Abstract
Background
Both dasatinib and nilotinib are approved frontline therapy for chronic myeloid leukemia, chronic phase (CML-CP) based on randomized trials compared to imatinib. However, no head to head comparison of dasatinib and nilotinib has been conducted in newly diagnosed CML-CP patients.
Method
We conducted a propensity score (PS) matched comparison of patients with CML-CP, who received front-line therapy by either dasatinib (N = 102) or nilotinib (N = 104) under the respective phase II trials conducted in parallel.
Result
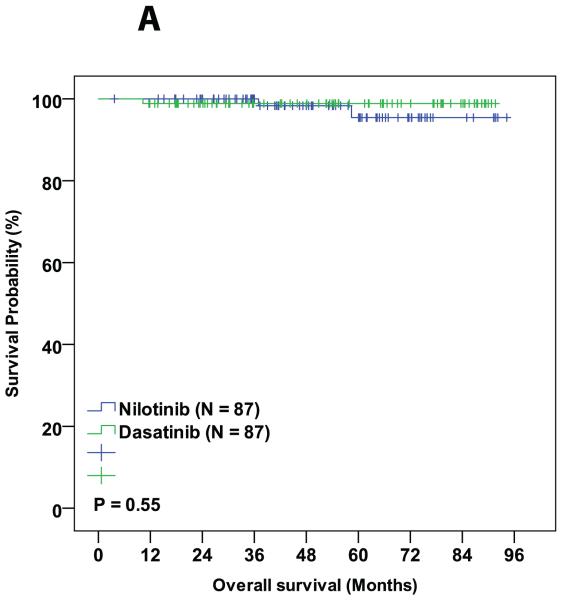
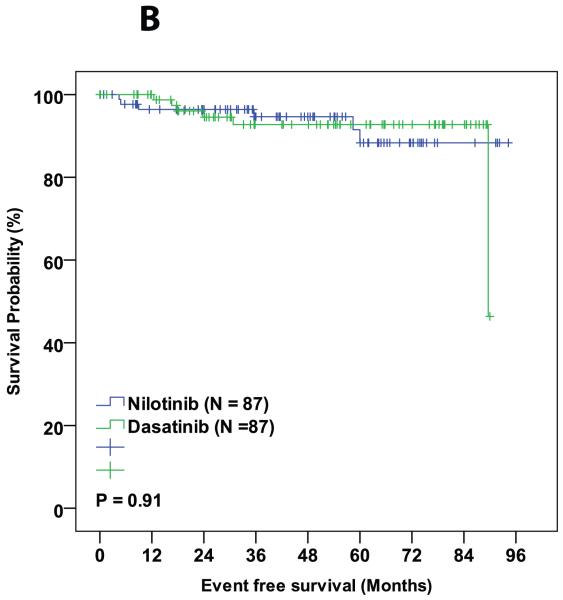
PS matching resulted in 87 patients from each trial to be matched for pre-treatment characteristics. The 3-month BCR-ABL1/ABL1 <10% rate was 93% with dasatinib and 94% with nilotinib (p=0.25), respectively. Rates of major molecular response (MMR) at 12 months were 77% and 85% (p=0.13), respectively, and MR4.5 at 36 months are 66% and 64% (p=0.96), respectively. All other clinically relevant response were similar between the two treatment cohorts. The 3-year probability of event-free survival was 89% in the dasatinib-treated patients and 87% for those treated with nilotinib (p=0.99). Corresponding 3-year overall survival probabilities were 99% and 93%, respectively (p=0.95). No statistical difference was observed between dasatinib and nilotinib treated groups in any of the other survival endpoints. Treatment discontinuation rate was also similar between the two cohorts (18% in dasatinib and 19% in nilotinib, P = 0.82).
Conclusion
In PS matched cohort of newly diagnosed CML-CP patients, dasatinib and nilotinib offer similar response and survival outcomes. Both drugs can be considered as reasonable standard of care options as a first line therapy in CML-CP patients.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative hematologic malignancy characterized by the presence of the Philadelphia chromosome, which results from a reciprocal translocation between chromosomes 9 and 22 [t(9;22)(q34;q11.2)].1 This translocation results in the generation of the BCR-ABL oncogene, a constitutively active fusion protein, which results in unregulated cell proliferation and reduced apoptosis. Introduction of BCR-ABL tyrosine kinase inhibitors (TKIs) in the treatment of CML has dramatically improved outcomes of CML patients. Currently, there are three approved TKIs as a frontline therapy for CML, namely imatinib, dasatinib and nilotinib. Compared to imatinib, the second generation TKIs, dasatinib and nilotinib, are 100-300 fold more potent and 10-100 fold more potent respectively in inhibiting BCR-ABL kinase activity.2 Previous randomized clinical trials demonstrated that dasatinib achieved faster and deeper responses in newly diagnosed chronic phase (CML-CP) patients than imatinib.3,4 Similarly, nilotinib achieved higher rates of MR4.5 and responses occurred faster. These resulted in a reduced incidence of progression to accelerated or blast phase compared to treatment with imatinib.5,6 However, no head to head comparison of dasatinib and nilotinib has been conducted in newly diagnosed CML-CP patients. In this study, we analyzed results of the two single-institution, single arm clinical trials conducted in parallel, one using dasatinib and the other nilotinib as frontline therapy for patients with CML-CP. We performed propensity score matching to adjust for pre-treatment confounding factors. This allowed us to compare the treatment efficacy and survival outcomes of dasatinib and nilotinib in clinically well-matched cohort.
Patients and Methods
Studied Patients
We studied patients with newly diagnosed CML-CP, who received frontline therapy under either one of two parallel single-arm, single-institution phase II trials (dasatinib trial: NCT00254423, N = 107 or nilotinib trial: NCT00129740, N = 104). Both study were conducted during the same period starting in 2005. Eligibility criteria of the two trials were nearly identical and are described previously.7,8 In brief, the following eligibility criteria were shared between the two trials: patients age 16 or older with CML-CP who were within 12 months of initial diagnosis and had received no or minimal prior therapy (defined as less than 30 days of prior interferon-alpha (with or without cytarabine) and/or other TKI (i.e. imatinib)); Eastern Cooperative Oncology Group (ECOG) performance status 0-2; adequate cardiac, renal and liver function. Patients with accelerated or blast phase (AP/BP) CML defined by MD Anderson Criteria were excluded from the dasatinib trial but eligible to the nilotinib trial; however all patients with accelerated phase are excluded from this analysis.9 Both treatment protocols were approved by the institutional review board at MD Anderson Cancer Center and informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Treatment
In the dasatinib trial (N = 107), dasatinib was given either 50mg orally twice a day (N = 30) or 100mg orally once a day (N =77). After 62 patients had been enrolled, the trial was amended and all subsequent patients were treated with 100 mg once daily. In the nilotinib trial (N = 104), nilotinib was administered at a dose of 400mg orally twice daily to all patients.
Follow-up evaluation
Follow-up bone marrow aspiration with conventional cytogenetics was performed in all study participants every 3 months in the first year and every 6-12 months thereafter. Cytogenetics was assessed by conventional cytogenetic analysis of bone marrow cells using the G-banding technique with analysis of at least 20 metaphases. Fluorescent in situ hybridization (FISH) on peripheral blood was used only when routine cytogenetic analysis was not successful (i.e. insufficient metaphases). Real-time polymerase chain reaction (PCR) for BCR-ABL transcripts was performed every 3 months for the first 12 months, then every 6 months.
Definition of response, survival endpoints and toxicity
For this analysis, the following responses were analyzed at 12 months and 36 months from the start of therapy: complete cytogenetic response (CCyR), major molecular response (MMR: BCR-ABL1/ABL1 ratio ≤0.1% by international scale [IS]10), MR4.0 (BCR-ABL1/ABL1 ratio ≤ 0.01% by IS), and MR4.5 (BCR-ABL1/ABL1 ratio ≤ 0.0032% by IS). Early response to therapy defined as BCR-ABL1/ABL1 <10% at 3 months was also assessed, as it has been shown to be clinically relevant.11,12 Overall survival (OS) was measured from the time treatment was started to the date of death from any cause at any time or date of last follow-up. Event free survival (EFS) was defined as the time from start of treatment to the date of any of the following events while on therapy: loss of complete hematologic response, loss of major cytogenetic response, progression to accelerated or blast phase, or death from any cause while on study. To complement the limitations of this definition, we also measured the failure-free survival (FFS) that accounts for treatment discontinuation from any reasons, in addition to the other events defined by EFS. Transformation free survival (TFS) was defined as the time from the start of therapy to the date of transformation to AP/BC while on therapy or to the date of last follow-up.
Statistical analysis
The chi-square or Fisher exact test was used to assess differences in categorical variables, and the Mann-Whitney U test was used to analyze continuous variables. The log-rank test was used to examine between-group differences in various survival outcomes. The propensity score (PS) for each patient was calculated by conducting multilogistic regression analysis against treatment category (dasatinib versus nilotinib) by entering the following variables: age, white blood cell (WBC) counts, serum lactate dehydrogenase (LDH) level, Sokal score13, serum creatinine, serum total bilirubin, and previous use of imatinib (<30 days).14 PS matching of the patient cohorts was then conducted using a caliper of 0.25 standard deviation15,16. More stringent caliper was also attempted but 0.25 gave the best matching model. Statistical analyses were performed using the R statistical programming language (version 3.1.3) and the SPSS software program (version 22; IBM Corporation, Armonk NY).
Results
Patient characteristics before matching
Clinical characteristics of the studied patients before matching are summarized in the Supplemental Table 1. Some statistically significant differences between the two cohorts were identified. Patients enrolled in the nilotinib trial had significantly higher serum lactate dehydrogenase (LDH) levels (P = 0.02), higher serum creatinine (P = 0.04), and trend toward higher white blood cell (WBC) count (P = 0.09). The number of patients who took imatinib for less than 30 days prior to the studies was significantly higher in dasatinib trial (P = 0.04).
Response to treatment and survival comparison before matching
The median follow up period was 54 months (95% CI: 48-60 months) and 49 months (95% CI: 41-58 months) for the dasatinib and nilotinib treated groups, respectively (P = 0.69). Clinically relevant treatment responses at each milestones were compared between the 2 trials before matching (Supplemental Table 2). There was a trend for a higher rate of BCR-ABL1 transcript < 10% at 3 months among patients treated with nilotinib (P = 0.08). No significant differences were observed in other relevant response criteria.
There were also no significant differences in the various 3-year survival endpoints between the two trials (Supplemental Table 3).
Patient characteristics in the matched cohort
Because of the clinical heterogeneity between the patients treated on each of the 2 trials, we performed propensity matching to control pre-treatment characteristics. PS matching resulted in matching of 87 patients in each trial. As shown in Table 1, pre-treatment characteristics were well matched between the two groups after PS matching.
Table 1.
Pretreatment characteristics of CML-CP patients treated with dasatinib and nilotinib after PS matching.
| Dasatinib (N = 87) | Nilotinib (N = 87) | P value | |
|---|---|---|---|
| Median age | 49 (19-79) | 47 (17-80) | 0.87 |
| Age ≥ 65 | 6 (7) | 6 (7) | 1.00 |
| Female | 37 (43) | 38 (44) | 0.85 |
| Sokal Score Group | |||
| Low | 69 (79) | 66 (76) | 0.78 |
| Intermediate | 14 (16) | 15 (17) | |
| High | 4 (5) | 6 (7) | |
| WBC, ×103/μL | 23.9 (0.8-193.0) | 39.8 (1.4-342.5) | 0.51 |
| HGB, g/dL | 11.9 (8.8-16.2) | 12.4 (8.9-15.8) | 0.65 |
| PLT, ×103/μL | 337 (86-1906) | 322 (73-1356) | 0.92 |
| BM blast % | 2 (0-6) | 2 (0-7) | 0.53 |
| BM blast ≥ 5 % | 3 (4) | 3 (4) | 1.00 |
| LDH, IU/L | 894 (393-3648) | 1097 (252-3467) | 0.37 |
| CRE, mg/dL | 0.9 (0.6-1.3) | 0.9 (0.6-1.3) | 0.27 |
| TBIL, mg/dL | 0.4 (0.2-3.4) | 0.4 (0.1-1.3) | 0.54 |
| ALB, mg/dL | 4.4 (3.7-5.5) | 4.4 (3.3-5.5) | 0.79 |
| AST, IU/L | 32 (14-121) | 36 (12-101) | 0.65 |
| ALT, IU/L | 25 (12-154) | 27 (11-84) | 0.94 |
| BCR-ABL1, IS | 14.1 (0.04-35.4) | 14.1 (0.01-35.4) | 0.68 |
| BCR-ABL1 type | |||
| b2a2 | 32 (37) | 34 (39) | 0.74 |
| b3a2 | 32 (37) | 37 (43) | |
| b3a3 | 1 (1) | 0 (0) | |
| b2a2 + b3a2 | 21 (24) | 15 (17) | |
| e1a2 | 1 (1) | 1 (1) | |
|
Previous use of
imatinib (<30 days) |
17 (19) | 14 (16) | 0.55 |
NOTE: The numbers are shown in either No. (%) or median (range) style; WBC, white blood cell count; HGB, hemoglobin; PLT, platelet count; BM, bone marrow; LDH, lactate dehydrogenase; CRE, creatinine; TBIL, total bilirubin; ALB, albumin; AST, aspartate transaminase; ALT, alanine transaminase; IS; international scale.
Response to the treatments in matched cohort
In the matched cohorts, median follow up of the dasatinib- and nilotinib-treated cohorts was 50.9 months (95% CI: 40.1-61.7) and 43.0 months (95% CI: 35.3-50.7), respectively (P = 0.56). Clinically relevant treatment responses at 3, 12, and 36 months milestone were compared between the two cohorts (Table 2). The 3-month BCR-ABL1/ABL1 <10% rate was 93% with dasatinib and 94% with nilotinib (p=0.25), respectively. Rates of MMR at 12 months were 77% and 85% (P = 0.13), respectively, and MR4.5 at 36 months were 66% and 64% (P = 0.96), respectively. All other response criteria analyzed were similarly equivalent between the two cohorts.
Table 2.
Treatment response among the matched patients with CML-CP treated with dasatinib and nilotinib.
| No. (%) | P value | ||
|---|---|---|---|
| Dasatinib (N = 87) |
Nilotinib (N = 87) |
||
| Response at 3 months | |||
| BCR/ABL1 <10% | 81 (93) | 82 (94) | 0.25 |
| Cumulative response at 12 months | |||
| MR4.0 | 42 (48) | 44 (51) | 0.88 |
| MR4.5 | 31 (36) | 37 (43) | 0.34 |
| MMR | 67 (77) | 74 (85) | 0.13 |
| CCyR | 82 (94) | 82 (94) | 0.51 |
| Cumulative response at 36 months | |||
| MR4.0 | 60 (69) | 62 (71) | 0.73 |
| MR4.5 | 57 (66) | 56 (64) | 0.96 |
| MMR | 77 (89) | 80 (92) | 0.17 |
| CCyR | 82 (94) | 82 (94) | 0.51 |
NOTE: CCyR: Complete cytogenetic response, MMR: Major molecular response (BCR-ABL1/ABL1 ratio ≤ 0.1% by international scale [IS]), MR4.0: Molecular response with 4 log reduction (BCR-ABL1/ABL1 ratio ≤ 0.01% by IS), MR4.5: Molecular response with 4.5 log reduction (BCR-ABL1/ABL1 ratio ≤ 0.0032% by IS)
Long-term outcomes in the matched cohort
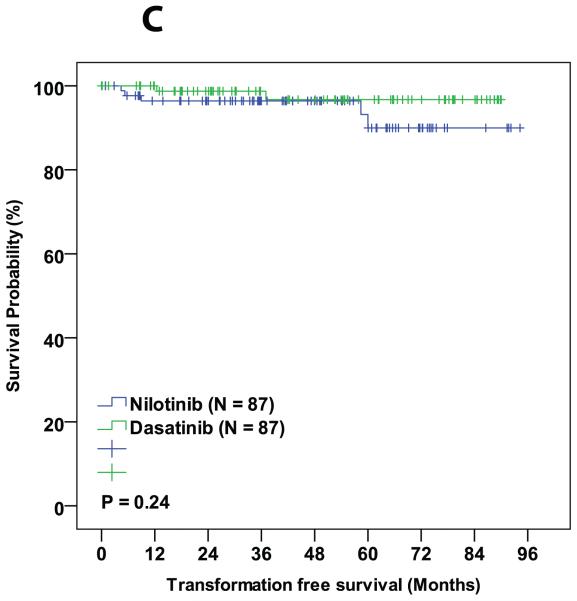
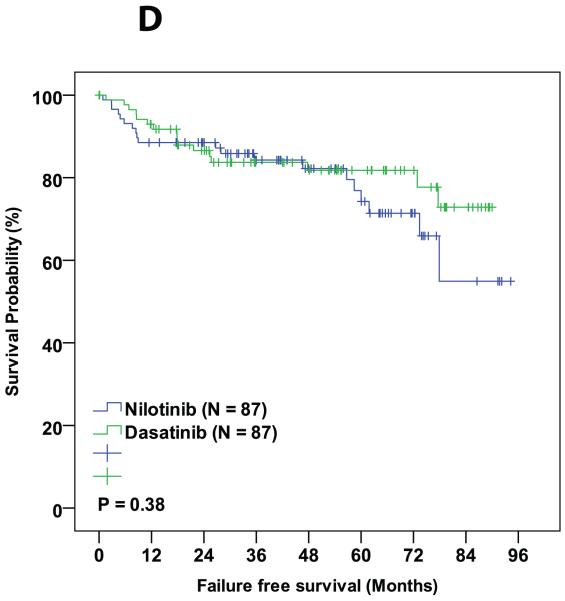
During the follow-up period, 3 patients died in the matched cohorts, 1 from the dasatinib cohort (died from a second malignancy while in MMR) and 2 from the nilotinib cohort (one died from pneumonia while in CMR and the other from unknown cause while in CCyR). Six patients progressed to AP/BC of which 2 were from dasatinib group and 4 were from nilotinib group. Sixteen (18%) patients in the dasatinib cohort and 17 (19%) in the nilotinib cohort discontinued therapy (P = 0.82). Reason for treatment discontinuation were toxicity (8 in dasatanib and 8 in nilotinib cohorts, respectively; P = 0.99), resistance to therapy (5 in dasatinib and 8 in nilotinib cohorts, respectively; P = 0.39), and financial (4 in dasatnib and 1 in nilotinib cohorts, respectively; P = 0.37,Table 4). The 3-year probability of event-free survival was 89% in the dasatinib-treated patients and 87% for those treated with nilotinib (P = 0.99). Corresponding 3-year overall survival probabilities were 99% and 93%, respectively (P = 0.95). No statistical difference was observed between dasatinib and nilotinib treated groups in any of the other survival endpoints (Table 3 and Figure 1A-D).
Table 4.
Treatment discontinuation
| Dasatinib (N = 87) | Nilotinib (N = 87) | |
|---|---|---|
| Treatment discontinuation, N(%) | 18 (21) | 20 (23) |
| Reason for discontinuation, N (% among discontinued patients) | ||
| Toxicity | 4 (22) | 6 (30) |
| Toxicity and resistance | 2 (11) | 0 (0) |
| Resistance/Progression | 6 (33) | 5 (25) |
| Death | 0 (0) | 1 (5) |
| Financial | 1 (6) | 4 (20) |
| Patient choice | 3 (17) | 1 (5) |
| Non-adherence | 2 (11) | 3 (15) |
Table 3.
Survival endpoints in matched cohort.
| Dasatinib | Nilotinib | P value | |
|---|---|---|---|
| Survival at 3 years (%) | |||
| OS | 99 | 93 | 0.95 |
| EFS | 89 | 87 | 0.99 |
| TFS | 95 | 89 | 0.28 |
| FFS | 74 | 63 | 0.71 |
NOTE: OS, overall survival; EFS, event free survival; FFS, failure free survival; TFS, transformation free survival.
Figure 1.
Comparison of various survival endpoints between patients who were treated with dasatinib and nilotinib in matched cohort. (A) Overall survival. (B) Event free survival. (C) Transformation free survival. (D) Failure free survival.
Adverse events in the matched cohort
Adverse events reported during the follow-up period were compared between dasatinib and nilotinib (Table 5). Significant differences in adverse event profile were observed between the two treatments. Overall, dasatinib was associated with higher incidence in cytopenia, respiratory symptoms, gastrointestinal related symptoms, neurological symptoms, and pleural effusion, whereas, nilotinib was associated with higher incidence in elevated liver enzymes and bilirubin levels. Two cardiovascular events were reported in patients treated with nilotinib, of which one patient experienced acute myocardial infarction and another patient with peripheral vascular disease.
Table 5.
Adverse events profiles in matched cohort.
| Any grade | Grade ≥ 3 | |||||
|---|---|---|---|---|---|---|
| Dasatinib (N = 87) | Nilotinib (N = 87) | P value | Dasatinib (N = 87) | Nilotinib (N = 87) | P value | |
| Hematological abnormality | ||||||
| Neutropenia | 7(8) | 8(9) | 0.5 | 4(5) | 3(3) | 0.5 |
| Anemia | 32(37) | 7(8) | <0.001 | 1(1) | 0(0) | 0.5 |
| Thrombocytopenia | 17(20) | 5(6) | 0.006 | 7(8) | 4(5) | 0.27 |
| Biochemical abnormality | ||||||
| Elevated Bilirubin | 3(3) | 59(68) | <0.001 | 0(0) | 7(8) | 0.007 |
| Elevated ALT | 10(12) | 47(54) | <0.001 | 0(0) | 1(1) | 0.5 |
| Elevated AST | 15(17) | 38(44) | <0.001 | 0(0) | 1(1) | 0.5 |
| Elevated ALP | 1(1) | 3(3) | 0.31 | 0(0) | 0(0) | NA |
| Hyperglycemia | 1(1) | 8(9) | 0.017 | 0(0) | 1(1) | 0.5 |
| Non-hematological AE | ||||||
| Fatigue | 63(72) | 38(44) | <0.001 | 11(13) | 1(1) | 0.002 |
| Weight loss | 2(2) | 1(1) | 0.5 | 0(0) | 0(0) | NA |
| Anorexia | 3(3) | 3(3) | 0.66 | 0(0) | 0(0) | NA |
| Flu like | 4(5) | 1(1) | 0.18 | 0(0) | 0(0) | NA |
| Headache | 42(48) | 18(21) | <0.001 | 4(5) | 0(0) | 0.06 |
| Mucositis | 6(7) | 2(2) | 0.14 | 0(0) | 0(0) | NA |
| Palpitations | 8(9) | 4(5) | 0.19 | 0(0) | 0(0) | NA |
| Prolonged QTc | 1(1) | 2(2) | 0.5 | 1(1) | 0(0) | 0.5 |
| Cough | 17(20) | 1(1) | <0.001 | 0(0) | 0(0) | NA |
| Dyspnea | 49(56) | 5(6) | <0.001 | 8(9) | 0(0) | 0.003 |
| Nausea | 42(48) | 20(23) | <0.001 | 1(1) | 1(1) | 0.751 |
| Vomiting | 24(28) | 6(7) | <0.001 | 0(0) | 0(0) | NA |
| Diarrhea | 51(59) | 8(9) | <0.001 | 4(5) | 0(0) | 0.06 |
| Constipation | 28(32) | 8(9) | <0.001 | 0(0) | 0(0) | NA |
| Pruritus | 8(9) | 22(25) | 0.005 | 0(0) | 0(0) | NA |
| Acne | 12(14) | 2(2) | 0.005 | 0(0) | 0(0) | NA |
| Erythema Multiforme | 4(5) | 1(1) | 0.18 | 0(0) | 0(0) | NA |
| Dizziness | 38(44) | 4(5) | <0.001 | 2(2) | 0(0) | 0.25 |
| Sensory neuropathy | 37(43) | 2(2) | <0.001 | 3(3) | 0(0) | 0.12 |
| Memory impairment | 39(45) | 2(2) | <0.001 | 5(6) | 0(0) | 0.029 |
| Edema | 37(43) | 1(1) | <0.001 | 0(0) | 0(0) | NA |
| Myalgia | 39(45) | 8(9) | <0.001 | 7(8) | 0(0) | 0.007 |
| Pleural effusion | 18(21) | 0(0) | <0.001 | 0(0) | 0(0) | NA |
| Cardiovascular event | 0(0) | 2(2) | 0.25 | 0(0) | 1(1) | 0.5 |
Discussion
In this study, we compared the efficacy and long-term outcomes of dasatinib and nilotinib as frontline therapy for patients with newly diagnosed CML-CP by comparing two phase 2 trials conducted in parallel. Raw comparison of the two trials showed that both treatment offers grossly equivalent response rate and survival outcomes, although there was a non-statistical trend toward faster response to achieve BCR/ABL < 10% at 3 months by nilotinib (P = 0.08). However, after propensity matching, this difference was not observed and there was no significant difference between dasatinib- and nilotinib-treated group in response rate and survival outcomes.
Dasatinib and nilotinib, both considered as second generation TKIs, are currently approved for both salvage and frontline therapy of patients with CML. Although both have been compared to imatinib in randomized trials, no direct comparison has been performed of these two agents in the salvage or frontline settings. In the DASISION phase 3 randomized trial, dasatinib 100 mg/day was compared to imatinib 400 mg/day as a frontline therapy for CML-CP patients.3 At 3-years, dasatinib induced cumulative rate of MMR, MR4, and MR4.5 in 69%, 35%, and 22%, respectively, while the same endpoints were achieved with imatinib in 55%, 22%, and 12%, respectively (P < 0.001, P = 0.0064, and P = 0.0007, respectively).11 Similarly, the ENESTnd trial compared nilotinib 600 mg/day and 800 mg/day to imatinib 400 mg/day as a frontline therapy for CML-CP patients. MMR, MR4.0, and MR4.5 at 3 years were 73%, 50%, and 32%, respectively, with nilotinib 600 mg/day, 70%, 44%, and 28%, respectively, with nilotinib 800 mg/day, all superior to the endpoints achieved by imatinib 400 mg/day (53%, 26%, and 15%, respectively).17 Both dasatinib and nilotinib induced earlier responses and were associated with fewer transformations to accelerated phase and blast phase compared to standard dose imatinib. Based on these results, both nilotinib and dasatinib were approved for frontline treatment in CML-CP patients by various regulatory agencies including US Food and Drug Administration (FDA). Today, having three possible options, treating physicians choose first-line TKIs based on several factors including but not limited to, efficacy, physician’s experience, toxicity profiles, patient comorbidities, and financial aspects.18
To date, no randomized trial has been conducted to compare dasatinib and nilotinib. Signorovitch et. al. previously conducted matching-adjusted indirect comparison of dasatinib and nilotinib using the published data from DASISION and ENESTnd trials.19-21 In their study, they reported that nilotinib was associated with significantly higher rates of MMR, MR4.0, and MR4.5 at 12 months and superior overall survival compared to dasatinib. However, this analysis included several limitations: 1) individual patient data were not available from the DASISION trial and the study used published data for comparison, 2) the study did not adjust for risk scores in the matching process, because ENESTnd study and DASISION study used different prognostic score, and 3) different ethnic groups of patients were treated in the 2 trials because ENESTnd accrued patients mostly from United States and United Kingdom, whereas DASISION study enrolled patients mainly from Asia, Australia, Europe, Russia and South America.
While there is no substitute for a head-to-head randomized trial, our study provides the “next best” comparative analysis of the available evidence for dasatinib and nilotinib as the initial treatment for patients with newly diagnosed CML-CP. The two trials compared in this study were conducted at the same institution in parallel. Patients were treated by the same group of investigators, following identical guidelines, and using the same laboratory for cytogentic and molecular analysis. All individual patient data were available from the two trials and we adjusted for multiple clinical parameters including Sokal risk scores. Further, we compared data of reported adverse events. Based on our results, both dasatinib and nilotinib provided excellent results in CML-CP patients and there was no evidence of a difference in clinically relevant treatment responses or long-term survival endpoints. However, there was significant difference in adverse event profiles between desatinib and nilotinib, while treatment discontinuation rate was similar between the two drugs. Collectively, our study suggests that both dasatinib and nilotinib are reasonable standard of care options for newly diagnosed CML-CP patients and choice of drug can be made based on other factors such as toxicity profiles, co-morbidities, physicians’ experience and treatment schedule.
There are some limitations in the current analysis. First, nilotinib was given as 400 mg twice daily and not 300 mg twice daily which is the currently approved dose for newly diagnosed CML-CP patients. Therefore, the response and outcomes may not accurately reflect the results expected from 300 mg twice daily. However, based on the analysis from ENESTnd trial, nilotinib 400 mg twice daily and 300 mg twice daily showed nearly equivalent response and survival outcomes.6 Therefore, we believe that we can still extrapolate our data to the real-world clinical decision-making. Second, PS matching cannot match factors that are not measurable or latent variables. Still, in the absence of a randomized trial, we believe our method provides the best available evidence for the comparison (suggesting based on our results, the likely equivalence) of dasatinib and nilotinib. Third, the current cohort is somewhat biased toward low risk patients. In the matched cohort, more than 70% of the patients were Sokal low risk. This is in contrast to DASSISION and ENESTnd trials by which 33% and 37%, respectively, was low risk patients. These difference likely account for the better outcome observed in the current analysis.
In conclusion, our results suggest that dasatinib and nilotinib offer similar response and survival outcomes in newly diagnosed CML-CP patients. Both drugs can be considered as reasonable standard of care options as a first line therapy in CML-CP patients. A prospective randomized trial to confirm these results is warranted.
Supplementary Material
Condensed abstract.
This propensity score matching analysis of parallel phase II trials found that both dasatinib and nilotinib offers equivalent response rate and long-term outcome in CML-CP patients when used as a frontline therapy.
This study suggests that both treatment options are reasonable choice as a frontline therapy and choice of drug can be made based on factors such as toxicity profiles, co-morbidities, physicians’ experience and treatment schedule.
Acknowledgements
The clinical trials analyzed in this report were supported in part by Bristol-Myers Squibb (BMS) (dasatinib) and Novartis (nilotinib), and by the MD Anderson Cancer Center Support Grant CA016672 (PI: Ronald DePinho) and Award Number P01 CA049639 (PI: Richard Champlin) from the National Cancer Institute.
Footnotes
Conflict of interest disclosures
JC receives research support from Ariad, BMS, Novartis, Pfizer and Teva, and is a consultant for Ariad, BMS, Novartis and Pfizer. NP receives research support and is a consultant for Novartis. EJ receives research support from Ariad, Pfizer, Teva and Novartis, and is a consultant for Ariad, BMS and Pfizer. FR receives research support from BMS. TK receives research support from BMS and is a consultant for Novartis.
Author contributions
KT conducted this analysis and wrote the manuscript. HK provided leadership, designed the clinical trails and treated patients. KS collected data. YY analyzed the results and wrote the manuscript. PJ and SJ collected data. FR, TK, NP, ND, GB, GGM, and EJ treated patients. JC designed, conducted the clinical trials, treated patients and analyzed the results used for this analysis; he also designed this study and reviewed and edited the manuscript.
All authors reviewed and approved the manuscript.
References
- 1.Nowell PC, Hungerford DA. Minute Chromosome in Human Chronic Granulocytic Leukemia. Science. 1960;132(3438):1497–1497. [Google Scholar]
- 2.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65(11):4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. The New England journal of medicine. 2010;362(24):2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119(5):1123–1129. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. The New England journal of medicine. 2010;362(24):2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9):841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 7.Cortes JE, Jones D, O'Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28(3):398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortes JE, Jones D, O'Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Dixon D, Keating MJ, et al. Characteristics of accelerated disease in chronic myelogenous leukemia. Cancer. 1988;61(7):1441–1446. doi: 10.1002/1097-0142(19880401)61:7<1441::aid-cncr2820610727>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Branford S, Fletcher L, Cross NC, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008;112(8):3330–3338. doi: 10.1182/blood-2008-04-150680. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour E, Kantarjian HM, Saglio G, et al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2014;123(4):494–500. doi: 10.1182/blood-2013-06-511592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain P, Kantarjian H, Nazha A, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: results with four tyrosine kinase inhibitor modalities. Blood. 2013;121(24):4867–4874. doi: 10.1182/blood-2013-03-490128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984;63(4):789–799. [PubMed] [Google Scholar]
- 14.D'Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in medicine. 1998;17(19):2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 15.Rosenbaum PR, Rubin DB. Constructing a Control-Group Using Multivariate Matched Sampling Methods That Incorporate the Propensity Score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 16.Lunt M. Selecting an appropriate caliper can be essential for achieving good balance with propensity score matching. American journal of epidemiology. 2014;179(2):226–235. doi: 10.1093/aje/kwt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. doi: 10.1038/leu.2012.134. [DOI] [PubMed] [Google Scholar]
- 18.Hughes T, White D. Which TKI? An embarrassment of riches for chronic myeloid leukemia patients. Hematology Am Soc Hematol Educ Program. 2013;2013:168–175. doi: 10.1182/asheducation-2013.1.168. [DOI] [PubMed] [Google Scholar]
- 19.Signorovitch J, Ayyagari R, Reichmann WM, Wu EQ, Chen L. Major molecular response during the first year of dasatinib, imatinib or nilotinib treatment for newly diagnosed chronic myeloid leukemia: a network meta-analysis. Cancer Treat Rev. 2014;40(2):285–292. doi: 10.1016/j.ctrv.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Signorovitch JE, Betts KA, Reichmann WM, et al. One-year and long-term molecular response to nilotinib and dasatinib for newly diagnosed chronic myeloid leukemia: a matching-adjusted indirect comparison. Current medical research and opinion. 2015;31(2):315–322. doi: 10.1185/03007995.2014.977992. [DOI] [PubMed] [Google Scholar]
- 21.Signorovitch JE, Wu EQ, Betts KA, et al. Comparative efficacy of nilotinib and dasatinib in newly diagnosed chronic myeloid leukemia: a matching-adjusted indirect comparison of randomized trials. Current medical research and opinion. 2011;27(6):1263–1271. doi: 10.1185/03007995.2011.576238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.