Abstract
Anesthetic photoaffinity ligands have had an increasing presence within anesthesiology research. These ligands mimic parent general anesthetics, and allow investigators to study anesthetic interactions with receptors and enzymes; identify novel targets; and determine distribution within biological systems. To date nearly all general anesthetics used in medicine have a corresponding photoaffinity ligand represented in the literature. In this review we examine all aspects of the current methodologies, including ligand design, characterization and deployment. Finally we offer points of consideration and highlight the future outlook as more photoaffinity ligands emerge within the field.
Introduction
Studying the interactions between general anesthetics and their macromolecular targets is crucial to the understanding of the biochemistry for these drugs. In recent years the application of photoaffinity labeling for this purpose has increased. Traditionally the objectives of this method are to allow researchers to identify the binding site(s) within established targets (micro-level) and, to a lesser extant, determine novel binding molecules and distribution within a given biological system (macro-level). Within anesthesia research there has been significant advancement in the development of photoaffinity ligands (PALs), from the initial use of neat halothane 1 to complex syntheses of bifunctional PALs allowing for affinity-based protein profiling2. The primary objective of this review is to feature current technologies and to discuss the application and results of anesthetic PALs target identifications.
Photoaffinity Ligand
In the current process of photoaffinity labeling, the chemical structure of the drug of interest (parent ligand) is modified to incorporate a photoreactive group that, upon ultraviolet (UV) irradiation, generates a highly reactive chemical intermediate. This intermediate then chemically “adducts” or “labels,” by covalent insertion to a bond in close proximity, such as solvent components or an occupied macromolecule. In the instance of a PAL bound to a macromolecule, the ligand adduct acts as a traceable modification that can be used to investigate ligand-target interactions and/or ligand distribution within the organ or tissue. A critical advantage that PALs provide for general anesthetics is that they mitigate the relative lower affinities, generally micromolar, associated with this particular class of drugs. They accomplish this by dramatically prolonging drug unbinding rates, thereby providing an irreversible snapshot of these otherwise transient interaction(s). On the other hand, the inherent high dissociation constants of anesthetics remains an experimental hurdle chiefly by adding to the difficultly in validation of specific binding sites in photoaffinity labeling studies. These concepts and current methodologies for validation of anesthetic-specific sites will be discussed later in the review. The following sections are devoted to the initial design and physicochemical characterization of an anesthetic PAL, both critical to the interpretation of anesthetic photoaffinity labeling results.
Anesthetic Photoaffinity Ligand
Design
Several factors are considered during the design of an anesthetic PAL; most notably that introduction of the photoreactive group into the anesthetic chemical structure does not significantly alter the biochemical and pharmacological properties of the parent drug. Diazirines are nearly universal as the photoreactive group incorpated within anesthetic PALs (Figure 1). With UV irradiation, diazirines undergo photoactivation, resulting in the release of an inert dinitrogen molecule and the generation of carbene chemical species that indiscriminately insert into the nearest molecule. The popularity of this particular photoreactive group can be largely attributed to the less damaging UV wavelength needed for photoactiviation, its relative stability and smaller size, and highly reactive intermediate product, the carbene. In combination, these attributes allow for less deviation of binding properties compared to the parent anesthetic. We direct the interested reader to previous reviews for further detailed discussion on the development and use of PALs and photoreactive groups 3–6.
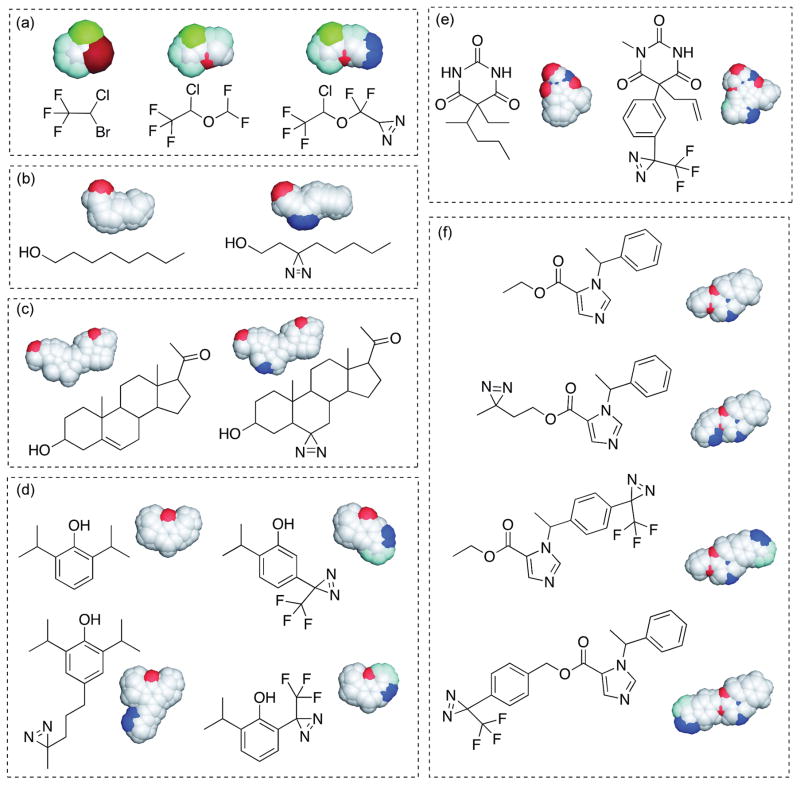
Figure 1.
Chemical and space-filling structures of general anesthetics and corresponding photoaffinity ligands. With the exception of halothane, a diazirine serves as the photoactive group. Halothane and Haloethers (a); halothane (left), isoflurane (middle), aziisoflurane (right). Alcohols (b): octanol (left), 3-azioctanol (right). Neurosteriods (c); pregnenolone (left), 6-azipregnenolone (right). Alkylphenols (d); propofol (top left), meta-azipropofol (top right), para-4-aziC5-propofol (bottom left), ortho-propofol diazirine (bottom right). Barbiturates (e); pentobarbital (left), mTFD-mephobarbital (right). Imidazoles (f); etomidate (top), azietomidate (upper middle), pTFD-etomidate (lower middle), TDBzl-etomidate (bottom)
In practice, the design and synthesis of a PAL to effectively mimic a general anesthetic can be arguably the most difficult process due to the challenging synthesis of stable molecules containing photoreactive groups, and the lack of in depth knowledge of molecular recognition elements between anesthetics and their important macromolecular targets. The small size and relatively featureless nature of most general anesthetics make any chemical modification, even a diazirine, a relatively large perturbation. As it stands, even the best-designed PAL requires considerable chemical deviation from the parent anesthetic structure (exception being halothane1) and alone cannot definitively determine a binding target and/or site for the parent drug.
Anesthetic Photoaffinity Ligand
Physicochemical Properties
After incorporation of the diazirine into the anesthetic chemical structure, the resulting changes in physicochemical properties require careful characterization before experimental deployment of the PAL. The stability and photactivation efficiency of the diazirine (or any incorporated photoreactive group) are unique to each developed PAL. The diazirine moeity should produce a distinctive and pronounced UV absorbance 4,7 that decays with increasing exposure to the appropriate UV wavelength 8,9. The rate of decay provides an estimate of photoactivation efficiency within the given buffer. A basic equation (Eq. 1) 10 can give the half-life of photoactivation (T1/2) with relationship to the intensity of the lamp (I0) applied, molar extinction coefficient (ε) and quantum yield of photoactivation (Ψ).
| 10 | (1) |
The chemical nature of the subsequent reactive intermediate generated by photoactivation is also unique to each PAL. The reactivity of the intermediate directly influences the propensity to covalently insert into nearby molecules or, depending on ligand chemical structure, itself (e.g. intramolecular reactions). For example, studies of 6-azipregnanolone, a PAL derivative for pregnanolone, reported that major photoactivation products were likely generated from internal rearrangement or bimolecular insertion reactions 11. As a result, some macromolecular sites would not be sufficiently represented to allow for detection due to low to zero yield of adducted intermolecular products. It could be argued that a propensity for intermolecular reactions decreases the probability for nonspecific labeling, but this ultimately depends on the relative reaction rates.
Assuming that a photoactivated ligand intermediate demonstrates sufficient probability for intermolecular interactions, it is generally considered that most carbenes do not demonstrate significant preference for covalent insertion, being capable of both nucleophilic and electrophilic attack 4. This includes to the solvent molecules, which under usual conditions are in far greater abundance than a target macromolecule. For example, the singlet carbene intermediate is readily quenched by covalent insertion into adjacent water molecules 12, and therefore presents another mechanism that deters nonspecific labeling. Similarly, amphipathic molecules, such as lipids and/or detergents, have also been shown to be readily adducted 6. Weiser et al. demonstrated that the tritiated propofol dervative [3H]meta-azipropofol, while showing preference towards synaptic dense regions (and therefore presumably protein), ubiquitously labeled whole rat brain, implying that lipids also act as a considerable photoadducted product 13.
Increasing evidence has suggested that proteins are a significant contributor to anesthetic mechanisms. As such, PALs have been applied toward understanding protein binding sites of anesthetics. Table 1 gives the list of photoadducted residues of halothane and diazirine-containing anesthetic PALs with protein targets. To our knowledge, there has not been a systematic investigation of an anesthetic PAL that conclusively demonstrates preferential insertion into selected amino acids within a protein or polypeptide. Indeed previous work has demonstrated that, while the efficiency (defined as mole of PAL per mole of stationary pure amino acid) of labeling of specific amino acids may be greater for a given PAL (e.g. Cys, Trp, His, Phe), a carbene can covalently insert into all amino acids even within a transmembrane domain 6,14. It should be noted that backbone atoms within a protein, not just side chains, can act as potential insertion sites. Indeed, previous evidence with aziisoflurane modification of apoferritin implies that backbone atoms (such as carbonyl oxygens) are suitable photoadduction sites 15.
Table 1.
Summary of adducted residues by anesthetic photoaffinity ligandss using amino acid sequncing methods
| Parent anesthetic | Anesthetic photoaffinity ligand | Amino acid sequencing methoda | Protein | Residue | Ref. |
|---|---|---|---|---|---|
| halothane | halothane | Edman degradation(14C) | apo-ferritin | Trp-15 | 58 |
| nAChR | αTyr-213, γTyr-111, δPhe-206 δTyr-228 | 43 | |||
| octanol | 3-azioctanol | Edman degradation (3H, 1H) | nAChR | αTyr-190, αTyr-198, αGlu-262, αHis-408, αCys-412 | 18 |
| MS/MS | adenylate kinase | His-36 | 59 | ||
| neural cell adhesion molecule L1 | Glu-33, Tyr-418 | 60 | |||
| protein kinase Cδ | Tyr-236, Lys-40, Glu-2 | 61 | |||
| protein kinase Cε | Tyr-176, Tyr-238, Tyr-250 | 62,63 | |||
| japanese firefly luciferase | Glu-313 | 36 | |||
| isoflurane | aziisoflurane | MS/MS | apo-ferritin | Arg-59 | 15 |
| lymphocyte function associated antigen-1 | Leu-135, Glu-137, Tyr-257, Leu-302, Lys-304, Lys-305 | 15,44 | |||
| platelet receptor integrin aIIbb3 | Asp-158, Lys-159 | 37 | |||
| etomidate | azietomidate | Edman degradation (3H) | nAChR | αTyr-98, αTyr-190, αGlu-262, αGlu-390, αCys-412, βAsp-268, δAsp-59, δSer-258, δCys-236, δSer-262, δGln-276 | 17,64 |
| GABAA receptor | α1Met-236, β1Met-286 | 65–67 | |||
| pTFD-etomidate | Edman degradation (3H) | nAChR | αLeu-251, αSer-252,αVal-255, αLeu-258, βLeu-257, βLeu-261, δLeu-265, δVal-269 | 19 | |
| TDBzl-etomidate | Edman degradation (3H) | nAChR | αLeu-251, αSer-252, αVal-255, γMet-299, δLeu-265, δVal-269, δLeu-272, δLeu-273, δGln-276 | 68 | |
| GABAA receptor | α1Cys-234, α1Met-236, β3Met-286, β3Cys-288, β3Val-290 | 69 | |||
| barbituate | (R)-(−)-mTFD-mephobarbital | Edman degradation(3H) | nAChR | αIle-231, αMet-242, αCys-412, βMet-249, βSer-254 βLeu-257, βVal-261, βLeu-265, γCys-252, γMet-299, δMet-257, δSer-258, δSer-262, δLeu-265, δVal-269 | 70 |
| GABAA receptor | α1Ala-291, α1Tyr-294, β3Met-227, β3Met-227, γ2Ser-301 | 49 | |||
| pregnanolone | 6-azipregnanolone | MS/MS | tubulin | Cys-354 | 11 |
| GABAA receptor | β3Phe-301 | 39 | |||
| propofol | meta-azipropofol | Edman degradation (3H, 1H) | nAChR | αSer-248, αSer-252, δArg-277, δPhe-232, δCys-236, δVal-269, δThr-274 | 71 |
| GABAA receptor | α1Met-236, α1Ile-239, β3Met-227, β3Met-286 | 72 | |||
| GLIC | Met-205, Tyr-254, Met-261, Asn-307 | 73 | |||
| MS/MS | apo-ferritin | Leu-24, Leu-81 | 28 | ||
| lymphocyte function associated antigen-1 | Ile-254, Tyr-257, Ile-258, Lys-287, Leu-302, Lys-304 | 74 | |||
| SIRT2 deacetylase | Tyr-139, Phe-190, Met-206 | 75 | |||
| VDAC | Gly-56, Val-184 | 53 | |||
| para-4-aziC5-propofol b | nAChR | 76 | |||
| ortho-propofol diazirine | MS/MS | human serum albumin | Lys-41, Trp-111, Lys-525, His-535, Lys-536 | 29 | |
| GABAA receptor | βHis-267 | 29 |
(nAChR) nicotinic acetylcholine receptor; (GABAA) γ-aminobutyric acid type A; (GLIC) gloeobacter ligand-gated ion channel; (SIRT2) sirtuin-2 ; (VDAC) voltage-dependent anion channel.
Noted in ( ) with Edman degradation amino acid sequencing method are the isotopes used for photoaffinity ligand detection.
para-4-aziC5-propofol was not applied in amino acid sequencing however displayed adduction to nAChR by gel electrophoresis and autoradiography
An exception to preferential residue labeling arises with aliphatic-diazirines and their increased predisposition to undergo diazo isomerization, an alternative intramolecular rearrangement. The diazo isomerization of the diazirine can lead to the generation of a carbocation, rather than a carbene, an intermediate that preferentially undergoes electrophilic attack of electron dense (nucleophilic) residues 7. The fraction of the PAL and its resulting products generated by this unwanted isomerization is specific to the chemical properties of the PAL with the event being debated for some aliphatic-diazirines 16. Regardless, the propensity of labeling Asp, Glu, His, and Tyr by azietomidate 17,18 led to the development of the etomidate derivatives pTFD-etomidate 19 and TDBzl-etomidate 20, both of which contain trifluoromethyl diazirine and trifluoromethylaryl diazirine respectively. These additional chemical groups chemically favor carbene generation, rather than diazo isomerization, leading to the desired indiscriminate covalent insertions 4,7,21. The synthetic changes of etomidate PALs resulted in a broader range of labeled residues, including hydrophobic residues 20,22,23. However whether these modifications were due to changes in photochemistry and/or to altered equilibrium binding due to the changes in chemical structure is unclear.
Anesthetic Photoaffinity Ligand
Biochemical and Pharmacological Activity
It is universally agreed that any novel anesthetic derivative, including PALs, requires a thorough investigation of biochemical and pharmacological activities to assure retention of parent drug characteristics. These studies can be placed in three basic groups; 1) equilibrium binding to anesthetic protein models, 2) isolated functional studies and 3) in vivo demonstration of pharmacological endpoints.
Similar equilibrium binding of the parent anesthetic and the PAL derivative to model proteins provides some evidence towards the retention of basic molecular recognition elements (hydrophobic forces, hydrogen bonding, van der Waals and electrostatic forces, etc.) between the parent drug and developed PAL. Well characterized anesthetic protein models previously crystalized in complex with the parent anesthetic at high resolution, such as apo-ferritin24,25 and human serum albumin 26,27, are often used.
The PAL should demonstrate similar functional effect(s) within an established protein target as the parent drug. For many micro-level studies, the targets considered include the cys-loop pentameric ligand gated ion channels, such as the nicotinic acetylcholine receptor (nAChR) and γ-amino butyric acid type A (GABAA) receptor. Functional characterization therefore requires electrophysiology. Within these studies investigators often observe changes in potency and/or efficacy dependent on the type and position of chemical modifications made to turn the parent ligand into a PAL, as observed with the meta- and ortho- trifluoromethyl-diazrine substitutions on propofol PALs 28,29. Similarly the different PAL derivatives of etomidate PALs show altered activity on nAChR, including approximately 5-fold differences in potency between azietomidate and pTFD-etomidate for inhibition 19,30. Because the contributions of these individual targets to the desired effect (anesthesia) is not known, it is not clear a priori how important these subtle changes in potency are for the interpretation of photoaffinity labeling data. Regardless, these studies aim to demonstrate that the ligand retains functional activity similar to the parent anesthetic. However this demonstration, while necessary, is not sufficient to indicate a common binding site in that the PAL may act as a functionally active ligand but at other site(s).
In addition to retention of activity in vitro, retention of in vivo activity is also a necessary form of validation of the anesthetic PAL. These studies usually include tadpole immobility assays or rodent loss of righting reflex within wild type and/or mutant models 31. Since the total mechanism by which any of the general anesthetics produce their in vivo endpoints remain unclear, the retention of in vivo activity alone is insufficient evidence to support binding and action at specific protein targets. An interesting demonstration of this is TDBzl-etomidate, an etomidate PAL derivative. TDBzl-etomidate demonstrates comparable potency for tadpole immobility and more potent potentiation of the GABAA receptor relative to the parent anesthetic 20. However unlike etomidate, this PAL acts as a positive modulator of nAChR, 19 indicating that caution is required when attempting to correlate in vivo potency and a shared binding site within a specific target macromolecule.
Each of the biochemical and pharmacological investigations can be extended to provide evidence to confirm the PALs’ capabilities to successfully insert into the bound targets after photoactivation. For example photoaffinity labeling of model proteins with halothane 1, isoflurane 15, or propofol 28,29 PAL derivatives have demonstrated insertion into residues lining the crystallographically confirmed site(s) of serum albumin or apo-ferritin. Irreversable enhancement of GABAA receptor gating and desensitization has been reported upon azietomidate photoactivation 32. Meta-azipropofol photoactivation in vivo has shown significant prolongation of emergence, nearly 10-fold, of tadpole immobility 33 suggesting successful covalent insertion into a sufficient mass or number of targets that contribute to an anesthetic endpoint. While the absence of this “optoanesthesia” feature does not negate the validity of a PAL, its presence is strong evidence for validity and utility. Evidence for reliable photoadduction can be considered just as important as the studies demonstrating retention of biochemical and pharmacological activity in that this characteristic is directly responsible for the identification of novel binding partners.
Micro-level Photoaffinity Labeling
The majority of studies in the current literature use anesthetic PALs to determine anesthetic binding sites within a specific preselected protein target, an approach we term micro-level studies. The identification and characterization of drug binding sites promotes increased understanding of the molecular mechanism(s) within pharmacological targets and, potentially, drug modifications to improve the drug safety profile.
Micro-level Target Detection
Methodologies
Two different methodologies of protein microsequencing are commonly used to identify protein binding sites photolabeled by a PAL. These include Edman degradation (ED) and mass spectrometry (MS), both of which determine binding sites to the amino acid level. Simplified schematics for both methods are shown in Figure 2a–b. ED and MS have been used extensively for multiple types of potential protein targets, soluble and insoluble, over the entire range of anesthetic PALs (Table 1) and much has been learned in terms of binding site location, specificity and mechanism of protein modulation. Depending on the characteristics and size of the investigated protein target, both ED and MS can become complex. For example, larger multimeric membrane proteins may require additional steps of precipitation, separation and protease digestions in order to achieve sufficient coverage of the sequence to confidently reveal or exclude adduction sites.
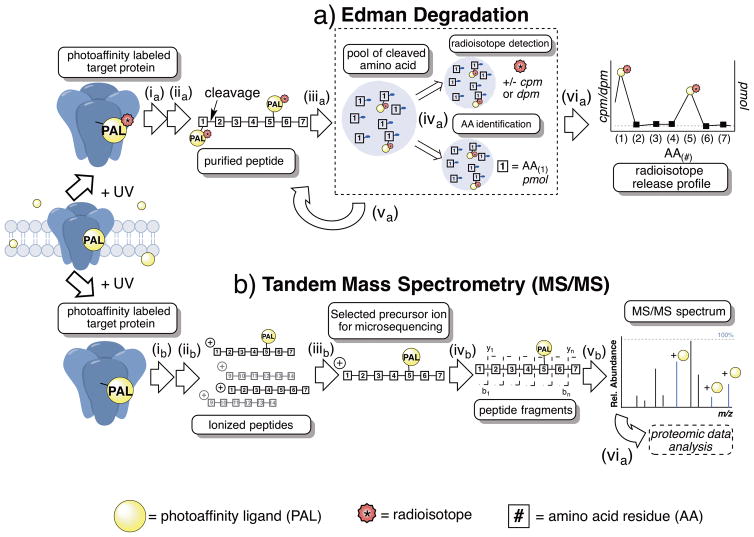
Figure 2.
Simplified schemes for the major methods used in micro-level anesthetic photoaffinity labeling; a) Edman degradation b) Tandem mass spectrometry (MS/MS). a) Edman degradation; ia) enriched target protein is photoaffinity labeled with radiolabeled (*) anesthetic photoaffinity ligand (PAL) and protein(s) are separated and digested into peptides; iia) peptides are isolated and rigorously purified; iiia) the first amino acid from the N-termini is cleaved from purified peptide via Edman reaction; iva) the cleaved amino acid is isolated, quantified and separated into two pools for (top) radioisotope detection by scintillation counting and (bottom) amino acid identification by chromatography; va) the cycle is repeated, gradually sequencing the entire purified peptide; via) the amino acid sequence is plotted against radioactivity (cpm, counts per minute, or dpm, disintegrations per minute) resulting in radioisotope release profile. b) Tandem mass spectrometry (MS/MS); ib) enriched target protein is photoaffinity labeled with anesthetic photoaffinity ligand (PAL) and protein(s) are separated and digested into peptides that are further separated by on- and/or offline chromatography methods; iib) peptides undergo ionization generally by electrospray ionization (ESI) or matrix-assisted laser desorption/ionization (MALDI); iiib) precursor ions are separated by mass/charge (m/z) within MS1 (aka. MS1 precursor ion); ivb) MS1 precursor ion undergoes mass fragmentation often by collision-induced dissociation (CID) resulting in largely b or y fragment ions; vb) mass spectrometry software are used for data acquisition, database search analysis and representation.
While the fundamental endpoint results of each method are identical (e.g. identities of the adducted amino acids), the final means to gather the results are notably different. For ED, individual amino acids are sequentially and chemically cleaved (e.g. the Edman reaction) from pools of purified peptide fragments from the digested target protein. The resulting pool of the cleaved amino acid is then separated into two smaller pools; one used for the identification of the amino acid using high-performance liquid chromatography, the other to detect whether this amino acid contains a radiolabeled PAL adduct. It should be noted that when a backbone atom is labeled by the radiolabeled PAL, the Edman reaction (or prior peptide cleavages) may be retarded. Although this has not been systematically studied, evidence in support of the possibility is the frequent observation that subsequent amino acid yield decreases after a radiolabeled amino acid.
In contrast, in the MS approach, the PAL adduct is detected by the change the label imparts to the molecular mass-to-charge (m/z) ratio of a peptide fragment. For amino acid level localization of the adduct, second level (MS/MS) or higher order data are required. Most often ion traps, quadrupole mass filters and mass analyzers are combined to the electrospray source for detection. The recent development of orbitrap and Fourier Transform Ion Cyclotron Resonance has resulted in very high mass accuracy, resolution, and dynamic range of detection for the MS method 34–36. These recent advances in MS technology and the non-dependence on radioactivity have contributed to the increased use of MS as a method for detection in photoaffinity labeling studies.
Micro-level Photoaffinity Labeling
Anesthestic-Specific Binding Site Validation
The protein binding sites identified by PAL labeling require several levels of validation to have confidence that the revealed site is the same as that of the parent anesthetic. According to pharmacological convention, specific photoaffinity labeling would be irreversible labeling by the PAL, within a saturable protein binding site. For the purposed of this review, we define anesthetic-specific photoaffinity labeling as when this same labeled saturable site would be shared by the parent anesthetic. In contrast, nonspecific photoaffinity labeling is a result of random adduction, such as to peripheral, solvent accessible, or lipid exposed regions of a protein. Nonspecific labeling could also occur with the migration of the reactive intermediate after photoactivation in solvent, lipid or protein matrix to a random and remote site. All these forms of labeling will occur over the course of a single experiment, but in general nonspecific labeling is challenging to detect in most conditions due to the large number of potential adduction sites, generally lower affinity and therefore lower occupancy, and random nature. Regardless, nonspecific labeling should have low reproducibility, so to categorize sites as “specific” requires several experiments.
Validation of an anesthetic-specific over a PAL-specific binding site is also required for a target protein. Notably, it should be recognized that both instances may contribute to a mechanism or component of “anesthesia,” however only parent anesthetic-specific adducted sites are of interest and relevance to clinical medicine. Generally the initial step includes reconciling the labeled residues against existing crystal structures or developed models to identify potential localization within a protein cavity, interface or pore site. The characteristics of these sites may indicate potential mechanisms as well as the likelihood for shared binding by the parent anesthetic. For example, the adduct site of aziisoflurane in platelet receptor integrin αIIbβ3 resided near a calcium binding site, a critical region for regulation of the protein. This proximity immediately suggests a potential mechanism for isoflurane-induced attenuation of platelet aggregation 37.
Another common form of validation is by mutagenesis of the adducted residue(s), followed by functional studies and perhaps repeat photoaffinity labeling experiments. These studies, while indirect, allow association of the photoadducted residue and site to functional activity of the parent anesthetic within the protein. However, common to all mutagenesis investigations, the change in residue might also alter protein structure or dynamics, altering the protein’s response to the anesthetic instead of changing the affinity for the binding site or altering protein structure preventing access to a regionally distinct binding site. Furthermore, since the photoadduction, and therefore site might be side chain independent, mutagenesis studies might not provide a clear interpretation.
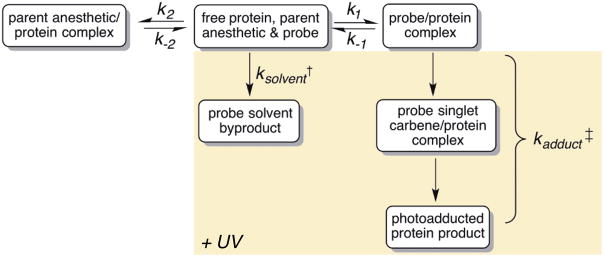
Finally direct evidence of a parent anesthetic-specific adduct site would be represented by successful inhibition of PAL labeling through competitive binding, or “protection,” by the parent anesthetic 10. Although intuitive, protection from photolabeling is complicated by the non-equilibrium nature of most photolabeling experiments. A kinetic mechanism for protection of a parent anesthetic-specific site from photoadduction is described in Figure 3, and is similar to that previously described for photoaffinity agents and other protection experiments for non-equilibrium systems 10,38. The model has simultaneous dependence on the two different affinities of the ligands (e.g. protecting ligand and PAL) for the protein target, and the photoreactivity of the PAL (which is highly dependent on the experimental conditions noted in section 2.2). Since the photolabeling event is irreversible, in contrast to the parent ligand, binding sites will be gradually depleted. Further, the PAL will also be gradually depleted by solvent labeling. Therefore, multiple consecutive and competing rates, unique to the target protein, protecting ligand and PAL, are present within a typical protection experiment. As a result, protection experiments require careful attention, otherwise, results can be misinterpreted.
Figure 3.
Schematic representation of the steps involved in the most basic mechanism of parent anesthetic protection of anesthetic photoaffinity labeling experiments. Highlighted region denotes the non-equilibrium reactions upon UV irradiation. †(ksolvent ) Rate of solvent quenching of the ligand, given that carbenes are readily quenched by water the rate is limited by photoactivation of the ligand and therefore a first order rate constant. ‡(kadduct ) Rate of photoadduction represents two consecutive first order reactions, the photoactivation of the ligand and the insertion by the singlet carbene into the protein.
Some physicochemical limitations of the PAL or the method for label detection may prevent quantitative assessment of protection. For example, a relatively high molar ratio of the parent anesthetic to PAL is generally required for protection (>100:1). However, such a high concentration may not be possible due to limitations in parent anesthetic solubility as reported previously 39, making interpretation of protection experiments a challenge. Quantitative detection by MS also poses limitations, particularly for label-free quantification that requires considerable protection for significance under most experimental conditions 40. However with continuing advancement of the technique, such as isobaric labeling methods 41,42, quantification of protection experiments by MS will be possible.
Micro-level Target Detection
Significant Findings
The potential for in-depth knowledge of the molecular mechanisms of anesthesia and/or drug side effects is reflected by the wide range of developed anesthetic PALs and their numerous micro-level investigations. In this section we will focus on selected significant findings for anesthetic PALs as examples of insights given by the technique. We direct the reader to our previous review, as well as to Table 1, 3 that provide a comprehensive overview of all developed anesthetic PALs and studied protein targets.
To date, two volatile anesthetic PALs have been deployed in micro-level investigations; neat halothane and aziisoflurane. Permitted by 14C-labeled halothane, ED identified halothane modification of extracellular and transmembrane domains within Torpedo nAChR 43. In particular, isoflurane protected halothane labeling of Tyr-228 within the δ subunit in a state-dependent manner. This finding highlighted a potential pocket formed within the receptor’s desensitized state that can accommodate either anesthetic and, through stabilization of the state, may contribute to the functional inhibition of nAChR. A similar trend of potential state-dependent binding was suggested by aziisoflurane labeling of lymphocyte function associated antigen-1 44. The labeling of Ile-135 and Glu-137 within the lymphocyte function associated antigen-1β1 domain by aziisoflurane suggested a closed state-dependent binding. The stabilization of this conformation by the volatile anesthetic may contribute to the impaired lymphocyte arrest and the antiinflammatory actions displayed by isoflurane 45–47.
The PAL derivative of mephobarbital, (R)-(−)-mTFD-mephobarbital, was shown to act as a particularly potent positive modulator of the GABAA receptor; approaching the potencies of etomidate and propofol 48. As such (R)-(−)-mTFD-mephobarbital was used to uncover binding sites of barbiturate anesthetics. When compared to azietomidate, photoaffinity labeling studies suggested distinct sets of sites within the GABAA receptor for the two anesthetics 49. Both (R)-(−)-mTFD-mephobarbital and azietomidate labeled intersubunit sites, and at a similar depth within the transmembrane region; however, the different anesthetics targeted different subunit interfaces. mTFD-mephobarbital selectively labeled α+/β- and γ+/β- while azietomidate selectively labeled the two β+/α-intersubunit sites with the GABAA receptors49. These studies demonstrate that the heterogeneity of anesthetic chemical structures may be reflected by different site locations within the same target. Indeed, with the likely joint use of multiple PALs, a progressive understanding of the molecular mechanisms, including state-dependent binding and structure-function relationships, for anesthetics within complex target proteins can be achieved.
Macro-level Photoaffinity Labeling
Macro-level photoaffinity labeling is a much less prevalent method compared to the application of micro-level photoaffinity labeling within anesthesiology research. Despite this, macro-level investigations are becoming recognized as a useful tool within chemical biology due to the increased need to uncover druggable targets, matched with the growing awareness of how promiscuous many drugs are. Anesthetics can be considered a prime example of the current paradox in drug development in that a there is an urgent need for optimized chemical designs in order to improve potency or decrease toxicity yet the lack of mechanistic knowledge prevents educated design. The following section presents an overview of selected studies and perspectives of anesthetic PALs in macro-level studies.
Macro-level Photoaffinity Labeling
Significant Findings
Previously the labeling of rat brain membranes with [3H]6-azipregnanolone provided an unbiased, affinity-based picture of neurosteroid targets within a complex biological system 50. Based on radioactivity of slices from a subsequent SDS-PAGE separation of proteins, specific labeling of a few gel bands was observed. Two proteins, tubulin and voltage-dependent anion channel-1 (VDAC-1), were subsequently identified and further investigated as potential protein targets. Micro-level studies found that tubulin was at Cys-354 11, a residue within the colchicine binding site, and consistent with the ability of 6-azipregnanolone and pregnanolone to inhibit tubulin polymerization 11. Other work using the non-clinical anesthetic PAL azidoanthracene has also implicated tubulin as a potential anesthetic target 51. Similarly further work on VDAC-1 found that it was unlikely to be an important target in the VDAC-1/GABAA receptor interaction pathway 52. VDAC-1 might however be responsible for alternative pathways and/or anesthetic side effects.
VDAC-1 was also identified as a specific anesthetic binding protein target in a macro-level investigation using [3H]meta-azipropofol 33. The subsequent micro-level investigation found that VDAC gating was modulated by propofol, and two binding sites were identified 53. In addition to VDAC-1, synaptosomal-associated protein- 25kDa (SNAP-25) was identified as a labeled protein within the macro-level investigation 54. The SNARE complex has potential as a target for anesthesia, with volatile anesthetics and propofol inhibiting neurotransmitter release by interactions with the complex 55,56. These few examples demonstrate how photoaffinity labeling has provided unexpected molecular targets that should provide opportunities for drug improvement when the associated physiology is understood.
Macro-level Photoaffinity Labeling
Perspectives
It is anticipated that many protein targets involved in anesthesia are low abundance integral membrane proteins such as ion channels and receptors. Both characteristics result in complications for macro-level detection using classical PAL techniques 50,54. However, over the past decade advancements in the photoaffinity labeling field have shown successful coupling of photoaffinity labeling and bioorthogonal reactions; for an excellent discussion of these developments we direct the reader to a 2012 review 57. Tandem anesthetic photoaffinity- click chemistry conjugation involves the additional incorporation of a biologically inert alkyne into the anesthetic PAL structure 2. The additional chemical group allows for affinity-based protein profiling of the anesthetic. This technology has numerous powerful applications from micro- to macro-level protein profiling and imaging investigations within complex systems.
General anesthetics are often considered to have low affinity for their targets relative to most other drugs. As such, the simplified view of “one drug, one target” for these drugs is exceedingly improbable. While the diversity of targets opens up new avenues for further development, it also presents significant challenges with respect to characterization and validation. Challenges associated with these macro-level investigations are similar to, if not greater than, micro-level investigations: sufficient PAL development and validation of identified protein targets. As the science moves towards systems biology and personalized medicine, the precise target-interaction profiles for each general anesthetic become increasingly necessary.
Conclusions
An ultimate goal of anesthesiology research is to understand the biochemistry that leads to pharmacological phenotypes displayed by anesthetics. The objective of this review was to present an overview of advancements and considerations in the use of anesthetic photoaffinity labeling as an experimental tool. Thus far anesthetic PALs have helped refine hypotheses and present new directions with respect to molecular targets. This knowledge should provide for educated improvements in drug design and allow for selective administration of an anesthetic to distinct populations (e.g., “personalized” anesthesia). The mechanisms of anesthesia have remained elusive for nearly two centuries and increasing evidence has suggested a highly complex process. In combination with other advancing techniques, we expect the use of anesthetic photoaffinity PALs to continue to be a significant tool in shedding light on this puzzle in medicine.
Acknowledgments
Funding: National Institutes of Health (GM055876, GM107117 ); National Science Foundation Graduate Research Fellowship (DGE-1321851).
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
Disclosures
Name: Kellie A. Woll
Contribution: Writing of the manuscript; generation of figures.
Attestation: Kellie A. Woll approved the final manuscript.
Name: William P. Dailey, PhD
Contribution: Writing of the manuscript.
Attestation: William P. Dailey approved the final manuscript.
Name: Grace Brannigan, PhD
Contribution: Writing of the manuscript, data collection and analysis.
Attestation: Grace Brannigan approved the final manuscript.
Name: Roderic G. Eckenhoff, MD
Contribution: Writing of the manuscript.
Attestation: Roderic G. Eckenhoff approved the final manuscript.
This manuscript was handled by: Gregory Crosby, MD
Contributor Information
Kellie A. Woll, Department of Anesthesiology & Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania; Department of Pharmacology, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania
William P. Dailey, Department of Chemistry, University of Pennsylvania School of Arts and Sciences, Philadelphia, Pennsylvania.
Grace Brannigan, Department of Physics, Rutgers University, Camden, New Jersey.
Roderic G. Eckenhoff, Department of Anesthesiology & Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia Pennsylvania.
References
- 1.Eckenhoff RG, Shuman H. Halothane binding to soluble proteins determined by photoaffinity labeling. Anesthesiology. 1993;79:96–106. doi: 10.1097/00000542-199307000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Woll KA, Pinch BP, Dailey WP, Eckenhoff RG. ‘Clickable’-Photoactive Propofol Analogue for the Identification of Anesthetic Targets. Biophys J. 2014;106:478A. [Google Scholar]
- 3.Weiser BP, Woll KA, Dailey WP, Eckenhoff RG. Mechanisms revealed through general anesthetic photolabeling. Curr Anesth Rep. 2014;4:57–66. doi: 10.1007/s40140-013-0040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubinsky L, Krom BP, Meijler MM. Diazirine based photoaffinity labeling. Bioorg Med Chem. 2012;20:554–70. doi: 10.1016/j.bmc.2011.06.066. Available at: <Go to ISI>://WOS:000299496100002. [DOI] [PubMed] [Google Scholar]
- 5.Martinu T, Dailey WP. Synthesis of carboalkoxychloro- and bromodiazirines. J Org Chem. 2004;69:7359–62. doi: 10.1021/jo040194r. [DOI] [PubMed] [Google Scholar]
- 6.Brunner J. New photolabeling and crosslinking methods. Annu Rev Biochem. 1993;62:483–514. doi: 10.1146/annurev.bi.62.070193.002411. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Burdzinski G, Kubicki J, Platz MS. Direct observation of carbene and diazo formation from aryldiazirines by ultrafast infrared spectroscopy. J Am Chem Soc. 2008;130:16134–5. doi: 10.1021/ja805922b. [DOI] [PubMed] [Google Scholar]
- 8.Das J. Photoincorporation of azialcohol to the C1B domain of PKCdelta is buffer dependent. J Photochem Photobiol B. 2009;95:185–8. doi: 10.1016/j.jphotobiol.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Seydel U, Gerace L. A 28,000-Da GDP/GTP-binding protein specific to the nuclear envelope. J Biol Chem. 1991;266:7602–8. [PubMed] [Google Scholar]
- 10.Bayley H. Photogenerated Reagents in Biochemistry and Molecular Biology. Elsevier; 1983. [Accessed October 16, 2015]. Available at: http://www.sciencedirect.com/science/article/pii/S0075753508704364. [Google Scholar]
- 11.Chen Z-W, Chen L-H, Akentieva N, Lichti CF, Darbandi R, Hastings R, Covey DF, Reichert DE, Townsend RR, Evers AS. A neurosteroid analogue photolabeling reagent labels the colchicine-binding site on tubulin: a mass spectrometric analysis. Electrophoresis. 2012;33:666–74. doi: 10.1002/elps.201100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Kubicki J, Peng H, Platz MS. Influence of solvent on carbene intersystem crossing rates. J Am Chem Soc. 2008;130:6604–9. doi: 10.1021/ja711385t. [DOI] [PubMed] [Google Scholar]
- 13.Weiser BP, Hall MA, Weinbren NL, Woll KA, Dailey WP, Eckenhoff MF, Eckenhoff RG. Macroscopic and Macromolecular Specificity of Alkylphenol Anesthetics for Neuronal Substrates. Sci Rep. 2015;5 doi: 10.1038/srep09695. Available at: http://dx.doi.org/10.1038/srep09695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigrist H, Muhlemann M, Dolder M. Philicity of amino-acid side-chains for photogenerated carbenes. J Photochem Photobiol B-Biology. 1990;7:277–87. [Google Scholar]
- 15.Eckenhoff RG, Xi J, Shimaoka M, Bhattacharji A, Covarrubias M, Dailey WP. Azi-isoflurane, a Photolabel Analog of the Commonly Used Inhaled General Anesthetic Isoflurane. Acs Chem Neurosci. 2010;1:139–45. doi: 10.1021/cn900014m. Available at: <Go to ISI>://WOS:000277980700007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das J. Aliphatic Diazirines as Photoaffinity Probes for Proteins: Recent Developments. Chem Rev. 2011;111:4405–17. doi: 10.1021/cr1002722. Available at: <Go to ISI>://WOS:000294699500001. [DOI] [PubMed] [Google Scholar]
- 17.Ziebell MR, Nirthanan S, Husain SS, Miller KW, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for H-3 azietomidate, a photoactivatable general anesthetic. J Biol Chem. 2004;279:17640–9. doi: 10.1074/jbc.M313886200. Available at: <Go to ISI>://WOS:000220870400093. [DOI] [PubMed] [Google Scholar]
- 18.Pratt MB, Husain SS, Miller KW, Cohen JB. Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem. 2000;275:29441–51. doi: 10.1074/jbc.M004710200. [DOI] [PubMed] [Google Scholar]
- 19.Hamouda AK, Stewart DS, Husain SS, Cohen JB. Multiple transmembrane binding sites for p-trifluoromethyldiazirinyl-etomidate, a photoreactive Torpedo nicotinic acetylcholine receptor allosteric inhibitor. J Biol Chem. 2011;286:20466–77. doi: 10.1074/jbc.M111.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Husain SS, Nirthanan S, Ruesch D, Solt K, Cheng Q, Li GD, Arevalo E, Olsen RW, Raines DE, Forman SA, Cohen JB, Miller KW. Synthesis of trifluoromethylaryl diazirine and benzophenone derivatives of etomidate that are potent general anesthetics and effective photolabels for probing sites on ligand-gated ion channels. J Med Chem. 2006;49:4818–25. doi: 10.1021/jm051207b. [DOI] [PubMed] [Google Scholar]
- 21.Moya-Barrios R, Cozens FL, Schepp NP. Absolute reactivity of halo(pyridyl)carbenes. J Org Chem. 2009;74:1148–55. doi: 10.1021/jo802132z. [DOI] [PubMed] [Google Scholar]
- 22.Husain SS, Stewart D, Desai R, Hamouda AK, Li SG, Kelly E, Dostalova Z, Zhou X, Cotten JF, Raines DE, Olsen RW, Cohen JB, Forman SA, Miller KW. p-Trifluoromethyldiazirinyl-etomidate: a potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels. J Med Chem. 2010;53:6432–44. doi: 10.1021/jm100498u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human alpha1beta3 gamma-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–47. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vedula LS, Brannigan G, Economou NJ, Xi J, Hall MA, Liu R, Rossi MJ, Dailey WP, Grasty KC, Klein ML, Eckenhoff RG, Loll PJ. A Unitary Anesthetic Binding Site at High Resolution. J Biol Chem. 2009;284:24176–84. doi: 10.1074/jbc.M109.017814. Available at: <Go to ISI>://WOS:000269380200031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R, Loll PJ, Eckenhoff RG. Structural basis for high-affinity volatile anesthetic binding in a natural 4-helix bundle protein. Faseb J. 2005;19:567–76. doi: 10.1096/fj.04-3171com. [DOI] [PubMed] [Google Scholar]
- 26.Eckenhoff RG, Petersen CE, Ha CE, Bhagavan NV. Inhaled anesthetic binding sites in human serum albumin. J Biol Chem. 2000;275:30439–44. doi: 10.1074/jbc.M005052200. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya AA, Curry S, Franks NP. Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J Biol Chem. 2000;275:38731–8. doi: 10.1074/jbc.M005460200. [DOI] [PubMed] [Google Scholar]
- 28.Hall MA, Xi J, Lor C, Dai S, Pearce R, Dailey WP, Eckenhoff RG. m-Azipropofol (AziPm) a photoactive analogue of the intravenous general anesthetic propofol. J Med Chem. 2010;53:5667–75. doi: 10.1021/jm1004072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip GM, Chen ZW, Edge CJ, Smith EH, Dickinson R, Hohenester E, Townsend RR, Fuchs K, Sieghart W, Evers AS, Franks NP. A propofol binding site on mammalian GABA receptors identified by photolabeling. Nat Chem Biol. 2013;22 doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, Olsen RW, Forman SA, Cohen JB, Miller KW. 2-(3-Methyl-3H-diaziren-3-yl)ethyl 1-(1-phenylethyl)-1H-imidazole-5-carboxylate: a derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–65. doi: 10.1021/jm020465v. [DOI] [PubMed] [Google Scholar]
- 31.Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, Eger EI., 2nd R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the gamma-aminobutyric acid receptor beta3 subunit. Anesth Analg. 2005;101:131–5. doi: 10.1213/01.ANE.0000153011.64764.6F. table of contents. [DOI] [PubMed] [Google Scholar]
- 32.Zhong HJ, Rusch D, Forman SA. Photo-activated azi-etomidate, a general anesthetic photolabel, irreversibly enhances gating and desensitization of gamma-aminobutyric acid type A receptors. Anesthesiology. 2008;108:103–12. doi: 10.1097/01.anes.0000296074.33999.52. Available at: <Go to ISI>://WOS:000251905400016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiser BP, Kelz MB, Eckenhoff RG. In vivo activation of azipropofol prolongs anesthesia and reveals synaptic targets. J Biol Chem. 2013;288:1279–85. doi: 10.1074/jbc.M112.413989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall AG, Hendrickson CL, Jackson GS. Fourier transform ion cyclotron resonance mass spectrometry: A primer. MASS Spectrom Rev. 1998;17:1–35. doi: 10.1002/(SICI)1098-2787(1998)17:1<1::AID-MAS1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 35.Han X, Aslanian A, Yates III., JR Mass spectrometry for proteomics. Curr Opin Chem Biol. 2008;12:483–90. doi: 10.1016/j.cbpa.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shanmugasundararaj S, Lehle S, Yamodo HI, Husain SS, Tseng C, Nguyen K, Addona GH, Miller KW. The location and nature of general anesthetic binding sites on the active conformation of firefly luciferase; a time resolved photolabeling study. PLoS One. 2012;7:e29854. doi: 10.1371/journal.pone.0029854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuki K, Bu W, Shimaoka M, Eckenhoff R. Volatile anesthetics, not intravenous anesthetic propofol bind to and attenuate the activation of platelet receptor integrin alphaIIbbeta3. PLoS One. 2013;8:e60415. doi: 10.1371/journal.pone.0060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagar S, Argikar UA, Tweedie DJ. Enzyme kinetics in drug metabolism: fundamentals and applications. Methods Mol Biol. 2014;1113:1–6. doi: 10.1007/978-1-62703-758-7_1. [DOI] [PubMed] [Google Scholar]
- 39.Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid Analog Photolabeling of a Site in the Third Transmembrane Domain of the beta 3 Subunit of the GABA(A) Receptor. Mol Pharmacol. 2012;82:408–19. doi: 10.1124/mol.112.078410. Available at: <Go to ISI>://WOS:000308070100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Wen Z, Washburn MP, Florens L. Refinements to label free proteome quantitation: how to deal with peptides shared by multiple proteins. Anal Chem. 2010;82:2272–81. doi: 10.1021/ac9023999. [DOI] [PubMed] [Google Scholar]
- 41.Chahrour O, Cobice D, Malone J. Stable isotope labelling methods in mass spectrometry-based quantitative proteomics. J Pharm Biomed Anal. 2015;113:2–20. doi: 10.1016/j.jpba.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Adams RM, Chourey K, Hurst GB, Hettich RL, Pan C. Systematic comparison of label-free, metabolic labeling, and isobaric chemical labeling for quantitative proteomics on LTQ Orbitrap Velos. J Proteome Res. 2012;11:1582–90. doi: 10.1021/pr200748h. [DOI] [PubMed] [Google Scholar]
- 43.Chiara DC, Dangott LJ, Eckenhoff RG, Cohen JB. Identification of nicotinic acetylcholine receptor amino acids photolabeled by the volatile anesthetic halothane. Biochemistry. 2003;42:13457–67. doi: 10.1021/bi0351561. [DOI] [PubMed] [Google Scholar]
- 44.Yuki K, Bu W, Xi J, Sen M, Shimaoka M, Eckenhoff RG. Isoflurane binds and stabilizes a closed conformation of the leukocyte function-associated antigen-1. FASEB J Off Publ Fed Am Soc Exp Biol. 2012;26:4408–17. doi: 10.1096/fj.12-212746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mobert J, Zahler S, Becker BF, Conzen PF. Inhibition of neutrophil activation by volatile anesthetics decreases adhesion to cultured human endothelial cells. Anesthesiology. 1999;90:1372–81. doi: 10.1097/00000542-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 46.Reutershan J, Chang D, Hayes JK, Ley K. Protective effects of isoflurane pretreatment in endotoxin-induced lung injury. Anesthesiology. 2006;104:511–7. doi: 10.1097/00000542-200603000-00019. [DOI] [PubMed] [Google Scholar]
- 47.Yuki K, Astrof NS, Bracken C, Yoo R, Silkworth W, Soriano SG, Shimaoka M. The volatile anesthetic isoflurane perturbs conformational activation of integrin LFA-1 by binding to the allosteric regulatory cavity. FASEB J Off Publ Fed Am Soc Exp Biol. 2008;22:4109–16. doi: 10.1096/fj.08-113324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savechenkov PY, Zhang X, Chiara DC, Stewart DS, Ge RL, Zhou XJ, Raines DE, Cohen JB, Forman SA, Miller KW, Bruzik KS. Allyl m-Trifluoromethyldiazirine Mephobarbital: An Unusually Potent Enantioselective and Photoreactive Barbiturate General Anesthetic. J Med Chem. 2012;55:6554–65. doi: 10.1021/jm300631e. Available at: <Go to ISI>://WOS:000306764600027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of Intersubunit General Anesthetic-binding Sites in the Transmembrane Domain of the Human alpha1beta3gamma2 gamma-Aminobutyric Acid Type A (GABAA) Receptor. J Biol Chem. 2013;288:19343–57. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darbandi-Tonkabon R, Hastings WR, Zeng CM, Akk G, Manion BD, Bracamontes JR, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. Photoaffinity Labeling with a neuroactive steroid analogue - 6-AZI-Pregnanolone labels voltage-dependent anion channel-1 in rat brain. J Biol Chem. 2003;278:13196–206. doi: 10.1074/jbc.M213168200. Available at: <Go to ISI>://WOS:000182189500078. [DOI] [PubMed] [Google Scholar]
- 51.Emerson DJ, Weiser BP, Psonis J, Liao Z, Taratula O, Fiamengo A, Wang X, Sugasawa K, Smith AB, 3rd, Eckenhoff RG, Dmochowski IJ. Direct modulation of microtubule stability contributes to anthracene general anesthesia. J Am Chem Soc. 2013;135:5389–98. doi: 10.1021/ja311171u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darbandi-Tonkabon R, Manion BD, Hastings WR, Craigen WJ, Akk G, Bracamontes JR, He Y, Sheiko TV, Steinbach JH, Mennerick SJ, Covey DF, Evers AS. Neuroactive steroid interactions with voltage-dependent anion channels: lack of relationship to GABA(A) receptor modulation and anesthesia. J Pharmacol Exp Ther. 2004;308:502–11. doi: 10.1124/jpet.103.058123. [DOI] [PubMed] [Google Scholar]
- 53.Weiser BP, Bu W, Wong D, Eckenhoff RG. Sites and functional consequence of VDAC-alkylphenol anesthetic interactions. [Accessed May 6, 2015];FEBS Lett. 2014 588:4398–403. doi: 10.1016/j.febslet.2014.10.009. Available at: http://www.sciencedirect.com/science/article/pii/S0014579314007443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiser BP, Kelz MB, Eckenhoff RG. In Vivo Activation of Azi-propofol Prolongs Anesthesia and Reveals Synaptic Targets. J Biol Chem. 2012;26:26. doi: 10.1074/jbc.M112.413989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herring BE, Xie Z, Marks J, Fox AP. Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol. 2009;102:1265–73. doi: 10.1152/jn.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herring BE. Etomidate and propofol inhibit the neurotransmitter release machinery at different sites (vol 589, pg 1103, 2011) J Physiol. 2011;589:4633. doi: 10.1113/jphysiol.2010.200964. Available at: <Go to ISI>://WOS:000295050800020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lapinsky DJ. Tandem photoaffinity labeling-bioorthogonal conjugation in medicinal chemistry. [Accessed October 16, 2015];Bioorg Med Chem. 2012 20:6237–47. doi: 10.1016/j.bmc.2012.09.010. Available at: http://www.sciencedirect.com/science/article/pii/S0968089612007080. [DOI] [PubMed] [Google Scholar]
- 58.Johansson JS, Scharf D, Davies LA, Reddy KS, Eckenhoff RG. A designed four-alpha-helix bundle that binds the volatile general anesthetic halothane with high affinity. Biophys J. 2000;78:982–93. doi: 10.1016/S0006-3495(00)76656-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Addona GH, Husain SS, Stehle T, Miller KW. Geometric isomers of a photoactivable general anesthetic delineate a binding site on adenylate kinase. J Biol Chem. 2002;277:25685–91. doi: 10.1074/jbc.M201303200. [DOI] [PubMed] [Google Scholar]
- 60.Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, Dou X, Chen S, Charness ME, Miller KW. An alcohol binding site on the neural cell adhesion molecule L1. Proc Natl Acad Sci U S A. 2008;105:371–5. doi: 10.1073/pnas.0707815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Das J, Addona GH, Sandberg WS, Husain SS, Stehle T, Miller KW. Identification of a general anesthetic binding site in the diacylglycerol-binding domain of protein kinase Cdelta. J Biol Chem. 2004;279:37964–72. doi: 10.1074/jbc.M405137200. [DOI] [PubMed] [Google Scholar]
- 62.Das J, Pany S, Rahman GM, Slater SJ. PKC epsilon has an alcohol-binding site in its second cysteine-rich regulatory domain. Biochem J. 2009;421:405–13. doi: 10.1042/BJ20082271. [DOI] [PubMed] [Google Scholar]
- 63.Pany S, Das J. Alcohol binding in the C1 (C1A+C1B) domain of protein kinase C epsilon. Biochim Biophys Acta. 2015;1850:2368–76. doi: 10.1016/j.bbagen.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chiara DC, Hong FH, Arevalo E, Husain SS, Miller KW, Forman SA, Cohen JB. Time-resolved photolabeling of the nicotinic acetylcholine receptor by [3H]azietomidate, an open-state inhibitor. Mol Pharmacol. 2009;75:1084–95. doi: 10.1124/mol.108.054353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li G-D, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a GABAA receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li GD, Chiara DC, Cohen JB, Olsen RW. Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type A receptors. J Biol Chem. 2009;284:11771–5. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li GD, Chiara DC, Cohen JB, Olsen RW. Numerous classes of general anesthetics inhibit etomidate binding to gamma-aminobutyric acid type A (GABAA) receptors. J Biol Chem. 2010;285:8615–20. doi: 10.1074/jbc.M109.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nirthanan S, Garcia G, 3rd, Chiara DC, Husain SS, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for TDBzl-etomidate, a photoreactive positive allosteric effector. J Biol Chem. 2008;283:22051–62. doi: 10.1074/jbc.M801332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiara DC, Dostalova Z, Jayakar SS, Zhou XJ, Miller KW, Cohen JB. Mapping General Anesthetic Binding Site(s) in Human alpha 1 beta 3 gamma-Aminobutyric Acid Type A Receptors with H-3 TDBzl-Etomidate, a Photoreactive Etomidate. Biochemistry. 2012;51:836–47. doi: 10.1021/bi201772m. Available at: <Go to ISI>://WOS:000300473500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamouda AK, Stewart DS, Chiara DC, Savechenkov PY, Bruzik KS, Cohen JB. Identifying barbiturate binding sites in a nicotinic acetylcholine receptor with [3H]allyl m-trifluoromethyldiazirine mephobarbital, a photoreactive barbiturate. Mol Pharmacol. 2014;85:735–46. doi: 10.1124/mol.113.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jayakar SS, Dailey WP, Eckenhoff RG, Cohen JB. Identification of Propofol Binding Sites in a Nicotinic Acetylcholine Receptor with a Photoreactive Propofol Analog. J Biol Chem. 2013;288:6178–89. doi: 10.1074/jbc.M112.435909. Available at: <Go to ISI>://WOS:000315820700014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jayakar SS, Zhou X, Chiara DC, Dostalova Z, Savechenkov PY, Bruzik KS, Dailey WP, Miller KW, Eckenhoff RG, Cohen JB. Multiple Propofol Binding Sites in a gamma-Aminobutyric Acid Type A Receptor (GABAAR) Identified Using a Photoreactive Propofol Analog. J Biol Chem. 2014;1:581728. doi: 10.1074/jbc.M114.581728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiara DC, Gill JF, Chen Q, Tillman T, Dailey WP, Eckenhoff RG, Xu Y, Tang P, Cohen JB. Photoaffinity labeling the propofol binding site in GLIC. Biochemistry. 2014;53:135–42. doi: 10.1021/bi401492k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuki K, Bu W, Xi J, Shimaoka M, Eckenhoff R. Propofol Shares the Binding Site with Isoflurane and Sevoflurane on Leukocyte Function-Associated Antigen-1. Anesth Analg. 2013;117 doi: 10.1213/ANE.0b013e3182a00ae0. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3844542/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weiser BP, Eckenhoff RG. Propofol inhibits SIRT2 deacetylase through a conformation-specific, allosteric site. J Biol Chem. 2015;290:8559–68. doi: 10.1074/jbc.M114.620732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stewart DS, Savechenkov PY, Dostalova Z, Chiara DC, Ge R, Raines DE, Cohen JB, Forman SA, Bruzik KS, Miller KW. p-(4-Azipentyl)propofol: A Potent Photoreactive General Anesthetic Derivative of Propofol. J Med Chem. 2011;54:8124–35. doi: 10.1021/jm200943f. Available at: <Go to ISI>://WOS:000297445400014. [DOI] [PMC free article] [PubMed] [Google Scholar]