed independently by 2 reviewers (WHL and YXD). Discrepancies among reviewers were resolved by discussions between the reviewers or by a third person (XN). Basic information obtained from each eligible trial included patient characteristics, numbers in each group, treatment doses, gender, median CD4 count, and so on. Data were reviewed to eliminate duplicate reports of the same trial.
2.5. Quality assessment and statistical analysis
The risk of bias of included trials was assessed by the Cochrane Collaboration's tool. The data were conducted on continuous and dichotomous outcomes and assessed by the meta-analytical techniques. The x2 and I2 tests were first calculated to assess the heterogeneity of the included trials. For P values more than 0.1, the assumption of homogeneity was valid, and the fixed-effects model was used; otherwise, data need to be dealt with the random-effects model because of the heterogeneity. Pooled risk ratios (RR) with 95% confidence intervals (95% CI) were calculated using either the fixed-effects model (M-H methods) or random-effects model (D-L methods). A 2-tailed P value of <0.05 suggested statistically significant. All calculations of this meta-analysis were performed by Review Manager (v.5.3). Also, we performed the comparisons of continuous variables using the independent t test from SPSS 22.
3. Results
3.1. Study characteristics and quality assessment
From a total of 489 unique studies identified using the search strategy (Supporting Fig. 1), we included 6 RCTs in this meta-analysis,[14–19] including 5888 patients. In total, 3239 or 2649 patients were enrolled into the TAF or TDF group, respectively. Two trials are randomized phase 2 studies,[15,16] and 4 trials are randomized, actively controlled, multicentre, phase 3 studies.[14,17–19] Patients enrolled in each trial come from different races with white, black, and Asian. Four trials[14–16,19] included patients who were treatment-naive participants. Among them, patients in 3 trials[14,16,19] were treated with once-daily oral tablets containing 150 mg elvitegravir(E), 150 mg cobicistat(C), 200 mg emtricitabine(F), and 10 mg tenofovir alafenamide or 300 mg tenofovir disoproxil fumarate, and patients in 1 trial[15] were treated with 400 mg darunavir, 150 mg cobicistat(C), 200 mg emtricitabine(F), and 10 mg tenofovir alafenamide or 300 mg tenofovir disoproxil fumarate. Remanent patients included 2 trials[17,18] were treatment-experienced participants who were either switch to TAF-containing regimens or to continue previous TDF-containing regimens. The characteristics of each study were listed in Supporting Table 1.
The quality assessment of included studies was performed using Cochrane Collaboration's tool with the outcome shown in Supporting Fig. 2. The percentages of low risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias were all >50% according to the description of each study. The outcome of risk of bias graph showed that there was low risk of bias in this meta-analysis.
3.2. Efficacy analysis
3.2.1. Virologic suppression
At week 48, 93.6% in the TAF group vs 91.2% in the TDF group were virally suppressed (HIV-1 RNA<50 copies/mL), and the rate of viral suppression for TAF was slightly better than that of TDF (RR,1.02; 95%CI:1.01–1.04; Fig. 1A) in the patients with HIV-1.
Figure 1.
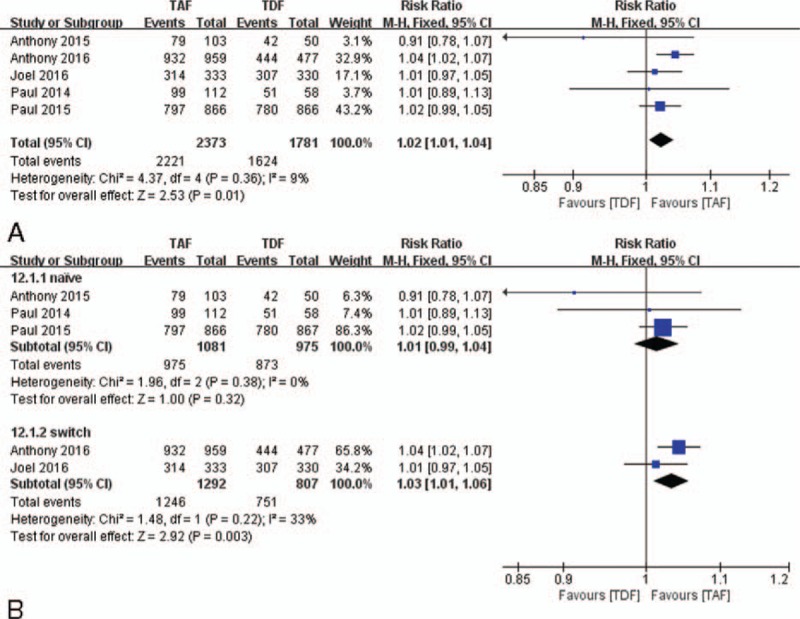
The rates of viral suppression and subgroup analysis compared TAF vs TDF at week 48. (A) viral suppression. (B) Subgroup analysis of viral suppression comparing naive and switch patients. TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate.
To determine whether TAF regimens susceptibilities were different between naive and experienced patients, we divided the eligible 6 trials into 2 subgroups, the treatment-naive group included 4 RCTs [14–16,19] and the treatment-experienced group included 2 RCTs,[17,18] and conducted subgroup analysis.
Subgroup analysis showed that the rates of virologic suppression were similar between TAF and TDF (TAF 90.2% vs TDF 89.5%; RR,1.01; 95%CI:0.99–1.04; Fig. 1B) in naive patients, whereas TAF had higher rate of virologic suppression than that of TDF in the experienced patients through week 48 (TAF 96.4% vs TDF 93.1%; RR, 1.03; 95%CI: 1.01–1.06; Fig. 1B).
3.2.2. Virologic failure with resistance
At week 48, 0.80% of all participants in the TAF group and 0.72% of all participants in the TDF group had virologic failure with resistance. The rate of resistance was similar between 2 groups (RR, 1.03; 95%CI: 0.58–1.83; Fig. 2A).
Figure 2.
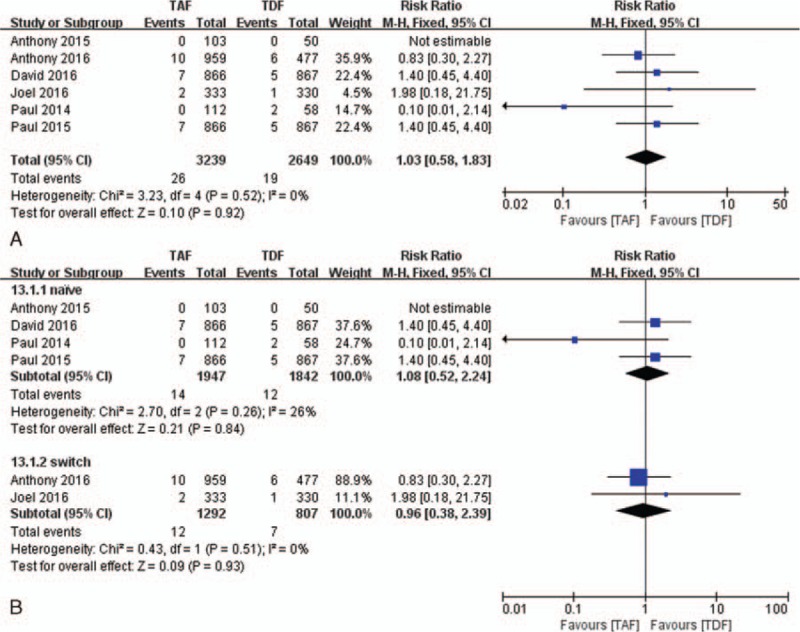
The rates of virologic failure with resistance and subgroup analysis compared TAF vs TDF at week 48. (A) Virologic failure with resistance. (B) Subgroup analysis of virologic failure with resistance comparing naive and switch patients. TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate.
In our subgroup analysis, the rates of resistance were not significantly different between the TAF group and the TDF group in naive patients (0.72% vs 0.65%; RR, 1.08; 95%CI: 0.52–2.24; Fig. 2B) as well as experienced patients (0.93% vs 0.87%; RR, 0.96; 95%CI: 0.38–2.39; Fig. 2B) through 48 weeks of treatment.
3.3. Safety analysis
3.3.1. Safety and tolerability
Five RCTs[14–18] reported adverse events during 48 weeks of therapy. The safety profiles of both TAF and TDF groups were similar, with 45.3% vs 48.1% patients reporting any treatment-emergent AE. No significant differences were observed in the rates of adverse reactions between the groups (RR,1.05; 95%CI:0.94–1.17; Fig. 3A).The most common adverse reactions were diarrhoea, nausea, headache, upper respiratory tract infection, nasopharyngitis, fatigue, cough, vomiting, arthralgia, rash, and pyrexia. Among main adverse reactions, TAF and TDF groups were similar in the rates of diarrhea, upper respiratory tract infection, nausea, and nasopharyngitis (see Supporting Figure 3 A–D). However, the TAF group had a higher rate of headache than that of the TDF group (see Supporting Figure 3E). In subgroup analysis, adverse event rates were similar between TAF and TDF groups in the naive patients (Fig. 3B) as well as experienced patients (Fig. 3B) through week 48.
Figure 3.
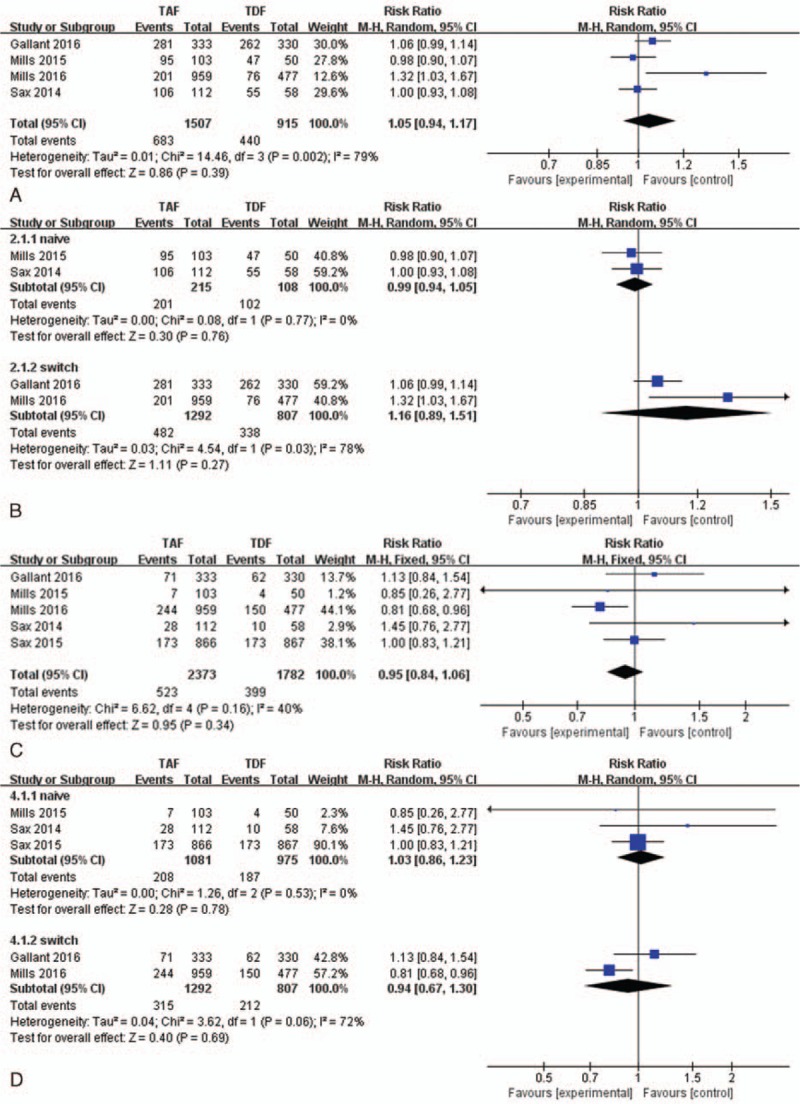
The rates of adverse events and subgroup analysis compared TAF vs TDF at week 48. (A) Adverse events of included trials. (B) Subgroup analysis of adverse events in the naive and switch patients. (C) Grade 3 or 4 laboratory abnormalities of included trials. (D) Subgroup analysis of grade 3 or 4 laboratory abnormalities in the naive and switch patients.TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate.
In total 22.0% of patients in the TAF group and 22.4% of patients in the TDF group had grade 3 or 4 laboratory abnormalities, which was similar between the groups (RR,0.95; 95%CI:0.84–1.06; Fig. 3C). In subgroup analysis, the rates of grade 3 or 4 laboratory abnormalities were similar between TAF and TDF groups in the naive patients (Fig. 3D) as well as experienced patients (Fig. 3D) through week 48. Laboratory abnormalities were usually mild to moderate in severity and resolved without study drug interruption. The fasting lipid parameters (see Supporting Table 2) in the TAF group increased from baseline in the TAF group compared with the TDF group at week 48. There were significant differences between the TAF group and the TDF group in the mean changes in total cholesterol (33 vs 12 mg/dL, P =0.014) and HDL (7 vs 3.3 mg/dL, P = 0.008), whereas no statistically significant differences between groups were observed in LDL (20 vs 4.5 mg/dL, P = 0.124), triglycerides (24 vs 1.5 mg/dL, P = 0.111), and total cholesterol/HDL ratio (0.1 vs 0.0, P = 0.435) in the naive patients. Moreover, for experienced patients, there were significant differences between the TAF group and the TDF group in the mean changes in total cholesterol (17 vs 1.5 mg/dL, P = 0.036) and triglyceride (10.5 vs –2 mg/dL, P = 0.002),whereas no significant differences between groups were observed in LDL (11 vs 1 mg/dL, P = 0.109), HDL(2 vs 0 mg/dL, P = 0.184),and total cholesterol/HDL ratio (0.15 vs 0.0, P = 0.095). During their studies, 5.7% of all participants in the TAF group and 4.0% of all participants in the TDF group started lipid-lowering drugs, and no statistical significant difference was found between the groups (see supporting Figure 3F). In addition, none of the serious adverse events that resulted in the deaths were deemed related to study drugs by the 6 RCTs.
3.3.2. Bone outcomes
Five RCTs [14–18] reported the information of changes in bone mineral density at 48 weeks, expressed as the median percent change from baseline. At week 48, 26.7% (269/1005) in the TAF group and 47.0%(414/881) in the TDF group had BMD declines of >3% from baseline at the spine, and 16.3%(162/995) in the TAF group and 50.1%(438/875) in the TDF group had BMD declines of >3% from baseline at the hip. Patients in the TAF group had significantly less reduction in bone mineral density than those in the TDF group through 48 weeks. Decrease in bone mineral density was significantly lower in the TAF group for both spine (RR, 0.56; 95%CI: 0.50–0.64; Fig. 4A) and hip (RR,0.33; 95%CI:0.28–0.38; Fig. 4B) compared to the TDF group.
Figure 4.

Bone and renal adverse events compared TAF vs TDF at week 48: (A) 48 weeks of declines of >3% from baseline in BMD at spine, (B) 48 weeks of declines of >3% from baseline in BMD at hip, (C) 48 weeks of renal events. BMD = bone mineral density, TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate.
Because of different parameters in reporting bone adverse events among the included studies, which we could not carry out meta-analysis for the safety of treatments, we summarized the bone events reported in each study in Supporting Table 3, and performed the comparisons of continuous data using independent t-test from SPSS between TAF group and TDF group. The mean percent BMD change from baseline was significantly less decrease in the TAF group at both the hip (–0.7% vs –3.25%, P = 0.005) and spine (−1.29% vs –3.28%, P = 0.002) as compared to the TDF group in the treatment-naive patients through week 48. Meanwhile, markers of bone turnover, procollagen Type 1 N-terminal propeptide (P1NP) and C-terminal telopeptide (CTx), increased less from baseline in the TAF group compared with the TDF group. At week 48, P1NP, a marker of bone formation, increased 6.9% from baseline for TAF vs 61.8% for TDF (P = 0.024), whereas CTx, a marker of bone resorption, increased 21.0% from baseline for TAF vs 76.2% for TDF (P = 0.003). Of note, for the treatment-experienced participants, bone mineral density increased in the TAF group but decreased in the patients who did not switch in the TDF group. The mean % BMD change from baseline was significantly increased in the TAF group at both the hip (1.30% vs −0.25%, P = 0.029) and spine (1.54% vs –0.32%, P = 0.036) as compared to the TDF group through week 48.
Additionally, 2 trials[14,17] reported information of fractures. However, 0.17% (2/1199) of patients in the TAF group and 0.75% (9/1197) of patients in the TDF group had fractures, which was deemed by the investigators to be the result of trauma and unrelated to the study drugs.
3.3.3. Renal outcomes
Data regarding therapy-related renal adverse events were available from 6 of the included trials.[14–19] However, 0.06%(2/3136) of patients in the TAF group and 0.62%(16/2599) of patients in the TDF group discontinued study drug because of renal adverse events through week 48. Patients in TAF group had a significantly lower rate in renal adverse events as compared with patients in the TDF group (RR,0.15; 95%CI:0.04–0.49; Fig. 4C).
For treatment-naive patients, the mean decrease in eGFR-CG from baseline was significantly lower with TAF than TDF (–4.93 mL/min vs –10.63 mL/min, respectively, P = 0.007) through week 48. Moreover, the mean increase in serum creatinine from baseline was 0.065 mg/dL for TAF vs 0.095 mg/dL for TDF (P = 0.051) (Table 1). Increases were observed at week 2 for each treatment group and remained stable through week 48. In addition, proteinuria (urine protein/creatinine ratio) and albuminuria (urine albumin/creatinine ratio) decreased from baseline for both groups, but no statistically significant differences were observed through 48 weeks. Renal tubular proteinuria (retinol binding protein/Cr and β-2-microglobulin/Cr) increased significantly in the TDF group, whereas significant declines or smaller increases were observed in the TAF group (P < 0.05 for all) (Table 1). Finally, no discontinuations of study drug in the TAF group through week 48, whereas 10 discontinuations in the TDF group because of renal adverse events.
Table 1.
Renal parameters change from baseline to week 48.
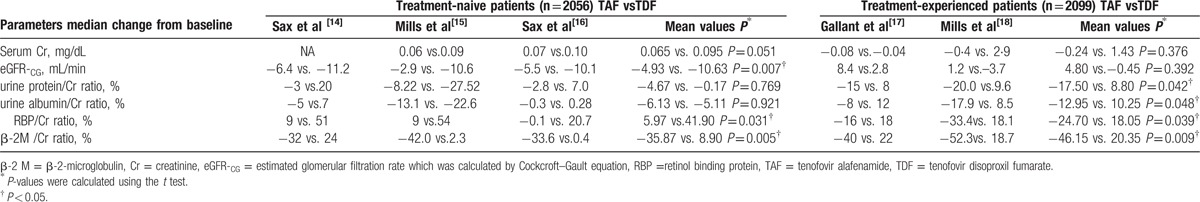
For the treatment-experienced patients, through week 48, the mean change from baseline in serum creatinine concentration in the TAF group was –0.24 mg/dL and 1.43 mg/dL in the TDF group (P = 0.376) (Table 1). Moreover, there were increases from baseline in eGFR-CG in the TAF group (4.8 mL/min) compared with minimal changes in the TDF group (–0.45 mL/min, P = 0.392). In addition, urine protein/creatinine ratio, urine albumin/creatinine ratio, retinol binding protein/ creatinine ratio, and β-2-microglobulin/creatinine ratio were significantly lower in patients who switched to TAF regimens. By contrast, patients who continued previous TDF-containing regimens had increases in each of these tests of proteinuria, with significant differences between the groups throughout week 48 (P < 0.05 for all; Table 1). Finally, 2 patients in the TAF group and 6 patients in the TDF group discontinued study treatment because of renal-related adverse events through week 48.
4. Discussion
This meta-analysis is the first study to compare the efficacy and safety of TAF versus TDF in the treatment of treatment-naive or treatment-experienced patients with HIV-1. Results from this study showed that both TAF-containing regimens and TDF-containing regimens demonstrated high and comparable rates of virologic suppression (90.2% in the TAF group and 89.5% in the TDF group) in the naive patients through 48 weeks of therapy. This study also showed that switching to the TAF-containing regimens was superior to continuing with the TDF containing regimens in maintaining virological suppression (96.4% in the TAF group and 93.1% in the TDF group) at 48 weeks in patients with HIV-1. The rate of virological failure with resistance was <1% of patients in both groups (0.80% in the TAF group and 0.72% in the TDF group), most commonly the nucleoside reverse transcriptase inhibitor resistance with M184 V.
Both treatments were generally well tolerated with few discontinuations due to adverse events or laboratory toxicities. The safety and tolerability appeared to be similar between the TAF group and the TDF group for the naive and experienced patients through 48 week of therapy. Total cholesterol in the TAF group were increased significantly as compared with the TDF group, whereas no significantly differences between both groups were found in total cholesterol/HDL ratio and low-density lipoprotein for the naive and experienced patients. The lower concentrations of tenofovir in plasma from TAF as compared with TDF, and the lipid-lowering effect of TFV[20,21] may explain the statistically significant increases in total cholesterol in the TAF group compared with the TDF group. Despite the differences in fasting lipid parameters, a similar proportion of participants compared TAF vs TDF group (5.7% vs 4.0%) started lipid-lowering drugs during study period.
A high proportion of patients with HIV are reported to have low BMD than age-matched HIV-uninfected controls.[23] Although TDF is a preferred treatment option for HIV-1 infection and one of the most widely prescribed antiretrovirals,[22] bone adverse events of TDF regimens is increasing concern.[10,23] In studies involving treatment-naive patients, initiation of ART has consistently been associated with a loss of BMD, and TDF-containing regimens lead to greater decline in bone density than regimens without TDF.[24–26] In this study, TAF regimens showed significantly smaller declines in hip and spine BMD than that of TDF regimens over 48 weeks for the naive patients. Meanwhile, less change in bone turnover markers in the TAF group than in the TDF group further supported the BMD findings. The mechanism of TDF-related reductions in BMD is not well understood but may be because of phosphate wasting and increased bone turnover by proximal tubule toxicity of TDF.[27,28] Of note, in this study, in patients who switched from TDF-containing regimens to TAF-containing regimens, increase in BMD at both hip and spine was significantly greater than that who continued previous TDF-containing regimens over 48 weeks, and mean percentage change in hip and spine BMD improved by 1.47% and 1.56%, respectively. Switching to the TAF regimen resulted in significant improvement of bone mineral density, which has the potential to be clinically significant to prevent bone diseases for patients with HIV infection. However, longer term follow up data are needed to evaluate the long-term clinical significance of this finding.
HIV-infected individuals are at increased risk for chronic kidney disease, as indicated by a decrease in eGFR or an increase in urinary protein excretion.[29–32] In the current study, for the treatment-naive patients, no one in the TAF group except 10 patients in the TDF group discontinued study drug because of renal adverse events through week 48. Although no participants discontinued study drug due to changes in creatinine (Cr) or eGFR, early small increases in SCr and then maintained stable through week 48 were observed in both groups, with no statistically significant differences. Therefore, this change was expected to be due to the known nonpathologic inhibitory effect of cobicistat on tubular creatinine secretion.[33] However, GFR-CG was reduced markedly in the TDF group than in the TAF group. The exact mechanism for this difference is not fully understood but may be associated with lower plasma TFV exposure with TAF compared to TDF. TFV in plasma is actively transported into the proximal renal tubular cell by organic anion transporter (OAT) 1 and OAT 3, but TAF is not a substrate for these transporters.[34] High doses of TFV has been suspected through its mitochondria toxicity in proximal renal tubular cells to cause TDF-related renal function decrement.[35] Meanwhile, the effect of TAF vs TDF on proximal renal function was assessed by urine albumin/Cr, urine protein/Cr, urine RBP/Cr, and urine β-2 M/Cr ratios. Increased urinary levels of these molecular weight proteins may indicate subclinical renal tubular cell dysfunction, because they are almost entirely removed through the ultrafiltrate and catabolized by the proximal tubule in the case of normal renal function.[36] In this study, There were no differences in urine albumin/Cr, urine protein/Cr; however, the markers of renal tubular proteinuria, urinary RBP/Cr and β-2 M/Cr ratios, were statistically significant differences between the TAF group and the TDF group. The values of urinary β-2 M/Cr and RBP/Cr ratios in the TDF group had greater increases in 5 or 7 times than that in the TAF group, respectively. However, patients in the TAF group had normal or mild-moderate elevations of these urine lower molecular weight proteins, which indicates that lower plasma TFV exposure with TAF has less effect than TDF on proximal renal tubular cell function.
In the study, switched to TAF regimens had significant efficacy advantages of improving renal function, including significantly decreases in urine albumin/Cr, urine protein/Cr, urine RBP/Cr, and urine β-2 M/Cr ratios as compared with patients who continued previous TDF-containing regimens over 48 weeks. Of note, urine albumin/Cr, urine protein/Cr in the TDF group in the naive patients had smaller decrease, but had significantly greater increase in experienced patients over 48 weeks. Higher urinary protein excretion has been associated with increased risk of mortality in HIV-infected individuals.[30–32] Therefore, we suggest that it improves the renal function by switching to TAF regimens, whereas the prolonged TDF use can worsen kidney function.
Our study had limitations. First, it has been reported that geographic, ethnic or HIV disease status (such as HIV-1 RNA concentration, CD4 cell count, as well as Estimated GFR, etc.) differences are possibly associated with agent efficacy. Second, 2 trials included in the study are phase 2 RCT with a small number of patients, which may influence our study on evaluating noninferiority. Thirdly, the difference of treatment regimens among included studies might affect the consistency of the results: in studies of naive patients, 3 studies[14,16,19] used fixed-dose of elvitegravir/cobicistat/emtricitabine, but 1 [15] used fixed-dose of darunavir/cobicistat/ emtricitabine. With respect to the studies of experienced patients, 1 compared the NRTI backbones emtricitabine/TAF or emtricitabine/TDF, and the other one used elvitegravir 150 mg, cobicistat 150 mg, emtricitabine 200 mg, and TAF 10 mg in fixed dose or maintained the previous TDF-containing regimen. Finally, when monitoring of the effect of ART on renal function in the HIV-1 patients, the estimated GFR in all included studies were calculated by CG equation which is not the most accurate formula in the case of the HIV population. Thus, we suggest that the eGFR would be calculated with more accurate formula such as the Chronic Kidney Disease Epidemiology Collaboration(CKD-EPI) equation in the future research.[37]
Overall, in this meta-analysis, at week 48, viral suppression rates were similar between TAF and TDF group (90.2% vs 89.5%) for the naïve patients. Interestingly, the rate was higher in patients who switched to TAF regimens compared with patients who continued previous TDF regimens (96.4% vs 93.1%). Both treatments were generally well tolerated with high barrier to resistance. Compared with TDF, TAF seemed to have advantages of improving renal and bone parameters for naive and experienced patients. Although longer-term follow-up are needed to evaluate the clinical significance associated with the benefits in bone and renal safety observed in TAF regimens, TAF-containing regimens is a promising HIV treatment option.
Supplementary Material
Footnotes
Abbreviations: β-2 M = β-2-microglobulin, ART=antiretroviral therapy, BMD = bone mineral density, Cr = creatinine, CTx = C-terminal telopeptide, eGFR-CG = estimated glomerular filtration rate which was calculated by Cockcroft–Gault equation, HDL = high density lipoprotein, LDL = low density lipoprotein, NRTIs =nucleoside and nucleotide reverse transcriptase inhibitors, P1NP = procollagen Type 1 N-terminal propeptide, RBP =retinol binding protein, TAF = tenofovir alafenamide, TDF = tenofovir disoproxil fumarate, TFV = tenofovir.
H-LW and XL contributed equally to this study and share first authorship.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998; 338:853–860. [DOI] [PubMed] [Google Scholar]
- 2.Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet 1998; 352:1725–1730. [DOI] [PubMed] [Google Scholar]
- 3.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med 2009; 360:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capeau J. Premature aging and premature age-related comorbidities in HIV-infected patients: facts and hypotheses. Clin Infect Dis 2011; 53:1127–1129. [DOI] [PubMed] [Google Scholar]
- 5.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. Available at http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf Accessed October 15, 2013. [Google Scholar]
- 6.Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis 2011; 57:773–780. [DOI] [PubMed] [Google Scholar]
- 7.Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral-naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr 2010; 55:49–57. [DOI] [PubMed] [Google Scholar]
- 8.VIREAD (Tenofovir Disoproxil Fumarate) Tablets, Powder. US Prescribing Information. Foster City, CA: CGSI; 2012. [Google Scholar]
- 9.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir–lamivudine versus tenofovir–emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–972. [DOI] [PubMed] [Google Scholar]
- 10.Schafer JJ, Manlangit K, Squires KE. Bone health and human immuno-deficiency virus infection. Pharmacotherapy 2013; 33:665–682. [DOI] [PubMed] [Google Scholar]
- 11.Van Rompay KK, Durand-Gasselin L, Brignolo LL, et al. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob Agents Chemother 2008; 52:3144–3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee WA, He GX, Eisenberg E, et al. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob Agents Chemother 2005; 49:1898–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birkus G, Kutty N, He GX, et al. Activation of 9-[(R)-2-[[(S)-[[(S)-1-(Isopropoxycarbonyl) ethyl]amino]phenoxyphosphinyl]-methoxy] propyl] adenine (GS-7340) and other tenofovir phosphonoamidate prodrugs by human proteases. Mol Pharmacol 2008; 74:92–100. [DOI] [PubMed] [Google Scholar]
- 14.Sax PE, Wohl D, Yin MT, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate, coformulated with elvitegravir, cobicistat, and emtricitabine, for initial treatment of HIV-1 infection: two randomised, double-blind, phase 3, non-inferiority trials. Lancet 2015; 385:2606–2615. [DOI] [PubMed] [Google Scholar]
- 15.Mills A, Crofoot G, Jr, McDonald C, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate in the first protease inhibitor-based single-tablet regimen for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2015; 69:439–445. [DOI] [PubMed] [Google Scholar]
- 16.Sax PE, Zolopa A, Brar I, et al. Tenofovir alafenamide vs. tenofovir disoproxil fumarate in single tablet regimens for initial HIV-1 therapy: a randomized phase 2 study. J Acquir Immune Defic Syndr 2014; 67:52–58. [DOI] [PubMed] [Google Scholar]
- 17.Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV 2016; 3:e158–165. [DOI] [PubMed] [Google Scholar]
- 18.Mills A, Arribas JR, Andrade-Villanueva J, et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis 2016; 16:43–52. [DOI] [PubMed] [Google Scholar]
- 19.Wohl D, Oka S, Clumeck N, et al. Brief report: a randomized, double-blind comparison of tenofovir alafenamide versus tenofovir disoproxil fumarate, each coformulated with elvitegravir, cobicistat, and emtricitabine for initial HIV-1 treatment: week 96 results. J Acquir Immune Defic Syndr 2016; 72:58–64. [DOI] [PubMed] [Google Scholar]
- 20.Behrens G, Maserati R, Rieger A, et al. Switching to tenofovir/emtricitabine from abacavir/lamivudine in HIV-infected adults with raised cholesterol: effect on lipid profiles. Antivir Ther 2012; 17:1011–1020. [DOI] [PubMed] [Google Scholar]
- 21.Tungsiripat M, Kitch D, Glesby MJ, et al. A pilot study to determine the impact on dyslipidemia of adding tenofovir to stable background antiretroviral therapy: ACTG 5206. AIDS 2010; 24:1781–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams I, Churchill D, Anderson J, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012. HIV Med 2012; 13 (suppl 2):1–85. [DOI] [PubMed] [Google Scholar]
- 23.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 24.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 2010; 51:963–972. [DOI] [PubMed] [Google Scholar]
- 25.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 2011; 203:1791–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin A, Moore C, Mallon PW, et al. Bone mineral density in HIV participants randomized to raltegravir and lopinavir/ritonavir compared with standard second line therapy. AIDS 2013; 27:2403–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fux CA, Rauch A, Simcock M, et al. Tenofovir use is associated with an increase in serum alkaline phosphatase in the Swiss HIV Cohort Study. Antivir Ther 2008; 13:1077–1082. [PubMed] [Google Scholar]
- 28.Leeming DJ, Alexandersen P, Karsdal MA, et al. An update on biomarkers of bone turnover and their utility in biomedical research and clinical practice. Eur J Clin Pharmacol 2006; 62:781–792. [DOI] [PubMed] [Google Scholar]
- 29.Szczech LA, Hoover DR, Feldman JG, et al. Association between renal disease and outcomes among HIV-infected women receiving or not receiving antiretroviral therapy. Clin Infect Dis 2004; 39:1199–1206. [DOI] [PubMed] [Google Scholar]
- 30.Choi A, Scherzer R, Bacchetti P, et al. Cystatin C, albuminuria, and 5-year all-cause mortality in HIV-infected persons. Am J Kidney Dis 2010; 56:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choi AI, Li Y, Deeks SG, et al. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation 2010; 121:651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyatt CM, Hoover DR, Shi Q, et al. Microalbuminuria is associated with all-cause and AIDS mortality in women with HIV infection. J Acquir Immune Defic Syndr 2010; 55:73–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.German P, Liu HC, Szwarcberg J, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr 2012; 61:32–40. [DOI] [PubMed] [Google Scholar]
- 34.Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther 2014; 19:687–692. [DOI] [PubMed] [Google Scholar]
- 35.Abraham P, Ramamoorthy H, Isaac B. Depletion of the cellular antioxidant system contributes to tenofovir disoproxil fumarate—induced mitochondrial damage and increased oxido-nitrosative stress in the kidney. J Biomed Sci 2013; 20:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Post FA, Wyatt CM, Mocroft A. Biomarkers of impaired renal function. Curr Opin HIV AIDS 2010; 5:524–530. [DOI] [PubMed] [Google Scholar]
- 37.Okparavero AA, Tighiouart H, Krishnasami Z, et al. Use of glomerular filtration rate estimating equations for drug dosing in HIV-positive patients. Antivir Ther 2013; 18:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


