Abstract
A quest for a systems-level neuroscientific basis of anesthetic-induced loss and return of consciousness has been in the forefront of research of the last two decades. Recent advances toward the discovery of underlying mechanisms have been achieved using experimental electrophysiology, multichannel electroencephalography, magnetoencephalography, and functional magnetic resonance imaging. By the careful dosing of various volatile and IV anesthetic agents to the level of behavioral unresponsiveness, both specific and common changes in functional and effective connectivity across large-scale brain networks have been discovered and interpreted in the context of how the synthesis of neural information might be affected during anesthesia. The results of most investigations to date converge toward the conclusion that a common neural correlate of anesthetic-induced unresponsiveness is a consistent depression or functional disconnection of lateral frontoparietal networks, which are thought to be critical for consciousness of the environment. A reduction in the repertoire of brain states may contribute to the anesthetic disruption of large-scale information integration leading to unconsciousness. In future investigations, a systematic delineation of connectivity changes with multiple anesthetics using the same experimental design and the same analytical method will be desirable. The critical neural events that account for the transition between responsive and unresponsive states should be assessed at similar anesthetic doses just below and above the loss or return of responsiveness. There will also be a need to identify a robust, sensitive, and reliable measure of information transfer. Ultimately, finding a behavior-independent measure of subjective experience that can track covert cognition in unresponsive subjects and a delineation of causal factors vs. correlated events will be essential to understand the neuronal basis of human consciousness and unconsciousness.
Introduction
General anesthetics have been in continuous clinical use since the first public demonstration of ether anesthesia at the Massachusetts General Hospital in 1846; the question of how these drugs work has persisted for almost as long. Although understanding the individual mechanistic pathways of each anesthetic drug is of scientific interest and might hold promise for the rational design of novel agents, we would suggest that the heart of the question relates to the common functional outcome achieved by structurally and pharmacologically diverse anesthetics. In the 19th century, ether, chloroform and nitrous oxide were the tools of the anesthetist. In the 21st century, an anesthesiologist or anesthetist could walk into an operating room and use (for example) propofol, sevoflurane, or ketamine to induce a desirable functional outcome that would activate the sequence of events required for insensate surgery. Note that it is the functional state, rather than the subjective state, induced by propofol, sevoflurane and ketamine that we regard as common. Behaviorally, this commonality is manifest as unresponsiveness to a command along with the assumption that, in the absence of neuromuscular blockade, the surgical patient is no longer conscious of the environment. We contend that a common functional mediator in the brain accounts for the common functional outcome in the operating room.
We acknowledge that the functional state defined by unresponsiveness could still be associated with disconnected consciousness. This leaves open the possibility of subjective experience that is generated internally (e.g., a dream state) rather than externally (e.g., by surgical events) (1,2). It is well known that anesthetic effects vary with respect to this “residual” subjective experience, with a variety of different states reported after different anesthetics that were titrated to a similar functional outcome such as unresponsiveness to a command (3).
It is important to recognize that we are not proposing a classical “unitary hypothesis,” which would imply that all anesthetics work through precisely the same mechanism (e.g., gamma-aminobutyric acid-mediated postsynaptic inhibition) to achieve precisely the same state (e.g., complete unconsciousness). We acknowledge fully that the molecular targets and mechanistic cascades of anesthetics are distinct, as are the accompanying subjective states. What we do propose, however, is that the anesthetic depression of a set of cortical brain regions, specifically, the lateral frontoparietal network, is a common mediator of the effect of anesthetic agents that accounts for the disruption of connected consciousness and does so by reducing the integration of neural information. It must be noted that the precise mechanism of frontoparietal depression is still unknown, with possibilities including corticocortical actions, thalamocortical actions, or a combination thereof.
In this article we review (1) the importance of information integration in consciousness, (2) early studies of network breakdown during anesthesia, (3) preclinical research demonstrating disrupted frontal-to-parietal connectivity during anesthesia, (4) translational and clinical research demonstrating disrupted frontal-to-parietal connectivity during anesthesia in humans, and (5) potential mechanisms for this functional disruption. A number of ideas presented here were previously presented in excellent reviews (4–14). Our intent is to provide a focused but comprehensive update on the subject matter with the inclusion of recent findings in support of our hypothesis. In addition, we expand on more recent studies that focus on the potential role of spatiotemporal fragmentation, which refers to the spatial organization and temporal coordination of neuronal activity that are observed during wakefulness but disturbed during general anesthesia.
Definitions
First, integration of neural information is to be understood as the general process that synthesizes sensory and other types of information from different parts of the brain to form a unified subjective experience. This article focuses on the lateral frontoparietal network, a group of frontal and parietal association regions in the dorsolateral cerebral cortex, that has been implicated to play important roles in integration, various cognitive functions, and consciousness (15,16). Its involvement in volatile anesthetic-induced unconsciousness was implied a decade ago (17) and since has been confirmed by numerous experiments in multiple species with all major classes of anesthetics (18–25) (Table 1). The critical role of this network is consistent with various pathological states of unconsciousness (25,26).
Table 1.
Electroencephalographic and fMRI Studies that found Altered Anterior-Posterior Brain Connectivity during Anesthetic-induced Unconsciousness
| Study | Participants | Anesthetic | Analytic Technique |
Comments |
|---|---|---|---|---|
| (54) John, 2001 | Surgical Patients |
Various | EEG coherence | Decrease in frontal- parietal and frontal- occipital |
| (140) Imas, 2005 | Rats | Halothane, Isoflurane, Desflurane |
Intracortical potentials, Transfer Entropy |
Decrease in frontal to parietal and occipital |
| (22) Lee, 2009 | Healthy Volunteers |
Propofol | EEG Evolution Map |
Disrupted frontoparietal feedback connectivity |
| (18) Boveroux, 2010 | Healthy Volunteers |
Propofol | fMRI BOLD correlation |
Decreased Default and Executive networks |
| (141) Stamatakis, 2010 |
Healthy Volunteers |
Propofol | fMRI BOLD correlation |
Increased Default Network connectivity |
| (142) Martuzzi, 2010 | Healthy Volunteers |
Sevoflurane | fMRI BOLD correlation |
Reduced temporal- parietal connectivity |
| (143) Schrouff, 2011 | Healthy Volunteers |
Propofol | fMRI BOLD correlation |
Reduced frontoparietal - temporal connectivity |
| (21) Ku, 2011 | Surgical Patients |
Propofol, Sevoflurane |
EEG Evolution Map, Symbolic Transfer Entropy |
Disrupted frontoparietal feedback connectivity |
| (89) Barrett, 2012 | Healthy Volunteers |
Propofol | EEG Granger Causality |
Increased bidirectional connectivity |
| (90) Nicolaou, 2012 | Surgical Patients |
Routine anesthesia |
EEG Granger Causality |
Increased frontoparietal connectivity |
| (81) Boly, 2012 | Healthy Volunteers |
Propofol | EEG Dynamic Causal Modeling |
Disrupted frontoparietal feedback connectivity |
| (86) Guldenmund, 2013 |
Healthy Volunteers |
Propofol | fMRI BOLD correlation |
Reduced connectivity in Default, Salience, and Executive networks |
| (23) Lee, Ku et al., 2013 |
Surgical Patients |
Ketamine, Propofol, Sevoflurane |
EEG Normalized Symbolic Transfer Entropy |
Disrupted frontoparietal feedback connectivity |
| (20) Jordan, 2013 | Healthy Volunteers |
Propofol | EEG Symbolic Transfer Entropy, fMRI correlation |
Disrupted anterior- posterior feedback; frontal-to-parietal best discriminator |
| (101) Lee et al., 2013 | Healthy Volunteers |
Propofol | EEG Directed Phase-lag Index |
Disrupted frontoparietal feedback connectivity; frontal and parietal hub reconfiguration |
| (91) Maksimow, 2014 | Healthy Volunteers |
Propofol | EEG Partial Directed Coherence |
Increase in frontal-to- occipital connectivity; decrease in reverse |
| (88) Liu, 2014 | Healthy Volunteers |
Propofol | fMRI BOLD correlation |
Increased Default (Precuneus) network |
| (83) Blain-Moraes, 2015 |
Healthy Volunteers |
Sevoflurane | EEG Phase-lag Index |
Disruption of fronto- occipital interaction |
| (85) Moon, 2015 | Healthy Volunteers; Model Brain Networks |
Sevoflurane; Selective Hub Disruption |
EEG Directed Phase-lag Index |
Disruption of frontoparietal feedback connectivity |
| (24) Muthukumaraswamy, 2015 |
Healthy Volunteers |
Ketamine | MEG Dynamic Causal Modeling |
Decreased frontoparietal effective connectivity; subanesthetic doses |
| (84) Palanca, 2015 | Healthy Volunteers |
Sevoflurane | fMRI BOLD correlation |
Reduced midline Default network |
| (144) Yanagawa, 2013 | Monkey | Propofol, Medetomidine, Ketamine |
ECoG Granger Causality |
Reduced prefrontal to temporal/visual interactions |
| (2) Warnaby, 2016 | Healthy volunteers |
Propofol | EEG and fMRI correlation |
Reduced insula to frontoparietal connectivity |
Second, in the article we use the word connectedness and connectivity in two different contexts. In the first sense, we refer to connected consciousness, the subjective experience or interaction of the patient with the environment. In the second sense, connectivity is applied to neuronal connectedness, e.g., the communication among neurons or brain regions. We propose that connected consciousness may require neuronal connectivity within the lateral frontoparietal network of the brain.
Furthermore, there are multiple types of connectivity discussed. For example, “functional” means that the connectivity measured between two brain regions may not be necessarily brought about by neuronal interactions along a direct axonal pathway but may be a result of intermediate relays or common input from a third source. Because these measures are based on the spatiotemporal correlation of electromagnetic or hemodynamic signals, they also may not reflect true causal influence of one brain region on another (which is referred to as “effective” connectivity). Importantly, our current understanding of the principles of neuronal information coding is incomplete and does not allow the deciphering of messages passed around in brain circuits. Nevertheless, both functional and effective connectivity represent useful surrogate measures of neural interactions and have already been providing groundbreaking discoveries of normal and pathological brain function. For example, using functional magnetic resonance imaging (fMRI) in stimulus or task-free (the latter being referred to as the “resting” state) conditions, several intrinsic brain networks involved in specific cognitive functions, such attention control, executive control, salience monitoring, etc. have been discovered. For further information, we kindly refer the reader to excellent reviews (27–29) and our Glossary of Concepts and Technical Terms (Appendix 1).
Consciousness and Information Integration
Since the 1990s, there has been a search for the neural correlates and neural causes of conscious experience (11,30–37). Research into the neural correlates of consciousness has focused on potential mechanism at multiple scales of organizational level ranging from quantum physical, to molecular, synaptic, neuronal circuit, and large-scale brain networks. The search for neuronal activity patterns, neuronal interactions, specific brain regions and large-scale networks that are critically important for being conscious has revealed numerous hypotheses and mechanisms, although relatively few have been confirmed by multiple investigations.
During the last few decades, intense neuroscience research utilizing noninvasive neuroimaging techniques in human subjects has revealed that cognitive functions are instantiated by various networks of interacting brain regions (38). In each region, neuronal ensembles form specialized information processors that share their computational results with other members of the network via ongoing interactions. Neuronal groups residing in multisensory and higher order association regions of the cerebral cortex are thought to play particularly important roles in analyzing and integrating information from multiple sources, including specific sensory regions of the brain. Importantly, the networks responsible for information processing and integration--and thus all sensory, motor, and cognitive functions--are transient, with dynamic formation and dissolution.
Our research programs have focused on topics related to information integration in the brain, a focus motivated by known phenomenological and neurobiological facts (4,39). The known phenomenological fact is that our perception of the world is unified, we do not; for example, experience the color, shape and warmth of the sun as disconnected elements, but rather as a singular whole. The known neurobiological fact is that the brain is subdivided into modules that independently process modalities (such as vision) and submodalities (such as color). To reconcile these subjective and objective facts, the brain must have mechanisms to synthesize the outputs of discrete neural processing in order to generate the unity of experience. Furthermore, if information synthesis is necessary for normal consciousness, it stands to reason that the interruption of this synthesis would be sufficient for unconsciousness (as in the case of general anesthesia).
An influential theory of consciousness related to information integration was developed by Tononi and Koch (33,40,41). Stated concisely, consciousness of a system is equivalent to its integrated information. Specifically, the larger the brain’s information capacity and the more integrated the information, the richer the conscious experience is. The theory is also cast in mathematical language that allows its empirical testing. For example, the information capacity of neuronal groups and their interaction as a measure of information integration can be assessed and compared in various conditions such as during wakefulness, sleep, and anesthesia. The spatiotemporal level, ranging from molecules to large-scale networks, at which information integration is to be assessed in the brain is yet unknown. Nevertheless, the introduction of a novel measure of brain complexity based on the framework of Integrated Information Theory has already shown great promise in separating conscious and unconscious states with multiple anesthetic agents, sleep-wake states, and pathological states of unconsciousness (42). Other approaches to measure brain complexity and information are being developed (43).
One potential mechanism of information integration in the brain is recurrent processing (also known as feedback, re-entrant, or reafferent processing) (44). This phrase denotes a neural signaling pathway that originates in higher-order cortical regions and modulates more primary processing regions. Recurrent processing has been found, by a variety of neuroscientific investigations and across a number of different regions in the brain, to be associated with conscious experience (26,45,46). This article focuses on recurrent processing in lateral cortices, a network that has been argued to be of central importance to the conscious experience of environmental stimuli such as surgery (47,48). This is in contrast to the medial frontoparietal system, which has been argued to be of central importance for endogenous conscious experience such as a dream state.
Measures of connectivity, which can be derived from neuroimaging or neurophysiological data, are often used as a surrogate for integrative processes in the brain. As noted, there are multiple types of “connectivity” that can be measured (49,50): (1) structural connectivity, defined as the anatomical connections between brain regions, (2) functional connectivity, defined as an instantaneous statistical covariation between the activity of brain regions, (3) directed connectivity, also defined as a statistical dependence, but with a comparison of local neuronal activity in one area to another area in the past, and (4) effective connectivity, defined as a causal relationship between the activities of different brain regions. Measures of directed connectivity (such as directed phase lag index (dPLI) (51), transfer entropy (52), and Granger causality (53) are often used to assess recurrent processing.
Early Studies of Anterior-Posterior Network Breakdown during Anesthesia
In a pioneering investigation, John et al. (54,55) studied 19-channel electroencephalograph (EEG) power and coherence in 176 surgical patients variously induced with etomidate, propofol or thiopental and maintained with one of three volatile anesthetics, N2O plus narcotics, or propofol. The data from all patients and protocols were combined in order to arrive at an anesthetic agent-invariant correlate of the state of unconsciousness. The authors found that the critical change in EEG that best correlated with the loss and return of responsiveness was a decrease in frontoparietal, frontal-occipital and interhemispheric 40 Hz gamma coherence (reflecting functional connectivity). Although directional influences were not studied at the time, this result was the first indication that a breakdown of anterior-posterior connectivity was a common feature of the unconscious state produced by various anesthetic agents. Since consciousness is preserved with one functioning hemisphere, the reduction of interhemispheric coherence is probably not a causally important factor in modulating the state of consciousness.
Within a few years of this study, White and Alkire (56) measured regional glucose utilization using positron emission tomography during halothane or isoflurane titrated to unresponsiveness and analyzed the data by structural equation modeling to estimate effective connectivity. The results suggested that the anesthetics produced both thalamocortical and corticocortical disconnection. Several investigations using functional imaging techniques questioned the importance of thalamocortical disconnection because the primary sensory regions of the cortex remained at least partially responsive to sensory stimuli (18,57–59) while the subjects were unconscious (behaviorally unresponsive). Using in vivo and slice recordings, Hentschke et al. suggested that the primary target of anesthetics was the cerebral cortex (60).
Anesthetic-Mediated Disruption of Recurrent Processing in Animal Models
A limitation of patient studies has been that dose-dependent anesthetic effects could not be easily studied and anesthesia induction during clinical care was generally too fast to allow the precise determination of critical changes in neural activities associated with the reversible transition between conscious and unconscious states. To overcome this limitation, preclinical studies of cortical neuronal interactions were conducted in chronically instrumented, freely moving animals under multiple finely graded steady-state anesthetic conditions.
frontoparietal In one such experiment, Imas et al. (17) estimated a surrogate for directional information transfer among frontal, parietal and occipital cortical sites using transfer entropy, a nonlinear, model-free measure of temporal interdependence of signals. Flash-induced intracortical potentials were recorded during wakefulness and graded levels of three volatile anesthetics (halothane, isoflurane and desflurane) and the data were combined in search of an agent-invariant correlate of unconsciousness. The results showed that the common effect of volatile anesthetics at an equivalent concentration that produced the loss of righting reflex was the preferential reduction of feedback transfer entropies in the frontoparietal, frontal-occipital, and parietal-occipital directions. At surgical levels of anesthesia, both feedforward and feedback transfers were significantly reduced. Of importance is that transfer entropies were derived from stimulus-induced potentials. Thus, they reflect the properties of a sensory processing stream in ascending (bottom-up) and descending (top-down) directions. Also, they were calculated from single-trial, wavelet-transformed gamma power that had been hypothesized to be important for feature binding and conscious perception (61,62). The hierarchical, recurrent connectivity of sensory systems has long been proposed as an essential substrate for conscious perception and conscious behavior (63–65). As suggested, the forward connections represent and analyze incoming sensory data, whereas the feedback projections provide attentional modulation, contextual selection, and interpretation of sensory information (66–68). The results of Imas et al. suggested that anesthetics may produce unconsciousness by interfering with the descending, top-down information stream, thereby preventing the conscious integration of sensory information.
Recently, Raz et al. (69) conducted animal studies that provided additional support for the role of top-down versus bottom-up sensory pathways in isoflurane anesthesia. They recorded stimulus-related local field potentials in various layers of the auditory cortex in vivo and in thalamocortical brain slices in vitro during auditory, visual, or thalamic stimulation. Recording of local field potentials in specific cortical laminae allows one to functionally identify ascending and descending corticocortical pathways because their synaptic targets are segregated to different cortical layers. Moreover, visual stimulation activates auditory cortex through cross-modal, descending pathways. At an isoflurane concentration associated with the loss of righting reflex, bottom-up responses to auditory stimuli were enhanced, whereas top-down responses to visual stimuli were reduced. Of note is that, unlike in humans, the primary visual evoked potentials are preserved in rodents during general anesthesia; therefore, the loss of cross-modal response was likely due to a suppression of a higher-order interaction rather than a differential vulnerability in primary sensory cortex. Despite the substantially different methodologies, Raz et al.’s results are highly complementary to those obtained with transfer entropy at gamma-frequency.
Another way to discriminate feedforward and feedback signaling is by segregating the temporal components of sensory evoked/induced responses. Evoked response refers to enhanced neuronal activity that is time-locked to the stimulus with relatively short latency, whereas induced response refers to neuronal activity that is temporally dispersed with usually long latency. Moreover, neural activities in primary visual cortex within the first 100 ms after stimulus presentation are associated with preconscious stimulus registration, whereas the subsequent, sustained activity reflects feedback from higher processing regions that is associated with conscious perception (45,70–72). Hudetz et al. (73) examined the concentration-dependent effect of desflurane on the flash-induced unit response in primary visual cortex of rats. As in Raz et al.’s more recent study, anesthetics did not attenuate the early (<100 ms) response after flash. However, the long-latency, sustained (150–1000 ms) response was significantly diminished in a concentration-dependent manner. Given that the long-latency response represents recurrent processing by an interaction with higher cortical regions, these results support the preferential effect of anesthetics on feedback signaling.
The proposed preferential reduction of anterior-posterior feedback or recurrent signaling by other types of anesthetics including IV agents has been less investigated in animal models. Pal et al. (74) recently reported a global reduction in long-range cortical gamma coherence after the infusion of ketamine that was reversible after the termination of the anesthetic. Ketamine has also been shown to suppress top-down effective connectivity, as determined by dynamic causal modeling, between medial prefrontal cortex and dorsal hippocampus (75).
Of mechanistic significance, a recent study by Reinhold et al. (76) found that optogenetic silencing of thalamic relay neurons in mice rapidly attenuated the sustained component of the visual evoked response suggesting that the long-lasting responses in visual cortex may arise from thalamocortical interactions as opposed to cortical recurrent circuits. Isoflurane anesthesia produced frequency-dependent filtering of thalamocortical transmission as previously indicated. However, because thalamocortical silencing was achieved by an activation of the thalamic reticular nucleus, a simultaneous depression of recurrent feedback from higher order association cortex cannot be completely excluded.
Anesthetic-Mediated Disruption of Recurrent Processing in Humans
Building on the work of Imas et al. (17,77), Lee et al. studied 10 young, healthy volunteers receiving bolus doses of propofol on two occasions and found that frontal-to-parietal directed connectivity was selectively suppressed in association with unconsciousness (22,78). Ku et al. and Lee et al. (21,23) extended this work to 18 surgical patients of varying ages and found that frontal-to-parietal connectivity (as measured by symbolic transfer entropy) was suppressed during propofol- and sevoflurane-induced unconsciousness, both directions of connectivity were suppressed during surgical anesthesia (as was also observed in rodent studies), and frontal-to-parietal connectivity significantly returned in association with consciousness (21,23). In support of the findings during recovery, connectivity across frontoparietal networks (together with the activation of brainstem-thalamus-anterior cingulate axis) has been found to correlate with the recovery of consciousness after exposure to dexmedetomidine and propofol (79). Importantly, selective inhibition of frontal-to-parietal connectivity has been shown after anesthetic doses of ketamine (23), despite distinct molecular mediators and distinct neurophysiology in the alpha and gamma bandwidths compared with propofol and sevoflurane. Although these studies were all correlative, the consistent and specific effect of diverse anesthetics on a network of known relevance to conscious processing holds mechanistic promise (80).
This series of studies on frontal-to-parietal directed connectivity was conducted with relatively rapid induction techniques and with low-resolution EEG. The findings, however, have been replicated with more controlled induction regimens and higher-resolution techniques. Boly et al. studied volunteers with high-density EEG and demonstrated selective disruption of frontal-to-parietal connectivity in association with stepwise induction of propofol-induced unconsciousness; effective connectivity was assessed using the technique of dynamic causal modeling (81). Jordan et al. studied volunteers with both high-density EEG and fMRI, demonstrating, without a priori assumptions, that disruption of frontal-to-parietal connectivity was the best discriminator (among various directional connectivity patterns) between consciousness and propofol-induced unconsciousness, as achieved by stepwise induction (20,82). Similarly, stepwise induction of sevoflurane-induced unconsciousness in volunteers being studied with high-density EEG was characterized by a breakdown of frontoparietal functional connectivity (as measured by phase lag index) (83,84) and frontal-to-parietal directional connectivity (as measured by dPLI) (85). Recently, combined EEG and magnetoencephalography in humans revealed a selective reduction of frontal-to-parietal connectivity by ketamine, as assessed by dynamic causal modeling (24). Although the doses of ketamine in this investigation were subanesthetic, reduction of feedback connectivity has been observed in past studies during infusion of the drug but prior to loss of responsiveness. Future studies of cortical connectivity at both subanesthetic and anesthetic doses of ketamine will be required. In summary, past findings using low-resolution techniques in surgical patients have been confirmed for propofol, sevoflurane and ketamine using carefully controlled methodologies and with high-resolution neuroimaging techniques.
Although directed connectivity is not usually assessed in fMRI studies, findings from several such investigations are nevertheless consistent with the EEG findings. For example, Boveroux et al. (18) found that a propofol-induced decrease in consciousness correlated with a decrease in both corticocortical and thalamocortical connectivity in frontoparietal networks (default mode and executive-control networks). As seen in several other studies, thalamocortical connectivity in low-level visual and auditory networks was preserved, but their cross-modal interaction was suppressed. This suggests that propofol-induced unconsciousness was linked to a breakdown of network connectivity between low-level sensory and high-level frontoparietal cortices. Similar conclusions were reached in follow up investigations (86).
Despite robust confirmation of disrupted frontal-to-parietal connectivity during anesthetic-induced unconsciousness, multiple studies have yielded conflicting results. Dexmedetomidine, a drug that arguably produces a reversible state of unresponsiveness, disrupted thalamocortical but not frontoparietal functional connectivity (87). Importantly, however, frontoparietal networks were metabolically depressed. Also, opposite changes in functional connectivity may occur among specific subregions of the same general brain structure (88). Moreover, the technique of Granger causality results in opposite connectivity findings (89,90), but different experimental protocols (91) and species (92) are also associated with disparate results. These data are important because they suggest, correctly, that there is no absolute correlation of a particular direction of information transfer with consciousness or unconsciousness. Indeed, directionality across frontoparietal networks during conscious experience depends on many conditions, including whether the eyes are opened and whether conscious percepts are real or imagined (93). However, comparison of directed connectivity techniques (dPLI, symbolic transfer entropy, and Granger causality) in model networks reveals that they all behave in a consistent fashion when coupling between nodes becomes sufficiently low (85).
Table 1 summarizes the main results of EEG and fMRI studies that reported an alteration (either increase or decrease) in some aspect of anterior-posterior brain connectivity during anesthesia titrated to unresponsiveness. The majority of studies support the reduction of anterior-posterior functional connectivity. Changes in directional or effective connectivity are specifically indicated when assessed. The effect of anesthetics, particularly that of propofol, on the medial Default Mode network is ambiguous. Connectivity results obtained with EEG, which is more sensitive to dorsolateral networks, are more uniform with the exception of those obtained using Granger causality.
Potential Mechanisms of Impaired Recurrent Processing in Frontoparietal Networks
It is critical to consider why there should be selective disruption of recurrent processing from frontal to posterior parietal cortices in the eyes-closed resting state. What mechanism could account for this asymmetry between anterior and posterior networks? When considering the effects of propofol, it would be tempting to hypothesize that the well-known phenomenon of anteriorization might be a contributor. The power of alpha oscillations shifts from occipital cortex to frontal cortex, with high coherence, in association with propofol-induced unconsciousness (94,95) that may be a result of thalamocortical interaction (96,97). It would be conceivable, therefore, that this highly coherent oscillation in the frontal region “locks out” communication volleys to more posterior networks. However, inhibition of frontal-to-parietal connectivity occurs during exposure to ketamine, which does not demonstrate anteriorization of alpha power and in fact decreases alpha power (98). Furthermore, a recent modeling study predicts that anteriorization is a consequence of disrupted feedback processing rather than a cause (99). This parallels studies in nonhuman primates suggesting that inhibition of feedback processes by general anesthetics precede the slow-wave phenotype (100).
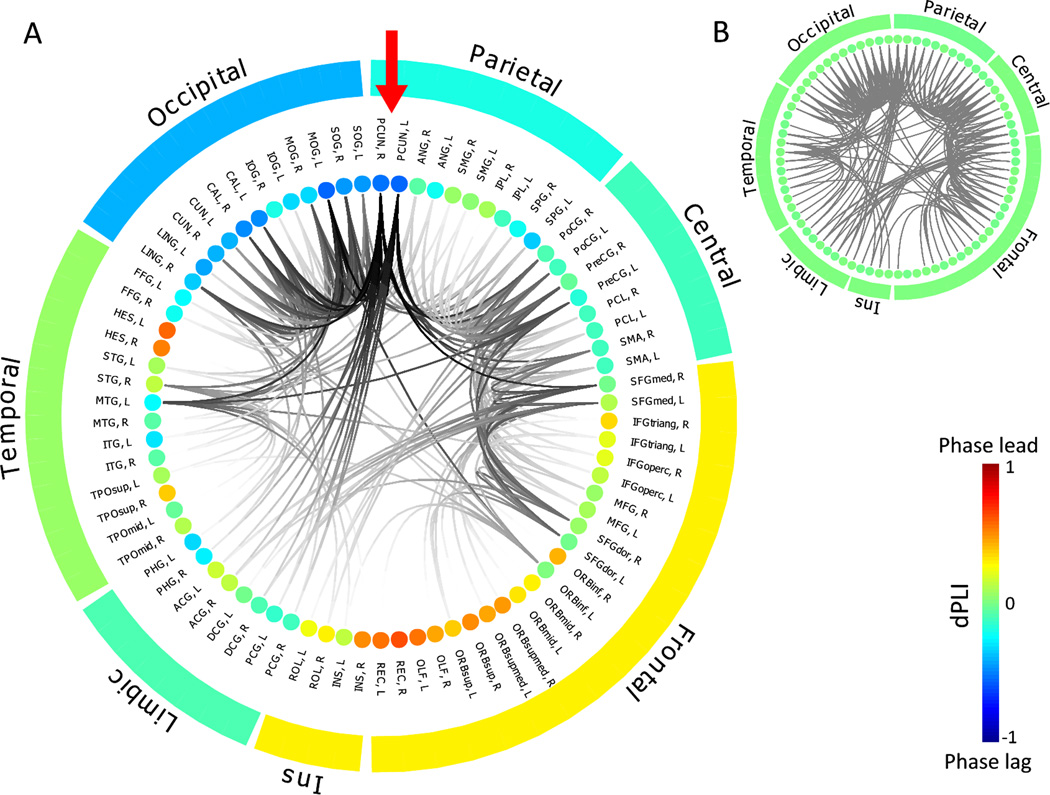
Studies by Lee et al. (101) and Moon et al. (85) suggest that changes in directional connectivity reflect changes in the underlying functional network topology during propofol- and sevoflurane-induced unconsciousness. After a bolus dose of propofol, highly connected “hubs” in the brain undergo a reconfiguration, with connectivity patterns in the posterior parietal cortex being disrupted (101). Much like an airport system, a disrupted hub would entail a reduction in incoming traffic, which is exactly what is observed in the form of reduced communication from frontal cortex to posterior parietal cortex. In a study that spanned mathematical analysis, simple networks, neuroanatomically informed network models and human brain networks reconstructed from high-density EEG, Moon et al. demonstrated that the more connections a network node has, the more incoming directed connections it attracts (85). When these highly connected hub nodes are computationally “lesioned,” the natural anterior-to-posterior connectivity that is present in the resting state becomes neutralized (Figure 1) in a way that recapitulates empirical EEG analysis from anesthetized patients. This suggests that empirical EEG findings during consciousness and anesthesia follow certain behaviors that could be predicted from more general network models and different experimental conditions could result in different patterns of directional connectivity because of the underlying differences in the topology of the network. Figure 1 (from Moon et al. (85)) illustrates the results of computational modeling of directional connectivity based on simulated alpha oscillations on a network of neuroanatomic nodes obtained by diffusion tensor imaging in the human brain. The simulation predicts a dominantly frontoparietal-directed connectivity in the resting, wakeful brain; the directionality of connections is neutralized after the selective removal of hub connections (mainly in the precuneus) to simulate the effect of anesthesia. However, Warnaby et al. (2) have recently suggested that anesthetic effects on the dorsal anterior insular cortex contribute to the fragmentation of the frontoparietal communication (2). Further work is clearly required.
Figure 1.
Neuroanatomically informed model of structural and directed connectivity in the human brain during resting state and simulated anesthesia. This is a representation of a human brain network, with structural connections between the neuroanatomical nodes determined by diffusion tensor imaging and neurophysiologic alpha oscillations simulated on that scaffolding. The anatomical connectivity of different brain regions is presented as ring plots together with average directed phase lag index (dPLI) value of the alpha oscillation for each region. The inset within the ringplot shows structural connections between nodes, highlighted by darker color if the node has a higher degree of connections. The nodes are aligned in groups: frontal lobe, central regions (including motor and somatosensory cortex), parietal lobe, occipital lobe, temporal lobe, limbic region, and Insula (Ins). Red arrow in (A) points to left and right precuneus, the most connected nodes and thus strongest hubs in the network. Color of each node shows the average dPLI values with respect to other nodes, from red (dPLI=1, a source of directed connectivity) to blue (dPLI=−1, a target of directed connectivity). Average dPLI for each group is also shown in color around the ring. In the resting state, frontal alpha oscillations lead and more posterior areas lag (A), which is also found empirically. After selective computational degradation of hub connections, performed in order to model anesthetic effects, the directionality from front to back is neutralized (B, with dPLI values now at 0 in green) as is also seen empirically with EEG. Figure adapted from (85) (open access, no permission needed).
In addition to these network-level considerations, there are also more conventional explanations for selective feedback inhibition during general anesthesia based on glutamatergic receptor function. It has been suggested that posterior-to-anterior feedforward pathways are mediated by AMPA receptors, while anterior-to-posterior feedback pathways are mediated by N-methyl-D-aspartate receptors (34). Indeed, in the visual system, feedforward and feedback processing can be dissociated with pharmacological manipulation of these receptor subtypes (46). In the frontoparietal system, dynamic causal modeling suggests that ketamine blocks both N-methyl-D-aspartate- and AMPA-mediated frontal-to-parietal connectivity (24).
The primary anatomical targets that mediate anesthetic interference with recurrent signaling in the brain remain unclear (13). Ultimately, the block of feedback connectivity associated with unconsciousness may be due to a loss of output from the sending (top) site, a disruption of input at the receiving (down) site, or both. The cause of either effect may be direct modulation of local neuronal/synaptic targets, in particular the distal dendrites of pyramidal cells (13), or indirectly, subcortical modulation of the feedback circuit (as in the thalamic silencing experiment described above). Moreover, connectivity changes may arise from specific target effects or from a distributed modification of interacting brain sites. The earliest effects on cortical activity and connectivity following loss of responsiveness with propofol and sevoflurane anesthesia appear to be in the frontal lobes (102,103). Langsjo et al. (79) saw an important connection between the frontoparietal network and a subcortical/limbic core constituted by regions in the brainstem, thalamus and anterior cingulate with propofol and dexmedetomidine. Likewise, a reduction in thalamocortical functional connectivity during sevoflurane-induced unconsciousness was pronounced in the anterior-posterior regions of the default mode network and ventral attention networks (84). Frontoparietal metabolic reductions were associated with central thalamic/pallidal suppression in brain-injured patients (104). Boly et al. (81) used dynamic causal modeling of source-reconstructed EEG data and found that the best explanation of their data was a decrease in backward connectivity from frontal to parietal cortices, while thalamocortical connectivity remained unchanged. The contradictory results may have been due to the simplification of treating the entire thalamus as a single structure, necessitating a more differentiated connectivity analysis of specific nuclei or nuclear groups of the thalamus. In that spirit, Liu et al. (105) observed preferential reduction of thalamocortical connectivity during propofol sedation in the nonspecific (intralaminar) nuclei of the thalamus, supporting the potential involvement of a generalized thalamic demodulation of intracortical connectivity.
Schiff (106) proposed a mesocircuit hypothesis as a mechanistic link between striatopallidal and cortical functional changes in brain-injured patients diagnosed with unresponsive wakefulness or minimally conscious states. In some of these patients, overt behavioral responsiveness is absent but there is an indication of preserved high-level covert cognitive functioning such as command following (107,108). As proposed, frontal lobe injuries including mesial frontal, basal forebrain, and intralaminar thalamic lesions, can result in central thalamic disruption of the frontoparietal cortical network (104). The mesocircuit mechanism may also apply, with some modifications, to the disconnection of frontoparietal networks and the consequent functional-cognitive dissociation during general anesthesia. In fact, predominant reductions in functional activation and cortical connectivity of the putamen were found during propofol-induced unresponsiveness (59), suggesting an alternative subcortical pathway or complement to central thalamic modulation of cortical connectivity consistent with the mesocircuit hypothesis.
Finally, another possible reason for disparate results is that most neuroimaging studies applied a factorial design for statistical comparison across several anesthetic states; most commonly wakefulness, light sedation, deep sedation (unresponsiveness), and recovery (notable exceptions are (57,79,87)). While this makes sense from a statistical point of view, the critical neuronal event(s) associated with the loss or return of consciousness should be identified by a planned comparison between the anesthetic dose that produces a maximally sedated but still responsive state and the dose that produces the minimally sedated but unresponsive state. In other words, changes that occur between wakefulness and light sedation should be excluded from the neural correlates of unconsciousness. Testing the described contrast places a greater demand on statistical power than does ANOVA, but it focuses on the critical neuronal event of interest that is precisely associated with the conscious state transition. Another recommended approach is to manipulate the state of consciousness by an exogenous stimulus at constant anesthetic dose/concentration (79). The latter may also help minimize the potential confound from neuronal changes associated with prerequisites or consequences of the true state transition (35,109).
Temporal Fragmentation and the State Repertoire
In this final section, we consider another possible mechanism of network disconnection, the disruption of temporal coordination of neuronal activity. It has been known that in both non-rapid-eye-movement sleep and anesthesia, the temporal pattern of local neuronal activity shifts from virtually continuous spiking activity (typical for wakefulness) to an intermittent pattern in which actively spiking “up” states alternate with electrically silent “down” states (110–112). That such a transition occurs upon loss of consciousness was demonstrated in three epilepsy patients undergoing cortical electrode removal (113). Following the bolus injection of propofol producing rapid loss of responsiveness, cortical neuronal activity became intermittent, occurring in phase with slow, large-amplitude electrocortical waves that were spatially disorganized over long distances. In light of current theories, such spatiotemporal fragmentation of neuronal activity is incompatible with normal information integration as required for conscious experience (114). In a subsequent rat study, a gradual uncoupling of single neuron spiking activity within a local region of visual cortex was observed at graded, steady-state levels of desflurane anesthesia (115). The latter results were obtained from a stepwise emergence protocol, from deep anesthesia to wakefulness, suggesting that the fragmentation of neuronal activity (1) was reversible upon withdrawal of the anesthetic, (2) occurred also in local circuits, and (3) was generalizable to at least two major classes of anesthetics as represented by propofol and desflurane. Given that the fragmentation of activity seems to occur in a graded, dose-dependent manner, its effects on information integration and the level of consciousness should also be graded (116). Nevertheless, such gradation occurs not as much in the level (e.g. state of arousal, vigilance), but in the temporal continuity of the stream of conscious experience. As indicated previously, repeated interruptions of the temporal sequence of sensory frames would prevent their contextual integration across the immediate past and present sensory frames, a phenomenon we called the “forgotten present” (39). A well-known phenomenon to illustrate this effect is the loss of subjective perception of motion when movie frames are presented at a rate below fusion frequency. We propose that a failure of temporal integration or binding in all sensory and other domains is generally inconsistent with subjective experience of any kind and thus, inconsistent with consciousness.
Moving on to large-scale, intrinsic networks, most EEG and fMRI studies have derived functional or effective connectivity from relatively long data samples (several minutes) representing steady-state conditions. However, there is growing evidence that intrinsic networks of the brain are highly dynamic and change continually on a time frame of minutes to seconds (117). Moreover, global EEG topographic patterns change even faster, on a time scale of approximately 100ms (118) that correlate with fMRI (119,120). This underlines the dynamic nature of cognition and ongoing mentation directed to either intrinsic or extrinsic sources. Few studies have attempted to capture the effect of anesthetics on the dynamics of spontaneous ongoing brain activity at this time scale (121). Such analyses can be achieved using sliding window (122) or point process (123) methods, or coactivation patterns (124). Hudetz et al. investigated the effect of propofol on the spontaneous fluctuations of blood-oxygen-level dependent (BOLD) activation patterns on a time scale of 1 second (125). Increasing the propofol dose to a level that corresponds to unconsciousness significantly reduced the temporal variance of BOLD coactivations, suggesting that fewer network patterns occurred in the unconscious condition. Similar results were recently obtained in propofol-sedated monkeys: resting-state functional connectivity patterns were less frequent and lacked anticorrelations (negative correlations) normally seen during wakefulness suggesting a loss of the rich functional dynamics in anesthesia (126).
The repertoire of network patterns or “states” that the brain can access over time is another essential determinant of the degree of information integration necessary for consciousness (116). The observed decrease of the temporal variance of BOLD coactivations indicates sparsification of distinct functional connectivity patterns over time (127). Moreover, the temporal fragmentation of neuronal activity, discussed in the previous section, may contribute to a loss of large BOLD coactivation patterns by hindering the coordination and communication of neuronal activities over long distances in the brain. An equivalent way of saying this is that brain states become stereotypical (10) or excessively stabilized (92).
Which states the brain selects from its repertoire is an interesting question. In a recent study, Hudson et al. (128) examined the temporal dynamics of multisite local field potentials in the rat brain during recovery from deep anesthesia. They found that neuronal activity patterns formed metastable states that persisted on the scale of minutes. In each case, the brain passed through a specific set of metastable states in an orderly fashion en route to consciousness, suggesting that the process of recovery required the brain to visit a precisely coordinated sequence of states.
In summary, both spatiotemporal fragmentation and the associated reduction in the brain’s state repertoire appear to predict a gradual diminution of conscious experience. Future work should test this hypothesis with other types of anesthetics and investigate the significance of directional connectivity changes in spatiotemporal fragmentation of neuronal activity across the cerebral cortex.
Alternative Theories
We have been advocating a corticocentric view of consciousness, which is consistent with the view that the contents of consciousness are represented in the cerebral cortex. Nevertheless, it has been known since the early 1950s that specific diencephalic and brainstem structures are absolutely essential for the conscious state (9,106). Accordingly, a great deal of research into the neural correlates of consciousness, wakefulness, and their modulation by anesthetic agents has focused on subcortical structures. Critically important components of the ascending arousal system along the midline in the pontomesencephalic reticular formation and the thalamus have been a major the focus of interest. Lesions of specific midline nuclei of the pons and the intralaminar thalamus invariably lead to coma while the stimulation of these sites can help regain consciousness (29,106,129–131). The role of the thalamus in mediating anesthetic effects on consciousness has been investigated in at least two aspects. One of these is the presumed impediment of thalamocortical sensory transmission of sensory signals due to anesthetic hyperpolarization of the modality-specific thalamic relay nuclei, an effect that led to the Thalamic Switch Theory (56). Such an effect may principally reduce the bandwith of sensory signal transmission, i.e., the highest frequency of neuron action potentials that can be transmitted via the thalamus, an effect that is most clearly seen in the somatosensory domain. However, given the relative preservation of cortical auditory and visual evoked responses (18,57,86), a complete thalamocortical disconnection of neuronal pathways seems unlikely. Nevertheless, anesthetic agents may alter thalamocortical neuronal interactions in subtle ways that may interfere with the ongoing information exchange between thalamus and cortex as well as among different cortical sites. Higher order thalamic nuclei relay sensory signals between primary and secondary cortical sensory areas (132) that could be more sensitive to anesthetic interference than the primary sensory regions. Agent-specific changes in the EEG are well described (133) and may be mediated by an alteration in thalamocortical interactions (96,97,134). Another possible role of the thalamus in mediating the effect of anesthetics may be associated with its role in the modulation of the overall level of spontaneous cortical activity (cortical arousal). The latter effect is executed mainly by the thalamic intralaminar (sometimes called nonspecific) nuclei that are enriched by the distinct group of calbindin-immunoreactive matrix cells known to be part of arousal circuits that modulate the level of wakefulness. Propofol appears to preferentially target these nuclei (105) while pharmacological activation can restore spontaneous movement in anesthetized animals. Similar, reanimation effects have been achieved by stimulating several other subcortical sites, particularly the ventral tegmental area (135–139). It is possible that the behavioral expression of pharmacological reanimation is a reflection of a restoration of connected consciousness while the status of subjective consciousness is unknown. Although anesthetic agents may alter cortical function by acting on various brain targets directly or indirectly, it is arguable that their ultimate effect on consciousness will likely depend on the resulting state of cortical function.
Conclusion and Future Directions
From the forgoing discussion, a few conclusions emerge. Investigations to date converge to the conclusion that, with various anesthetic agents, a depression or disconnection of lateral frontoparietal networks appears to be a consistent correlate of disrupted connected consciousness, clinically observed as unresponsiveness.
As to future work, a more systematic delineation of directional connectivity changes with multiple anesthetics using the same experimental design and the same analytical method will be desirable. In all cases, the critical neural connectivity changes between responsive and unresponsive states at similar anesthetic doses should be assessed. There is a need for more theoretical/analytical work to identify a robust, sensitive, and reliable index of neural communication that is commonly accepted and applicable to various empirical measurement modalities. Finding a behavior-independent measure of subjective experience that can track the possible presence of covert cognition in overtly unresponsive individuals should be a major step toward the determination of the neural correlates of consciousness and unconsciousness. Finally, a delineation of causal factors versus correlated events will be necessary. This may require explicit, selective manipulation of neuronal communication along specific pathways to modulate the state of consciousness in human subjects under anesthesia.
Finally, our proposition focused on connected consciousness, an aspect that can be tracked with sensory-behavioral measures as afforded by clinical assessment. Consciousness in a more general sense, including internal sensations, feelings, mentation, etc., depends on the functionality of multiple brain systems, not only the frontoparietal network. Likewise, complete unconsciousness, the absence of all subjective experience that one may call oblivion, may depend on a more global disruption of communication in multiple brain networks. Future studies may address the neuronal basis of consciousness and its disruption by anesthetics in a more general sense.
Acknowledgments
Research reported in this publication was supported by the Institute of General Medical Sciences of the National Institutes of Health, Bethesda, MD under award numbers R01GM056398, R01GM103894, R01GM098578, R01GM111293 and by the James S. McDonnell Foundation, St. Louis, MO under award number 220020419. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and the McDonnell Foundation.
Appendix 1. Glossary of Concepts and Technical terms
Consciousness
The state in which subjective experience of any kind (sensory, volitional, emotional, visceral, etc.) is possible. It can be distinct from wakefulness.
Wakefulness
An autonomic state usually characterized by eye opening and behavioral responsiveness. It does not necessarily imply the presence of consciousness (e.g., wakefulness can occur in a vegetative state).
Awareness
The content of consciousness, what one is conscious of.
Anesthesia awareness
The postoperative recollection or memory of a stimulus or event consciously experienced during intended general anesthesia.
Connected consciousness
A state in which the subject consciously perceives environmental stimuli.
Disconnected consciousness
A state in which the awareness of external environmental stimuli and events is absent but during which there can still be conscious content (e.g., a dream state).
Functional connectivity
The temporal covariation of two signals (electroenecephalogram, local field potential, blood oxygen-dependent functional magnetic resonance imaging, etc.) that implies direct or indirect interdependence of underlying neuronal activity.
Effective connectivity
The covariation of two signals with a time lag in a well-defined temporal order that suggests (but does not demonstrate) causal influence.
Granger causality
A statistical measure of causal interdependence of two signals based on the predictability of one signal from the other.
Transfer Entropy
A nonlinear measure of causal interdependence of two signals based on the statistical predictability of one signal from the other.
Phase Lag Index
A robust measure of statistical interdependence of signals based on the consistent nonzero phase difference between two signals.
Dynamic Causal Modeling
A Bayesian statistical procedure to infer the effective connectivity of brain regions based on the selection from biologically plausible alternative models.
Default Mode Network
A collection of functionally connected cortical regions that exhibits relatively high baseline activity in the absence of exogenous stimulation or the subject’s engagement to task.
Footnotes
The authors declare no conflicts of interest.
Disclosures
Name: Anthony G. Hudetz, DBM, PhD
Contribution: Manuscript preparation.
Attestation: Approved the final manuscript.
Name: George A. Mashour, MD, PhD
Contribution: Manuscript preparation.
Attestation: Approved the final manuscript.
This manuscript was handled by: Gregory Crosby, MD
Contributor Information
Anthony G. Hudetz, Department of Anesthesiology, Center for Consciousness Science, University of Michigan, Ann Arbor, Michigan.
George A. Mashour, Department of Anesthesiology, Center for Consciousness Science, University of Michigan, Ann Arbor, Michigan.
References
- 1.Sanders RD, Tononi G, Laureys S, Sleigh JW. Unresponsiveness not equal unconsciousness. Anesthesiology. 2012;116:946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warnaby CE, Seretny M, Mhuircheartaigh RN, Rogers R, Jbabdi S, Sleigh J, Tracey I. Anesthesia-induced Suppression of Human Dorsal Anterior Insula Responsivity at Loss of Volitional Behavioral Response. Anesthesiology. 2016 doi: 10.1097/ALN.0000000000001027. [DOI] [PubMed] [Google Scholar]
- 3.Noreika V, Jylhankangas L, Moro L, Valli K, Kaskinoro K, Aantaa R, Scheinin H, Revonsuo A. Consciousness lost and found: subjective experiences in an unresponsive state. Brain Cogn. 2011;77:327–334. doi: 10.1016/j.bandc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Mashour GA. Cognitive unbinding: a neuroscientific paradigm of general anesthesia and related states of unconsciousness. Neurosci Biobehav Rev. 2013;37:2751–2759. doi: 10.1016/j.neubiorev.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boly M, Sanders RD, Mashour GA, Laureys S. Consciousness and responsiveness: lessons from anaesthesia and the vegetative state. Curr Opin Anaesthesiol. 2013;26:444–449. doi: 10.1097/ACO.0b013e3283628b5d. [DOI] [PubMed] [Google Scholar]
- 6.Bonhomme V, Boveroux P, Brichant JF, Laureys S, Boly M. Neural correlates of consciousness during general anesthesia using functional magnetic resonance imaging (fMRI) Arch Ital Biol. 2012;150:155–163. doi: 10.4449/aib.v150i2.1242. [DOI] [PubMed] [Google Scholar]
- 7.Nallasamy N, Tsao DY. Functional connectivity in the brain: effects of anesthesia. Neuroscientist. 2011;17:94–106. doi: 10.1177/1073858410374126. [DOI] [PubMed] [Google Scholar]
- 8.Franks NP, Zecharia AY. Sleep and general anesthesia. Can J Anaesth. 2011;58:139–148. doi: 10.1007/s12630-010-9420-3. [DOI] [PubMed] [Google Scholar]
- 9.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–2650. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boly M, Seth AK, Wilke M, Ingmundson P, Baars B, Laureys S, Edelman DB, Tsuchiya N. Consciousness in humans and non-human animals: recent advances and future directions. Front Psychol. 2013;4:625. doi: 10.3389/fpsyg.2013.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhrig L, Dehaene S, Jarraya B. Cerebral mechanisms of general anesthesia. Ann Fr Anesth Reanim. 2014;33:72–82. doi: 10.1016/j.annfar.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K. The role of dendritic signaling in the anesthetic suppression of consciousness. Anesthesiology. 2015;122:1415–1431. doi: 10.1097/ALN.0000000000000673. [DOI] [PubMed] [Google Scholar]
- 14.Langsjo JW, Revonsuo A, Scheinin H. Harnessing anesthesia and brain imaging for the study of human consciousness. Curr Pharm Des. 2014;20:4211–4224. [PubMed] [Google Scholar]
- 15.Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 16.Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett. 2005;387:145–150. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- 19.Hudetz AG. General anesthesia and human brain connectivity. Brain Connect. 2012;2:291–302. doi: 10.1089/brain.2012.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan D, Ilg R, Riedl V, Schorer A, Grimberg S, Neufang S, Omerovic A, Berger S, Untergehrer G, Preibisch C, Schulz E, Schuster T, Schroter M, Spoormaker V, Zimmer C, Hemmer B, Wohlschlager A, Kochs EF, Schneider G. Simultaneous electroencephalographic and functional magnetic resonance imaging indicate impaired cortical top-down processing in association with anesthetic-induced unconsciousness. Anesthesiology. 2013;119:1031–1042. doi: 10.1097/ALN.0b013e3182a7ca92. [DOI] [PubMed] [Google Scholar]
- 21.Ku SW, Lee U, Noh GJ, Jun IG, Mashour GA. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS One. 2011;6:e25155. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee U, Kim S, Noh GJ, Choi BM, Hwang E, Mashour GA. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009;18:1069–1078. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of frontal-parietal communication by ketamine, propofol, and sevoflurane. Anesthesiology. 2013;118:1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthukumaraswamy SD, Shaw AD, Jackson LE, Hall J, Moran R, Saxena N. Evidence that Subanesthetic Doses of Ketamine Cause Sustained Disruptions of NMDA and AMPA-Mediated Frontoparietal Connectivity in Humans. J Neurosci. 2015;35:11694–11706. doi: 10.1523/JNEUROSCI.0903-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noirhomme Q, Soddu A, Lehembre R, Vanhaudenhuyse A, Boveroux P, Boly M, Laureys S. Brain connectivity in pathological and pharmacological coma. Front Syst Neurosci. 2010;4:160. doi: 10.3389/fnsys.2010.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S, Friston K. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332:858–862. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- 27.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckner RL. The brain's default network: origins and implications for the study of psychosis. Dialogues Clin Neurosci. 2013;15:351–358. doi: 10.31887/DCNS.2013.15.3/rbuckner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannawi Y, Lindquist MA, Caffo BS, Sair HI, Stevens RD. Resting brain activity in disorders of consciousness: a systematic review and meta-analysis. Neurology. 2015;84:1272–1280. doi: 10.1212/WNL.0000000000001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raichle ME. The neural correlates of consciousness: an analysis of cognitive skill learning. Philos Trans R Soc Lond B Biol Sci. 1998;353:1889–1901. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- 32.Baars BJ, Laureys S. One, not two, neural correlates of consciousness. Trends Cogn Sci. 2005;9:269. doi: 10.1016/j.tics.2005.04.008. author reply 70. [DOI] [PubMed] [Google Scholar]
- 33.Tononi G, Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- 34.Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Aru J, Bachmann T, Singer W, Melloni L. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev. 2012;36:737–746. doi: 10.1016/j.neubiorev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Rees G. Neural correlates of consciousness. Ann N Y Acad Sci. 2013;1296:4–10. doi: 10.1111/nyas.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann T. On the brain-imaging markers of neural correlates of consciousness. Front Psychol. 2015;6:868. doi: 10.3389/fpsyg.2015.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci. 2010;14:277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Hudetz AG. Suppressing Consciousness: mechanisms of general anesthesia. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25:196–204. [Google Scholar]
- 40.Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tononi G, Koch C. Consciousness: here, there and everywhere? Philos Trans R Soc Lond B Biol Sci. 2015;370 doi: 10.1098/rstb.2014.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, Casarotto S, Bruno MA, Laureys S, Tononi G, Massimini M. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med. 2013;5:198ra05. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- 43.Schartner M, Seth A, Noirhomme Q, Boly M, Bruno MA, Laureys S, Barrett A. Complexity of Multi-Dimensional Spontaneous EEG Decreases during Propofol Induced General Anaesthesia. PLoS One. 2015;10:e0133532. doi: 10.1371/journal.pone.0133532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edelman GM, Gally JA. Reentry: a key mechanism for integration of brain function. Front Integr Neurosci. 2013;7:63. doi: 10.3389/fnint.2013.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Super H, Spekreijse H, Lamme VA. Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) Nat Neurosci. 2001;4:304–310. doi: 10.1038/85170. [DOI] [PubMed] [Google Scholar]
- 46.Self MW, Kooijmans RN, Super H, Lamme VA, Roelfsema PR. Different glutamate receptors convey feedforward and recurrent processing in macaque V1. Proc Natl Acad Sci U S A. 2012;109:11031–11036. doi: 10.1073/pnas.1119527109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanhaudenhuyse A, Demertzi A, Schabus M, Noirhomme Q, Bredart S, Boly M, Phillips C, Soddu A, Luxen A, Moonen G, Laureys S. Two distinct neuronal networks mediate the awareness of environment and of self. J Cogn Neurosci. 2011;23:570–578. doi: 10.1162/jocn.2010.21488. [DOI] [PubMed] [Google Scholar]
- 48.Demertzi A, Soddu A, Laureys S. Consciousness supporting networks. Curr Opin Neurobiol. 2013;23:239–244. doi: 10.1016/j.conb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. doi: 10.1089/brain.2011.0008. [DOI] [PubMed] [Google Scholar]
- 50.Friston K, Moran R, Seth AK. Analysing connectivity with Granger causality and dynamic causal modelling. Curr Opin Neurobiol. 2013;23:172–178. doi: 10.1016/j.conb.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stam CJ, van Straaten EC. Go with the flow: use of a directed phase lag index (dPLI) to characterize patterns of phase relations in a large-scale model of brain dynamics. Neuroimage. 2012;62:1415–1428. doi: 10.1016/j.neuroimage.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 52.Schreiber T. Measuring information transfer. Phys Rev Lett. 2000;85:461–464. doi: 10.1103/PhysRevLett.85.461. [DOI] [PubMed] [Google Scholar]
- 53.Seth AK, Edelman GM. Distinguishing causal interactions in neural populations. Neural Comput. 2007;19:910–933. doi: 10.1162/neco.2007.19.4.910. [DOI] [PubMed] [Google Scholar]
- 54.John ER, Prichep LS, Kox W, Valdes-Sosa P, Bosch-Bayard J, Aubert E, Tom M, diMichele F, Gugino LD. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10:165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- 55.John ER. The neurophysics of consciousness. Brain Res Brain Res Rev. 2002;39:1–28. doi: 10.1016/s0165-0173(02)00142-x. [DOI] [PubMed] [Google Scholar]
- 56.White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Lauer KK, Ward BD, Rao SM, Li SJ, Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012;33:2487–2498. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Plourde G, Belin P, Chartrand D, Fiset P, Backman SB, Xie G, Zatorre RJ. Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology. 2006;104:448–457. doi: 10.1097/00000542-200603000-00011. [DOI] [PubMed] [Google Scholar]
- 59.Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hentschke H, Schwarz C, Antkowiak B. Neocortex is the major target of sedative concentrations of volatile anaesthetics: strong depression of firing rates and increase of GABAA receptor-mediated inhibition. Eur J Neurosci. 2005;21:93–102. doi: 10.1111/j.1460-9568.2004.03843.x. [DOI] [PubMed] [Google Scholar]
- 61.Tallon-Baudry C, Bertrand O, Delpuech C, Pernier J. Stimulus specificity of phase-locked and non-phase-locked 40 Hz visual responses in human. J Neurosci. 1996;16:4240–4249. doi: 10.1523/JNEUROSCI.16-13-04240.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dehaene S, Sergent C, Changeux JP. A neuronal network model linking subjective reports and objective physiological data during conscious perception. Proc Natl Acad Sci U S A. 2003;100:8520–8525. doi: 10.1073/pnas.1332574100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cauller LJ, Kulics AT. The neural basis of the behaviorally relevant N1 component of the somatosensory-evoked potential in SI cortex of awake monkeys: evidence that backward cortical projections signal conscious touch sensation. Exp Brain Res. 1991;84:607–619. doi: 10.1007/BF00230973. [DOI] [PubMed] [Google Scholar]
- 64.Cauller L. Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav Brain Res. 1995;71:163–170. doi: 10.1016/0166-4328(95)00032-1. [DOI] [PubMed] [Google Scholar]
- 65.Jackson ME, Cauller LJ. Neural activity in SII modifies sensory evoked potentials in SI in awake rats. Neuroreport. 1998;9:3379–3382. doi: 10.1097/00001756-199810260-00008. [DOI] [PubMed] [Google Scholar]
- 66.Shao Z, Burkhalter A. Different balance of excitation and inhibition in forward and feedback circuits of rat visual cortex. J Neurosci. 1996;16:7353–7365. doi: 10.1523/JNEUROSCI.16-22-07353.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- 68.Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- 69.Raz A, Grady SM, Krause BM, Uhlrich DJ, Manning KA, Banks MI. Preferential effect of isoflurane on top-down vs. bottom-up pathways in sensory cortex. Front Syst Neurosci. 2014;8:191. doi: 10.3389/fnsys.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Del Cul A, Baillet S, Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5:e260. doi: 10.1371/journal.pbio.0050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Evoked brain responses are generated by feedback loops. Proc Natl Acad Sci U S A. 2007;104:20961–20966. doi: 10.1073/pnas.0706274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. 2007;27:2858–2865. doi: 10.1523/JNEUROSCI.4623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hudetz AG, Vizuete JA, Imas OA. Desflurane selectively suppresses long-latency cortical neuronal response to flash in the rat. Anesthesiology. 2009;111:231–239. doi: 10.1097/ALN.0b013e3181ab671e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pal D, Hambrecht-Wiedbusch VS, Silverstein BH, Mashour GA. Electroencephalographic coherence and cortical acetylcholine during ketamine-induced unconsciousness. British Journal of Anaesthesia. 2015;114(6):979–989. doi: 10.1093/bja/aev095. Br J Anaesth 2015; 115 Suppl 1:i77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moran RJ, Jones MW, Blockeel AJ, Adams RA, Stephan KE, Friston KJ. Losing control under ketamine: suppressed cortico-hippocampal drive following acute ketamine in rats. Neuropsychopharmacology. 2015;40:268–277. doi: 10.1038/npp.2014.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reinhold K, Lien AD, Scanziani M. Distinct recurrent versus afferent dynamics in cortical visual processing. Nat Neurosci. 2015;18:1789–1797. doi: 10.1038/nn.4153. [DOI] [PubMed] [Google Scholar]
- 77.Imas OA, Ropella KM, Wood JD, Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–221. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 78.Hudetz AG. Feedback suppression in anesthesia. Is it reversible? Conscious Cogn. 2009;18:1079–1081. doi: 10.1016/j.concog.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 79.Langsjo JW, Alkire MT, Kaskinoro K, Hayama H, Maksimow A, Kaisti KK, Aalto S, Aantaa R, Jaaskelainen SK, Revonsuo A, Scheinin H. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012;32:4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sleigh JW. The study of consciousness comes of age. Anesthesiology. 2013;118:1245–1246. doi: 10.1097/ALN.0b013e318291031f. [DOI] [PubMed] [Google Scholar]
- 81.Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, Ledoux D, Bonhomme V, Brichant JF, Tononi G, Laureys S, Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mashour GA. Consciousness and the 21st century operating room. Anesthesiology. 2013;119:1003–1005. doi: 10.1097/ALN.0b013e3182a7cad1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blain-Moraes S, Tarnal V, Vanini G, Alexander A, Rosen D, Shortal B, Janke E, Mashour GA. Neurophysiological correlates of sevoflurane-induced unconsciousness. Anesthesiology. 2015;122:307–316. doi: 10.1097/ALN.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Palanca BJ, Mitra A, Larson-Prior L, Snyder AZ, Avidan MS, Raichle ME. Resting-state Functional Magnetic Resonance Imaging Correlates of Sevoflurane-induced Unconsciousness. Anesthesiology. 2015;123:346–356. doi: 10.1097/ALN.0000000000000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moon JY, Lee U, Blain-Moraes S, Mashour GA. General relationship of global topology, local dynamics, and directionality in large-scale brain networks. PLoS Comput Biol. 2015;11:e1004225. doi: 10.1371/journal.pcbi.1004225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guldenmund P, Demertzi A, Boveroux P, Boly M, Vanhaudenhuyse A, Bruno MA, Gosseries O, Noirhomme Q, Brichant JF, Bonhomme V, Laureys S, Soddu A. Thalamus, brainstem and salience network connectivity changes during propofol-induced sedation and unconsciousness. Brain Connect. 2013;3:273–285. doi: 10.1089/brain.2012.0117. [DOI] [PubMed] [Google Scholar]
- 87.Akeju O, Loggia ML, Catana C, Pavone KJ, Vazquez R, Rhee J, Contreras Ramirez V, Chonde DB, Izquierdo-Garcia D, Arabasz G, Hsu S, Habeeb K, Hooker JM, Napadow V, Brown EN, Purdon PL. Disruption of thalamic functional connectivity is a neural correlate of dexmedetomidine-induced unconsciousness. Elife. 2014;4:e04499. doi: 10.7554/eLife.04499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X, Li SJ, Hudetz AG. Increased precuneus connectivity during propofol sedation. Neurosci Lett. 2014;561:18–23. doi: 10.1016/j.neulet.2013.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barrett AB, Murphy M, Bruno MA, Noirhomme Q, Boly M, Laureys S, Seth AK. Granger causality analysis of steady-state electroencephalographic signals during propofol-induced anaesthesia. PLoS One. 2012;7:e29072. doi: 10.1371/journal.pone.0029072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicolaou N, Hourris S, Alexandrou P, Georgiou J. EEG-based automatic classification of 'awake' versus 'anesthetized' state in general anesthesia using Granger causality. PLoS One. 2012;7:e33869. doi: 10.1371/journal.pone.0033869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maksimow A, Silfverhuth M, Langsjo J, Kaskinoro K, Georgiadis S, Jaaskelainen S, Scheinin H. Directional connectivity between frontal and posterior brain regions is altered with increasing concentrations of propofol. PLoS One. 2014;9:e113616. doi: 10.1371/journal.pone.0113616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solovey G, Alonso LM, Yanagawa T, Fujii N, Magnasco MO, Cecchi GA, Proekt A. Loss of Consciousness Is Associated with Stabilization of Cortical Activity. J Neurosci. 2015;35:10866–10877. doi: 10.1523/JNEUROSCI.4895-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dentico D, Cheung BL, Chang JY, Guokas J, Boly M, Tononi G, Van Veen B. Reversal of cortical information flow during visual imagery as compared to visual perception. Neuroimage. 2014;100:237–243. doi: 10.1016/j.neuroimage.2014.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004;50:257–266. doi: 10.1159/000079981. [DOI] [PubMed] [Google Scholar]
- 95.Purdon PL, Pierce ET, Mukamel EA, Prerau MJ, Walsh JL, Wong KF, Salazar-Gomez AF, Harrell PG, Sampson AL, Cimenser A, Ching S, Kopell NJ, Tavares-Stoeckel C, Habeeb K, Merhar R, Brown EN. Electroencephalogram signatures of loss and recovery of consciousness from propofol. Proc Natl Acad Sci U S A. 2013;110:E1142–E1151. doi: 10.1073/pnas.1221180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ching S, Cimenser A, Purdon PL, Brown EN, Kopell NJ. Thalamocortical model for a propofol-induced alpha-rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:22665–22670. doi: 10.1073/pnas.1017069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vijayan S, Ching S, Purdon PL, Brown EN, Kopell NJ. Thalamocortical mechanisms for the anteriorization of alpha rhythms during propofol-induced unconsciousness. J Neurosci. 2013;33:11070–11075. doi: 10.1523/JNEUROSCI.5670-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blain-Moraes S, Lee U, Ku S, Noh G, Mashour GA. Electroencephalographic effects of ketamine on power, cross-frequency coupling, and connectivity in the alpha bandwidth. Front Syst Neurosci. 2014;8:114. doi: 10.3389/fnsys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou DW, Mowrey DD, Tang P, Xu Y. Percolation Model of Sensory Transmission and Loss of Consciousness Under General Anesthesia. Phys Rev Lett. 2015;115:108103. doi: 10.1103/PhysRevLett.115.108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu X, Yanagawa T, Leopold DA, Chang C, Ishida H, Fujii N, Duyn JH. Arousal transitions in sleep, wakefulness, and anesthesia are characterized by an orderly sequence of cortical events. Neuroimage. 2015;116:222–231. doi: 10.1016/j.neuroimage.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee H, Mashour GA, Noh GJ, Kim S, Lee U. Reconfiguration of network hub structure after propofol-induced unconsciousness. Anesthesiology. 2013;119:1347–1359. doi: 10.1097/ALN.0b013e3182a8ec8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heinke W, Fiebach CJ, Schwarzbauer C, Meyer M, Olthoff D, Alter K. Sequential effects of propofol on functional brain activation induced by auditory language processing: an event-related functional magnetic resonance imaging study. Br J Anaesth. 2004;92:641–650. doi: 10.1093/bja/aeh133. [DOI] [PubMed] [Google Scholar]
- 103.Deshpande G, Kerssens C, Sebel PS, Hu X. Altered local coherence in the default mode network due to sevoflurane anesthesia. Brain Res. 2010;1318:110–121. doi: 10.1016/j.brainres.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fridman EA, Beattie BJ, Broft A, Laureys S, Schiff ND. Regional cerebral metabolic patterns demonstrate the role of anterior forebrain mesocircuit dysfunction in the severely injured brain. Proc Natl Acad Sci U S A. 2014;111:6473–6478. doi: 10.1073/pnas.1320969111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu X, Lauer KK, Ward BD, Li SJ, Hudetz AG. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monti MM, Vanhaudenhuyse A, Coleman MR, Boly M, Pickard JD, Tshibanda L, Owen AM, Laureys S. Willful modulation of brain activity in disorders of consciousness. N Engl J Med. 2010;362:579–589. doi: 10.1056/NEJMoa0905370. [DOI] [PubMed] [Google Scholar]
- 108.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 109.Bachmann T, Hudetz AG. It is time to combine the two main traditions in the research on the neural correlates of consciousness: C = L × D. Front Psychol. 2014;5:940. doi: 10.3389/fpsyg.2014.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kasanetz F, Riquelme LA, Murer MG. Disruption of the two-state membrane potential of striatal neurones during cortical desynchronisation in anaesthetised rats. J Physiol. 2002;543:577–589. doi: 10.1113/jphysiol.2002.0024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT. Cyclic and sleep-like spontaneous alternations of brain state under urethane anaesthesia. PLoS One. 2008;3:e2004. doi: 10.1371/journal.pone.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic 'up' states fragments of wakefulness? Trends Neurosci. 2007;30:334–342. doi: 10.1016/j.tins.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–E3386. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mashour GA. Fragmenting consciousness. Proc Natl Acad Sci U S A. 2012;109:19876–19877. doi: 10.1073/pnas.1217595109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vizuete JA, Pillay S, Ropella KM, Hudetz AG. Graded defragmentation of cortical neuronal firing during recovery of consciousness in rats. Neuroscience. 2014;275:340–351. doi: 10.1016/j.neuroscience.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oizumi M, Albantakis L, Tononi G. From the phenomenology to the mechanisms of consciousness: Integrated Information Theory 3.0. PLoS Comput Biol. 2014;10:e1003588. doi: 10.1371/journal.pcbi.1003588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koenig T, Prichep L, Lehmann D, Sosa PV, Braeker E, Kleinlogel H, Isenhart R, John ER. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage. 2002;16:41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- 119.Britz J, Van De Ville D, Michel CM. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage. 2010;52:1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 120.Musso F, Brinkmeyer J, Mobascher A, Warbrick T, Winterer G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. Neuroimage. 2010;52:1149–1161. doi: 10.1016/j.neuroimage.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 121.Hutchison RM, Hutchison M, Manning KY, Menon RS, Everling S. Isoflurane induces dose-dependent alterations in the cortical connectivity profiles and dynamic properties of the brain's functional architecture. Hum Brain Mapp. 2014;35:5754–5775. doi: 10.1002/hbm.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang C, Liu Z, Chen MC, Liu X, Duyn JH. EEG correlates of time-varying BOLD functional connectivity. Neuroimage. 2013;72:227–236. doi: 10.1016/j.neuroimage.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Criticality in large-scale brain FMRI dynamics unveiled by a novel point process analysis. Front Physiol. 2012;3:15. doi: 10.3389/fphys.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu X, Chang C, Duyn JH. Decomposition of spontaneous brain activity into distinct fMRI co-activation patterns. Front Syst Neurosci. 2013;7:101. doi: 10.3389/fnsys.2013.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hudetz AG, Liu X, Pillay S. Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connect. 2015;5:10–22. doi: 10.1089/brain.2014.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barttfeld P, Uhrig L, Sitt JD, Sigman M, Jarraya B, Dehaene S. Signature of consciousness in the dynamics of resting-state brain activity. Proc Natl Acad Sci U S A. 2015;112:887–892. doi: 10.1073/pnas.1418031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee U, Oh G, Kim S, Noh G, Choi B, Mashour GA. Brain networks maintain a scale-free organization across consciousness, anesthesia, and recovery: evidence for adaptive reconfiguration. Anesthesiology. 2010;113:1081–1091. doi: 10.1097/ALN.0b013e3181f229b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hudson AE, Calderon DP, Pfaff DW, Proekt A. Recovery of consciousness is mediated by a network of discrete metastable activity states. Proc Natl Acad Sci U S A. 2014;111:9283–9288. doi: 10.1073/pnas.1408296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–272. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 130.Schiff ND. Central thalamic deep-brain stimulation in the severely injured brain: rationale and proposed mechanisms of action. Ann N Y Acad Sci. 2009;1157:101–116. doi: 10.1111/j.1749-6632.2008.04123.x. [DOI] [PubMed] [Google Scholar]
- 131.Schiff ND. Central thalamic deep brain stimulation for support of forebrain arousal regulation in the minimally conscious state. Handb Clin Neurol. 2013;116:295–306. doi: 10.1016/B978-0-444-53497-2.00024-3. [DOI] [PubMed] [Google Scholar]
- 132.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 133.Purdon PL, Sampson A, Pavone KJ, Brown EN. Clinical Electroencephalography for Anesthesiologists: Part I: Background and Basic Signatures. Anesthesiology. 2015;123:937–960. doi: 10.1097/ALN.0000000000000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gili T, Saxena N, Diukova A, Murphy K, Hall JE, Wise RG. The thalamus and brainstem act as key hubs in alterations of human brain network connectivity induced by mild propofol sedation. J Neurosci. 2013;33:4024–4031. doi: 10.1523/JNEUROSCI.3480-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chemali JJ, Van Dort CJ, Brown EN, Solt K. Active emergence from propofol general anesthesia is induced by methylphenidate. Anesthesiology. 2012;116:998–1005. doi: 10.1097/ALN.0b013e3182518bfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kenny JD, Taylor NE, Brown EN, Solt K. Dextroamphetamine (but Not Atomoxetine) Induces Reanimation from General Anesthesia: Implications for the Roles of Dopamine and Norepinephrine in Active Emergence. PLoS One. 2015;10:e0131914. doi: 10.1371/journal.pone.0131914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Solt K, Cotten JF, Cimenser A, Wong KF, Chemali JJ, Brown EN. Methylphenidate actively induces emergence from general anesthesia. Anesthesiology. 2011;115:791–803. doi: 10.1097/ALN.0b013e31822e92e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Solt K, Van Dort CJ, Chemali JJ, Taylor NE, Kenny JD, Brown EN. Electrical stimulation of the ventral tegmental area induces reanimation from general anesthesia. Anesthesiology. 2014;121:311–319. doi: 10.1097/ALN.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Taylor NE, Chemali JJ, Brown EN, Solt K. Activation of D1 dopamine receptors induces emergence from isoflurane general anesthesia. Anesthesiology. 2013;118:30–39. doi: 10.1097/ALN.0b013e318278c896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 2005;102:937–947. doi: 10.1097/00000542-200505000-00012. [DOI] [PubMed] [Google Scholar]
- 141.Stamatakis EA, Adapa RM, Absalom AR, Menon DK. Changes in resting neural connectivity during propofol sedation. PLoS One. 2010;5:e14224. doi: 10.1371/journal.pone.0014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2010;49:823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, Bruno MA, Laureys S, Phillips C, Pelegrini-Issac M, Maquet P, Benali H. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 144.Yanagawa T, Chao ZC, Hasegawa N, Fujii N. Large-scale information flow in conscious and unconscious states: an ECoG study in monkeys. PLoS One. 2013;8:e80845. doi: 10.1371/journal.pone.0080845. [DOI] [PMC free article] [PubMed] [Google Scholar]