Abstract
Background
Hydroxyurea (HU) induces dose-dependent increased fetal hemoglobin (HbF) for sickle cell disease (SCD). Large deviation from historical personal best (PBest) HbF, a clinic-based version of maximum dose, may identify a subset with suboptimal HU adherence over time.
Procedure
Retrospective clinical data from youth ages 10–18 years prescribed HU at two centers were extracted from medical records at three time points: pre-HU initiation, PBest and a recent assessment. Decrease from PBest HbF of 20% or more at recent assessment despite stable dosing was designated as high deviation from PBest. Acute hospital use was compared between 1-year periods, pre-HU and ±6 months for PBest and recent assessment. Groups were compared using descriptive and bivariate nonparametric statistics.
Results
Seventy-five youth, mean HU duration 5.9 years, met eligibility criteria. Mean ages of HU initiation, PBest and recent assessment were 8.0, 10.9 and 13.9 years, respectively. Despite stable dosing, average HbF of 19.5% at PBest overall declined by 31.8% at recent assessment. PBest HbF declined by 11.7 and 40.1% in two groups, the latter comprised 70.7% of the sample, had lower pre-HU and recent HbF and higher dosing. They experienced more urgent hospital use during the year framing recent assessment than during PBest; these findings were supported by sensitivity analysis.
Conclusions
Decline from PBest HbF is a novel approach to assess HU effectiveness, is common among youth and may represent suboptimal adherence. Larger prospective studies using additional adherence measures are needed to confirm our approach of tracking HbF deviation over time and to define an appropriate cutoff.
Keywords: fetal hemoglobin, hydroxyurea, medication adherence, sickle cell disease
1 INTRODUCTION
Induction of fetal hemoglobin (HbF) is a major therapeutic effect of hydroxyurea (HU) for sickle cell disease (SCD)1,2 and is highly dose-dependent.1,3 Through clinical trials, stable maximum tolerated dose (MTD) been defined by standard criteria using threshold levels of cytopenias for neutrophils and other blood cell lineages.2,4,5 Long-term trials of children taking HU were launched by HUG-KIDS and other trials.3,5 Under stable dosing,6 steady HU-induced HbF was demonstrated for up to 12 years of use7,8 including through adolescence.3
Poor medication adherence commonly occurs in chronic disease management across several common chronic conditions, and is generally defined as below 80% of daily medication use.9 Among children with chronic medical conditions, adolescents have especially high rates of poor adherence,10–15 especially those from underresourced communities.16 For SCD, analysis of two statewide Medicaid databases for HU pharmacy refill revealed frequent poor adherence to daily HU across broad user ages, and was associated with tempered clinical benefit and increased healthcare costs.17,18 Poor pediatric HU adherence has also been reported using parent self-report and pharmacy possession.19–22
Standardized biomarkers such as HbA1C in diabetes, drug levels or other objective measures typically provide reliable measures of treatment adherence compared with patient self-report.10,11,19,23,24 Biomarkers permit comparisons across patients and populations and avoid logistical barriers to obtaining drug refill data outside of studies. In contrast, an absolute biomarker for the use of HU does not exist. The short half-life of HU precludes steady state drug monitoring.25 Other hematologic markers also are highly impacted by HU but tend to be highly dynamic.3 For example, HU-enhanced mean corpuscular volume (MCV)2 is also affected by the rate of hemolysis and reticulocyte count,1,3 while absolute neutrophil count (ANC) often fluctuates.25 Absolute increment of HbF induction varies by pharmacokinetic and genetic factors,1,26,27 limiting its use as a biomarker. In contrast, dose-sensitive HbF induction requires consistent HU use and is itself a drug target [reviewed in reference (2)], underscoring its potential as a biomarker to assess adherence.
As no uniform level of HbF induction exists, we hypothesized that the deviation from a child’s historical “personal best (PBest),” the HbF level at stable maximum weight-based dose, could serve as an individualized marker for HU adherence. PBest may differ from HbF at MTD, as outside of clinical trials some patients may never reliably take daily HU and not reach target physiologic MTD.2,4 Using a clinic-based sample from two independent SCD centers in New York City, this retrospective analysis was undertaken with the following objectives: (i) assess deviation from PBest HbF over time in youth prescribed HU in clinic settings; and (ii) to examine the relationship between PBest HbF and urgent hospital use. We focused on youth between 10 and 18 years because disease control for youth with chronic conditions often declines during adolescence.28 In this analysis, patients may potentially have been less adherent if the decrease in HbF from PBest was substantially below the range of biologic alterations in HU response seen in adherent children assessed in clinical trials over time.17,19,20 A specific cutoff for deviation of PBest was tested as a preliminary step in determining the utility of this approach.
2 METHODS
This study was conducted under protocol approval by the institutional review boards at both participating institutions. Waiver of consent was obtained for complete case ascertainment for this retrospective analysis. Patient data were obtained from electronic health records.
Collaboration between the two sites was based on comparable clinical indications for HU (nearly all for recurrent pain crisis and/or acute chest syndrome) and similarities in age ranges and ethnic backgrounds of the patient populations. Nearly all of the data were collected prior to 2015, with most pre-HU data from 2011 or earlier. HU dosing was based on strategies for treatment and cytopenia toxicities modeled from pediatric clinical trials for an ANC target of 2000–4000.6,29 Both sites also followed toxicity targets for hematologic toxicity of platelet, hemoglobin (Hb) and reticulocyte sufficiency, adjusting for dose by body weight, per pediatric clinical standards for HU therapy over the past several years.2,4,6,7 HbF values were routinely collected during well clinic visits two to four times annually. Renal and hepatic serum chemistries were assessed at least twice yearly. Abnormal tests resulted in holding the HU dose as per standard protocol.16 Data were collected for current and recently treated patients, most of whom received care within the previous 3 years and whose recent assessment was in 2014. Demographic data were limited to sex, race/ethnicity, age and duration of HU therapy.
Eligible youth had HbSS or HbS-Beta0 thalassemia and were regularly prescribed HU. Laboratory data were analyzed at three time points: immediately prior to HU initiation, PBest and a recent assessment. A child’s PBest was defined as the highest HbF value while on HU at a stable maximum dose per weight (mg/kg/day). Recent assessment was defined as the most recent HbF determination, accompanied by six additional months for clinical data collection (described below). All laboratory values were collected during a clinically well period of time and at least 3 months after a transfusion. For this study, HbF values were reviewed over the years while on HU therapy. HbF levels within 3 months of each time point were reviewed to avoid selection of outlier values. HU dose, HbF by percent of total Hb (%), MCV (excluding patients with HbS-Beta0 thalassemia), Hb, absolute reticulocyte count (ARC), white blood count (WBC) and ANC were also recorded at each time point.
Urgent hospital use was defined by the number of urgent care visits [emergency room (ER) and admissions] and total length of hospital stay (LOS) for each patient during three 1-year periods: the year prior to HU initiation and ±6 months spanning the PBest and recent assessment (depicted in Fig. 1). Urgent hospital use was validated through review of hospital records to ensure that visits were SCD-related encounters and to exclude nonurgent visits such as prescheduled surgical procedures.2 To assess the clinical impact of changing HbF, study inclusion required minimum gaps in time between the three data collection points to avoid overlap. PBest HbF was at least 6 months following HU initiation. Avoiding overlap between time periods of PBest and recent assessment required a minimum of 12 months separation. We chose to include additional 3 months or more for a total time gap of at least 15 months.
FIGURE 1.
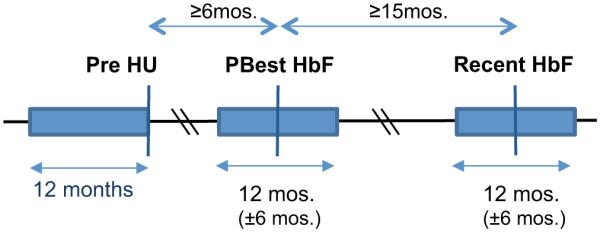
Scheme for clinical data collection
As a first step in assessing an appropriate metric for estimating adherence using deviation from PBest HbF, we defined high deviation as 20% or more deviation from PBest at the recent clinical assessment: [(PB-recent)/PB ×100]. This proportion was selected, as it represents the converse of the standard minimum of 80% used to define medication adherence.17,20–22,24,28 Moreover, this cutoff in deviation of HbF was generally larger than that seen in cohorts of HU users who were deemed adherent within long-term clinical trials.24
Data were analyzed using descriptive statistics stratified by site and deviation (high or low) from PBest. Groups were compared using chi-square and Wilcoxon rank-sum tests. For change in urgent hospital use and HU dose, paired data were analyzed using the Wilcoxon signed-rank test. Data were analyzed using SAS 9.3 statistical software (Cary, NC). A sensitivity analysis30 was performed around the definition of 20% deviation from PBest using cutoffs of 25 and 15% to test the robustness of study findings. Using these alternate thresholds, we assessed the proportion of subjects meeting the criterion for high PBest deviation, whether there were differences by gender or site, and the relationship between deviation and resource use.
3 RESULTS
3.1 Sample characteristics
A total of 109 patients of the two-site sample met eligibility criteria of sickle type, age and HU use. Of these, 75 (37 from site 1 and 38 from site 2) met the additional requirement of an at least 15-month gap between PBest and recent assessment. All patients were at least 3 years of age at HU initiation and all but 6 were 4 years of age or older. Three patients had HbSB0 thalassemia. Clinical data were unavailable for four patients at the time of PBest and for an additional 24 for the year pre-HU. Missing data were due to transfer from another hospital, switch from transfusion therapy to HU or incomplete electronic records. Periodic renal and hepatic blood testing indicated stable function during the study period. Ethnicity differed by site, with more Latino/multirace patients at site 1 (57.9 vs. 32.4%, P = 0.03) (Table 1).
TABLE 1.
Characteristics of the sample on HU, stratified by site and by deviation from PBest
| Stratification by site | Total (N = 75) |
Site 1 (N = 38) |
Site 2 (N = 37) |
P value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Race/ethnicity (%): Hispanic/multirace | 34 | 45.3 | 22 | 57.9 | 12 | 32.4 | 0.03 |
|
| |||||||
| Non-African American (Hispanic/multirace) | 35 | 46.1 | 57.9 | 13 | 34.2 | 0.04 | |
|
| |||||||
| Gender: female (%) | 34 | 45.3 | 19 | 50.0 | 15 | 40.5 | 0.41 |
|
| |||||||
| Suboptimally adherent (>20% deviation from PBest HbF) | 53 | 70.7 | 25 | 65.8 | 28 | 75.7 | 0.35 |
|
| |||||||
| Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||
| Current age (years) | 14.0 | 2.7 | 13.9 | 3.1 | 14.2 | 2.3 | 0.54 |
|
| |||||||
| Age at HU initiation (years) | 8.0 | 3.4 | 7.7 | 4.1 | 8.5 | 2.3 | 0.10 |
|
| |||||||
| Age at PBest (years) | 10.9 | 3.0 | 10.6 | 3.5 | 11.2 | 2.3 | 0.25 |
|
| |||||||
| HU duration (years) | 5.9 | 3.1 | 6.2 | 3.4 | 5.6 | 2.8 | 0.47 |
|
| |||||||
| Time to PBest (years) (N = 65) | 2.8* | 2.5 | 3.0 | 2.8 | 2.7 | 2.1 | 0.81 |
|
| |||||||
| Time from PBest to recent (years) | 3.1 | 1.6 | 3.6 | 1.5 | 2.9 | 1.7 | 0.15 |
|
| |||||||
| HU dose at PBest (mg/kg/day) | 24.4 | 4.1 | 23.3 | 4.3 | 25.8 | 3.5 | 0.02 |
|
| |||||||
| Recent HU dose (mg/kg/day) | 24.3 | 4.2 | 22.8 | 4.8 | 25.7 | 2.7 | 0.004 |
|
| |||||||
| Stratification by deviation from PBest HbF | Low deviation (N = 22) | High deviation (N = 53) | P value | ||||
| N | Mean | SD | N | Mean | SD | ||
|
| |||||||
| Race/ethnicity: Hispanic/multirace | 11 | 50.0 | 23 | 43.4 | 0.60 | ||
|
| |||||||
| Gender: female (%) | 9 | 40.9 | 25 | 47.2 | 0.62 | ||
|
| |||||||
| Recent age (years) | 22 | 14.5 | 2.7 | 53 | 13.8 | 2.8 | 0.42 |
|
| |||||||
| Age at HU initiation (years) | 22 | 8.8 | 3.7 | 49 | 7.7 | 3.2 | 0.11 |
|
| |||||||
| Age at PBest (years) | 22 | 12.0 | 3.0 | 53 | 10.5 | 2.9 | 0.06 |
|
| |||||||
| HU duration (years) | 22 | 5.7 | 3.5 | 49 | 3.0 | 1.0 | 0.27 |
|
| |||||||
| Time from PBest to recent (years) | 22 | 2.5 | 1.0 | 53 | 3.3 | 1.7 | 0.01 |
|
| |||||||
| HU dose at PBest† | 22 | 22.9 | 3.9 | 51 | 25.1 | 4.1 | 0.02 |
|
| |||||||
| Recent HU dose (mg/kg/day)† | 22 | 22.8 | 3.4 | 52 | 24.9 | 4.4 | 0.03 |
Median 1.7 years.
By paired analysis: no differences in dose between two time points for either adherence group or the total sample. Bold font for P values: where significant as P < 0.05.
3.2 Analysis of HU use
Average age of HU initiation, PBest and recent assessment of the sample were 8.0, 10.9 and 14.0 years, respectively, with no differences by site (Table 1). Average duration of HU therapy was 5.9 years. Time from HU initiation to PBest averaged 2.8 years (median 1.7 years). HbF% from baseline to PBest averaged an increase of 11.7% (Table 2) and is comparable to changes reported elsewhere.1,4,7,26 Mean HU dosing at PBest was lower at site 1 (23.3 vs. 25.8 mg/kg/day, P = 0.02) and at recent assessment (22.8 vs. 25.7 mg/kg/day, P = 0.004) (Table 2). To account for possible changes to HU dosing in clinical practice based on key clinical trials,1,3,29 mean HU doses before and since 2012 were compared and found to be unchanged.
TABLE 2.
Laboratory values for pre-HU, PBest and recent assessment by deviation from PBest
| Low deviation from PBest (N = 22) |
High deviation from PBest (N = 53) |
P value | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Pre HU | |||||||
|
| |||||||
| HbF (%) | 19 | 10.7 | 5.7 | 41 | 6.8 | 4.8 | 0.009 |
|
| |||||||
| Hb (gm/dl) | 18 | 8.0 | 1.0 | 39 | 8.0 | 1.0 | 0.92 |
|
| |||||||
| MCV (fl)* | 19 | 83.5 | 6.2 | 44 | 83.9 | 6.7 | 0.77 |
|
| |||||||
| ARC (×109 |−1) | 19 | 266 | 85 | 39 | 352 | 137 | 0.02 |
|
| |||||||
| WBC (×109 |−1) | 18 | 12.5 | 2.4 | 39 | 15.5 | 5.5 | 0.03 |
|
| |||||||
| ANC (×109 |−1) | 18 | 5.4 | 2.2 | 39 | 6.5 | 4.7 | 0.81 |
|
| |||||||
| PBest | |||||||
|
| |||||||
| HbF (%) | 22 | 21.9 | 5.1 | 53 | 18.5 | 6.9 | 0.07 |
|
| |||||||
| HbF increase: pre-HU to PBest | 20 | 11.4 | 5.7 | 43 | 11.8 | 5.8 | 0.94 |
|
| |||||||
| Hb (gm/dl) | 22 | 9.6 | 1.4 | 53 | 9.0 | 1.2 | 0.26 |
|
| |||||||
| MCV(fl)* | 21 | 99.4 | 10.3 | 51 | 98.9 | 10.5 | 0.81 |
|
| |||||||
| ARC (×109 |−1) | 22 | 162 | 96 | 53 | 172 | 86 | 0.52 |
|
| |||||||
| WBC (×109 |−1) | 22 | 7.6 | 2.4 | 53 | 9.5 | 4.4 | 0.04 |
|
| |||||||
| ANC (×109 |−1) | 22 | 3.7 | 1.6 | 52 | 4.4 | 3.5 | 0.72 |
|
| |||||||
| Recent assessment | |||||||
|
| |||||||
| HbF (%) | 21 | 19.1 | 4.5 | 51 | 11.4 | 5.5 | <0.001 |
|
| |||||||
| HbF deviation from PBest (%) | 21 | 11.7 | 5.3 | 51 | 40.1 | 17.5 | <0.001 |
|
| |||||||
| Hb (gm/dl) | 21 | 9.5 | 1.2 | 51 | 8.6 | 1.2 | 0.005 |
|
| |||||||
| MCV(fl)* | 21 | 99.6 | 11.6 | 51 | 95.0 | 12.2 | 0.12 |
|
| |||||||
| ARC (×109 |−1) | 22 | 194 | 76 | 51 | 276 | 134 | 0.02 |
|
| |||||||
| WBC (×109 |−1) | 21 | 7.7 | 2.0 | 51 | 10.5 | 3.7 | 0.002 |
|
| |||||||
| ANC (×109 |−1) | 21 | 3.6 | 1.4 | 51 | 6.8 | 13.4 | 0.02 |
Excludes three patients with HbS-Beta0 thalassemia.
Bold font for P values: where significant as P < 0.05.
3.3 Analysis by high or low deviation from PBest
At recent assessment, overall average deviation from PBest was 31.8%. Only 29.3% of the sample remained within 20% of their PBest HbF. The proportion of patients with high deviation was similar at each site (64.9% at site 1 and 73.7% at site 2). PBest deviation did not differ by gender, race/ethnicity or age (Table 2). Other similar features between the two groups were age of HU initiation, duration of therapy, time to reach PBest (Table 1) and the increment of HU-induced HbF (11.4 vs. 11.8, P = 0.94) (Table 2). PBest HbF was somewhat higher in the low-deviation group (21.9 vs. 18.5, P = 0.07). Paradoxically, HU doses at PBest and recent assessment were higher in the group with more deviation. By paired analysis for each patient, HU doses between the two time points did not significantly differ for the entire sample or within each deviation group (data not shown).
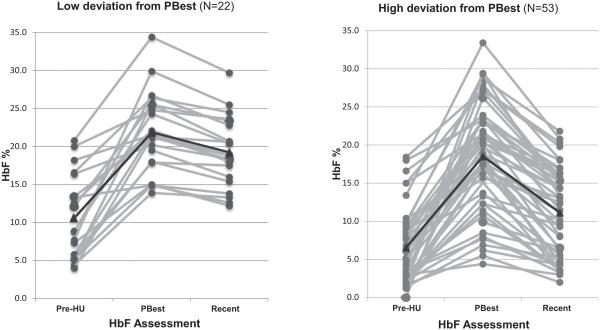
Deviation from PBest HbF varied widely between the two groups, both by percentage and absolute decrease (Table 2 and Fig. 2). The average deviation from PBest HbF was 3.4-fold lower in the low-deviation group: 11.7% compared with 40.1% in the group with high deviation (P < 0.001). Of the three HbF levels assessed, pre-HU HbF was significantly higher in the low-deviation group (10.7 vs. 6.8%, P = 0.004). HbF was also higher in this group at the recent assessment (19.1 vs. 11.4%, P < 0.001) (Table 2). At recent assessment, concordant differences between deviation groups were seen in other HU-induced laboratory effects: Hb, ARC, WBC and ANC. Notably, deviation from PBest in the entire sample was inversely correlated with MCV at recent assessment, with higher deviation from PBest associated with lower MCV (r = –0.44, P < 0.001).
FIGURE 2.
HbF levels at three time points by deviation from PBest (low or high). The black line indicates the average value at each time point
3.4 Urgent hospital use
Urgent care visits (ER visits plus hospital admissions) and total LOS varied widely by subject (Table 3). Hospital data during PBest and recent assessment represented nearly the complete sample (N = 70 or 71 of 75 patients). As a group, patients with low deviation from PBest had longer mean LOS during the year of PBest (7.4 vs. 1.9 days, P = 0.03) (Table 3) and higher median LOS (not shown), and a lower proportion of patients with zero hospitalizations (45.4 vs. 67.4%; data not shown). Despite this baseline difference in severity, during the year of recent assessment, no differences in hospital use by group were seen, and a higher proportion of patients with low deviation lacked hospitalizations (68.2 vs. 49.0%).
TABLE 3.
Urgent hospital use (ER visits and hospitalizations) over 1-year periods stratified by deviation from PBest
| Low deviation from PBest (N = 22) |
High deviation from PBest (N = 48) |
P value | |||||
|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | ||
| Urgent hospital use: pre-HU | |||||||
|
| |||||||
| ER + hospitalizations | 13 | 2.6 | 3.0 | 28 | 3.2 | 2.8 | 0.79 |
|
| |||||||
| Total LOS | 13 | 5.9 | 9.2 | 28 | 7.5 | 7.3 | 0.11 |
|
| |||||||
| Urgent hospital use: year of PBest (YrPB) | |||||||
|
| |||||||
| ER + hospitalizations | 22 | 2.3 | 2.2 | 48 | 1.4 | 1.5 | 0.08 |
|
| |||||||
| Total LOS | 22 | 7.4 | 10.5 | 49 | 1.9 | 3.9 | 0.03 |
|
| |||||||
| Urgent hospital use: recent year (YrRecent ) | |||||||
|
| |||||||
| ER + hospitalizations | 22 | 2.2 | 4.5 | 48 | 2.5 | 3.1 | 0.81 |
|
| |||||||
| Total LOS | 22 | 6.8 | 17.6 | 49 | 7.1 | 11.3 | 0.28 |
|
| |||||||
| Change in urgent hospital use: pre-HU − YrPB | |||||||
|
| |||||||
| ER + hospitalizations | 13 | −0.2 | 1.9 | 28 | −1.5 | 2.2 | Low deviation P = 0.36; high deviation P<0.001 |
|
| |||||||
| Total LOS | 13 | 1.9 | 7.7 | 28 | −5.4 | 6.7 | Low deviation P = 0.88; high deviation P<0.001 |
|
| |||||||
| Change in urgent hospital use: YrPB − YrRecent | |||||||
|
| |||||||
| ER + hospitalizations | 22 | −0.05 | 3.8 | 48 | 1.2 | 2.8 | Low deviation P = 0.52; high deviation P=0.01 |
|
| |||||||
| Tot a l LOS | 22 | −0.6 | 13.5 | 49 | 5.2 | 10.4 | Low deviation P = 0.48; high deviation P=0.001 |
Bold font for P values: where significant as p < 0.05.
Most importantly, paired data for individual patients showed differences in urgent resource use by deviation group between the time periods from PBest to current assessment. For youth with low deviation, urgent hospital use was unchanged. In contrast, urgent hospital encounters and LOS increased significantly for youth with high deviation at recent assessment (P = 0.01 and 0.001, respectively). Paired differences in urgent use at each time point for each patient are depicted in the Supplementary Figure S1.
In the smaller data set comparing urgent hospital use between pre-HU and PBest (N = 41), paired data for the youth with high deviation demonstrated less urgent use (N = 28; P = 0.001). Average urgent use for the small number of youth with low deviation (N = 13) was unchanged. Overall, deviation from PBest HbF modestly correlated (r = 0.24, P = 0.04) with change in urgent hospital use.
3.5 Sensitivity analysis
When alternative thresholds for deviation from PBest were employed, 56% (deviation >25%) and 78.7% (deviation >15%) of youth in our sample met criteria for high deviation. In both cases, there were no differences in deviation group by site or gender (data not shown). Supplementary Table S1 examines urgent hospital use and its change from pre-HU use to PBest and from PBest to recent assessment across the range of deviation cut points. For youth with low deviation, no significant changes were found in urgent hospital visits or total LOS from pre-HU to year of PBest or from year of PBest to recent assessment. Compared with the year prior to HU initiation, youth with high deviation during the year of PBest had fewer urgent visits (−1.4 ± 2.1, −1.5 ± 2.2, both Ps < 0.01) and LOS (−4.8 ± 6.6, −5.0 ± 6.6, P < 0.01 for both analyses) using the cutoff of 15 and 25%, respectively. Compared with the year of PBest, youth with high deviation had greater urgent visits (1.1 ± 2.7, 1.3 ± 3.1, both Ps < 0.01) and LOS (5.0 ± 10.0, 5.5 ± 10.7, both Ps < 0.01) across cutoff values.
4 CONCLUSION
This retrospective clinic-based analysis from two academic sites in New York City examines changes in induced HbF over time as a novel approach to assess medication effectiveness under conditions of standard clinical care. We focused on the deviation from maximum HUinduced HbF to a recent assessment to examine the utility of PBest as a potential biomarker for adherence in youth ages 10–18 years for whom urgent clinical data could be evaluated. To pilot this approach, we used a cutoff of at least 20% decline from PBest HbF to recent assessment to define high deviation. Using this metric, two readily discernible groups were found with substantial differences in mean deviation from PBest HbF. By sensitivity analysis, the relationship between high deviation and resource use was robust to a range of cutoff value assumptions. By our categorization and despite stable HU dosing, high deviation from PBest was found in over two thirds of youth in our sample, the same proportion of patients found to be suboptimally adherent as calculated by medication possession for a statewide sample.17 In contrast, the group with low deviation had only minor changes in HbF over time, comparable to the modest degree of variation reported in long-term pediatric clinical studies.7,8
Two distinct data types support our preliminary approach to define suboptimal adherence: (i) Highly concordant differences between the two groups were found at recent assessment in multiple other HU-sensitive hematologic parameters, as expected from differing HU exposure at recent assessment but not at PBest. These differences were observed despite stable HU dosing. (ii) Only the group with high deviation had significantly greater urgent hospital use during the year surrounding their recent assessment compared to their year of PBest. In contrast, patients with low deviation had stable urgent care use during those two periods. In addition, HU dosing was unchanged for each patient, while routine renal and hepatic testing did not identify alterations or toxicity that may have altered HbF response to HU.
No specific demographic or treatment-related factors correlated with PBest deviation, including age, ethnicity, gender, PBest HbF or duration of HU therapy. The lack of correlation suggests the importance of patient- or family-specific factors, as was reported for adolescents with pediatric Type 1 diabetes.31 For example, perhaps patients with low deviation felt better in response to drug, thereby improving their confidence in the value of its use. Variation in parental certainty of HU benefit may also affect support for its use.16 Higher prescribed HU doses were seen in the high-deviation group at each of the two time points, perhaps reflecting less drug exposure for dose-sensitive cytopenias.
Several possible explanations for differences in deviation from PBest may be speculated. Higher deviation may have resulted in a larger decline in HbF among those who started with lower HbF, leading to greater deviation from highest HU-induced HbF. However, a striking gap was observed in deviation from PBest between the two groups. These differences exceed the differences seen in pre-HU levels, suggesting distinct patient groups not directly related to pre-HU HbF level. Alternatively, frequent decline in HU-induced HbF could reflect diminished marrow regenerative capacity or other limits to HU effect on stress erythropoiesis.32,33 A biologic explanation is less likely, as a relative—although not absolute—maintenance of HU-induced HbF was observed in 30% of youth in our sample, and has been reported in prospective pediatric multi-year analyses under stable dosing.3,7,8
Variability in urgent hospital use reflects the high degree of heterogeneity in intermittent acute disease manifestations, modest sample size and observations related to clinical HU effects made only over two 1-year periods. The low-deviation group overall experienced more urgent hospital care at PBest, suggesting that some of these patients may have had a more severe clinical phenotypes or more variation despite higher HbF pre-HU and at PBest. At recent assessment, urgent use increased only for high-deviation group. Our findings suggest that fewer severe symptoms at PBest in the high-deviation group may have resulted in lower incentive to adhere to a HU regimen. Variation from alpha thalassemia trait or other disease modifiers may have affected the distribution of acute symptoms.
In some youth, PBest may be only a relative marker of adherence. Some patients in this clinic-based cohort may never have attained complete daily HU use. The greater delay between HU initiation and PBest seen in this sample contrasts to reports in clinical trials.1,34 Prolonged time to reach PBest likely reflects the differences in a clinic-based approach to treatment, perhaps in addition to the challenges of pediatric care using capsules designed for adult therapy. Modest though significant difference was seen in HU dose between the two sites and between the two groups. Incomplete exposure to daily HU and its dose-dependent cytopenias may underlie modestly higher dosing in the high-deviation patients.
The prevalence of high deviation was not surprising. Previous reports have documented comparable low daily usage of penicillin and other regularly prescribed medications,22 and specifically HU among children20,35 and adults with SCD.17,18 Minority race/ethnicity and lower socioeconomic status are risk factors for disparities in medication adherence among children with chronic disease36–38 and can be associated with worse disease outcome.37 Our sample was selected for high risk of suboptimal adherence, based on features associated with barriers to medication use in other chronic conditions, including age and ethnic mismatch between patients and providers. Additional barriers may have included widespread parental concerns about drug toxicity and uncertainties about HU medical benefit.36,39
Biomarkers are used in clinical management of other chronic conditions to monitor the balance of treatment effect and toxicity, such as biologic targets or drug levels used in diabetes mellitus, lupus erythematosus or renal transplant recipients [recently reviewed in reference (10)10]. Lack of a uniform target for HU induction may create challenges to medication dosing, such as we observed in the high-deviation group.
The primary limitations of this study were the retrospective design, use of historical clinical data outside of a structured trial, measures limited to laboratory data, use of single points of laboratory assessment and modest sample size. Heterogeneity of clinic-based approach to HU dosing compared to trial-based time to MTD HbF6,34 likely reflects a combined effect of patient and provider preference, evolving clinical practice for HU use and limited pediatric dosing options using commercially available HU capsules.40 Single HbF determinations were used for each time point and may not fully represent changes in short- or long-term daily HU use. Nonetheless, these values had been obtained while patients were clinically well and with stable HU dosing, excluded outlier values and correlated with other simultaneously obtained laboratory data. HbF tracking has recently been used to assess impact of an intervention to improve adherence.41 Beyond PBest, addition of a second key biomarker should be explored, such as increments in MCV. Beyond laboratory data and clinical correlation, adherence measures such as pharmacy refill or pill counting were not obtainable retrospectively. Physiologic explanations for falling HbF such as decreasing bone marrow erythropoietic capacity or changes in pharmacodynamics could not be excluded. Acute healthcare utilization was the only measure of disease severity, and was limited to the hospital affiliated with the site where the child received care. Genetic analysis of HU-induced HbF was not performed.1,26 Fewer patients had pre-HU clinical data available for analysis, rendering those data less robust, and are not the primary focus of this report. Recently, larger increments in induced HbF were reported in clinical trials using lower toxicity standards for cytopenias.1,7
Our clinic-based findings suggest different HbF trajectories between the two groups defined by PBest deviation, correlate with acute hospital use and resemble adherence patterns described in adolescents with other major chronic illnesses.10–13 In the absence of a uniform HbF treatment target, deviation from PBest HbF may be a useful biomarker for HU adherence for adolescent patients in clinical settings. Larger prospective studies using additional adherence measures are needed to confirm our approach of tracking HbF deviation to assess HU use and to define the appropriate cutoff. Our findings are consistent with reports based on state claims database or pharmacy refills of widespread suboptimal HU adherence and missed dose-dependent benefits among adolescents.17 Collectively, these findings underscore the need for intervention trials to improve medication self-management in adolescents with SCD41,42 (e.g. clinical trial NCT02029742).
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by 5R21NR013745 (P.I.s NSG and AMS). Additional support was provided by 5UL1TR000040 (P.I. Henry Ginsberg, Columbia University).
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
ABBREVIATIONS
- ANC
absolute neutrophil count
- ARC
absolute reticulocyte count
- ER
emergency room
- HbF
fetal hemoglobin
- HU
hydroxyurea
- LOS
length of hospital stay
- MCV
mean corpuscular volume
- PBest
personal best
- WBC
white blood count
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Ware RE, Despotovic JM, Mortier NA, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of hydroxyurea treatment for children with sickle cell anemia. Blood. 2011;118(18):4985–4991. doi: 10.1182/blood-2011-07-364190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGann PT, Ware RE. Hydroxyurea for sickle cell anemia: What have we learned and what questions still remain? Curr Opin Hematol. 2011;18(3):158–165. doi: 10.1097/MOH.0b013e32834521dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman SA, Schultz WH, Davis JS, et al. Sustained long-term hematologic efficacy of hydroxyurea at maximum tolerated dose in children with sickle cell disease. Blood. 2004;103(6):2039–2045. doi: 10.1182/blood-2003-07-2475. [DOI] [PubMed] [Google Scholar]
- 4.Kinney TR, Helms RW, O’Branksi EE, et al. Safety of hydroxyurea in children with sickle cell anemia: Results of the HUG-KIDS study, a phase I/II trial. Pediatric Hydroxyurea Group. Blood. 1999;94(5):1550–1554. [PubMed] [Google Scholar]
- 5.Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: The HUSOFT extension study. Blood. 2005;106(7):2269–2275. doi: 10.1182/blood-2004-12-4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ware RE. Optimizing hydroxyurea therapy for sickle cell anemia. Hematology Am Soc Hematol Educ Program. 2015;2015(1):436–443. doi: 10.1182/asheducation-2015.1.436. [DOI] [PubMed] [Google Scholar]
- 7.Hankins JS, Aygun B, Nottage K, et al. From infancy to adolescence: Fifteen years of continuous treatment with hydroxyurea in sickle cell anemia. Medicine (Baltimore) 2014;93(28):e215. doi: 10.1097/MD.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferster A, Tahriri P, Vermylen C, et al. Five years of experience with hydroxyurea in children and young adults with sickle cell disease. Blood. 2001;97(11):3628–3632. doi: 10.1182/blood.v97.11.3628. [DOI] [PubMed] [Google Scholar]
- 9.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 10.McGrady ME, Hommel KA. Medication adherence and health care utilization in pediatric chronic illness: A systematic review. Pediatrics. 2013;132(4):730–740. doi: 10.1542/peds.2013-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: Prevention and intervention. Curr Opin Pediatr. 2010;22(4):405–411. doi: 10.1097/MOP.0b013e32833a46a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai M, Oppenheimer JJ. Medication adherence in the asthmatic child and adolescent. Curr Allergy Asthma Rep. 2011;11(6):454–464. doi: 10.1007/s11882-011-0227-2. [DOI] [PubMed] [Google Scholar]
- 13.Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. doi: 10.1093/pch/13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salema NE, Elliott RA, Glazebrook C. A systematic review of adherence-enhancing interventions in adolescents taking long-term medicines. J Adolesc Health. 2011;49(5):455–466. doi: 10.1016/j.jadohealth.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Quittner AL, Zhang J, Marynchenko M, et al. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest. 2014;146(1):142–151. doi: 10.1378/chest.13-1926. [DOI] [PubMed] [Google Scholar]
- 16.Anderson BJ. Who forgot? The challenges of family responsibility for adherence in vulnerable pediatric populations. Pediatrics. 2012;129(5):e1324–1325. doi: 10.1542/peds.2012-0526. [DOI] [PubMed] [Google Scholar]
- 17.Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86(3):273–277. doi: 10.1002/ajh.21968. [DOI] [PubMed] [Google Scholar]
- 18.Ritho J, Liu H, Hartzema AG, Lottenberg R. Hydroxyurea use in patients with sickle cell disease in a Medicaid population. Am J Hematol. 2011;86(10):888–890. doi: 10.1002/ajh.22134. [DOI] [PubMed] [Google Scholar]
- 19.Thornburg CD, Calatroni A, Telen M, Kemper AR. Adherence to hydroxyurea therapy in children with sickle cell anemia. J Pediatr. 2010;156(3):415–419. doi: 10.1016/j.jpeds.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh KE, Cutrona SL, Kavanagh PL, et al. Medication adherence among pediatric patients with sickle cell disease: A systematic review. Pediatrics. 2014;134(6):1175–1183. doi: 10.1542/peds.2014-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau HS, de Boer A, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 22.Patel NG, Lindsey T, Strunk RC, DeBaun MR. Prevalence of daily medication adherence among children with sickle cell disease: A 1-year retrospective cohort analysis. Pediatr Blood Cancer. 2010;55(3):554–556. doi: 10.1002/pbc.22605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costedoat-Chalumeau N, Amoura Z, Hulot JS, et al. Adherence to treatment in systemic lupus erythematosus patients. Best Pract Res Clin Rheumatol. 2013;27(3):329–340. doi: 10.1016/j.berh.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. doi: 10.1002/pds.1230. discussion 575–577. [DOI] [PubMed] [Google Scholar]
- 25.Nevin J, Myers L, Osunkwo I, Kanter J. A retrospective study to assess the utility of frequent laboratory monitoring of pediatric patients with sickle cell disease on hydroxyurea. J Pediatr Hematol Oncol. 2014;36(3):e180–e184. doi: 10.1097/MPH.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 26.Green NS, Ender KL, Pashankar F, et al. Candidate sequence variants and fetal hemoglobin in children with sickle cell disease treated with hydroxyurea. PLoS ONE. 2013;8(2):e55709. doi: 10.1371/journal.pone.0055709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green NS, Barral S. Emerging science of hydroxyurea therapy for pediatric sickle cell disease. Pediatr Res. 2014;75(1–2):196–204. doi: 10.1038/pr.2013.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Helgeson VS, Reynolds KA, Siminerio L, Escobar O, Becker D. Parent and adolescent distribution of responsibility for diabetes selfcare: Links to health outcomes. J Pediatr Psychol. 2008;33(5):497–508. doi: 10.1093/jpepsy/jsm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Hematol Oncol Clin North Am. 2010;24(1):199–214. doi: 10.1016/j.hoc.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thabane L, Mbuagbaw L, Zhang S, et al. A tutorial on sensitivity analyses in clinical trials: The what, why, when and how. BMC Med Res Methodol. 2013;13:92. doi: 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naranjo D, Mulvaney S, McGrath M, Garnero T, Hood K. Predictors of self-management in pediatric type 1 diabetes: Individual, family, systemic, and technologic influences. Curr Diab Rep. 2014;14(11):544. doi: 10.1007/s11892-014-0544-7. [DOI] [PubMed] [Google Scholar]
- 32.Platt OS. Hydroxyurea for the treatment of sickle cell anemia. N EnglJ Med. 2008;358(13):1362–1369. doi: 10.1056/NEJMct0708272. [DOI] [PubMed] [Google Scholar]
- 33.Mabaera R, West RJ, Conine SJ, et al. A cell stress signaling model of fetal hemoglobin induction: What doesn’t kill red blood cells may make them stronger. Exp Hematol. 2008;36(9):1057–1072. doi: 10.1016/j.exphem.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Thornburg CD, Rogers ZR, Jeng MR, et al. Adherence to study medication and visits: Data from the BABY HUG trial. Pediatr Blood Cancer. 2010;54(2):260–264. doi: 10.1002/pbc.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brandow AM, Panepinto JA. Hydroxyurea use in sickle cell disease: The battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Rev Hematol. 2010;3(3):255–260. doi: 10.1586/ehm.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McQuaid EL, Everhart RS, Seifer R, et al. Medication adherence among Latino and non-Latino white children with asthma. Pediatrics. 2012;129(6):e1404–e1410. doi: 10.1542/peds.2011-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: A Children’s Oncology Group study. JAMA Oncol. 2015;1(3):287–295. doi: 10.1001/jamaoncol.2015.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: A Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. doi: 10.1182/blood-2014-01-552166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer. 2013;60(4):653–658. doi: 10.1002/pbc.24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandow AM, Jirovec DL, Panepinto JA. Hydroxyurea in children with sickle cell disease: Practice patterns and barriers to utilization. Am J Hematol. 2010;85(8):611–613. doi: 10.1002/ajh.21749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Estepp JH, Winter B, Johnson M, Smeltzer MP, Howard SC, Hankins JS. Improved hydroxyurea effect with the use of text messaging in children with sickle cell anemia. Pediatr Blood Cancer. 2014;61(11):2031–2036. doi: 10.1002/pbc.25177. [DOI] [PubMed] [Google Scholar]
- 42.Creary SE, Gladwin MT, Byrne M, Hildesheim M, Krishnamurti L. A pilot study of electronic directly observed therapy to improve hydroxyurea adherence in pediatric patients with sickle-cell disease. Pediatr Blood Cancer. 2014;61(6):1068–1073. doi: 10.1002/pbc.24931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.