Abstract
Background
Prior studies have shown that etomidate modulates γ-aminobutyric acid type A (GABAA) receptors by binding at the β+ – α− subunit interface within the transmembrane domain of receptors that incorporate β2 or β3 subunits. Introducing an asparagine-to-methionine (N265M) mutation at position 265 of the β3 subunit, which sits within the etomidate binding site, attenuates the hypnotic effect of etomidate in vivo. It was reported recently that the photoactivatable barbiturate R–mTFD-MPAB also acts on GABAA receptors primarily by binding to a homologous site at the γ - β interface. Given this difference in drug binding sites established by in vitro experiments, we hypothesized that the β3-N265M mutant mice would not be resistant to the anesthetic effects of R–mTFD-MPAB in vivo, whereas the same mutant mice would be resistant to the anesthetic effects of R-etomidate.
Methods
We measured the effects of IV injection of etomidate and R–mTFD-MPAB on loss and recovery of righting reflex in wild type mice and in mice carrying the β3-N265M mutation.
Results
Etomidate–induced hypnosis, as measured by duration of loss of righting reflex, was attenuated in the N265M knock-in mice, confirming prior results. By contrast, recovery of balance and coordinated movement, as measured by the ability to maintain all four paws on the ground, was unaffected by the mutation. Neither hypnosis nor impairment of coordinated movement produced by the barbiturate R–mTFD-MPAB was affected by the mutation.
Conclusions
The findings confirmed our hypothesis that mutating the etomidate binding site would not alter the response to the barbiturate R-mTFD-MPAB. Furthermore, we confirmed prior studies indicating that etomidate-induced hypnosis is mediated in part by β3-containing receptors. We also extended prior findings by showing that etomidate-impaired balance and coordinated movement are not mediated by β3–containing receptors, thus implicating β2-containing receptors in this end point.
Introduction
It is widely accepted that many anesthetic drugs generate their effects by acting on γ-aminobutyric acid type A receptors (GABAARs)(1-3). GABAARs are the most abundant inhibitory receptor in the central nervous system (CNS). They are members of the Cys-loop ligand-gated ion channel superfamily that consists of five subunits arranged pseudo-symmetrically around a central ion pore. There are 16 different but highly homologous GABAAR subunits (α1–6, β1–3, γ1–3, δ, ɛ, θ, π)(4). Although all pentameric configurations that occur naturally are not thoroughly established, the most common arrangement reading anti-clockwise around the central ion pore, as viewed from the extracellular side, is thought to be β–α–β–α–x, where x might be any subunit except possibly α. In practice, the most common fifth subunit in GABAARs in the CNS are β, γ and δ.
R-etomidate is a general anesthetic that acts on GABAARs with high affinity and enantioselectivity. Importantly, its potency is subunit–dependent; GABAARs containing a β1 subunit are insensitive to R-etomidate whereas those with β2 or β3 subunits are sensitive (5). These observations show that general anesthetics can act selectively on certain subtypes of GABAARs. Furthermore, it was discovered that mutating a single residue in both β2 and β3 subunits (N256S and N265M, respectively) could render GABAARs containing them insensitive to etomidate (6). Introduction of each of these mutations into mice (knock-in mice) has provided a tool for examining the contributions of β2– and β3–containing GABAARs to the many components underlying the state of general anesthesia (7,8). Subsequently, photolabeling with etomidate derivatives in heterologously expressed GABAARs has identified the binding site for R-etomidate within the transmembrane domain at the interface between the β3 and α1 subunits (9). Based on homology models of the GABAAR and the structure of a β3 homopentameric GABAAR (10), the photolabeled residues and β3 N265 site all reside within the β+ – α− subunit interface and within ~10 Å of each other (Figure 1), suggesting they constitute the etomidate binding site.
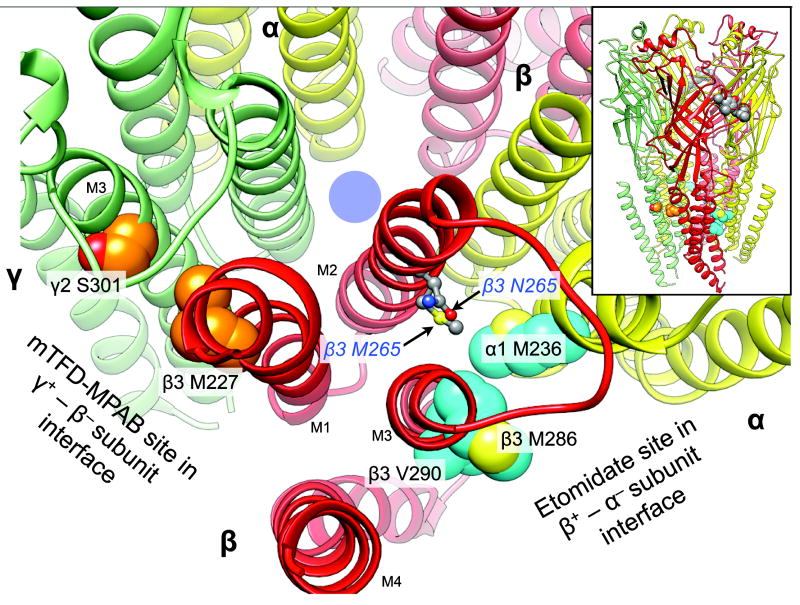
Figure 1.
The location of the N265M mutation relative to that of the etomidate and R–mTFD-MPAB sites on a homology model of α1β3γ2L GABAARs. The backbone of the receptor is represented as ribbons with relevant residues in space filled mode. The five subunits of the GABAAR are arranged around a central ion–conducting pore (blue circle) and colored as follows: α1, yellow; β3, red, and γ2 green. The main figure shows a cross section of the transmembrane domain with parts of the extracellular domain removed for clarity. Residues photolabeled by etomidate derivatives or R–mTFD-MPAB are shown with cyan and orange carbons respectively. Other atoms are colored conventionally (carbons grey; oxygens red; nitrogens blue, and sulfurs yellow). The residues at β3 265 are represented as ball and stick. The inset at top right shows the whole pentamer with a cluster of space filled agonist–associated residues in the extracellular domain in the β3+ – α1− subunit interface (α1 Phe-65; β3 Tyr-157 & -205, Phe-200). The homology model is based on the GluCl chloride channel (3RHW.pdb) (11,22). The intracellular domain is not modeled because no structural information is available.
On the other hand, it was shown in 2013 that the photoactivatable barbiturate R–mTFD-MPAB also acts on GABAARs, but primarily by binding at the γ - β interface, with a 60-fold preference over R-etomidate’s site at the β+ – α−interface (11). Thus, R–mTFD-MPAB differs in vitro from etomidate in interacting exclusively with GABAARs that contain a γ subunit and by acting at a single subunit interface that is homologous to, but separate from, the etomidate site. Given this difference in drug binding sites established by in vitro experiments, we hypothesized that in in vivo experiments, the β3-N265M mutant mice would not be resistant to the anesthetic effects of R–mTFD-MPAB, whereas the same mutant mice would be resistant to the anesthetic effects of R-etomidate, as shown in previous work (7,12).
Materials and Methods
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Eighth Edition, 2011) and were approved by the University of Wisconsin Institutional Animal Care and Use Committee, Madison, Wisconsin. All efforts were made to minimize animal suffering and reduce the number of animals used.
Male and female offspring of heterozygous breeding pairs homozygous for the asparagine-to-methionine point mutation at GABAAR β3 subunit position 265 (β3-N265M) as well as wild-type (WT) controls were used for this study. Mice were housed in an animal care facility with continuous access to standard mouse chow and water. Twelve hour light-dark cycles were maintained. Mice were genotyped using DNA from tail tips, amplified by polymerase chain reaction.
Etomidate (Tocris Bioscience, Bristol, UK) was dissolved in sterile saline to 5mg/ml stock solution. R–mTFD-MPAB (13)was dissolved in dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO) to 5mg/ml stock solution. These stock solutions were further diluted with additional saline or DMSO on the day of injections to working solutions of a concentration such that an injection volume of 2.5ml/kg would deliver the desired dose of drug while keeping the volume of DMSO as low as possible and consistent among mice while injecting R-mTFD-MPAB.
Injections were performed on mice aged 38-120 days. Drug was prepared on the day of injections as described above. The individual performing the injections was blinded to the genotype of the mice being injected. Mice were weighed and an appropriate volume of drug was drawn up in a 1 ml syringe (BD Biosciences, San Jose, CA). Mice were restrained and injected in a lateral tail vein using a 30-gauge ½-inch needle (BD Biosciences, San Jose, CA). Mice were immediately removed from the restraint device and placed on their backs to check for righting reflex. Once a mouse lost righting reflex (which was generally immediately) a timer was started and the mouse was placed in an empty housing container and observed continuously. Stimulation was provided every 30 seconds in the form a gentle nudge. The time at which the mouse regained its righting reflex, i.e. was able to maintain an upright posture on all four paws, was recorded, and is referred to as the time (in seconds) until “return of righting reflex” (RORR). Some mice made an initial attempt to regain an upright stance but instead fell to the opposite side, usually repeatedly, resulting in the appearance of a “rolling motion.” If this rolling occurred, the time at which a mouse began to undertake these attempts at righting was also recorded, and is referred to as the “return of attempted righting” (ROAR). The “duration of anesthesia” was defined as the time until RORR or time until ROAR.
Doses used for etomidate injections were 2.5, 5, and 10mg/kg. Doses used for R–mTFD-MPAB injections were 5, 7.5, and 10mg/kg. Five additional mice (3 WT and 2 mutant) were injected with 2.5ml/kg of plain DMSO and observed for loss of righting reflex, which did not occur.Repeat injections were performed on mice only if tail veins appeared viable after initial injection. Any repeat injection was performed at a minimum of 4 days after the prior injection.
No a priori statistical power calculation was conducted to guide sample size. Instead, the sample size was based on our previous experience with these methods. For the experiments, N = 76 animals were observed in N = 98 conditions with 20 animals used in two conditions, and one animal used in three conditions. For the analyses, the responses from the animals were treated as independent of one another (i.e., that an animal’s response at one dose was independent of another dose). To examine differences between mutant and WT animals at each dose, a Mann-Whitney test was applied with a Bonferroni correction applied to adjust for the fact that three comparisons were made for each drug. Medians [IQR] were used to report the data, with median differences and bootstrapped 95%CI of these differences used to index the degree of difference between each group. All analyses were conducted with R statistical software (R Foundation for Statistical Computing, Vienna, Austria). Where appropriate, all analyses were two-tailed, and p < 0.05 was used to interpret statistical significance.
Results
Intravenous injection of etomidate (2.5-10 mg/kg) to WT mice produced a dose-dependent loss of righting reflex (LORR), with larger doses leading to a greater duration of anesthesia (Figure 2A). Similarly, injection of MPAB (5-10 mg/kg) to WT mice produced a dose-dependent LORR (Figure 2B). Because the highest dose of MPAB (10 mg/kg) caused a very prolonged duration of anesthesia and, in many cases, death upon injection, it was administered only a limited number of times and these data were not included in statistical analysis.
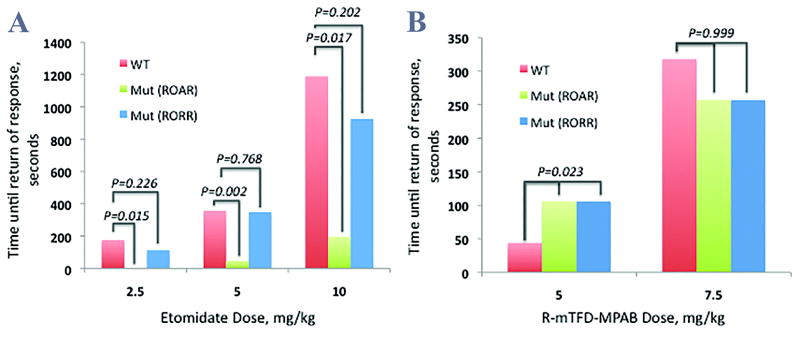
Figure 2.
The contrasting dependence of the duration of anesthesia for R-etomidate and R–mTFD-MPAB on the N265M mutation in mice. Median time to return of righting reflex (RORR) and attempted righting (ROAR) of wild-type (WT) and mutant N265M mice receiving (A) etomidate at doses of 2.5, 5, and 10 mg/kg and (B) R–mTFD-MPAB at doses of 5 and 7.5 mg/kg.
Intravenous injection of etomidate (2.5-10 mg/kg) to mutant mice also produced a dose-dependent LORR. However, these mice displayed unique behavior on emergence from anesthesia. Before being able to maintain all four paws on the ground these mice would attempt to right themselves, but, lacking the apparent coordination to do so, would fall to the opposite side in what was deemed a “rolling” behavior. WT mice did not display this behavior, nor did either genotype after injection with MPAB. This behavior was deemed ROAR as opposed to the classic RORR in which mice are able to maintain all 4 paws in contact with the ground.
Mutant mice receiving etomidate had significantly shorter duration of anesthesia, defined as the time until ROAR or RORR, than their WT counterparts (Figure 2A). At the 2.5 mg/kg dose, 7/8 mutant animals did not lose righting reflex, while 8/8 WT animals were observed to sleep for a median [IQR] duration of 175 [144] seconds. The median difference (95%CI) between the two groups was 175 (119, 344), p = 0.015. At the 5.0 mg/kg dose, the mutant animals slept for 46 [29] seconds, while the WT slept for 357.5 [84.25] seconds, median difference: 311.5 (95%CI: 273, 381), p = 0.0021. At the 10.0 mg/kg dose, the mutant animals slept for 195 [63] seconds, while the WT slept for 1189 [1086.5] seconds, median difference: 994 (95%CI: 586,2464), p = 0.017.
Although the duration of anesthesia was shorter for mutant than WT mice, there was no statistical difference between genotypes in time to recovery of balance and coordination. The 2.5, 5, and 10mg/kg doses yielded median RORR times of 113.5 [137.75], 348 [157], and 926 [20] seconds, respectively. When compared to WT mice, the median difference for the 2.5mg/kg dose was 61.5 (95%CI: -93.5, 218.4), p=0.226. The median difference for 5mg/kg was 9.5 (95%CI: -141,163), p=0.768, and for the 10mg/kg dose was 263 (95%CI: -101,1221), p=0.202.
For mice receiving MPAB at the 5mg/kg dose, mutant mice had a median [IQR] RORR time of 106 [85.25] seconds, while WT mice had a median RORR time of 44 [33] seconds (Figure 2B). The median difference was -62 (95%CI: -114.6—2.87), p = 0.023. A single mouse died immediately after injection of the 5mg/kg dose. For the 7.5mg/kg dose, mutant mice had a median RORR time of 257 [203.25] seconds, while WT mice had a median RORR time of 318 [427.25] seconds. The median difference of 61 (95%CI: -160.5-257.5) was not statistically significantly different (p = 0.999). There were 3 deaths associated with the 7.5mg/kg dose of MPAB. Two of these occurred immediately after injection while the other occurred the night after injection.
Five (3 mutant, 2 WT) of the first nine mice injected with 10mg/kg of MPAB died either shortly after injection or later on the day of injection, thus injections of this dose were discontinued. No data from any mouse that died in conjunction with injection of MPAB were used in data analysis. Because there was only an n = 2 for each genotype injected with the 10mg/kg dose, these data were also not included in analysis.
Discussion
Our results clearly show that the LORR caused by IV injection of R–mTFD-MPAB is insensitive to the β3 N265M mutation whereas the potency of R-etomidate is dramatically shifted in these mutant animals. This finding indicates that R–mTFD-MPAB does not cause general anesthesia by acting on the etomidate site in the β+ – α− interfaces of GABAARs.
All functional combinations of GABAAR subunits are thought to contain β+ – α− interfaces, because the GABA binding site is located at this interface in the extracellular domain, nearly 50 Å from the etomidate site in the transmembrane domain (14). The action of R-etomidate is dependent on the subtype of β subunit. Receptors with β1 subunits, which constitute a minority of GABAARs in the CNS (4), are relatively insensitive, whereas those with β2 and β3 subunits are equally sensitive (5). The type of α subunit is less important for affinity, although the magnitude of current enhancement may vary (5). Thus, β1–containing receptors appear to play little if any role in etomidate–induced anesthesia.
Observations from this study do not provide any additional information about how exactly R–mTFD-MPAB causes anesthesia, but four lines of evidence support the hypothesis that it causes anesthesia by acting on GABAARs that contain γ-subunits (11,13). First, in an equilibrium LORR study of tadpoles immersed in R–mTFD-MPAB solutions, the EC50 was found to be 3.7μM. Second, this concentration is comparable to that which enhances currents induced by low concentrations of GABA in α1β3γ2 GABAARs in oocytes (EC50 is 2.1 μM). Third, the enantiomer, S–mTFD-MPAB, is much less potent both in tadpoles and action on GABAARs(13). Fourth, R–mTFD-MPAB photolabels α1β3γ2 GABAARs in the γ2+ – β3− Interfaces at the level of the transmembrane domain at a site that is homologous to the etomidate site in the β+ – α− Interfaces (11).
All synaptic GABAA receptors are thought to contain γ2 subunits due to their critical role in targeting receptors to the synapse, and GABAA receptors lacking γ2 are thought to be exclusively extrasynaptic. On the other hand, not all γ2–containing GABAARs are synaptic. Thus, R–mTFD-MPAB and etomidate will both act on native synaptic receptors, but they will only act together on a subset of extrasynaptic GABAA receptors that contain γ subunits. Thus, we might expect the physiological processes underlying the global anesthetic state to differ between the two agents. For example, δ-subunit containing extrasynaptic receptors have been implicated in etomidate’s action (15), but are unlikely to be important in R–mTFD-MPAB’s because γ2 Ser-301 in the R–mTFD-MPAB binding site is homologous with δ Trp-299, a residue that is large enough to sterically hinder binding. Indeed, site–directed mutagenesis to a tryptophan is commonly used for this purpose (16,17).
An unexpected observation during these experiments was the unique behavior of mutant mice receiving etomidate. To our knowledge, this rolling behavior has not been reported previously. Reported studies in which β3-N265M mice received etomidate (7,12) indeed showed decreased times until RORR in mutants but little detail was given as to what the authors defined as RORR, or any subjective differences in behavior during experiments. Previous studies of genetically modified mice that lack specific GABAAR subunits, or that carry mutations rendering specific subunits insensitive to anesthetics, have shown that specific endpoints depend upon certain subsets of GABAARs (1,15). For example, immobility (impaired hindlimb withdrawal reflex) produced by etomidate and propofol is mediated by GABAARs that incorporate β3 subunits (7). By contrast, sedation (reduced spontaneous motor activity) is mediated by GABAARs that incorporate β2 subunits (8). Similarly, memory impairment produced by a low dose of etomidate (reduced fear conditioning to context) depends upon modulation of GABAARs that incorporate β2 subunits together with α5 subunits (18-20). Our present finding that LORR is shorter in mice that carry the β3-N265M mutation is consistent with previous studies showing that hypnosis depends in part on GABAARs that incorporate β3 subunits (7,12). Although N265M mice attempted to right themselves sooner than WT mice, our novel observation that β3-N265M mice did not recover the ability to maintain an upright posture on all four paws until the same time as WT mice indicates that impaired coordination or balance induced by etomidate does not depend upon β3 subunits, so presumably is produced by modulation of β2 subunits, since GABAA receptors that incorporate β1 subunits are relatively insensitive to etomidate.
During experimentation an attempt was made to blind the injector/observer to prevent bias as much as possible. After each round of injections, however, the observer was unblinded for data tabulation. The unique behavior of mutants receiving etomidate thus diminished the blinding process during later rounds of injections.
A weakness of the present study is the apparently limited therapeutic range for R–mTFD-MPAB. Injections of the 10mg/kg dose were halted due to a large proportion of post-injection deaths from the drug, and 12.5% of animals died after successful injection with the next highest dose (7.5mg/kg). Prior experiments (data not shown) indicated limited efficacy with doses as low as 2.5-3mg/kg, making the therapeutic window for R–mTFD-MPAB in mice quite narrow. This was not an issue for our purposes because we sought only to compare two genotypes receiving similar doses and not to establish a dose-response curve.
The primary aim of this study was to further test the hypothesis that R–mTFD-MPAB binds to the GABAAR at a site remote from the site of etomidate binding. The data we obtained largely support the hypothesis, because mice with the β3-N265M mutation were not resistant to the anesthetic effects of R–mTFD-MPAB. For reasons unclear at this time, mutant mice receiving 5mg/kg of R–mTFD-MPAB had in fact a significantly longer (two-fold) time to RORR. In addition, the observed differences in emergence behaviors in mutant versus WT mice receiving etomidate raises further questions as to the location, composition, and function of the GABAAR. Further research is needed on the subunit–dependence of R–mTFD-MPAB’s action on GABAARs because it also photolabeled to a lesser degree the α1+ – β3− subunit interface (11). However, the ability to compare the in vivo actions of R-etomidate and R–mTFD-MPAB clearly holds promise both for finding the common pathways by which they both exert anesthesia and for teasing apart GABAAR-mediated contributions to the different components of anesthetic action. Such an endeavor could eventually lead to the development of agents that act more selectively on the CNS to the benefit of patients.
Acknowledgments
This research was supported by grants from the National Institute of General Medical Sciences GM 58448 (KWM) and GM 101497 (RAP). Additional support came from the Department of Anesthesia, Critical Care & Pain Medicine, Massachusetts General Hospital, as well as the Department of Anesthesiology, University of Wisconsin, Madison.
The authors wish to thank Professor Karol S. Bruzik and Dr. Pavel Y. Savechenkov of the Department of Medicinal Chemistry and Pharmacology, University of Illinois, Chicago for providing the R–mTFD-MPAB. Additional thanks to Drs. Rachel Jurd and Uwe Rudolph for providing the genetically modified mice that we studied.
Figure 1 was produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081) (21) with a homology model kindly supplied by David C. Chiara.
This manuscript was handled by: Gregory Crosby, MD
Footnotes
The authors declare no conflicts of interest.
Disclosures
Name: Corey A. Amlong, MD, MS
Contribution: Dr. Amlong contributed to experimental design, conduct of the experiments, analysis, creation of figures, and writing and editing of this manuscript.
Attestation: Dr. Amlong approved the final manuscript. Dr. Amlong attests to the integrity of the original data and the analysis reported in this manuscript. Dr. Amlong is the archival author.
Name: Mark G. Perkins, BS
Contribution:Mr. Perkins contributed to the animal husbandry and conduct of the experiments.
Attestation: Mr. Perkins approved the final manuscript.
Name: Timothy T. Houle, PhD
Contribution:Dr. Houle performed the statistical analysis.
Attestation: Dr. Houle approved the final manuscript.
Name: Keith W. Miller, D.Phil
Contribution:Prof. Miller contributed to the conception, creation of figures, and writing and editing of this manuscript.
Attestation: Prof. Miller approved the final manuscript. Prof Miller attests to the integrity of the original data and the analysis reported in this manuscript.
Name: Robert A. Pearce, MD, PhD
Contribution:Dr. Pearce contributed to experimental design, analysis, and writing and editing of this manuscript.
Attestation: Dr. Pearce approved the final manuscript. Dr. Pearce attests to the integrity of the original data and the analysis reported in this manuscript.
Contributor Information
Corey A. Amlong, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
Mark G. Perkins, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
Timothy T. Houle, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Keith W. Miller, Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts.
Robert A. Pearce, Department of Anesthesiology, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin.
References
- 1.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nature reviews. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 2.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med. 2003;348:2110–24. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- 3.Olsen RW, Li G-D. GABAA receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation. Can J Anaesth. 2011;58:206–15. doi: 10.1007/s12630-010-9429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen RW, Sieghart W. GABA A receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–8. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type A receptor. BrJPharmacol. 1997;120:749–56. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11031–6. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB Journal. 2003;17:250–2. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, Myers J, Sur C, Howell O, Rutter AR, Atack J, Macaulay AJ, Hadingham KL, Hutson PH, Belelli D, Lambert JJ, Dawson GR, McKernan R, Whiting PJ, Wafford KA. Sedation and anesthesia mediated by distinct GABA(A) receptor isoforms. J Neurosci. 2003;23:8608–17. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human alpha1beta3 gamma-aminobutyric acid type A receptors with [(3)H]TDBzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–47. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller PS, Aricescu AR. Crystal structure of a human GABAA receptor. Nature. 2014;512:270–5. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic binding sites in the transmembrane domain of the human alpha1beta3gamma2 GABAA receptor. The Journal of biological chemistry. 2013;288:19343–57. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liao M, Sonner JM, Husain SS, Miller KW, Jurd R, Rudolph U, Eger EI., 2nd R (+) etomidate and the photoactivable R (+) azietomidate have comparable anesthetic activity in wild-type mice and comparably decreased activity in mice with a N265M point mutation in the gamma-aminobutyric acid receptor beta3 subunit. Anesthesia and analgesia. 2005;101:131–5. doi: 10.1213/01.ANE.0000153011.64764.6F. [DOI] [PubMed] [Google Scholar]
- 13.Savechenkov PY, Zhang X, Chiara DC, Stewart DS, Ge R, Zhou X, Raines DE, Cohen JB, Forman SA, Miller KW, Bruzik KS. Allyl m-trifluoromethyldiazirine mephobarbital: an unusually potent enantioselective and photoreactive barbiturate general anesthetic. Journal of medicinal chemistry. 2012;55:6554–65. doi: 10.1021/jm300631e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller PS, Smart TG. Binding, activation and modulation of Cys-loop receptors. Trends Pharmacol Sci. 2010;31:161–74. doi: 10.1016/j.tips.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Bonin RP, Orser BA. GABA(A) receptor subtypes underlying general anesthesia. Pharmacol Biochem Behav. 2008;90:105–12. doi: 10.1016/j.pbb.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Amin J. A single hydrophobic residue confers barbiturate sensitivity to gamma-aminobutyric acid type C receptor. Mol Pharmacol. 1999;55:411–23. [PubMed] [Google Scholar]
- 17.Findlay GS, Ueno S, Harrison NL, Harris RA. Allosteric modulation in spontaneously active mutant gamma-aminobutyric acidA receptors. Neurosci Lett. 2001;305:77–80. doi: 10.1016/s0304-3940(01)01646-9. [DOI] [PubMed] [Google Scholar]
- 18.Cheng VY, Martin LJ, Elliott EM, Kim JH, Mount HT, Taverna FA, Roder JC, Macdonald JF, Bhambri A, Collinson N, Wafford KA, Orser BA. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–20. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarnowska ED, Rodgers FC, Oh I, Rau V, Lor C, Laha KT, Jurd R, Rudolph U, Eger EI, 2nd, Pearce RA. Etomidate blocks LTP and impairs learning but does not enhance tonic inhibition in mice carrying the N265M point mutation in the beta3 subunit of the GABA(A) receptor. Neuropharmacology. 2015;93:171–8. doi: 10.1016/j.neuropharm.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodgers FC, Zarnowska ED, Laha K, Engin E, Zeller A, Keist R, Rudolph U, Pearce RA. Etomidate impairs long term potentiation in vitro by targeting α5-GABAARs on non-pyramidal cells. Journal of Neuroscience. 2015 doi: 10.1523/JNEUROSCI.0315-15.2015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 22.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]