Abstract
OBJECTIVES
To measure short-term changes in physical and cognitive function and emotional well-being of older adults receiving intensive chemotherapy for acute myeloid leukemia (AML).
DESIGN
Prospective observational study.
SETTING
Single academic institution.
PARTICIPANTS
Individuals aged 60 and older with newly diagnosed AML who received induction chemotherapy (N = 49, mean age 70 ± 6.2, 56% male).
MEASUREMENTS
Geriatric assessment (GA) was performed during inpatient examination for AML and within 8 weeks after hospital discharge after induction chemotherapy. Measures were the Pepper Assessment Tool for Disability (activity of daily living, instrumental activity of daily living (IADL), mobility questions), Short Physical Performance Battery (SPPB), grip strength, Modified Mini-Mental State examination, Center for Epidemiologic Studies Depression Scale, and the Distress Thermometer. Changes in GA measures were assessed using paired t-tests. Analysis of variance models were used to evaluate relationships between GA variables and change in function over time.
RESULTS
After chemotherapy, IADL dependence worsened (mean 1.4 baseline vs 2.1 follow-up, P < .001), as did mean SPPB scores (7.5 vs 5.9, P = .02 for total). Grip strength also declined (38.9 ± 7.7 vs 34.2 ± 10.3 kg, P < .001 for men; 24.5 ± 4.8 vs 21.8 ± 4.7 kg, P = .007 for women). No significant changes in cognitive function (mean 84.7 vs 85.1, P = .72) or depressive symptoms (14.0 vs. 11.3, P = .11) were detected, but symptoms of distress declined (5.0 vs 3.2, P < .001). Participants with depressive symptoms at baseline and follow-up had greater declines in SPPB scores those without at both time points.
CONCLUSIONS
Short-term survivors of intensive chemotherapy for AML had clinically meaningful declines in physical function. These data support the importance of interventions to maintain physical function during and after chemotherapy. Depressive symptoms before and during chemotherapy may be linked to potentially modifiable physical function declines.
Keywords: leukemia, elderly, function, depression, cognition
Acute myelogenous leukemia (AML) largely affects older adults and requires intensive chemotherapy for cure.1 The optimal therapy for older adults with AML is unclear because age-related outcomes are consistently worse than in younger adults.2–4 Poor outcomes for older adults are due to alterations in tumor biology (resistance to standard therapies) and individual characteristics (lower treatment tolerance).5–7 While selected older adults benefit from standard intensive therapies, they are at risk of greater treatment-associated toxicity than younger adults, which can negatively affect functional independence and quality of life.8 Despite the challenges associated with intensive therapy, for many older adults, it may provide the best opportunity for prolonged disease-free survival. Rather than developing treatment strategies based on chronological age alone, new strategies are needed to individualize assessment and direct supportive care interventions to improve treatment tolerance and benefit.9,10
Use of geriatric assessment (GA) in the context of AML therapy may be an important strategy that can help individualize care for older adults. Studies in heterogeneous cancer populations have shown its utility as a predictive tool for chemotherapy toxicity.11,12 GA also demonstrates the effect of multiple concurrent vulnerabilities on outcomes. This information is critical to inform person-centered decision-making and to develop interventions to improve treatment tolerance and outcomes. In older adults with AML deemed fit to receive intensive induction chemotherapy, it was reported that impaired physical performance and impaired cognition were independently associated with shorter survival.6 Retrospective studies also support the utility of GA to predict survival of older adults with AML.13
Few studies have used GA as an outcome assessment to better understand the effect of treatment on physical and cognitive function, depressive symptoms, and distress. Studies specifically evaluating physical function and quality of life of older AML survivors suggest that the greatest decrements in function may occur shortly after induction therapy, with somewhat lesser functional recovery in older than younger patients.14–17 A major concern when considering post-remission treatments for older adults is the greater likelihood that they will not tolerate effective therapies after induction because of declines in functional status or acquired comorbidities. In trials, up to 20% of older adults who achieve remission for AML receive no post-remission therapy, as is standard in younger adults.18 Inability to administer adequate post-remission therapy may contribute to worse age-related outcomes in individuals with AML. Measuring the effect of treatment on multiple domains of function may provide needed information to design better supportive care interventions that will optimize treatment tolerance. This approach could improve physical function and quality of life and enhance opportunities for older adults to receive optimal anticancer therapy.
The current analysis presents follow-up data from a previously reported prospective cohort of older adults with AML.6,19 It was hypothesized that receipt of intensive chemotherapy would result in clinically significant declines in physical function in survivors. The objectives of this report are to describe short-term changes in physical function, cognitive function, depressive symptoms, and distress using GA in older inpatients receiving chemotherapy for AML and to identify risk factors for decline in function after induction therapy.
METHODS
Study Population
This was a prospective single-institution study of 74 older adults with AML to evaluate the predictive utility of GA for survival and to assess the effect of chemotherapy on GA measures after treatment. Eligibility criteria included age 60 and older, newly diagnosed AML, and being hospitalized for intensive standard-dose induction chemotherapy. Details of the study cohort have been previously published.6
Procedures
Geriatric assessment was performed during inpatient examination for AML (pre-induction chemotherapy) and postinduction chemotherapy during outpatient evaluation for post-remission therapies (typically within 8 weeks of discharge from induction hospitalization). These time points were chosen as clinically relevant assessment periods for treatment decisions. GA measures have been described in detail previously.6,19 Briefly, they included the Pepper Assessment Tool for Disability (PAT-D; includes self-reported activities of daily living (ADLs), instrumental activities of daily living (IADLs), and mobility questions; range 1–5; higher scores indicate worse function),19–21 Short Physical Performance Battery (SPPB;22–24 includes timed 4-m walk, chair stands, standing balance; range 0–12; higher scores indicate better function), grip strength,25 Modified Mini-Mental State (3MS) examination,26,27 Center for Epidemiologic Studies Depression Scale (CESD), 28,29 Distress Thermometer,30,31 and Hematopoietic Stem Cell Comorbidity Index (HCT-CI).32 In this observational study, participants received usual supportive care, which includes physical therapy when ordered by the treating physician on an as-needed basis. Psychosocial counseling was also available on an as-needed basis in response to physician referral for symptoms.
Analysis Cohort
The analysis cohort comprised participants who survived induction therapy and had a GA performed in the clinic within 8 weeks of discharge from induction hospitalization during evaluation for post-remission therapy.
Outcomes
Outcomes were ADL, IADL, SPPB (total and component), grip strength, 3MS, CES-D, and Distress Thermometer change scores. The percentage of subjects determined to be impaired using standard cutoffs for these measures6 were also compared at baseline and follow-up.
Covariates
Covariates in this analysis included demographic characteristics (age, sex, race, education) and clinical data available from the baseline visit. Clinical data included laboratory data, such as hemoglobin, lactate dehydrogenase (LDH), and white blood cell (WBC) count; participant characteristics, such as comorbidity (HCT-CI score), Eastern Cooperative Oncology Group (ECOG) performance score, and body mass index (BMI); tumor characteristics, such as prior myelodysplastic syndrome (MDS) and cytogenetic risk group; and treatment type. Complete remission was defined as a morphological leukemia-free state, including less than 5% blasts in the bone marrow, no blasts with Auer rods, no persistent extramedullary disease, and inclusive of individuals with incomplete platelet count recovery (<100,000) who were transfusion independent. 33 Information on discharge to a skilled nursing facility was captured in medical record review.
Statistics
Descriptive statistics (means and frequencies) were used to characterize participants at baseline and follow-up. Baseline participant characteristics of those who complete the follow-up assessment were compared with the characteristics of those who did not using t-tests (age, hemoglobin, LDH, WBC count, BMI, ECOG score, 3MS score, CES-D, distress, gait speed, PAT-D, PAT-D ADLs, PAT-D IADLs, PAT-D Mobility, SPPB, grip strength) and chi-square or Fisher exact tests (sex, race, education, risk group, MDS, individual comorbidities, chemotherapy type). Significance of changes in GA measures was assessed using paired t-tests. To explore the relationship between changes in GA measures and participant characteristics, change in the measure was first regressed on the baseline value plus potential predictors of interest and covariates (age, sex, risk group, MDS, hemoglobin, WBC count, chemotherapy type, CES-D, 3MS, distress, ECOG), considering each variable one at a time. Any variables with P-values of .25 or less were then entered into multivariable models (see Results). To explore the relationship between change in GA measures and achieving complete remission after induction, t-tests were used to compare change in each measure according to remission status.
To explore the effect of change in depression score on change in SPPB, participants were divided into four groups based on their level of depressive symptoms at baseline and follow-up. Next, two-way mixed-effects analysis of variance was used to model the effect of time (baseline or follow-up) and these groups on SPPB measured at two time points. Contrasts were used to estimate the change within a group and to test whether the change differed according to group. The same approach was applied to the effect of change in cognition on change in SPPB. All analyses were performed in SAS version 9.3 (SAS Institute, Inc., Cary, NC). A two-sided alpha level of .05 was used to indicate statistical significance.
RESULTS
Of the 74 older adults who received intensive induction chemotherapy and had a baseline GA, 54 survived induction and were evaluated for postremission therapy, whereas 16 died during induction, and four were discharged to hospice. Of the 54 evaluable for follow-up, 49 completed the posttreatment GA (5 were not assessed because of scheduling conflicts). Baseline characteristics of the follow-up cohort (n = 49) are presented in Table 1. At baseline, participants had a mean age of 70.0 ± 6.2, with a slight male predominance. Most had a good oncology (ECOG ≤ 1) performance status as rated by the treating physician, with a low prevalence of major comorbid conditions. Most had intermediate- or poor-risk cytogenetics. The follow-up cohort (n = 49) differed at baseline from those who were not assessed at follow-up (n = 25) by having a lower BMI (mean BMI 28.1 vs 31.1 kg/m2, P = .04) and better cognitive function, physical performance, and ECOG scores.
Table 1.
Characteristics of Older Adults Undergoing Chemotherapy for Acute Myelogenous Leukemia (N = 49)
| Characteristic | Value |
|---|---|
| Demographic, n (%) | |
| Age at initiation of therapy, median (IQR) | 68.6 (9.2) |
| Age at initiation of therapy, n (%) | |
| 60–69 | 28 (57.1) |
| 70–79 | 17 (34.7) |
| ≥80 | 4 (8.2) |
| Male, n (%) | 28 (57.1) |
| White, n (%) | 47 (95.9) |
| Education level, n (%)a | |
| <High school | 8 (17.0) |
| High school | 12 (25.5) |
| ≥College | 27 (57.5) |
| Clinical | |
| Hemoglobin, g/dL, median (IQR) | 9.4 (2.2) |
| Lactate dehydrogenase, U/L, median (IQR) | 226.0 (187.0) |
| White cell count/μL, median (IQR) | 4.0 (19.6) |
| Body mass index, kg/m2, median (IQR) | 27.3 (5.8) |
| ECOG score ≤1, n (%)b | 40 (83.3) |
| Prior myelodysplastic syndrome, n (%) | 12 (24.5) |
| Cytogenetic risk group, n (%)c | |
| Favorable | 2 (4.2) |
| Intermediate | 32 (66.7) |
| Poor | 14 (29.2) |
| Coronary artery disease, n (%) | 9 (18.4) |
| Chronic obstructive pulmonary disease, n (%) | 5 (10.2) |
| Diabetes mellitus, n (%) | 10 (20.4) |
| Congestive heart failure, n (%) | 1 (2.0) |
| Comorbidity score (Hematopoietic Cell Transplantation Comorbidity Index), median (IQR) | 1.0 (2.0) |
| Chemotherapy, n (%)d | |
| Anthracycline + cytarabine + etoposide | 8 (16.3) |
| Anthracycline + cytarabine | 29 (59.2) |
| Other | 12 (24.5) |
| Achieved remission, n (%) | 43 (87.8) |
| Discharged to skilled nursing facility (vs home), n (%) | 4 (8.2) |
Education level was unavailable for two subjects.
Eastern Cooperative Oncology Group (ECOG) score was unavailable for one subject.
Cytogenetic test results were unavailable for one subject. Favorable risk group inv(16), t(8;21), t(15;17). Intermediate risk group includes normal, +8, +6, −Y, del (12p). Poor risk group includes −5/5q−,−7/7q−,abn (11q23) and complex aberrant karyotype, ≥3 abnormalities.
Chemotherapy doses administered were within standard ranges for anthracycline dosing (daunorubicin 45–60 mg/m2, idarubicin 12–20 mg/m2) and cytarabine dosing (100–200 mg/m2), per protocol.
IQR = interquartile range.
Participants in the follow-up cohort reported more limitations in physical function after induction chemotherapy than at baseline (Table 2). In addition, clinically significant declines in physical performance were detected at follow-up. For SPPB component scores, significant declines were detected in gait speed and balance. Grip strength also declined significantly in men and women after induction. Higher ECOG performance scores at follow-up indicated physicians’ perception of functional decline. There were no significant changes in cognitive function or depressive symptoms from baseline to follow-up. Symptoms of distress decreased after induction.
Table 2.
Baseline and Follow-Up Geriatric Assessment Scores of Older Adults Undergoing Induction Chemotherapy for Acute Myelogenous Leukemia (N = 49)
| Assessment | Baseline | Follow-Up | Change | P-Value |
|---|---|---|---|---|
| Cognition: 3MS (range 0–100, impairment <80) | 84.7 ± 8.8 | 85.1 ± 9.4 | 0.4 ± 7.4 | .72 |
| Psychological function | ||||
| CES-D (range 0–60, impairment >16) | 13.9 ± 11.6 | 11.4 ± 10.7 | −2.5 ± 11.7 | .15 |
| Distress thermometer (range 0–10, impairment ≥4) | 5.0 ± 3.1 | 3.1 ± 3.1 | −1.9 ± 3.5 | <.001 |
| Physical function | ||||
| PAT-D (range 1–5, impairment>1) at the time of treatment | ||||
| Activity of daily living subscale | 1.3 ± 0.6 | 1.4 ± 0.5 | 0.2 ± 0.5 | .02 |
| Instrumental activity of daily living subscale | 1.4 ± 0.7 | 2.1 ± 1.0 | 0.8 ± 1.1 | <.001 |
| Mobility subscale | 2.1 ± 1.2 | 2.8 ± 1.4 | 0.7 ± 1.1 | <.001 |
| SPPB (range 0–12, impairment ≤9) | 7.6 ± 4.0 | 5.8 ± 4.3 | −1.8 ± 5.0 | .02 |
| Gait speed, m/s | 0.8 ± 0.2 | 0.9 ± 0.3 | 0.1 ± 0.2 | .19 |
| Balance score | 3.1 ± 1.5 | 2.5 ± 1.7 | −0.6 ± 1.9 | .03 |
| Gait speed score | 2.7 ± 1.6 | 1.9 ± 1.8 | −0.8 ± 2.1 | .01 |
| Chair stand score | 1.8 ± 1.4 | 1.4 ± 1.5 | −0.4 ± 1.8 | .12 |
| Grip strength, kg | ||||
| Male | 40.1 ± 7.2 | 34.8 ± 10.2 | −5.3 ± 4.6 | <.001 |
| Female | 24.6 ± 5.0 | 21.7 ± 4.8 | −2.9 ± 4.0 | .007 |
| ECOG | 1.1 ± 0.5 | 1.4 ± 0.65 | 0.3 ± 0.8 | .006 |
Sample size variability for specific reported outcomes based on available data is as follows: Modified Mini-Mental State Exam (3MS) (n = 46), Center for Epidemiologic Studies Depression Scale (CES-D) (n = 48), distress thermometer (n = 48), Pepper Assessment Tool for Disability (PAT-D) (n = 43), walking speed calculation for Short Physical Performance Battery (SPPB) (n = 38), grip strength (n = 40), Eastern Cooperative Oncology Group (ECOG) (n = 47). For 3MS, SPPB, and grip strength, higher scores reflect better function. For CES-D, distress thermometer, and PAT-D, higher scores reflect worse function.
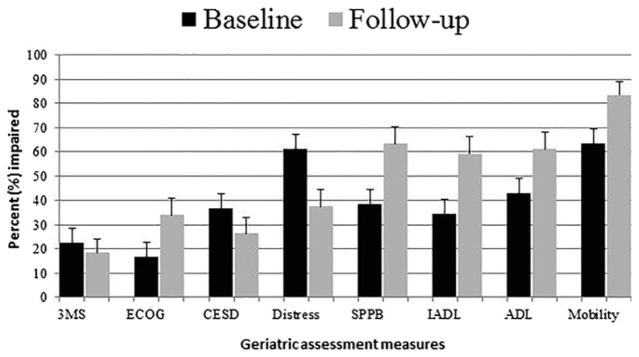
Figure 1 shows the percentage of participants who met criteria for impairment on each GA measure, highlighting the greater prevalence of impaired physical function at follow-up. For example, 38.8% of the cohort had impaired physical performance (SPPB score ≤ 9) at baseline, increasing to 63.3% at follow-up (P = .01).
Figure 1.
Percentage of participants with acute myelogenous leukemia with impairment in geriatric assessment measures at baseline and after induction therapy (N = 49). Impairment is defined as scores on each measure as follows: Modified Mini-Mental State (3MS) examination <77; Eastern Cooperative Oncology Group (ECOG) >1; Center for Epidemiologic Studies Depression Scale (CES-D) >16; Distress >4; Short Physical Performance Battery (SPPB) <9; Pepper Assessment Tool for Disability (PAT-D) instrumental activity of daily living (IADL) subscale >1; PAT-D activity of daily living (ADL) subscale >1; PAT-D Mobility subscale >1.
Exploratory analyses were conducted to identify baseline characteristics associated with change in physical function. The most-consistent characteristics associated with decline in physical function were related to unfavorable tumor biology and intensity of treatment. Specifically, decline in ADL score was significantly associated with unfavorable cytogenetic risk group (P = .05) and receipt of the most-intense chemotherapy regimen (cytarabine + daunorubicin + etoposide) (P = .03) after adjustment for baseline ADL score, BMI, ECOG score, and sex. Decline in IADL score was significantly associated with higher BMI (P = .03) and unfavorable cytogenetic risk score (P = .01) after adjusting for baseline IADL score, BMI, risk group, and chemotherapy type. Improvement in the SPPB balance subscale was positively associated with higher baseline 3MS score (P = .05) after adjustment for baseline balance score, ECOG score, CES-D score, and cytogenetic risk group. Decline in SPPB gait speed score was associated with receipt of the most-intense chemotherapy type (P = .02) after adjustment for baseline gait score, ECOG, 3MS, and CES-D score. There was no significant association between change in GA measures and remission status (data not shown).
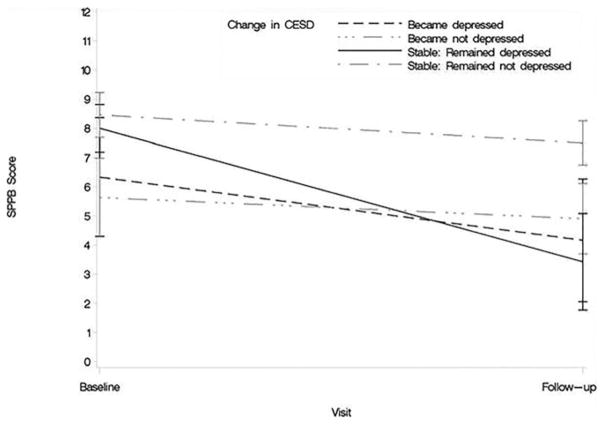
Additional exploratory analyses were conducted to investigate whether change in depression or cognitive impairment modified change in physical performance (SPPB) from baseline to follow-up. Figure 2 illustrates the relationship between clinically meaningful depressive symptoms and change in physical performance. Participants with depressive symptoms (CES-D ≥ 16) at baseline and follow-up had a significant decline in SPPB score (mean 8.0 (standard error 1.5) at baseline to 3.4 (1.5) at follow-up, P = .03), but those without depressive symptoms at either time point did not (8.5 (0.8) at baseline to 7.5 (0.8) at follow-up, P = .41). Likewise, participants who screened positive for cognitive impairment (3MS ≤ 77) at both time points had a significant decline in SPPB score, whereas those without cognitive impairment did not. Participants with cognitive impairment at baseline and at follow-up had a greater decline in SPPB score (8.0 (1.7) at baseline to 3.6 (1.7) at follow-up, P = .07) than those without cognitive impairment at either time point (8.4 (0.7) at baseline to 6.9 (0.7) at follow-up, P = .12).
Figure 2.
Relationship between depressive symptoms and change in physical performance (N = 49). Higher scores on the Short Physical Performance Battery (SPPB) indicate better physical performance. CES-D = Center for Epidemiologic Studies Depression.
DISCUSSION
In this study of older adults with AML, intensive chemotherapy was associated with clinically meaningful declines in self-reported and objectively measured physical function after induction chemotherapy. By contrast, receipt of induction chemotherapy did not worsen scores on screening measures of cognitive function, depression, or distress. Finally, exploratory analyses suggest that presence of persistent depressive symptoms may modify the risk of functional decline, thereby identifying a subset of individuals who may be at particularly high risk of physical deconditioning during therapy. These observations can help address informational needs of individuals with AML and their families related to the short-term consequences of treatment. They can also serve as a basis for design of supportive care interventions to mitigate the negative effect of induction chemotherapy on physical function in older adults.
Preventing or mitigating the negative consequences of functional decline is a pressing need in older adults being treated for AML and may be a critical component to improving outcomes. Potential consequences of short-term functional decline include poor quality of life, greater dependence, risk of secondary medical complications such as falls, greater healthcare use, and limitations in subsequent cancer treatment options to maintain a durable remission. These consequences, individually or together, could increase mortality risk for older adults. Greater mortality was reported in elderly adults with poor functional recovery after a cancer diagnosis and after a hospitalization. 34,35 Measuring the effect of treatment on function is a first step in determining optimal supportive care for older adults with AML. The current study addresses a gap in the literature, because cancer treatment clinical trials do not routinely measure functional outcomes.36
The findings of a negative short-term effect of chemotherapy on physical functioning are consistent with observational studies in older adults with cancer with various tumor types. A multi-institutional study investigated the effect of first-line chemotherapy; of 364 individuals with cancer aged 70 and older, 16.7% developed disability (dependence in ADLs) with the first cycle of treatment.37 A case–control analysis of more than 1,700 cancer cases showed that self-reported functional status declined during the first year after diagnosis, and older age was an independent predictor of decreased function.38 The current findings are also consistent with documented loss of functional independence in older adults who require hospitalization for medical illness.39
Standard induction therapy for AML differs from delivery of chemotherapy in other cancer settings in part because of the intensity of chemotherapy delivered over a period of weeks. Treatment results in prolonged myelosuppression and typically requires approximately 4–6 weeks of inpatient supportive care to treat infectious complications and provide on-going blood product support. Given this, it would be expected that older adults treated intensively for AML might be at even greater risk of functional decline than those treated for other cancers. Two well-designed observational studies specifically evaluated the trajectory of objectively measured physical performance over time after intensive chemotherapy in individuals with AML stratified according to age.14,17 The first study included 38 older adults with a short-term follow-up similar to that of the current study cohort,17 and the second study investigated functional trajectory over 1 year.14 Performance measures assessed were grip strength, repeat chair stands, and 2-minute walk test for endurance. In the study investigating short-term change in physical performance, consistent with the current results, older adults experienced decline in grip strength after induction. Repeated chair stand testing was stable, similar to the current SPPB chair stand component score, but 2-minute walk time improved, suggesting improved endurance in younger and older adults after induction chemotherapy.
Although the 2-minute walk was not measured in the current study, an average decline in gait speed score was observed. A possible explanation for some differences in results includes differences in performance tests used. For example, the SPPB includes assessment of balance, which declined significantly and contributed to decline in the total score. The scoring of the SPPB and its components also captures a score of 0 for those participants who cannot perform tests, rather than excluding them from analysis because of missing data. In addition, it is likely that there is a difference in participant selection. The cohort included individuals who may have been less fit at baseline, some of whom may not have been able to perform a 2-minute walk test at study entry. The exploratory data also show the effect of treatment type and tumor biology on function, and these factors may also have differed between the study populations. These data underscore the importance of collecting standardized functional assessment measures in clinical trials to facilitate cross-study comparisons.
The current study adds to the literature by providing an opportunity to explore measurable participant characteristics at baseline that may affect risk of functional decline. This is the first study of older adults with AML to include assessment of cognition and emotional health in the context of physical function assessment after treatment. An important finding in the exploratory analyses is the suggestion that depressed mood modifies the risk of functional decline. Although participants were (on average) less likely to report depressive symptoms after treatment, in those with persistent depressive symptoms, physical performance declined markedly after therapy. This is consistent with the findings in older adults without AML showing an independent association between baseline depressive symptoms and ADL decline after the first cycle of chemotherapy.37 These results are also intuitive clinically. Adults with persistent depressive symptoms may be less likely to engage in self-directed or supervised physical activity while hospitalized, increasing the risk of deconditioning. Results for individuals with persistent cognitive impairment showed a similar trend, although it did not achieve statistical significance. A relationship between cognitive function and physical function has been well described in other settings,40,41 again highlighting the importance of considering concurrent and interrelated vulnerabilities in the design of supportive care interventions.
These results could inform the design of supportive care interventions targeting maintenance of physical function. There is precedence in the setting of bone marrow transplantation (in which high-intensity therapy is also delivered in the inpatient setting) to support the utility of structured physical activity during receipt of chemotherapy to minimize declines in function.42 Several randomized studies have suggested that exercise during treatment may minimize functional decline and improve quality of life.42–44 The underlying premise is that prevention of functional decline may be more effective than attempts to recondition after decline has occurred. Physical activity interventions are feasible for adults with AML, with some evidence to support benefits in quality of life and physical functioning, 45–50 although few studies have focused on older adults. This analysis can further inform intervention design for older adults with AML in several ways. First, the data suggest that the most vulnerable individuals are in greatest need of interventions to maintain and improve function; these individuals need to be identified early. Second, if depressive symptoms are not addressed in the context of physical activity interventions, benefits of any intervention may be suboptimal. Optimal physical activity studies may require testing multitargeted interventions based on an individual’s risk profile to maximize functional outcomes. Potential interventions for depressive symptoms, such as referral to psychosocial oncology professionals, prescription of antidepressant medications, and education on the benefits of physical activity on depressive symptoms, could enhance the effectiveness of a physical activity program for at-risk individuals.
Beyond the research implications of this analysis, the results suggest a role for geriatricians in the decision-making process and management of older adults with AML who are considering intensive chemotherapy. Geriatric consultation to assist in management of individuals who have screened positive for depressive symptoms or cognitive impairment could facilitate strategies to minimize the negative functional consequences of these impairments during treatment. For example, assistance with delirium prevention strategies for adults who screen positive for cognitive impairment could be a high-yield enhancement to standard oncology supportive care.
This study has several limitations. First, it was a single-institution study, which limits generalizability and requires validation. Second, the sample size was small, limiting power to detect differences in assessment measures over time and ability to conduct exploratory analyses. For example, small differences in mean cognitive function after chemotherapy may not achieve significance in a sample of this size. Multiplicity of significance tests may lead to higher Type 1 error rates than the nominal P-value, and results should be interpreted accordingly. In addition, this study included a single time point for follow-up assessment, so longitudinal changes could not be observed over time. Nonetheless, this time point is a clinically relevant assessment point after induction.
This is the first study to assess physical function after induction chemotherapy in the context of cognition and emotional health using a geriatric assessment approach. The results provide an opportunity to explore relationships between these vulnerabilities to inform interventions. The use of validated measures of physical function, cognition, depression, and distress add to the literature. In addition, inclusion of self-reported and objectively measured physical function is a strength, because these approaches provide complementary information. This study builds upon prior work specifically supporting the utility of the SPPB as a measure that may predict outcomes and be used as an outcome to quantify functional changes over time during treatment. Finally, the study included a well-described cohort of elderly adults who received relatively homogenous treatment and had remarkably complete follow-up for such a vulnerable population with high morbidity and mortality.
In summary, older adults treated intensively for AML are at risk of clinically meaningful decline in physical function after induction chemotherapy. Additional vulnerabilities detected using a GA, such as presence of depressive symptoms, may identify subsets of individuals who are at particularly high risk of deconditioning. Novel supportive care interventions that account for the interrelationships between vulnerabilities detected using GA are needed to optimize function during and after intensive therapies.
Acknowledgments
Presented in part at the annual meeting of the 12th International Society of Geriatric Oncology, October 25–27, 2012, Manchester, United Kingdom.
We thank Karen Klein, MA, ELS (Research Support Core, Wake Forest University Health Sciences) for her editorial contributions to this manuscript. All individuals who contributed significantly to this work are listed and have provided written approval.
Conflict of interest: The authors have no conflicts of interest to report. Dr. Klepin was funded by the American Society of Hematology (ASH)–Association of Specialty Professors (ASP) Junior Faculty Scholar Award in Clinical and Translational Research (supported by ASH, Atlantic Philanthropies, John A. Hartford Foundation, Association of Specialty Professors), the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30 AG-021332), Paul Beeson Career Development Award in Aging Research (K23AG038361; supported by National Institute on Aging, American Federation for Aging Research, John A. Hartford Foundation, and Atlantic Philanthropies), Gabrielle’s Angel Foundation for Cancer Research and Wake Forest Baptist Comprehensive Cancer Center’s National Cancer Institute Cancer Center Support Grant P30CA012197.
Author Contributions: Klepin: study concept and design, data and subject acquisition, analysis and interpretation of data, preparation of manuscript. Pardee, Ellis, Berenzon, Powell: data and subject acquisition, interpretation of data, preparation of manuscript. Tooze, Danhauer, Rao, Wildes: analysis and interpretation of data, preparation of manuscript. Mihalko: study concept and design, analysis and interpretation of data, preparation of manuscript. Kritchevsky, Williamson, Powell: study concept and design, interpretation of data, editing of manuscript.
Sponsor’s Role: The funders were not involved in any aspect of study design, data acquisition, data analysis, interpretation of data, or manuscript preparation.
References
- 1. [Accessed July 30, 2015];SEER Cancer Statistics Review 1975–2009. [on-line]. Available at http://seercancergov/publications/
- 2.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Ravandi F, O’brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–4429. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Etienne A, Esterni B, Charbonnier A, et al. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109:1376–1383. doi: 10.1002/cncr.22537. [DOI] [PubMed] [Google Scholar]
- 6.Klepin HD, Geiger AM, Tooze JA, et al. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287–4294. doi: 10.1182/blood-2012-12-471680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wedding U, Rohrig B, Klippstein A, et al. Impairment in functional status and survival in patients with acute myeloid leukaemia. J Cancer Res Clin Oncol. 2006;132:665–671. doi: 10.1007/s00432-006-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El-Jawahri AR, Abel GA, Steensma DP, et al. Health care utilization and end-of-life care for older patients with acute myeloid leukemia. Cancer. 2015;121:2840–2848. doi: 10.1002/cncr.29430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juliusson G. Most 70- to 79-year-old patients with acute myeloid leukemia do benefit from intensive treatment. Blood. 2011;117:3473–3474. doi: 10.1182/blood-2010-11-321737. [DOI] [PubMed] [Google Scholar]
- 10.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: A population-based study. Haematologica. 2012;97:1916–1924. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2011;118:3377–3386. doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherman AE, Motyckova G, Fega KR, et al. Geriatric assessment in older patients with acute myeloid leukemia: A retrospective study of associated treatment and outcomes. Leuk Res. 2013;37:998–1003. doi: 10.1016/j.leukres.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alibhai SM, Breunis H, Timilshina N, et al. Quality of life and physical function in adults treated with intensive chemotherapy for acute myeloid leukemia improve over time independent of age. J Geriatr Oncol. 2015;6:262–271. doi: 10.1016/j.jgo.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Alibhai SM, Leach M, Gupta V, et al. Quality of life beyond 6 months after diagnosis in older adults with acute myeloid leukemia. Crit Rev Oncol Hematol. 2009;69:168–174. doi: 10.1016/j.critrevonc.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Alibhai SM, Leach M, Kermalli H, et al. The impact of acute myeloid leukemia and its treatment on quality of life and functional status in older adults. Crit Rev Oncol Hematol. 2007;64:19–30. doi: 10.1016/j.critrevonc.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Mohamedali H, Breunis H, Timilshina N, et al. Older age is associated with similar quality of life and physical function compared to younger age during intensive chemotherapy for acute myeloid leukemia. Leuk Res. 2012;36:1241–1248. doi: 10.1016/j.leukres.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Stone RM, Berg DT, George SL, et al. Postremission therapy in older patients with de novo acute myeloid leukemia: A randomized trial comparing mitoxantrone and intermediate-dose cytarabine with standard-dose cytarabine. Blood. 2001;98:548–553. doi: 10.1182/blood.v98.3.548. [DOI] [PubMed] [Google Scholar]
- 19.Klepin HD, Geiger AM, Tooze JA, et al. The feasibility of inpatient geriatric assessment for older adults receiving induction chemotherapy for acute myelogenous leukemia. J Am Geriatr Soc. 2011;59:1837–1846. doi: 10.1111/j.1532-5415.2011.03614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ettinger WH, Jr, Burns R, Messier SP, et al. A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST) JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 21.Katz S. Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55A:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Rantanen T, Volpato S, Ferrucci L, et al. Handgrip strength and cause-specific and total mortality in older disabled women: Exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- 26.Bland RC, Newman SC. Mild dementia or cognitive impairment: The Modified Mini-Mental State examination (3MS) as a screen for dementia. Can J Psychiatry. 2001;46:506–510. doi: 10.1177/070674370104600604. [DOI] [PubMed] [Google Scholar]
- 27.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 28.Beekman AT, Deeg DJ, Van Limbeek J, et al. Criterion validity of the Center for Epidemiologic Studies Depression scale (CES-D): Results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27:231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 29.Nelson CJ, Cho C, Berk AR, et al. Are gold standard depression measures appropriate for use in geriatric cancer patients? A systematic evaluation of self-report depression instruments used with geriatric, cancer, and geriatric cancer samples. J Clin Oncol. 2010;28:348–356. doi: 10.1200/JCO.2009.23.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell AJ. Pooled results from 38 analyses of the accuracy of distress thermometer and other ultra-short methods of detecting cancer-related mood disorders. J Clin Oncol. 2007;25:4670–4681. doi: 10.1200/JCO.2006.10.0438. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell AJ. Short screening tools for cancer-related distress: A review and diagnostic validity meta-analysis. J Natl Compr Canc Netw. 2010;8:487–494. doi: 10.6004/jnccn.2010.0035. [DOI] [PubMed] [Google Scholar]
- 32.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 34.Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171–2179. doi: 10.1111/j.1532-5415.2008.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehl M, Lu X, Silliman R, et al. Decline in physical functioning in first 2 years after breast cancer diagnosis predicts 10-year survival in older women. J Cancer Surviv. 2013;7:20–31. doi: 10.1007/s11764-012-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Holt B, Breems DA, Berna BH, et al. Various distinctive cytogenetic abnormalities in patients with acute myeloid leukaemia aged 60 years and older express adverse prognostic value: Results from a prospective clinical trial. Br J Haematol. 2007;136:96–105. doi: 10.1111/j.1365-2141.2006.06403.x. [DOI] [PubMed] [Google Scholar]
- 37.Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol. 2013;31:3877–3882. doi: 10.1200/JCO.2012.47.7430. [DOI] [PubMed] [Google Scholar]
- 38.Petrick JL, Reeve BB, Kucharska-Newton AM, et al. Functional status declines among cancer survivors: Trajectory and contributing factors. J Geriatr Oncol. 2014;5:359–367. doi: 10.1016/j.jgo.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 40.Ojagbemi A, D’Este C, Verdes E, et al. Gait speed and cognitive decline over 2 years in the Ibadan Study of Aging. Gait Posture. 2015;41:736–740. doi: 10.1016/j.gaitpost.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65A:1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarden M, Baadsgaard MT, Hovgaard DJ, et al. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009;43:725–737. doi: 10.1038/bmt.2009.27. [DOI] [PubMed] [Google Scholar]
- 43.Baumann FT, Kraut L, Schule K, et al. A controlled randomized study examining the effects of exercise therapy on patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:355–362. doi: 10.1038/bmt.2009.163. [DOI] [PubMed] [Google Scholar]
- 44.Dimeo FC, Tilmann MH, Bertz H, et al. Aerobic exercise in the rehabilitation of cancer patients after high dose chemotherapy and autologous peripheral stem cell transplantation. Cancer. 1997;79:1717–1722. [PubMed] [Google Scholar]
- 45.Alibhai SM, O’Neill S, Fisher-Schlombs K, et al. A clinical trial of supervised exercise for adult inpatients with acute myeloid leukemia (AML) undergoing induction chemotherapy. Leuk Res. 2012;36:1255–1261. doi: 10.1016/j.leukres.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klepin HD, Danhauer SC, Tooze JA, et al. Exercise for older adult inpatients with acute myelogenous leukemia: A pilot study. J Geriatr Oncol. 2011;2:11–17. doi: 10.1016/j.jgo.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alibhai SM, O’Neill S, Fisher-Schlombs K, et al. A pilot phase II RCT of a home-based exercise intervention for survivors of AML. Support Care Cancer. 2014;22:881–889. doi: 10.1007/s00520-013-2044-8. [DOI] [PubMed] [Google Scholar]
- 48.Chang PH, Lai YH, Shun SC, et al. Effects of a walking intervention on fatigue-related experiences of hospitalized acute myelogenous leukemia patients undergoing chemotherapy: A randomized controlled trial. J Pain Symptom Manage. 2008;35:524–534. doi: 10.1016/j.jpainsymman.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 49.Bergenthal N, Will A, Streckmann F, et al. Aerobic physical exercise for adult patients with haematological malignancies. Cochrane Database Syst Rev. 2014;11:CD009075. doi: 10.1002/14651858.CD009075.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Smith-Turchyn J, Richardson J. A systematic review on the use of exercise interventions for individuals with myeloid leukemia. Support Care Cancer. 2015;23:2435–2446. doi: 10.1007/s00520-015-2752-3. [DOI] [PubMed] [Google Scholar]