Abstract
Background
Intervention studies among individuals in diverse community settings are needed to reduce health disparities in colorectal cancer (CRC) screening and mortality rates. The current study compared the efficacy of two intervention conditions promoting CRC screening among Black individuals.
Methods
Black individuals (aged 50-75, N=330) were recruited in community settings in four Tampa Bay counties. Following consent and a baseline interview which assessed sociodemographic and health-related variables, participants received a culturally-targeted CRC photonovella booklet plus fecal immunochemical test (FIT) kit or a standard CRC screening brochure plus FIT kit. The primary outcome was FIT kit screening uptake.
Results
FIT screening uptake at 6 months was 86.7% overall (90.3% in the brochure group and 81.9% in the photonovella group). Controlling for baseline between group differences, there was no influence of intervention on FIT kit uptake (p=.756). Significant predictors of not returning a FIT kit included being unable to work (p=.010), having higher religious belief scores (p=.015), and living further from the cancer center (p=.015).
Conclusions
Providing FIT kits and educational print materials to Black individuals in community settings resulted in high rates of CRC screening. The study also identified subgroups of participants who were less likely to return a FIT kit and provides insight for future interventions.
Keywords: colorectal cancer, cancer screening, intervention trial, culturally-targeted, minority health
Introduction
Among men and women, colorectal cancer (CRC) is the third leading cancer in both incidence and mortality in the United States.1 Completion of CRC screening tests at intervals specified by national guidelines can reduce CRC incidence and mortality.1,2 Multiple screening modalities with varied screening intervals exist for those at average CRC risk (i.e., colonoscopy every 10 years, flexible sigmoidoscopy every 5 years, double-contrast barium enema every 5 years, computed tomographic colonography every 5 years, stool DNA test, annual fecal occult blood test [FOBT], or annual fecal immunochemical test [FIT]).1,2
Blacks have the highest CRC incidence and mortality compared to other racial and ethnic groups.1 At the current rate of about 56%1, screening rates among Blacks fall well below the National Colorectal Cancer Roundtable 80% target goal by 20183,4 and the Healthy People 2020 goals of 70.5%5. Screening rates are also lower among socioeconomically disadvantaged populations, foreign-born individuals, and those with certain psychosocial characteristics (e.g., lack of perceived susceptibility). 1,6-8
Based upon these disparities, as well as the increasingly ethnically-diverse Black population in the United States and Florida specifically9-13 a culturally-targeted intervention was designed to promote CRC screening among US-born and foreign-born Blacks who were not currently up-to-date with CRC screening. The rationale for selecting a photonovella approach was based on our formative work which revealed community members wanted printed, portable educational materials that were relevant to their cultural characteristics.14,15 As background, a photonovella tells a story using photos and limited text to present concepts (in this case, CRC screening). They are often more engaging, empowering, and entertaining than a traditional educational brochures. Photonovellas aim to tell a story that matches the cultural and literacy level preferences of the intended audience.
Culturally-targeted and tailored interventions have been studied among diverse groups to promote a number of health behaviors.16-21 One study to increase FOBT-uptake among rural African-American women found that women in the culturally-targeted and self-empowering group were significantly more likely to return a FOBT kit than did individuals in other intervention groups.18 Women in the modified cultural group were also significantly more likely to complete an FOBT than those in the traditional group.18 Prior literature about culturally-targeted studies have suggested that targeting may be more effective with certain subgroups (based upon personal factors such as acculturation),19 whereas other culturally-targeted studies have found no significant differences between groups.21,22
The current study tested the efficacy of a culturally-targeted photonovella plus FIT kit intervention (referred to hereafter as Photonovella+FIT) to increase screening uptake among Blacks who were not up-to-date with CRC screening compared to a standard Centers for Disease Control and Prevention “Screen for Life” brochure (not targeted for Blacks) plus FIT kit (referred to hereafter as Brochure+FIT). The study addressed the following research questions:
Are there intervention group differences in FIT kit return?
What sociodemographic and health-related variables are associated with not returning a FIT kit?
The primary hypothesis was that Photonovella+FIT would be associated with greater uptake compared to Brochure+FIT. This manuscript reports initial uptake results and evaluates intervention group differences and factors associated with intervention effects.
Methods
Participants received one of two interventions: Photonovella+FIT or Brochure+FIT. The current study used community-based participatory research methods. A community advisory board (CAB) comprised of three lay advisors and a primary care provider gave input on the photonovella design. The storyline, content, graphics, and photos were informed by our prior work,14,15 conceptualized with the help of the CAB, and tested with community members in an iterative development process. With low literacy and visual appeal in mind, the photonovella featured bright colors, photos and graphics, and a conversational dialog. To increase appeal for individuals who immigrated from or had ties to Caribbean nations, the cover depicted a map including Florida and the Caribbean and flags of the United States and multiple Afro-Caribbean nations. Educational information on intestinal anatomy, CRC, and CRC screening tests were illustrated through graphics, text boxes, and dialog. Educational messages addressed constructs of the Preventive Health Model (PHM) framework including susceptibility, salience and coherence of CRC screening, self-efficacy, response efficacy, and barriers (e.g., fear of finding an abnormal result).6,23-25 Participants in both conditions were provided with a FIT kit, written and verbal instructions, and a postage paid envelope to return the kit for processing.
Study sample
The University of South Florida Institutional Review Board approved the HIPPA-compliant study procedures. Eligible participants included those who: 1) self-identified as Black or African-American, 2) were aged 50-75, 3) were not up-to-date per guidelines, 4) were at average risk with no CRC symptoms, 5) were willing to provide at least two forms of contact information and the contact information of a secondary individual, and 6) could speak, read, and write English. Individuals at increased CRC risk due to having one first-degree relative with CRC diagnosed age ≤60, ≥2 first-degree relatives with CRC, or a personal history of CRC, adenomas, or inflammatory bowel disease were not eligible.
Procedure
The study was conducted in four Tampa Bay area counties divided into two contiguous regions of comparable population size separated by an interstate highway. One region was randomly assigned Photonovella+FIT and the other received Brochure+FIT. To reduce risk of intervention contamination, all individuals living in a given region received the same intervention and research assistants were randomly assigned to work in one region.
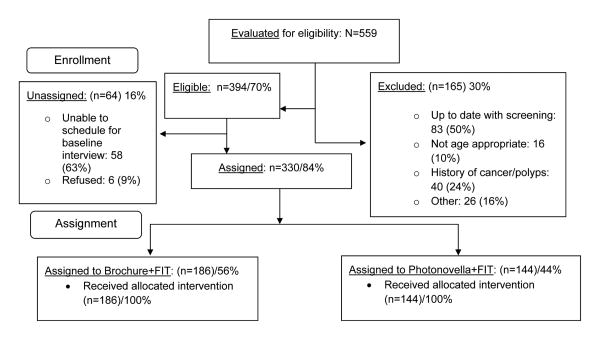
Recruitment details are reported elsewhere (Davis et al., under review). Briefly, participants were recruited through passive (e.g., newspaper and online ads), active (i.e., face-to-face intercepts), and snowball (i.e., peer referrals by previously-enrolled participants) methods. Of 559 individuals evaluated for eligibility, 394 were eligible and 330 were enrolled and received an intervention (Figure 1). Based upon the geographic location of their residence, forty-four percent (N=144) were enrolled in Photonovella+FIT.
Figure 1. CONSORT diagram.
A brief questionnaire was used to determine study eligibility. Following informed consent among those eligible and interested, but prior to intervention delivery, research assistants conducted baseline in-person interviews assessing demographics, CRC awareness, prior CRC screening behavior, and health beliefs. Participants received a $20 gift card for baseline interview completion, requiring 45-60 minutes. Next, participants were provided Photonovella+FIT or Brochure+FIT. Reminder letters were mailed to participants who had not returned the FIT kit 2-4 weeks after study entry. In addition, a postcard was sent to all participants in March as part of CRC Awareness Month. Returned FIT kits were processed by the cancer center's clinical lab. Participants returning a FIT kit were called and mailed a physician-signed results letter.
Measures
Screening uptake
Screening uptake was based on receipt of a completed FIT kit by the study team within 180 days of enrollment (no or yes). Time to FIT kit return was calculated based on date FIT kit was provided (study enrollment date) and date completed FIT kit was received.
PHM variables
The PHM questionnaire consists of 7 subscales each measuring a validated multi-item construct with all items using a 5-point response scale (1=“strongly disagree” to 5=“strongly agree”) (see Table 1).15,24-27 These constructs have been previously used in research with African Americans.26,27 Perceived susceptibility assessed participants' perceived chances of developing CRC or polyps. Response efficacy assessed participants' beliefs about whether CRC can be detected early through screening and whether removal of CRC polyps can prevent CRC. Salience and coherence measured participants' beliefs regarding importance and salience of CRC screening. The last item was reverse coded for scoring. Cancer worry assessed the extent to which participants worried about having positive screening results. Social influence measured perceived social support for CRC screening from family/friends and healthcare provider and desire to comply with family/friends and healthcare provider support for CRC screening. Self-efficacy measured confidence in one's ability to complete a FIT kit.28 Religious beliefs assessed the extent to which these beliefs influence one's health behaviors (e.g., belief that one's health is in God's hands).
Table 1. Description of health-related belief measures.
| Measure name and source | Number of items | Cronbach's alpha in current study | Cronbach's alpha in prior studies | Alpha citations |
|---|---|---|---|---|
| Perceived susceptibility25 | 3 | 0.83 | 0.53 to 0.86 | 15,25-27 |
| Response efficacy25 | 2 | 0.54 | 0.47 to 0.68 | 24,26,27 |
| Salience and coherence25 | 4 | 0.69 | 0.60 to 0.91 | 24,26,27 |
| Cancer worry25 | 2 | 0.65 | 0.52 to 0.69 | 24,26,27 |
| Social influence25 | 4 | 0.70 | 0.47 to 0.68 | 24,26,27 |
| Self-efficacy25 | 6 | 0.74 | 0.88 | 28 |
| Religious beliefs25 | 5 | 0.63 | -- | -- |
| Cancer fatalism8,32,33 | 15 | 0.84 | 0.88 | 34,35 |
Health literacy
The 8-item version of the Rapid Estimate of Adult Literacy–Revised measured health literacy.29,30 Participants were asked to read and pronounce health terms from a list.29,30 One point was given for each item pronounced correctly.
Awareness
Four separate yes-no items adapted from the NCI's Health Information National Trends Survey31 assessed whether participants had previously heard of double contrast barium enema, stool blood test, sigmoidoscopy, and colonoscopy. Responses of “yes” were coded as one point. No points were given for responses of “no” or “I don't know.” Nine additional items assessed CRC and CRC screening knowledge. One point was given for each correct response. An awareness score was calculated by summing the points for all thirteen items.
Cancer fatalism
Cancer fatalism was measured with the 15-item Powe Fatalism Inventory to assess participants' beliefs as to whether death is inevitable when cancer has been diagnosed.8,32-35 Participants respond either “yes” or “no”; one point is given for each “yes” response. Higher scores indicate higher levels of fatalism.
Healthcare experiences
Participants responded to three separate yes-no items regarding whether they had ever previously completed a CRC screening test, had a regular physician, and received an annual physical exam.
Residential distance from cancer center
Although FIT kits were mailed to the cancer center and no visits were required, we hypothesized that participants living in closer proximity might be more familiar with the institution hosting the study and therefore more likely to return FIT kits. Participants' residential addresses were utilized to calculate distance in miles from the cancer center.
Sociodemographic variables
Participants provided their age, gender, racial heritage (i.e., African-American [US-born Black American] vs. foreign country [Caribbean, Haitian, or Other]), ethnicity, employment status, education level, marital status, income, and health insurance status. Employment status included four categories: 1) unable to work/disabled; 2) employed/self-employed; 3) not employed outside the home (unemployed for >1year/unemployed for <1year/student/homemaker); or 4) retired.
Statistical analysis
Statistical analyses were conducted using SAS software (version 9.4[TS1M1], 2012, Cary, NC). T-tests and chi-square analyses using exact method with Monte Carlo estimation were conducted to examine intervention group differences for sociodemographic and health-related variables. Those variables with a group difference p-value < .10 were included as potential confounding variables in primary analyses assessing group differences on FIT kit return. Analyses of predictors of FIT kit return first used univariate logistic regression. Significant univariate predictors were further assessed using multivariable logistic regression. A two-sided p-value of <0.05 was considered statistically significant for all analyses.
Results
Participant characteristics
Participant characteristics are displayed in Table 2. The following nine variables assessed at baseline exhibited group differences at p<.10 and were considered potential confounds: gender, racial heritage, education, employment status, annual physical exam, prior CRC screening, residential distance from cancer center, health literacy, and salience and coherence.
Table 2. Descriptive statistics for sociodemographic and health-related variables at enrollment.
| Variables (Discrete) | Total N (%) |
Brochure N (%) |
Photonovella N (%) |
p-value |
|---|---|---|---|---|
|
| ||||
| Gender | ||||
| Male | 173 (52) | 88 (47) | 85 (59) | 0.036 |
| Female | 157 (48) | 98 (53) | 59 (41) | |
|
| ||||
| Racial heritage | ||||
| African-American | 308 (93) | 168 (90) | 140 (97) | 0.014 |
| Caribbean/Haitian/Other | 22 (7) | 18 (10) | 4 (3) | |
|
| ||||
| Ethnicity | ||||
| Hispanic | 9 (3) | 7 (4) | 2 (1) | 0.308 |
| Non-Hispanic | 321 (97) | 179 (96) | 142 (99) | |
|
| ||||
| Marital status | ||||
| Married/Partnered | 102 (31) | 55 (30) | 47 (33) | 0.745 |
| Divorced/Separated/Widowed | 118 (36) | 66 (36) | 52 (36) | |
| Never married/Single | 110 (33) | 65 (35) | 45 (31) | |
|
| ||||
| Employment | ||||
| Employed | 132 (40) | 82 (44) | 50 (35) | 0.006 |
| Not employed | 80 (24) | 48 (26) | 32 (22) | |
| Retired | 51 (15) | 31 (17) | 20 (14) | |
| Unable to work | 67 (20) | 25 (13) | 42 (29) | |
|
| ||||
| Education | ||||
| Less than HS/GED | 55 (17) | 22 (12) | 33 (23) | 0.050 |
| HS/GED | 112 (34) | 65 (35) | 47 (33) | |
| Some College | 107 (32) | 67 (36) | 40 (28) | |
| College Graduate/Post-Graduate | 56 (17) | 32 (17) | 24 (17) | |
|
| ||||
| Health insurance | ||||
| No | 143 (43) | 80 (43) | 63 (44) | 0.911 |
| Yes | 187 (57) | 106 (57) | 81 (56) | |
|
| ||||
| Income | ||||
| Less than $10,000 | 122 (37) | 60 (32) | 62 (43) | 0.388 |
| $10,000-$25,000 | 99 (30) | 59 (32) | 40 (28) | |
| $25,001-$35,000 | 30 (9) | 20 (11) | 10 (7) | |
| $35,001-$50,000 | 36 (11) | 22 (12) | 14 (10) | |
| $50,001-$75,000 | 20 (6) | 13 (7) | 7 (5) | |
| $75,001-$100,000 | 4 (1) | 3 (2) | 1 (1) | |
| $100,001+ | 5 (2) | 2 (1) | 3 (2) | |
|
| ||||
| Prior CRC screening test | ||||
| No | 234 (71) | 122 (66) | 112 (78) | 0.027 |
| Yes | 94 (29) | 62 (34) | 32 (22) | |
|
| ||||
| Regular healthcare provider | ||||
| No | 133 (40) | 70 (38) | 63 (44) | 0.258 |
| Yes | 196 (60) | 116 (62) | 80 (56) | |
|
| ||||
| Have an annual physical exam | ||||
| No | 143 (44) | 72 (39) | 71 (49) | 0.074 |
| Yes | 184 (56) | 111 (61) | 73 (51) | |
| Variable (Continuous) | Total Mean (SD) |
Brochure Mean (SD) |
Photonovella Mean (SD) |
p-value |
|---|---|---|---|---|
| Age | 56.4 (5.1) | 56.4 (4.9) | 56.5 (5.3) | 0.831 |
| Residential distance from CC (miles) | 15.7 (13.2) | 9.8 (10.9) | 4.8 (2.8) | <0.001 |
| Health literacy | 5.4 (2.7) | 5.9 (2.5) | 4.8 (2.8) | <0.001 |
| Awareness | 7.0 (2.2) | 7.1 (2.0) | 6.8 (2.4) | 0.149 |
| Salience and coherence | 19.0 (1.7) | 18.7 (1.9) | 19.4 (1.1) | <0.001 |
| Religious beliefs | 12.5 (5.1) | 12.7 (4.9) | 12.3 (5.3) | 0.435 |
| Perceived susceptibility | 9.0 (3.2) | 8.9 (3.2) | 9.0 (3.1) | 0.847 |
| Response efficacy | 8.8 (1.5) | 8.7 (1.5) | 8.8 (1.6) | 0.563 |
| Cancer worry | 5.1 (2.5) | 5.1 (2.5) | 5.0 (2.6) | 0.673 |
| Social influence | 15.6 (3.9) | 15.3 (3.9) | 16.0 (4.0) | 0.107 |
| Self-efficacy | 28.4 (2.7) | 28.5 (2.5) | 28.2 (3.0) | 0.372 |
| Cancer fatalism | 3.9 (3.2) | 4.0 (3.2) | 3.8 (3.2) | 0.547 |
Note. N = 330. HS = high school; CRC = colorectal cancer; SD = standard deviation; CC = cancer center.
Percentage totals may not equal 100% due to rounding and/or missing data.
The chi-square using exact method with Monte Carlo estimation was used for categorical variables. T-test was used for continuous variables.
FIT kit uptake
FIT kit return within 6 months for the total sample was 86.7%: 81.9% in Photonovella+FIT and 90.3% in Brochure+FIT. The influence of intervention was assessed using logistic regression in a model including the nine potential confounds (see above, N=324, χ2(13)=31.9, p=.003). Intervention was not a significant predictor of FIT kit return (AOR=1.07, CI=0.45-2.53, p=.881). Employment status significantly predicted FIT kit return in this model (p=.044). Those unable to work/disabled had a lower return rate (73%) than the other 3 employment categories (> 87%). Number of miles lived from the cancer center was a marginally significant predictor (p=.066), with those living closer to the cancer center more likely to return the FIT kit.
Among those returning a FIT kit, the median number of days to return was 9.5 days in Photonovella+FIT and 11 days in Brochure+FIT (p=.19). Time-to-event analyses revealed no significant effect of intervention group (log-rank p=0.17).
Factors Associated with Not Returning a FIT Kit
Given the high rate of return, we assessed factors associated with failure to return the FIT kit. Table 3 presents the six sociodemographic and health beliefs variables that significantly predicted failure to return the FIT kit. These predictors were further assessed using backward stepwise procedures. The final model included three predictors: greater residential distance from cancer center (AOR: 1.03, CI: 1.01-1.05, p=.015), stronger religious beliefs (AOR: 1.09, CI: 1.02-1.16, p=.015) and employment status (p=.010; disabled/unable to work vs. not-employed AOR: .15, CI: .05-.46).
Table 3. Significant predictors of failure to return the FIT kit (13.3%).
| Univariate, Unadjusted | Multivariable, Adjusted | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor | OR | 95% CI | p-value | OR | 95% CI | p-value |
|
| ||||||
| Greater residential distance from cancer center (miles) (vs. less residential distance from cancer center) | 1.03 | 1.01-1.05 | .006 | 1.03 | 1.01-1.05 | .015 |
|
| ||||||
| Lower health literacy (vs. higher) | 1.18 | 1.06-1.33 | .003 | |||
|
| ||||||
| Lower awareness score (vs. higher) | 1.19 | 1.04-1.37 | .014 | |||
|
| ||||||
| Higher religious beliefs score (vs. lower) | 1.08 | 1.02-1.15 | .012 | 1.09 | 1.02-1.16 | .015 |
|
| ||||||
| No prior CRC screening (vs. prior CRC screening) | 2.33 | 1.00-5.44 | .050 | |||
|
| ||||||
| Employment status | ||||||
| Unable to work | 1.00 | .003 | 1.00 | .010 | ||
| Employed | 0.38 | 0.18-0.80 | 0.49 | 0.23-1.08 | ||
| Not employed | 0.14 | 0.05-0.45 | 0.15 | 0.05-0.46 | ||
| Retired | 0.36 | 0.13-1.00 | 0.45 | 0.16-1.26 | ||
Note: OR = odds ratio; CI = confidence interval; CRC = colorectal cancer.
Health literacy, awareness, religious beliefs, and residential distance from cancer center in miles were each continuous variables, whereas prior colorectal cancer screening and employment status were categorical variables.
Significant univariate predictors were further assessed using multivariable logistic regression.
Adjusted models included the following variables as potential confounds: gender, racial heritage, education, employment status, annual physical exam, prior CRC screening, residential distance from cancer center, health literacy, and salience and coherence.
Non-significant univariate analyses results not shown.
Additional clinical findings
Of the 286 completed FIT kits, 13 kits produced abnormal results (4.5%). Nine individuals completed diagnostic colonoscopies, three are pending colonoscopies, and one individual refused to complete a diagnostic colonoscopy. Of individuals with verified colonoscopy results, one was diagnosed with a rectal cancer, four had polyps (number of polyps ranging from 1-20), and three had no polyps.
Discussion
Our study resulted in overall FIT kit uptake that exceeded Healthy People goals5 and the 80% by 2018 national goal3,4 with 86.7% of participants in the total sample returning a completed FIT kit. This is especially notable given that all participants were not current with CRC screening at enrollment, and prior screening status did not predict uptake. The high CRC screening uptake rate in our study suggests addressing access barriers (providing FIT kits) and offering print educational information may increase CRC screening rates among this population. To our knowledge, only one other CRC screening educational intervention paired with FIT kit has achieved this level of CRC screening (Davis, et al., under review). Few other CRC screening interventions have achieved more than 70% screening rates among average CRC risk individuals not up-to-date with screening guidelines; these were more labor-intensive patient navigation interventions as compared to our low-intensity print intervention.21,36-38
Our results showed no statistically significant differences in screening uptake or time to FIT kit return between intervention groups. These findings are consistent with results of a culturally-targeted patient navigation intervention for colonoscopy receipt among African-Americans that achieved a 76% screening rate with no differences between groups.21 In that study, 80% of those in the standard condition completed colonoscopy versus 74% and 76% in the two culturally-targeted intervention arms.21 Although not culturally-targeted, another trial compared three conditions with each including an FOBT kit and found no differences across groups.23 As in our study, the lack of group differences may be partially due to the provision of education and actionable access to screening tests in all conditions. Studies showing marked statistically significant group differences tend to be those comparing intervention versus “usual care or no intervention”.39-42 It is important to note that addressing the barrier of access to screening by providing a free FIT kit may have been more important than the independent influence of culturally-targeting the educational materials. In both arms, the FIT kits played an important role in screening completion. However, cultural targeting was still important as our community partners prefer and see significant value in the locally developed, engaging photonovella booklet, and want to disseminate the photonovella through their organizations.
Significant predictors of not returning a FIT kit included being unable to work, living farther from the cancer center, and having higher religious belief scores. Individuals who were not employed/students/homemakers were 54% more likely to return their FIT kits compared to those who were disabled/unable to work. In prior studies, individuals needing help with daily tasks and those identifying as disabled were less likely to complete an FOBT compared to individuals who did not need help.43,44 Level of disability, which was not assessed in our study, may be a screening barrier.45 In addition, as hypothesized, living farther from the cancer center was associated with being less likely to return a FIT kit. It is unclear why this factor is related to kit return. It is plausible that individuals living closer to the cancer center were more familiar with the cancer center hosting the study. Finally, with regard to higher religious beliefs predicting failure to return a FIT kit, results might be expected given that the scale assesses the belief that CRC screening is not necessary due to one's health being in God's hands. However, a prior CRC screening study among African-Americans found that religiosity was positively associated with colonoscopy.46
This study has a number of strengths. First, the study tested a culturally-targeted intervention in a diverse group of Blacks. Second, participants were enrolled using a variety of methods from the community rather than clinical setting; 40% of participants did not have a regular PCP, and therefore, may lack the opportunity to receive a provider's CRC screening recommendation. Although not tested, it is possible participants had greater trust in the research due to being recruited in community settings. Third, the primary outcome was based on objective return of completed FIT kits. Fourth, both groups were provided print materials and FIT kits which allowed testing of the unique contribution of cultural targeting. Finally, completing face-to-face interviews led to minimal missing data.
Limitations
Study limitations should also be discussed. First, randomization based upon residential geographic location likely contributed to different accrual rates and hence different group sample sizes as well as baseline differences between groups. This issue was addressed by adjusting for these variables in the primary analysis. Second, despite the various recruitment methods used, the number of individuals reporting foreign-born Black racial heritage status (7%) was small, but similar to national levels of foreign-born Blacks (8.7%).13 Third, the low refusal rate (Figure 1) may suggest selection bias; our enrolled sample may be highly-motivated thus accounting for high FIT kit uptake rates. Finally, the current study was conducted among Blacks living in a single geographic region which limits generalizability. Nevertheless, this study represents a pragmatic trial that can be readily translated into evidence-based public health practice and large scale dissemination of FIT kits through community events.
Future directions
Future analyses will examine repeat rates of FIT kit completion and group differences at 12- and 24-month post-intervention. Given the success of providing education and a FIT kit in a community sample, this strategy could be tested among other medically-underserved populations, in other languages, and on mHealth platforms. Future interventions might also target those disabled and with higher religious beliefs.
Conclusions
Our results suggest a promising strategy to increase CRC screening rates among Black individuals in the community who are not currently up-to-date with screening. Findings suggest printed education materials paired with free FIT kit delivery may help address health equity and promote action.
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Cancer Society, the National Cancer Institute, or the National Institutes of Health.
Funding sources: The study was funded by Research Scholar Grant Award RSGT-11-012-01-CPPB (PI: C.K. Gwede) from the American Cancer Society. The efforts of Drs. Christy and Davis were supported by grant R25CA090314-12 (PI: P. B. Jacobsen) from the National Cancer Institute. This work was also supported in part by the Biostatistics Core and the Survey Methods Core at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (NIH/NCI Grant Number: P30-CA076292).
Footnotes
Conflict of interest statement: The authors do not have any conflicts of interest to declare.
Author contributions: Dr. Christy had a role in data analysis/interpretation, wrote the article, and approved the final version. Ms. Williams and Ms. Govindaraju had a role in data acquisition, revised the article, and approved the final version. Dr. Davis had a role in data acquisition, data analysis/interpretation, revised the article, and approved the final version. Ms. Zhao and Dr. Sutton had a role in data analysis/interpretation, revised the article, and approved the final version. Drs. Quinn, Vadaparampil, and Shibata had a role in conceptualization/study design, revised the article, and approved the final version. Dr. Lin had a role in conceptualization/study design, data analysis/interpretation, revised the article, and approved the final version. Drs. Roetzheim and Meade had a role in conceptualization/study design, data acquisition, revised the article, and approved the final version. Dr. Gwede had a role in conceptualization/study design, funding acquisition, data acquisition, data analysis/interpretation, revised the article, and approved the final version.
References
- 1.American Cancer Society. Colorectal Cancer Facts & Figure 2014-2016. 2014 Available from URL: http://www.cancer.org/acs/groups/content/documents/document/acspc-042280.pdf.
- 2.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 3.National Colorectal Cancer Roundtable. [January 4, 2016];Tools & Resources – 80% by 2018. 2015 Available from URL: http://nccrt.org/tools/80-percent-by-2018/
- 4.Smith RA, Manassaram-Baptiste D, Brooks D. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. [January 4, 2016];HealthyPeople.gov 2020 Topics & Objectives: Cancer. 2014 Available from URL: http://www.healthypeople.gov/2020/topics-objectives/topic/cancer.
- 6.Myers RE, Ross E, Jepson C, et al. Modeling adherence to colorectal cancer screening. Prev Med. 1994;23:142–151. doi: 10.1006/pmed.1994.1020. [DOI] [PubMed] [Google Scholar]
- 7.Bynum SA, Davis JL, Green BL, Katz RV. Unwillingness to participate in colorectal cancer screening: examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. Am J Health Promot. 2012;26:295–300. doi: 10.4278/ajhp.110113-QUAN-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs. 1995;18:385–392. [PubMed] [Google Scholar]
- 9.United States Census Bureau. The foreign-Born population in the United States: 2010. Washington, DC: United States Census Bureau; 2010. 2012. [Google Scholar]
- 10.United States Census Bureau. The black population: 2010. Washington, DC: United States Census Bureau; 2011. [Google Scholar]
- 11.United States Census Bureau. United States Census 2000: The foreign-born population: 2000 Census 2000 Brief. Washington, DC: United States Census Bureau; 2001. [Google Scholar]
- 12.U.S. Census Bureau. United States Census 2000: The black population: 2000 Census 2000 Brief. Washington, DC: United States Census Bureau; 2001. [Google Scholar]
- 13.Anderson M. A rising share of the U S black population is foreign born; 9 percent are immigrants; and while most are from the Caribbean, Africans drive recent growth. Washington, D.C: Pew Research Center; 2015. [Google Scholar]
- 14.Gwede CK, Jean-Francois E, Quinn GP, et al. Perceptions of colorectal cancer among three ethnic subgroups of US blacks: a qualitative study. J Natl Med Assoc. 2011;103:669–680. doi: 10.1016/s0027-9684(15)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwede CK, William CM, Thomas KB, et al. Exploring disparities and variability in perceptions and self-reported colorectal cancer screening among three ethnic subgroups of U. S. Blacks. Oncol Nurs Forum. 2010;37:581–591. doi: 10.1188/10.ONF.581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreuter MW, Lukwago SN, Bucholtz RD, Clark EM, Sanders-Thompson V. Achieving cultural appropriateness in health promotion programs: Targeted and tailored approaches. Health Educ Behav. 2003;30:133–146. doi: 10.1177/1090198102251021. [DOI] [PubMed] [Google Scholar]
- 17.Kreuter MW, Strecher VJ, Glassman B. One size does not fit all: The case for tailoring print materials. Ann Behav Med. 1999;21:276–283. doi: 10.1007/BF02895958. [DOI] [PubMed] [Google Scholar]
- 18.Powe BD. Promoting fecal occult blood testing in rural African American women. Cancer Pract. 2002;10:139–146. doi: 10.1046/j.1523-5394.2002.103008.x. [DOI] [PubMed] [Google Scholar]
- 19.Wang JH, Schwartz MD, Brown RL, et al. Results of a randomized controlled trial testing the efficacy of a culturally targeted and a generic video on mammography screening among chinese-american immigrants. Cancer Epidemiol Biomarkers Prev. 2012;21:1923–1932. doi: 10.1158/1055-9965.EPI-12-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon EJ, Feinglass J, Carney P, et al. A culturally targeted website for Hispanics/Latinos about living kidney donation and transplantation: A randomized controlled trial of increased knowledge. Transplantation. 2015 doi: 10.1097/TP.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 21.Jandorf L, Braschi C, Ernstoff E, et al. Culturally targeted patient navigation for increasing african americans' adherence to screening colonoscopy: A randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2013;22:1577–1587. doi: 10.1158/1055-9965.EPI-12-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Breitkopf CR, Dawson L, Grady JJ, Breitkopf DM, Nelson-Becker C, Snyder RR. Intervention to improve follow-up for abnormal Papanicolaou tests: A randomized clinical trial. Health Psychol. 2014;33:307–316. doi: 10.1037/a0032722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110:2083–2091. doi: 10.1002/cncr.23022. [DOI] [PubMed] [Google Scholar]
- 24.Vernon SW, Myers RE, Tilley BC. Development and validation of an instrument to measure factors related to colorectal cancer screening adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–832. [PubMed] [Google Scholar]
- 25.Vernon SW, Myers RE, Tilley BC, Li S. Factors associated with perceived risk in automotive employees at increased risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:35–43. [PubMed] [Google Scholar]
- 26.Tiro JA, Vernon SW, Hyslop T, Myers RE. Factorial validity and invariance of a survey measuring psychosocial correlates of colorectal cancer screening among African Americans and Caucasians. Cancer Epidemiol Biomarkers Prev. 2005;14:2855–2861. doi: 10.1158/1055-9965.EPI-05-0217. [DOI] [PubMed] [Google Scholar]
- 27.McQueen A, Tiro JA, Vernon SW. Construct validity and invariance of four factors associated with colorectal cancer screening across gender, race, and prior screening. Cancer Epidemiol Biomarkers Prev. 2008;17:2231–2237. doi: 10.1158/1055-9965.EPI-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flight IH, Wilson CJ, McGillivray J, Myers RE. Cross-cultural validation of the preventive health model for colorectal cancer screening: an Australian study. Health Educ Behav. 2010;37:724–736. doi: 10.1177/1090198110364107. [DOI] [PubMed] [Google Scholar]
- 29.Bass PF, 3rd, Wilson JF, Griffith CH. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18:1036–1038. doi: 10.1111/j.1525-1497.2003.10651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis TC, Long SW, Jackson RH, et al. Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 31.HINTS. [January 2, 2009];Health Informational National Trends Survey (HINTS) 2009 Available from URL: http://cancercontrol.cancer.gov/hints/questions.jsp.
- 32.Powe BD. Perceptions of cancer fatalism among African Americans: the influence of education, income, and cancer knowledge. J Natl Black Nurses Assoc. 1994;7:41–48. [PubMed] [Google Scholar]
- 33.Powe BD. Cancer fatalism among African-Americans: a review of the literature. Nurs Outlook. 1996;44:18–21. doi: 10.1016/s0029-6554(96)80020-0. [DOI] [PubMed] [Google Scholar]
- 34.Powe BD. Cancer fatalism among elderly Caucasians and African Americans. Oncol Nurs Forum. 1995;22:1355–1359. [PubMed] [Google Scholar]
- 35.Powe BD, Finnie R. Cancer fatalism: the state of the science. Cancer Nurs. 2003;26:454–465. doi: 10.1097/00002820-200312000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Cavanagh MF, Lane DS, Messina CR, Anderson JC. Clinical case management and navigation for colonoscopy screening in an academic medical center. Cancer. 2013;119:2894–2904. doi: 10.1002/cncr.28156. [DOI] [PubMed] [Google Scholar]
- 37.Ma GX, Shive S, Tan Y, et al. Community-based colorectal cancer intervention in underserved Korean Americans. Cancer Epidemiol. 2009;33:381–386. doi: 10.1016/j.canep.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Horne HN, Phelan-Emrick DF, Pollack CE, et al. Effect of patient navigation on colorectal cancer screening in a community-based randomized controlled trial of urban African American adults. Cancer Causes Control. 2015;26:239–246. doi: 10.1007/s10552-014-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen BH, McPhee SJ, Stewart SL, Doan HT. Effectiveness of a controlled trial to promote colorectal cancer screening in Vietnamese Americans. Am J Public Health. 2010;100:870–876. doi: 10.2105/AJPH.2009.166231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldman SN, Liss DT, Brown T, et al. Comparative effectiveness of multifaceted outreach to initiate colorectal cancer screening in community health centers: A randomized controlled trial. J Gen Intern Med. 2015;30:1178–1184. doi: 10.1007/s11606-015-3234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritvo PG, Myers RE, Paszat LF, et al. Personal navigation increases colorectal cancer screening uptake. Cancer Epidemiol Biomarkers Prev. 2015;24:506–511. doi: 10.1158/1055-9965.EPI-14-0744. [DOI] [PubMed] [Google Scholar]
- 42.Charlton ME, Mengeling MA, Halfdanarson TR, et al. Evaluation of a home-based colorectal cancer screening intervention in a rural state. J Rural Health. 2014;30:322–332. doi: 10.1111/jrh.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber MF, Banks E, Ward R, Sitas F. Population characteristics related to colorectal cancer testing in New South Wales, Australia: results from the 45 and Up Study cohort. J Med Screen. 2008;15:137–142. doi: 10.1258/jms.2008.008050. [DOI] [PubMed] [Google Scholar]
- 44.Frederiksen BL, Jørgensen T, Brasso K, Holten I, Osler M. Socioeconomic position and participation in colorectal cancer screening. Br J Cancer. 2010;103:1496–1501. doi: 10.1038/sj.bjc.6605962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merten JW, Pomeranz JL, King JL, Moorhouse M, Wynn RD. Barriers to cancer screening for people with disabilities: a literature review. Disabil Health J. 2015;8:9–16. doi: 10.1016/j.dhjo.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Brittain K, Murphy VP. Sociocultural and health correlates related to colorectal cancer screening adherence among urban African Americans. Cancer Nurs. 2015;38:118–124. doi: 10.1097/NCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]