Abstract
Background
Alcohol use disorders are associated with single nucleotide polymorphisms in GABRA2, the gene encoding the GABAA receptor α2-subunit in humans. Deficient GABAergic functioning is linked to impulse control disorders, intermittent explosive disorder, and to drug abuse and dependence, yet it remains unclear if α2-containing GABAA receptor sensitivity to endogenous ligands is involved in excessive alcohol drinking.
Methods
Male wild-type C57BL/6J and point-mutated mice rendered insensitive to GABAergic modulation by benzodiazepines (H101R), allopregnanolone or THDOC (Q241M), or high concentrations of ethanol (S270H/L277A) at α2-containing GABAA receptors were assessed for their binge-like, moderate or escalated chronic drinking using drinking in the dark, continuous access and intermittent access to alcohol protocols, respectively. Social approach by mutant and wild-type mice in forced alcohol abstinence was compared to approach by EtOH-naïve controls. Social deficits in forced abstinence were treated with allopregnanolone (0, 3.0, 10.0 mg/kg, i.p.) or midazolam (0, 0.56, 1.0 mg/kg, i.p.).
Results
Mice with benzodiazepine-insensitive α2-containing GABAA receptors (H101R) escalated their binge-like drinking. Mutants harboring the Q241M point-substitution in Gabra2 showed blunted chronic intake in the continuous and intermittent access protocols. S270H/L277A mutants consumed excessive amounts of alcohol but, unlike wild-types, they did not show forced abstinence-induced social deficits.
Conclusions
These findings suggest a role for: 1.) H101 for species-typical binge-like drinking, 2.) Q241 for escalated chronic drinking, and 3.) S270 and/or L277 for the development of forced abstinence-associated social deficits. Clinical findings report reduced BZD-binding sites in the cortex of dependent patients; the present findings suggest a specific role for BZD-sensitive α2-containing receptors. In addition, amino acid residue 241 in Gabra2 is necessary for positive modulation and activation of GABAA receptors by allopregnanolone and THDOC; we postulate that neurosteroid action on α2-containing receptor may be necessary for escalated chronic ethanol intake.
Keywords: Gabra2, alcohol use disorder, binge-like drinking, alcohol forced abstinence, chronic alcohol drinking
More than half of American adults consume alcohol at least once a year; yet, only 7% of the population will receive a diagnosis of an alcohol use disorder (AUD). Deficits in inhibitory transmission, particularly in the prefrontal cortex, may increase the risk of developing an AUD and have been linked to impulse control disorders, intermittent explosive disorder, and to drug abuse and dependence (Volkow et al., 1993; Best et al., 2002; Coccaro et al., 2007; Davidson et al., 2000; Heinz et al., 2011). Within the central nervous system, fast synaptic inhibition is mediated, in part, by GABAA receptors comprised of 2α, 2β, and 1γ subunits surrounding a ligand-gated chloride ion channel (Olsen and Sieghart 2008). Heterogeneity in GABAA receptor composition can determine sensitivity to endogenous and exogenous receptor modulators including benzodiazepines, neurosteroids, ethanol and general anesthetics (Belelli and Lambert 2005; Farrant and Nusser 2005; Olsen and Sieghart 2008).
Numerous human genetics studies identify a link between alcohol dependence and single-nucleotide polymorphisms (SNPs) in GABRA2, the gene encoding the GABAA receptor α2-subunit protein (Covault et al., 2004; Edenberg et al., 2004; Bierut et al., 2010; Li et al., 2014). Minor allelic variants of these SNPs appear to be inherited together within haplotype blocks in the GABRA2 gene (Covault et al., 2004; Fehr et al., 2006; Enoch et al., 2009). Guided by research on the association between allelic variants in the human genome and alcohol dependence, the present work employs preclinical genetic mouse models to clarify whether alterations in α2-containing GABAA receptor sensitivity to alcohol, select neurosteroids, or to benzodiazepines may play a functional role in escalating binge-like or chronic, escalated alcohol consumption.
A major limitation in the field of alcohol research is the enigmatic site of action for clinically relevant doses of alcohol. Using in vitro techniques, studies have identified the α4βδ or α6βδ GABAA subtypes for potentiation of inhibitory currents by low, physiologically relevant concentrations of alcohol: yet, to date, there is no universal agreement on these findings (Suzdak et al., 1986; Mehta and Ticku 1988; Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Borghese et al., 2006a; but White et al., 1990; Mihic et al., 1994; Homanics et al., 1997; Borghese and Harris 2007). A second approach is to use rodent models of voluntary alcohol consumption to evaluate the behavior of mice harboring targeted mutations. Although behavior is far-removed from the possible receptor site of action, in the absence of selective pharmacological tools, behavioral studies can reveal which receptor domains may be necessary for non-selective drugs like alcohol to elicit specific behavioral effects.
By introducing targeted point-substitutions in the α2-subunit protein sequence, three mutant mouse strains have been generated with α2-containing GABAA receptors that are insensitive to modulation by benzodiazepines (in vitro: Wieland et al., 1992; Benson et al., 1998; in vivo: Low et al., 2000), modulation and activation by allopregnanolone and tetrahydrodeoxycorticosterone (in vitro: Hosie et al., 2006, 2009), or modulation by high concentrations of ethanol (in vitro: Mihic et al., 1997; Borghese et al., 2006b; in vivo: Homanics et al., 2005; Blednov et al., 2011; Werner et al., 2011). Assessing these mutant mice for binge-like, moderate and escalated chronic alcohol consumption may clarify the relationship between α2-containing GABAA receptor sensitivity to positive modulators and escalated alcohol consumption.
Methods
Animals
Mutant H101R mice were homozygous for a histidine to arginine point-substitution in Gabra2, conferring insensitivity to benzodiazepines at α2-containing GABAA receptors. H101R mutants were initially backcrossed for fifteen generations to a wild-type C57BL/6J (wt; Jackson Laboratories, Bar Harbor, ME) background to establish a line that is congenic with wt mice. Therefore, experimental H101R mutants and wt mice were generated from filial homozygous breeding pairs.
Experimental mutant S270H/L277A mice were homozygous for a serine to histidine mutation and a gain-of-function leucine to alanine point-substitution in Gabra2, rendering them insensitive to some effects of ethanol while maintaining near-normal GABA-responding (Werner et al., 2011). Mutant S270H/L277A mice were bred to a C57BL/6J background for at least six generations at Jackson Laboratories (stock number: 012942), and for two generations in the Tufts Psychopharmacology lab (Medford, MA). Experimental neurosteroid-insensitive Q241M mutants, bred to a C57BL/6J background, were homozygous for a glutamine to methionine point-substitution in Gabra2. Homozygous S270H/L277A and Q241M point-mutants and their wt counterparts were bred from heterozygous pairs. Tail samples were collected for genotyping by PCR (Transnetyx, Inc.). Data analyses revealed no differences between ethanol consumption by wt mice generated from heterozygous crosses and wt mice bred from homozygous pairs; therefore all wt groups were collapsed for subsequent analyses and for data portrayal. See Table 1 for all experimental group ns.
Table 1.
| Experimental group ns | ||||
|---|---|---|---|---|
|
| ||||
| Wt | H101R | Q241M | S270H/L277A | |
| Drinking in the dark (DID) | n =10 | 9 | 9 | 10 |
|
| ||||
| DID with lickometer | n =5 | 5 | 9 | 8 |
|
| ||||
| 2-bottle choice DID | n =9 | 8 | - | - |
|
| ||||
| Intermittent access to alcohol (IAA) | n = 11 | 10 | 10 | 10 |
|
| ||||
| Social approach | ||||
| IAA forced abstinence | n = 10 | 9 | 10 | 10 |
| EtOH-naïve | n = 9 | 11 | 9 | 11 |
|
| ||||
| Continuous access to alcohol (CAA) | n = 9 | 8 | 9 | 9 |
|
| ||||
| Ascending concentrations of EtOH (3–20%) | n = 8 | 9 | 9 | 10 |
|
| ||||
| Ascending concentrations of sucrose or quinine | n =8 | 4 | 5 | 7–8& |
One mouse euthanized following sucrose testing
At eight-to-ten weeks, experimental mutant and wt males were housed singly to assess individual alcohol or tastant solution intake. Adult wild-type female C57BL/6J mice (n=20) were ovariectomized (OVX) and used as social stimulus mice for social approach testing during forced abstinence from alcohol. All mice were housed in clear polycarbonate cages (28×17×14 cm) lined with pine shavings within a temperature-controlled mouse vivarium (21±1 °C, 30–40% humidity) that was kept on a 12-h photocycle (lights off 0700h). Experimental males and OVX mice received unrestricted access to rodent chow (Purina LabDiet 5001, PMI Nutrition International, Brentwood, MO). With the exception of males assigned to the drinking in the dark protocol, mice received continuous access to tap water. During assessments of fluid intake, solutions were presented in 50 mL centrifuge tubes (Nalgene). Each centrifuge tube was fitted with a rubber stopper (No. 5, Fisher Scientific, Agawam, MA) and a sipper-tube containing two ball bearings (Ancare Corp., Bellmore, NY) to prevent unintentional fluid loss. All animals were cared for in accordance with the National Research Council’s Guide for the Care and Use of Laboratory Animals (8th ed., 2011) and protocols were approved by the Institutional Animal Care and Use Committee of Tufts University.
Binge-like drinking: drinking in the dark
Adult mutant H101R, S270H/L277A, Q241M and wt males were assessed for binge-like ethanol intake in their home cages according to the four-day, drinking in the dark (DID) procedure outlined by Rhodes et al., (2005). Three hours into the dark photoperiod, water bottles were replaced with a single 50 mL centrifuge tube containing 20% EtOH (w/v). On days 1–3, mice received 2-hr access to 20% EtOH after which EtOH was replaced with water for the remaining 22-hr. On day 4, binge-like intake was measured over the course of an extended, four-hr access period. Blood samples were then promptly collected from the submandibular vein, centrifuged at 4°C and plasma (5 μL) was analyzed for blood ethanol concentration (BEC) using the AM-1 Analox Analyzer (Analox Instruments USA; Lunenburg, MA). Binge-like drinking was operationally defined as a pattern of alcohol consumption resulting in a BEC exceeding 80 mg/dL within 4 h.
In an adaptation of the DID protocol, H101R, S270H/L277A, Q241M and wt males were evaluated for their pattern of 20% EtOH binge-like intake using a contact lickometer setup. Each experimental male’s home cage was fitted with a custom-made stainless steel panel; sipper-tubes were lowered through a hole in the right side of each panel for fluid presentation. Stainless steel mesh flooring was secured to the bottom of each panel to form a raised platform. To drink, mice stood on this mesh platform and made tongue-contact with the metal sipper-tube, thereby closing a circuit. The mesh platform and sipper-tube were each connected to a contact lickometer controller (MedAssociates; model ENV-250B) which transmitted signals to a MED-PC interface; a lick was recorded each time a closed circuit was detected (detection threshold: >0.001 ms interlick interval). All mice were habituated to the lickometer setup for three days with free access to tap water and rodent chow prior to the four-day DID procedure. Blood was collected immediately after 4-hour access to 20% EtOH on the fourth day of the DID protocol for BEC analysis.
In a second adaptation of DID, we aimed to determine if escalated binge-like drinking in the classic protocol was due to involuntary alcohol intake in the absence of water. According to this adaptation, mice had access to two centrifuge tubes of water for 22h or 20h per day. For the first three days, one water tube was exchanged with 20% EtOH, signifying the beginning of the two-hour, two-bottle choice access period. On the final, binge day, mice received four-hour access to water and 20% EtOH. Because only H101R mutants escalated their binge-like drinking in the one-bottle DID test, only these mutants and wt mice were assessed in this protocol.
For all drinking experiments, mice were weighed daily and centrifuge tubes were weighed prior to and after the EtOH or tastant access period to determine intake volume (mL; assuming 1g=1mL). Alcohol consumption was calculated as grams of EtOH consumed according to body weight (g/kg) and as percent preference. To control for unintentional fluid loss, bottle measurements were recorded from an empty cage. These values were subtracted from each mouse’s mL intake to account for leakage during the fluid access period.
Intermittent and continuous access to ethanol
Eight-to-ten-week old mutant H101R, S270H/L277A, Q241M and wt males were assessed for chronic voluntary ethanol consumption according to the intermittent access (IA) procedure as explained previously by Hwa et al., (2015). In short, three hours into the dark phase on Mondays, Wednesdays and Fridays, mice received two-bottle choice, 24-hr access to tap water and 20% EtOH. On all other days, mice were presented with two centrifuge tubes filled with tap water. To control for side-preference, EtOH presentation alternated between the right and left side of the cage lid. This intermittent schedule of alcohol presentation has been shown to induce escalated EtOH consumption by C57BL/6J wt males (20–25 g/kg/24h; Hwa et al., 2011). To contrast with the escalated levels of alcohol intake observed using the intermittent access procedure, separate adult mutant and wt males either received continuous access to 20% EtOH and water for six weeks (Fig S1).
Ascending concentrations of ethanol, sucrose or quinine
Ascending concentrations of EtOH (3, 6, 10, 20% w/v) and water were presented to adult male mutant and wt mice. Each concentration was made available for four consecutive days with presentation alternating sides daily (3% EtOH and water on days 1–4, 6% on days 5–8, 10% on days 9–12, and 20% on days 13–16). Four-day individual intake averages were calculated for each concentration to account for any side preference.
To determine if preference for palatable and aversive tastants differed between mutant lines, male mutant and wt mice were tested for their sucrose (10, 30, 100 mM) and quinine (0.1, 0.3 mM) intake. Ascending concentrations of the tastant solution and water were presented for four days per concentration as detailed for EtOH above. After the final day of access to the highest concentration of sucrose, mice received two weeks of water prior to receiving the lowest concentration of quinine.
Social approach in forced alcohol abstinence
Male mutant and wt mice either received intermittent access to 20% EtOH or access to two bottles of tap water for 16 consecutive weeks (Fig S1, S5). During week seven, mice were habituated to intraperitoneal (i.p.) injections and were evaluated for side preference in a three-chamber apparatus. During side preference screens, the male mouse was placed in the center chamber of the three-chamber apparatus; after a 5-min habituation, the two side doors were opened and the mouse was permitted to explore all three chambers for 10-min. Two ethanol-drinking experimental mice (one wt, one H101R) and two EtOH-naïve control mice (one H101R, one Q241M) were excluded from the final social approach data analyses due to a persistent side preference (i.e. mice repeatedly spent more than 60% of their total test time in either the right or left chamber; for final ns, see Table 1).
From weeks eight to sixteen, mice were tested weekly for social approach toward a novel OVX stimulus mouse six-to-eight hours after IA 20% EtOH was replaced with water (i.e. during forced alcohol abstinence). Males were randomly assigned a novel OVX female each social approach test day and no two males received the same female stimulus animal on the same day. As the male habituated to the central chamber, his assigned OVX stimulus mouse was placed in a wire mesh cage in either the right or left chamber (Fig 2SA). The EtOH forced abstinence or EtOH-naïve male was held within the central chamber of a three-chamber apparatus for a 5-min habituation period. Following habituation, the male received an injection and was returned to the central chamber (Fig S2B). Thirteen minutes later, the doors on either side of the central chamber were lifted, allowing the male to move freely between the central chamber, the chamber with the OVX stimulus mouse, and a third chamber with an empty stimulus cage during a 10-min social approach test. EthoVision XT software tracked the male and recorded his total distance travelled (cm) and the duration of time he spent within the social approach zone. The social approach zone was defined as the region extending 2.25 cm past the radius of the occupied stimulus cage.
For the initial social approach test, ethanol-drinking experimental males in forced abstinence and ethanol-naïve controls were treated systemically with vehicle (half received 20% βCD, half received 0.9% saline). To establish which genotypes demonstrated social deficits in forced abstinence, two-way analyses of variance (ANOVA) were conducted within each genotype (forced EtOH abstinence vs. EtOH-naïve; saline vs. 20% βCD vehicle on the first day).
To recover social approach behavior, mice were tested weekly in the three-chamber apparatus following intraperitoneal injections of midazolam (MDZ) or allopregnanolone (ALLO) in an injection volume of 1 mL/100 grams of body weight. Allopregnanolone (3α-hydroxy-5α-pregnan-20-one; Steraloids, Inc.) was dissolved in a vehicle of 20% (2-hydroxypropyl)-β-cyclodextrin (βCD; Sigma-Aldrich) and midazolam HCl (Sigma-Aldrich) was dissolved in 0.9% saline vehicle. EtOH-naïve and forced EtOH abstinence mutant and wt mice received drug doses in a randomized order according to a mixed, factorial design; each mouse was tested six times for social approach following injections of ALLO (3.0, 10.0 mg/kg), MDZ (0.56, 1.0 mg/kg), and their respective vehicles. Stable levels of drinking were maintained from week four through week sixteen of intermittent access to alcohol (Fig S5).
Statistical analyses
Ethanol intake (g/kg) data collected from DID experiments were analyzed using two-way repeated measures analyses of variance (2-way RM ANOVA; genotype x day). For mice receiving continuous (CA) or intermittent access (IA) to alcohol, individual mean intake (g/kg) and percent ethanol intake data for 18 ethanol-access days were analyzed by 2-way ANOVA to detect interactions between protocol and genotype. To identify genotype-associated differences in the progression of IA or CA drinking, 2-way RM ANOVA (genotype x week) were also run on individual daily intake (g/kg) and alcohol preference values averaged by week.
For all significant 2-way ANOVA, Dunnett’s test was used to compare treatment levels to a control condition (for DID, CA, IA drinking experiments: wt x mutant; for social approach and locomotion in forced abstinence: wt x mutant, vehicle x drug dose; for ascending concentrations of EtOH: 10% EtOH x all other concentrations, wt x mutant; for sucrose or quinine concentrations: lowest concentration x all other concentrations, wt x mutant).
Results
Escalated binge-like drinking by H101R mutants
Gabra2 H101R mutant mice escalated their four-hour binge-like drinking compared to wild-types in the drinking in the dark (DID) protocol (Fig 1A, 1B). Two-way RM ANOVA (genotype x day) of EtOH intake (g/kg/2 or 4hr) revealed main effects of genotype (F(3,102)=77.67, p<0.001) and day (F(3, 34)=13.32, p<0.001). Compared with their two-hour access intake, mice consumed significantly more when they received four-hour access to EtOH on the final day of the DID protocol.
Fig 1.
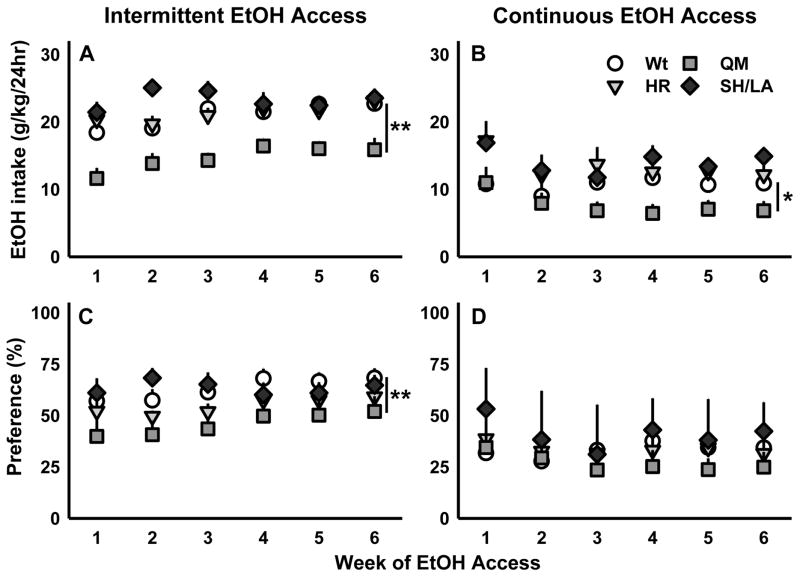
Wild-type (Wt) and α2-subunit point-mutated male mice: H101R (HR), Q241M (QM), and S270H/L277A (SH/LA) in models of binge-like ethanol drinking (A, B, C), chronic escalated drinking (D), or chronic moderate drinking (E). Four-hour binge 20% EtOH intake on the last day of the drinking in the dark (DID) protocol either without (A) or with the lickometer setup (B); BECs (mg/dL) were determined on the final day of DID after four-hour EtOH access (C); data are shown as genotype mean ± SEM with each point representing individual animals; dashed line indicates the operational definition of binge-like drinking as a pattern of consumption yielding BECs >80 mg/dL. Daily average ethanol intake (g/kg) with intermittent access to 20% EtOH (D) and daily average intake (g/kg) with continuous access to 20% EtOH (E). Data (A, B, D, E) are shown as mean + SEM with each bar representing a different group of animals; *p<0.05, **p<0.01 compared to wild-type, ##p<0.01 compared to intermittent access
In separate mice, four-hour DID licking data were collected using a lickometer setup and analyzed in ten-minute time bins (Fig S4). Two-way RM ANOVA (time bin x genotype) identified a main effect of time bin (F(24, 600)=7.144, p<0.001) with the greatest number of licks occurring in the first ten-minutes of EtOH access. Two-way ANOVA of EtOH intake data in the lickometer setup identified an interaction between genotype and day (F(9,66)=2.28, p=0.027) driven by increased drinking by H101R mice compared to wild-types on the binge day (Fig 1B), but not on prior, two-hour access days (data not shown).
Blood was collected from wt and mutant mice assigned to single-bottle DID experiments. A one-way ANOVA of BEC (mg/dL) by genotype revealed a non-significant trend (p=0.062) of reduced BECs in Q241M mutants (M=73.93±12.3) and slightly higher than average BECs in H101R mutants (M=127.43±18.72) as compared to wild-types (Fig 1C). With the exception of Q241M mutants, all genotypes satisfied the requirement of >80 mg/dL for binge-like drinking. A simple linear regression equation (BEC = 52.3+0.136*(licks within final hour)) was able to predict significant variability in BEC values according to the number of licks within the final hour of four-hour binge-like drinking (F(1,28) =12.01, p=0.002; R2 = 0.3).
Two-way ANOVA (genotype x DID protocol) detected a significant interaction (F(1, 32)=9.53, p=0.004). H101R mutants in the original, single-bottle DID experiment consumed more EtOH (g/kg) as compared to those assessed for two-bottle choice DID; in contrast, wt mice consumed similar amounts regardless of DID protocol (Fig S3). In contrast with the original DID protocol findings, there was no significant difference in 4-hour binge intake (g/kg) between wt and H101R mice that were evaluated in a two-bottle choice DID protocol. Despite consuming comparable g/kg EtOH, H101R mice had a significantly higher alcohol preference (%) as compared to wt controls (1-way ANOVA; (F(1, 15)=6.32, p=0.024); wt: M=84.30±6.53; H101R: M=57.81±8.04). High preference for alcohol was driven by low water consumption by H101R mice and contributed to significantly lower total fluid intake by these mutants (1-way ANOVA; (F(1,15)=6.20, p=0.025)).
Reduced chronic ethanol intake by Q241M mutants
Mice that received intermittent rather than continuous access to alcohol consumed significantly greater amounts of ethanol (F(1, 68)=157.9, p<0.001; Fig 1D, 1E). Two-way ANOVA also detected a significant main effect of genotype on EtOH intake (F(3, 68)=24.16, p<0.001); compared to wt mice, Gabra2 Q241M mutants consumed less 20% IA EtOH while S270H/L277A mutants consumed more (Fig 1D).
Daily individual EtOH intake values (g/kg) were averaged by week for mice with intermittent access to EtOH. Two-way RM ANOVA of these data revealed a significant genotype by week interaction (F(15, 185)=2.13, p=0.01), and main effects of genotype (F(3, 37)=33.27, p<0.001) and week (F(5, 185)=9.15, p<0.001). While wt mice consistently consumed more EtOH than Q241M mutants, both wt and Q241M mice consumed progressively more EtOH per day for the first three weeks (Fig 2A). EtOH intake values stabilized for all genotypes following the third week of intermittent access. The significant difference between ethanol intake (g/kg) by wt and Q241M mice remained consistent following week three of intermittent alcohol drinking (F(3, 37) = 22.22, p<0.001); drug administration and social approach testing from weeks eight to sixteen of IA did not impact ethanol intake (Fig S5).
Fig 2.
Daily 20% EtOH intake (g/kg) for the first six weeks that wild-type (Wt) and mutant Gabra2 H101R (HR), Q241M (QM), and S270H/L277A (SH/LA) males received access to either intermittent (A) or continuous 20% EtOH access (B). Average daily preference for 20% EtOH over water is displayed in percent for mice receiving chronic intermittent (C) or continuous access (D) to EtOH. Data are shown as mean + SEM; *p<0.05, **p<0.01 compared to wild-type
Similarly, Q241M mutants consumed less EtOH than wild-types in the continuous access experiment, driving a main effect of genotype (F(3, 31)=9.94, p<0.001; Fig 2B). In contrast with mice that received intermittent access to alcohol, those with continuous access reduced their drinking, generating significantly lower intake values following the second week of drinking (main effect of week: (F(5, 155)=5.72, p<0.001; Fig 2B). Therefore, it appears that, regardless of the drinking protocol, mice require 9–14 days of alcohol access for their daily EtOH intake (g/kg) to stabilize. Two-way RM ANOVA on average total daily volume intake (mL water + mL EtOH) values revealed no effect of genotype (wt vs. mutant genotypes) or of chronic alcohol access protocol (CA vs. IA).
Daily drinking data were also analyzed as % EtOH preference (calculated as: mL EtOH intake/mL total fluid intake * 100). For mice with intermittent EtOH access, 2-way RM ANOVA detected significant main effects of genotype (F(3, 37)=8.33, p<0.001) and week (F(5, 185)=5.15, p<0.001) on % EtOH preference. As revealed by post-hoc analyses, Q241M mice had significantly lower preference for EtOH as compared to wild-types, and, for all genotypes, average daily % EtOH preference stabilized following the third week of intermittent access (Fig 2C). Analysis of continuous access % EtOH preference data identified main effects of genotype (F(3, 31)=3.39, p=0.03) and week (F(5, 155)=2.96, p=0.014). The main effect of genotype was driven by a difference between the Q241M and S270H/L277A mutants; yet, no mutant line differed appreciably from wt controls. As seen with g/kg intake data from mice given continuous access to alcohol, % EtOH preference stabilized during the third week of continuous access drinking (Fig 2D).
Social approach in forced alcohol abstinence
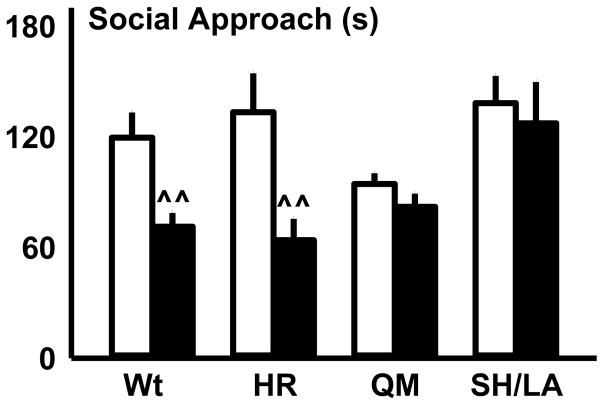
On the first day of social approach testing, mice in forced abstinence and EtOH-naïve controls received either 0.9% saline or 20% βCD vehicle. These social approach data were analyzed within genotype by two-way ANOVA (forced abstinence vs. EtOH-naïve; saline vs. 20% βCD on the first day) to establish whether mice in forced abstinence exhibited deficits in social approach as compared to their EtOH-naïve counterparts. This analysis revealed that wt mice in forced abstinence spent significantly less time in the social approach zone as compared to EtOH-naïve wt controls (F(1, 16)=14.347, p=0.002; Fig 3). Likewise, H101R mutants that were in forced abstinence from alcohol spent significantly less time in the social approach zone compared to EtOH-naïve H101R mutants (F(1, 15)=9.164, p=0.008; Fig 3). Conversely, there was no difference in social approach behavior between Q241M or S270H/L277A mutants in forced abstinence and their EtOH-naïve counterparts (Fig 3). These initial analyses guided subsequent treatments with MDZ and ALLO to reverse abstinence-associated social deficits observed in wt and H101R mice.
Fig 3.
Wild-type (Wt) and H101R (HR), Q241M (QM) and S270H/L277A (SH/LA) mutant mice were EtOH-naïve or received intermittent alcohol access (IAA). Forced abstinence-associated deficits in social behavior were determined by comparing vehicle-treated EtOH-naïve mice (white bars) with vehicle-treated mice in forced EtOH abstinence (black bars). Wt and HR mice showed reduced social approach in forced abstinence from EtOH; data are depicted as mean + SEM; ^^ p<0.01 within genotype comparisons between vehicle-treated EtOH-naïve and IAA mice in forced EtOH abstinence
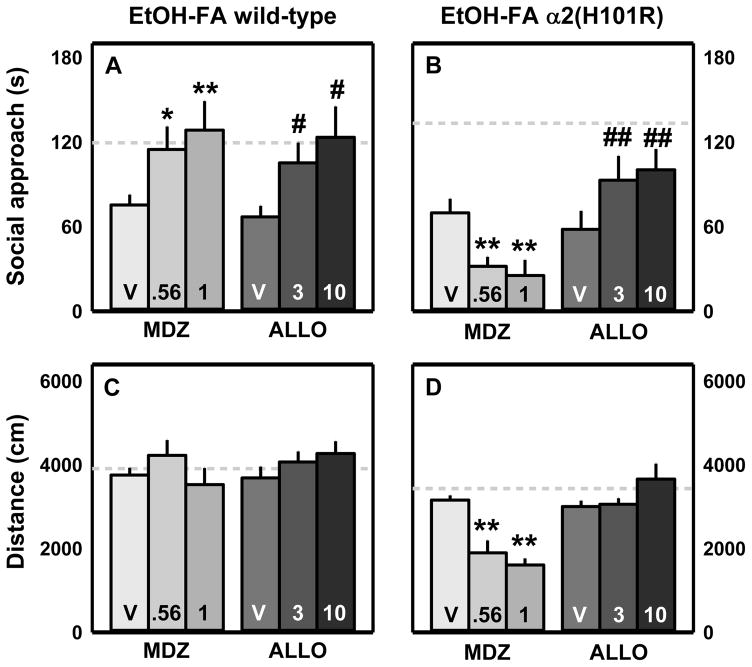
Because six-week intermittent access drinking differed by genotype and was predicted to impact behavior in forced abstinence, social approach was analyzed with one-way RM ANOVA by genotype. Social approach after MDZ or ALLO treatment was compared to behavior after 0.9% saline or 20% βCD administration, respectively. These analyses revealed significant treatment effects in wt mice with the 0.56 and 1.0 mg/kg doses of MDZ and the 3.0 and 10.0 mg/kg doses of ALLO increasing social approach in forced abstinence as compared to their respective vehicles (MDZ: (F(2, 18)=8.241, p=0.003); ALLO: (F(2, 18)=5.22, p=0.016); Fig 4A). For H101R mice, there was a significant effect of MDZ (F(2, 16)=6.403, p=0.009) with both doses reducing social approach time (Fig 4B). Since the data were not normally distributed, Friedman RM ANOVA on ranks was conducted on ALLO data for H101R mice to reveal a significant effect of the drug treatment (χ2(2)=13.56, p=0.001). As compared to 20% βCD, both the 3.0 and 10.0 mg/kg doses of ALLO increased social approach in forced abstinence from alcohol (Fig 4B).
Fig 4.
Wild-type and H101R mutants maintained on intermittent alcohol access were treated with doses of midazolam (MDZ; 0.56 and 1.0 mg/kg) or allopregnanolone (ALLO; 3.0 and 10.0 mg/kg) to recover forced abstinence (FA)-associated deficits in social approach behavior. Vehicle (V) was 0.9% saline or 20% β-cyclodextrin for MDZ and ALLO, respectively. Social approach data (A, B) and distance travelled data (C, D) are shown as mean + SEM; for each genotype, dotted lines denote average social approach time or distance travelled by vehicle-treated EtOH-naïve mice; *p<0.05, **p<0.01 saline vehicle vs. MDZ; #p<0.05, ##p<0.01 β-cyclodextrin vehicle vs. ALLO
Distance travelled during social approach testing in forced abstinence was used as a potential metric of chronic drinking-induced motor impairments (Knapp et al. 2005) and for ALLO- or MDZ-induced sedation. Neither wt nor H101R mutants showed motor impairment due to forced abstinence as revealed by one-way ANOVA between EtOH-naïve and EtOH-forced abstinence mice. This suggests that the social approach protocol used in the present study allowed for independent measurements of social avoidance and locomotor behavior. Additional one-way RM ANOVA or Friedman RM ANOVA were run within genotype to detect drug-treatment effects on motor activity during forced abstinence. There was no effect of drug treatment on locomotor behavior in wt mice in forced abstinence (Fig 4C). However one-way RM ANOVA did detect a significant effect of treatment in H101R mutants (F(2, 16)=14.771, p<0.001) with reduced distance travelled following treatment with MDZ (0.56 or 1.0 mg/kg; Fig 4D). This suggests that reduced social approach at this dose was associated with increased sedation.
To establish whether there was an effect of genotype on social approach, a one-way ANOVA was conducted on data collected from EtOH-naïve, vehicle-treated mice. Q241M mice showed a trend toward reduced social approach compared to wt animals. These mutants may be insensitive to the anxiolytic effects of endogenous allopregnanolone; therefore, EtOH-naïve Q241M mice and EtOH-naïve wt controls were included in subsequent social approach tests following ALLO administration. These EtOH-naïve mice were also tested for their sensitivity to the anxiolytic effects of MDZ.
Two-way RM ANOVA on social approach by EtOH-naïve Q241M and wt mice treated with MDZ revealed a significant effect of drug treatment (F(2, 36)=4.88, p=0.013) and an interaction between genotype and drug administration (F(2, 36)=4.75, p=0.015). Midazolam (1.0 mg/kg) treatment increased social approach time by EtOH-naïve Q241M mice to levels that were comparable to EtOH-naïve wt controls (Fig S6A, S6B). Conversely, there was no effect of ALLO treatment or genotype on approach behavior. Midazolam treatment interacted with genotype (F(2,36)=4.12, p=0.025), producing a significant reduction in distance travelled by EtOH-naïve wt mice, but not by Q241M mutants (Fig S6C, S6D). Allopregnanolone treatment also interacted with genotype (F(2,36)=10.584, p<0.001); however, this interaction was driven by increased distance travelled by wt mice and reduced locomotion by Q241M mutants (Fig S6C, S6D).
Concentration-dependent ethanol, sucrose or quinine preference
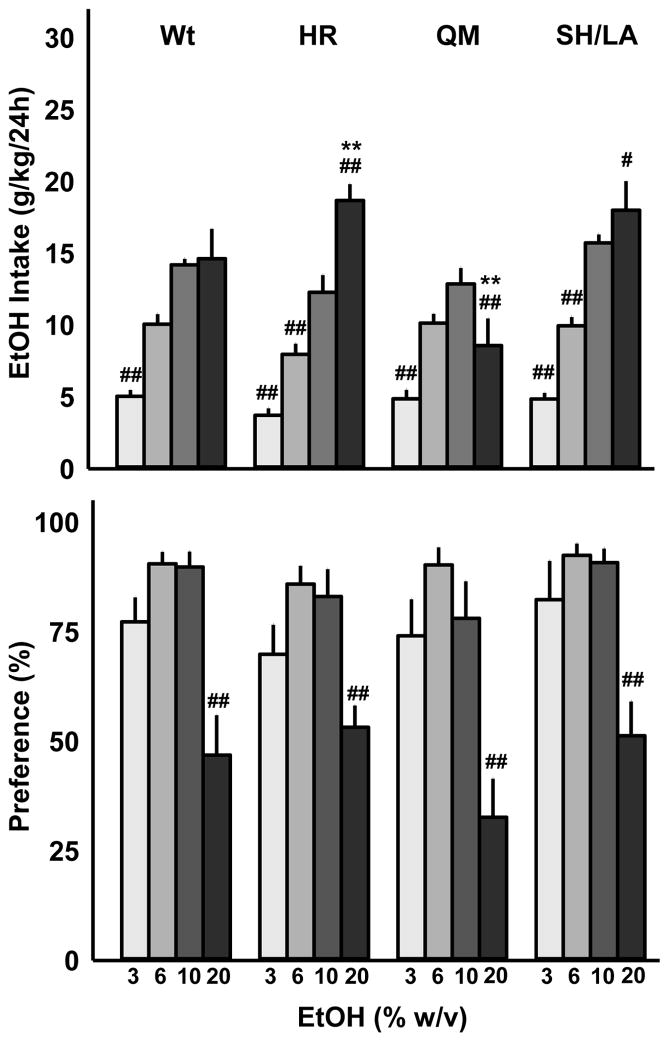
Two-way RM ANOVA was used to detect an interaction between genotype and either intake or percent preference for a specific concentration of ethanol. Analysis of intake data (g/kg) revealed a significant interaction (F(9,96)=11.28, p<0.001) and a main effect of concentration (F(3, 96)=102.31, p<0.001). Post-hoc comparisons were conducted as 10% vs. 3, 6, or 20% EtOH (w/v). All genotypes consumed more 10% EtOH (w/v) as compared to the 3% solution while only the H101R and S270H/L277A mutants consumed more 20% than the 10% concentration. Conversely, Q241M mice drank considerably less 20% EtOH (Fig 5). As compared with wild-types, H101R mice consumed significantly more 20% EtOH while Q241M mutants consumed significantly less 20% EtOH (Fig 5). Two-way RM ANOVA of % EtOH preference data detected a significant effect of EtOH concentration (F(3,96)=82.77, p<0.001) which was due to reduced preference for the 20% EtOH (w/v) solution regardless of genotype (Fig 5). Two-way RM ANOVA on total daily fluid intake (mL EtOH + mL H2O) revealed a significant main effect of concentration (F(3, 96)=40.89, p<0.001) and an interaction between genotype and EtOH concentration (F(9,96) = 4.535, p<0.001). All mice consumed the most total fluid upon receiving access to 3% EtOH and water; however, only Q241M mice did not show increased total volume intake when offered 20% EtOH and water. This suggests that mice may adjust their water intake based on their g/kg EtOH consumption; because 20% EtOH intake by Q241M mice was low, they did not increase their water intake like wt, H101R and S270H/L277A mutants.
Fig 5.
Wild-type (Wt) and H101R (HR), Q241M (QM), and S270H/L277A (SH/LA) mutant males accessed ascending concentrations of alcohol (3, 6, 10, 20% w/v) and water. Data are shown as mean + SEM for EtOH intake (g/kg; A) and percent preference (B); #p<0.05, ##p<0.01 compared to 10% EtOH; **p<0.01 compared to wild-type
Two-way RM ANOVA was also used to determine if there was a significant interaction between genotype and preference for ascending concentrations of sucrose solution (10, 30, 100 mM). A main effect of concentration (F(2, 42)=44.04, p<0.001; Fig S7) was associated with preference for the 30 and 100 mM sucrose solutions as compared to the 10 mM sucrose concentration. Two-way RM ANOVA revealed a main effect of quinine (F(1,20)=29.13, p<0.001) with all genotypes avoiding the high, 0.3 mM quinine solution (Fig S7). Analysis of total daily fluid intake (mL sucrose solution + mL water) revealed a main effect of sucrose concentration (F(2, 42)=69.98, p<0.001) resulting from greater volumetric intake when mice were given access to 100 mM sucrose and water. Interestingly, a similar effect was observed when mice received 0.3 mM quinine and water; all genotypes significantly increasing their total fluid intake (mL quinine solution + mL water; F(1, 20)=19.81, p<0.001). While increased volumetric intake during sucrose testing was associated with substantial100 mM sucrose solution intake, the increase in fluid consumed when mice received access to 0.3 mM quinine was driven by elevated water consumption.
Discussion
The present study highlights the following findings: mutant mice with BZD-insensitive α2-containing GABAA receptors escalated their binge-like alcohol intake; conversely, chronic intermittent drinking by H101R mutants was indistinguishable from wild-type mice. Rendered insensitive to ALLO and THDOC at α2-containing GABAA receptors, Q241M mutants consumed less than wild-types in the chronic drinking protocols. Finally, mice harboring the S270H/L277A mutations in the Gabra2 protein sequence consumed the same amount of alcohol as wild-type mice; yet, unlike wild-type mice, these mutants did not show disrupted social approach in forced abstinence from chronic, excessive alcohol intake.
Human and rodent studies have revealed a correlation between reduced GABAA receptor BZD-binding sites and alcohol-dependence (Freund 1980; Freund and Ballinger 1988; Volkow et al., 1993, 1995; Gilman et al., 1996; Lingford-Hughes et al., 1998; Laukkanen et al., 2013; but Korpi et al., 1992). By evaluating H101R mutant mice, we provide evidence to suggest that BZD-insensitivity - or a yet unknown functional change caused by the mutation - can promote excessive binge-like alcohol consumption when mice are not given the choice between ethanol and water. In a two-bottle choice drinking in the dark procedure (2BC DID), H101R mutants maintained wild-type-like levels of EtOH consumption; these values were significantly lower than those achieved by H101R mutants with access to EtOH only. In contrast with wild-types, 2BC did not elicit equal volumetric EtOH and water drinking by H101R mutant mice (EtOH preference means; H101R: 84.3%; wt: 57.81%). Interestingly, this disparity was not observed in chronic 2BC protocols despite similar EtOH (g/kg) intake between H101R and wild-type mice. These findings may indicate that, in H101R mutants, the presentation of a non-EtOH-containing bottle is sufficient to diminish limited-access drinking. It is possible that single-bottle access to EtOH may drive disinhibited intake and that providing an alternative is sufficient to disrupt escalated drinking. Future lickometer assessments will see if presentation of a second bottle disrupts the pattern of licking during the four-hour DID access period. In addition, work should address how other environmental enrichments may disrupt single-bottle EtOH drinking and whether this effect is specific to H101R mutants.
To clarify the present findings, future work must establish how α2(H101R)-containing GABAA receptors respond to ethanol. In recombinant receptors, the α2(H101R) mutation produces a rightward shift of the GABA dose-response curve (Benson et al.,1998), which might suggest a reduced sensitivity to GABA in vivo. However, at least in central and lateral/basolateral amygdala, the amplitudes of extracellularly evoked inhibitory postsynaptic currents are unchanged by the α2(H101R) mutation, which is consistent with no change in GABAergic functions in the absence of benzodiazepines (Marowsky et al. 2004). Until we have a more complete understanding of how the α2(H101R) mutation alters the properties of GABAA receptors throughout the brain, we cannot formally exclude the possibility that increased binge-like drinking by H101R mutants may reflect altered GABA sensitivity and/or potentiation of GABA-induced currents by ethanol.
Animals rendered selectively insensitive to ALLO and THDOC at α2-containing GABAA receptors reduced their drinking in a protocol that models chronic escalated ethanol intake. The Q241M mutation impedes neurosteroid positive modulator binding to the membrane-bound modulatory site of the α2-subunit (Hosie et al., 2006). Because both low-concentration potentiation and activation by high concentrations of neurosteroids require this modulatory site, these mutants should be insensitive to all neurosteroid action at α2-containing GABAA receptors. Indeed, in vitro dose-effect curves do show that this single amino acid substitution can block ALLO-potentiation of GABA currents (Hosie et al., 2009). A number of studies demonstrate that ALLO or its synthetic analog, ganaxolone, can increase responding for alcohol and can escalate alcohol intake (Janak and Gill 2003; Nie and Janak 2003; Ramaker et al., 2014). Yet, studies also provide evidence for reduced drinking with regional increases in ALLO or following ALLO or ganaxolone treatment (Besheer et al., 2010; Cook et al., 2014; Ramaker et al., 2015); conflicting evidence may reflect dose- and time-dependent effects of ALLO and/or an interaction between ALLO and history of alcohol consumption (Janak et al., 1998; Ford et al., 2005; Ramaker et al., 2011). In the present investigation, Q241M mutants showed reduced alcohol intake beginning with their first day of access, indicating that neurosteroids may need to act on α2-containing GABAA receptors for alcohol to have its rewarding effects. One hypothesis is that ALLO or THDOC initially binds to the membrane-bound GABAA receptor modulatory site to induce a conformational change in the receptor. This may subsequently render an extracellular site more accessible to ethanol, leading to receptor positive modulation. Additional studies need to establish whether or not α2(Q241M)-containing receptors are sensitive to ethanol-potentiation of GABA-induced currents. The possibility that ethanol reward value may be altered in Q241M mutants must be addressed directly in studies comparing alcohol-reinforced responding by mutants to responding by wild-type controls.
During a chronic intermittent access to alcohol procedure, wild-type, H101R, and S270H/L277A mice all consumed ~20 g/kg/24 hr for six weeks. Despite consistently drinking substantial amounts of alcohol, S270H/L277A mutants did not show wild-type-like deficits in social behavior during forced abstinence from alcohol. These mutants harbor two mutations, one to block potentiation by ethanol and the other is a gain-of-function mutation that normalizes GABA sensitivity (Homanics et al., 2005; Borghese et al., 2006b). To identify the specific substitution that affects behavior in forced abstinence, it would be necessary to pair the S270H mutation with an alternative gain-of-function mutation; if these mutants were to behave like the present S270H/L277A mice, then social deficits in forced abstinence may specifically involve serine at location 270 in the α2-subunit protein sequence.
In agreement with earlier work, the present findings demonstrate similar binge-like ethanol intake by mutant male S270H/L277A and wild-type mice (Blednov et al. 2011). However, drinking by S270H/L277A mutants may exceed that of wild-types when animals receive intermittent access to a high concentration of ethanol (i.e. 20% EtOH (w/v); this difference is not evident when animals receive 15% EtOH (v/v; Blednov et al. 2011). S270H/L277A mutants may be less sensitive to some of the aversive effects of alcohol as supported by their escalated chronic ethanol consumption, insensitivity to conditioned taste aversion (Blednov et al. 2011) and intact social approach behavior in forced abstinence from chronic alcohol. Future studies need to clarify whether these mice are selectively insensitive to the aversive effects of ethanol or if they also show deficits in their sensitivity to reward.
High-risk alcohol dependence–associated GABRA2 allelic variants do not affect primary protein sequence in humans, and therefore, mutant mice with amino acid substitutions do not serve as humanized preclinical models to assess alcohol dependence risk. The present study does, however, provide insight regarding how alcohol or endogenous ligands may interact with the GABAA receptor α2-subunit protein to either increase or reduce drinking. Because previous studies using Gabra2 null mutants did not reveal any differences in ethanol intake (Dixon et al., 2012), we chose to use mice harboring targeted amino acid substitutions to address the role of precise GABAA receptor modulatory sites in consumption. Although the action of alcohol on these mutated receptors is not fully characterized, we speculate that α2-containing GABAA receptor sensitivity to benzodiazepines, neurosteroids, or another presently unidentified endogenous ligand may influence specific patterns of drinking. To determine if the present preclinical findings translate to alcohol-dependent patients, clinical studies should investigate individuals with the high-risk GABRA2 haplotype for their sensitivity to benzodiazepines, allopregnanolone and THDOC. Interestingly, recent clinical findings suggest that high AUD-risk GABRA2 SNPs may occur in spans of sequence that regulate GABAA receptor gene expression during a specific perinatal period (Lieberman et al., 2015). Altered expression of receptor subunits during development may change GABAA receptor composition and sensitivity to endogenous modulators. Future research addressing the potential regulatory role of AUD-associated GABRA2 SNPs may guide the development of pharmacogenetic tools to aid in the diagnosis and treatment of alcohol use disorders.
Supplementary Material
Acknowledgments
The project described was supported by Award Numbers R01AA013983 to KAM from the National Institute on Alcohol Abuse and Alcoholism and R01MH080006 to UR from the National Institute of Mental Health.
This work was funded by NIH grants R01 AA013983 (Klaus A. Miczek, Ph.D.) and R01 MH080006 (Uwe Rudolph, M.D.). We would like to thank J. Thomas Sopko, Vallent Lee, Alexandra Barkin, John Auld, Henry Butler, Mark Z. Vrana, Kelly Burke, and Jill Kelly for their excellent contributions.
Footnotes
Conflict of Interest The authors declare no conflict of interest.
The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Mental Health or the National Institutes of Health.
References
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acid A receptors rendered diazepam-insensitive by point-mutated alpha-subunits. Febs Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lindsay TG, O’Buckley TK, Hodge CW, Morrow AL. Pregnenolone and ganaxolone reduce operant ethanol self-administration in alcohol-preferring p rats. Alcohol Clin Exp Res. 2010;34:2044–2052. doi: 10.1111/j.1530-0277.2010.01300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best M, Williams JM, Coccaro EF. Evidence for a dysfunctional prefrontal circuit in patients with an impulsive aggressive disorder. Proc Natl Acad Sci USA. 2002;99:8448–8453. doi: 10.1073/pnas.112604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierut LJ, Agrawal A, Bucholz KK, Doheny KF, Laurie C, Pugh E, Fisher S, Fox L, Howells W, Bertelsen S, Hinrichs AL, Almasy L, Breslau N, Culverhouse RC, Dick DM, Edenberg HJ, Foroud T, Grucza RA, Hatsukami D, Hesselbrock V, Johnson EO, Kramer J, Krueger RF, Kuperman S, Lynskey M, Mann K, Neuman RJ, Nothen MM, Nurnberger JI, Porjesz B, Ridinger M, Saccone NL, Saccone SF, Schuckit MA, Tischfield JA, Wang JC, Rietschel M, Goate AM, Rice JP. A genome-wide association study of alcohol dependence. Proc Natl Acad Sci USA. 2010;107:5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Borghese CM, McCracken ML, Benavidez JM, Geil CR, Osterndorff-Kahanek E, Werner DF, Iyer S, Swihart A, Harrison NL, Homanics GE, Harris RA. Loss of ethanol conditioned taste aversion and motor stimulation in knockin mice with ethanol-insensitive alpha 2-containing GABA(A) receptors. J Pharmacol Exp Ther. 2011;336:145–154. doi: 10.1124/jpet.110.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Storustovu SI, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. The delta subunit of gamma-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther. 2006a;316:1360–1368. doi: 10.1124/jpet.105.092452. [DOI] [PubMed] [Google Scholar]
- Borghese CM, Werner DF, Topf N, Baron NV, Henderson LA, Boehm SL, Blednov YA, Saad A, Dai S, Pearce RA, Harris RA, Homanics GE, Harrison NL. An isoflurane- and alcohol-insensitive mutant GABA(A) receptor alpha(1) subunit with near-normal apparent affinity for GABA: Characterization in heterologous systems and production of knockin mice. J Pharmacol Exp Ther. 2006b;319:208–218. doi: 10.1124/jpet.106.104406. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey MS, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biol Psychiatry. 2007;62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Cook JB, Werner DF, Maldonado-Devincci AM, Leonard MN, Fisher KR, O’Buckley TK, Porcu P, McCown TJ, Besheer J, Hodge CW, Morrow AL. Overexpression of the steroidogenic enzyme cytochrome p450 side chain cleavage in the ventral tegmental area increases 3 alpha,5 alpha-thp and reduces long-term operant ethanol self-administration. J Neurosci. 2014;34:5824–5834. doi: 10.1523/JNEUROSCI.4733-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am J Med Genet B. 2004;129B:104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation - A possible prelude to violence. Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Dixon CI, Walker SE, King SL, Stephens DN. Deletion of the gabra2 gene results in hypersensitivity to the acute effects of ethanol but does not alter ethanol self administration. PLoS One. 2012;7:e47135. doi: 10.1371/journal.pone.0047135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei XL, Tian HJ, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Hodgkinson CA, Yuan QP, Albaugh B, Virkkunen M, Goldman D. GABRG1 and GABRA2 as independent predictors for alcoholism in two populations. Neuropsychopharmacology. 2009;34:1245–1254. doi: 10.1038/npp.2008.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fehr C, Sander T, Tadic A, Lenzen KP, Anghelescu I, Klawe C, Dahmen N, Schmidt LG, Szegedi A. Confirmation of association of the GABRA2 gene with alcohol dependence by subtype-specific analysis. Psychiatric Genet. 2006;16:9–17. doi: 10.1097/01.ypg.0000185027.89816.d9. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Phillips TJ, Finn DA. Neurosteroid modulators of GABAA receptors differentially modulate ethanol intake patterns in male C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1630–1640. doi: 10.1097/01.alc.0000179413.82308.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund G. Benzodiazepine receptor loss in brains of mice after chronic alcohol consumption. Life Sci. 1980;27:987–992. doi: 10.1016/0024-3205(80)90109-5. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WE. Decrease of benzodiazepine receptors in frontal-cortex of alcoholics. Alcohol. 1988;5:275–282. doi: 10.1016/0741-8329(88)90065-1. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Adams K, JohnsonGreene D, Junck L, Kluin KJ, Brunberg J, Martorello S, Lohman M. Positron emission tomographic studies of cerebral benzodiazepine-receptor binding in chronic alcoholics. Ann Neurol. 1996;40:163–171. doi: 10.1002/ana.410400207. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat Rev Neurosci. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Elsen FP, Ying SW, Jenkins A, Ferguson C, Sloat B, Yuditskaya S, Goldstein PA, Kralic JE, Morrow AL, Harrison NL. A gain-of-function mutation in the GABA(A) receptor produces synaptic and behavioral abnormalities in the mouse. Genes Brain and Behav. 2005;4:10–19. doi: 10.1111/j.1601-183X.2004.00090.x. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, Mi Z, Wang X, Grayson DR, Firestone LL. Gene knockout of the alpha 6 subunit of the gamma-aminobutyric acid type A receptor: Lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997;5:588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA(A) receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABA(A) receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent Escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa LS, Nathanson AJ, Shimamoto A, Tayeh JK, Wilens AR, Holly EN, Newman EL, DeBold JF, Miczek KA. Aggression and increased glutamate in the mPFC during withdrawal from intermittent alcohol in outbred mice. Psychopharmacology. 2015;232:2889–902. doi: 10.1007/s00213-015-3925-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Redfern JEM, Samson HH. The reinforcing effects of ethanol are altered by the endogenous neurosteroid, allopregnanolone. Alcohol Clin Exp Res. 1998;22:1106–1112. [PubMed] [Google Scholar]
- Janak PH, Michael Gill T. Comparison of the effects of allopregnanolone with direct GABAergic agonists on ethanol self-administration with and without concurrently available sucrose. Alcohol. 2003;30:1–7. doi: 10.1016/s0741-8329(03)00068-5. [DOI] [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Breese GR. Modulation of ethanol withdrawal-induced anxiety-like behavior during later withdrawals by treatment of early withdrawals with benzodiazepine/gamma-aminobutyric acid ligands. Alcohol Clin Exp Res. 2005;29:553–563. doi: 10.1097/01.alc.0000158840.07475.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Uusi-oukari M, Wegelius K, Casanova MF, Zito M, Kleinman JE. Cerebellar and frontal cortical benzodiazepine receptors in human alcoholics and chronically alcohol-drinking rats. Biol Psychiatry. 1992;31:774–786. doi: 10.1016/0006-3223(92)90309-n. [DOI] [PubMed] [Google Scholar]
- Laukkanen V, Storvik M, Hakkinen M, Akamine Y, Tupala E, Virkkunen M, Tiihonen J. Decreased GABA(A) benzodiazepine binding site densities in postmortem brains of cloninger type 1 and 2 alcoholics. Alcohol. 2013;47:103–108. doi: 10.1016/j.alcohol.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Li DW, Sulovari A, Cheng C, Zhao HY, Kranzler HR, Gelernter J. Association of gamma-aminobutyric acid A receptor alpha 2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology. 2014;39:907–918. doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman R, Kranzler HR, Joshi P, Shin D-G, Covault J. GABRA2 alcohol dependence risk allele is associated with reduced expression of chromosome 4p12 GABAA subunit genes in human neural cultures. Alcohol Clin Exp Res. 2015;39:1654–64. doi: 10.1111/acer.12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJA, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. Br JPsychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Marowsky A, Fritschy J, Vogt K. Functional mapping of GABAA receptor subtypes in the amygdala. Eur J Neurosci. 2004;20:1281–89. doi: 10.1111/j.1460-9568.2004.03574.x. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Ethanol potentiation of gabaergic transmission in cultured spinal-cord neurons involves gamma-aminobutyric acid-a-gated chloride channels. J Pharmacol Exp Ther. 1988;246:558–564. [PubMed] [Google Scholar]
- Mihic SJ, Whiting PJ, Harris RA. Anesthetic concentrations of alcohols potentiate GABA(A) receptor-mediated currents - lack of subunit specificity. Eur J Pharmacol. 1994;268:209–214. doi: 10.1016/0922-4106(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MA, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Newman EL, Smith KS, Takahashi A, Chu A, Hwa LS, Chen Y, DeBold JF, Rudolph U, Miczek KA. alpha 2-containing GABA(A) receptors: a requirement for midazolam-escalated aggression and social approach in mice. Psychopharmacology. 2015;232:4359–4369. doi: 10.1007/s00213-015-4069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie H, Janak PH. Comparison of reinstatement of ethanol- and sucrose-seeking by conditioned stimuli and priming injections of allopregnanolone after extinction in rats. Psychopharmacology. 2003;168:222–228. doi: 10.1007/s00213-003-1468-0. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International union of pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Fretwell AM, Finn DA. Alteration of ethanol drinking in mice via modulation of the GABA(A) receptor with ganaxolone, finasteride, and gaboxadol. Alcohol Clin Exp Res. 2011;35:1994–2007. doi: 10.1111/j.1530-0277.2011.01551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Ford MM, Phillips TJ, Finn DA. Differences in the reinstatement of ethanol seeking with ganaxolone and gaboxadol. Neuroscience. 2014;272:180–187. doi: 10.1016/j.neuroscience.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaker MJ, Strong-Kaufman MN, Ford MM, Phillips TJ, Finn DA. Effect of nucleus accumbens shell infusions of ganaxolone or gaboxadol on ethanol consumption in mice. Psychopharmacology. 2015;232:1415–1426. doi: 10.1007/s00213-014-3777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong Q, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric-acid receptor-mediated chloride transport in rat-brain synaptoneurosomes. Proc Natl Acad Sci USA. 1986;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Hitzemann R, Pappas N, Burr G, Pascani K, Wong C, Fowler JS, Wolf AP. Regional brain metabolic response to lorazepam in subjects at risk for alcoholism. Alcohol Clin Exp Res. 1995;19:510–516. doi: 10.1111/j.1530-0277.1995.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Hitzemann R, Fowler JS, Wolf AP, Pappas N, Biegon A, Dewey SL. Decreased cerebral response to inhibitory neurotransmission in alcoholics. Am J Psychiatry. 1993;150:417–422. doi: 10.1176/ajp.150.3.417. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha(4)beta(3)delta and alpha(6)beta(3)delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner DF, Swihart A, Rau V, Jia F, Borghese CM, McCracken ML, Iyer S, Fanselow MS, Oh I, Sonner JM, Eger EI, Harrison NL, Harris RA, Homanics GE. Inhaled anesthetic responses of recombinant receptors and knockin mice harboring alpha2(S270H/L277A) GABA(A) receptor subunits that are resistant to isoflurane. J Pharmacol Exp Ther. 2011;336:134–144. doi: 10.1124/jpet.110.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G, Lovinger DM, Weight FF. Ethanol inhibits nmda-activated current but does not alter gaba-activated current in an isolated adult mammalian neuron. Brain Research. 1990;507:332–336. doi: 10.1016/0006-8993(90)90292-j. [DOI] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABA-A receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.