Abstract
Pain, the hallmark complication of sickle cell disease (SCD), is largely managed with opioid analgesics in the United States, but comprehensive data regarding the long-term use of opioids in this patient population is lacking. The pain medication prescription records from a cohort of 203 SCD patients were analyzed. Twenty-five percent were not prescribed opioid medications while 47% took only short-acting opioids, 1% took only long-acting opioids, and 27% took a combination of short-acting and long-acting opioids. The median (interquartile range) daily opioid dose was 6.1 mg (1.7-26.3 mg) of oral morphine equivalents, which is lower than the published opioid use among patients with other pain syndromes. The dose of opioids correlated with the number of admissions due to vaso-occlusive crisis (VOC) (r=0.53, p<0.001). When the patients were grouped into quartiles based on daily dose opioid use, a logistic regression model showed that history of avascular necrosis (AVN) (OR 2.87, 95% CI: 1.37-6.02, p=0.005), 25-OHD levels (OR 0.59, 95% CI: 0.38-0.93, p=0.024) and total bilirubin concentration (OR 0.64, 95% CI: 0.42-0.99, p=0.043) were independently associated with opioid use quartiles. In conclusion, doses and types of opioid medications used by adult SCD patients vary widely. Our findings implicate AVN and lower vitamin D levels as factors associated with higher opioid use. They also suggest an association of higher bilirubin levels, possibly suggesting higher hemolytic rate, with lower opioid use.
Keywords: sickle cell disease, opioid, pain medicine, pattern, avascular necrosis
Introduction
Sickle cell disease (SCD) is caused by homozygous or compound heterozygous mutations in the beta-globin genes that affect 25 million people worldwide (1, 2). In the United States, approximately 100,000 people are affected and the majority of the cost, estimated at $2.4 billion annually, is attributed to frequent emergency room (ER) visits and hospitalizations due to recurrent vaso-occlusive pain episodes (3-5). Pain, the hallmark complication of SCD, is a complex manifestation that includes a combination of chronic, acute and neuropathic pain (6), and that impairs quality of life (7). In the United States, sickle cell pain is managed mainly using opioid analgesics, especially in the adult population (1). The opioid dose requirement varies among SCD patients and often exceeds treatment guidelines derived from other pain syndromes, particularly during a vaso-occlusive crisis (VOC) (8, 9). Failure to achieve adequate pain control during VOC is one of the major reasons for admission (10). Despite their important role in pain management regimens, comprehensive data regarding the long-term use of opioids in adult SCD patients is lacking. The purpose of this study is to investigate the patterns of opioid use in SCD to help us better understand pain mechanisms and management strategies.
Methods
Patient population and data collection
Three hundred and fifty-nine adults (age ≥ 18 years old) with the diagnosis of SCD followed at the University of Illinois Hospital (UIH) Comprehensive Sickle Cell Center between July 2010 and August 2014 were enrolled in a prospective, natural history study of SCD. A total of 203 patients continued following up with the center for 12 months after the enrollment (the study period) and had outpatient prescription records. Medication prescribing records during the 12 months after enrollment were obtained from a pharmacy prescription reporting system at UIH. The pharmacy prescription records reported the types and quantities of medications being prescribed, the names of the patients, the dates of prescription, and prescriber’s information. Any enrollees who were prescribed any type or dose of NSAIDs during the 12-month study period were also recorded. Patient demographic information, laboratory data, and hospital admissions were collected from the electronic medical record charting system, Cerner PowerChart. The study was approved by the Institutional Review Board prior to the initiation of chart review.
Opioid medication dose conversion
The oral opioid medication doses were converted to oral morphine equivalents (OME) based on the following ratio (11): 1 mg codeine = 0.13 mg OME, 1 mg hydrocodone = 1 mg OME, 1 mg hydromorphone = 5 mg OME, 1 mg methadone = 4.7 mg OME, 1 mg tramadol = 0.2 mg OME, 1 mg oxycodone = 1.5 mg OME, 1 mg tapentadol = 0.4 mg OME. Transdermal fentanyl was converted based on 1 mcg/hr = 3 mg OME/daily.
Statistical Analysis
Patients were divided into quartiles based on the daily dose of opioids. Univariate analyses of patient characteristics were conducted using Spearman correlation for continuous variables and the Cochran–Mantel–Haenszel trend test for categorical variables. Stepwise ordinal logistic regression analysis was used to identify independent clinical correlates of daily opioid use categories. SYSTAT 13 (Systat Software Corporation, Chicago, IL, USA) and SAS 9.3 (SAS Institute Inc. Cary, NC, USA) were used for the analyses.
Results
The 203 patients analyzed in this study included 153 hemoglobin (Hb) SS or Sβ0-thalassemia, 37 Hb SC, and 13 Sβ+-thalassemia patients. The median age was 35 years (interquartile range [IQR] 25-45 years), and 37% were males. Seventy-seven patients (38%) were receiving hydroxyurea therapy. One hundred and fifty-two patients (75%) required opioid-type pain medications- 47% took short-acting opioids alone, 1% took only long-acting opioids, and 27% took short and long-acting opioids. Seventy-seven patients (38%) also used nonsteroidal anti-inflammatory drugs (NSAIDs), among which 17 patients (8%) took NSAIDs without opioids. The median (IQR) daily opioid dose of all patients was 2.6 mg (0-12.4 mg) oral morphine equivalents (OME). Among patients who were prescribed opioids, the median (IQR) daily opioid dose was 6.1 mg (1.7-26.3 mg) OME. Seventy-one percent of all patients used less than 10 mg OME daily, whereas 13% used more than 50 mg OME daily (Figure 1).
Figure 1.
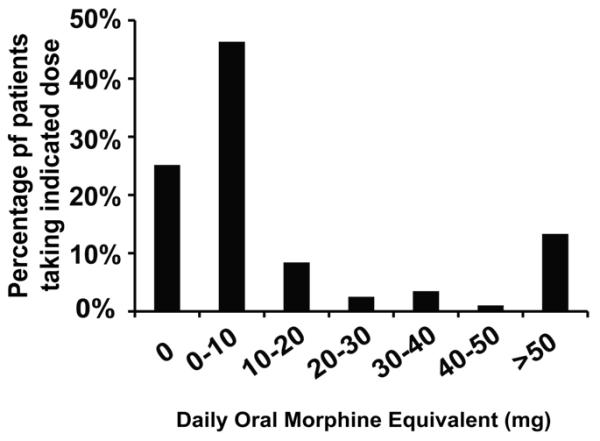
Frequency of Opioid Use in All SCD Patients.
Acetaminophen-hydrocodone was the most commonly prescribed short-acting medication, used by 55% of patients prescribed opioids, followed by acetaminophen-codeine (29%) and immediate-release (IR) morphine (21%) (Supplementary Figure 1). Among long-acting opioids, extended-release (ER) morphine was used by 28% of the patients, while methadone (7%), fentanyl patch (4%), and oxycodone ER (3%) were prescribed less frequently.
The number of hospital admissions due to VOC had a significant correlation with the opioid dosage in a Spearman correlation model (r=0.53, p<0.001), suggesting that high-dose opioid users tend to utilize healthcare resources more often. To study the demographic and clinical variables that might influence the opioid use, the patients were grouped into quartiles based on the daily dose of opioids and patient characteristics were compared among the quartiles (Table 1). The high dose opioid users tended to have a history of AVN (p<0.001), lower total bilirubin concentration (p=0.007), and indirect bilirubin concentration (p=0.008). When a multivariate ordinal logistic regression analysis was conducted to evaluate the independent clinical correlates of opioid use in 145 subjects, history of AVN, lower 25-OHD level concentration, and lower total bilirubin concentration were independently associated with higher opioid use quartiles (Table 2).
Table 1. Patient Characteristics Based on Daily Opioid Use.
Median (interquartile range) or percent is shown. Patient characteristics were compared using Spearman correlation analysis for continuous variables or Cochran trend test for categorical variables.
| Daily opioid dose quartile | N | 1 | N | 2 | N | 3 | N | 4 | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Daily opioid dose (mg OME) | 51 | 0 (0-0) | 51 | 1.1 (0.7-1.7) | 51 | 6.3 (3.7-8.8) | 50 | 57.3 (28.8-111.8) | <0.001 |
| Gender (%male) | 51 | 45 | 51 | 22 | 51 | 41 | 50 | 40 | 0.9 |
| Age (yo) | 51 | 30 (21-46) | 51 | 31 (23-42) | 51 | 39 (28-45) | 50 | 36 (28-45) | 0.03 |
| SCD genotype (%SS/Sbeta0) | 51 | 77 | 51 | 75 | 51 | 71 | 50 | 80 | 0.81 |
| On hydroxyurea (%) | 51 | 24 | 51 | 28 | 51 | 51 | 50 | 50 | 0.001 |
| History of AVN (%) | 50 | 6 | 50 | 22 | 51 | 22 | 50 | 40 | <0.001 |
| 25-OHD (ng/mL) | 46 | 19 (11-27) | 49 | 16 (9-23) | 51 | 15 (9-23) | 47 | 14 (8-26) | 0.06 |
| WBC ( × 109/L) | 49 | 8.4 (7.0-10.5) | 51 | 8.8 (7.2-11.4) | 51 | 9.0 (6.9-11.6) | 50 | 9.2 (7.1-12.6) | 0.24 |
| Hemoglobin (g/dL) | 49 | 9.0 (7.9-10.5) | 51 | 9.0 (8.0-10.1) | 51 | 8.7 (7.9-10.3) | 50 | 9.1 (7.6-10.7) | 0.79 |
| Hemoglobin F (%) | 34 | 3.8 (1.5-8.5) | 35 | 4.4 (2.1-7.9) | 44 | 5.4 (2.3-11.6) | 39 | 5.9 (3.3-11.2) | 0.02 |
| Absolute reticulocyte (×109/L) | 44 | 283 (218-420) | 45 | 318 (198-432) | 47 | 322(188-426) | 42 | 273 (181-354) | 0.26 |
| Reticulocyte (%) | 44 | 11.5 (6.1-14.9) | 45 | 11.6 (5.8-14.7) | 47 | 11.7 (6.2-18.0) | 42 | 9.3 (6.5-14.0) | 0.49 |
| MCV (fl) | 49 | 90 (84-102) | 51 | 90 (82-101) | 51 | 94 (87-109) | 50 | 92 (81-102) | 0.36 |
| Total bilirubin (mg/dL) | 42 | 2.7 (1.8-6.1) | 40 | 2.3 (1.6-4.1) | 35 | 2.0 (1.4-2.5) | 36 | 1.8 (1.2-3.3) | 0.007 |
| Direct bilirubin (mg/dL) | 9 | 0.6 (0.3-0.9) | 16 | 0.4 (0.3-0.5) | 12 | 0.5 (0.4-0.6) | 7 | 0.4 (0.3-0.5) | 0.36 |
| Indirect bilirubin (mg/dL) | 9 | 3.0 (1.7-7.3) | 16 | 1.9 (1.2-3.3) | 12 | 1.8 (1.1-3.7) | 7 | 1.2 (0.9-1.3) | 0.008 |
| LDH (u/L) | 13 | 428 (354-509) | 18 | 282 (255-321) | 11 | 373 (249-427) | 14 | 330 (220-392) | 0.14 |
| AST (u/L) | 42 | 37 (27-64) | 40 | 37 (29-52) | 35 | 30 (23-44) | 36 | 35 (27-51) | 0.17 |
| ALT (u/L) | 42 | 23 (15-29) | 40 | 24 (16-24) | 35 | 18 (16-28) | 36 | 23 (14-35) | 0.64 |
Table 2.
Clinical Correlates of Opioid Dose Quartiles
| Correlate | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| History of AVN | 2.87 | 1.37-6.02 | 0.005 |
| 25-OHD Concentration | 0.59 | 0.38-0.93 | 0.024 |
| Total Bilirubin Concentration | 0.64 | 0.42-0.99 | 0.043 |
A stepwise ordinal logistic regression analysis was performed of quartiles of daily opioid. The covariates originally placed into the analysis were age, SS/Sbeta0 SCD genotype, history of AVN, 25-OHD levels, AST, and total bilirubin. History of AVN, 25-OHD levels, and total bilirubin remained in the regression model after covariates with P > 0.10 were eliminated from the model in a stepwise manner. The 25-OHD and total bilirubin concentrations were log transformed. N=145.
Common laboratory tests that may reflect hemolysis include indirect bilirubin, AST, LDH, reticulocyte percent and absolute reticulocyte count (12, 13). In this data set, the total bilirubin concentration appears to reflect hemolysis, as the total bilirubin concentration correlated more strongly with the indirect bilirubin fraction (n=44, r=0.99) than the direct bilirubin fraction (n=44, r=0.53) and with AST (n=152, r=0.51) than ALT (n=152, r=0.27) levels. Furthermore, total bilirubin also correlated significantly with LDH (n=49, r=0.37, p=0.009), reticulocyte percent (n=137, r=0.60, p<0.001), and the absolute reticulocyte count (n=137, r=0.56, p<0.001). In the ordinal logistic regression model, the indirect bilirubin concentration could replace the total bilirubin concentration even though the model included less participants (n=43, OR 0.37, 95% CI: 0.16-0.85, p=0.020) but the direct bilirubin concentration could not (n=43, OR 0.42, 95% CI: 0.13-1.33, p=0.14).
Discussion
Opioid-type medications are the main component of pain management regimens in SCD in the United States (6, 14), but the literature on the patterns of opioid use is limited (15-17), especially in the adult population. In this study, the opioid use was comprehensively evaluated based on the prescribing records in an adult sickle cell cohort. Our findings show that 47% of patients took short-acting opioids alone, whereas 27% took both short and long-acting opioids. Among the patients who were prescribed opioids, the median dose of daily opioids was relatively low at 6.1 mg OME. In addition to opioids, a fraction of patients also took NSAIDs routinely. Patients with the highest frequency of hospital admissions due to VOC received the highest daily opioid doses. A history of AVN, lower 25-OHD and lower total bilirubin were also independently associated with higher opioid requirements.
We found that the patients taking more oral pain medications tend to seek medical care more frequently as judged by the frequency of hospital admissions for VOC. A recognized complication of patients on chronically high doses of opioids is the development of opioid tolerance (18). This presents a challenge to healthcare providers to manage pain adequately, especially acute pain during VOC, since the dose requirement often exceeds treatment guidelines. In the outpatient setting, we found that 25% of SCD patients do not routinely use opioids (Figure 1) and 71% use less than 10 mg OME daily (Figure 1). The median dose was 6.1 mg (1.7-26.3 mg) OME daily among patients who were prescribed opioids. In comparison, a recent study showed that patients with chronic non-cancer pain (CNCP) consumed a median dose of 75 mg OME per day, and only 8.8% of them used less than 20 mg OME daily (19). The average opioid use among CNCP patients ranges from 20 to 180 mg OME daily (19-24) and among cancer patients ranges from 30-167 mg OME daily (25-29) (Table 3).
Table 3.
Opioid Use in Different Chronic Pain Conditions.
| Conditions | Daily Opioid Use (OME, mg) | N | Country or region |
References |
|---|---|---|---|---|
|
Sickle cell disease
(SCD) |
Median (IQR): 6.1 (1.7-26.3) | 203 | US | This study |
|
Chronic non-cancer
pain (CNCP) |
Mean (SD): 58 (79) | 11,989 | Germany | (24) |
| Median (IQR): 75 (36-145) | 1417 | Australia | (19) | |
| Median (IQR): 180 (60-501) | 145 | Canada | (22) | |
| Median (Range): 20.3 (5-72.1) | 60 | US | (21) | |
| Mean (SD): 118 (149) | 466 | US | (23) | |
| Cancer pain | Median: 45 | 91 | US | (25) |
| Median (IQR): 120 (45-270) | 198 | US | (26) | |
| Mean (SD): 167 (170) | 123 | Egypt | (28) | |
| Median (Range): 30 (20-60) | 371 | Portugal | (29) |
A systematic literature search on all articles published from January 2012 to October 2015 was performed in November 2015 using Pubmed. The keyword “morphine equivalent” was used.
The result was derived from the total consumption of opioids.
The results presented in this study are based on the prescribing records of adult SCD patients in a comprehensive sickle cell care center at UIH. The majority of the pain medications used by the enrolled patients were prescribed by the physicians in the center, but some patients obtain pain medication prescriptions upon hospital/ER discharge or from other institutions. In a quality improvement study, the UIH opioid medication prescription records were compared to the dispensing records of the Illinois Prescription Monitoring Program (ILPMP), which is a reliable resource to track the use of controlled substances in Illinois and neighboring states including Indiana and Wisconsin. Our prescription records had a significant correlation (r over 0.9) with the dispensing records of ILPMP. When the daily opioid doses were compared between the dispensing records from ILPMP and the UIH prescription records, the UIH prescription records under-estimated opioid use by approximately 40%. This needs to be taken into consideration when interpreting the results of this study.
Several clinical correlates of high opioid use were identified in this study. AVN is a relatively prevalent complication among the SCD patients (30), resulting in severe chronic pain and disability, and it negatively affects health-related quality of life (31). Hemolysis and vaso-occlusion are two important aspects of the pathogenesis of SCD (32). A vaso-occlusive mechanism is believed to be linked to AVN and pain crises, which is consistent with our finding, whereas hemolysis is associated with complications such as pulmonary hypertension, stroke, and priapism, and is less related to vaso-occlusive pain crisis (32). In keeping with this perspective, the total bilirubin concentration, a marker for hemolysis, had a negative association with opioid use. Indirect bilirubin concentration was only available in a limited number of patients, and it showed a similar negative relationship with opioid use in the univariate analysis (Table 1) and the multivariate analysis. The other significant correlate, lower 25-OHD levels, has been linked to the frequency of vaso-occlusive crises in previous reports (33, 34). The use of hydroxyurea was found to correlate positively with high dose of opioid use in this cohort (Table 1). However, hydroxyurea is only indicated for patients with more VOC admissions, which may confound the findings. Therefore, hydroxyurea use is not included in the multivariate analysis (Table 2).
The prevalence of Hb SC genotype in this cohort is slightly lower than the reported estimates in the U.S. (3). Potential causes include that Hb SC patients may not seek medical care as frequently as Hb SS/Sbeta0 patients, and may be under-represented in this cohort. We did not observe the more severe sickling type of SCD (Hb SS or Sβ0-thalassemia) to be associated with high dose of opioids (Table 1). The prevalence of AVN, the identified opioid use correlate, is comparable between Hb SS/Sβ0 and SC patients in our cohort and in the published literature (35, 36). In addition, vitamin D deficiency, another opioid use correlate, is not associated with the SCD genotype (37). This may explain the similar usage of opioids between the Hb SS/Sβ0 and SC patients. Older patients seemingly utilized more opioids when the quartiles 3 and 4 were compared to the quartiles 1 and 2 (Table 1), although age was not a factor in the multivariate analysis (Table 2). The longer life expectancy in the Hb SC patients (38) may also contribute to the similar usage of opioids despite milder disease.
This study showed that the prevalence of NSAID use such as ibuprofen and naproxen in the SCD patients was as high as 38%, even without considering medications purchased over the counter without a prescription, which is higher than the prevalence of regular NSAID users in the general U.S. population of 12% (39). NSAID use in SCD is of concern because of the potential to cause or exacerbate renal complications (40-43). Acetaminophen-hydrocodone (55%) and acetaminophen-codeine (29%) were among the most commonly used short-acting pain medications in this patient population. The combination of acetaminophen with opioids provides the benefit of dual analgesic effect, but also presents potential adverse effects such as liver-toxicity requiring regular monitoring of liver function (44). The doses of acetaminophen prescribed at UIH do not exceed the 4 gram daily maximum dose (45).
Our study is not without limitations. The results are based on analysis of prescribing records, which may under-estimate the pain medication use, especially medications also available over the counter. We focused on patients who continued following up with the sickle cell center 12 months after enrollment in a registry, which may exclude some patients with mild disease. Additionally, patients with aggressive disease may seek medical care mainly through frequent inpatient admissions rather than outpatient office visits. Despite these potential limitations, our report showed similar percentage of long-acting and short-acting opioid use in sickle cell patients as a previous report based on pain diaries (17). We did not collect information on priapism, leg ulcers, length of hospital visits, or number of ED visits in this study, which should be addressed in further studies.
Supplementary Material
Acknowledgements
Dr. Santosh Saraf is supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under Award Number K23HL125984. Dr. Robert Molokie is supported by grant number 1R01HL078536 from the National Institutes of Health, National Heart Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or the Department of Veterans Affairs.
References
- 1.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–30. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 2.Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. 2008;86:480–7. doi: 10.2471/BLT.06.036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38:S512–21. doi: 10.1016/j.amepre.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Lanzkron S, Carroll CP, Haywood C., Jr. The burden of emergency department use for sickle-cell disease: an analysis of the national emergency department sample database. Am J Hematol. 2010;85:797–9. doi: 10.1002/ajh.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84:323–7. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 6.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–56. doi: 10.1182/blood-2012-04-383430. [DOI] [PubMed] [Google Scholar]
- 7.Anie KA, Grocott H, White L, Dzingina M, Rogers G, Cho G. Patient self-assessment of hospital pain, mood and health-related quality of life in adults with sickle cell disease. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon LR. Pain management in adults with sickle cell disease in a medical center emergency department. J Natl Med Assoc. 2010;102:1025–32. doi: 10.1016/s0027-9684(15)30729-x. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe P, Martinovich Z, Buckley B, Schmelzer A, Paice JA. Safety of an ED High-Dose Opioid Protocol for Sickle Cell Disease Pain. J Emerg Nurs. 2015;41:227–35. doi: 10.1016/j.jen.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25. doi: 10.1002/ajh.20336. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. 2014. [DOI] [PubMed]
- 12.Minniti CP, Sable C, Campbell A, Rana S, Ensing G, Dham N, Onyekwere O, Nouraie M, Kato GJ, Gladwin MT, Castro OL, Gordeuk VR. Elevated tricuspid regurgitant jet velocity in children and adolescents with sickle cell disease: association with hemolysis and hemoglobin oxygen desaturation. Haematologica. 2009;94:340–7. doi: 10.3324/haematol.13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barcellini W, Fattizzo B. Clinical Applications of Hemolytic Markers in the Differential Diagnosis and Management of Hemolytic Anemia. Dis Markers. 2015;2015:635670. doi: 10.1155/2015/635670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stuart MJ, Nagel RL. Sickle-cell disease. Lancet. 2004;364:1343–60. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 15.Yoon SL, Black S. Comprehensive, integrative management of pain for patients with sickle-cell disease. J Altern Complement Med. 2006;12:995–1001. doi: 10.1089/acm.2006.12.995. [DOI] [PubMed] [Google Scholar]
- 16.Dampier C, Ely E, Brodecki D, O'Neal P. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002;24:643–7. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Smith WR, McClish DK, Dahman BA, Levenson JL, Aisiku IP, de ACV, Bovbjerg VE, Roberts JD, Penberthy LT, Roseff SD. Daily home opioid use in adults with sickle cell disease: The PiSCES project. J Opioid Manag. 2015;11:243–53. doi: 10.5055/jom.2015.0273. [DOI] [PubMed] [Google Scholar]
- 18.Dumas EO, Pollack GM. Opioid tolerance development: a pharmacokinetic/pharmacodynamic perspective. AAPS J. 2008;10:537–51. doi: 10.1208/s12248-008-9056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell G, Nielsen S, Larance B, Bruno R, Mattick R, Hall W, Lintzeris N, Cohen M, Smith K, Degenhardt L. Pharmaceutical Opioid Use and Dependence among People Living with Chronic Pain: Associations Observed within the Pain and Opioids in Treatment (POINT) Cohort. Pain Med. 2015 doi: 10.1111/pme.12773. [DOI] [PubMed] [Google Scholar]
- 20.Chang SC, Ma CC, Lee CT, Hsieh SW. Pharmacoepidemiology of chronic noncancer pain patients requiring chronic opioid therapy: A nationwide population-based study. Acta Anaesthesiol Taiwan. 2015 doi: 10.1016/j.aat.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Morasco BJ, Cavanagh R, Gritzner S, Dobscha SK. Care management practices for chronic pain in veterans prescribed high doses of opioid medications. Fam Pract. 2013;30:671–8. doi: 10.1093/fampra/cmt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Busse JW, Mahmood H, Maqbool B, Maqbool A, Zahran A, Alwosaibai A, Alshaqaq E, Persaud N, Cooper L, Carol A, Sumpton J, McGinnis E, Rosenbaum D, Lidster N, Buckley DN. Characteristics of patients receiving long-term opioid therapy for chronic noncancer pain: a cross-sectional survey of patients attending the Pain Management Centre at Hamilton General Hospital, Hamilton, Ontario. CMAJ Open. 2015;3:E324–30. doi: 10.9778/cmajo.20140126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham JL, Hayes SE, Townsend CO, Laures HJ, Hooten WM. Associations between spousal or significant other solicitous responses and opioid dose in patients with chronic pain. Pain medicine (Malden, Mass ) 2012;13:1034–9. doi: 10.1111/j.1526-4637.2012.01434.x. [DOI] [PubMed] [Google Scholar]
- 24.Marschall U, L'Hoest H, Radbruch L, Hauser W. Long-term opioid therapy for chronic non-cancer pain in Germany. Eur J Pain. 2015 doi: 10.1002/ejp.802. [DOI] [PubMed] [Google Scholar]
- 25.Arthur J, Yennurajalingam S, Nguyen L, Tanco K, Chisholm G, Hui D, Bruera E. The routine use of the Edmonton Classification System for Cancer Pain in an outpatient supportive care center. Palliat Support Care: 2014:1–8. doi: 10.1017/S1478951514001205. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen LM, Rhondali W, De la Cruz M, Hui D, Palmer L, Kang DH, Parsons HA, Bruera E. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage. 2013;45:506–16. doi: 10.1016/j.jpainsymman.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vigano A, Bruera E, Suarez-Almazor ME. Age, pain intensity, and opioid dose in patients with advanced cancer. Cancer. 1998;83:1244–50. [PubMed] [Google Scholar]
- 28.Alsirafy SA, Galal KM, Abou-Elela EN, Ibrahim NY, Farag DE, Hammad AM. The use of opioids at the end-of-life and the survival of Egyptian palliative care patients with advanced cancer. Ann Palliat Med. 2013;2:173–7. doi: 10.3978/j.issn.2224-5820.2013.10.02. [DOI] [PubMed] [Google Scholar]
- 29.Pina P, Sabri E, Lawlor PG. Characteristics and associations of pain intensity in patients referred to a specialist cancer pain clinic. Pain Res Manag. 2015;20:249–54. doi: 10.1155/2015/807432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, Bellevue R, Koshy M, Moohr JW, Smith J. Sickle cell disease as a cause of osteonecrosis of the femoral head. N Engl J Med. 1991;325:1476–81. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- 31.Dampier C, Lieff S, LeBeau P, Rhee S, McMurray M, Rogers Z, Smith-Whitley K, Wang W. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55:485–94. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osunkwo I, Ziegler TR, Alvarez J, McCracken C, Cherry K, Osunkwo CE, Ofori-Acquah SF, Ghosh S, Ogunbobode A, Rhodes J, Eckman JR, Dampier C, Tangpricha V. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. Br J Haematol. 2012;159:211–5. doi: 10.1111/bjh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osunkwo I, Hodgman EI, Cherry K, Dampier C, Eckman J, Ziegler TR, Ofori-Acquah S, Tangpricha V. Vitamin D deficiency and chronic pain in sickle cell disease. Br J Haematol. 2011;153:538–40. doi: 10.1111/j.1365-2141.2010.08458.x. [DOI] [PubMed] [Google Scholar]
- 35.Mukisi-Mukaza M, Saint Martin C, Etienne-Julan M, Donkerwolcke M, Burny ME, Burny F. Risk factors and impact of orthopaedic monitoring on the outcome of avascular necrosis of the femoral head in adults with sickle cell disease: 215 patients case study with control group. Orthop Traumatol Surg Res. 2011;97:814–20. doi: 10.1016/j.otsr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Akinyoola AL, Adediran IA, Asaleye CM. Avascular necrosis of the femoral head in sickle cell disease in Nigeria: a retrospective study. Niger Postgrad Med J. 2007;14:217–20. [PubMed] [Google Scholar]
- 37.Goodman BM, 3rd, Artz N, Radford B, Chen IA. Prevalence of vitamin D deficiency in adults with sickle cell disease. J Natl Med Assoc. 2010;102:332–5. doi: 10.1016/s0027-9684(15)30605-2. [DOI] [PubMed] [Google Scholar]
- 38.Elmariah H, Garrett ME, De Castro LM, Jonassaint JC, Ataga KI, Eckman JR, Ashley-Koch AE, Telen MJ. Factors associated with survival in a contemporary adult sickle cell disease cohort. Am J Hematol. 2014;89:530–5. doi: 10.1002/ajh.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23:43–50. doi: 10.1002/pds.3463. [DOI] [PubMed] [Google Scholar]
- 40.Nath KA, Hebbel RP. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol. 2015;11:161–71. doi: 10.1038/nrneph.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, Klug PP. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330:1639–44. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- 42.Hamideh D, Alvarez O. Sickle cell disease related mortality in the United States (1999-2009) Pediatr Blood Cancer. 2013;60:1482–6. doi: 10.1002/pbc.24557. [DOI] [PubMed] [Google Scholar]
- 43.Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol. 2014;70:1159–72. doi: 10.1007/s00228-014-1734-6. [DOI] [PubMed] [Google Scholar]
- 44.Kaye AM, Kaye AD, Lofton EC. Basic concepts in opioid prescribing and current concepts of opioid-mediated effects on driving. Ochsner J. 2013;13:525–32. [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Department of Health and Human Services FaDA. Center for Drug Evaluation and Research (CDER) Organ-Specific Warnings: Internal Analgesic, Antipyretic, and Antirheumatic Drug Products for Over-the -Counter Human Use — Labeling for Products That Contain Acetaminophen Guidance for Industry. 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


