Abstract
Attention-Deficit/Hyperactivity disorder (ADHD) is the most commonly diagnosed mental health disorder in childhood and persists into adulthood in up to 65% of cases. ADHD is associated with adverse outcomes such as the ability to gain and maintain employment (Kessler et al. 2009; Kupper et al. 2012), and is associated with an increased risk for substance abuse (Groenman et al. 2013; Upadhyaya 2008; Wilens et al. 1995), obesity (Albayrak et al. 2013; Cortese et al. 2008; Nazar et al. 2012; Nazar et al. 2014), workplace injuries (Breslin and Pole 2009; Hodgkins et al. 2011; Swensen et al. 2004), and traffic accidents (Barkley and Cox 2007; Barkley et al. 1993; Jerome et al. 2006a; Jerome et al. 2006b; Merkel et al. 2013). A majority of diagnosed children have motor deficits, however few studies have examined motor deficits in young adults. This study provides a novel examination of visuomotor control of grip force in young adults with and without ADHD. Participants were instructed to maintain force production over a 20-second trial with and without real-time visual feedback about their performance. The results demonstrated that when visual feedback was available, adults with ADHD produced slightly higher grip force than controls. However, when visual feedback was removed, adults with ADHD had a faster rate of decay of force, which was associated with ADHD symptom severity and trait impulsivity. These findings suggest that there may be important differences in the way that adults with ADHD integrate visual feedback during continuous motor tasks. These may account for some of the motor impairments reported in children with ADHD. These deficits could result from (1) dysfunctional sensory motor integration and/or (2) deficits in short-term visuomotor memory.
Keywords: grip force, motor control, visual feedback, attention-deficit/hyperactivity disorder (ADHD)
Attention deficit hyperactivity disorder (ADHD) is one of the most common childhood-onset neuropsychiatric disorders and persists into adulthood in up to 65% of cases (Faraone et al. 2006). In addition to the core behavioral features, motor impairments are reported in 30% to 50% of patients (Barkley 1998; Fliers et al. 2008; Gillberg 1998; Kadesjo and Gillberg 1998) and up to 50% of pediatric patients have comorbid developmental coordination disorder (Gillberg et al. 2004; Kadesjo and Gillberg 1999; Pitcher et al. 2003). Motor symptoms reported in children with ADHD include unintentional and unnecessary movements (Cole et al. 2008; Macneil et al. 2011; Mostofsky et al. 2003; O'Brien et al. 2010), impaired timing (Rubia et al. 2003; Zelaznik et al. 2012), fine motor deficits (Piek et al. 1999; Pitcher et al. 2003), and illegible handwriting (Adi-Japha et al. 2007; Brossard-Racine et al. 2015; Racine et al. 2008). Motor deficits in children are associated with poor adaptive functioning in home life, socialization, and self-direction (Wang et al. 2011) and the psychosocial outcomes of childhood ADHD with motor impairments is a significant problem. Thus, although the motor characteristics of childhood ADHD are recognized, there is a significant gap in the literature quantifying motor deficits in adults with ADHD. Recent work demonstrates increased postural sway (Hove et al. 2015), deficits in sensorimotor timing (Valera et al. 2010), and deficits in visuomotor adaptation (Kurdziel et al. 2015) in adults with ADHD. The present work provides a precise, novel, and quantitative analysis of continuous force output in adults with ADHD.
In spite of the importance of motor problems in adult ADHD, the sensorimotor deficits that underlie these problems remain poorly understood. A subset of children with ADHD have comorbid sensory modulation disorder (Cascio 2010; Mangeot et al. 2001), a deficit in regulation and organization of the degree, intensity, and nature of a response to sensory stimuli (Lane et al. 2000; McIntosh et al. 1999). This is notable because sensory processing is paramount for the development of motor skills. Therefore, the previously mentioned deficits in motor control may be related to deficits in processing or integrating sensory feedback. Deficits in several domains of visual processing have been reported in adults with ADHD. These include problems with visual attention and selection (Cross-Villasana et al. 2015; Li et al. 2012), and deficits orienting to relevant stimuli (Tegelbeckers et al. 2015). Consequently, impaired visually guided movement in ADHD may reflect deficits in visuomotor processing, planning or executing movements, activating muscles, or in storing and retrieving the internal action representation used to guide motor output. The current study is a novel examination of how adults with ADHD use visual feedback to control ongoing movement in a precision grip force task.
Precision gripping is an important task for activities of daily living, especially eating, writing, and self-care. The visuomotor control of precision grip is associated with activation in a distributed neural system including parietal cortex, dorsolateral prefrontal cortex, premotor cortex, supplementary motor area, primary motor cortex, the nuclei of the basal ganglia, motor regions of the cerebellum, and visual motion areas such as V3 and V5 (Coombes et al. 2011; Neely et al. 2013a; Vaillancourt et al. 2006). In particular, the parietal and motor cortices are paramount for processing sensory feedback and generating motor commands to stay on task (Vaillancourt et al. 2006) and the basal ganglia are critical for the planning and parameterization of grip force (Prodoehl et al. 2009). Since visuomotor control of grip force is reliant on such a widely distributed system, it is vulnerable to deficits in any one of these regions. This is important because neuroimaging, neuropsychological, neurochemical, and genetic studies are converging on a neural systems-based anatomy of ADHD wherein dysfunction is thought to affect the cortex, basal ganglia, thalamus, and cerebellum (Makris et al. 2009) – all major players in visuomotor control processes (Coombes et al. 2011; Neely et al. 2013a; Vaillancourt et al. 2006). Consequently, it is possible that motor impairments in ADHD are related to difficulty integrating visual feedback for control of an ongoing movement. Such a discovery would provide important insights to the neurobiology of ADHD.
In addition to identifying deficits in force control, we sought to link observed deficits in force control to self-reported symptoms of ADHD. This is important because there continues to be a debate about how to best identify adult ADHD. The DSM-5 categorizes ADHD as a neurodevelopmental disorder that begins in childhood and is characterized by lifelong symptoms. Diagnosis in adults can be challenging because a limited developmental perspective is available and retrospective reports are often unreliable (Mannuzza et al. 2002). Self-report of symptoms can be administered via questionnaires wherein individuals rate the frequency with which they experience ADHD symptoms. One such questionnaire is the short form of the Conners’ Adult ADHD Rating Scale (CAARS S:S) (Conners 1999). The CAARS has four factor-derived subscales: Inattention/Memory Problems, Hyperactivity/Restlessness, Impulsivity/Emotional Lability, and Problems with Self-Concept. In addition, the scale contains an ADHD Index that provides an aggregate measure of impairment. T-scores on the CAARS have a mean of 50 and a standard deviation of 10 and interpretation of the CAARS is meant to be done by examination of the pattern of elevated scale scores, such that the greater the number of scales with T-scores above 65, the greater the likelihood of moderate to severe problems (Conners 1999). We elected to use the CAARS self-report because it provides a psychometrically rigorous set of behavioral dimensions, it has been validated for use in North America, and it is easy to use in laboratory-based studies. The current study sought to evaluate whether deficits in force control were associated with scores on the Inattention/Memory Problems – a dimension or the ADHD Index of the CAARS S:S. We hypothesized that adults with ADHD would have difficulty maintaining accurate and ongoing force production when they have discontinuous visual feedback, as is often the case in real life. We reasoned that a deficit in force control could be related to failure to attend to the visual feedback, failure to create an accurate representation of the goal, and/or failure of working memory to retain the task goal. Thus, we further hypothesized that force production would be related scores on the Inattention/Memory Problems dimension of the CAARS.
A third motivation for this study was to examine how the behavioral construct of impulsivity is related to motor output. We posit that impulsive individuals act in the absence of a motor plan, or a less-developed motor plan that may not be accurate for the task. To evaluate this hypothesis, all participants completed the Barratt Impulsiveness Scale (BIS-11) to assess trait impulsivity. The BIS-11 is a 30-item psychometric tool for impulsivity (Patton et al. 1995) that can be divided into six first-order factors: attention, cognitive instability, motor impulsiveness, perseverance, self-control, and cognitive complexity. We hypothesized that impulsivity related to the factor of attention may reflect failures to plan in advance of movement onset. This failure to plan could result from inattention to relevant visual stimuli or an impulsive strategy to respond first and plan later. Thus, we hypothesized that force production would be related to scores on the attention factor of the BIS-11 scale.
Method
Participants
Forty individuals, with a mean age of 20.43 ± 1.87 years, were recruited through local flyers and radio advertisement in the Centre County Region. 20 had self-reported ADHD (12 females) and 20 were age- and sex-matched healthy controls (see Table 1). Participants were recruited through advertisement in the State College area. We recruited two groups of participants: those who self-identified as having been given a diagnosis of ADHD persisting into adulthood and those who had never been given a diagnosis of ADHD. Participants were excluded if they reported a musculoskeletal disorder, history of head injury, or neurologic/seizure disorder. No participants were taking medications known to affect motor control at the time of testing, including antipsychotics, or anticonvulsants (Reilly et al. 2008). Participants taking stimulant medication withheld their morning dose for an average medication washout of 24 hours. Written informed consent was obtained from all participants after a complete description of the study was provided. All procedures were approved by the Institutional Review Board at The Pennsylvania State University and were consistent with the 1964 Declaration of Helsinki.
Table 1.
Participant characteristics
| Variables | Group | ||
|---|---|---|---|
| Control | ADHD | Significant Group Differences | |
| Sample size | 20 | 20 | |
| Right-handed | 18 | 20 | |
| Females | 13 | 12 | |
| Age, yrs | 20.4 (1.87) | 20.45 (1.90) | ADHD=HC, t(38) = 0.08, p = .934 |
| MVC, right pinch grip | 35.25 (15.37) | 34.36 (8.46) | |
| CAARS-S:S | |||
| A. Inattention/Memory Problems | 47.1 (8.08) | 64.65 (13.31) | ADHD>HC, t(38) = 5.04, p < .001 |
| B. Hyperactivity/Restlessness | 48.1 (8.93) | 60.6 (9.61) | ADHD>HC, t(38) = 4.26, p < .001 |
| C. Impulsivity/Emotional Lability | 41.9 (3.93) | 51.25 (10.53) | ADHD>HC, t(38) = 3.72, p = .001 |
| D. Problems with Self-Concept | 43.6 (7.13) | 47.3 (17.57) | ADHD>HC, t(38) = 0.86, p = .40 |
| E. ADHD Index | 44.7 (6.84) | 58.65 (16.27) | ADHD>HC, t(38) = 3.53, p = .001 |
| BIS-11 | |||
| First Order Factors | |||
| 1. Attention | 9.1 (2.59) | 16.05 (2.46) | ADHD>HC, t(38) = 8.70, p < .001 |
| 2. Motor Impulsiveness | 14.8 (3.59) | 17.45 (4.30) | ADHD>HC, t(38) = 2.12, p = .04 |
| 3. Self-control | 11.4 (3.28) | 15.65 (3.82) | ADHD>HC, t(38) = 3.78, p = .001 |
| 4. Cognitive Complexity | 10.95 (2.09) | 12.15 (2.96) | ADHD>HC, t(38) = 1.48, p = .15 |
| 5. Perseverance | 6.9 (2.17) | 8.2 (2.40) | ADHD>HC, t(38) = 1.80, p = .08 |
| 6. Cognitive Instability | 5.95 (2.28) | 7.8 (2.12) | ADHD>HC, t(38) = 2.66, p < .01 |
| Second Order Factors | |||
| 1. Attentional Impulsiveness | 15.05 (4.44) | 23.85 (4.12) | ADHD>HC, t(38) = 6.50, p < .001 |
| 2. Motor Impulsiveness | 21.7 (5.09) | 25.65 (5.52) | ADHD>HC, t(38) = 2.35, p = .02 |
| 3. Non-planning Impulsiveness | 22.35 (4.72) | 27.8 (5.44) | ADHD>HC, t(38) = 3.38, p = .002 |
Measures
Symptoms of ADHD were assessed with the self-report short form (S:S) of the CAARS. The CAARS-S:S has 26 items and 6 subscales. There are four factor-derived subscales: Inattention/Memory Problems, Hyperactivity/Restlessness, Impulsivity/Emotional Lability, and Problems with Self-Concept. The remaining two subscales are the ADHD Index and an Inconsistency Index. Trait impulsivity was assessed with the Barratt Impulsiveness Scale (BIS-11), a 30-item psychometric tool for impulsivity (Patton et al. 1995). The BIS-11 assesses the behavioral construct of impulsivity, which is a multi-dimensional construct (Patton et al. 1995). Patton and colleagues (1995) demonstrated that the BIS-11 can be divided into six first-order factors: attention, cognitive instability, motor impulsiveness, perseverance, self-control, and cognitive complexity.
Handedness was assessed with the Edinburgh Handedness Inventory (Oldfield 1971). This 10-item inventory asks participants to indicate which hand they would use to complete common tasks, such as striking a match, throwing a ball, or using scissors. Handedness was determined using a laterality quotient (LQ = (R−L)/(R+L) * 100), where a score of 100 reflected complete right-hand dominance, and a score of -100 reflected complete left-hand dominance.
Grip Force Task
The precision grip task studied here has been employed in previous investigations to understand how the neural control of voluntary movement is impaired in Autism Spectrum Disorders (ASD) (Mosconi et al. 2015; Wang et al. 2015), Parkinson’s disease (Vaillancourt et al. 2001) and atypical parkinsonian disorders (Neely et al. 2013b), and essential tremor (Neely et al. 2015; Poon et al. 2011). Visual stimuli were presented on a 102 cm (40-inch) Samsung television screen with resolution 1920 × 1080 and a 120 Hz refresh rate. Participants were seated upright in a chair (JedMed Straight Back Chair, St. Louis, MO) a horizontal distance of 127 cm from the screen. The forearm of the dominant arm rested in a relaxed position at approximately 100° of flexion on an adjustable non-tilting hospital table. The room was dimly lit to limit glare and reflection on the screen. As shown in Figure 1A, participants held a custom-designed Bragg-grating fiber-optic precision grip force transducer using a precision grip with the dominant hand (Neuroimaging Solutions, Gainesville, FL). The force transducer was calibrated and had a resolution of 0.025 N. Data was collected at 62.5 Hz using customized code written in LabVIEW (National Instruments, Austin, TX) via a sm130 Dynamic Optical Sensing Interrogator (Micron Optics, Atlanta, GA).
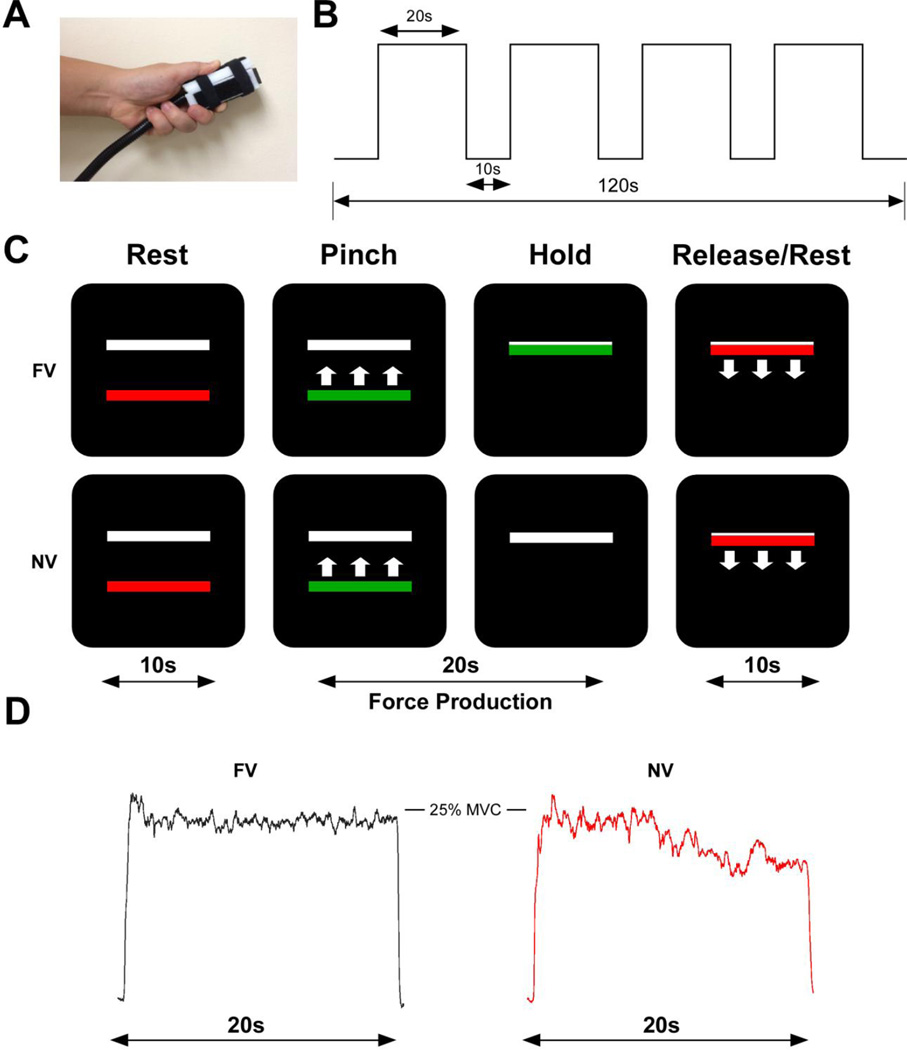
Figure 1. Participants completed a full-vision and a no-vision precision grip task.
A: The precision grip apparatus held between the thumb and index finger. B: The experimental procedure was 120 seconds in length. Each block of 20 seconds of force was separated by 10 seconds of rest. C: The visual display contained two horizontal bars presented against a black background. The target bar (white) was stationary and the red/green force bar provided real-time visual feedback. In the full-vision (FV) task, visual feedback was available for the duration of the trial. In the no-vision (NV) task, the force bar disappeared for the last 12 seconds of the trial. E: Exemplar raw force data is shown for the FV (black) and NV (red) trials.
Procedures
Before the experimental task, each participant’s maximum voluntary contraction (MVC) was measured using a pinch grip dynamometer (Lafayette Hydraulic Pinch Gauge, Model J00111, Lafayette, IN). The average of three-five second trials determined each participant’s maximum voluntary contraction (MVC) in Newtons. During the task, participants viewed two horizontal bars: a red/green force bar which moved up with increasing force and down with decreasing force, and a white, static target bar. The target bar was set at 25% of each participant’s MVC. The onset of force was cued by the force bar turning green and offset of force was cued by the color red. Participants were instructed to produce force as quickly and as accurately as possible at the time of the color change from red to green and to keep the green bar at the target force level for the duration of the 20 second trial, until offset of force was cued by a change to red. As shown in Figure 1B, each experimental trial started and ended with 10 seconds of rest and included four 20-second trials of force with 10 seconds of rest in between each trial. During full vision trials, the moving force bar remained visible for the whole trial, providing real-time visual feedback about performance. As shown in Figure 1C, during the no-vision trials, the force bar disappeared for the last 12 seconds of the trial and participants were instructed to continue producing force at the target level until the trial ended. Participants completed one run of full-vision and one run of no-vision trials. The order of each run was counterbalanced across participants. All participants completed a practice session to become familiar with the timing and force output requirements of the task.
Grip Force Data Analysis
The force time series data was digitally filtered using a tenth-order Butterworth filter with a 15 Hz low-pass cut-off frequency. Visual inspection of force output was performed and four time-points were determined for each trial: onset of force, beginning and end of force production, and offset of force. The 10-second periods of rest between each trial were removed from data analysis. The remainder of the data was averaged into 80-one second time bins to account for the four 20-second trials of force. Mean force and standard deviation of force were calculated for each 1-second time bin. In addition, mean force and standard deviation for the entire force interval in the full-vision condition were calculated. Last, mean rate of change during the ramp up to target and the mean rate of change during the decrease to baseline were determined for the start and end of each full-vision trial. All calculations were conducted with custom algorithms in MATLAB.
Statistical analysis
The primary goal of this work was to determine the relationship between rate of force decay in the no-vision trials and self-reported ADHD symptoms. Since symptom severity exists on a dimension and is not necessarily categorical in nature, we first conducted a categorical analysis to identify patterns in adults with ADHD and subsequently conducted a dimensional analysis with continuous ADHD relevant measures. We used multilevel models to analyze mean force output in the no-vision trials, fitting a three-level model with time nested within blocks, which in turn were nested within participants. Decay of force over time was modeled with linear and quadratic random effects for time. Person-level covariates included either scores on the CAARS S:S, scores on the BIS-11, or a self-report yes/no question about lifetime diagnosis for ADHD. In particular, we elected to use the CAARS S:S subscales for Inattention/Memory Problems and the ADHD Index, as well as the BIS-11 first-order factor for Attention. Models were fit in R (R Core Team, 2013) using the lmer function. The scale scores for the CAARS and BIS were centered at zero. We started by fitting a random intercepts only model, with linear fixed effects for time and an interaction with the appropriate person-level covariates. Models adding linear and quadratic random effects for time showed substantial improvement in fit. We made similar comparisons in the full vision comparison. Specifically, we fit the above random effects models to the first 3 seconds of the trial (onset), the first 10 seconds, and the last 10 seconds – representing the first and second half of each trial.
Results
Participants
All but two participants were confirmed to be right-hand dominant using the Edinburgh Handedness Inventory. The results for the left-handed individuals did not differ from those who were right-handed and thus they were included in the analysis. Table 1 shows the demographic and clinical information for each group. The univariate ANOVA for age revealed that the groups did not differ significantly (p > 0.5). However, as reported in Table 1, and consistent with the diagnostic strategy, the groups were significantly different on all subscales of the CAARS S:S and the BIS-11. Pinch grip MVC for the dominant hand did not differ between groups, t(38) = −0.18, p = .861.
Grip Force
Figure 2 displays the mean force output for each group as a function of time, for each visual condition. We first discuss the results for the full vision condition. Mean force and standard deviation of force was submitted to a mixed model ANOVA for time (12-1-second bins) by group (ADHD, Control). The results for mean force revealed a main effect of group, F(1, 28) = 7.66, p < .001, such that individuals with ADHD (25.00% MVC SD 0.48% MVC) produced greater force than controls (24.44% MVC SD 0.85% MVC) across the last 12 seconds of the trial. No effect of time, or interaction for time by group, was observed. The results for standard deviation of force did not reveal any main effects or interactions.
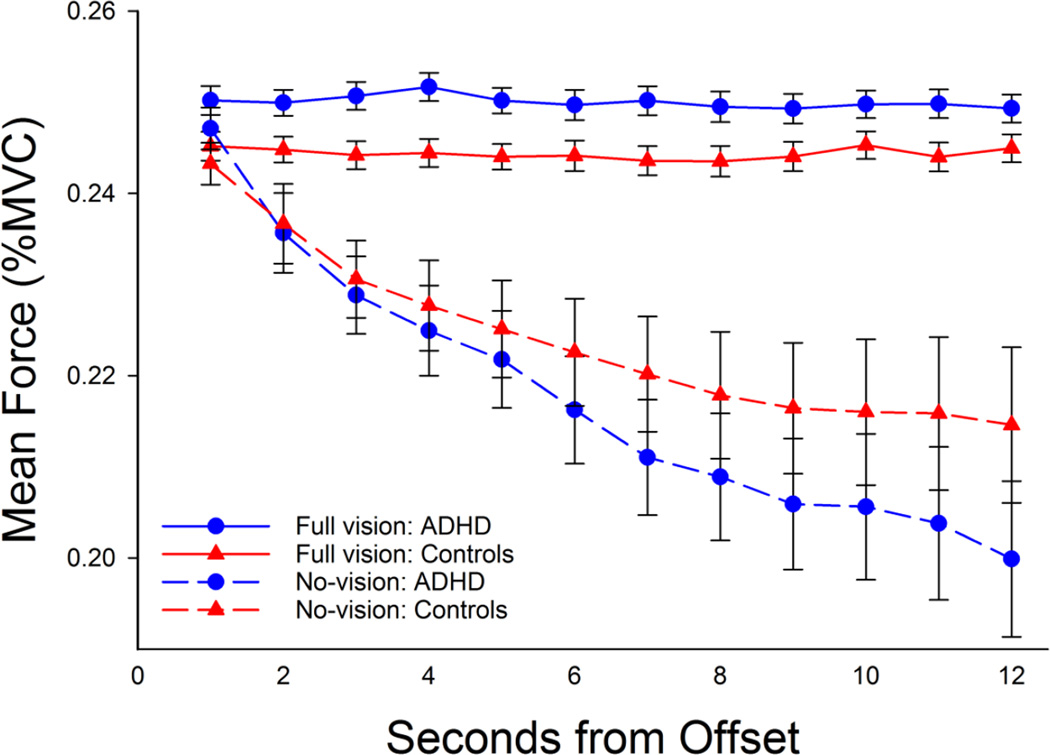
Figure 2. Mean force as a function of visual condition, group, and time. Error bars represent standard error of the mean.
As shown in Figure 2, individuals with ADHD showed a decrease in force output as a function of time. Mean force and standard deviation of force was submitted to a mixed model ANOVA for time (12-1-second bins) by group (ADHD, Control). The results for mean force revealed a main effect of time, F(11, 418) = 47.31, p < .05, and a time by group interaction, F(11, 418) = 2.53, p = .004. We elected to decompose this interaction by use of multilevel models, reported in the following paragraphs. The results for standard deviation of force output yielded a main effect of time, F(11, 418) = 2.29, p = .010, such that variability decreased linearly as a function of time (highest order polynomial, linear, F(1, 38) = 8.83, p = .005). A main effect of group or interaction for time by group was not observed.
Results of the multilevel models for the no-vision condition are given in Table 2. All models showed a strong negative trend for time as participants reduced grip force output when visual feedback was removed. Using diagnostic group as a categorical predictor, we observed significant fixed effects for linear (t = 4.83, p < .001) and quadratic (t = 6.13, p < .001) change over time. We also observed an interaction between group and linear time with the ADHD group showing a steeper decline (t = 2.01, p < .05). The predicted curves for each diagnostic group based on Model A in Table 2 are shown in Figure 2; consistent with our prediction, the self-report of ADHD persisting into adulthood was associated with a faster rate of decay of force (blue line) compared to the control group (red line).
Table 2.
| Model A: time, group | |||
| Fixed Effect | Coefficient | SE |
t- value |
| Intercept | 22.170 | 0.509 | 43.53 |
| Time | −0.268 | 0.056 | −4.83 |
| Group | −0.692 | 0.649 | −1.07 |
| Time2 | 0.027 | 0.004 | 6.13 |
| Time by group | −0.152 | 0.075 | −2.01 |
| Model B: time, CAARS-Inattention/Memory Problems | |||
| Fixed Effect | Coefficient | SE |
t- value |
| Intercept | 23.130 | 1.367 | 16.928 |
| Time | −0.344 | 0.041 | −8.39 |
| CAARS-Inattention/Memory Problems | −0.023 | 0.023 | −1.00 |
| Time2 | 0.027 | 0.004 | 6.12 |
| Time by CAARS-Inattention/Memory Problems | −0.005 | 0.003 | −1.68 |
| Model C: time, CAARS-ADHD Index | |||
| Fixed Effect | Coefficient | SE |
t- value |
| Intercept | 23.080 | 1.257 | 18.35 |
| Time | −0.344 | 0.041 | −8.38 |
| CAARS-ADHD Index | −0.024 | 0.023 | −1.05 |
| Time2 | 0.027 | 0.004 | 6.13 |
| Time by CAARS-ADHD Index | −0.004 | 0.003 | −1.38 |
| Model D: time, BIS-Attention | |||
| Fixed Effect | Coefficient | SE |
t- value |
| Intercept | 23.240 | 1.021 | 22.75 |
| Time | −0.344 | 0.041 | −8.57 |
| BIS-Attention | −0.113 | 0.075 | −1.50 |
| Time2 | 0.027 | 0.004 | 6.13 |
| Time by BIS-Attention | −0.019 | 0.009 | −2.21 |
The results and interpretation were similar when inattention/working memory problems were indexed dimensionally instead of categorically, using the continuous CAARS and BIS scales as predictors (see Table 2). The BIS Attention scale showed a significant interaction with linear time (t = 2.21, p < .05). Although the CAARS Inattention/Memory Problems and ADHD index sub-scales were in the predicted direction, they were not statistically significant (t = 1.68, p > .05; t = 1.38, p > .05 respectively).
Discussion
The present study investigated visuomotor control of grip force in healthy young adults and young adults who self-identified as having a diagnosis of ADHD persisting into adulthood. Our sampling technique yielded a group of young adults with mild to moderate ADHD symptoms as assessed by the CAARS. Grip force was measured in two tasks: a full-vision task wherein visual feedback was available for 20 seconds and a no-vision task wherein visual feedback was removed for the last 12 seconds of the task. There are three novel findings. First, when visual feedback was available, adults with ADHD produced slightly higher grip force than controls in the last 12 seconds of the force interval. Second, when visual feedback was removed, adults with ADHD had a steeper rate of decay of force. Third, the rate of decay of force was associated with ADHD symptom severity and trait impulsivity. These findings suggest that there are important differences in the way that adults with ADHD integrate visual feedback during continuous motor tasks. These may account for some of the motor impairments reported in children with ADHD.
When visual feedback was available, adults with ADHD produced slightly more force than controls during the last 12 seconds of the trial. This interval encompasses the steady-state portion wherein participants must integrate real-time visual feedback to continuously maintain isometric force. Continuous motor tasks rely on sensory feedback mechanisms to a greater degree than discrete motor tasks, such that individuals can continuously adjust their motor output to stay on target (Deutsch and Newell 2001; Deutsch and Newell 2003). In a previous study using a similar hand-grip task, 30 seconds of visually guided force production was associated with brain activity in right-lateralized regions including dorsolateral prefrontal cortex, ventral premotor cortex, and inferior parietal lobe (IPL) (Neely et al. 2013a). Importantly, these regions are implicated in the continuous online control of action (Desmurget et al. 1999; Desmurget and Grafton 2000), especially for tasks that require continuous visuospatial information. Although young adults with ADHD produced slightly more force than controls, it is noteworthy that they were more accurate than controls. That is, the task required 25% MVC and the mean force for adults with ADHD was 25.00% compared to 24.44% for controls. Although this difference is statistically significant it does not suggest that individuals with ADHD have difficulty integrating visual feedback for continuous control of force output.
In contrast to the full vision condition wherein visual feedback about performance was always available; in the no-vision condition, visual feedback was removed for the last 12 seconds of each trial. In the absence of visual feedback, healthy young adults decrease force output gradually as a function of time (Vaillancourt and Russell 2002). Vaillancourt and Russell suggest that the reduction in force output is a consequence of the limits of short-term visuomotor memory. As motor memory decays, a similar reduction in the activity of motor neurons occurs, leading to decreased force output (Vaillancourt and Russell 2002). Importantly, the reduction in force output does not occur until 0.5 – 1.5 s after visual feedback has been removed, an interval much longer than the time in which proprioceptive and cutaneous feedback impact force output (Johansson and Westling 1984). The results of the current study demonstrate that visuomotor memory may decay at a faster rate in adults with ADHD compared to healthy controls, evidenced by a steeper rate of decay of force output.
The finding that adults with ADHD do not have difficulty producing appropriate force when visual feedback is available, yet demonstrate a steeper rate of decay when visual feedback becomes unavailable may be attributed to ADHD-related deficits in short-term visuomotor memory. That is, individuals with ADHD may be more reliant on visual feedback to guide continuous motor output. A growing body of literature that suggests that individuals with ADHD have deficits in error detection and feedback processing to adjust subsequent behavior (Castellanos and Tannock 2002; Plessen et al. 2015; Shiels and Hawk 2010; Spinelli et al. 2011). In addition, impairments in visual processing, such as depth perception, peripheral vision, and visual search, have been reported in young adults with ADHD (Kim et al. 2014). We propose that such deficits, coupled with problems detecting errors and processing feedback, would make it difficult to store, retrieve, or maintain an accurate internal representation of a motor goal, a function of working memory. Indeed, meta-analyses in children with ADHD demonstrate a particular impairment in visuospatial working memory (Martinussen et al. 2005; Willcutt et al. 2005) and a meta-analysis in adults with ADHD reports deficits in both phonological and visuospatial working memory (Alderson et al. 2013).
Clinical associations
One of the goals of this work was to examine the relationship between symptom severity and the rate of force decay in the absence of vision. Four iterations of multilevel models demonstrated a consistent relationship between self-reported symptoms and a faster rate of decay of force output. Group membership (assigned by self-identification by the participant) and the BIS-11 subscale for Attention predicted a faster rate of decay. Although the CAARS subscales for Inattention/Memory Problems and ADHD Index these did not reach conventional levels of significance, the models are in the same direction – increased symptom severity was associated with a faster rate of decay. Together, these results suggest that attention and working memory processes may be related to the maintenance of continuous grip force output in the absence of visual feedback. Indeed, inattention is a core feature of ADHD and working memory deficits have frequently been reported in the literature (Martinussen et al. 2005; Rapport et al. 2008; Willcutt et al. 2005). Working memory operations require a distributed network of brain regions, including dorsolateral prefrontal cortex, premotor cortex, dorsal cingulate cortex, and posterior parietal cortex (D'Esposito 2007) and these are key regions in the pathophysiology of ADHD (Makris et al. 2009).
Conclusions, limitations, and future directions
Our findings should be viewed in light of several limitations. The goal of this work was to report an initial evaluation of how adults with ADHD use visual feedback and visuomotor memory to guide precision force output. Other sensory modalities, such as tactile and proprioceptive feedback, should be examined to determine whether sensory processing differences occur in ADHD and, further, how these differences may impact continuous and discrete motor output. In addition, it is important to note that young adults volunteering for a study in a University setting typically have limited access to their pediatric medical records. We did not want to increase the burden to participants by requesting such records. Consequently, for this first investigation of adult ADHD from our group, we relied on a self-identification strategy followed by self-report of impairment using the CAARS. On one hand, the lack of a clinical diagnosis from a clinician may be viewed as a limitation. On the other hand, however, our work demonstrates that differences in motor output are clear even in moderate ADHD. Future studies from our group will employ more rigorous measures to evaluate current symptomatology.
In conclusion, we demonstrate that adults with ADHD do not have difficulty integrating visual feedback for real-time control of grip force; however, when visual feedback is removed, their grip force output decays as a function of time. The precision grip task studied here was examined in a college-attending population and our ADHD group was characterized by moderate, not severe, impairment. This suggests that our task is a sensitive index of motor control. We aim to extend our work to a community population of young adults with ADHD who may be experiencing more adverse symptoms. An important avenue for future work will be identifying patterns of performance across a greater range of severity to ensure generalization of results and discovery of the ideal amount of sensory feedback useful for the ongoing control of movement among adults with ADHD.
Figure 3. Multilevel models with linear and quadratic time effects.
Predicted regression lines for mean force as a function of seconds from the removal of visual feedback. Blue line represents self-reported ADHD group. Red line represents healthy controls. The separating lines reflect the group by time interaction shown in Table 2 (Model A).
Acknowledgments
This study was supported by NIH NCATS TR000126 and NIH R01 MH084947.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- Adi-Japha E, Landau YE, Frenkel L, Teicher M, Gross-Tsur V, Shalev RS. ADHD and dysgraphia: underlying mechanisms Cortex. a journal devoted to the study of the nervous system and behavior. 2007;43:700–709. doi: 10.1016/s0010-9452(08)70499-4. [DOI] [PubMed] [Google Scholar]
- Albayrak O, et al. Common obesity risk alleles in childhood attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2013;162:295–305. doi: 10.1002/ajmg.b.32144. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Kasper LJ, Hudec KL, Patros CH. Attention-deficit/hyperactivity disorder (ADHD) and working memory in adults: a meta-analytic review. Neuropsychology. 2013;27:287–302. doi: 10.1037/a0032371. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Attention-deficit hyperactivity disorder. Scientific American. 1998;279:66–71. doi: 10.1038/scientificamerican0998-66. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Cox D. A review of driving risks and impairments associated with attention-deficit/hyperactivity disorder and the effects of stimulant medication on driving performance. Journal of safety research. 2007;38:113–128. doi: 10.1016/j.jsr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, DuPaul GJ, Shelton TL. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: a 3- to 5-year follow-up survey. Pediatrics. 1993;92:212–218. [PubMed] [Google Scholar]
- Breslin FC, Pole JD. Work injury risk among young people with learning disabilities and attention-deficit/hyperactivity disorder in Canada. Am J Public Health. 2009;99:1423–1430. doi: 10.2105/AJPH.2008.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brossard-Racine M, Shevell M, Snider L, Belanger SA, Julien M, Majnemer A. Persistent Handwriting Difficulties in Children With ADHD After Treatment With Stimulant Medication. J Atten Disord. 2015;19:620–629. doi: 10.1177/1087054712461936. [DOI] [PubMed] [Google Scholar]
- Cascio CJ. Somatosensory processing in neurodevelopmental disorders. J Neurodev Disord. 2010;2:62–69. doi: 10.1007/s11689-010-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- Cole WR, Mostofsky SH, Larson JC, Denckla MB, Mahone EM. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71:1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners KC, Erhardt D, Sparrow E. Conners' Adult ADHD Rating Scales. North Tonawanda, NY: Multi-Health Systems Inc; 1999. [Google Scholar]
- Coombes SA, Corcos DM, Vaillancourt DE. Spatiotemporal tuning of brain activity and force performance. Neuroimage. 2011;54:2226–2236. doi: 10.1016/j.neuroimage.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, et al. Attention-deficit/hyperactivity disorder (ADHD) and obesity: a systematic review of the literature. Crit Rev Food Sci Nutr. 2008;48:524–537. doi: 10.1080/10408390701540124. [DOI] [PubMed] [Google Scholar]
- Cross-Villasana F, et al. The Speed of Visual Attention and Motor-Response Decisions in Adult Attention-Deficit/Hyperactivity. Disorder Biol Psychiatry. 2015;78:107–115. doi: 10.1016/j.biopsych.2015.01.016. [DOI] [PubMed] [Google Scholar]
- D'Esposito M. From cognitive to neural models of working memory. Philos Trans R Soc Lond B Biol Sci. 2007;362:761–772. doi: 10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Deutsch KM, Newell KM. Age differences in noise and variability of isometric force production. J Exp Child Psychol. 2001;80:392–408. doi: 10.1006/jecp.2001.2642. [DOI] [PubMed] [Google Scholar]
- Deutsch KM, Newell KM. Deterministic and stochastic processes in children's isometric force variability. Dev Psychobiol. 2003;43:335–345. doi: 10.1002/dev.10140. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine. 2006;36:159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Fliers E, et al. Motor coordination problems in children and adolescents with ADHD rated by parents and teachers: effects of age and gender. J Neural Transm. 2008;115:211–220. doi: 10.1007/s00702-007-0827-0. [DOI] [PubMed] [Google Scholar]
- Gillberg C. Hyperactivity, inattention and motor control problems: prevalence, comorbidity and background factors. Folia Phoniatr Logop. 1998;50:107–117. doi: 10.1159/000021456. [DOI] [PubMed] [Google Scholar]
- Gillberg C, et al. Co-existing disorders in ADHD -- implications for diagnosis and intervention. European child & adolescent psychiatry. 2004;13(Suppl 1):I80–I92. doi: 10.1007/s00787-004-1008-4. [DOI] [PubMed] [Google Scholar]
- Groenman AP, et al. Substance use disorders in adolescents with attention deficit hyperactivity disorder: a 4-year follow-up study. Addiction. 2013;108:1503–1511. doi: 10.1111/add.12188. [DOI] [PubMed] [Google Scholar]
- Hodgkins P, Montejano L, Sasane R, Huse D. Risk of injury associated with attention-deficit/hyperactivity disorder in adults enrolled in employer-sponsored health plans: a retrospective analysis. Prim Care Companion CNS Disord. 2011;13 doi: 10.4088/PCC.10m01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hove MJ, Zeffiro TA, Biederman J, Li Z, Schmahmann J, Valera EM. Postural sway and regional cerebellar volume in adults with attention-deficit/hyperactivity disorder. Neuroimage Clin. 2015;8:422–428. doi: 10.1016/j.nicl.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: a literature review, meta-analysis and critique. J Can Acad Child Adolesc Psychiatry. 2006a;15:105–125. [PMC free article] [PubMed] [Google Scholar]
- Jerome L, Segal A, Habinski L. What we know about ADHD and driving risk: a literature review, meta-analysis and critique. J Can Acad Child Adolesc Psychiatry. 2006b;15:105–125. [PMC free article] [PubMed] [Google Scholar]
- Johansson RS, Westling G. Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res. 1984;56:550–564. doi: 10.1007/BF00237997. [DOI] [PubMed] [Google Scholar]
- Kadesjo B, Gillberg C. Attention deficits and clumsiness in Swedish 7-year-old children. Dev Med Child Neurol. 1998;40:796–804. doi: 10.1111/j.1469-8749.1998.tb12356.x. [DOI] [PubMed] [Google Scholar]
- Kadesjo B, Gillberg C. Developmental coordination disorder in Swedish 7-year-old children. J Am Acad Child Adolesc Psychiatry. 1999;38:820–828. doi: 10.1097/00004583-199907000-00011. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Lane M, Stang PE, Van Brunt DL. The prevalence and workplace costs of adult attention deficit hyperactivity disorder in a large manufacturing firm. Psychol Med. 2009;39:137–147. doi: 10.1017/S0033291708003309. [DOI] [PubMed] [Google Scholar]
- Kim S, Chen S, Tannock R. Visual function and color vision in adults with Attention-Deficit/Hyperactivity. Disorder J Optom. 2014;7:22–36. doi: 10.1016/j.optom.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper T, Haavik J, Drexler H, Ramos-Quiroga JA, Wermelskirchen D, Prutz C, Schauble B. The negative impact of attention-deficit/hyperactivity disorder on occupational health in adults and adolescents. Int Arch Occup Environ Health. 2012;85:837–847. doi: 10.1007/s00420-012-0794-0. [DOI] [PubMed] [Google Scholar]
- Kurdziel LB, Dempsey K, Zahara M, Valera E, Spencer RM. Impaired visuomotor adaptation in adults with ADHD. Exp Brain Res. 2015;233:1145–1153. doi: 10.1007/s00221-014-4190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane CA, Fischman MG, Hart MA, Reeve TG. Manipulations of sensory information: a test of the hypothesis of redundancy of knowledge of results. Percept Mot Skills. 2000;91:1106–1112. doi: 10.2466/pms.2000.91.3f.1106. [DOI] [PubMed] [Google Scholar]
- Li X, et al. Atypical pulvinar-cortical pathways during sustained attention performance in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2012;51:1197–1207. e1194. doi: 10.1016/j.jaac.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macneil LK, Xavier P, Garvey MA, Gilbert DL, Ranta ME, Denckla MB, Mostofsky SH. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology. 2011;76:622–628. doi: 10.1212/WNL.0b013e31820c3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeot SD, Miller LJ, McIntosh DN, McGrath-Clarke J, Simon J, Hagerman RJ, Goldson E. Sensory modulation dysfunction in children with attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2001;43:399–406. doi: 10.1017/s0012162201000743. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Klein DF, Bessler A, Shrout P. Accuracy of adult recall of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 2002;159:1882–1888. doi: 10.1176/appi.ajp.159.11.1882. [DOI] [PubMed] [Google Scholar]
- Martinussen R, Hayden J, Hogg-Johnson S, Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. [DOI] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, Hagerman RJ. Sensory-modulation disruption, electrodermal responses, and functional behaviors. Dev Med Child Neurol. 1999;41:608–615. doi: 10.1017/s0012162299001267. [DOI] [PubMed] [Google Scholar]
- Merkel RL, Jr, et al. Comparison of On-Road Driving Between Young Adults With and Without ADHD. J Atten Disord. 2013 doi: 10.1177/1087054712473832. [DOI] [PubMed] [Google Scholar]
- Mosconi MW, Mohanty S, Greene RK, Cook EH, Vaillancourt DE, Sweeney JA. Feedforward and feedback motor control abnormalities implicate cerebellar dysfunctions in autism spectrum disorder. J Neurosci. 2015;35:2015–2025. doi: 10.1523/JNEUROSCI.2731-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky SH, Newschaffer CJ, Denckla MB. Overflow movements predict impaired response inhibition in children with ADHD. Perceptual and motor skills. 2003;97:1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Nazar BP, Pinna CM, Suwwan R, Duchesne M, Freitas SR, Sergeant J, Mattos P. ADHD Rate in Obese Women With Binge Eating and Bulimic Behaviors From a Weight-Loss Clinic. J Atten Disord. 2012 doi: 10.1177/1087054712455503. [DOI] [PubMed] [Google Scholar]
- Nazar BP, Suwwan R, de Sousa Pinna CM, Duchesne M, Freitas SR, Sergeant J, Mattos P. Influence of attention-deficit/hyperactivity disorder on binge eating behaviors and psychiatric comorbidity profile of obese women. Comprehensive psychiatry. 2014;55:572–578. doi: 10.1016/j.comppsych.2013.09.015. [DOI] [PubMed] [Google Scholar]
- Neely KA, Coombes SA, Planetta PJ, Vaillancourt DE. Segregated and overlapping neural circuits exist for the production of static and dynamic precision grip force. Hum Brain Mapp. 2013a;34:698–712. doi: 10.1002/hbm.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KA, et al. Functional Brain Activity Relates to 0–3 and 3–8 Hz Force Oscillations in Essential Tremor. Cereb Cortex. 2015;25:4191–4202. doi: 10.1093/cercor/bhu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely KA, et al. Force control deficits in individuals with Parkinson's disease, multiple systems atrophy, and progressive supranuclear palsy. PLoS One. 2013b;8:e58403. doi: 10.1371/journal.pone.0058403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JW, Dowell LR, Mostofsky SH, Denckla MB, Mahone EM. Neuropsychological profile of executive function in girls with attention-deficit/hyperactivity disorder. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2010;25:656–670. doi: 10.1093/arclin/acq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Piek JP, Pitcher TM, Hay DA. Motor coordination and kinaesthesis in boys with attention deficit-hyperactivity disorder. Developmental medicine and child neurology. 1999;41:159–165. doi: 10.1017/s0012162299000341. [DOI] [PubMed] [Google Scholar]
- Pitcher TM, Piek JP, Hay DA. Fine and gross motor ability in males with ADHD. Developmental medicine and child neurology. 2003;45:525–535. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- Plessen KJ, et al. Reduced error signalling in medication-naive children with ADHD: associations with behavioural variability and post-error adaptations. J Psychiatry Neurosci. 2015;40:140353. doi: 10.1503/jpn.140353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon C, Robichaud JA, Corcos DM, Goldman JG, Vaillancourt DE. Combined measures of movement and force variability distinguish Parkinson's disease from essential tremor. Clin Neurophysiol. 2011;122:2268–2275. doi: 10.1016/j.clinph.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev. 2009;33:900–908. doi: 10.1016/j.neubiorev.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine MB, Majnemer A, Shevell M, Snider L. Handwriting performance in children with attention deficit hyperactivity disorder (ADHD) J Child Neurol. 2008;23:399–406. doi: 10.1177/0883073807309244. doi:23/4/399 [pii] 10.1177/0883073807309244 [doi] [DOI] [PubMed] [Google Scholar]
- Rapport MD, Alderson RM, Kofler MJ, Sarver DE, Bolden J, Sims V. Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. J Abnorm Child Psychol. 2008;36:825–837. doi: 10.1007/s10802-008-9215-y. [DOI] [PubMed] [Google Scholar]
- Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain and cognition. 2008;68:415–435. doi: 10.1016/j.bandc.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubia K, Noorloos J, Smith A, Gunning B, Sergeant J. Motor timing deficits in community and clinical boys with hyperactive behavior: the effect of methylphenidate on motor timing. J Abnorm Child Psychol. 2003;31:301–313. doi: 10.1023/a:1023233630774. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW., Jr Self-regulation in ADHD: the role of error processing. Clin Psychol Rev. 2010;30:951–961. doi: 10.1016/j.cpr.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Vasa RA, Joel S, Nelson TE, Pekar JJ, Mostofsky SH. Variability in post-error behavioral adjustment is associated with functional abnormalities in the temporal cortex in children with ADHD. J Child Psychol Psychiatry. 2011;52:808–816. doi: 10.1111/j.1469-7610.2010.02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swensen A, Birnbaum HG, Ben Hamadi R, Greenberg P, Cremieux PY, Secnik K. Incidence and costs of accidents among attention-deficit/hyperactivity disorder patients. J Adolesc Health. 2004;35:346, e341–e349. [PubMed] [Google Scholar]
- Tegelbeckers J, Bunzeck N, Duzel E, Bonath B, Flechtner HH, Krauel K. Altered salience processing in attention deficit hyperactivity disorder. Hum Brain Mapp. 2015;36:2049–2060. doi: 10.1002/hbm.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya HP. Substance use disorders in children and adolescents with attention-deficit/hyperactivity disorder: implications for treatment and the role of the primary care physician Prim Care Companion. J Clin Psychiatry. 2008;10:211–221. doi: 10.4088/pcc.v10n0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Mayka MA, Corcos DM. Intermittent visuomotor processing in the human cerebellum, parietal cortex, and premotor cortex. J Neurophysiol. 2006;95:922–931. doi: 10.1152/jn.00718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Russell DM. Temporal capacity of short-term visuomotor memory in continuous force production. Exp Brain Res. 2002;145:275–285. doi: 10.1007/s00221-002-1081-1. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Slifkin AB, Newell KM. Visual control of isometric force in Parkinson's disease. Neuropsychologia. 2001;39:1410–1418. doi: 10.1016/s0028-3932(01)00061-6. [DOI] [PubMed] [Google Scholar]
- Valera EM, et al. Neural substrates of impaired sensorimotor timing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:359–367. doi: 10.1016/j.biopsych.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Huang TH, Lo SK. Motor ability and adaptive function in children with attention deficit hyperactivity disorder Kaohsiung. J Med Sci. 2011;27:446–452. doi: 10.1016/j.kjms.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Wang Z, Magnon GC, White SP, Greene RK, Vaillancourt DE, Mosconi MW. Individuals with autism spectrum disorder show abnormalities during initial and subsequent phases of precision gripping. J Neurophysiol. 2015;113:1989–2001. doi: 10.1152/jn.00661.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Prince J. Pharmacotherapy of adult attention deficit/hyperactivity disorder: a review. J Clin Psychopharmacol. 1995;15:270–279. doi: 10.1097/00004714-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zelaznik HN, Vaughn AJ, Green JT, Smith AL, Hoza B, Linnea K. Motor timing deficits in children with Attention-Deficit/Hyperactivity disorder. Human movement science. 2012;31:255–265. doi: 10.1016/j.humov.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]