Abstract
Persistent cocaine-induced neuroadaptations within the cortico-striatal circuitry might be related to elevated risk of relapse observed in human addicts even after months or years of drug-free abstinence. Identification of these neuroadaptations may lead development of novel, neurobiologically-based treatments of relapse. In the current study, 12 adult male Sprague-Dawley rats self-administered cocaine (or received yoked-saline) for 2 weeks followed by 3 weeks of home-cage abstinence. At this point, we analyzed expression of proteins involved in regulation of Gαi- and Gαq-protein signaling in the dorsal striatum (dSTR). Animals abstinent from chronic cocaine showed decreased expression of regulator of G-protein signaling 2 (RGS2) and RGS4, as well as upregulation of RGS9. These data, together with the increased ratio of Gαq-to-Gαi proteins indicated, ‘sensitized’ Gαq signaling in the dSTR of abstinent cocaine animals. To evaluate activation of Gαq signaling during relapse, another group of abstinent cocaine animals (and yoked saline controls, 22 rats together) was re-introduced to the cocaine context and PKC-mediated phosphorylation in the dSTR was analyzed. Re-exposure to the cocaine context triggered cocaine seeking and increase in phosphorylation of cellular PKC substrates, including phospho-ERK and phospho-CREB. In conclusion, this study demonstrates persistent dysregulation of RGS proteins in the dSTR of abstinent cocaine animals that may produce an imbalance in local Gαq-to-Gαi signaling. This imbalance might be related to augmented PKC-mediated phosphorylation during relapse to cocaine-seeking. Future studies will address whether selective targeting of RGS proteins in the dSTR can be utilized to suppress PKC-mediated phosphorylation and relapse to cocaine-seeking.
Keywords: relapse, addiction, G-protein, ERK, CREB
Graphical Abstract
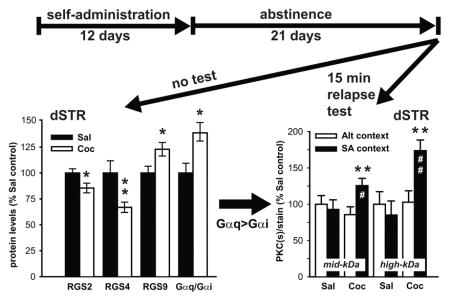
Cocaine self-administration followed by abstinence alters protein levels of G-proteins and negative regulators of G-protein signaling: RGS2/4 and 9 in the dorsal striatum. Re-exposure to cocaine context (relapse test) augments PKC-mediated phosphorylation in the same tissue. Possible imbalance in Gαq>Gαi signaling in the dorsal striatum might be related to relapse.
INTRODUCTION
Cocaine addiction is a compulsive and chronically relapsing drug-taking disorder (O’Brien, 2001). The risk of relapse remains high even after months or years of drug-free abstinence and represents a major challenge in successful treatment of drug addiction in humans (Mendelson and Mello, 1996). In animal studies, relapse can be modeled by reintroducing animals with a history of cocaine self-administration to the context previously associated with drug intake. Drug-associated context (with or without discrete cues) is then sufficient to trigger cocaine-seeking even after prolonged periods of drug-free abstinence, akin to relapse events in human addicts (for reviews see: Crombag et al., 2008, Marchant et al, 2015).
Neural circuitry recruited during relapse to drug-seeking depends on prior extinction training and on the type of cues available during relapse. Thus, cue-induced cocaine-seeking following extinction requires activation of the dorsomedial prefrontal cortex (dmPFC), nucleus accumbens (NAc) or the basolateral amygdala (BLA), while context-induced seeking following abstinence depends on activation of the dorsal striatum (dSTR; McLaughlin and See, 2003, Fuchs et al., and 2006). Specifically, pharmacological inhibition of the dSTR (by local infusion of baclofen+muscimol) attenuated context-induced cocaine-seeking following a 2–3 week abstinence (Fuchs et al., 2006, See et al., 2007, Pacchioni et al., 2011). Under the same experimental conditions, neither PFC, BLA or NAc were found to be involved (Fuchs et al., 2006, See et al., 2007). The role of dSTR in context-induced cocaine-seeking after abstience is further supported by findings of pronounced neuronal activation, as judged indirectly by phosphorylation of ERK1/2 mitogen-activated protein kinase (Edwards et al., 2011) and induction of immediate early genes (Zavala et al., 2007, Hearing et al., 2008) observed in this region immediately after relapse; or directly by functional imaging of dSTR during responding to cocaine-associated (contextual) cues (Volkow et al., 2006, Wong et al., 2006, Liu et al., 2013).
Few animal and human studies, that explored neural mechanisms in the dSTR regulating cocaine-seeking, highlighted the role of local dopamine or glutamate receptors (Vanderschuren et al., 2005, Wong et al., 2006, Belin and Everitt, 2008). However, the persistent character of cocaine-seeking and context-induced neural activation in the dSTR cannot be easily explained by changes in dopamine or glutamate receptor numbers in this brain region, as none of the post-cocaine changes detected persisted over prolonged periods of abstinence (Ben-Shahar et al., 2007, Knackstedt et al., 2014).
In the current study, we proposed to investigate one of the cellular mechanisms by which chronic cocaine could modify dopamine and glutamate receptor function without altering total receptor numbers, that is - dysregulation of receptor G-protein signaling. Indeed, at least two studies suggested that cocaine self-administration alters G-protein signaling of D2-like dopamine receptors in the striatum that can be detected even after an extended drug-free period (Bowers et al., 2004, Frankowska et al., 2013). And further, Bowers et al. (2004) demonstrated that both post-cocaine changes in (ventral)striatal G-protein signaling and persistence of cocaine-seeking can be attributed to upregulation of an accessory protein to G-protein signaling, termed AGS3. Besides AGS3, a strong case can be made for the potential relationship between drug-seeking and another group of accessory proteins termed “regulators of G-protein signaling” (RGS). First, RGS proteins limit the duration and intensity of Gαi- and Gαq-coupled receptor signaling, including that of dopamine D2 receptors and metabotropic glutamate receptor subtype 5 (mGlu5; Saugstad et al., 1998, Cabrera-Vera et al., 2004, Min et al., 2012, Schwendt et al., 2012). As such, RGS proteins in the striatum can mimic the action of D2 and mGlu5 antagonists that are known to suppress cocaine-seeking (e,g. see Khroyan et al., 2000, Gal and Gyertyan, 2006, Kumaresan et al., 2009, Knackstedt et al., 2014). Second, several RGS proteins (such as RGS2, RGS4, and RGS9) are enriched in the striatum, when compared to other brain regions (e.g. see Gold et al., 1997, Grafstein-Dunn et al., 2001). Third, treatment with psychostimulants often leads to dynamic changes in RGS protein expression in the striatum (for review see: Chen and Hemby, 2014). And fourth, recent evidence indicates an association of functional RGS gene polymorphisms with addiction in humans (e.g. RGS4; Ho et al., 2010).
Taken together, the purpose of the current study was to investigate whether chronic cocaine produces lasting changes in the expression of proteins participating in receptor-mediated G-protein signaling (G-proteins and RGS proteins) in the dSTR. And further, whether post-cocaine dysregulation of these proteins coincides with augmented activation of downstream protein kinases in the dSTR during context-induced relapse to cocaine-seeking.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC, USA) weighing 275–300 g at the time of delivery were individually housed in a temperature- and humidity-controlled vivarium on a reversed 12 h light-dark cycle. Rats received ad libitum water throughout the study and 20–25 g of standard rat chow (Harlan, Indianapolis, IN, USA) daily until self-administration stabilized, at which time animals were given ad libitum access to chow. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and were performed in accordance with the Guide for the Care and Use of Laboratory Animals. Experiment 1 used 12 rats; none were removed from the study. Experiment 2 used 22 rats, 2 of which were removed from the study due to a loss of catheter patency.
Drugs
Cocaine hydrochloride was obtained from the NIDA Controlled Substances Program (Research Triangle Institute, NC, USA). Cocaine was dissolved in saline (0.9% sodium chloride) to a final concentration of 4 mg/ml (0.25 mg/infusion).
Catheter surgery
Animals were surgically implanted with jugular vein catheters as described previously with few modifications (Knackstedt et al., 2014). Briefly, rats were anesthetized with a mixture of ketamine and xylazine (66 mg/kg and 1.33 mg/kg i.p.), followed by Equithesin (0.5 ml/kg i.p.). In addition, Ketorolac (2.0 mg/kg, i.p.) was given pre- and post-operatively as an analgesic. An antibiotic solution of cefazolin (10 mg/0.1 ml; Schein Pharmaceuticals, Florham Park, NJ) was given along with 0.1 ml 70 U/ml heparinized saline post-surgery and during recovery. During self-administration, rats received an infusion (0.1 ml) of 10 U/ml heparinized saline (Elkins-Sinn, Cherry Hill, NJ) before each session. After each session, catheters were flushed with cefazolin and 70 U/ml heparinized saline. Catheter patency was periodically verified with methohexital sodium (10 mg/ml i.v.; Eli Lilly, Indianapolis, IN, USA), a short-acting barbiturate that produces a rapid loss of muscle tone when administered intravenously.
Cocaine self-administration, abstinence and relapse procedures
Following at least 5 days of recovery from surgery, rats were randomly assigned to either cocaine or yoked-saline groups and underwent self-administration procedures using standard operant chambers (30 × 24 × 30 cm; Med Associates, St. Albans, VT) equipped with two retractable levers. Animals assigned to cocaine group received response- (active lever presses) contingent infusions of cocaine (0.25 mg/50μl infusion) on a FR1 schedule of reinforcement. Each infusion was followed by a 20-second ‘time-out’ period, during which no stimulus cues were presented. Presses on the inactive lever were not reinforced, but were recorded. Yoked-saline rats received infusions of saline contingent upon the cocaine infusions received by the animal in the paired chamber. Daily sessions (2 hr/day) continued until meeting the criterion of 10 or more infusions for 12 days. Following self-administration, rats underwent 20 days of abstinence. During days 1–10 of abstinence, animals were handled daily for 5 min only. During days 11–20 of abstinence, animals were relocated into a mouse cage (alternate environment, Alt) for 2 hours daily. On day 21 of abstinence, animals were either euthanized (Experiment 1) or were placed into a self-administration chamber (Experiment 2) for a 15 minute context-primed relapse test, during which levers were extended but presses did not produce drug or cues, or were placed for 15 minutes in the alternative (Alt) environment. All animals in Experiment 2 were euthanized at the end of the 15 minute context exposure. In both experiments, animals were euthanized by rapid decapitation, brains extracted, snap-frozen and stored at −80C until processed for immunoblotting analysis as described below.
Western Blotting
Frozen brains were trimmed in the cryostat to prepare 2-mm-thick coronal slices corresponding to coordinates +2.7 / +0.7 mm anterior to bregma in the rat brain atlas (Paxinos and Watson 2007). Dorsal striatal tissue was then bilaterally dissected from these sections using a 2.5 mm Harris Uni-Core micropuncher. Total tissue protein was extracted from dSTR, separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Membranes were blocked in 5% milk/Tris-buffered saline and probed overnight at 4°C with a primary antibody diluted in 5% milk/Tris-buffered saline containing 0.1% Tween 20. The following primary antibodies were used in this study: CREB (MAB5432), phospho-Ser133-CREB (06-519), Gq/11α (AB1643), pan-PKC (05-983), RGS4 (ABT17) - all from EMD Millipore (Billerica, MA); Gαi-1 (sc-391), Gαo (sc-387), PLCβ1 (sc-9050), RGS2 (sc-9103), - all from Santa Cruz Biotechnology (Santa Cruz, CA); phospho-Thr202/Tyr204-ERK1/2 (9101s), ERK1/2 (9102), phospho-(Ser) PKC Substrate (2261) - all from Cell Signaling Technology (Danvers, MA), and RGS9-2 (Martemyanov et al., 2005). After the incubation with an appropriate HRP-conjugated secondary antiserum (Jackson Immuno Research, West Grove, PA), immunoreactive bands on the membranes were detected by ECL+ chemiluminescence reagents on an X-ray film (GE Healthcare, Piscataway, NJ). For the PKC substrate antibody, the entire lane was scanned so that our assessment would be based on multiple substrates. Equal loading and transfer of proteins were confirmed by stripping and reprobing of the same membranes for calnexin (an intracellular protein that was not altered by any experimental treatment) or normalized to a protein pattern visualized by standard Coomassie stain. The integrated band density of each protein sample was measured using NIH Image J software (http://rsb.info.nih.gov/ij/). Occasionally a sample was excluded from densitometric analysis if high background or irregular band staining was observed in developed immunoblots.
Statistical analysis
Total cocaine intake received during self-administration (in Experiment 1 vs 2) and group differences in active and inactive lever presses during context-induced relapse test (Experiment 2) were analyzed by one-way ANOVA followed by Tukey’s post hoc test. Immunoblotting data, represented by integrated density of individual bands, were normalized for the density of calnexin immunoreactivity within the same sample, analyzed by independent sample t-tests (Experiment 1) or two-way ANOVA (Experiment 2) followed by Tukey’s post hoc tests, and expressed as the percentage of the yoked-saline controls. Sigma Stat (Systat Software, San Jose, CA) software was used for statistical analysis of all data. A value of p < 0.05 was considered statistically significant.
RESULTS
Experiment 1: Protein levels of RGS and G proteins in the dSTR following cocaine self-administration and 21 days of abstinence
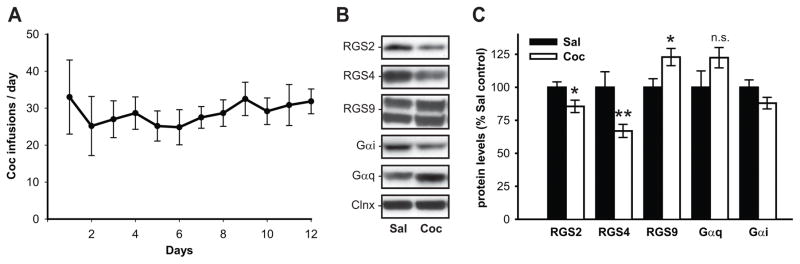
Animals successfully acquired cocaine self-administration, receiving 28.3 ± 0.8 daily infusions on average, and their mean total cocaine intake over 12 days was 215.2 ± 31 mg/kg (Fig. 1A). Following self-administration, the animals underwent a period of drug-free abstinence. The animals were euthanized on the last day of abstinence (day 21) and total protein lysates were prepared from the dSTR and analyzed by immunoblotting. Representative images illustrating immunoblotting signals for the analyzed RGS and G-proteins in the dSTR are displayed on Figure 1B. Quantitative analysis of immunoblotting data revealed that history of cocaine self-administration and abstinence downregulated protein levels of RGS2 (t(12)=2.34, p<0.05) and RGS4 (t(12) = 3.82, p<0.01), while upregulating RGS9 (t(11) = −2.48, p<0.05; Fig. 2C). On the other hand, cocaine did not significantly alter the levels of Gαq (t(11) = −1.54, n.s.) and Gαi (t(11)= 1.72, n.s.) proteins (Fig. 2C). There was also no significant effect of treatment on protein levels of Gαo, and on downstream Gαq targets: PLCβ1 and PKC in the dSTR (data not shown). As RGS proteins that limit Gαq (RGS2) and Gαq/Gαi (RGS4) signaling were decreased and RGS protein that selectively limits Gαi signaling (RGS9) was increased, a potential imbalance in Gαq/Gαi signaling in the dSTR of Saline vs. Cocaine animals was investigated. Animals with a history of cocaine self-administration and abstinence showed increased Gαq/Gαi protein expression ratio (t(12) = −2.27, p<0.05) when compared to their saline counterparts (data not shown).
Fig. 1. Changes in protein levels of RGS proteins and G-proteins in the dSTR of rats with history of cocaine self-administration and 21 days of abstinence.
(A) Mean daily infusions (±SEM) during 12 days of cocaine self-administration. (B) Representative immunoblots of analyzed proteins as detected in the dSTR of cocaine and yoked-saline rats. (C) Quantitative analysis of RGS protein and G-protein levels in dSTR of cocaine and yoked-saline rats. Data represent the mean protein levels ±SEM expressed as a percentage of yoked-Sal controls (n = 6/group)* = p < 0.05, ** = p < 0.01 vs Sal.
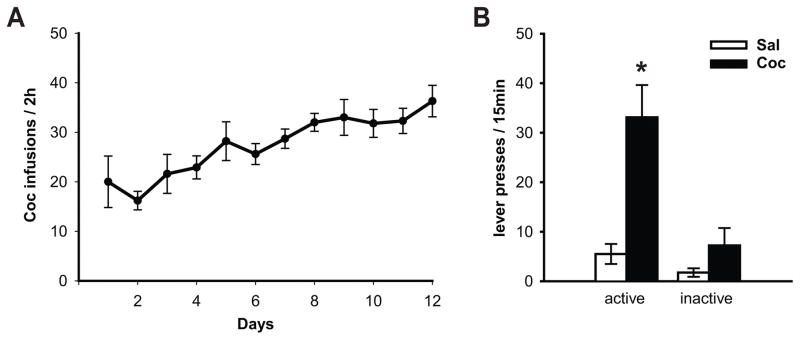
Fig. 2. Re-exposure to cocaine-paired context (15 min relapse test) following 21 days of abstinence elicits robust cocaine-seeking.
(A) Mean daily infusions (±SEM) during 12 days of cocaine self-administration. (B) Re-exposure to self-administration context for 15 minutes elicited robust drug-seeking behavior in cocaine animals when compared to their yoked-saline counterparts. Data represent the mean active and inactive lever presses ±SEM (n = 5–7/group). * = p < 0.05 Coc-active vs Sal-active.
Experiment 2: Post-abstinence context-induced cocaine-seeking and PKC-mediated phosphorylation in the dSTR
Animals successfully acquired cocaine self-administration, receiving 28.1 ± 1.8 daily infusions on average, and their mean total cocaine intake over 12 days was 209.2 ± 16 mg/kg (Fig. 2A). This intake was not statistically different from the mean total intake in Experiment 1 (data not shown). After the completion of the self-administration regimen and 21 days of abstinence, cocaine and yoked-saline animals were subdivided into two groups that were either exposed to the self-administration (SA) context or the alternative (Alt) context. There were no statistical differences in prior cocaine intake between Coc-SA and Coc-Alt animals (data not shown). On day 21 of abstinence, animals were either exposed to the self-administration chamber for the context-induced relapse test or placed in the alternative context. One-way ANOVA analysis of lever pressing revealed a main effect of condition (F(1,17) = 7.48, p<0.01; Fig. 2B). The mean (±SEM) number of active lever responses during the 15 minute test was significantly greater in cocaine-treated animals than yoked-saline rats (Coc-active vs. Sal-active; p<0.05). Inactive lever pressing during the test was not significantly different between cocaine and yoked-saline animals. Immediately after the end of test, animals were euthanized and dSTR tissue was processed for immunoblotting to analyze protein phosphorylation.
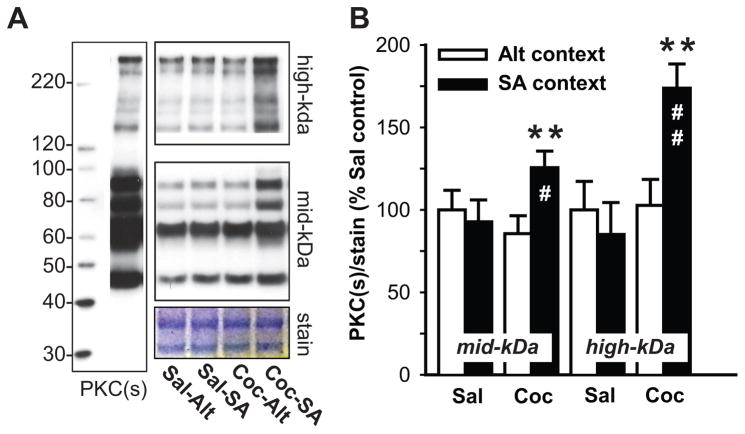
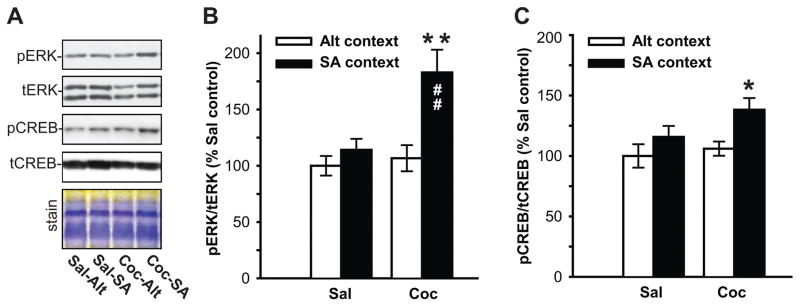
Changes in RGS and G-proteins characterized in Experiment 1 indicate the possibility of ‘sensitized’ Gαq-mediated signaling in the dSTR of animals with prior cocaine experience. One of the well-established consequences of Gαq signaling is activation of protein kinase C (PKC) followed by PKC-mediated phosphorylation of various cellular substrates. Therefore to assess whether cocaine animals show augmented Gαq signaling in the dSTR upon re-exposure to cocaine context, overall PKC activity (PKC-mediated phosphorylation) was evaluated by immunoblotting, using antibody that selectively detects any Ser residues phosphorylated by PKC. Due to differences in signal intensity of bands detected by phospho-(Ser) PKC substrate antibody large-size (>100kDa kDa molecular weight, high-kDa) and mid-size (<100kDa molecular weight, mid-kDa) PKC substrates were analyzed separately (Fig. 3A). No main effect of cocaine or the context on phosphorylation of mid-kDa substrates was detected, however there was a significant cocaine x context interaction (F(1,18) = 4.91, p<0.05). Post-hoc analysis revealed that re-exposure to the cocaine context (Coc-SA animals) increased phosphorylation of mid-kDa PKC substrates when compared to cocaine animals that were never re-introduced to the cocaine-context (Coc-Alt; p<0.01), and also when compared to yoked-saline animals re-exposed to the self-administration chamber (Sal-SA; p<0.05; Fig. 3B). With regards to high-kDa PKC substrates, a main effect of cocaine (F(1,18) = 7.33, p<0.01) and cocaine x context interaction was detected (F(1,18) = 6.50, p<0.05). Similarly to phosphorylation of mid-kDa substrates, exposure to the cocaine context increased phosphorylation of high-kDa PKC substrates in Coc-SA animals when compared to both Coc-Alt (p<0.01) and Sal-SA (p<0.01) animals (Fig. 3B). Next, we investigated phosphorylation of extracellular signal-regulated kinase (ERK1/2) and cAMP response element-binding protein (CREB, Fig. 3A), two known substrates of Gαq(RGS2/RGS4)-to-PKC intracellular signaling pathway (Yan et al., 1997), that have also been implicated in persistent drug-seeking (e.g. (Lu et al., 2005, Hoffmann et al., 2012). Analysis of normalized phospho-ERK1/2 signal in the dSTR revealed a main effect of both cocaine (F(1,17) = 7.44, p< 0.05) and context (F(1,17) = 5.19, p<0.05), but no interaction. Post-hoc analysis revealed that re-exposure to the cocaine context increased phospho-ERK1/2 levels in Coc-SA when compared to (Coc-Alt; p<0.01) and (Sal-SA; p<0.01) animals (Fig. 3B). As CREB is one of the downstream targets of ERK1/2 activation, it was not surprising that we found an analogous upregulation of CREB phosphorylation by re-exposure to cocaine context in animals with a history of cocaine self-administration. Phospho-CREB analysis in the dSTR revealed a main effect of cocaine (F(1,17) = 4.63, p<0.05), though not the context (F(1,17) = 4.18, p=0.057), with post-hoc analysis showing upregulation of phospho-CREB levels in the dSTR of Coc-SA vs. Coc-Alt (p<0.05) and Sal-SA (p<0.05) animals (Fig. 3B). Total levels of ERK1/2 and CREB in the dSTR were not altered in any of the treatment groups (data not shown).
Fig. 3. Re-exposure to cocaine-paired context (15 min relapse test) following 3 weeks of abstinence induces phosphorylation of PKC substrates in the dSTR.
(A) Representative immunoblots showing an overall pattern of PKC-mediated protein phosphorylation in the dSTR across treatment groups as detected by antibody recognizing phosphorylated substrates of the PKC kinase. Coomassie stain of the membrane confirms equal protein loading across samples. (B) Quantitative analysis large (high-kDa) and medium-size (mid-kDa) proteins phosphorylated by PKC in the dSTR of cocaine and yoked-saline animals immediately after 15 minute exposure to self-administration or alternative context. Data represent the mean ±SEM of phosphorylated/total protein ratios expressed as a percentage of Sal controls. (n = 5–7/group). * * = p < 0.05, **= p < 0.01 Coc SA context vs Coc-Alt context, # = p < 0.05, ## = p < 0.01 Coc-SA context vs. Sal-SA context.
DISCUSSION
The present study describes downregulation of RGS2, RGS4 and upregulation of RGS9 proteins in the dSTR of animals with a history of cocaine self-administration and 21 days of abstinence (Fig. 1). In addition, an increased ratio of Gαq-to-Gαi G-proteins was also detected in the dSTR. Next, a brief re-exposure to the cocaine-paired context following 21 days of abstinence elicited robust drug-seeking behavior (Fig. 2) accompanied by generalized activation of PKC-mediated phosphorylation in the dSTR (Fig. 3), as well as induction of ERK1/2 and CREB phosphorylation in the same tissue (Fig. 4). The potential significance of the RGS protein dysregulation in the dSTR in terms of cellular mechanisms mediating post-abstinence relapse to drug-seeking is discussed below.
Fig. 4. Re-exposure to cocaine-paired context (for 15 mins) following 3 weeks of abstinence induces phosphorylation of ERK1/2 and CREB in the dSTR.
(A) Representative immunoblots of (phospho)-ERK and (phospho)-CREB signal in the dSTR across treatment groups. Coomassie stain of the membrane confirms equal protein loading across samples. Quantitative analysis of phospho-CREB (B) and phospho-ERK1/2 (C) signal in the dSTR of cocaine and yoked-saline animals immediately after 15 minute exposure to self-administration or alternative context. Data represent the mean ±SEM of phosphorylated/total protein ratios expressed as a percentage of Sal controls (n = 5–7/group). * = p < 0.05, **= p < 0.01 Coc-SA context vs Coc-Alt context, ## = p < 0.01 Coc-SA context vs. Sal-SA context.
Although the effects of psychostimulants on gene expression of several RGS proteins has been well documented, most of the studies describe dynamic and short-lived gene expression changes analogous to that of immediate early genes (for review see: Chen and Hemby, 2014). However, only changes in gene/protein expression (in this case of RGS proteins) that outlast the acute withdrawal period are thought to be relevant for persistent cocaine-seeking and relapse. To our knowledge there are only two studies that evaluated RGS protein gene expression in the striatum following extended withdrawal from chronic psychostimulant administration. Using a non-contingent cocaine administration paradigm and 28 days of withdrawal, Eipper-Mains et al. (2013) found a decrease in RGS2 and RGS4 mRNA in mouse ventral striatum. Under conditions identical to the current study, we found a decrease in RGS4 mRNA levels in the dSTR following cocaine self-administration and 21 days of abstinence (Schwendt et al., 2007). In the current study, we extended these findings to include three RGS proteins enriched in the striatum: RGS2, RGS4 and RGS9, and measured their protein levels. The importance of measuring protein (and not just mRNA) is based on observations that the activity and abundance of RGS proteins depends on post-transcriptional regulation that includes degradation (Davydov and Varshavsky, 2000, Posokhova et al., 2010), and post-translational modifications (Jones, 2004).
In general, RGS proteins limit the activity of Gαi- and Gαq-proteins, although different RGS proteins display a selective preference for Gαi vs. Gαq subtypes. As for RGS proteins studied here, RGS2 preferentially inhibits Gαq proteins, RGS4 inhibits both Gαq and Gαi proteins, and RGS9 selectively inhibits Gαi proteins (Hollinger and Hepler, 2002). Therefore a decrease in RGS2 and RGS4 coupled with an increase in RGS9, as observed here in the dSTR of animals abstinent from cocaine self-administration (Fig. 1), might produce an imbalance between Gαq and Gαi inhibition (Gαq being relatively less inhibited than Gαi). This effect is further amplified by an increased ratio of Gαq-to-Gαi protein expression also observed in this study. Next, we investigated whether this ‘sensitized’ Gαq signaling in the dSTR can be revealed as increased activation of downstream targets during relapse to cocaine-seeking. One of the well-characterized consequences of stimulating Gαq-mediated signaling is activation of PKC (measured here as overall PKC-mediated phosphorylation of its cellular substrates). Increased activation of PKC detected in the dSTR after the relapse test (Fig. 3) is in agreement with disinhibited Gαq-mediated signaling and supports the role of the Gαq-PKC pathway in dSTR mechanisms mediating cocaine-seeking following abstinence. Though we did not address the identity of the PKC subtype involved, recent evidence suggests that the activation of the PKCγ subtype in the ventral striatum mediates the reinstatement of extinguished cocaine seeking (Schmidt et al., 2013 and 2015).
Next, we investigated phosphorylation of ERK1/2 and CREB kinases in the dSTR. ERK1/2 is a mitogen-activated protein kinase phosphorylated upon activation of Gαq-(RGS2/RGS4)-to-PKC signaling pathway (Yan et al., 1997, Anger et al., 2007), while CREB is a transcription factor (also) activated by ERK1/2-mediated signaling. In general, activation (phosphorylation) of striatal ERK1/2 and CREB is important for regulation of cocaine reward and cocaine-seeking (for review see: Zhai et al., 2008). Here we show a context-specific ERK and CREB phosphorylation in the dSTR of abstinent cocaine animals that occurred only upon exposure to the cocaine-paired context, but not in response to the neutral, alternate context (Fig. 4). Since increased ERK and CREB phosphorylation is followed by induction of immediate early gene expression (Girault et al., 2007), our data are in agreement with context-specific activation of immediate early genes in the dSTR observed following the post-abstinence relapse test (Hearing et al., 2008).
What remains to be determined is the identity of neurotransmitters and receptors in the dSTR that are involved in mediating relapse to cocaine-seeking after abstinence and PKC-mediated phosphorylation. Previous studies in animals and human addicts highlighted the role of dopamine in the dSTR in cue-controlled cocaine-seeking or craving (Ito et al., 2002, Vanderschuren et al., 2005, Volkow et al., 2006, Wong et al. 2006). It has been proposed that emergence of cocaine-seeking habits is tied to the gradual recruitment of dSTR circuitry over the course of drug self-administration, and that this recruitment depends on serial dopamine connections between the ventral and the dorsal STR (Belin and Everitt, 2008). Indeed, relapse to cocaine-seeking following extended cocaine self-administration and 2 week abstinence increased dopamine levels in the dSTR (Gabriele et al., 2012). In contrast to dopamine, the same study failed to detect changes in dSTR glutamate. Also, in our recent study, blockade of mGlu5 glutamate receptors in the dSTR had no effect on relapse to cocaine-seeking following 3 weeks of abstinence (Knackstedt et al., 2014). Although the role of dSTR glutamate in persistent cocaine-seeking cannot be completely ruled out (see: Vanderschuren et al., 2005), current evidence does not support the role of dSTR glutamate in relapse.
With regards to identity of receptors in the dSTR possibly involved in relapse, preliminary evidence supports the role of local D1 dopamine receptors in context-induced cocaine- (Dr. Ronald See, personal communication) and heroin-seeking (Bossert et al., 2009). Although D1 receptor–mediated effects typically depend on activation of Gαs-proteins, D1 receptors alone, or D1–D2 heterodimers can also activate Gαq-proteins (Rashid et al., 2007, Nishi et al., 2011). Importantly, recent evidence suggests that D1-to-Gαq signaling in the striatum can mediate behavioral effects of D1 receptor agonists (Medvedev et al., 2013). In relation to our findings, it is possible that downregulation of RGS2 or RGS4 disinhibits D1-toGαq (or D1/D2-to-Gαq) signaling, while upregulation of RGS9 attenuates D2-to-Gαi signaling (Cabrera-Vera et al., 2004) and that both of these events are related to ‘priming’ of dSTR to drug-associated cues (context) in abstinent cocaine animals.
It should be noted that contingency of delivered cocaine itself might have had an effect on post-cocaine changes in RGS and G-proteins observed in the current study. Indeed, response-contingent cocaine administration (self-administration group) was shown to produce distinct striatal neuroadaptations when compared to response-independent (yoked) delivery (e.g.: Stefanski et al., 2007, Frankowska et al., 2013, Pomierny-Chamiolo et al., 2015). However this is not a generalized pattern, as in other instances the modality of cocaine exposure did not have effects on post-cocaine pattern of striatal gene/protein expression changes (e.g.: Graziella De Montis et al., 1998, Ziółkowska et el., 2006, Larson et al., 2010). For example, both passive cocaine administration (repeated injections; Yuferov et al., 2003, Eipper-Mains et al., 2013) and cocaine self-administration (Schwendt et al., 2007) decreased RGS4 mRNA levels in the dSTR. However, future studies on regulation of RGS proteins by cocaine will need to utilize experimental design that allows for comparison between pure pharmacological effects of cocaine vs. motivational aspects of cocaine- and reward-seeking (e.g. yoked-cocaine group, sucrose self-administering group).
In conclusion, the present study identified dysregulation of RGS2, RGS4 and RGS9 in the dSTR proteins of animals with a history of cocaine self-administration and abstinence that coincided with augmented PKC, ERK and CREB activation during post-abstinence relapse. Future studies will evaluate whether genetic or pharmacological manipulation of RGS proteins, aimed at the reversal of cocaine-induced changes, also display the ability to suppress relapse to drug-seeking. This is important as targeting RGS proteins could be used to reverse maladaptive plasticity and to develop new treatments for neuropsychiatric disorders (Gerber et al., 2016).
Acknowledgments
We thank Phong Do, Adrian Gomez and Katie Nicolas for technical assistance and Lori Knackstedt for valuable comments on this manuscript. This research was supported by National Institute on Drug Abuse (NIDA) grants DA025846 (MS), and NIH grant C06 RR015455.
References
- Anger T, Klintworth N, Stumpf C, Daniel WG, Mende U, Garlichs CD. RGS protein specificity towards Gq- and Gi/o-mediated ERK 1/2 and Akt activation, in vitro. Journal of biochemistry and molecular biology. 2007;40:899–910. doi: 10.5483/bmbrep.2007.40.6.899. [DOI] [PubMed] [Google Scholar]
- Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Keeley P, Cook M, Brake W, Joyce M, Nyffeler M, Heston R, Ettenberg A. Changes in levels of D1, D2, or NMDA receptors during withdrawal from brief or extended daily access to IV cocaine. Brain Res. 2007;1131:220–228. doi: 10.1016/j.brainres.2006.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Wihbey KA, Pickens CL, Nair SG, Shaham Y. Role of dopamine D(1)-family receptors in dorsolateral striatum in context-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2009;206:51–60. doi: 10.1007/s00213-009-1580-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–281. doi: 10.1016/s0896-6273(04)00159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Vera TM, Hernandez S, Earls LR, Medkova M, Sundgren-Andersson AK, Surmeier DJ, Hamm HE. RGS9-2 modulates D2 dopamine receptor-mediated Ca2+ channel inhibition in rat striatal cholinergic interneurons. Proc Natl Acad Sci U S A. 2004;101:16339–16344. doi: 10.1073/pnas.0407416101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–3243. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hemby S. Dysregulation of RGS Proteins by Psychostimulants. J Addiction Prevention. 2014;2:7. [Google Scholar]
- Davydov IV, Varshavsky A. RGS4 is arginylated and degraded by the N-end rule pathway in vitro. J Biol Chem. 2000;275:22931–22941. doi: 10.1074/jbc.M001605200. [DOI] [PubMed] [Google Scholar]
- Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW. Emergence of context-associated GluR(1) and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol. 2011;16:450–457. doi: 10.1111/j.1369-1600.2010.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eipper-Mains JE, Kiraly DD, Duff MO, Horowitz MJ, McManus CJ, Eipper BA, Graveley BR, Mains RE. Effects of cocaine and withdrawal on the mouse nucleus accumbens transcriptome. Genes, brain, and behavior. 2013;12:21–33. doi: 10.1111/j.1601-183X.2012.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankowska M, Marcellino D, Adamczyk P, Filip M, Fuxe K. Effects of cocaine self-administration and extinction on D2 -like and A2A receptor recognition and D2 -like/Gi protein coupling in rat striatum. Addict Biol. 2013;18:455–466. doi: 10.1111/j.1369-1600.2012.00452.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci. 2006;26:3584–3588. doi: 10.1523/JNEUROSCI.5146-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Gabriele A, Pacchioni AM, See RE. Dopamine and glutamate release in the dorsolateral caudate putamen following withdrawal from cocaine self-administration in rats. Pharmacol Biochem Behav. 2012;103:373–379. doi: 10.1016/j.pbb.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal K, Gyertyan I. Dopamine D3 as well as D2 receptor ligands attenuate the cue-induced cocaine-seeking in a relapse model in rats. Drug Alcohol Depend. 2006;81:63–70. doi: 10.1016/j.drugalcdep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Gerber KJ, Squires KE, Hepler JR. Roles for Regulator of G Protein Signaling Proteins in Synaptic Signaling and Plasticity. Mol Pharmacol. 2016;89:273–286. doi: 10.1124/mol.115.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Gold SJ, Ni YG, Dohlman HG, Nestler EJ. Regulators of G-protein signaling (RGS) proteins: region-specific expression of nine subtypes in rat brain. J Neurosci. 1997;17:8024–8037. doi: 10.1523/JNEUROSCI.17-20-08024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstein-Dunn E, Young KH, Cockett MI, Khawaja XZ. Regional distribution of regulators of G-protein signaling (RGS) 1, 2, 13, 14, 16, and GAIP messenger ribonucleic acids by in situ hybridization in rat brain. Brain research Molecular brain research. 2001;88:113–123. doi: 10.1016/s0169-328x(01)00038-9. [DOI] [PubMed] [Google Scholar]
- Graziella De Montis M, Co C, Dworkin SI, Smith JE. Modifications of dopamine D1 receptor complex in rats self-administering cocaine. Eur J Pharmacol. 1998;362:9–15. doi: 10.1016/s0014-2999(98)00731-6. [DOI] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct Funct. 2008;213:215–227. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AM, MacKay RK, Dodd PR, Lewohl JM. Association of polymorphisms in RGS4 and expression of RGS transcripts in the brains of human alcoholics. Brain Res. 2010;1340:1–9. doi: 10.1016/j.brainres.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann HM, Nadal R, Vignes M, Ortiz J. Chronic cocaine self-administration modulates ERK1/2 and CREB responses to dopamine receptor agonists in striatal slices. Addict Biol. 2012;17:565–575. doi: 10.1111/j.1369-1600.2011.00353.x. [DOI] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacological reviews. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, Everitt BJ. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci. 2002;22:6247–6253. doi: 10.1523/JNEUROSCI.22-14-06247.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TL. Role of palmitoylation in RGS protein function. Methods in enzymology. 2004;389:33–55. doi: 10.1016/S0076-6879(04)89003-7. [DOI] [PubMed] [Google Scholar]
- Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294:680–687. [PubMed] [Google Scholar]
- Knackstedt LA, Trantham-Davidson HL, Schwendt M. The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol. 2014;19:87–101. doi: 10.1111/adb.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, Pierce RC. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson EB, Akkentli F, Edwards S, Graham DL, Simmons DL, Alibhai IN, Nestler EJ, Self DW. Striatal regulation of ΔFosB, FosB, and cFos during cocaine self-administration and withdrawal. J Neurochem. 2010;115:112–122. doi: 10.1111/j.1471-4159.2010.06907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Chefer S, Lu H, Guillem K, Rea W, Kurup P, Yang Y, Peoples L, Stein EA. Dorsolateral caudate nucleus differentiates cocaine from natural reward-associated contextual cues. Proc Natl Acad Sci U S A. 2013;110:4093–4098. doi: 10.1073/pnas.1207531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–32. doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martemyanov KA, Yoo PJ, Skiba NP, Arshavsky VY. R7BP, a novel neuronal protein interacting with RGS proteins of the R7 family. J Biol Chem. 2005;280:5133–5136. doi: 10.1074/jbc.C400596200. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Medvedev IO, Ramsey AJ, Masoud ST, Bermejo MK, Urs N, Sotnikova TD, Beaulieu JM, Gainetdinov RR, Salahpour A. D1 dopamine receptor coupling to PLCbeta regulates forward locomotion in mice. J Neurosci. 2013;33:18125–18133. doi: 10.1523/JNEUROSCI.2382-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. The New England journal of medicine. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Min C, Cheong SY, Cheong SJ, Kim M, Cho DI, Kim KM. RGS4 exerts inhibitory activities on the signaling of dopamine D2 receptor and D3 receptor through the N-terminal region. Pharmacological research. 2012;65:213–220. doi: 10.1016/j.phrs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Shuto T. Mechanisms for the modulation of dopamine d(1) receptor signaling in striatal neurons. Frontiers in neuroanatomy. 2011;5:43. doi: 10.3389/fnana.2011.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien C. Drug addiction and drug abuse. In: Hardman JLL, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. pp. 621–642. [Google Scholar]
- Pacchioni AM, Gabriele A, See RE. Dorsal striatum mediation of cocaine-seeking after withdrawal from short or long daily access cocaine self-administration in rats. Behav Brain Res. 2011;218:296–300. doi: 10.1016/j.bbr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomierny-Chamiolo L, Miszkiel J, Frankowska M, Pomierny B, Niedzielska E, Smaga I, Fumagalli F, Filip M. Withdrawal from cocaine self-administration and yoked cocaine delivery dysregulates glutamatergic mGlu5 and NMDA receptors in the rat brain. Neurotox Res. 2015;27:246–258. doi: 10.1007/s12640-014-9502-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posokhova E, Uversky V, Martemyanov KA. Proteomic identification of Hsc70 as a mediator of RGS9-2 degradation by in vivo interactome analysis. Journal of proteome research. 2010;9:1510–1521. doi: 10.1021/pr901022m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;104:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugstad JA, Marino MJ, Folk JA, Hepler JR, Conn PJ. RGS4 inhibits signaling by group I metabotropic glutamate receptors. J Neurosci. 1998;18:905–913. doi: 10.1523/JNEUROSCI.18-03-00905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Kimmey BA, Arreola AC, Pierce RC. Group I metabotropic glutamate receptor-mediated activation of PKC gamma in the nucleus accumbens core promotes the reinstatement of cocaine seeking. Addict Biol. 2015;20:285–296. doi: 10.1111/adb.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Schassburger RL, Guercio LA, Pierce RC. Stimulation of mGluR5 in the accumbens shell promotes cocaine seeking by activating PKC gamma. J Neurosci. 2013;33:14160–14169. doi: 10.1523/JNEUROSCI.2284-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendt M, Hearing MC, See RE, McGinty JF. Chronic cocaine reduces RGS4 mRNA in rat prefrontal cortex and dorsal striatum. Neuroreport. 2007;18:1261–1265. doi: 10.1097/WNR.0b013e328240507a. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Sigmon SA, McGinty JF. RGS4 overexpression in the rat dorsal striatum modulates mGluR5- and amphetamine-mediated behavior and signaling. Psychopharmacology (Berl) 2012;221:621–635. doi: 10.1007/s00213-011-2606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 2007;194:321–331. doi: 10.1007/s00213-007-0850-8. [DOI] [PubMed] [Google Scholar]
- Stefański R, Ziółkowska B, Kuśmider M, Mierzejewski P, Wyszogrodzka E, Kołomańska P, Dziedzicka-Wasylewska M, Przewłocki R, Kostowski W. Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system. Brain Res. 2007;1157:1–10. doi: 10.1016/j.brainres.2007.04.074. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, Brasić JR, Kimes AS, Maris MA, Kumar A, Contoreggi C, Links J, Ernst M, Rousset O, Zukin S, Grace AA, Lee JS, Rohde C, Jasinski DR, Gjedde A, London ED. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–27. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- Yan Y, Chi PP, Bourne HR. RGS4 inhibits Gq-mediated activation of mitogen-activated protein kinase and phosphoinositide synthesis. J Biol Chem. 1997;272:11924–11927. doi: 10.1074/jbc.272.18.11924. [DOI] [PubMed] [Google Scholar]
- Yuferov V, Kroslak T, Laforge KS, Zhou Y, Ho A, Kreek MJ. Differential gene expression in the rat caudate putamen after “binge” cocaine administration: advantage of triplicate microarray analysis. Synapse. 2003;48:157–169. doi: 10.1002/syn.10198. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H, Li Y, Wang X, Lu L. Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement. Cellular and molecular neurobiology. 2008;28:157–172. doi: 10.1007/s10571-007-9240-3. [DOI] [PubMed] [Google Scholar]
- Ziółkowska B, Stefański R, Mierzejewski P, Zapart G, Kostowski W, Przewłocki R. Contingency does not contribute to the effects of cocaine self-administration on prodynorphin and proenkephalin gene expression in the rat forebrain. Brain Res. 2006;1069:1–9. doi: 10.1016/j.brainres.2005.11.042. [DOI] [PubMed] [Google Scholar]