Abstract
Objectives
To determine whether lifestyle factors measured late in life could compress the disabled period towards the end of life.
Design
Community-based cohort study of older adults followed from 1989 to 2015.
Setting
Four US communities.
Participants
Men and women ages 65 and older (N= 5248, mean age 72.7 ± 5.5 years, 57% women, 15.2% minority) who were living in the community, not wheel-chair dependent and able to give informed consent at baseline.
Measurements
Multiple lifestyle factors including smoking, alcohol consumption, physical activity, diet, body mass index (BMI), social networks and social support were measured at baseline. Activities of Daily Living (ADL) were assessed at baseline and throughout follow-up. Years of Life (YoL) was defined as years until death. Years of Able Life (YAL) was defined as years without any ADL difficulty. YAL/YoL%, the proportion of life lived able, was used to indicate the relative compression/expansion of the disabled period.
Results
The average duration of disabled years was 4.5 (out of 15.4 mean YoL) for women and 2.9 (out of 12.4 mean YoL) for men. In a multivariable model, obesity (compared to normal BMI) was associated with 7.3% (95% CI, 5.4–9.2) lower YAL/YoL%. Scores in the lowest quintile of the Alternate Healthy Eating Index (compared to highest) were associated with a 3.7% (95% CI, 1.6–5.9) lower YAL/YoL%. Every 25 blocks walked in a week was associated with 0.5% (95% CI, 0.3–0.8) higher YAL/YoL%.
Conclusion
The effects of healthy lifestyle factors on the proportion of future life lived free of disability indicate that the disabled period can be compressed, given the right combination of these factors.
Keywords: disability, older adults, lifestyle, active life expectancy
INTRODUCTION
The duration of the disabled period near the end of life has enormous personal and societal implications.1 A disabled older adult experiences a poorer quality of life2 and has poorer health outcomes including frequent hospital admissions3 and a higher risk for mortality.4,5 Years lived with disability contribute substantially to Medicare costs6 which are already projected to increase due to the aging of the baby-boom generation. Informal caregiving for disabled older adults, primarily by family members, result in considerable invisible cost.7 Despite these implications, and the obvious public health need to compress this terminal morbid period, we have limited evidence regarding the factors that influence the length and proportion of the disabled period near the end of life.
The average length of the disabled period in populations and its change over time in the context of increasing life expectancy has been the subject of much debate.8 Central to this debate is the compression of morbidity paradigm which argues that healthy lifestyle factors can postpone the onset of disease and disability, compressing the morbid period.9 Postponement of disease and disability by a healthy lifestyle is well known, however, healthy lifestyle factors can also postpone death. Therefore, compression of morbidity can occur only if factors postpone morbidity to a greater degree than death.10 It is therefore important to distinguish and delineate the effects of lifestyle factors on the onset of disease and disability from their effect on the life span in order to understand their potential to compress morbidity.
The Cardiovascular Health Study, a cohort of older adults followed for over 25 years, provides a unique opportunity in aging research to assess health and morbidity near the end of the life span. In this study, we used disability as our measure of morbidity and examined the differential effect of multiple lifestyle factors measured late in life on the health span and life span of CHS participants. Health span was measured as the duration of life free of disability as observed in the study, defined as years of life without any reported difficulty in activities of daily living and referred to as Years of Able Life (YAL). Life span was measured as the total Years of Life (YoL) in the study. Additionally, we describe the association of lifestyle factors with the YAL/YoL%, an estimate of the proportion of observed years without disability, in order to determine whether any of these factors are associated with a relative compression or expansion of the disabled period prior to death.
METHODS
Study design and participants
The Cardiovascular Health Study (CHS) is a longitudinal study of cardiovascular risk factors in 5888 adults aged 65 and older at baseline. Participants were recruited from a random sample of age-eligible Medicare beneficiaries and household members in four US communities: Sacramento County, California; Forsyth County, North Carolina; Washington County, Maryland and Allegheny County, Pennsylvania.11,12 Eligible participants were not institutionalized or wheelchair dependent, did not require a proxy for consent, were not under treatment for cancer at the time of enrollment and were expected to remain in their location for at least three years. The study enrolled 5201 participants in 1990 and added 687 African-Americans in 1993. Participants provided written informed consent and the protocol was approved by the institutional review boards at each participating center.
Participants completed an extensive interview and examination at the field centers at baseline. After enrollment, participants were seen annually, and were contacted by telephone at 6-month intervals until 1999. Since 1999, participants have been contacted every 6 months by telephone to ascertain health status including disability, and were invited to participate in an in-person visit in 2005–06.
Years of Life (YoL), Years of Able Life (YAL) and the Proportion of Life Lived Able (YAL/YoL %)
Study participants were followed for a total of 25 years. Deaths were identified at 6 month contacts and from obituaries, medical records, proxy interviews, death certificates and a search of the National Death Index. Follow-up for vital status was 100% complete. YoL was defined as each person’s number of years from baseline to death. We estimated remaining YoL for 544 (9.2%) CHS participants who were still alive at the end of follow-up using regression equations based on age, sex, baseline ADL and self-rated health, as described elsewhere.13
Self-reported difficulties in Activities of Daily Living (ADL; eating, bathing, toileting, dressing, getting out of bed or chair, and walking around the home) were assessed annually through 1999 and every six months thereafter. YAL was defined as the number of observed years without any ADL difficulty. From 2000 to 2004, the questions regarding ADLs addressed change in difficulty rather than difficulty status. To create a complete data set of ADL difficulty every 6 months, we imputed ADL difficulty status at the half year intervals between 1990 and 1999, and at all of the time points from 2000 to 2004. For all other time points, 0.3% to 12.5% of ADL data were missing and were also imputed. This method of imputation of CHS data has been previously described.14,15 Remaining YAL for 544 participants who survived to the end of follow-up were imputed using regression equations.13 Subsequently, YAL/YoL% was calculated to represent the relative proportion of the observed life span which was lived free of ADL difficulty.
Lifestyle factors
Lifestyle factors were assessed at baseline. Smoking and alcohol consumption were self-reported. Participants were considered to be former alcohol drinkers if they were non-drinkers at baseline and reported 1) having stopped alcohol consumption in the past five years and/or 2) ever drank five or more drinks of any kind of alcohol almost every day. Leisure time activity (kilocalories/week) was assessed using the modified Minnesota leisure-time activities questionnaire,16 and a weighted sum of kilocalories expended in physical activity was calculated and log transformed. The highest intensity of reported activity was used to categorize the exercise intensity of participants to high, moderate, low or none.17 Distances walked were assessed by self-report of blocks walked in the previous week. Dietary habits were assessed for the original cohort alone using the picture-sort National Cancer Institute food frequency questionnaire.18 The Alternate Healthy Eating Index (AHEI) was calculated from these data consistent with previous studies19 and categorized into quintiles. Standardized techniques were used to measure height and weight. Body Mass Index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Social support was measured using a six-item version of the Interpersonal Support Evaluation List,20 and social networks (size, closeness and frequency of contacts) were measured using the 10-item Lubben social network scale.21
Potential confounders
Potential confounders considered included socio-demographic factors (age, sex, race, education, income and marital status) and baseline health indicators (self-reported health, change in weight, difficulties in instrumental activities of daily living, chronic health conditions, and cognition). These were selected based on their established association with lifestyle, onset of disability and life expectancy. Sex, age, race, education, income, marital status, self-rated health, weight change of more than 10 pounds in the previous year, arthritis and cancer were self-reported at baseline. Difficulties in Instrumental Activities of Daily Living (IADL) were self-reported in six domains (telephone use, shopping, preparing food, performing light household work, performing heavy household work, and managing finances). Cognition was measured using the Mini Mental Status Examination.22 Retaining methods used previously in CHS, we defined hypertension as self-report of high blood pressure accompanied by medication use or an average seated blood pressure of ≥160/95.23 Borderline hypertension was defined as a systolic blood pressure from 140 to 159 or a diastolic from 90 to 94. Diabetes was classified as fasting blood glucose ≥126 mg/dL or use of anti-glycemic medication, and impaired fasting glucose was classified as glucose 110 to 125 mg/dL.24 Angina, myocardial infarction, heart failure, peripheral artery disease, stroke, and transient ischemic attack were identified using self-report and hospitalization records and adjudicated as previously described.25 Depression was assessed using the 10-item version of the Center for Epidemiologic Studies Depression Scale.26 Missing values for lifestyle factors and potential confounders were imputed as described previously.27 Data were available for 5248 participants after imputation.
Statistical analysis
Mean YoL, YAL and YAL/YoL% were computed by categories of each baseline lifestyle factor or confounder. Means and 95% confidence intervals of YoL, YAL, disabled years and YAL/YoL% were computed for each age and sex group. We used a confounder-adjusted multi-variable linear regression model to examine the effect of lifestyle factors on YAL, YoL and the YAL/YoL%. As diet was assessed only in the original cohort, sample size for this model was 4581. Results from this model were compared to that from a model excluding the diet variable (n=5248) and as the results were similar, the model with the diet variable is presented. Because of the large number of comparisons, we report p-values of <0.001 as statistically significant.
Additionally, to illustrate the potential for optimizing YAL, YoL and YAL/YoL%, we compared the difference between an optimal (healthy) lifestyle versus an unhealthy lifestyle using predicted values from statistical models. We calculated the predicted mean values of YAL, YoL and YAL/YoL% in gender and race-specific models that pre-specified values for all other socio-demographic variables and lifestyle variables excluding diet (n=5248). Socio-demographic variables were fixed at typical values (70 years of age, high school/GED education, currently married, and income <$25,000). The healthy lifestyle was defined by the following characteristics: never smoking, 1–7 drinks/week, BMI 18–25, high exercise intensity, 2300 Kcal exercise (~75th percentile), 48 blocks walked (~75th percentile), social network score of 38 (~75th percentile), and social support score 6 (~25th percentile). The unhealthy lifestyle was defined by the following: current smoking, ≥14 drinks/week, BMI≥30, no exercise intensity, 375 Kcal exercise (~25th percentile), 6 blocks walked (~ 25th percentile), social network score 28 (~25th percentile), and social support score 9 (~75th percentile). This comparison would help to illustrate the values of YAL, YoL and YAL/YOL% that could potentially be achieved by having all the specified healthy lifestyle factors, as compared to values achieved when all the specified multiple unhealthy factors exist, among individuals in different race and gender groups.
RESULTS
Baseline characteristics
At baseline, the mean (SD) participant age was 72.7 (5.5) years, 57% were women and 15.2% were black. At the end of follow-up, 90.8 % of the participants had died. Distribution of baseline characteristics and the unadjusted mean YAL, YoL and YAL/YoL% by these characteristics are shown in Table 1. For example, there were 2992 women who averaged 15.4 YoL, 10.9 YAL, and who spent 65.6 % of their YoL in the able state. Men in the study (n= 2256) averaged 12.4 YoL, 9.5 YAL and were able for 70.9% of their YoL. The average number of disabled years was 2.9 years for men and 4.5 years for women. After adjustment for all of the demographic variables in Table 1, each lifestyle measure was significantly associated with each of the outcomes at p<.001 except smoking and YAL/YoL% (p=.007) and social support and YoL (p=.006).
Table 1.
Baseline Characteristics of CHS Participants, With Mean Years of Life (YoL), Mean Years of Able Life (YAL), and Mean YAL/YoL% in Each Category (n=5248).
| Characteristic | N (%) or Mean (SD) |
Mean (SD) YoL |
Mean (SD) YAL |
Mean (SD) YAL/YoL% |
|---|---|---|---|---|
| Socio-demographic factors | ||||
| Sex | ||||
| Male | 2256 (43) | 12.4 (7.2) | 9.5 (7.0) | 70.9 (24.8) |
| Female | 2992 (57) | 15.4 (7.3) | 10.9 (7.4) | 65.6 (26.4) |
| Age | 72.7 (5.5) | |||
| 65–69 | 1816 (34.6) | 17.5 (7.5) | 13.7 (7.8) | 74.5 (22.8) |
| 70–74 | 1687 (32.1) | 14.5 (6.8) | 10.6 (6.8) | 69.5 (24.7) |
| 75–79 | 1071 (20.4) | 11.4 (5.6) | 7.6 (5.5) | 62.9 (26.4) |
| 80–84 | 497 (9.5) | 9.0 (4.9) | 5.2 (4.0) | 55.8 (27.3) |
| ≥85 | 177 (3.4) | 6.7 (4.1) | 3.4 (3.4) | 47.8 (31.0) |
| Racea | ||||
| White | 4449 (84.8) | 14.2 (7.4) | 10.5 (7.3) | 69.1 (25.1) |
| Black | 799 (15.2) | 13.5 (7.3) | 9.0 (7.3) | 60.7 (29.0) |
| Education | ||||
| ≥College | 541 (10.3) | 15.1 (7.6) | 11.5 (7.7) | 72.2 (24.4) |
| Some College/Vocational | 1724 (32.9) | 14.7 (7.3) | 10.9 (7.3) | 70.0 (24.6) |
| High School/GED | 1452 (27.7) | 14.8 (7.4) | 11.0 (7.4) | 70.0 (24.9) |
| <High School | 1531 (29.2) | 12.6 (7.1) | 8.5 (6.7) | 61.9 (27.7) |
| Income | ||||
| ≥$25,000 | 2091 (39.8) | 15.5 (7.6) | 12.0 (7.6) | 73.2 (23.0) |
| <$25,000 | 3157 (60.2) | 13.2 (7.1) | 9.2 (6.9) | 64.3 (27.0) |
| Marital Status | ||||
| Currently Married | 3529 (67.2) | 14.7 (7.4) | 11.0 (7.4) | 70.2 (24.8) |
| Not Married | 1719 (32.8) | 13.0 (7.2) | 8.9 (6.8) | 63.0 (27.3) |
| Health factors | ||||
| Self-Reported Health | ||||
| Excellent | 721 (13.7) | 16.9 (7.2) | 13.8 (7.5) | 78.6 (19.9) |
| Very Good | 1272 (24.2) | 15.6 (7.4) | 12.2 (7.3) | 74.7 (21.4) |
| Good | 1961 (37.4) | 14.1 (7.2) | 10.2 (6.9) | 68.6 (24.3) |
| Fair | 1094 (20.8) | 11.6 (6.8) | 7.2 (6.1) | 56.9 (28.3) |
| Poor | 200 (3.8) | 8.6 (6.3) | 3.9 (4.5) | 38.2 (28.4) |
| Number of IADL difficulties | 0.35 ± 0.72 | |||
| 0 | 3910 (74.5) | 14.9 (7.3) | 11.5 (7.2) | 73.4 (22.0) |
| 1 | 1024 (19.5) | 12.4 (7.0) | 7.6 (6.3) | 56.3 (27.3) |
| ≥2 | 314 (6) | 9.7 (6.8) | 4.3 (5.3) | 36.1 (30.5) |
| MMSE Score | 27.5 ± 2.7 | |||
| 29–30 | 2367 (45.1) | 15.8 (7.3) | 12.0 (7.4) | 72.7 (23.0) |
| 27–28 | 1687 (32.1) | 13.9 (7.4) | 10.0 (7.2) | 67.5 (26.2) |
| <27 | 1194 (22.8) | 11.3 (6.7) | 7.3 (6.2) | 58.8 (28.3) |
| Weight Change | ||||
| No Changes | 3728 (71) | 14.5 (7.3) | 10.8 (7.3) | 70.4 (24.7) |
| Gained and Lost >10 lbs | 354 (6.7) | 14.5 (7.7) | 10.0 (7.4) | 64.2 (27.1) |
| Gained >10 lbs | 471 (9) | 14.1 (7.1) | 9.4 (6.8) | 62.1 (27.2) |
| Lost >10 lbs | 695 (13.2) | 12.1 (7.3) | 8.1 (6.8) | 60.1 (28.3) |
| Arthritis | ||||
| No | 2536 (48.3) | 14.4 (7.5) | 11.3 (7.4) | 74.1 (22.5) |
| Yes | 2712 (51.7) | 13.9 (7.3) | 9.3 (7.0) | 62.0 (27.4) |
| Cancer | ||||
| No | 4503 (85.8) | 14.3 (7.4) | 10.5 (7.3) | 68.3 (25.6) |
| Yes | 745 (14.2) | 12.9 (7.2) | 9.1 (6.9) | 65.3 (27.3) |
| Hypertension | ||||
| Normal | 2178 (41.5) | 15.5 (7.5) | 11.9 (7.6) | 72.2 (23.9) |
| Borderline | 745 (14.2) | 13.8 (7.2) | 10.1 (7.2) | 68.2 (25.8) |
| Hypertensive | 2325 (44.3) | 13.0 (7.1) | 8.9 (6.7) | 63.7 (27.0) |
| Diabetes | ||||
| Normal | 3766 (71.8) | 14.9 (7.4) | 11.1 (7.4) | 69.8 (25.1) |
| Impaired Fasting Glucose | 619 (11.8) | 14.0 (7.4) | 10.1 (7.1) | 67.5 (25.3) |
| Diabetes | 863 (16.4) | 10.9 (6.6) | 7.0 (5.8) | 59.8 (27.9) |
| Angina | ||||
| No | 4399 (83.8) | 14.7 (7.4) | 10.9 (7.4) | 69.0 (27.3) |
| Yes | 849 (16.2) | 11.2 (6.5) | 7.4 (5.8) | 61.8 (27.3) |
| Myocardial Infarction | ||||
| No | 4756 (90.6) | 14.5 (7.4) | 10.6 (7.3) | 68.3 (25.7) |
| Yes | 492 (9.4) | 10.3 (6.4) | 7.1 (5.9) | 63.6 (27.5) |
| Congestive Heart Failure | ||||
| No | 5026 (95.8) | 14.4 (7.3) | 10.6 (7.3) | 68.7 (25.4) |
| Yes | 222 (4.2) | 7.9 (5.8) | 4.3 (4.4) | 48.9 (30.0) |
| Peripheral Artery Disease | ||||
| No | 5112 (97.4) | 14.3 (7.4) | 10.4 (7.3) | 68.2 (25.7) |
| Yes | 136 (2.6) | 8.5 (5.3) | 5.3 (4.9) | 56.5 (30.4) |
| Stroke | ||||
| No | 5033 (95.9) | 14.4 (7.4) | 10.5 (7.3) | 68.5 (25.5) |
| Yes | 215 (4.1) | 8.8 (5.8) | 5.1 (4.9) | 53.0 (30.2) |
| Transient ischemic attack | ||||
| No | 5102 (97.2) | 14.2 (7.4) | 10.4 (7.3) | 68.1 (25.8) |
| Yes | 146 (2.8) | 10.2 (6.3) | 6.4 (5.5) | 59.2 (27.9) |
| Chronic Obstructive Pulmonary Disease | ||||
| No | 4559 (86.9) | 14.3 (7.4) | 10.6 (7.3) | 68.9 (25.4) |
| Yes | 689 (13.1) | 12.7 (7.2) | 8.4 (6.7) | 61.3 (27.9) |
| Depression Score | 4.6 ± 4.6 | |||
| 0–1 | 1511 (28.8) | 14.7 (7.4) | 11.7 (7.4) | 75.4 (21.5) |
| 2–6 | 2390 (45.5) | 14.3 (7.4) | 10.5 (7.2) | 68.8 (25.0) |
| 7+ | 1347 (25.7) | 13.2 (7.4) | 8.4 (6.9) | 57.7 (28.5) |
| Lifestyle factors | ||||
| Smoking Status | ||||
| Never | 2440 (46.5) | 15.0 (7.5) | 10.8 (7.6) | 66.8 (26.6) |
| Former | 2196 (41.8) | 13.7 (7.3) | 10.1 (7.1) | 69.4 (24.9) |
| Current | 612 (11.7) | 12.4 (7.0) | 8.9 (6.7) | 66.5 (26.1) |
| No. of Alcoholic Drinks/Week | ||||
| Never | 2127 (40.5) | 13.9 (7.1) | 9.7 (7.0) | 64.2 (27.1) |
| Former | 469 (8.9) | 11.3 (7.3) | 7.9 (6.8) | 62.6 (27.9) |
| <1 | 1019 (19.4) | 14.7 (7.5) | 11.0 (7.4) | 70.6 (24.4) |
| (1,7) | 913 (17.4) | 15.0 (7.5) | 11.5 (7.5) | 72.2 (23.4) |
| (7,14) | 312 (5.9) | 15.0 (7.7) | 11.5 (7.7) | 72.0 (24.1) |
| ≥14 | 408 (7.8) | 14.4 (7.3) | 11.0 (7.3) | 73.2 (23.1) |
| Physical Activity (Kcal) | 1734 ± 2055 | |||
| ≥Kcal 2300 | 1314 (25) | 15.7 (7.2) | 12.3 (7.3) | 74.8 (21.7) |
| Kcal 375–2299 | 2618 (49.9) | 14.3 (7.4) | 10.5 (7.2) | 69.5 (24.4) |
| Kcal 0–374 | 1316 (25.1) | 12.2 (7.2) | 7.8 (6.8) | 57.7 (29.2) |
| Exercise Intensity | ||||
| High | 543 (10.3) | 16.6 (7.2) | 13.3 (7.6) | 76.2 (22.6) |
| Moderate | 1793 (34.2) | 15.0 (7.3) | 11.1 (7.2) | 70.6 (23.9) |
| Low | 2466 (47) | 13.5 (7.3) | 9.7 (7.0) | 66.9 (25.8) |
| None | 446 (8.5) | 11.1 (7.0) | 6.6 (6.5) | 52.0 (30.4) |
| Blocks Walked | ||||
| >48 | 1300 (24.8) | 15.4 (7.3) | 12.2 (7.3) | 76.4 (20.2) |
| 7–48 | 2433 (46.4) | 14.7 (7.3) | 11.0 (7.2) | 70.5 (23.7) |
| 0–6 | 1515 (28.9) | 12.1 (7.2) | 7.6 (6.6) | 56.2 (29.3) |
| Alternate Healthy Eating Index | ||||
| 52–80.5 | 904 (19.7) | 16.8 (7.2) | 13.1 (7.3) | 75.2 (21.2) |
| 43–51.5 | 890 (19.4) | 14.9 (7.2) | 11.0 (7.1) | 70.3 (23.8) |
| 35–42.5 | 942 (20.6) | 14.2 (7.3) | 10.4 (7.2) | 68.1 (25.3) |
| 27–34.5 | 899 (19.6) | 13.3 (7.3) | 9.6 (7.1) | 67.2 (26.3) |
| 5.5–26.5 | 946 (20.7) | 12.1 (7.1) | 8.5 (6.7) | 64.4 (27.6) |
| Body Mass Indexb | ||||
| <18.5 | 81 (1.5) | 10.6 (6.8) | 7.4 (6.0) | 63.4 (26.4) |
| 18.5–24.99 | 1994 (38) | 13.9 (7.5) | 10.5 (7.4) | 70.9 (24.3) |
| 25–26.99 | 1061 (20.2) | 14.2 (7.4) | 10.7 (7.3) | 69.9 (25.0) |
| 27–29.99 | 1109 (21.1) | 14.5 (7.3) | 10.6 (7.3) | 67.9 (25.3) |
| ≥30 | 1003 (19.1) | 14.5 (7.2) | 9.3 (7.0) | 60.0 (28.6) |
| Social Network Score | 32.4 ± 7.4 | |||
| ≥38 | 1312 (25) | 15.4 (7.4) | 11.6 (7.4) | 71.4 (23.4) |
| 28–37 | 2647 (50.4) | 14.3 (7.4) | 10.5 (7.3) | 68.7 (25.4) |
| <28 | 1289 (24.6) | 12.6 (7.1) | 8.6 (6.8) | 62.6 (28.3) |
| Social Support Score | 8.26 ± 2.60 | |||
| <7 | 1738 (33.1) | 14.4 (7.4) | 10.7 (7.3) | 69.9 (25.0) |
| [7,9) | 1630 (31.1) | 14.5 (7.4) | 10.7 (7.3) | 69.4 (24.7) |
| ≥9 | 1880 (35.8) | 13.6 (7.4) | 9.5 (7.2) | 64.7 (27.3) |
Abbreviations: CHS, Cardiovascular Health Study; GED, General education development test; IADL, Instrumental activities of daily living; Kcal, Kilocalories; MMSE, Mini-mental state examination.
Non-Black minorities were included with Whites as they were very few (American Indian/Alaskan Native N=12, Asian/Pacific Islander N=4, Other = 4).
The overweight category (BMI 25.0 to 29.9) was subdivided into two groups as this category held a large proportion of the participants (41.3%)
Trends in disabled years
The number of observed years of total and able life was lower for older participants, reflecting their earlier death during the course of the study and consequently, shorter follow-up period (Table 2). However, the number of disabled years was similar across baseline age groups among men as well as among women, indicating the terminal nature of the disabled period. Consequently, the YAL/YoL% was lower among older age groups among both men and women. Women in all age groups had more disabled years than men in corresponding age groups.
Table 2.
Mean Observed Years of Life (YoL), Years of Able Life (YAL), Years of Disability, and YAL/YoL% in Different Baseline Age Groups, for Women and Men.
| Age at baseline | N | Mean observed YoL (a) |
Mean observed YAL (b) |
Mean Observed Years of Disability (a–b) |
YAL/YoL% (b/a*100) |
||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | ||
| Women(n=2992) | |||||||||
| 65 to 69 years | 1126 | 18.6 | 18.2, 19.0 | 14.2 | 13.8, 14.7 | 4.4 | 4.2, 4.6 | 72.9 | 71.6, 74.3 |
| 70 to 74 years | 930 | 15.7 | 15.3, 16.2 | 11.0 | 10.6, 11.4 | 4.7 | 4.5, 5.0 | 66.4 | 64.8, 68.1 |
| 75 to 79 years | 609 | 12.2 | 11.7, 12.7 | 7.7 | 7.3, 8.2 | 4.5 | 4.2, 4.8 | 59.6 | 57.5, 61.8 |
| 80 to 84 years | 241 | 10.1 | 9.5, 10.7 | 5.4 | 4.9, 5.9 | 4.7 | 4.3, 5.2 | 51.3 | 47.9, 54.7 |
| 85+ years | 86 | 7.6 | 6.6, 8.5 | 3.5 | 2.6, 4.3 | 4.1 | 3.4, 4.8 | 41.5 | 34.8, 48.2 |
| Men (n=2256) | |||||||||
| 65 to 69 years | 690 | 15.7 | 15.1, 16.3 | 12.8 | 12.2, 13.4 | 2.9 | 2.7, 3.1 | 77.1 | 75.4, 78.7 |
| 70 to 74 years | 757 | 13.1 | 12.6, 13.6 | 10.1 | 9.6, 10.6 | 3.0 | 2.8, 3.2 | 73.3 | 71.7, 75.0 |
| 75 to 79 years | 462 | 10.3 | 9.8, 10.8 | 7.4 | 6.9, 7.8 | 2.9 | 2.7, 3.2 | 67.1 | 64.8, 69.4 |
| 80 to 84 years | 256 | 7.9 | 7.4, 8.5 | 5.1 | 4.6, 5.6 | 2.8 | 2.5, 3.2 | 60.1 | 56.8, 63.4 |
| 85+ years | 91 | 5.8 | 5.1, 6.6 | 3.3 | 2.7, 3.9 | 2.5 | 2.0, 3.0 | 53.7 | 47.5, 59.9 |
Abbreviations: CI, Confidence interval
Lifestyle factors and YAL, YoL, YAL/YoL%
After adjusting for socio-demographic and baseline health variables, as well as other lifestyle factors, smoking was associated with fewer years of life (3.5 years less), and fewer years of able life (3.1 years less) (Table 3). Former smokers had fewer YoL (1.3 years less) and fewer YAL (1.2 years less) when compared to non-smokers. However, the YAL/YoL percentages among smokers as well as former smokers were not significantly different from that of non-smokers. Former drinkers had fewer YoL when compared to non-drinkers, but there was no significant difference in the YAL or YAL/YoL%. Current drinkers, on the other hand, did not seem to be significantly different from non-drinkers in terms of YAL, YoL and YAL/YoL%. Higher energy expenditure (2.7 fold) in Kilocalories was associated with more YAL (borderline significance), albeit with a small effect size (0.3 years). Exercise intensity was no longer significantly associated with YAL, YoL or YAL/YoL% but those who walked more blocks per week had significantly greater YAL/YoL%. Every 25 blocks walked per week was associated with 0.5% higher YAL/YoL%. Compared to the highest quintile of the AHEI, which is indicative of a healthy diet, lower quintiles were associated with significantly fewer YoL and YAL. Participants in the worst quintile of AHEI had 1.5 years fewer YoL, 1.5 years fewer YAL and 3.7% lower YAL/YoL%. Using the conservative p-value cut-off of <0.001, the latter difference would be of borderline statistical significance. Underweight participants (BMI < 18.5) had significantly fewer YoL (3 years less) compared to those with normal BMI, but their YAL and YAL/YoL% were not significantly different. Participants who were obese (BMI ≥ 30) had a significantly lower proportion of able years (7.3% less) when compared to those with normal BMI, but the higher YoL and lower YAL associated with this category did not reach statistical significance. Social networks and social support were not found to be associated with YoL, YAL or YAL/YoL%.
Table 3.
Lifestyle Factors and Years of Life (YoL), Years of Able Life (YAL), and YAL/YoL %, Linear Regression Modela, n= 4581b
| Factors | YOL
|
YAL
|
YAL/YOL percentage
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | p-valuec | Coefficient | 95% CI | p-valuec | Coefficient | 95% CI | p-valuec | |
| Smoking | <0.001 | <0.001 | 0.048 | ||||||
| Never | Ref | – | – | Ref | – | – | Ref | – | – |
| Former | −1.3 | (−1.6, −0.9) | <0.001 | −1.2 | (−1.5, −0.8) | <0.001 | −0.9 | (−2.3, 0.5) | 0.203 |
| Current | −3.5 | (−4.1, −2.9) | <0.001 | −3.1 | (−3.6, −2.5) | <0.001 | −2.6 | (−4.8, −0.5) | 0.016 |
|
| |||||||||
| Alcohol Consumption | 0.016 | 0.071 | 0.107 | ||||||
| Never | Ref | – | – | Ref | – | – | Ref | – | – |
| Former | −1.1 | (−1.8, −0.4) | 0.001 | −0.7 | (−1.3, −0.03) | 0.041 | −0.8 | (−3.2, 1.6) | 0.500 |
| <1 drink/week | 0 | (−0.5, 0.5) | 0.951 | 0.3 | (−0.2, 0.7) | 0.236 | 1.9 | (0.2, 3.6) | 0.027 |
| (1,7) drinks/week | 0.3 | (−0.3, 0.8) | 0.322 | 0.4 | (−0.1, 0.9) | 0.147 | 0.3 | (−1.5, 2.2) | 0.735 |
| (7,14) drinks/week | −0.05 | (−0.8, 0.7) | 0.890 | −0.1 | (−0.9, 0.6) | 0.774 | −1.5 | (−4.2, 1.3) | 0.287 |
| ≥14 drinks/week | 0.1 | (−0.6, 0.8) | 0.778 | 0.2 | (−0.5, 0.8) | 0.598 | 0.5 | (−2.0, 2.9) | 0.695 |
|
| |||||||||
| Blocks walked (per 25 blocks) | 0 | (−0.08, 0.08) | 0.972 | 0.1 | (−0.02, 0.14) | 0.128 | 0.5 | (0.3, 0.8) | <0.001 |
|
| |||||||||
| Exercise Intensity | 0.027 | 0.009 | 0.719 | ||||||
| High | 0.6 | (0.01, 1.2) | 0.047 | 0.8 | (0.2, 1.4) | 0.011 | 0.3 | (−1.9, 2.4) | 0.817 |
| Moderate | 0.4 | (−0.04, 0.8) | 0.074 | 0.2 | (−0.2, 0.6) | 0.257 | −0.7 | (−2.1, 0.8) | 0.369 |
| Low | Ref | – | – | Ref | – | – | Ref | – | – |
| None | 1.1 | (−0.1, 2.3) | 0.084 | 1.2 | (0.0, 2.4) | 0.049 | −0.9 | (−5.3, 3.5) | 0.693 |
|
| |||||||||
| Total Energy expenditured (Kcal/week) |
0.2 | (0.1, 0.4) | 0.003 | 0.3 | (0.1, 0.4) | 0.001 | 0.5 | (−0.1, 1.1) | 0.096 |
|
| |||||||||
| Alternate Healthy Eating Index | <0.001 | <0.001 | 0.011 | ||||||
| 52–80.5 | Ref | ||||||||
| 43–51.5 | −1.1 | (−1.7, −0.6) | <0.001 | −1.2 | (−1.7, −0.6) | <0.001 | −1.6 | (−3.6, 0.4) | 0.112 |
| 35–42.5 | −1.1 | (−1.7, −0.6) | <0.001 | −1.1 | (−1.6, −0.5) | <0.001 | −2.9 | (−4.8, −0.9) | 0.005 |
| 27–34.5 | −1.2 | (−1.8, −0.6) | <0.001 | −1.2 | (−1.7, −0.6) | <0.001 | −2.6 | (−4.7, −0.5) | 0.014 |
| 5.5–26.5 | −1.5 | (−2.2, −0.9) | <0.001 | −1.5 | (−2.1, −0.9) | <0.001 | −3.7 | (−5.9, −1.6) | 0.001 |
|
| |||||||||
| Body Mass Index | <0.001 | 0.001 | <0.001 | ||||||
| <18.5 | −3.0 | (−4.3, −1.6) | <0.001 | −2.1 | (−3.5, −0.8) | 0.002 | 0.8 | (−4.2, 5.8) | 0.761 |
| (18.5, 24.99) | Ref | – | Ref | – | Ref | – | |||
| (25, 26.99) | 0.2 | (−0.2, 0.7) | 0.342 | −0.06 | (−0.5, 0.4) | 0.800 | −2.0 | (−3.6, −0.3) | 0.021 |
| (27, 29.99) | 0.6 | (0.2, 1.1) | 0.009 | 0.02 | (−0.4, 0.5) | 0.934 | −2.8 | (−4.5, −1.1) | 0.001 |
| ≥30 | 0.6 | (0.1, 1.2) | 0.020 | −0.7 | (−1.2, −0.2) | 0.008 | −7.3 | (−9.2, −5.4) | <0.001 |
|
| |||||||||
| Social Network Score | 0.02 | (−0, 0.05) | 0.099 | 0.02 | (−0.01, 0.04) | 0.263 | 0.02 | (−0.1, 0.1) | 0.725 |
|
| |||||||||
| Social Support Scoree | −0.01 | (−0.08, 0.07) | 0.870 | −0.04 | (−0.1, 0.03) | 0.303 | −0.2 | (−0.5, 0.04) | 0.097 |
Abbreviations: CI, Confidence interval; Kcal, Kilocalories; Ref, Reference
All lifestyle factors were included in a single regression model, and were adjusted for sex, age, race, education, income and marital status, self-reported health, difficulties in Instrumental Activities of Daily Living, weight change, cognition and chronic conditions (arthritis, cancer, hypertension, diabetes, angina, myocardial infarction, congestive heart failure, peripheral arterial disease, stroke, transient ischemic attack, chronic obstructive pulmonary disease and depression).
The sample size is smaller as the diet variable was not available for the entire sample. Results from a model with the larger sample size (n=5248) but excluding the diet variable were similar.
To minimize false positive results due to multiple testing, the p-value cut-off of <0.001 was used.
This variable was natural log transformed and the coefficients should be interpreted as the increase in YoL, YAL and YAL/YoL% associated with a 2.72 fold increase in energy expenditure measured in kilocalories/week.
A higher social support score indicates lower social support.
Figure 1 displays the predicted mean values of YAL, YoL and YAL/YoL% from statistical models that pre-specified values of a healthy and unhealthy lifestyle. At comparable values of socio-demographic factors, a healthy lifestyle differed considerably from an unhealthy lifestyle in terms of YAL, YoL, disabled years and YAL/YoL% and was associated with an absolute and relative compression of the disabled period in all race and gender groups.
Figure 1.
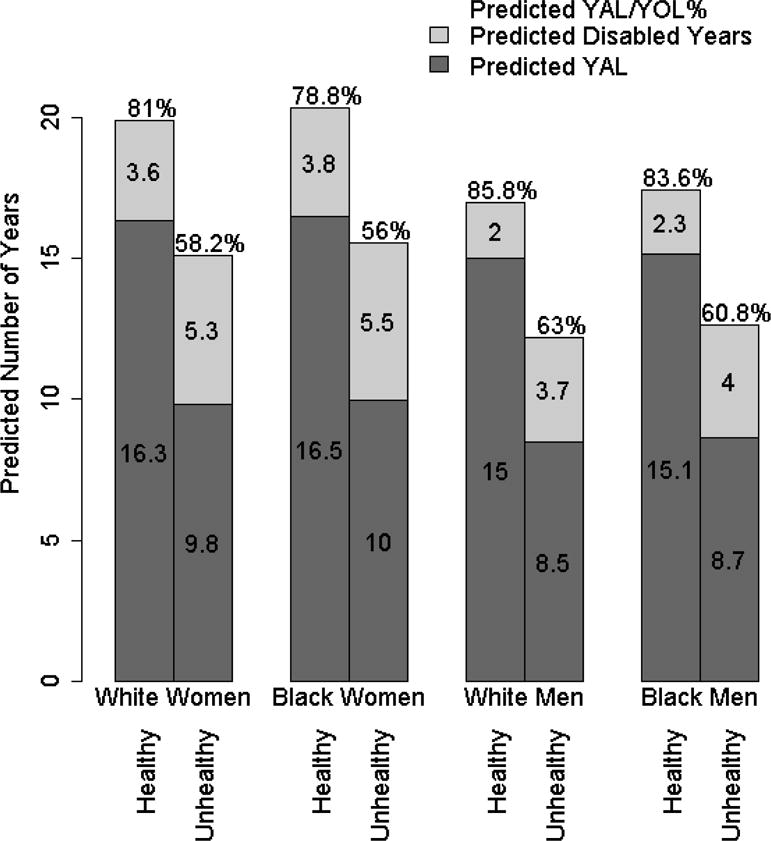
Predicted Number of YAL, Disabled Years, YoL, and YAL/YoL% in Different Race and Gender Groups With Healthy and Unhealthy Lifestyles.
Abbreviations: YAL, Years of able life; YOL, Years of life
DISCUSSION
In this community-based cohort of 5248 older adults who were recruited at an average age of 73 years and followed up for 25 years, the average number of disabled years was about 2.9 years for men and 4.5 years for women. Multiple lifestyle factors were significantly associated with the observed total and able years of life as well as the proportion of observed life lived without disability. Greater distances walked and better diet quality were associated with a relative compression of the disabled period. Obesity was associated with a relative expansion of the disabled period. Smoking was associated with a loss of both able and total years.
More than three decades ago, several contradicting theories of population aging were proposed to explain how increasing life expectancy might influence the duration of the morbid period.9,28,29 While most of the research evaluating the theories have examined population trends in active life expectancy over time30–32, there has been limited information on factors that influence the duration of this period at the individual level. Our results provide epidemiological evidence for the plausibility of the compression of morbidity theory by demonstrating the potential for lifestyle factors to compress or expand the disabled period near the end of life. As demonstrated in Figure 1, the presence of varying combinations of these factors in older individuals could determine the absolute and relative duration of disability in individuals. Populations could compress or expand morbidity depending on the prevalence of risk factors and favorable factors. These results therefore have both clinical and broader public health implications.
Quantifying the morbid period has been difficult, given the dearth of observed data; demographic research on active life expectancies has provided the best estimates until now. Twenty two years ago, Guralnik et al. estimated mean disabled life expectancies of 1.5 years (White men), 1.6 years (Black men), 2.8 years (White women), and 3.0 years (Black women) at age 75 using life table methods.33 We used observed data rather than life tables and found the disabled period to be longer, but differences in methodology between the studies make it difficult to draw conclusions about a change over 22 years. Precise measurements of the terminal morbid period would be possible if cohorts of older adults are followed till extinction. Estimates of life expectancy are more readily available and indicate that observed values from CHS are generalizable to the US population. Survival rates in CHS have been compared to rates from US census data and found to be similar.34 The YoL values in CHS have been compared to life expectancy from the US life tables and found to have good agreement.13
The impact of lifestyle on health and life span is well known; what does our research contribute? We have provided evidence for the effect of a late life lifestyle on the actual duration and proportion of the terminal disabled period. This is a critical addition to the existing evidence on the effects of lifestyle on onset of disease, disability and mortality. For example, smoking is a well-known hazard for early death,35,36 however, the effect of smoking on disabled years in old age has not been clearly elucidated. Smoking has been shown to reduce active life expectancy in older adult populations,37,38 but these were estimates based on life tables and did not take multiple confounding factors into account. Our study provides evidence that even among smokers who avoided an early death, smoking continues to have a large impact on longevity and the length of the able period. Current alcohol consumption was not related to YoL or YAL though former drinkers had the poorest outcomes, which is consistent with previous reports that older adults stop drinking when they become ill.39,40
A high level of physical activity among older adults is associated with a reduced duration of disability before death37 and is a strong predictor for dying without disability.41 Our findings are consistent with the literature and provide evidence of the association of physical activity with disabled years. A healthy diet, as defined by the AHEI, is associated with a lower risk for chronic disease19 and mortality.42 Our findings indicate that a healthy diet among older adults can potentially contribute to reducing the disabled period.
The effect of obesity on mortality declines with age43,44 and it does not seem to be as poor a prognostic factor among the old as in the young. Nevertheless, obese older adults have been shown to have greater disability rates45 and a shorter active life expectancy.46 Our finding that obesity is associated with an expansion of the disabled period has important ramifications in the context of rising obesity rates among the older adult population.47 Underweight older adults, known to be at high risk for poorer health outcomes48, had the highest risk for reduced total and able life span, among all BMI categories in our study.
The main strength of our study is the 25 year follow-up of a large, well characterized, community-based sample of older adults with a mean age of 73 at cohort entry, which provided observed data on disability late in life. The non-availability of such data has been a deterrent to drawing conclusions about this period. ‘Years of Able Life’ incorporates health and survival, and is a robust measure of successful aging. It has an edge over ‘time to death’—the outcome measure in most survival analyses—by accounting for the quality of survival, and it remains a powerful and efficient primary outcome measure.49 In other studies, Active Life Expectancy has been defined using the endpoint “loss of independence in ADL”, and therefore may incorporate a period where older adults experience difficulties in these activities but do not obtain assistance. As defined here, YAL represents a fully able period where participants experience no difficulty in ADL and is hence a more accurate measure of the duration of good physical function.
Several limitations should be kept in mind while examining these results. Disability and health behaviors were self-reported and the subjectivity of self-report must be kept in mind, although self-report remains the gold standard of disability assessment. Health behaviors were assessed at baseline and behavior patterns could have changed over time influencing the outcome. However, we were able to characterize participants at a time when lifestyle had not been extensively altered by disease and the baseline assessment is therefore more likely to represent the lifestyle over a greater part of the adult lifespan. Also, as measurement of the outcomes began at baseline, updating the lifestyle measures would have resulted in issues of reverse causality. It is also possible that earlier (e.g., mid-life) lifestyle, correlated with the baseline lifestyle measured in the study, may be driving the findings in this report. We also recognize that residual confounding may be present despite the multiple confounders that have been adjusted for.
Conclusions
Among older adults, the mean duration of the disabled period is about 2.9 years for men and 4.5 years for women. Lifestyle factors may potentially compress or expand this period, independent of their effect on life expectancy. While the increasing obesity levels in this age group can herald a ‘disability epidemic’, the promotion of healthy lifestyle factors can potentially reduce the public health burden due to disability as more adults reach old age.
Acknowledgments
Funding Source: This research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA) and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827. A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Sponsors Role: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Mini Jacob conceived and designed the study, interpreted results, wrote the first draft and revised the manuscript. Laura Yee conducted the analysis, interpreted results, wrote the statistical analysis section and reviewed the manuscript. Anne Newman, Paula Diehr and Alice Arnold conceived and designed the study, acquired data, designed and supervised the analysis, interpreted results and reviewed the manuscript. Paulo Chaves, Calvin Hirsch, and David Siscovick conceived and designed the study, acquired data, interpreted results and reviewed the manuscript. Stephen Thielke interpreted results and reviewed the manuscript. Liana Del Gobbo contributed to the analysis, interpreted results and reviewed the manuscript.
Disclaimer: The findings and conclusions in this article are those of the authors, who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the National Institutes of Health. Therefore, no statement in this article should be construed as an official position of the National Institutes of Health.
Additional Contributions: We thank Jon Kilner, MS, MA (Pittsburgh, PA, USA) for editorial support. He received financial compensation for his contribution.
References
- 1.Katz S, Branch LG, Branson MH, et al. Active life expectancy. N Engl J Med. 1983;309:1218–1224. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Zack MM, Krahn GL, et al. Health-related quality of life among older adults with and without functional limitations. Am J Public Health. 2012;102:496–502. doi: 10.2105/AJPH.2011.300500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Depalma G, Xu H, Covinsky KE, et al. Hospital readmission among older adults who return home with unmet need for ADL disability. Gerontologist. 2013;53:454–461. doi: 10.1093/geront/gns103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hennessy S, Kurichi JE, Pan Q, et al. Disability Stage is an Independent Risk Factor for Mortality in Medicare Beneficiaries Aged 65 Years and Older. PM R. 2015 doi: 10.1016/j.pmrj.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forman-Hoffman VL, Ault KL, Anderson WL, et al. Disability status, mortality, and leading causes of death in the United States community population. Med Care. 2015;53:346–354. doi: 10.1097/MLR.0000000000000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubitz J, Cai L, Kramarow E, et al. Health, life expectancy, and health care spending among the elderly. N Engl J Med. 2003;349:1048–1055. doi: 10.1056/NEJMsa020614. [DOI] [PubMed] [Google Scholar]
- 7.Joo H, Dunet DO, Fang J, et al. Cost of informal caregiving associated with stroke among the elderly in the United States. Neurology. 2014;83:1831–1837. doi: 10.1212/WNL.0000000000000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mor V. The compression of morbidity hypothesis: A review of research and prospects for the future. J Am Geriatr Soc. 2005;53(9 Suppl):S308–309. doi: 10.1111/j.1532-5415.2005.53496.x. [DOI] [PubMed] [Google Scholar]
- 9.Fries JF. Aging, natural death, and the compression of morbidity. N Engl J Med. 1980;303:130–135. doi: 10.1056/NEJM198007173030304. [DOI] [PubMed] [Google Scholar]
- 10.Fries JF. Measuring and monitoring success in compressing morbidity. Ann Intern Med. 2003;139(5 Pt 2):455–459. doi: 10.7326/0003-4819-139-5_part_2-200309021-00015. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: Design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 12.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 13.Diehr P, Diehr M, Arnold AM, et al. Predicting Future Years of Life, Health, and Functional Ability: A Healthy Life Calculator for Older Adults. UW Biostatistics Working Paper Series. 2015 doi: 10.1177/2333721415605989. http://biostats.bepress.com/uwbiostat/paper407. [DOI] [PMC free article] [PubMed]
- 14.Longstreth WT, Jr, Diehr PH, Yee LM, et al. Brain imaging findings in elderly adults and years of life, healthy life, and able life over the ensuing 16 years: The Cardiovascular Health Study. J Am Geriatr Soc. 2014;62:1838–1843. doi: 10.1111/jgs.13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehr P, Patrick DL, Spertus J, et al. Transforming self-rated health and the SF-36 scales to include death and improve interpretability. Med Care. 2001;39:670–680. doi: 10.1097/00005650-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Taylor HL, Jacobs DR, Jr, Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 17.Siscovick DS, Fried L, Mittelmark M, et al. Exercise intensity and subclinical cardiovascular disease in the elderly. The Cardiovascular Health Study. Am J Epidemiol. 1997;145:977–986. doi: 10.1093/oxfordjournals.aje.a009066. [DOI] [PubMed] [Google Scholar]
- 18.Kumanyika S, Tell GS, Fried L, et al. Picture-sort method for administering a food frequency questionnaire to older adults. J Am Diet Assoc. 1996;96:137–144. doi: 10.1016/S0002-8223(96)00042-9. [DOI] [PubMed] [Google Scholar]
- 19.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: Moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 20.Heitzmann CA, Kaplan RM. Assessment of methods for measuring social support. Health Psychol. 1988;7:75–109. doi: 10.1037//0278-6133.7.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Lubben JE. Assessing social networks among elderly populations. Fam Community Health. 1988;11:42–52. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: The Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 24.Pearte CA, Furberg CD, O’Meara ES, et al. Characteristics and baseline clinical predictors of future fatal versus nonfatal coronary heart disease events in older adults: The Cardiovascular Health Study. Circulation. 2006;113:2177–2185. doi: 10.1161/CIRCULATIONAHA.105.610352. [DOI] [PubMed] [Google Scholar]
- 25.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 26.Andresen EM, Malmgren JA, Carter WB, et al. Screening for depression in well older adults: Evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 27.Arnold AM, Kronmal RA. Multiple imputation of baseline data in the cardiovascular health study. Am J Epidemiol. 2003;157:74–84. doi: 10.1093/aje/kwf156. [DOI] [PubMed] [Google Scholar]
- 28.Gruenberg EM. The failures of success. Milbank Mem Fund Q Health Soc. 1977;55:3–24. [PubMed] [Google Scholar]
- 29.Manton KG. Changing concepts of morbidity and mortality in the elderly population. Milbank Mem Fund Q Health Soc. 1982;60:183–244. [PubMed] [Google Scholar]
- 30.Cai L, Lubitz J. Was there compression of disability for older Americans from 1992 to 2003? Demography. 2007;44:479–495. doi: 10.1353/dem.2007.0022. [DOI] [PubMed] [Google Scholar]
- 31.Crimmins EM, Beltran-Sanchez H. Mortality and morbidity trends: Is there compression of morbidity? J Gerontol B Psychol Sci Soc Sci. 2011;66:75–86. doi: 10.1093/geronb/gbq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Chen G, Song X, et al. Trends in disability-free life expectancy among Chinese older adults. J Aging Health. 2009;21:266–285. doi: 10.1177/0898264308328978. [DOI] [PubMed] [Google Scholar]
- 33.Guralnik JM, Land KC, Blazer D, et al. Educational status and active life expectancy among older blacks and whites. N Engl J Med. 1993;329:110–116. doi: 10.1056/NEJM199307083290208. [DOI] [PubMed] [Google Scholar]
- 34.Newman AB, Sachs MC, Arnold AM, et al. Total and cause-specific mortality in the cardiovascular health study. J Gerontol A Biol Sci Med Sci. 2009;64:1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peto R, Lopez AD, Boreham J, et al. Mortality from smoking worldwide. Br Med Bull. 1996;52:12–21. doi: 10.1093/oxfordjournals.bmb.a011519. [DOI] [PubMed] [Google Scholar]
- 36.Jha P, Ramasundarahettige C, Landsman V, et al. 21st-century hazards of smoking and benefits of cessation in the United States. N Engl J Med. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 37.Ferrucci L, Izmirlian G, Leveille S, et al. Smoking, physical activity, and active life expectancy. Am J Epidemiol. 1999;149:645–653. doi: 10.1093/oxfordjournals.aje.a009865. [DOI] [PubMed] [Google Scholar]
- 38.Nusselder WJ, Looman CW, Marang-van de Mheen PJ, et al. Smoking and the compression of morbidity. J Epidemiol Community Health. 2000;54:566–574. doi: 10.1136/jech.54.8.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukamal KJ, Psaty BM, Rautaharju PM, et al. Alcohol consumption and risk and prognosis of atrial fibrillation among older adults: The Cardiovascular Health Study. Am Heart J. 2007;153:260–266. doi: 10.1016/j.ahj.2006.10.039. [DOI] [PubMed] [Google Scholar]
- 40.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: Explaining the U-shaped curve. Lancet. 1988;2:1267–1273. doi: 10.1016/s0140-6736(88)92890-5. [DOI] [PubMed] [Google Scholar]
- 41.Leveille SG, Guralnik JM, Ferrucci L, et al. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999;149:654–664. doi: 10.1093/oxfordjournals.aje.a009866. [DOI] [PubMed] [Google Scholar]
- 42.Akbaraly TN, Ferrie JE, Berr C, et al. Alternative Healthy Eating Index and mortality over 18 y of follow-up: Results from the Whitehall II cohort. Am J Clin Nutr. 2011;94:247–253. doi: 10.3945/ajcn.111.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Diehr P, Bild DE, Harris TB, et al. Body mass index and mortality in nonsmoking older adults: The Cardiovascular Health Study. Am J Public Health. 1998;88:623–629. doi: 10.2105/ajph.88.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarnell JW, Patterson CC, Thomas HF, et al. Comparison of weight in middle age, weight at 18 years, and weight change between, in predicting subsequent 14 year mortality and coronary events: Caerphilly Prospective Study. J Epidemiol Community Health. 2000;54:344–348. doi: 10.1136/jech.54.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jenkins KR. Obesity’s effects on the onset of functional impairment among older adults. Gerontologist. 2004;44:206–216. doi: 10.1093/geront/44.2.206. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds SL, Saito Y, Crimmins EM. The impact of obesity on active life expectancy in older American men and women. Gerontologist. 2005;45:438–444. doi: 10.1093/geront/45.4.438. [DOI] [PubMed] [Google Scholar]
- 47.Fakhouri TH, Ogden CL, Carroll MD, et al. Prevalence of obesity among older adults in the United States, 2007–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- 48.Diehr P, O’Meara ES, Fitzpatrick A, et al. Weight, mortality, years of healthy life, and active life expectancy in older adults. J Am Geriatr Soc. 2008;56(1):76–83. doi: 10.1111/j.1532-5415.2007.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diehr P, Patrick DL, Burke GL, et al. Survival versus years of healthy life: Which is more powerful as a study outcome? Control Clin Trials. 1999;20:267–279. doi: 10.1016/s0197-2456(98)00062-2. [DOI] [PubMed] [Google Scholar]


