Abstract
Objectives
To evaluate whether the Hospital Admission Risk Profile (HARP) score is associated with skilled nursing or acute rehabilitation facility discharge following an acute hospitalization.
Design
Retrospective cohort study
Setting
One inpatient unit of a rural, academic medical center
Participants
Hospitalized patients 70 years or older from October 1, 2013 to June 1, 2014
Measurements
Patient age at the time of admission, modified Folstein Mini-Mental Status Exam score, and self-reported instrumental activities of daily living two weeks prior to admission were used to calculate a HARP score. The primary predictor was HARP score and the primary outcome was discharge disposition (home, facility, or deceased). Multivariate analysis evaluated the association between HARP score and discharge disposition adjusting for age, sex, comorbidities, and length of stay.
Results
Four hundred twenty eight patients, admitted from home, were screened and categorized by HARP score as low (162 [37.8%]), intermediate (157 [36.7%]), or high (109 [25.5%]). Patients with high HARP scores were significantly more likely to be discharged to a facility compared to those with low HARP scores (55% vs. 20%; p<0.001). After adjustment, patients with high compared to low HARP scores were over 4 times more likely to be discharged to a facility (OR 4.58, 95% CI 2.42–8.66).
Conclusion
Among a population of older hospitalized adults, the HARP score (using readily available admission information) identifies patients at increased risk for skilled nursing or acute rehabilitation facility discharge. Early patient identification for potential facility discharges may allow for targeted interventions to prevent functional decline, improve informed shared decision-making about post-acute care needs, and expedite discharge planning.
Keywords: Hospital Admission Risk Profile, discharge disposition, hospitalization, skilled nursing facility
INTRODUCTION
Older adults hospitalized for an acute illness are at high risk for functional decline which, in medical patients, is strongly associated with an increased risk of readmissions.1–4 Functional decline often necessitates transfer to a skilled nursing facility (SNF) or acute rehabilitation facility upon discharge from the hospital.5 Early identification of hospitalized patients at-risk for functional decline may allow for the implementation of targeted measures to prevent or mitigate the deleterious effects of hospitalization.
Previous studies have identified predictors of nursing home admission or long-term care placement for older adults, but are largely based on community-based non-hospitalized patients.6,7 Highly predictive variables include multiple activities of daily living (ADL) dependencies, cognitive impairment, and prior nursing home utilization.8 Yet, a paucity of literature describes risk factors for facility transfers at the time of an acute hospital admission using patient-reported or electronic health record information typically obtained during the admissions process. Administrative data, including the 36-item Short Form Health Survey (SF-36)9, Charlson co-morbidity score10, or healthcare utilization prior to admission,11,12 have been used, but such data often are collected following discharge and not available in real-time to be used in a clinical capacity to impact patient care.
The Hospital Admission Risk Profile (HARP) is a simple and easy to use instrument that identifies patients at risk for functional decline during a hospitalization using information that is readily obtained during the admission process.13 In the original validation study, patients in the high HARP score cohort had a higher rate of ADL decline and were more likely to be residents of a facility three months after discharge.13 The relationship between admission HARP score and discharge disposition from an acute hospitalization has, to our knowledge, not been examined. The objective of this current study is to evaluate the association of the HARP score with facility discharges in hospitalized older adults.
METHODS
Study Setting and Participants
This study was performed at Dartmouth-Hitchcock Medical Center, a rural, academic 396-bed tertiary care hospital located in Lebanon, New Hampshire. Dartmouth-Hitchcock Medical Center serves a population of 1.5 million people from a circumscribed area of rural New Hampshire and Vermont and has approximately 25,000 discharges annually.14 Patients included in this study were aged ≥70 years and admitted to a single 35 bed internal medicine inpatient unit from October 1, 2013 to June 1, 2014. Geriatric admission screenings were implemented in this unit as a component of a larger quality improvement initiative to improve care for hospitalized older adults using a team of geriatric trained licensed nursing assistants (LNA). The HARP score was used to identify patients at increased risk for functional decline who would receive increased mobilization and activities from the geriatric LNAs while hospitalized. Patients 70 years or older were selected as this was the age range used for the initial validation study for the HARP score.13 All hospitalized patients were internal medicine and medicine subspecialty patients managed by a hospitalist service covered by both resident and non-resident physicians. Patients were excluded from the study if a complete geriatric screening (described below) was not completed upon admission due to an inability to obtain information from the patient or family members, patient transfer to another service or unit, or patient discharge prior to completion of the admission screening. Patients who were admitted from a facility (which included skilled nursing facilities and acute rehabilitation facilities) were excluded from data analysis as these patients had a high likelihood of returning to a facility after discharge. The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College and granted a waiver for signed informed consent. Patients and families were given an information sheet upon enrollment describing the collection of data and the quality improvement initiative that was implemented and given the option to not participate or have their data recorded in the database.
Primary Predictor (HARP)
Enrolled patients received a geriatric screening after admission to the unit by a specially trained geriatric licensed nursing assistant. The geriatric screening included the patient’s age in years at the time of hospital admission, patient or family self-reported activities of daily living and instrumental activities of daily living (ADL and IADL)15 two weeks prior to admission, living situation prior to admission (home, home with assistance, assisted living facility, nursing home/skilled nursing facility, transfer from outside hospital), cognitive screening using the Folstein Mini-Mental Status Exam (MMSE),16 and patient’s desired discharge disposition. The screenings were reviewed by a supervising geriatric nurse practitioner who could address any concerns identified with the admission screenings. Based on the original Sager HARP protocol, the patient’s age at the time of admission, modified Folstein MMSE, and self or family reported IADLs two weeks prior to admission were used by the LNA to calculate a HARP score for each patient.17 The modified Folstein MMSE omits the language items and the IADLs evaluated included managing finances, taking medications, use of the telephone, shopping, transportation, housekeeping, and food preparation.17,18 Patients with complete dependence in all ADL categories were included in this study but were excluded from the original HARP validation study (as patients with complete ADL dependence had no potential to decline in ADL function)17.
Covariates
The patients’ electronic medical record numbers were recorded in a secure database and used to query our data warehouse to obtain additional patient data. Data obtained resided on secure institutional servers maintained in accordance with Dartmouth-Hitchcock security standards. The recorded data represented demographic information (age, gender), clinical information (body mass index (BMI) at the time of admission, comorbidities), and hospitalization information (length of stay and discharge disposition). Hospitalization length of stay was calculated from the documented discharge and admission dates. As our institution is the sole provider of tertiary acute care and the largest provider of outpatient services including primary care in the region, we obtained comorbidity information from internal billing data. Comorbidities were based on internal billing codes using International Classification of Disease, Ninth Edition (ICD-9) and Current Procedural Terminology (CPT®) codes as described below for inpatient and outpatient visits and dichotomized (present/absent). A patient was noted to have a specific comorbidity if they had two or more occurrences of a diagnosis code over any period of time with at least one diagnosis code within the last 24 months, or ≥1 applicable CPT® code at any time. Defined comorbidities included asthma, coronary artery disease (CAD), cancer, chronic obstructive pulmonary disease (COPD), diabetes mellitus, heart failure, hypertension, renal disease, and ischemic vascular disease. Comorbidity identification and BMI data was validated via manual chart review by members of the study team.
Primary outcome: Discharge disposition
The primary outcome assessed was discharge disposition after the index hospitalization, categorized as (1) home (which included patients discharged home with or without visiting nurses (VNA) or other home services such as skilled physical or occupational therapy) or assisted living facilities, (2) facility which included skilled nursing facilities, acute rehabilitation facilities, and swing bed transfers to community hospitals, or (3) deceased. In our local region, there are no long term acute care (LTAC) facilities to include in the discharge disposition.
Statistical Analyses
Continuous data are presented as means ± standard deviations, and categorical data as counts and percentages. Using the calculated HARP score on admission to the hospital, based on the original protocol published by Sager13, patients were assigned to three cohorts – low (HARP score 0–1), intermediate (HARP score 2–3) and high (HARP score 4–5) groups. An analysis of variance assessed differences between HARP group and continuous variables, and Cochran-Mantel-Haenszel tests for discrete variables. The primary outcome was discharge disposition defined as home (including assisted living facilities), facility (including skilled nursing facilities, acute rehabilitation facilities, and swing bed transfers to community hospital), or deceased. Our primary predictor was HARP group (low HARP = referent). Multiple logistic regression models were created after adjusting for age, sex, comorbidities, and length of stay. We present odds ratios with 95% confidence intervals. All statistical tests were two-sided, and P-values <0.05 were considered significant. All analyses were performed using STATA v.12 (College Station, TX).
The geriatric screenings including the HARP scores were recorded in a database on a separate password protected network drive as the institution’s electronic health record did not have appropriate data fields for entry and the institution did not allow LNAs to document in the progress notes section of the electronic health record.
RESULTS
A total of 592 patients were initially enrolled. One hundred eighteen patients were excluded due to incomplete data fields or admission screenings and 46 patients admitted from a facility were excluded from further data analysis as these patients had a high likelihood of returning back to a facility after discharge. For the 428 included hospitalized patients, 162 (37.8%) had a low HARP score, 157 (36.7%) had an intermediate score and 109 (25.5%) had a high score. Mean age of the cohort was 80.5±7.2 years, 49.3% were female, and 99.8% were admitted from home. Patients in the high HARP group were more likely to be female (60.6%) and older (86.2 ± 7.3 years) compared to the low and intermediate groups.
Baseline characteristics are presented in Table 1. There were no differences among the HARP groups in the total number of comorbidities (mean 3.57±1.81). No individual comorbidity was significantly different among the three groups except for a diagnosis of cancer which was more prevalent in the low and intermediate groups. BMI was lower in the high HARP group compared to the low HARP group (25.62kg/m2 ±6.25 vs. 27.67 kg/m2±6.53; p=0.016). Table 2 summarizes hospitalization length of stay and discharge disposition for the overall and three HARP cohorts. There were similar hospital lengths of stay (overall average 8.4±23.3 days; p=0.466) and similar inpatient mortality rates (overall 1.6%). Patients in the high HARP score group, as compared to those in the intermediate or low groups, were significantly more likely to be discharged to a facility (55% vs. 36% vs. 20%; p<0.001). Table 3 displays the multivariate analysis of admission factors associated with a facility discharge by admission HARP score group. After adjustment for age, sex, comorbidity score, and length of stay, patients in the high HARP score group were 4.6 times more likely to be discharged to a facility compared to the referent, the low HARP score group (OR 4.58, 95% CI: 2.42–8.66).
Table 1.
Baseline Demographic Characteristics of Study (N=428 patients)
| Overall Cohort |
HARP Score | |||||
|---|---|---|---|---|---|---|
| Low (0–1) | Intermediate (2–3) | High (4–5) | P-value | |||
| Number | 428 | 162 (37.8%) | 157 (36.7%) | 109 (25.5%) | ||
| Female (%) | 49.3% | 41.9% | 49.0% | 60.6% | 0.011 | |
| Age, years | 80.5±7.20 | 76.35±4.6 | 80.9±6.5 | 86.2±7.3 | <0.001 | |
| Number of comorbidities |
3.57±1.81 | 3.36±1.83 | 3.97±1.80 | 3.31±1.74 | 0.99 | |
| Asthma | 38 (8.9%) | 15 (9.3%) | 13 (8.3%) | 10 (9.2%) | 0.95 | |
| CAD | 163 (38.1%) | 58 (35.8%) | 69 (43.9%) | 36 (33.0%) | 0.14 | |
| Cancer | 134 (31.3%) | 58 (35.8%) | 55 (35.0%) | 21 (19.3%) | 0.007 | |
| COPD | 123 (28.7%) | 48 (29.6%) | 51 (32.5%) | 24 (22.0%) | 0.17 | |
| Diabetes Mellitus | 142 (33.2%) | 47 (29.0%) | 60 (38.2%) | 35 (32.1%) | 0.21 | |
| Heart Failure | 142 (33.2%) | 48 (29.6%) | 60 (38.2%) | 34 (31.2%) | 0.23 | |
| Hypertension | 350 (81.8%) | 125 (77.2%) | 136 (86.6%) | 89 (81.7%) | 0.09 | |
| Renal disease | 169 (39.5%) | 56 (34.6%) | 73 (46.5%) | 40 (36.7%) | 0.7 | |
| Vascular disease | 269 (62.9%) | 90 (55.6%) | 107 (68.2%) | 72 (66.1%) | 0.05 | |
| BMI, kg/m2 | 26.96 ± 6.64 | 27.67±6.53 | 27.15±6.92 | 25.62±6.25 | 0.016 | |
Note: All values represented are mean ± standard deviation or counts (percent).
BMI=Body Mass Index, CAD=Coronary Artery Disease, COPD=Chronic Obstructive Pulmonary Disease, HARP=Hospital Admission Risk Profile
Table 2.
Hospitalization Length of Stay (LOS), Inpatient Mortality, and Discharge Disposition in the Overall Cohort and by Hospital Admission Risk Profile (HARP) Score For Patients Not Admitted From a Facility
| HARP Score | |||||
|---|---|---|---|---|---|
| Overall | Low (0–1) | Intermediate (2–3) | High (4–5) | P-value | |
| n=428 | n=162 | n=157 | n=109 | ||
| LOS (days) | 8.4±23.3 | 10.2±33.4 | 7.1±10.1 | 7.79±18.2 | 0.466 |
|
Discharge disposition |
|||||
| Home | 271 (63.3%) | 126 (77.8%) | 97 (61.8%) | 47 (43.1%) | <0.001 |
| Facility | 150 (35.0%) | 33 (20.4%) | 57 (36.3%) | 60 (55%) | <0.001 |
| Deceased | 7 (1.6%) | 2 (1.2%) | 3 (1.9%) | 2 (1.8%) | * |
Note: HARP=Hospital Admission Risk Profile, LOS=Length of stay for hospitalization, *=sample size too small in cells to test differences
Table 3.
Multivariate Analysis of Admission Factors Associated With Facility Discharge by Hospital Admission Risk Profile (HARP) Score
| Low HARP (95% CI) |
Intermediate HARP (95% CI) |
High HARP (95% CI) |
Age (95% CI) |
Sex (95% CI) |
Co-morbidities (95% CI) |
Length of stay (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Model 1 | Referent | 2.26 (1.37–3.74) | 4.91 (2.86–8.43) | - | - | - | - |
| Model 2 | Referent | 2.18 (1.29–3.69) | 4.51 (2.40–8.49) | 1.00 (0.97–1.04) | 1.13 (0.74–1.72) | - | - |
| Model 3 | Referent | 2.14 (1.26–3.64) | 4.51 (2.40–8.48) | 1.01 (0.97–1.04) | 1.13 (0.74–1.73) | 1.03 (0.91–1.15) | - |
| Model 4 | Referent | 2.21 (1.29–3.78) | 4.58 (2.42–8.66) | 1.01 (0.98–1.04) | 1.15 (0.75–1.77) | 1.03 (0.92–1.16) | 1.01 (0.99–1.02) |
Note: All values listed are represented as odds ratios (95% confidence intervals).
Model 1 - unadjusted, Model 2 - adjusted for age, sex, Model 3 - adjusted for age, sex, comorbidity score, Model 4 - adjusted for age, sex, comorbidity score, and length of stay.
DISCUSSION
The HARP score, which is calculated using a patient’s age, cognitive status and self-reported IADLs at the time of admission, predicts patients at high risk for discharge to a facility. While the HARP score has previously been reported to be associated with loss of ADL function at discharge and facility placement three months after discharge13, to our knowledge, this is the first study to evaluate discharge disposition with this simple and practical tool.
Using information that is readily available at admission, the HARP score strongly predicts high risk patients for discharge to a facility. These findings build upon previously described tools such as the Discharge Decision Support Tool (D2S2) and the Early Screen for Discharge Planning (ESDP) that identify patients who need increased discharge planning services or referrals for post-acute care but do not specifically address discharge location.19,20 Early identification of high risk patients creates an opportunity to promote targeted, evidence-based interventions including physical and occupational therapy, early mobilization21, and specific inpatient geriatric care initiatives included within Acute Care for the Elderly (ACE) units21–23 or Hospital Elder Life Programs (HELP)24 to help prevent functional decline and potentially prevent discharges to facilities. Additionally, early identification of a potential need for facility discharge during a hospitalization may prompt environmental modifications at home, training and education of home caregivers, and the addition of home health services and assistive devices that would allow for home discharges in patients who otherwise may have been discharged to a facility.
Identifying high risk patients has the potential to better expedite discharge planning during the hospitalization. Often, patients are referred to facilities only after resolution of acute medical issues and who, as a result, remain hospitalized several days while awaiting facility bed offers. Investment of care management services can be initiated at an earlier stage of the hospitalization if individuals are determined to be at high risk of needing increased post-acute care. As such, transfers could potentially occur at an earlier stage during a hospitalization leading to reduced lengths of stay and avoiding potential iatrogenic complications from prolonged inpatient stays. Early identification of possible facility-based discharges could also allow for greater shared decision-making between inpatient care teams and patients and families by moving the conversation of post-discharge care needs to an earlier point in the hospital course.
The study has a number of limitations. First, the analysis was performed on a relatively small sample of patients who were hospitalized in one medical unit in a single academic institution with a largely rural, white patient population. External validity is limited and replication of this study at other institutions with more urban or diverse patient populations would be helpful to confirm these findings. Second, self- and family-reported IADL information was used to calculate the HARP score including no objective or performance-based functional assessments to validate or augment this assessment. Hospitalized older adults tend to overestimate ADL function25 which could have potentially led to the incorrect classification of patients into a lower HARP score group. Third, other potential patient factors and admission diagnoses could be more powerful predictors of facility discharges during an acute hospitalization that were not specifically measured or evaluated for this study. For example, certain admission diagnoses such as hip fractures are associated with a very high likelihood of facility discharge upon admission yet patients may have low HARP scores if they had a high functional status before the fracture. In addition to patient-based factors, contextual factors such as the presence of an able caregiver at home to provide assistance following discharge were not measured but could add to the precision of the HARP and also help inform post-discharge planning.
In comparing this study population to the original HARP study by Sager et al13 which was carried out in six primarily urban hospitals, it must be noted that this study occurred in a rural setting which may be associated with increased facility utilization when compared to urban populations.26 In the original HARP study, average patient age (79±6.2 development cohort, 80±6.1 validation cohort) and hospitalization length of stay (8.7 days development, 8.1 validation) were similar to our overall study population (80.5±7.2 age, 8.4±23.2 day length of stay). Our patient population had a higher percentage in the high HARP score group (25.5% vs. 22.8% development and 17.7% validation) that may suggest an older, more frail population but may also reflect the increase in the acuity of inpatient care since the original publication of the Sager study in 1996.
The findings of this study suggest several areas of future research and improvement opportunities. One is whether the routine use of the HARP score in inpatient care can increase the delivery of targeted physical and occupational therapy and specialized geriatric care to increase home discharges and decrease hospitalization length of stay in high risk patients. The other is whether inclusion of additional patient factors, such as the presence of an able caregiver and social support at home, or testing with brief performance based functional assessments which do not rely on self-report, can improve the precision of the HARP score in identifying patients at high risk for discharge to facilities.
In conclusion, the use of the HARP score on admission, using patient information that is readily obtained during a typical admission assessment, can identify patients at higher risk for facility discharge. Early patient identification for potential facility discharges may allow for targeted interventions to prevent functional decline, improve informed shared decision-making about post-acute care needs, and may expedite discharge planning.
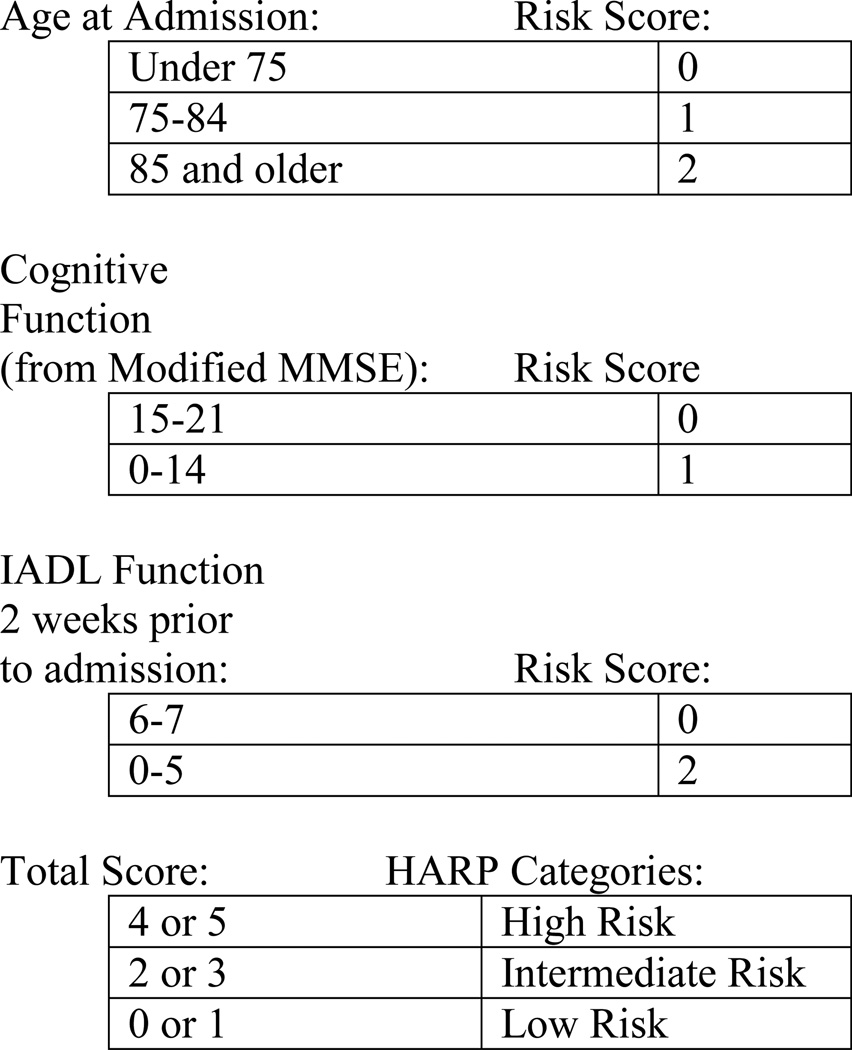
Figure 1. Hospital Admission Risk Profile (HARP) Scoring System Used to Calculate HARP Score.
Note: From Sager 1996. Figure based on Appendix 113
MMSE = Mini-Mental Status Exam, IADL = Instrumental Activities of Daily Living
Acknowledgments
Conflict of Interest: This work was partially funded through work supported by Stephen Liu’s participation in the Practice Change Leaders for Aging and Health Program sponsored by the Atlantic Philanthropies and the John A. Hartford Foundation. Stephen Liu is a consultant for The Oak Group International, Wellesley, MA. This consulting work was not related to the design, methods, analysis or preparation of this manuscript.
Dr. Batsis receives funding from Health Resources Services Administration (UB4HP19206-01-00) for medical geriatric teaching, the Junior Faculty Career Development Award, the Department of Medicine, Dartmouth-Hitchcock Medical Center, and the Dartmouth Centers for Health and Aging
Dr. Bartels receives funding from the National Institute of Mental Health (K12 HS0217695 (AHRQ), NIMH: T32 MH073553, R01 MH078052, R01 MH089811; R24 MH102794 CDC U48DP005018
Support was also provided by the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Sponsor’s Role: None
Appendix
Conflict of Interest Disclosures
| Elements of Financial/Personal Conflicts |
Stephen Liu | John Batsis | Stephen Bartels |
Rebecca Masutani, John Mecchella, Justin Montgomery, Yu Yan |
||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation |
X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
Authors can be listed by abbreviations of their names.
For “yes” x mark(s): give brief explanation below:
Footnotes
work was performed while at Dartmouth-Hitchcock Medical Center
Author Contributions: Liu: study concept and design, data analysis, results interpretation, drafting, revision. Yu: data abstraction, data analysis, results interpretation, drafting, revision. Mecchella: data abstraction, data analysis, results interpretation, drafting, revision. Montgomery: study concept and design, data analysis, results interpretation, drafting, revision. Masutani, data analysis and abstraction, results interpretation, revision. Bartels: study concept and design, data analysis, results interpretation, revision. Batsis: study concept and design, data analysis, results interpretation, drafting, revision.
References
- 1.Sager MA, Franke T, Inouye SK, et al. Functional outcomes of acute medical illness and hospitalization in older persons. Archives of Internal Medicine. 1996;156:645–652. [PubMed] [Google Scholar]
- 2.Hirsch CH, Sommers L, Olsen A, Mullen L, Winograd CH. The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38:1296–1303. doi: 10.1111/j.1532-5415.1990.tb03451.x. [DOI] [PubMed] [Google Scholar]
- 3.Boyd CM, Ricks M, Fried LP, et al. Functional decline and recovery of activities of daily living in hospitalized, disabled older women: the Women's Health and Aging Study I. J Am Geriatr Soc. 2009;57:1757–1766. doi: 10.1111/j.1532-5415.2009.02455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. Journal of hospital medicine : an official publication of the Society of Hospital Medicine. 2014;9:277–282. doi: 10.1002/jhm.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helvik AS, Skancke RH, Selbaek G, Engedal K. Nursing home admission during the first year after hospitalization - the contribution of cognitive impairment. PloS one. 2014;9:e86116. doi: 10.1371/journal.pone.0086116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong JH, Mitchell OS, Koh BS. Disaggregating activities of daily living limitations for predicting nursing home admission. Health services research. 2015;50:560–578. doi: 10.1111/1475-6773.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman SM, Steinwachs DM, Rathouz PJ, Burton LC, Mukamel DB. Characteristics predicting nursing home admission in the program of all-inclusive care for elderly people. The Gerontologist. 2005;45:157–166. doi: 10.1093/geront/45.2.157. [DOI] [PubMed] [Google Scholar]
- 8.Gaugler JE, Duval S, Anderson KA, Kane RL. Predicting nursing home admission in the U.S: a meta-analysis. BMC Geriatr. 2007;7:13. doi: 10.1186/1471-2318-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 11.Louis Simonet M, Kossovsky MP, Chopard P, Sigaud P, Perneger TV, Gaspoz JM. A predictive score to identify hospitalized patients' risk of discharge to a post-acute care facility. BMC health services research. 2008;8:154. doi: 10.1186/1472-6963-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairchild DG, Hickey ML, Cook EF, et al. A prediction rule for the use of postdischarge medical services. J Gen Intern Med. 1998;13:98–105. doi: 10.1046/j.1525-1497.1998.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sager MA, Rudberg MA, Jalaluddin M, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. Journal of the American Geriatrics Society. 1996;44:251–257. doi: 10.1111/j.1532-5415.1996.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 14.Dartmouth-Hitchcock Medical Center, Facts and Figures. [Accessed September 14, 2015]; at http://www.dartmouth-hitchcock.org/about_dh/facts_and_figures.html. [Google Scholar]
- 15.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Sager MA, Rudberg MA, Jalaluddin M, et al. Hospital admission risk profile (HARP): identifying older patients at risk for functional decline following acute medical illness and hospitalization. J Am Geriatr Soc. 1996;44:251–257. doi: 10.1111/j.1532-5415.1996.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 18.Graf C. The Hospital Admission Risk Profile (HARP) Medsurg nursing : official journal of the Academy of Medical-Surgical Nurses. 2008;17:437–438. [PubMed] [Google Scholar]
- 19.Holland DE, Harris MR, Leibson CL, Pankratz VS, Krichbaum KE. Development and validation of a screen for specialized discharge planning services. Nursing research. 2006;55:62–71. doi: 10.1097/00006199-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Bowles KH, Holmes JH, Ratcliffe SJ, Liberatore M, Nydick R, Naylor MD. Factors identified by experts to support decision making for post acute referral. Nursing research. 2009;58:115–122. doi: 10.1097/NNR.0b013e318199b52a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox MT, Sidani S, Persaud M, et al. Acute care for elders components of acute geriatric unit care: systematic descriptive review. J Am Geriatr Soc. 2013;61:939–946. doi: 10.1111/jgs.12282. [DOI] [PubMed] [Google Scholar]
- 22.Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A Randomized Trial of Care in a Hospital Medical Unit Especially Designed to Improve the Functional Outcomes of Acutely Ill Older Patients. New England Journal of Medicine. 1995;332:1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 23.Hung WW, Ross JS, Farber J, Siu AL. Evaluation of the Mobile Acute Care of the Elderly (MACE) service. JAMA internal medicine. 2013;173:990–996. doi: 10.1001/jamainternmed.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inouye SK, Bogardus ST, Jr, Baker DI, Leo-Summers L, Cooney LM., Jr The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48:1697–1706. doi: 10.1111/j.1532-5415.2000.tb03885.x. [DOI] [PubMed] [Google Scholar]
- 25.Sager MA, Dunham NC, Schwantes A, Mecum L, Halverson K, Harlowe D. Measurement of activities of daily living in hospitalized elderly: a comparison of self-report and performance-based methods. J Am Geriatr Soc. 1992;40:457–462. doi: 10.1111/j.1532-5415.1992.tb02011.x. [DOI] [PubMed] [Google Scholar]
- 26.Dubay LC. Comparison of rural and urban skilled nursing facility benefit use. Health care financing review. 1993;14:25–37. [PMC free article] [PubMed] [Google Scholar]