Abstract
Over the last decade the important concept has emerged that microglia, similar to other tissue macrophages, assume different phenotypes and serve several effector functions, generating the theory that activated microglia can be organized by their pro-inflammatory or anti-inflammatory and repairing functions. Importantly, microglia exist in a heterogenous population and their phenotypes are not permanently polarized into two categories; they exist along a continuum where they acquire different profiles based on their local environment. In Parkinson’s disease (PD), neuroinflammation and microglia activation are considered neuropathological hallmarks, however their precise role in relation to disease progression is not clear, yet represent a critical challenge in the search of disease-modifying strategies. This review will critically address current knowledge on the activation states of microglia as well as microglial phenotypes found in PD and in animal models of PD, focusing on the expression of surface molecules as well as pro-inflammatory and anti-inflammatory cytokine production during the disease process. While human studies have reported an elevation of both pro- or anti-inflammatory markers in the serum and CSF of PD patients, animal models have provided insights on dynamic changes of microglia phenotypes in relation to disease progression especially prior to the development of motor deficits. We also review recent evidence of malfunction at multiple steps of NFκB signaling that may have a causal interrelationship with pathological microglia activation in animal models of PD. Finally, we discuss the immune-modifying strategies that have been explored regarding mechanisms of chronic microglial activation.
Keywords: microglia, neurodegeneration, neuroinflammation, Parkinson's, immune, neuroprotection
1. Introduction
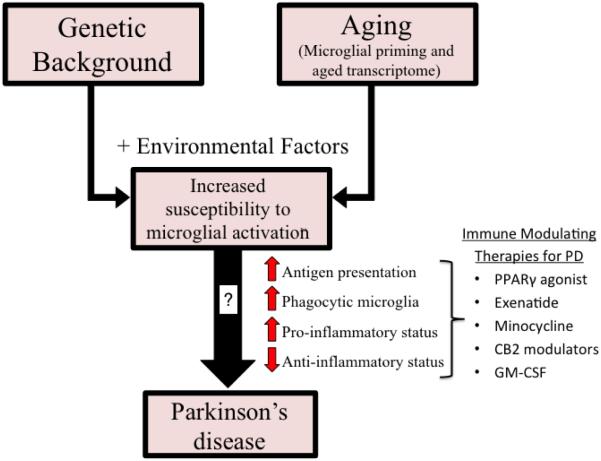
The pathological hallmarks of Parkinson’s disease (PD) are a loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SN) and the presence of proteinaceous inclusions termed Lewy bodies and neurites. In addition, numerous studies have highlighted a potential role for neuroinflammation in PD, the hallmark of which is microgliosis (Tansey and Goldberg, 2010). Microglia are the resident immune cells in the brain derived from progenitors originating in the yolk sac during embryogenesis (Alliot et al., 1999). They serve various functions that include eliminating and remodeling synapses during development, surveying the environment to maintain homeostasis, and responding to injury to limit tissue damage, initiate repair processes, and promote neuronal survival. On the other hand, in response to an insult or in various neurodegenerative disorders microglia can proliferate, display morphological changes, alter their gene expression and surface markers to those that are characteristic of chronic activation. Together this response is termed microgliosis, yet it is not clear if microglia play a beneficial or detrimental role in the neurodegenerative process. Similarly, it is not well understood if microglia solely contribute to PD as a pathogenic response that facilitates disease or as a disease-initiating component. Although microglia are the primary cell in the brain responsible for immune surveillance, under pathological conditions, peripheral macrophages and other immune cells can traffic across a disrupted blood brain barrier (BBB) and most likely contribute to the role of neuroinflammation in PD. A new and growing area of PD research is now focused on understanding whether microglia exert a protective function via production of neurotrophic factors, or contribute to neuronal death by producing inflammatory factors that are toxic to DA and other vulnerable neurons. In order to address the impact of microglia on neurodegeneration in PD, whether protective or deleterious, we must better understand the behavior of microglia during disease progression. We posit that the microglial phenotypes in the aging brain become further altered in PD giving rise to a prevalence of pro-inflammatory microglia, which likely contribute to progressive neuronal loss (Figure 1). To support our view we will discuss the complex microglial phenotypes found in PD and in animal models of PD and the immune-modifying strategies that have been explored regarding mechanisms of chronic microglial activation.
Figure 1. Potential role of inflammation in Parkinson’s disease pathogenesis.
Results of microglial phenotypes have varied across studies and PD patients. Genetic background and aging, along with other environmental factors (ie: illness) impact the susceptibility of microglia activation leading to a lack of homeostatic support (increased antigen presentation, phagocytic microglia, pro-inflammatory status and decreased anti-inflammatory status) to the surrounding tissue that may further influence the pathogenesis of Parkinson’s disease. Multiple immune modulating therapies are currently being investigated for their effect on the phenotypes and effector functions of activated microglia and may represent promising approaches in the treatment of PD.
1.1 Microglia Activation States: complex and heterogeneous phenotypes
Over the last decade the important concept has emerged that microglia, similar to other tissue macrophages, assume different phenotypes and perform specific effector functions depending on the precise nature of the stimulus, its intensity and duration (Olah et al., 2011; Perry et al., 2007; Tansey and Goldberg, 2010). Activation of microglia through various pattern recognition receptors such as toll-like receptors (TLRs) leads to the synthesis of a range of different cytokines, inflammatory mediators, growth factors and cell surface molecules. These secondary effects of microglial activation have been used to classify them as either pro- or anti-inflammatory. However it is crucial to understand that activated microglia exist in a heterogenous population and that their phenotypes are not necessarily polarized into two categories, either pro- or anti-inflammatory, but instead exist along a continuum where they acquire different profiles based on their local environment. And as the brain ages, a progressive pro-inflammatory status also referred to as inflammaging manifests increasing the risk of age-related disorders and leading to microglial priming that may increase the propensity to develop neurodegenerative diseases such as PD (Perry and Holmes, 2014).
Microglia undergo morphological changes when they shift from a quiescent to an activated state. Morphologically, resting, quiescent microglia have long ramified processes with small cell bodies in vivo, while activated microglia take on an amoeboid shape with shorter processes and larger cell bodies. Functionally, one way that microglia remain in a quiescent state is via the interaction between the glycoprotein CD200 located on surrounding neurons and the receptor CD200R located on microglia (Hoek et al., 2000). There are multiple other ligand-receptor pairs that make up an important system for cross-talk between neurons and microglia including CD45-CD22 and fractalkine-CX3CR1. Recent evidence suggests that morphological changes should not be interpreted as a sign of activation but instead that the microglia are impaired and can no longer efficiently perform their intrinsic functions (Heppner et al., 2015). Microglia Aβ-binding scavenger receptors that are largely involved in Aβ plaque clearance were downregulated in aged Alzheimer’s disease mice (Hickman et al., 2008), suggesting alterations in microglial receptors may be a process that coincides with a shift in morphology. Furthermore, in a mouse model of Alzheimer’s disease, the phagocytic capacity of microglia was reduced and inversely correlated to the Aβ plaque burden, suggesting that microglia function declined with severity of disease (Krabbe et al., 2013). Similar defective phagocytic activity was described in aged mice whereby primary microglia isolated from aged mice had less effectively taken up exosome associated α-synuclein oligomers compared to microglia from young mice (Bliederhaeuser et al., 2015). On the contrary, results from other studies in animal models of PD have suggested long-term phagocytic activation of microglia (Barcia et al., 2013; Rodriguez et al., 2007). These studies used immunohistochemical techniques to highlight presence of phagocytic markers, thus potentially suggesting that some microglia in the heterogeneous population maintain their phagocytic capacity in late stages of the disease but the presence of phagocytic markers does not necessarily correlate with in vivo phagocytic capacity. Therefore we must be cautioned to evaluate more than the morphological changes in order to understand the functions of the microglia and their relationship with neurodegeneration in PD.
The term “activated microglia” is an oversimplified term that lacks information about the heterogeneous nature of activated microglia and their various and complex effector functions. Activated macrophages also have diverse functions that historically have been categorized by their distinct patterns of secreted factors and cell surface markers following stimuli such as cytokines. The original concept of macrophage polarization described an M1 stage as cytotoxic and maintaining a pro-inflammatory phenotype, while a M2 stage was associated with repair and healing or an anti-inflammatory phenotype (Mackaness, 1962; Mills et al., 2000). Stereotypical M1 phenotypes occur when challenged with LPS and IFN-γ which are produced in large quantities during bacterial and viral infections, respectively, leading to the production of inflammatory cytokines such as tumor necrosis factor (TNF), interlukin-6 (IL-6), IL-12, and IL-1β and lead to the upregulation of cell surface markers such as major histocompatibility complex-II (MHC-II) and CD86 (Martinez et al., 2006; Nau et al., 2002). The M2 state is divided into 3 different sub-classes each having distinct activation mechanisms: M2a, M2b, and M2c (Biswas and Mantovani, 2010; Edwards et al., 2006; Martinez and Gordon, 2014; Stout et al., 2005). Macrophages stimulated by IL-4 adopt an M2a-like phenotype that functions to suppress inflammation. Alternatively, M2b responses control selective phagocytosis and regulate inflammation. This response is stimulated by TLR ligands that become activated by the fusion of Fc gamma receptors (FcγRII) and immunoglobulin G (IgG) complexes. Lastly, the M2c phenotype has been implicated in tissue remodeling and immunoregulation and is stimulated by the binding of IL-10 to its receptor and can be enhanced by glucocorticoids (Gratchev et al., 2008). The proposed distinct macrophage activation states became controversial in 2011 when activation pathways were found to overlap, suggesting that states of activation may be intermittent and dynamic. A recent publication authored by 25 senior investigators proposes new nomenclature to describe the complex landscape of macrophage responses to various stimuli (Martinez and Gordon, 2014; Murray et al., 2014).
Based on the similarities between microglia and macrophages, microglia activation responses were also initially classified using M1/M2 polarization terminology. Based on the emergence of new cutting-edge technologies such as single-cell RNA sequencing and cytometry time-of-flight (CyTOF) mass spectrometer for high-speed acquisition of highly multi-parametric single cell data it has become clear that describing the states of microglia activation by the M1 or classical activation and M2 or alternative activation phenotypes is not accurate. The phenotypic responses of microglia do not necessarily mimic those of macrophages. For example, IL-4 stimulus promotes CD163 and CD206 expression in macrophages but not in microglia (Mittelbronn, 2014). Furthermore, the responsiveness of primary human microglia to LPS and IFN-γ is minimal to absent depending on culture conditions in contrast to the robust induction of TNF and CCR7 observed in monocyte-derived macrophages. Moreover, primary microglia produce increased CD163 and CCL18 after stimulation with dexamethasone, while the response of monocyte-derived macrophages is largely restricted to increases in CD163 (Melief et al., 2012).
Although microglia can be manipulated in vitro to elicit polarized responses, the in vivo profiling of microglia activation is more complex and much less clear with distinctions by species and/or location. Anatomical regional differences have been reported for microglia frequency with greater density found in the telencephalon, while within the same region, more microglia are found in myelinated areas compared to non-myelinated (Lawson et al., 1990; Mittelbronn et al., 2001). In relation to PD, early reports describe, within the less dense mescencephalon, that microglia are more numerous in the SN, particularly in the pars reticulata, compared to adjacent structures (Lawson et al., 1990). Interestingly, microglial density is positively correlated to LPS-induced neurodegeneration suggesting regional differences in cell responsiveness (Kim et al., 2000). Potentially contributing to their functional response, variability in microglia basal gene expression exists among brain regions. For example, TNF mRNA is greater in microglia freshly isolated from the SN compared to the hippocampus of Wistar rats, suggesting that microglial profiles may contribute to regional vulnerability and susceptibility of neurons in a diseased state such as PD (Doorn et al., 2015). This idea is further supported by the non-uniform transcriptional identity of aged microglia across brain regions. Recent investigations have shown that microglia isolated from aged mice lose their younger, more distinct gene expression profile with greatest changes in found in the hippocampus which may result in a lack of homeostatic support to the surrounding tissue and further influence the location dependent susceptibility to neurodegenerative disorders (Grabert et al., 2016). Future studies to better understand regional functionality of microglia should be conducted using the translating ribosome affinity purification (TRAP) methodology (Heiman et al., 2008). Furthermore, microglial surface markers also have demonstrated regional diversity. Using flow cytometry, isolates from gray matter (occipital cortex) had increased levels of CD45 compared to white matter (corpus callosum), suggesting that gray matter microglia may be in a more immunosuppressed state (Melief et al., 2012). Understanding region-specific differences may provide insight into the contribution of microglial responsiveness to disease and their vast capacity to produce a spectrum of responses versus merely polarized responses.
2. Microglia in Parkinson’s disease (PD)
Research into disequilibrium and complexity of microglial activation responses became an area of intense focus in hope to identify a potential mechanism that could contribute to neuroinflammation and potentially underlie PD pathogenesis. The idea was that identifying neuroinflammatory profiles in the brains of PD patients may help define potential targets for anti-inflammatory neuroprotective therapies and could help determine the success of immunotherapies (Sanchez-Guajardo et al., 2013; Tansey and Goldberg, 2010). However, the challenges of such targeted therapies require sensitive methods to detect microglial activation states and phenotypic changes which remains a challenging undertaking given these factors are likely to display complex temporal associations with PD depending on Braak disease stage, rate of disease progression and duration of disease. In order to move forward and conquer these challenges we will discuss current understandings of the activation states of microglia in PD as determined by post-mortem histological evaluation and in vivo imaging and the microglia phenotypes found in PD tissues and biofluids.
2.1 Evidence for Microglia Activation in PD
Multiple studies have described reactive microglial in postmortem brain samples of PD patients. Specifically, major histocompatibility complex class II (MHC-II) immunoreactive microglia were identified in areas of the SN and striatum in PD patients and in patients with induced parkinsonism from a self-administered analog of synthetic meperidine analogue known as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) that resulted from faulty chemistry and was responsible for “frozen addicts” with extreme parkinsonian bradykinesia (Imamura et al., 2003; Langston et al., 1999; McGeer et al., 1988). Located in the vicinity of the few remaining nigral DA neurons, these microglia displayed morphologies characteristic of activated and phagocytic cells, similar to those seen in aging (Jyothi et al., 2015), and displayed increased HLA immunoreactivity with some microglia containing visible evidence of phagocytized neuromelanin, the characteristic pigment of nigral DA neurons (Langston et al., 1999). Interestingly, Croisier et al. found an inverse correlation of CD68 immunoreactivity with disease duration, and suggested that elevated phagocytosis was associated with ongoing tissue destruction, decreasing in final disease stages when it was no longer functionally relevant (Croisier et al., 2005). These and other associations between the presence of activated microglia and advanced parkinsonian stages as well as the presence of α-synuclein aggregation suggest a significant contribution of microglial activation to the progressive pathological process in PD. However, given that most of these observations were taken at the end stage of disease, it remains unclear whether the activation of inflammatory responses and microgliosis could play a detrimental or beneficial role at earlier stages of the neuropathology. In either case, the involvement of the immune system and potentially microglial activation in the pathogenesis of PD has been supported by a large genome-wide association study (GWAS) that identified a single nucleotide polymorphism (SNP) in the human leukocyte antigen (HLA-DRA) gene, which encodes HLA-DR antigens that are expressed by antigen-presenting cells such as microglia, as a genetic risk factor for late-onset PD (Hamza et al., 2010).
Evidence for the ongoing activation of microglia in PD has been confirmed using positron emission tomography (PET) imaging. Radioligands such as 11C-PK11195, 11C-PBR, or 18F-FEPPA selectively bind to peripheral benzodiazepine receptors (PBR), more recently termed translocator proteins (TSPO), which generally have low expression in a healthy brain, but are greatly enhanced in the presence of neuroinflammation and therefore widely accepted as a measurement of activated microglia (Banati et al., 1997; Papadopoulos et al., 2006). Widespread microglial activation in PD as reflected by increased binding of 11C-PK11195 was reported in the pons, basal ganglia and frontal and temporal cortex of PD patients compared to age-matched normal controls (Gerhard et al., 2006). Yet longitudinal evaluation of 11C-PK11195 binding potential 18-28 months later did not show significant changes in radioligand binding regardless of the significant increases in UPDRS scores, suggesting that PBR binding does not correlate with disease progression. However, other studies have demonstrated a positive correlation in the levels of 11C-PK11195 in the midbrain with the UPDRS in early stage PD patients (Ouchi et al., 2005), and although not significant, trends of higher levels of 11C-PK11195 were found in advanced stages of PD compared to unmedicated patients within a year of their diagnosis (Bartels et al., 2010). Diffuse binding of 11C-PK11195 has also been described in PD patients with a 25-30% increase in the temporal and occipital cortical regions (Edison et al., 2013); whereas, other studies demonstrated selective binding potential increases in the SN and putamen (Iannaccone et al., 2013) or the midbrain (Ouchi et al., 2005). While these disparate findings may partly reflect the large variability of neuroinflammation in PD patients, the largest inherent limitation of these studies is that quantitative interpretations of binding signal are confounded by large inter-individual variability in binding affinity due to genetic polymorphisms in TSPO (Owen et al., 2012; Yoder et al., 2013); therefore, caution should be used when comparing between individuals in cross-sectional studies. Even in a study that controlled for the rs6971 polymorphism, 18F-FEPPA binding was not significant in the striatum of PD patients compared to controls, yet interestingly within the PD group demonstrated trends of increased TSPO expression in high compared to mixed affinity binders (Koshimori et al., 2015). The most conservative interpretation of these findings is that the progressive degeneration in PD may not be associated with increasing microgliosis, suggesting there is not a parallel relationship and instead there may exist a threshold of microglial activation or dysfunction that continues to contribute to cell death. Yet, it also leaves open the possibility that microglia phenotypes fluctuate along with disease progression. However, PBR ligands are unlikely to distinguish across the spectrum of microglia activation states, therefore nowadays it is not possible to evaluate microglia phenotype in the brain and make a clear correlation with different stages of PD progression in parkinsonian patients. Clearly it would be advantageous to develop PET radioligands that can detect microglial phenotypes or functional behavior. Furthermore, development of radioligands for α-synuclein aggregation would allow comparisons between Lewy pathology and not only DA neuron dysfunction but also microglial activation.
Future application of microglial imaging to evaluate patterns of temporal activation in PD are needed. Animal models of ischemia have demonstrated microglial temporal activation and distribution shifts. Mice induced with focal cerebral ischemia show CD68 expressing microglia bordering the ischemic core 24 hours after lesion, but then migrated into the core by 7 days post-ischemic lesion (Perego et al., 2011), suggesting that phagocytic behavior increased by 7 days because nerve loss was irreversible. However, little is known about the exact mechanisms by which microglia influence the outcome of disease, especially a progressive neurodegenerative disorder.
2.2 Microglia phenotypes in PD
Given the complex profile of microglia, the presence of reactive microglia alone does not define their helpful or harmful role in PD neuropathology. Evaluation of soluble factors such as cytokines in brain tissue, cerebral spinal fluid (CSF) or serum from PD patients may provide hints to the pathogenic impact of microglia activation (Table 1). Elevated levels of cytokines including TNF-α, IL-6 and IL-1β were reported in CSF and striatal samples of individuals with PD (Blum-Degen et al., 1995; Mogi et al., 1994a; Mogi et al., 1994b; Mogi et al., 2007; Muller et al., 1998). Additionally with the use of immunohistochemistry, nitric oxide synthase, cyclo-oxygenases 1 and 2 (COX1 and COX2) were found elevated in amoeboid microglia from PD samples of the SN (Knott et al., 2000), while microglia in the putamen (Imamura et al., 2003) or SN (Hunot et al., 1999) of PD patients were also immunoreactive for cytokines such as IL-6, TNF-α and IL-1β. More recently, evaluation of CSF from PD patients compared to a reference healthy control group revealed no significant differences in the inflammatory markers Flt3 ligand and fractalkine (Shi et al., 2011) or C-reactive protein (CRP), IL-6, TNF-α, eotaxin, interferon gamma-induced protein-10 (IP-10), monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory protein-1β (MIP-1β) (Lindqvist et al., 2013). Interestingly, and perhaps not surprisingly, increased CSF levels of CRP correlated with non-motor symptoms including depression and fatigue, while MCP-1 expression was significantly associated with depression (Lindqvist et al., 2013), suggesting a role for cytokine production in non-motor disease symptoms with significant inter-individual variability of inflammatory phenotypes in relation to disease pathology. The interaction of neuroinflammation and non-motor symptoms is further supported by a significant correlation between 11C-PK11195 binding in the cortical regions and cognitive impairment in both PD patients with dementia and Alzheimer patients (Fan et al., 2015). Another cytokine transforming growth factor-beta (TGF-β), known to inhibit microglia activation, has been observed in the brains and CSF of PD patients (Mogi et al., 1995; Vawter et al., 1996). Therefore, it is possible that adaptive changes in the brain occur at some point along disease progression to induce a protective microglia phenotype, which would account for pro- and anti-inflammatory cytokines co-existence in CSF. To date, the relationship between aggregated or soluble α-synuclein and CSF inflammatory markers has not been evaluated in-depth in PD patients.
Table 1.
Cytokines detected in brain tissue, cerebral spinal fluid (CSF) or serum of Parkinson’s disease patients. Results are in comparison with control patients. CRP, C reactive protein; EGF, Epidermal growth factor; FACIT, functional assessment of chronic illness therapy; HADS, hospital anxiety and depression scale; IFN-γ, interferon-gamma; IP-10, interferon gamma-induced protein-10; MCP-1, monocyte chemotactic protein-1; MIP-1β, macrophage inflammatory protein-1β; NT-proCNP, N-terminal Pro-C-type natriuretic peptide; SN, substantia nigra; TGF- β, transforming growth factor β; TNF, tumor necrosis factor
| Tissue or Biofluid |
Number of PD patients (M;F) |
Mean age of PD (years) |
Hoehn & Yahr |
duration of disease (years) |
Number of controls (M;F) |
Mean age of controls (years) |
Results | Comments | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Caudate and putamen |
5M;4F | 72 | - | - | 6M;5F | 71 | Caudate/Putamen: ↑ IL-1β, IL-6, TNF-α Caudate only: ↑ EGF |
No significant changes found in the cerebral cortex of PD compared to controls. |
Mogi et al., 1994a |
| Caudate and putamen |
9 | No mean defined (75 - 91) |
- | - | 8 | No mean defined (75 - 91) |
↑ TNF-α | Mogi et al., 1994b | |
| Caudate and putamen |
5M;4F | 72 | - | - | 6M;5F | 71 | ↑ TGF-β1 | Mogi et al., 1995 | |
| Caudate, Putamen and SN |
12M;3F | 69.7 | - | 16.9 | 6M; 8F | 60.8 | Caudate/Putamen/SN: ↑ IFNγ and NF- κBp65 Caudate: ↑ p53 (apoptosis related protein) |
Similar levels of INFγ,NF-κBp65, and p53 in cerebellum and frontal cortex of PD and controls. |
Mogi et al., 2007 |
|
| |||||||||
| 6M;9F | 58 | - | - | 6M;10F | 46 | ↑ TNF-α | Mogi et al., 1994b | ||
| 12M;10F | 61 ± 1.54 | 1-3 | 0.5 - 3 | 6M;6F | 61 ± 4.15 |
↑ IL-1β, IL-6 | No changes in CSF IL-2. No significant differences found in plasma. Measurements taken off medication. |
Blum-degen et al., 1995 | |
| CSF | 7M;7F | 68 | - | - | 10M;3F | 46 | ↑ TGF-β1 | Evaluations completed on ventricular CSF. |
Mogi et al., 1995 |
| 22M;8F | 73.8 ± 1.4 | - | - | 11M;5F | 72.7 ± 3.1 |
↑ TGF-β1 and TGF- β2 |
Evaluations completed on ventricular CSF |
Vawter et al., 1996 | |
| 8M;4F | 57.5 ± 2.6 | - | - | ↑ IL-6 | Evaluated de novo patients. IL-6 negatively correlated with UPDRS scores. |
Muller et al., 1998 | |||
| 44M;27F (non- demented) |
64.1 ± 10.5 |
1.9 ± 0.8 |
6.4 ± 10.5 |
14M;19F | 65.8 ± 8.8 |
No significant differences in CRP, IL- 6, TNF-α, eotaxin, IP- 10, MCP-1, and MIP- 1β ↑ CRP (compared to non-demented PD and controls) |
CRP and MCP-1 correlated with HADS depression score. CRP correlated with FACIT score (fatigue) |
Lindqvist et al., 2013 | |
| 12M;4F (demented) |
72 ± 5.8 | 3.1 ± 0.9 |
15.8 ± 6.5 |
||||||
| 93M;33F | 63.8 ± 10.4 |
- | - | 76M;61F | 58.9 ± 18.4 |
No significant difference in fractalkine |
Frackalkine/Aβ1- 42 positively correlated to UPDRS score and H&Y. |
Shi et al., 2013 | |
|
| |||||||||
| 17M;14F | 61.5 ± 2.8 | 2-4 | 6.9 ± 1 | 10M;10F | 67 ± 3.2 | ↑ TNF-α, IL-6, IFNγ, IL-10, IL-4, IL-2 |
Significant increases (1.5x) in TBARS (lipid peroxidation) compared to controls. |
Brodacki et al., 2008 | |
| Serum | 7M;10F | 65.3 ± 13.1 (without levodopa) |
70.6% <3 |
2.8 ± 3.9 | 8M;15F | 60 ± 7.8 | No significant difference in IL-6 |
Negative correlation found between serum IL-6 and activities of daily living scale in levodopa group. |
Hofmann et al., 2009 |
| 15M;8F | 66.5 ± 8.8 (with levodopa) |
47.8% <3 |
8.1 ± 6.1 | ||||||
| 30M;30F | 59.17 ± 15.48 |
2.35 ± 0.83 |
11.27 ± 10.69 |
12M;12F | 64 ± 5.8 | ↑ TNF-α, NT- proCNP |
Positive correlation between IL-6 and IL-1β; IL-6 and H&Y stage in PD. |
Koziorowski et al., 2012 | |
In the plasma, inflammatory cytokines have been found and correlated to PD severity as determined by Hoehn and Yahr staging (Brodacki et al., 2008; Hofmann et al., 2009; Koziorowski et al., 2012). Another study evaluated a total of 17 cytokines and chemokines in plasma of PD patients that were homozygous carriers of the rs3129882 G SNP, but found no changes with the exception of increased CCL-3, also known as MIP-1α (Kannarkat et al., in press). The rs3129882 SNP is located in the first intron on the HLA-DRA gene and homozygous carriers of the high-risk G allele have elevated baseline and inducibility of MHC-II expression on antigen presenting cells, suggesting that cellular immune phenotypes may be an alternative marker to the soluble markers found in biofluids.
Based on the above reports, conclusions of microglial phenotypes throughout the different stages of PD are difficult not only because of the limited number of studies and the effect of method and timing of collection, but are also likely distorted by an exceeding number of patients who die in late stages of the disease. A recent study examined mRNA levels of cytokines and mediators of the immune response PD patients across stages and found increased expression of IL-6 signal transducer in the SN of patients in stage 1-2 while at later stages of the disease the same marker was downregulated (Garcia-Esparcia et al., 2014). This observation further reveals the complex responses dependent on the stage of PD-related pathology; yet again the low number of samples collected from patients in early stage PD and the inability to corroborate their findings at the protein level limited this study. Moreover, long-term L-DOPA therapy or antidepressant therapy often administered to patients in late disease stage, may affect immune responses in opposite directions, adding a factor of complication to human studies (Barnum et al., 2008; Baumeister et al., 2015; Lindqvist et al., 2013). Unfortunately, these limitations restrict our ability to fully understand the state of microglia during progressive neurodegeneration and α-synuclein deposition. Future evaluation of microglial gene expression in post-mortem brains of PD patients who died at different stages of disease may aid investigators to better define microglial phenotypes. It is becoming increasingly clear that in many diseases, microglia largely express complex gene expression signatures (Butovsky et al., 2014; Moore et al., 2015; Pisanu et al., 2014; Tang and Le, 2015), and mixed phenotypes most likely reflect a dynamic state or may be simply due to the presence of a heterogeneous population of microglia during disease. Until human studies can be conducted to evaluate the neuroinflammatory interaction with neurodegeneration and other histopathological hallmarks like Lewy Body formation, conclusions about these relationships will have to be drawn from animal models of the disease.
In summary, it remains to be established whether the activation of inflammatory responses and reactive microglia could play a beneficial role at some stage during the progression of PD in humans. As described above, human studies have reported an elevation of both pro- and anti-inflammatory cytokines in the serum and CSF of PD patients, suggesting that pro- and anti-inflammatory microglia may coexist in the PD brain and complex changes in microglia phenotypes are likely to maintain and exacerbate PD neuropathology (Brodacki et al., 2008; Mogi et al., 1995; Mogi et al., 1994a; Mogi et al., 1996; Rentzos et al., 2009). Noteworthy, in Alzheimer's disease and in the elderly brain an imbalance of pro- and anti-inflammatory microglia phenotypes have been reported (Hoozemans et al., 2006; Jimenez et al., 2008). Therefore, it is reasonable to speculate that there is an array of activated microglia that coalesce in the PD brain and potentially drive disease progression via cytokine release (Tansey and Goldberg, 2010). Improvements in single-cell phenotyping and development of PET ligands for neuroimaging that can distinguish specific phenotypes among the vast array of heterogeneous microglia profiles will be needed to resolve these unanswered questions.
3. Microgliosis in models of PD-like degeneration
It is generally believed that stress signals produced by damaged neurons or aggregated misfolded proteins released by dead neurons activate microglia, which in turn lead to secondary degeneration, being responsible for the massive neurodegeneration in PD. Animal models have added pivotal insights to the understanding of microglia involvement in PD neuropathology. Neurotoxins such as MPTP and 6-hydroxydopamine (6-OHDA) induce different degrees of microgliosis in lesioned areas. Moreover, a number of pro-inflammatory stimuli have been used to activate microglia in order to mediate DA neuron toxicity including lipopolysaccharide (LPS), α-synuclein, and inflammatory cytokines. While all models induce some degree of activated microglia surrounding damaged DA neurons, they display differences in the time-course of microglia activation in response to the toxic stimulus. Moreover, different results have been reported on the sequence of toxic events in DA areas, where microglia activation may either precede neuronal damage or be a secondary event, depending on the animal model investigated (Frank-Cannon et al., 2009; Ramsey and Tansey, 2014). Differences mostly relate to the species used and to the primary trigger of degeneration, that is whether a neurotoxin or an inflammatory insult is delivered. Moreover, the protocol chosen to administer the neurotoxin, either an acute or chronic protocol, thereby determining the time-course of the degenerative process, will also influence microgliosis progression. Since microglia may display different phenotypes and perform specific effector functions depending both on the type and strength of the stimulus, a careful evaluation of the choice of animal model is pivotal in the experimental design so that it is suitable for the purpose of the study.
3.1 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) models
Monkey studies have consistently reported HLA-DR-stained reactive microglia in the SN of MPTP-injected monkeys years after neurotoxin injection, suggesting that an ongoing and long-lasting microgliosis was triggered and persisted in the absence of MPTP (Barcia et al., 2004; Hurley et al., 2003; McGeer et al., 2003; Vazquez-Claverie et al., 2009). Moreover, a similar extent of microgliosis was reported after subacute or chronic MPTP administration, which was independent from the extent of neuronal loss in the SN (Vazquez-Claverie et al., 2009). In rodents, the majority of studies aimed at investigate microgliosis in PD have been performed in acute models of MPTP toxicity. Pioneer studies performed in mice administered acute doses of MPTP reported early and transient presence of reactive microglia in the SN, which preceded neuronal loss and disappeared within a few weeks after toxin exposure (Czlonkowska et al., 1996; Kohutnicka et al., 1998; Kurkowska-Jastrzebska et al., 1999; Liberatore et al., 1999; Smeyne and Jackson-Lewis, 2005; Vazquez-Claverie et al., 2009). More recent studies have investigated reactive microglia in chronic regimens of MPTP administration, which more closely reproduces the progressive nature of neurodegeneration and symptom development observed in PD neuropathology. These models show an early time course of microglia activation in the SN that is detectable in the absence of nigral cell loss in the premotor phase of the neuropathology when non-motor symptoms such as olfactory dysfunction have been observed (Rodriguez et al., 2007; Schintu et al., 2009). Along with the chronic administration regimen, reactive microglia proliferate as the DA degeneration progresses, and persist at least six months after discontinuation of MPTP. Moreover, in presence of advanced nigral degeneration, microglial cells are closely associated with apoptotic cells, suggesting an active role in the scavenging of dying neurons (Meredith, 2005).
3.2 6-Hydroxydopamine (6-OHDA) models
6-OHDA injections induce strong microglial activation in the SN or the striatum; yet microglia activation has been described as an early, simultaneous or delayed event with respect to DA degeneration depending on the site of 6-OHDA injection (Marinova-Mutafchieva et al., 2009; Sanchez-Pernaute et al., 2004; Walsh et al., 2011). After intrastriatal 6-OHDA infusion, reactive microglia were detectable histologically in the striatum days after terminal degeneration (Walsh et al., 2011), while microgliosis in the SN was absent or elicited subsequently to DA degeneration (Depino et al., 2003; Henry et al., 2009; Walsh et al., 2011). In contrast, microgliosis preceding DA degeneration was detected in both the striatum and SN of rats intrastriatally treated with 6-OHDA (Armentero et al., 2006). Interestingly, when the neurotoxin was infused directly into axons, microgliosis preceded striatal terminal degeneration and occurred together with nigral degeneration (Walsh et al., 2011). Evaluation of 3H-PK11195 autoradiography from rats administered unilateral intrastriatal 6-OHDA showed early strong microgliosis in the striatum starting 3 days after toxin delivery, but delayed and progressive increases in radiotracer density in the SN, synchronous with the timeline of degeneration (Maia et al., 2012). Overall, studies performed in toxin-based models support early and enduring microglia activation in the course of DA neuronal loss.
3.3 Lipopolysaccharide (LPS) models
The bacterial endotoxin LPS administered either intranigrally or peripherally, induces a potent activation of microglia and secretion of pro-inflammatory factors which precede the degeneration of the nigrostriatal DA pathway, and is therefore used to investigate direct neuroinflammation-induced dopamine loss (Castano et al., 1998; Dutta et al., 2008). Depending upon the dose of LPS administration, the resulting DA neurodegeneration has been described as rapid or delayed and progressive. Acute LPS intranigral infusion in C57BL/6 mice or rats induced rapid activation of microglial cells and degeneration of dopamine neurons in the SN within 7 days, which remained stable up to one year (Arai et al., 2004; Herrera et al., 2000). Subacute or chronic infusion of low-dose LPS into rodent brain triggered a rapid and persistent activation of microglia, followed by a delayed and gradual loss of nigral DA neurons that began at 3-4 weeks, reaching 70% of neuronal loss by 10 weeks (Gao et al., 2002; McCoy et al., 2006; Tanaka et al., 2013). When LPS was administered as a single intraperitoneal injection, it caused rapid microglia activation and increased brain TNF, that remained elevated for 10 months followed by delayed and progressive loss of nigral DA neurons starting at 7 months after LPS treatment that reached 47% loss at 10 months (Qin et al., 2007). Therefore, data from the LPS models indicate that neuroinflammation may be both an initiating stimulus and a self-propelling mechanism of progressive neuron degeneration.
3.4 α-synuclein models
A variety of α-synuclein-based models have been developed in an attempt to replicate the α-synucleinopathy observed in PD patients. These models include overexpression of wild type human α-synuclein or mutant A53T human α-synuclein, and transgenic mice overexpressing truncated human α-synuclein in DA neurons, as extensively reviewed (Dawson et al., 2010; Giasson et al., 2002; Magen and Chesselet, 2010; Masliah et al., 2000; Wakamatsu et al., 2008). Although none of these models replicate the main hallmarks of PD, such as progressive and severe loss of DA neurons and motor deficits, they have greatly contributed to the understanding of α-synuclein-induced toxicity. Alternative models include mice overexpressing human wild type α-synuclein under the Thy-1 promoter (Chesselet et al., 2012) or the tyrosine hydroxylase promoter (Su et al., 2008), and viral vector driven α-synuclein overexpression in rodents (Decressac et al., 2012; Lo Bianco et al., 2002; Sanchez-Guajardo et al., 2010; Van der Perren et al., 2015; Yamada et al., 2004) and primates (Eslamboli et al., 2007). Viral vector-based models show a progressive degeneration of nigrostriatal neurons that is associated with gradual motor impairment (Decressac et al., 2012; Van der Perren et al., 2015). Independent of the extent of nigrostriatal degeneration, α-synuclein models offer the opportunity to study pathologic mechanisms specific to early disease stage, and to elucidate mechanisms of cellular dysfunction which precede cell death (Chesselet et al., 2012), including inflammatory responses and the relationship between α-synuclein accumulation and subsequent inflammation (Allen Reish and Standaert, 2015; Gao et al., 2008; Gao et al., 2011a). In Thy-1 α-synuclein mice an early and progressive increase of activated microglia and inflammatory cytokine TNF was specifically observed in striatum and SN that preceded dopamine fiber damage (Watson et al., 2012). Similarly, one-month old TH α-synuclein mice displayed an increase of Iba1-positive ameboid shaped microglia in the SN and increased expression of TNF in both the SN and striatum (Su et al., 2008). As mentioned above, different forms of wild type or mutated α-synuclein have been overexpressed in the rodent brain, which were shown to affect microglia activation in different ways. LPS induced a persistent microgliosis, production of inflammatory molecules, and a progressive degeneration of nigrostriatal neurons when administered to transgenic mice overexpressing human A53T mutant α-synuclein, but not in wild type α-synuclein mice, suggesting that microglia bridge neuroinflammation and α-synuclein-induced cellular damage in mediating chronic degeneration in PD (Gao et al., 2011a). Moreover, studies in α-synuclein overexpressing mice and primates have been pivotal to demonstrate that levels of α-synuclein-induced neuronal pathology, either in presence or absence of DA degeneration, strictly modulate the phenotype of activated microglia (Barkholt et al., 2012; Sanchez-Guajardo et al., 2010).
3.5 LRRK2 models
Cells or mice bearing a genetic mutation of the leucine-rich repeat kinase 2 (LRRK2) gene have provided direct evidence of the causal relationship between neuroinflammation and DA neuron degeneration in late-onset PD. LRRK2 expression is upregulated in activated microglia under pathological conditions, whereas LRRK2 ablation or inhibition prevents a full inflammatory response, resulting in attenuated inflammatory cytokine production following LPS, reduced nuclear factor (NF)-κB transcriptional activity, and reduced microglial morphological activation, chemotactic and phagocytic activity (Kim et al., 2012; Marker et al., 2012; Moehle et al., 2012). Missense mutations in LRRK2 are the most common cause of both genetic and idiopathic PD where disease penetrance is variable (Healy et al., 2008). The G2019S LRRK2 mutation is the most frequent mutation responsible for dominantly inherited PD, and is associated with increased kinase activity. PD patients with the G2019S LRRK2 mutation present clinical and pathological phenotypes nearly indistinguishable from idiopathic disease (Paisan-Ruiz et al., 2004; West et al., 2005; Zimprich et al., 2004). Remarkably, LRRK2 KO rats were resistant to DA degeneration when challenged by either intracranial LPS or α-synuclein overexpression, while LRRK2 overexpression accelerated the progression of neuropathology in A53T α-synuclein transgenic mice (Daher et al., 2014; Lin et al., 2009). Of note, one study reported an age-dependent partial loss of nigral neurons and damage to the nigrostriatal system in mice carrying the G2019S LRRK2 mutation (Ramonet et al., 2011). Overall, results from transgenic LRRK2 mutant mice suggest that there is another contributing factor in the brain environment leading to the progressive neurodegeneration in PD. Therefore, inhibition of LRRK2 protects from DA cell loss by preventing the recruitment of activated microglia and potentially infiltrating myeloid cells, while LRRK2 overexpression causes neuronal damage, placing the neuroinflammatory response center stage among causal factors of neurodegeneration in this PD model.
Recently, mice carrying null mutation for the c-Rel gene, a regulatory subunit of the transcriptional regulator of inflammation NFκB, have been characterized and suggested to be a useful model of progressive inflammation-induced nigral degeneration (Baiguera et al., 2012). c-Rel KO mice develop an age-dependent PD-like progressive neurodegeneration and accumulation of aggregated α-synuclein with activation of CD11b-positive microglial cells in the SN, highlighting NFκB as a potential downstream target for the regulation of microglial activation.
4. Microglia activation states in animal models of PD-like degeneration
As described above, PET imaging studies in human subjects have revealed that the extent of microglia activation does not increase as the clinical features of the disease progress (Gerhard et al., 2006). In line with this evidence, the extent of reactive microglia in monkeys was similar after a subacute or a chronic MPTP regimen and appeared independent from the lesion extent in the SN (Hurley et al., 2003; Vazquez-Claverie et al., 2009). These findings suggest that progressive degeneration of DA neurons and terminals may not be due to progressively increasing microglial proliferation, but leave open the possibility that changes in the microglia phenotype occur along with disease progression that confer to them a permanent and increasing neurotoxicity for DA neurons that cannot be appreciated using the current PET imaging methodologies.
4.1 Pro-inflammatory microglia
In an attempt to explore changes in microglia phenotype in PD and their time-course, a number of studies have investigated the expression of surface immune molecules in microglial cells in animal models. These pioneer studies have revealed that the time-course of expression displayed by these molecules does not necessarily reflect the time-course of microglia activation during disease progression, suggesting that dynamic changes in the phenotype of activated microglia are occurring in experimental models of PD. Following an acute MPTP protocol in mice, an early upregulation of MHC-I and ICAM-I were found in reactive microglia on days 1-7 post treatment, while MHC-II was transiently upregulated on days 7-14, then became undetectable on day 21 and up to 6 months after the lesion; these findings provided the first suggestion of dynamic changes in microglia effector function in an animal model of PD (Kurkowska-Jastrzebska et al., 1999; Yasuda et al., 2007). Primary murine ventral midbrain cultures exposed to media with elevated IFN-γ from microglia stimulated with LPS, α-synuclein, or A53T mutated α-synuclein had significantly heightened proportions of MHC-I expression compared to the same cultures either plated with control media from microglia that were not stimulated or exposed directly to these stimuli, or compared to primary cortical and striatal cultures exposed to the same stimulated media. Furthermore, induction of MHC-I on murine catecholamine neurons by factors (IFN-γ) released from stimulated microglia triggered extensive neuronal death, suggesting that stimulated microglia can selectively promote MHC-I presentation in SN catecholamine neurons (Cebrian et al., 2014). In mice intravenously injected with peripheral bone-marrow-derived cells, chronic MPTP induced an early microglia proliferation greatest in areas of rich DA innervation such as the anterior, medial and posterior striatum, which began to express the marker of phagocytosis CD68 only at later stages of degeneration (Rodriguez et al., 2007). Following 6-OHDA injection into the medial forebrain bundle (MFB), an increase in OX-42 and MHC-II immunoreactivity was detected as early as day 1 post-lesion, while levels of CD68 started to increase after 3 days, reaching maximal levels 9-13 days post-lesion (Marinova-Mutafchieva et al., 2009). Interestingly, upregulation of CD68 was coincident with dopaminergic degeneration in a microglia subpopulation located near dopaminergic neurons (Kurkowska-Jastrzebska et al., 1999). Therefore, phagocytic mechanisms may be responsible for microglia-mediated secondary neuronal degeneration, being mostly upregulated in presence of intense tissue destruction, which is in line with human findings (Cebrian et al., 2014; Croisier et al., 2005). In support of this concept, similar results were reported after medial forebrain bundle (MFB) transection, where early microglia activation was followed by a delayed upregulation of CD68 that occurred together with DA degeneration (Cho et al., 2006). Although interpretation of data obtained from toxin-based models is undermined by the diverse induction of microglia activation depending on the model and protocol used (as discussed above), overall these studies strongly suggest that microglia phenotypes are modulated in a manner that depends on the degree of DA damage/degeneration in their microenvironment, perhaps first characterized by an antigen-presentation effector function, then followed by the induction of a heightened pro-inflammatory phenotype, followed by phagocytic phenotype in the more advanced stages of degeneration.
Studies in rats and primates overexpressing viral vector driven human α-synuclein have provided convincing evidence that microglia acquire different phenotypes and display different effector functions at early versus late stages of neurodegeneration (Barkholt et al., 2012; Sanchez-Guajardo et al., 2010). In rats overexpressing viral driven human α-synuclein, microglia assumed different morphology and phenotype depending on the level of α-synuclein expression and the related degree of α-synuclein-induced DA degeneration (Sanchez-Guajardo et al., 2010). Upon exposure to levels of human α-synuclein that cause neuronal damage but not cell death perhaps modeling early disease stages, microglia displayed an antigen-presenting phenotype with increased MHC-II expression, in contrast to the more macrophage-like morphology with increased CD68 expression when exposed to human α-synuclein levels that induced DA degeneration (Sanchez-Guajardo et al., 2010). Similar results were reported in primates, where human α-synuclein overexpression induced a long-term microglia polarization (Barkholt et al., 2012). Importantly, in this study human A53T mutant α-synuclein overexpression induced neurodegeneration that resulted in nigral dopaminergic cell death, while wild type human α-synuclein overexpression induced by recombinant adeno-associated virus injections resulted in degeneration that was limited to DA fibers. In addition, although the extent of activated microglia was similar across primates that received α-synuclein compared to controls, microglia polarization into a macrophage-like morphology was more pronounced in the A53T over-expressing animals (Barkholt et al., 2012). The significance of this is unclear but highlights the complexity of the molecular interactions between different forms of α-synuclein and brain microglia that shapes microglial responses.
In addition to cell surface molecules, a number of studies have investigated soluble factors such as cytokines and chemokines, following administration of different neurodegeneration-inducing neurotoxins and inflammagens, highlighting their role in DA degeneration and revealing dynamic changes in the production of these molecules coincident with the neurodegenerative process. Genes involved in the inflammatory response including cytokines, chemokines and pro-inflammatory enzymes, contain in their promoter region the DNA binding site for the transcription factor NFκB, a master regulator of inflammatory responses (Hayden and Ghosh, 2004). In the SN of PD patients and of MPTP-intoxicated monkeys and mice, a robust expression of the p65 subunit of NFκB has been described in the nucleus and cytoplasm of glial cells (Ghosh et al., 2007; Hunot et al., 1997; Mondal et al., 2012). Accordingly, the concentration of a number of inflammatory cytokines is elevated in either CSF or affected brain regions in PD patients, and pro-inflammatory cytokines such as TNF and IFN-γ were found elevated years after neurotoxin injection in MPTP-treated monkeys (Barcia et al., 2011; Nagatsu et al., 2000). Emphasizing the cardinal role of inflammatory cytokines in DA degeneration, blocking of soluble TNF by intranigral administration of TNF dominant negative variants rescued DA neurons from intrastriatal 6-OHDA toxicity (Harms et al., 2011; McCoy et al., 2006; McCoy et al., 2008), while transgenic mice carrying homozygous mutant alleles for the TNF receptors or lacking TNF were completely protected against the DA neurotoxicity of MPTP, indicating that the pro-inflammatory cytokine TNF is an obligatory component of DA neurodegeneration (Ferger et al., 2004; Sriram et al., 2002). Moreover, overexpression of TNF in the SN through adenoviral vector was sufficient to cause nigral degeneration, while IL-1β overexpression exacerbated 6-OHDA induced nigral degeneration and microglia activation (De Lella Ezcurra et al., 2010; Pott Godoy et al., 2008). Interestingly, in TNF and IFN-γ KO mice, MPTP caused microglia activation, but cells displayed limited phenotypical changes, suggesting that these cytokines were necessary for full microglia activation (Barcia et al., 2011; Harms et al., 2012). An increase of mRNA and protein for inflammatory cytokines, such as IL-1β, TNF and IL-6, has been consistently reported after acute MPTP exposure together with an increase of inducible nitric oxide synthase (iNOS) levels, suggesting that reactive microglia acquired an early pro-inflammatory phenotype in this model (Bian et al., 2009; Liberatore et al., 1999; Lofrumento et al., 2011; Pattarini et al., 2007; Yasuda et al., 2008). Interestingly, such increase of inflammatory cytokines was followed by a delayed upregulation of the microglial receptor CX3CR1 for the chemokine fractalkine CX3CL, presumably as a compensatory mechanism to limit neuronal damage and decrease levels of CX3CL (Cardona et al., 2006; Pattarini et al., 2007). In the chronic MPTP model, progressive dopaminergic degeneration was associated with an early and persistent increase of TNF, which preceded neuronal degeneration, followed by a later rise of IL-1β (Luchtman et al., 2009; Pisanu et al., 2014). In the rat intrastriatal 6-OHDA model, reactive microglia was observed early after toxin infusion, while an increase of IL-1β mRNA, but not protein, was reported 30 days after infusion, suggesting a different temporal profile of microglia activation relative to cytokine production in this model (Depino et al., 2003; Luchtman et al., 2009; Pisanu et al., 2014). Interestingly, in non-human primates administered chronic low doses of MPTP, serum levels of IL-6, TNF-β, IL-16 or IL-8 did not fluctuate significantly in either asymptomatic or parkinsonian monkeys, but increases in TNF and IFN-γ were observed in monkeys displaying parkinsonism (Barcia et al., 2011).
In summary, studies in animal models of PD suggest that overproduction of inflammatory cytokines is an early and persistent event that is associated with microglia activation from the early stages of injury, preceding and likely driving microglia polarization towards a phagocytic, CD68-expressing phenotype present in later stages of neurodegeneration. Increased phagocytic function clearly is not a detrimental feature per se, however the upregulation of phagocytosis in microglial cells which have mostly assumed a pro-inflammatory phenotype in earlier stages of the disease is likely to contribute to progressive neuronal loss.
4.2 Anti-inflammatory microglia
Despite evidence in humans, the expression of anti-inflammatory molecules has been poorly described in experimental models of PD. As a consequence, a pivotal yet unresolved question is whether microglia neurotoxicity is driven by the switch from a beneficial neuroprotective phenotype to one in which microglia are chronically activated to secrete pro-inflammatory cytokines that enhance apoptotic cell death cascades in neurons. Chronic MPTP treatment in mice has been reported to increase pro- and decrease anti-inflammatory markers in microglia, creating a ratio of inflammatory mediators that may perpetuate and accelerate the course of disease (Pisanu et al., 2014; Rojo et al., 2010). Specifically, expression of pro-inflammatory cytokines as well as COX-2 and iNOS was increased in parkinsonian mice, while expression of anti-inflammatory cytokines such as IL-4, and surface markers including CD206, YM-1, Arg-1 and FIZZ-1 were decreased in the SN, suggesting that the toxin-induced degeneration of DA neurons was associated with a skewed microglia polarization and prevalence of pro-inflammatory microglia (Pisanu et al., 2014; Rojo et al., 2010). Moreover, while upregulation of pro-inflammatory cytokines was early and persistent during the chronic MPTP treatment in mice, downregulation of anti-inflammatory cytokines and CD206 was delayed and associated with appearance of motor symptoms and extensive degeneration of the SN (Pisanu et al., 2014). Therefore, while both pro- and anti-inflammatory phenotypes of microglia co-existed in the early phase of the disease, this balance slowly gave way to a prevalence of pro-inflammatory microglia in the late stages of degeneration.
Additional insights come from LRRK2 transgenic mice. Primary microglia from mice overexpressing the PD-linked LRRK2 R1441G mutation exhibited increased expression and secretion of inflammatory cytokines (TNF, IL-1β, IL-12) upon LPS stimulation compared to wild type microglia, while production of IL-10 was decreased. In addition, reduced expression of the pro-inflammatory marker iNOS was detected in CD68-positive microglia in LRRK2 KO mice compared to wild type mice. Moreover, conditioned medium from LPS-stimulated microglia from LRRK2 R1441G transgenic mice induced significant cell death when added to neuronal cultures, suggesting that enhanced neuroinflammation and a prevalent pro-inflammatory phenotype may be a key mechanism underlying neuroinflammation in LRRK2-associated PD (Gillardon et al., 2012). Although molecular mechanisms regulating the skewed microglia polarization in PD are currently under investigation, they remain largely unknown.
4.3 Role and regulation of NFκB in microglia activation states
Recent studies have highlighted a role for aberrant NFκB activation in PD pathogenesis, suggesting that enhanced NFκB activation may sustain the activated state of pro-inflammatory microglia. Persistent NFκB activation in both neurons and glia has been associated with progressive neurodegeneration in PD (Ghosh et al., 2007; Glass et al., 2010; Hunot et al., 1997; Mogi et al., 2007), while inhibition of the NFκB pathway has been shown to confer neuroprotection in animal models of PD by dampening inflammatory responses (Flood et al., 2011; Ghosh et al., 2007; Tran et al., 2008). Conversely, NFκB activity in neurons may promote neuronal survival (Mattson et al., 1997), suggesting that the source of NFκB may dictate its overall function, yet this distinction is still not clear.
NFκB is expressed in neurons and glia but its role has been better characterized in glia where it regulates the expression of inflammatory cytokines but also growth factors and effector enzymes in response to activation of many receptors involved in immunity (Grinberg-Bleyer et al., 2015; O'Neill and Kaltschmidt, 1997; Panet et al., 2001; Tansey and Goldberg, 2010). Therefore, NFκB plays a crucial role in maintaining cellular homeostasis, and the activation of NFκB signaling is tightly controlled (Grinberg-Bleyer et al., 2015; Ruland, 2011). NFκB is normally inactive in the cytoplasm in a complex with IκB family proteins. External stimuli lead to the activation of IκB kinases (IKK) complex, which then phosphorylates IκB allowing its subsequent degradation in the proteasome, and releasing NFκB, which translocates to the nucleus where it binds to specific sequences in the promoter or enhancer regions of target genes (Baldwin, 2001; Hayden and Ghosh, 2004). Relevant to PD pathogenesis, NFκB signaling may take place through either the classical or alternative pathway (Bonizzi and Karin, 2004). In the classical pathway, binding of activating molecules including TNF or LPS to their respective receptors causes the activation of the IKKβ subunit (also called NEMO), leading to translocation of the NFκB dimer p65/p50 or p65/c-Rel to the nucleus, where they transcriptionally activate pro-inflammatory genes (Grinberg-Bleyer et al., 2015). In the non-canonical pathway, binding of NFB inducers such as LTβ, BAFF and CD40 to their receptors causes the activation of the IKKα subunit, which lead to phosphorylation of the precursor IkB protein p100 and its proteasomal processing into p52, which translocates to the nucleus upon dimerization with RelB, where it activates growth and survival genes that may be of particular importance in neurons during inflammatory stress.
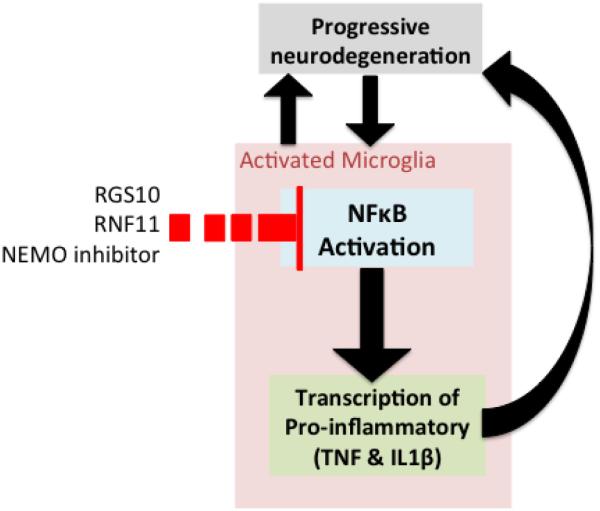
In recent years, the NFκB activation pathway has been investigated in detail in relation to PD pathogenesis. One hypothesis that has emerged is that in PD a skewed activation of the canonical NFκB pathway may dominate over a more balanced stimulation of both the canonical and non-canonical pathways, leading to microglia polarization and DA degeneration. Of note, NFκB has been recognized as a key pathway in α-synuclein dependent activation of phagocytic microglia and increased production of inflammatory cytokines (Fellner et al., 2013). We will further discuss evidence for the protective effect of NFκB key regulators in animal models of PD including regulator of G-protein signaling 10 (RGS10), RING finger protein 11 (RNF11), and NEMO (Figure 2).
Figure 2. Microglial NFκΒ regulation of immune responses in animal models of PD.
Malfunctions at multiple steps of NFκB signaling have been argued to have a causal interrelationship with pathological microglia leading to increased pro-inflammatory gene expression and further contributing to neurodegeneration. Regulator of G-protein signaling 10 (RGS10), RING finger protein 11 (RNF11), and NEMO inhibitor exert negative regulation on NFκΒ signaling producing a dampened immune response and reduced neurodegeneration.
4.3.1 Regulator of G-protein Signaling-10 (RGS10)
Recently RGS10, a microglia-enriched GTPase activating protein (GAP), has been recognized as a key negative regulator of NFκB activity and microglia/macrophage polarization in inflammatory conditions (Fellner et al., 2013; Lee et al., 2013; Lee et al., 2011; Lee et al., 2008). RGS10-null mice display increased microglial burden, while RGS10-null microglia/macrophages treated with LPS display a pro-inflammatory phenotype, with increased production of inflammatory cytokines over anti-inflammatory phenotype markers as compared to wild type cells, and enhanced cytotoxicity on DA neurons (Lee et al., 2013; Lee et al., 2011). Interestingly, RGS10-null microglia showed higher levels of p50/p65 NFkB subunits both in resting and TNF-stimulated conditions, suggesting a dysregulated NFκB activation, with canonical pathway prevailing over the alternative pathway (Lee et al., 2011). Moreover, in vivo gene transfer of RGS10 into the rat SN reduced microgliosis and protected against 6-OHDA neurotoxin-induced death of DA neurons, while RGS10-null macrophages exerted higher cytotoxicity on DA MN9D neuroblastoma cells, suggesting a link between NFκB dysregulation, prevalence of pro-inflammatory microglia and nigral degeneration (Lee et al., 2013; Lee et al., 2011).
4.3.2 Ring Finger Protein 11 (RNF11)
As mentioned above, NFκB is expressed in glia as well as neurons, where it can either mediate inflammation-induced toxicity or promote the expression of survival genes. RNF11, in association with A20 ubiquitin-editing complex, is a key negative regulators of NFκB signaling pathway, whose role has been demonstrated in neurons and more recently in microglia (Dalal et al., 2012; Pranski et al., 2013). In microglia RNF11 expression levels were inversely correlated to NFκB activation, in that RNF11 silencing increased NFκB activation, inflammatory molecules production, and inflammation-mediated cytotoxicity, whereas RNF11 overexpression conferred protection against LPS-induced cell cytotoxicity through an inhibition of the inflammatory response, suggesting that RNF11 may be a potential target for modulating inflammatory responses in neurodegenerative diseases (Dalal et al., 2012). Similar results were observed in TNF-stimulated neurons in culture, where RNF11 silencing resulted in enhanced p65RelA nuclear translocation and heightened inflammatory responses (Pranski et al., 2013). Interestingly, targeted knockdown of RNF11 in nigral DA neurons of 6-OHDA-induced hemiparkinsonian rats, was associated with increased NFκB activity and promoted DA cell survival, while RNF11 overexpression inhibited NFκB activity and increased dopaminergic cell loss (Pranski et al., 2013). Of note, RNF11 suppression was associated with enhanced expression of antioxidants GSS and SOD1, neurotrophic factor BDNF and the anti-apoptotic factor Bcl2, whose transcription is believed to rely on non-canonical NFκB pathway activation. Overall these studies highlight the dual role of NFκB in neuroprotection, and emphasize the importance of tight regulation of NFκB pathway activation within the context of neuroinflammation during neurodegenerative conditions.
4.3.3 NFκB essential modulator (NEMO)
The selective NEMO inhibitor compound A is a small molecule that specifically blocks the catalytic activity of IKKβ, and therefore the activation of the p50/p65 NFκB heterodimer (May et al., 2000). Compound A inhibited the production of pro-inflammatory factors by LPS-activated microglia and p65 nuclear translocation, and protected DA neurons from LPS-induced degeneration in vivo (Zhang et al., 2010). Accordingly, another IKK-targeted molecule, the peptide corresponding to the IKK NEMO-binding domain (NBD), reduced nigral activation of NFκB, suppressed microglial activation in the SN, protected nigrostriatal neurons, and improved motor performance in MPTP-treated mice and monkeys, while suppressing inflammatory molecule production (Ghosh et al., 2007; Mondal et al., 2012).
Taken together, these pre-clinical studies strongly suggest that selective targeting of the canonical NFκB activation pathway may afford protection against neurodegeneration in PD (Ghosh et al., 2007; Zhang et al., 2010). More specifically, the identification of selective inhibitors of the canonical pathway, which may target specific components like IKKβ and IKKγ but not molecules belonging to the non-canonical pathway like IKKα or the P100/p52, may represent therapeutic agents in the treatment of chronic inflammation-induced neurotoxicity in PD.
5. Clinical studies to target microglia activation with immune-modulating drugs
It is reasonable to speculate that different phenotypes of activated microglia co-exist in the PD brain, given that functionally distinct microglia have been reported in Alzheimer's disease and in the aging brain (Hoozemans et al., 2006; Jimenez et al., 2008). Therefore, it is possible that the dynamic interactions between microglia and other pathogenic components drive PD progression via excessive cytokine release (Tansey and Goldberg, 2010). For these reasons, microglia polarization has been proposed to be a reasonable target for neuroprotective therapies by several researchers (Sanchez-Guajardo et al., 2013; Tansey and Goldberg, 2010). The recognition that pro-inflammatory microglia are likely to play a critical role in responses that hasten neuronal death has prompted great interest in testing anti-inflammatory drugs as disease-modifying agents in PD. Non-steroidal anti-inflammatory drugs (NSAIDs) have provided positive results in preclinical models; however epidemiological studies have yielded conflicting results on the extent to which chronic intake of these drugs reduce the risk of PD (Bower et al., 2006; Chen et al., 2005; Esposito et al., 2007; Gao et al., 2011b; Noyce et al., 2012). More recent understanding of the complex immune-regulatory function of microglia in PD, and the recognition of phenotypes with diverse effector functions in the course of this pathology, have suggested that strategies aimed at modulating rather than inhibiting microglia activation may represent a promising therapeutic approach in the treatment of PD (Tansey and Goldberg, 2010).
5.1. Peroxisome proliferator receptor (PPAR) gamma (PPAR-γ)
PPAR-γ is a member of the nuclear hormone receptor superfamily. This receptor was first characterized in adipose tissue as a key regulator of lipid and glucose metabolism through modulation of gene expression. Endogenous ligands include oxidized fatty acids, unsaturated fatty acids, 15-deoxy-Δ-prostaglandin J2 (15d-PGJ2). Synthetic ligands are some non-steroidal anti-inflammatory drugs such as ibuprofen, fenoprofen, indomethacin, and thiazolidinediones (TZDs) including pioglitazone and rosiglitazone, that are FDA approved drugs for the treatment of type II diabetes mellitus (T2DM) (Carta, 2013). More recently, the main role of PPAR-γ in the regulation of immune responses and neuroinflammation, has gained increasing attention and led to the investigation of synthetic agonists as a therapeutic against pathologies with a neuroinflammatory component, such as neurodegenerative diseases. As a result, preclinical studies have consistently reported the neuroprotective efficacy of PPAR-γ agonists in different PD models in rodents as well as in primates (Carta et al., 2011; Dehmer et al., 2004; Schintu et al., 2009; Swanson et al., 2011). A phase II clinical trial with the agonist pioglitazone, tested as a disease modifying agent in early PD, has failed to confirm preclinical results (NCT01280123). However, several issues mostly related to the PK profile of this drug, including the scarce BBB permeability, leave uncertain whether the drug sufficiently reached the target, suggesting the need for further clinical evaluation of PPAR-γ agonists in PD (Carta and Simuni, 2015; NETPD-Investigators, 2014).
PPAR-γ is highly expressed in peripheral immune cells, including macrophages, lymphocytes, and dendritic cells, as well as in immune cells in the brain. Besides microglia, astrocytes, oligodendrocytes and neurons also express PPAR-γ (Bernardo et al., 2000; Varga et al., 2011). Gene expression is regulated by PPAR-γ through mechanisms involving transactivation, where the PPAR-γ/retinoid × receptor heterodimer binds to the PPAR-responsive elements (PPREs) in the promoter region of target genes (Ricote and Glass, 2007). Alternatively, inhibition of gene expression may occur via transrepression mechanisms, that are independent from DNA-binding, involving physical sequestration of activated transcriptional factors, including NFkB through a direct interaction with the p65 subunit (Ricote and Glass, 2007). Relevant to the present discussion, modulation of target genes involved in the inflammatory response includes: suppression of pro-inflammatory cytokines and induction of anti-inflammatory molecules, upregulation of antioxidant enzymes, upregulation of scavenger receptors such as CD36 (Ballesteros et al., 2014; Carta, 2013; Martin et al., 2012; Reddy et al., 2008). Targeting PPAR-γ is known to modulate the phenotype of peripheral macrophages via the above cited mechanisms. Hence, a number of studies have shown that PPAR-γ modulates the production of both pro- and anti-inflammatory cytokines by these cells, boosting the anti-inflammatory benefit while suppressing the harmful macrophage phenotype (Odegaard et al., 2007; Pascual et al., 2007; Satoh et al., 2010; Straus and Glass, 2007; Szanto et al., 2010). In contrast, in infected peripheral macrophages the natural PPAR-γ ligand 15dPGJ2 increased both anti-inflammatory polarization and phagocytic activity of these cells (Penas et al, 2013). We have recently shown in a chronic MPTP mouse model of PD, that PPAR-γ agonists administered in a neuroprotective regimen, hampered pro-inflammatory microglia while boosting an anti-inflammatory phenotype, suggesting that the immunomodulatory properties may contribute to the disease modifying potential of PPAR-γ agonists in PD (Pisanu et al., 2014).
5.2 Glucagon-like peptide-1 (GLP-1)
GLP-1 is a target for neuroprotection and has elicited much interest in recent years (Aviles-Olmos et al., 2013; Holscher, 2014). GLP-1 is an incretin hormone which facilitates insulin release in hyperglycemic episodes and overcome insulin desensitization, and GLP-1 mimetics are a successful strategy to treat T2DM (Holscher, 2014). Specifically, the GLP-1 synthetic agonist exenatide has shown neuroprotective properties in multiple animal models of PD (Bertilsson et al., 2008; Harkavyi et al., 2008; Kim et al., 2009; Li et al., 2009; Rampersaud et al., 2012). Exenatide has been recently evaluated in a proof of concept study using a single-blind trial design, where 45 patients with moderate PD were monitored for disease progression, and randomly assigned to receive subcutaneous exenatide for 12 months or to act as controls. In this pilot study, exenatide group suggested clinically relevant improvements across motor and cognitive measures as compared with the control group (NCT01174810) (Aviles-Olmos et al., 2013; Foltynie and Aviles-Olmos, 2014). The mechanism underlying GLP-1-mediated neuroprotection is still a matter of controversy. As a growth factor, GLP-1 neuroprotective effects may be partly due to classic growth factor effects such as increased expression of genes involved in cell growth and repair, increase of cell metabolism, and inhibition of apoptosis (Holscher, 2014). In addition, accumulating evidence indicates that GLP-1 mimetics display anti-inflammatory properties, which may largely support the neuroprotective action of these compounds, by suppressing the expression of inflammatory molecules as exemplified by the inhibition of IL-1β production in LPS-stimulated astrocytes treated with GLP-1 (Iwai et al., 2006). In mouse macrophages, exenatide treatment suppressed LPS-induced mRNA expression of TNF-α, monocyte chemoattractant protein-1, and nuclear translocation of p65, while in human monocytes it reduced the expression of CD11b (Arakawa et al., 2010). In MPTP-intoxicated mice, exenatide prevented the MPTP induced microglia activation and expression of TNF-α, IL-1β and MMP-3 mRNA in the SN (Kim et al., 2009). A recent study showed that increase of GLP-1 levels obtained by the delivery of dipeptidyl peptidase (DPP)-4 inhibitor saxagliptin, resulted in neuroprotection in the rotenone rat model of dopaminergic degeneration, and decreased the rotenone-induced inflammatory response, by reducing NF-κΒ activation, iNOS, TNF-α, ICAM-1 and myeloperoxidase (Nassar et al., 2015).
5.3 Minocycline
Minocycline is a tetracycline derivative used for treating a variety of infections. Besides antimicrobial effects, minocycline was shown to exert neuroprotective and anti-inflammatory activity in preclinical studies in the MPTP model of PD (Du et al., 2001; Thomas and Le, 2004; Wu et al., 2002). In vitro, minocycline inhibited MPP(+)-induced neurotoxicity and iNOS expression via suppression of p38 MAPK phosphorylation, but neuroprotection was afforded only in the presence of glial cells (Du et al., 2001). In MPTP-treated mice, minocycline inhibited MPTP-induced activation of microglia and production of IL-1β and iNOS in the SN (Wu et al., 2002). A Phase II futility trial in early untreated PD patients and an 18-month follow-up study indicated that minocycline could not be rejected as futile and did present safety concerns; it therefore received consideration for definitive Phase III trials in order to determine whether it had disease-modifying activity on the long term progression of PD (Investigators, 2006, 2008).
5.4 Cannabinoid receptor 2 (CB2)
Cannabinoids act as a major signaling regulator of various neurotransmitters and have anti-inflammatory properties (Giuffrida et al., 1999; Gomez-Galvez et al., 2015; Romero et al., 1996). The role of the cannabinoid system is thought to be dependent on which receptor is activated, CB1 or CB2. Earlier therapeutic studies focused on altering CB1 activity because of its high expression in the central nervous system, specifically in the basal ganglia, and its hypokinesia effect on levodopa induced motor complications (Fox et al., 2002; Morgese et al., 2007; Sieradzan et al., 2001). However, the therapeutic potential of CB1 antagonists is controversial due to inconsistent results including a clinical trial testing rimonabant, a CB1 antagonist, which did not demonstrate any antiparkinsonian effects on either the severity of motor deficits or levodopa-induced dyskinesias (Mesnage et al., 2004). Whereas, CB2 receptors are highly expressed on activated microglia and are upregulated in the SN of PD patients (Gomez-Galvez et al., 2015). Various studies have selectively blocked CB2 receptors to modulate the microglial activation state leading to neuroprotection in a mouse model of PD (Gomez-Galvez et al., 2015) and mice with mild traumatic brain injury (Reiner et al., 2015). Both studies demonstrate a reduction in pro-inflammatory mediators such as TNFα, IL-1 β, IL-6, IL-10 following CB2 antagonism in primary human microglial cells (Reiner et al., 2015) or intrastriatal LPS-treated mice (Gomez-Galvez et al., 2015). Although there are currently no clinical trials modulating CB2 activity, pre-clinical work demonstrates its great promise as a therapeutic target.
5.5 Granulocyte-macrophage colony-stimulating factor (GM-CSF)
GM-CSF is an immune modulator that has been safely used to reduce infections in immunosuppressed patients following chemotherapy by increasing myeloid progenitor proliferation, and more recently has shown to induce regulatory T cells (Tregs), which are dysfunctional in PD (Buchsel et al., 2002; Rowin et al., 2012; Sheng et al., 2011). GM-CSF treatment demonstrated neuroprotective properties in MPTP- (Kosloski et al., 2013) and paraquat-treated mice (Mangano et al., 2011), with increases in Tregs and downregulation of CD11b+ microglia, suggesting a role for GM-CSF to effect the pro-inflammatory nature of microglia. After the success of GM-CSF in animal models of PD, a clinical trial is currently recruiting patients to evaluate the safety of an engineered GM-CSF called Leukine (sargamostrim) in idiopathic PD (NCT01882010).
6. Concluding remarks
The idea that microglia may assume a spectrum of phenotypes and serve multiple effector functions has revolutionized the classical view of “activated microglia” as an oversimplified concept pointing to the need for a deeper characterization of their heterogeneous nature in relation with disease progression. In light of this novel view a growing area of research is now focusing on understanding whether activated microglia may exert a protective function at some stage of PD via production of neurotrophic factors, or contribute to neuronal death by producing inflammatory factors that are toxic to DA and other vulnerable neurons, and whether they influence typical pathological mechanisms such as α-synuclein aggregation and propagation. Yet is likely that the age-associated changes in inflammatory responses, along with genetic and environmental factors, give rise to a prevalence of pro-inflammatory microglia in PD, which likely contribute to progressive neuronal loss.
Studies in biofluids from PD patients have shown an overproduction and coexistence of both pro- and anti-inflammatory cytokines, yet understanding the time course and degree to which microglia produce these mediators may provide hints to therapeutic immunomodulatory targets and identify appropriate candidates for successful intervention. Moreover, human studies are limited by an exceeding number of patients who die in late stages of the disease, and by a therapeutic history which may have affected disease-related immune responses, undermining the possibility to understand the complex dynamic of microglia states in the different stages of PD. Post mortem studies have demonstrated the presence of activated and phagocytic cells in the vicinity of remaining nigral DA neurons, suggesting the contribution of microglia to the progression of neurodegeneration, yet leaving undetermined whether they could play a beneficial role at earlier stages of the neuropathology. While imaging studies have provided evidence of ongoing, persistent activation of microglia in PD by detection of TSPO ligand binding, techniques that would distinguish different phenotypes of activated microglia are needed in the future for a better understanding of microglia phenotype in the brain particularly during the early stages of PD.
Animal studies have permitted a closer and more specific look at the activation state of microglia in relation to the extent of neurodegeneration, and have given the possibility to follow dynamic changes in phenotype and function along with disease progression. According to the concept that microglia may display different phenotypes depending both on the type and strength of the stimulus, a comparative look at animal studies reveals that progression of microgliosis and microglia polarization is to some extent dependent upon the animal model and species, whether a toxin or inflammatory stimulus has been administered, and the regimen of administration. This peculiarity has offered the great possibility to dissect and manipulate the inflammatory process at different stages of degeneration. Overall, studies conducted in parkinsonian monkeys and toxin-based models support an early microglia activation in the course of DA neuronal loss, while inflammation-based models as well as LRRK2 transgenic mice have added the pivotal concept that neuroinflammation may be both an initiating stimulus and a self-propelling mechanism of progressive neuron degeneration. Studies based on α-synuclein overexpression have disclosed the relationship between the accumulation of different forms of α-synuclein and subsequent inflammation, and have been pivotal to understand that levels of α-synuclein-induced neuronal pathology strictly modulate the phenotype of activated microglia, inducing different phenotypes and effector functions at early versus late stages of neurodegeneration. A careful evaluation of cytokines content in the different PD models has attributed a specific pathological role to pro-inflammatory cytokines in disease progression, suggesting that an early and persistent overproduction of detrimental cytokines may precede microglia polarization towards the phagocytic phenotype expressing high levels of CD68, which characterize later stages of intense neurodegeneration, likely contributing to spreading of neuronal loss. On the other hand, a still scarce number of studies have described the expression and production of anti-inflammatory molecules from activated microglia. These studies have shown that markers typically associated with a beneficial phenotype were gradually decreased in the SN, and that the increased production of pro-inflammatory mediators was associated with motor impairment and massive cell death. All together animal studies lead to the suggestion that inflammation-driven DA neuron degeneration may be underpinned by a gradual process of skewed microglia polarization, with prevalence of pro-inflammatory microglia beginning early in the course of the disease, and further polarization to phagocytic phenotype which sustains massive cell destruction and its progression toward late end-stages. Further studies in this direction will be needed to fully address the still unresolved question whether microglia neurotoxicity is driven by the switch from a beneficial neuroprotective phenotype to one in which microglia are chronically activated to secrete harmful pro-inflammatory cytokines that enhance apoptotic cell death cascades in neurons.
From a mechanistic perspective, enhanced NFκB activation may hold a role in pathologically activated state of pro-inflammatory microglia, given the evidence that inhibition of the NFκB pathway confer neuroprotection in animal models of PD by dampening inflammatory responses. Based on this assumption, the NFκB activation pathway has been profoundly dissected and investigated in relation to PD pathogenesis. The emerging hypothesis is that in PD a skewed activation of the canonical NFκB pathway may dominate over a more balanced stimulation of both the canonical and non-canonical pathways, leading to microglia polarization and DA degeneration. Several studies support this concept by showing that manipulation of specific molecules belonging to the canonical NFκB signaling including regulator of G-protein signaling 10 (RGS10), RING finger protein 11 (RNF11), and NEMO can drive protection or degeneration effects in animal models of PD, leading to the hope that selective regulators of the canonical NFκB pathway may represent therapeutic agents in the treatment of chronic inflammation-induced neurotoxicity in PD.
Based on advancing knowledge on the spectrum microglia phenotypes, chronic microglia activation is proposed as a reasonable target for neuroprotective therapies in PD. Testing of drugs with anti-inflammatory and/or immunomodulatory properties such PPAR-γ agonists, CB2 modulators, the GLP-1 synthetic agonist exenatide, subclinical dosage of antibiotic minocycline and the immunomodulator GM-CSF goes in this direction. All of these drugs have shown beneficial results in animal models of PD when administered in the early stages of degeneration and some are now being tested in clinical trials. Given that all evidence points to neuroinflammation being present in the early stages of the degenerative process, it will be critical to test the efficacy of these drugs in patient populations prior to significantly advanced disease in order to maximize chances for success in modifying the course of disease progression.
Highlights.
microglia exist in a heterogenous population and their phenotypes are not permanently polarized into two categories
neuroinflammation and microglia activation are considered neuropathological hallmarks, however their precise role in relation to disease progression is not clear
animal models have provided insights on dynamic changes of microglia phenotypes in relation to disease progression especially prior to the development of motor deficits microglial phenotypes in the aging brain become further altered in PD giving rise to a prevalence of pro-inflammatory microglia, which likely contribute to progressive neuronal loss
malfunction at multiple steps of NFκB signaling that may have a causal interrelationship with pathological microglia activation in animal models of PD
Acknowledgments
We thank members of the Carta and Tansey labs for useful discussions. Funding for the authors of this review came from NIH/NINDS R01NS072467-05 (MGT), the Michael J. Fox Foundation for Parkinson’s Research (MGT), NIH/NIEHS 2T32ES012870-11 (VJ), The Perry & Ruby Stevens Foundation (ARC) and Fondazione Banco di Sardegna U629.2013/AI.553 MGB (ARC).
Abbreviation List
- BBB
blood-brain barrier
- CSF
cerebral spinal fluid
- COX1
cyclo-oxygenase 1
- COX2
cyclo-oxygenase 2
- CRP
C reactive protein
- DA
dopamine
- EGF
Epidermal growth factor
- FACIT
functional assessment of chronic illness therapy
- FcγRII
Fc gamma receptor II
- HADS
hospital anxiety and depression scale
- IFN-γ
interferon-gamma
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- IL-12
interleukin-12
- IP-10
interferon gamma-induced protein-10
- LRRK2
leucine-rich repeat kinase 2
- LPS
lipopolysaccharide
- MHC-II
major histocompatibility complex-II
- MCP-1
monocyte chemotactic protein-1
- MIP-1β
macrophage inflammatory protein-1β
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NFκB
nuclear factor kappa beta
- NT-proCNP
N-terminal Pro-C-type natriuretic peptide
- PET
positron emission tomography
- RNF11
Ring Finger Protein 11
- SN
substantia nigra
- TGF- β
transforming growth factor β
- TNF
tumor necrosis factor
- TLR
toll-like receptor
- 6-OHDA
6-hydroxydopamine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen Reish HE, Standaert DG. Role of alpha-Synuclein in Inducing Innate and Adaptive Immunity in Parkinson Disease. J Parkinsons Dis. 2015;5:1–19. doi: 10.3233/JPD-140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Arai H, Furuya T, Yasuda T, Miura M, Mizuno Y, Mochizuki H. Neurotoxic effects of lipopolysaccharide on nigral dopaminergic neurons are mediated by microglial activation, interleukin-1beta, and expression of caspase-11 in mice. J Biol Chem. 2004;279:51647–51653. doi: 10.1074/jbc.M407328200. [DOI] [PubMed] [Google Scholar]
- Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentero MT, Levandis G, Nappi G, Bazzini E, Blandini F. Peripheral inflammation and neuroprotection: systemic pretreatment with complete Freund's adjuvant reduces 6-hydroxydopamine toxicity in a rodent model of Parkinson's disease. Neurobiol Dis. 2006;24:492–505. doi: 10.1016/j.nbd.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, Whitton P, Wyse R, Isaacs T, Lees A, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123:2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, Morelli M, Ingrassia R, Benarese M, Porrini V, et al. Late-onset Parkinsonism in NFkappaB/c-Rel-deficient mice. Brain. 2012;135:2750–2765. doi: 10.1093/brain/aws193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr. Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros I, Cuartero MI, Pradillo JM, de la Parra J, Perez-Ruiz A, Corbi A, Ricote M, Hamilton JA, Sobrado M, Vivancos J, et al. Rosiglitazone-induced CD36 up-regulation resolves inflammation by PPARgamma and 5-LO-dependent pathways. J Leukoc Biol. 2014;95:587–598. doi: 10.1189/jlb.0613326. [DOI] [PubMed] [Google Scholar]
- Banati RB, Myers R, Kreutzberg GW. PK ('peripheral benzodiazepine')--binding sites in the CNS indicate early and discrete brain lesions: microautoradiographic detection of [3H]PK11195 binding to activated microglia. J Neurocytol. 1997;26:77–82. doi: 10.1023/a:1018567510105. [DOI] [PubMed] [Google Scholar]
- Barcia C, Ros CM, Annese V, Gomez A, Ros-Bernal F, Aguado-Yera D, Martinez-Pagan ME, de Pablos V, Fernandez-Villalba E, Herrero MT. IFN-gamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson's disease. Cell Death Dis. 2011;2:e142. doi: 10.1038/cddis.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcia C, Ros CM, Ros-Bernal F, Gomez A, Annese V, Carrillo-de Sauvage MA, Yuste JE, Campuzano CM, de Pablos V, Fernandez-Villalba E, Herrero MT. Persistent phagocytic characteristics of microglia in the substantia nigra of long-term Parkinsonian macaques. J Neuroimmunol. 2013;261:60–66. doi: 10.1016/j.jneuroim.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Barcia C, Sanchez Bahillo A, Fernandez-Villalba E, Bautista V, Poza YPM, Fernandez-Barreiro A, Hirsch EC, Herrero MT. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia. 2004;46:402–409. doi: 10.1002/glia.20015. [DOI] [PubMed] [Google Scholar]
- Barkholt P, Sanchez-Guajardo V, Kirik D, Romero-Ramos M. Long-term polarization of microglia upon alpha-synuclein overexpression in nonhuman primates. Neuroscience. 2012;208:85–96. doi: 10.1016/j.neuroscience.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Barnum CJ, Eskow KL, Dupre K, Blandino P, Jr., Deak T, Bishop C. Exogenous corticosterone reduces L-DOPA-induced dyskinesia in the hemi-parkinsonian rat: role for interleukin-1beta. Neuroscience. 2008;156:30–41. doi: 10.1016/j.neuroscience.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, Leenders KL. [11C]-PK11195 PET: quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson's disease? Parkinsonism Relat Disord. 2010;16:57–59. doi: 10.1016/j.parkreldis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Baumeister D, Ciufolini S, Mondelli V. Effects of psychotropic drugs on inflammation: consequence or mediator of therapeutic effects in psychiatric treatment? Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-4044-5. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. Eur J Neurosci. 2000;12:2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Patrone C, Zachrisson O, Andersson A, Dannaeus K, Heidrich J, Kortesmaa J, Mercer A, Nielsen E, Ronnholm H, Wikstrom L. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson's disease. J Neurosci Res. 2008;86:326–338. doi: 10.1002/jnr.21483. [DOI] [PubMed] [Google Scholar]
- Bian MJ, Li LM, Yu M, Fei J, Huang F. Elevated interleukin-1beta induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine aggravating dopaminergic neurodegeneration in old male mice. Brain Res. 2009;1302:256–264. doi: 10.1016/j.brainres.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- Bliederhaeuser C, Grozdanov V, Speidel A, Zondler L, Ruf WP, Bayer H, Kiechle M, Feiler MS, Freischmidt A, Brenner D, et al. Age-dependent defects of alpha-synuclein oligomer uptake in microglia and monocytes. Acta Neuropathol. 2015 doi: 10.1007/s00401-015-1504-2. [DOI] [PubMed] [Google Scholar]
- Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer's and de novo Parkinson's disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bower JH, Maraganore DM, Peterson BJ, Ahlskog JE, Rocca WA. Immunologic diseases, anti-inflammatory drugs, and Parkinson disease: a case-control study. Neurology. 2006;67:494–496. doi: 10.1212/01.wnl.0000227906.99570.cc. [DOI] [PubMed] [Google Scholar]
- Brodacki B, Staszewski J, Toczylowska B, Kozlowska E, Drela N, Chalimoniuk M, Stepien A. Serum interleukin (IL-2, IL-10, IL-6, IL-4), TNFalpha, and INFgamma concentrations are elevated in patients with atypical and idiopathic parkinsonism. Neurosci Lett. 2008;441:158–162. doi: 10.1016/j.neulet.2008.06.040. [DOI] [PubMed] [Google Scholar]
- Buchsel PC, Forgey A, Grape FB, Hamann SS. Granulocyte macrophage colony-stimulating factor: current practice and novel approaches. Clin J Oncol Nurs. 2002;6:198–205. doi: 10.1188/02.CJON.198-205. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carta AR. PPAR-gamma: therapeutic prospects in Parkinson's disease. Curr Drug Targets. 2013;14:743–751. doi: 10.2174/1389450111314070004. [DOI] [PubMed] [Google Scholar]
- Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E. Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: new evidences on neuroprotection in a progressive Parkinson's disease model. Neuroscience. 2011;194:250–261. doi: 10.1016/j.neuroscience.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Carta AR, Simuni T. Thiazolidinediones under preclinical and early clinical development for the treatment of Parkinson's disease. Expert Opin Investig Drugs. 2015;24:219–227. doi: 10.1517/13543784.2015.963195. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. J Neurochem. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Cebrian C, Zucca FA, Mauri P, Steinbeck JA, Studer L, Scherzer CR, Kanter E, Budhu S, Mandelbaum J, Vonsattel JP, et al. MHC-I expression renders catecholaminergic neurons susceptible to T-cell-mediated degeneration. Nat Commun. 2014;5:3633. doi: 10.1038/ncomms4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson's disease: the Thy1-aSyn ("Line 61") mice. Neurotherapeutics. 2012;9:297–314. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BP, Song DY, Sugama S, Shin DH, Shimizu Y, Kim SS, Kim YS, Joh TH. Pathological dynamics of activated microglia following medial forebrain bundle transection. Glia. 2006;53:92–102. doi: 10.1002/glia.20265. [DOI] [PubMed] [Google Scholar]
- Croisier E, Moran LB, Dexter DT, Pearce RK, Graeber MB. Microglial inflammation in the parkinsonian substantia nigra: relationship to alpha-synuclein deposition. J Neuroinflammation. 2005;2:14. doi: 10.1186/1742-2094-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson's disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- Daher JP, Volpicelli-Daley LA, Blackburn JP, Moehle MS, West AB. Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A. 2014;111:9289–9294. doi: 10.1073/pnas.1403215111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal NV, Pranski EL, Tansey MG, Lah JJ, Levey AI, Betarbet RS. RNF11 modulates microglia activation through NF-kappaB signalling cascade. Neurosci Lett. 2012;528:174–179. doi: 10.1016/j.neulet.2012.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66:646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lella Ezcurra AL, Chertoff M, Ferrari C, Graciarena M, Pitossi F. Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol Dis. 2010;37:630–640. doi: 10.1016/j.nbd.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45:939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- Depino AM, Earl C, Kaczmarczyk E, Ferrari C, Besedovsky H, del Rey A, Pitossi FJ, Oertel WH. Microglial activation with atypical proinflammatory cytokine expression in a rat model of Parkinson's disease. Eur J Neurosci. 2003;18:2731–2742. doi: 10.1111/j.1460-9568.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- Doorn KJ, Breve JJ, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ, van Dam AM. Brain region-specific gene expression profiles in freshly isolated rat microglia. Front Cell Neurosci. 2015;9:84. doi: 10.3389/fncel.2015.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, et al. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta G, Zhang P, Liu B. The lipopolysaccharide Parkinson's disease animal model: mechanistic studies and drug discovery. Fundam Clin Pharmacol. 2008;22:453–464. doi: 10.1111/j.1472-8206.2008.00616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edison P, Ahmed I, Fan Z, Hinz R, Gelosa G, Ray Chaudhuri K, Walker Z, Turkheimer FE, Brooks DJ. Microglia, amyloid, and glucose metabolism in Parkinson's disease with and without dementia. Neuropsychopharmacology. 2013;38:938–949. doi: 10.1038/npp.2012.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslamboli A, Romero-Ramos M, Burger C, Bjorklund T, Muzyczka N, Mandel RJ, Baker H, Ridley RM, Kirik D. Long-term consequences of human alpha-synuclein overexpression in the primate ventral midbrain. Brain. 2007;130:799–815. doi: 10.1093/brain/awl382. [DOI] [PubMed] [Google Scholar]
- Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson's disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Fan Z, Aman Y, Ahmed I, Chetelat G, Landeau B, Ray Chaudhuri K, Brooks DJ, Edison P. Influence of microglial activation on neuronal function in Alzheimer's and Parkinson's disease dementia. Alzheimers Dement. 2015;11:608–621 e607. doi: 10.1016/j.jalz.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Fellner L, Irschick R, Schanda K, Reindl M, Klimaschewski L, Poewe W, Wenning GK, Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-alpha) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- Flood PM, Qian L, Peterson LJ, Zhang F, Shi JS, Gao HM, Hong JS. Transcriptional Factor NF-kappaB as a Target for Therapy in Parkinson's Disease. Parkinsons Dis. 2011;2011:216298. doi: 10.4061/2011/216298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltynie T, Aviles-Olmos I. Exenatide as a potential treatment for patients with Parkinson's disease: first steps into the clinic. Alzheimers Dement. 2014;10:S38–46. doi: 10.1016/j.jalz.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Fox SH, Henry B, Hill M, Crossman A, Brotchie J. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord. 2002;17:1180–1187. doi: 10.1002/mds.10289. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson's disease. Environ Health Perspect. 2011a;119:807–814. doi: 10.1289/ehp.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011b;76:863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Esparcia P, Llorens F, Carmona M, Ferrer I. Complex deregulation and expression of cytokines and mediators of the immune response in Parkinson's disease brain is region dependent. Brain Pathol. 2014;24:584–598. doi: 10.1111/bpa.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Ischiropoulos H, Lee VM, Trojanowski JQ. The relationship between oxidative/nitrative stress and pathological inclusions in Alzheimer's and Parkinson's diseases. Free Radic Biol Med. 2002;32:1264–1275. doi: 10.1016/s0891-5849(02)00804-3. [DOI] [PubMed] [Google Scholar]
- Gillardon F, Schmid R, Draheim H. Parkinson's disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience. 2012;208:41–48. doi: 10.1016/j.neuroscience.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Galvez Y, Palomo-Garo C, Fernandez-Ruiz J, Garcia C. Potential of the cannabinoid CB receptor as a pharmacological target against inflammation in Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, Freeman TC, Summers KM, McColl BW. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016 doi: 10.1038/nn.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratchev A, Kzhyshkowska J, Kannookadan S, Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E, Muller-Molinet I, Gooi L, Goerdt S. Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. J Immunol. 2008;180:6553–6565. doi: 10.4049/jimmunol.180.10.6553. [DOI] [PubMed] [Google Scholar]
- Grinberg-Bleyer Y, Dainichi T, Oh H, Heise N, Klein U, Schmid RM, Hayden MS, Ghosh S. Cutting Edge: NF-kappaB p65 and c-Rel Control Epidermal Development and Immune Homeostasis in the Skin. J Immunol. 2015;194:2472–2476. doi: 10.4049/jimmunol.1402608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson's disease. Nat Genet. 2010;42:781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson's disease. J Neuroinflammation. 2008;5:19. doi: 10.1186/1742-2094-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Barnum CJ, Ruhn KA, Varghese S, Trevino I, Blesch A, Tansey MG. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson's disease. Mol Ther. 2011;19:46–52. doi: 10.1038/mt.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms AS, Lee JK, Nguyen TA, Chang J, Ruhn KM, Trevino I, Tansey MG. Regulation of microglia effector functions by tumor necrosis factor signaling. Glia. 2012;60:189–202. doi: 10.1002/glia.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Healy DG, Wood NW, Schapira AH. Test for LRRK2 mutations in patients with Parkinson's disease. Pract Neurol. 2008;8:381–385. doi: 10.1136/jnnp.2008.162420. [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry V, Paille V, Lelan F, Brachet P, Damier P. Kinetics of microglial activation and degeneration of dopamine-containing neurons in a rat model of Parkinson disease induced by 6-hydroxydopamine. J Neuropathol Exp Neurol. 2009;68:1092–1102. doi: 10.1097/NEN.0b013e3181b767b4. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358–372. doi: 10.1038/nrn3880. [DOI] [PubMed] [Google Scholar]
- Herrera AJ, Castano A, Venero JL, Cano J, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28:8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Hofmann KW, Schuh AF, Saute J, Townsend R, Fricke D, Leke R, Souza DO, Portela LV, Chaves ML, Rieder CR. Interleukin-6 serum levels in patients with Parkinson's disease. Neurochem Res. 2009;34:1401–1404. doi: 10.1007/s11064-009-9921-z. [DOI] [PubMed] [Google Scholar]
- Holscher C. Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol. 2014;221:T31–41. doi: 10.1530/JOE-13-0221. [DOI] [PubMed] [Google Scholar]
- Hoozemans JJ, Veerhuis R, Rozemuller JM, Eikelenboom P. Neuroinflammation and regeneration in the early stages of Alzheimer's disease pathology. Int J Dev Neurosci. 2006;24:157–165. doi: 10.1016/j.ijdevneu.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debre P, Agid Y, Dugas B, Hirsch EC. FcepsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, production of nitric oxide and tumor necrosis factor-alpha in glial cells. J Neurosci. 1999;19:3440–3447. doi: 10.1523/JNEUROSCI.19-09-03440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley SD, O'Banion MK, Song DD, Arana FS, Olschowka JA, Haber SN. Microglial response is poorly correlated with neurodegeneration following chronic, low-dose MPTP administration in monkeys. Exp Neurol. 2003;184:659–668. doi: 10.1016/S0014-4886(03)00273-5. [DOI] [PubMed] [Google Scholar]
- Iannaccone S, Cerami C, Alessio M, Garibotto V, Panzacchi A, Olivieri S, Gelsomino G, Moresco RM, Perani D. In vivo microglia activation in very early dementia with Lewy bodies, comparison with Parkinson's disease. Parkinsonism Relat Disord. 2013;19:47–52. doi: 10.1016/j.parkreldis.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson's disease brains. Acta Neuropathol. 2003;106:518–526. doi: 10.1007/s00401-003-0766-2. [DOI] [PubMed] [Google Scholar]
- Investigators, N.N.-P. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- Investigators, N.N.-P. A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55:352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Jimenez S, Baglietto-Vargas D, Caballero C, Moreno-Gonzalez I, Torres M, Sanchez-Varo R, Ruano D, Vizuete M, Gutierrez A, Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer's disease: age-dependent switch in the microglial phenotype from alternative to classic. J Neurosci. 2008;28:11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyothi HJ, Vidyadhara DJ, Mahadevan A, Philip M, Parmar SK, Manohari SG, Shankar SK, Raju TR, Alladi PA. Aging causes morphological alterations in astrocytes and microglia in human substantia nigra pars compacta. Neurobiol Aging. 2015;36:3321–3333. doi: 10.1016/j.neurobiolaging.2015.08.024. [DOI] [PubMed] [Google Scholar]
- Kannarkat GT, Cook D, Lee J, Chang J, Chung J, Sandy E, Paul K, Ritz B, Bronstein J, Factor S, et al. Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson's Disease: An Observational and Case-Control Study. NPJ Parkinson’s Disease. doi: 10.1038/npjparkd.2015.2. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Yang MS, Choi D, Kim JH, Kim HS, Seol W, Choi S, Jou I, Kim EY, Joe EH. Impaired inflammatory responses in murine Lrrk2-knockdown brain microglia. PLoS One. 2012;7:e34693. doi: 10.1371/journal.pone.0034693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson's disease. J Endocrinol. 2009;202:431–439. doi: 10.1677/JOE-09-0132. [DOI] [PubMed] [Google Scholar]
- Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- Kohutnicka M, Lewandowska E, Kurkowska-Jastrzebska I, Czlonkowski A, Czlonkowska A. Microglial and astrocytic involvement in a murine model of Parkinson's disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Immunopharmacology. 1998;39:167–180. doi: 10.1016/s0162-3109(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Koshimori Y, Ko JH, Mizrahi R, Rusjan P, Mabrouk R, Jacobs MF, Christopher L, Hamani C, Lang AE, Wilson AA, et al. Imaging Striatal Microglial Activation in Patients with Parkinson's Disease. PLoS One. 2015;10:e0138721. doi: 10.1371/journal.pone.0138721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE. GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J Neuroimmunol. 2013;265:1–10. doi: 10.1016/j.jneuroim.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziorowski D, Tomasiuk R, Szlufik S, Friedman A. Inflammatory cytokines and NT-proCNP in Parkinson's disease patients. Cytokine. 2012;60:762–766. doi: 10.1016/j.cyto.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, Miller KR, Prokop S, Kettenmann H, Heppner FL. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8:e60921. doi: 10.1371/journal.pone.0060921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the nor mal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- Lee JK, Chung J, Kannarkat GT, Tansey MG. Critical role of regulator G-protein signaling 10 (RGS10) in modulating macrophage M1/M2 activation. PLoS One. 2013;8:e81785. doi: 10.1371/journal.pone.0081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Chung J, McAlpine FE, Tansey MG. Regulator of G-protein signaling-10 negatively regulates NF-kappaB in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats. J Neurosci. 2011;31:11879–11888. doi: 10.1523/JNEUROSCI.1002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, McCoy MK, Harms AS, Ruhn KA, Gold SJ, Tansey MG. Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. J Neurosci. 2008;28:8517–8528. doi: 10.1523/JNEUROSCI.1806-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberatore GT, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WG, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, et al. Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson's-disease-related mutant alpha-synuclein. Neuron. 2009;64:807–827. doi: 10.1016/j.neuron.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P. alpha -Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson's disease. Proc Natl Acad Sci U S A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofrumento DD, Saponaro C, Cianciulli A, De Nuccio F, Mitolo V, Nicolardi G, Panaro MA. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation. 2011;18:79–88. doi: 10.1159/000320027. [DOI] [PubMed] [Google Scholar]
- Luchtman DW, Shao D, Song C. Behavior, neurotransmitters and inflammation in three regimens of the MPTP mouse model of Parkinson's disease. Physiol Behav. 2009;98:130–138. doi: 10.1016/j.physbeh.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- Magen I, Chesselet MF. Genetic mouse models of Parkinson's disease The state of the art. Prog Brain Res. 2010;184:53–87. doi: 10.1016/S0079-6123(10)84004-X. [DOI] [PubMed] [Google Scholar]
- Maia S, Arlicot N, Vierron E, Bodard S, Vergote J, Guilloteau D, Chalon S. Longitudinal and parallel monitoring of neuroinflammation and neurodegeneration in a 6-hydroxydopamine rat model of Parkinson's disease. Synapse. 2012;66:573–583. doi: 10.1002/syn.21543. [DOI] [PubMed] [Google Scholar]
- Mangano EN, Peters S, Litteljohn D, So R, Bethune C, Bobyn J, Clarke M, Hayley S. Granulocyte macrophage-colony stimulating factor protects against substantia nigra dopaminergic cell loss in an environmental toxin model of Parkinson's disease. Neurobiol Dis. 2011;43:99–112. doi: 10.1016/j.nbd.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Marinova-Mutafchieva L, Sadeghian M, Broom L, Davis JB, Medhurst AD, Dexter DT. Relationship between microglial activation and dopaminergic neuronal loss in the substantia nigra: a time course study in a 6-hydroxydopamine model of Parkinson's disease. J Neurochem. 2009;110:966–975. doi: 10.1111/j.1471-4159.2009.06189.x. [DOI] [PubMed] [Google Scholar]
- Marker DF, Puccini JM, Mockus TE, Barbieri J, Lu SM, Gelbard HA. LRRK2 kinase inhibition prevents pathological microglial phagocytosis in response to HIV-1 Tat protein. J Neuroinflammation. 2012;9:261. doi: 10.1186/1742-2094-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HL, Mounsey RB, Mustafa S, Sathe K, Teismann P. Pharmacological manipulation of peroxisome proliferator-activated receptor gamma (PPARgamma) reveals a role for anti-oxidant protection in a model of Parkinson's disease. Exp Neurol. 2012;235:528–538. doi: 10.1016/j.expneurol.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K. Activation of NF-kappaB protects hippocampal neurons against oxidative stress-induced apoptosis: evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res. 1997;49:681–697. doi: 10.1002/(SICI)1097-4547(19970915)49:6<681::AID-JNR3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- May MJ, D'Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289:1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- Melief J, Koning N, Schuurman KG, Van De Garde MD, Smolders J, Hoek RM, Van Eijk M, Hamann J, Huitinga I. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia. 2012;60:1506–1517. doi: 10.1002/glia.22370. [DOI] [PubMed] [Google Scholar]
- Meredith SC. Protein denaturation and aggregation: Cellular responses to denatured and aggregated proteins. Ann N Y Acad Sci. 2005;1066:181–221. doi: 10.1196/annals.1363.030. [DOI] [PubMed] [Google Scholar]
- Mesnage V, Houeto JL, Bonnet AM, Clavier I, Arnulf I, Cattelin F, Le Fur G, Damier P, Welter ML, Agid Y. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson disease. Clin Neuropharmacol. 2004;27:108–110. doi: 10.1097/00002826-200405000-00003. [DOI] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mittelbronn M. The M1/M2 immune polarization concept in microglia: a fair transfer? Neuroimmunol Neuroinflammation. 2014;1:2. [Google Scholar]
- Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101:249–255. doi: 10.1007/s004010000284. [DOI] [PubMed] [Google Scholar]
- Moehle MS, Webber PJ, Tse T, Sukar N, Standaert DG, DeSilva TM, Cowell RM, West AB. LRRK2 inhibition attenuates microglial inflammatory responses. J Neurosci. 2012;32:1602–1611. doi: 10.1523/JNEUROSCI.5601-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Narabayashi H, Riederer P, Nagatsu T. Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson's disease. Neurosci Lett. 1995;193:129–132. doi: 10.1016/0304-3940(95)11686-q. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994a;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neurosci Lett. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994b;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Kondo T, Mizuno Y, Nagatsu T. p53 protein, interferon-gamma, and NF-kappaB levels are elevated in the parkinsonian brain. Neurosci Lett. 2007;414:94–97. doi: 10.1016/j.neulet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K. Testing NF-kappaB-based therapy in hemiparkinsonian monkeys. J Neuroimmune Pharmacol. 2012;7:544–556. doi: 10.1007/s11481-012-9377-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CS, Ase AR, Kinsara A, Rao VT, Michell-Robinson M, Leong SY, Butovsky O, Ludwin SK, Seguela P, Bar-Or A, Antel JP. P2Y12 expression and function in alternatively activated human microglia. Neurol Neuroimmunol Neuroinflamm. 2015;2:e80. doi: 10.1212/NXI.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgese MG, Cassano T, Cuomo V, Giuffrida A. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–119. doi: 10.1016/j.expneurol.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Blum-Degen D, Przuntek H, Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson's disease. Acta Neurol Scand. 1998;98:142–144. doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson's disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- Nassar NN, Al-Shorbagy MY, Arab HH, Abdallah DM. Saxagliptin: a novel antiparkinsonian approach. Neuropharmacology. 2015;89:308–317. doi: 10.1016/j.neuropharm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc Natl Acad Sci U S A. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETPD-Investigators 2014 annual meetings. Ann Neurol. 2014;76(Suppl 18):S1–S254. doi: 10.1002/ana.24247. [DOI] [PubMed] [Google Scholar]
- Noyce AJ, Bestwick JP, Silveira-Moriyama L, Hawkes CH, Giovannoni G, Lees AJ, Schrag A. Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann Neurol. 2012;72:893–901. doi: 10.1002/ana.23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Red Eagle A, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah M, Biber K, Vinet J, Boddeke HW. Microglia phenotype diversity. CNS Neurol Disord Drug Targets. 2011;10:108–118. doi: 10.2174/187152711794488575. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson's disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, Lopez de Munain A, Aparicio S, Gil AM, Khan N, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Panet H, Barzilai A, Daily D, Melamed E, Offen D. Activation of nuclear transcription factor kappa B (NF-kappaB) is essential for dopamine-induced apoptosis in PC12 cells. J Neurochem. 2001;77:391–398. doi: 10.1046/j.1471-4159.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–409. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Pascual G, Ricote M, Hevener AL. Macrophage peroxisome proliferator activated receptor gamma as a therapeutic target to combat Type 2 diabetes. Expert Opin Ther Targets. 2007;11:1503–1520. doi: 10.1517/14728222.11.11.1503. [DOI] [PubMed] [Google Scholar]
- Pattarini R, Smeyne RJ, Morgan JI. Temporal mRNA profiles of inflammatory mediators in the murine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine model of Parkinson's disease. Neuroscience. 2007;145:654–668. doi: 10.1016/j.neuroscience.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174. doi: 10.1186/1742-2094-8-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Perry VH, Holmes C. Microglial priming in neurodegenerative disease. Nat Rev Neurol. 2014;10:217–224. doi: 10.1038/nrneurol.2014.38. [DOI] [PubMed] [Google Scholar]
- Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, Carta AR. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson's disease. Neurobiol Dis. 2014;71:280–291. doi: 10.1016/j.nbd.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Pott Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson's disease. Brain. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pranski EL, Dalal NV, Sanford CV, Herskowitz JH, Gearing M, Lazo C, Miller GW, Lah JJ, Levey AI, Betarbet RS. RING finger protein 11 (RNF11) modulates susceptibility to 6-OHDA-induced nigral degeneration and behavioral deficits through NF-kappaB signaling in dopaminergic cells. Neurobiol Dis. 2013;54:264–279. doi: 10.1016/j.nbd.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, et al. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One. 2011;6:e18568. doi: 10.1371/journal.pone.0018568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud N, Harkavyi A, Giordano G, Lever R, Whitton J, Whitton P. Exendin-4 reverts behavioural and neurochemical dysfunction in a pre-motor rodent model of Parkinson's disease with noradrenergic deficit. Br J Pharmacol. 2012;167:1467–1479. doi: 10.1111/j.1476-5381.2012.02100.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ramsey CP, Tansey MG. A survey from 2012 of evidence for the role of neuroinflammation in neurotoxin animal models of Parkinson's disease and potential molecular targets. Exp Neurol. 2014;256:126–132. doi: 10.1016/j.expneurol.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy RC, Narala VR, Keshamouni VG, Milam JE, Newstead MW, Standiford TJ. Sepsis-induced inhibition of neutrophil chemotaxis is mediated by activation of peroxisome proliferator-activated receptor-{gamma} Blood. 2008;112:4250–4258. doi: 10.1182/blood-2007-12-128967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Heldt SA, Presley CS, Guley NH, Elberger AJ, Deng Y, D'Surney L, Rogers JT, Ferrell J, Bu W, et al. Motor, visual and emotional deficits in mice after closed-head mild traumatic brain injury are alleviated by the novel CB2 inverse agonist SMM-189. Int J Mol Sci. 2015;16:758–787. doi: 10.3390/ijms16010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentzos M, Nikolaou C, Andreadou E, Paraskevas GP, Rombos A, Zoga M, Tsoutsou A, Boufidou F, Kapaki E, Vassilopoulos D. Circulating interleukin-10 and interleukin-12 in Parkinson's disease. Acta Neurol Scand. 2009;119:332–337. doi: 10.1111/j.1600-0404.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Alvarez-Erviti L, Blesa FJ, Rodriguez-Oroz MC, Arina A, Melero I, Ramos LI, Obeso JA. Bone-marrow-derived cell differentiation into microglia: a study in a progressive mouse model of Parkinson's disease. Neurobiol Dis. 2007;28:316–325. doi: 10.1016/j.nbd.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Rojo AI, Innamorato NG, Martin-Moreno AM, De Ceballos ML, Yamamoto M, Cuadrado A. Nrf2 regulates microglial dynamics and neuroinflammation in experimental Parkinson's disease. Glia. 2010;58:588–598. doi: 10.1002/glia.20947. [DOI] [PubMed] [Google Scholar]
- Romero J, Garcia-Palomero E, Fernandez-Ruiz JJ, Ramos JA. Involvement of GABA(B) receptors in the motor inhibition produced by agonists of brain cannabinoid receptors. Behav Pharmacol. 1996;7:299–302. doi: 10.1097/00008877-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Rowin J, Thiruppathi M, Arhebamen E, Sheng J, Prabhakar BS, Meriggioli MN. Granulocyte macrophage colony-stimulating factor treatment of a patient in myasthenic crisis: effects on regulatory T cells. Muscle Nerve. 2012;46:449–453. doi: 10.1002/mus.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruland J. Return to homeostasis: downregulation of NF-kappaB responses. Nat Immunol. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Barnum CJ, Tansey MG, Romero-Ramos M. Neuroimmunological processes in Parkinson's disease and their relation to alpha-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro. 2013;5:113–139. doi: 10.1042/AN20120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Guajardo V, Febbraro F, Kirik D, Romero-Ramos M. Microglia acquire distinct activation profiles depending on the degree of alpha-synuclein neuropathology in a rAAV based model of Parkinson's disease. PLoS One. 2010;5:e8784. doi: 10.1371/journal.pone.0008784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Pernaute R, Ferree A, Cooper O, Yu M, Brownell AL, Isacson O. Selective COX-2 inhibition prevents progressive dopamine neuron degeneration in a rat model of Parkinson's disease. J Neuroinflammation. 2004;1:6. doi: 10.1186/1742-2094-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh N, Shimatsu A, Himeno A, Sasaki Y, Yamakage H, Yamada K, Suganami T, Ogawa Y. Unbalanced M1/M2 phenotype of peripheral blood monocytes in obese diabetic patients: effect of pioglitazone. Diabetes Care. 2010;33:e7. doi: 10.2337/dc09-1315. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Garau A, Carboni E, Carta AR. Progressive dopaminergic degeneration in the chronic MPTPp mouse model of Parkinson's disease. Neurotox Res. 2009;16:127–139. doi: 10.1007/s12640-009-9061-x. [DOI] [PubMed] [Google Scholar]
- Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN. GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol. 2011;240-241:65–73. doi: 10.1016/j.jneuroim.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieradzan KA, Fox SH, Hill M, Dick JP, Crossman AR, Brotchie JM. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology. 2001;57:2108–2111. doi: 10.1212/wnl.57.11.2108. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson's disease. Brain Res Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O'Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson's disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, Kemnitz JW, Johnson JA, Emborg ME. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8:91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto A, Balint BL, Nagy ZS, Barta E, Dezso B, Pap A, Szeles L, Poliska S, Oros M, Evans RM, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Ishii A, Ohtaki H, Shioda S, Yoshida T, Numazawa S. Activation of microglia induces symptoms of Parkinson's disease in wild-type, but not in IL-1 knockout mice. J Neuroinflammation. 2013;10:143. doi: 10.1186/1742-2094-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Le WD. Minocycline: neuroprotective mechanisms in Parkinson's disease. Curr Pharm Des. 2004;10:679–686. doi: 10.2174/1381612043453162. [DOI] [PubMed] [Google Scholar]
- Tran TA, McCoy MK, Sporn MB, Tansey MG. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J Neuroinflammation. 2008;5:14. doi: 10.1186/1742-2094-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Perren A, Toelen J, Casteels C, Macchi F, Van Rompuy AS, Sarre S, Casadei N, Nuber S, Himmelreich U, Osorio Garcia MI, et al. Longitudinal follow-up and characterization of a robust rat model for Parkinson's disease based on overexpression of alpha-synuclein with adeno-associated viral vectors. Neurobiol Aging. 2015;36:1543–1558. doi: 10.1016/j.neurobiolaging.2014.11.015. [DOI] [PubMed] [Google Scholar]
- Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812:1007–1022. doi: 10.1016/j.bbadis.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Dillon-Carter O, Tourtellotte WW, Carvey P, Freed WJ. TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson's disease in ventricular cerebrospinal fluid. Exp Neurol. 1996;142:313–322. doi: 10.1006/exnr.1996.0200. [DOI] [PubMed] [Google Scholar]
- Vazquez-Claverie M, Garrido-Gil P, San Sebastian W, Izal-Azcarate A, Belzunegui S, Marcilla I, Lopez B, Luquin MR. Acute and chronic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administrations elicit similar microglial activation in the substantia nigra of monkeys. J Neuropathol Exp Neurol. 2009;68:977–984. doi: 10.1097/NEN.0b013e3181b35e41. [DOI] [PubMed] [Google Scholar]
- Wakamatsu M, Ishii A, Iwata S, Sakagami J, Ukai Y, Ono M, Kanbe D, Muramatsu S, Kobayashi K, Iwatsubo T, Yoshimoto M. Selective loss of nigral dopamine neurons induced by overexpression of truncated human alpha-synuclein in mice. Neurobiol Aging. 2008;29:574–585. doi: 10.1016/j.neurobiolaging.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Walsh S, Finn DP, Dowd E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson's disease in the rat. Neuroscience. 2011;175:251–261. doi: 10.1016/j.neuroscience.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, Effros RB, Chesselet MF. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol. 2012;237:318–334. doi: 10.1016/j.expneurol.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H. Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson's disease. J Neurochem. 2004;91:451–461. doi: 10.1111/j.1471-4159.2004.02728.x. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Shimoda T, Uno K, Tateishi N, Furuya S, Yagi K, Suzuki K, Fujita S. The effects of MPTP on the activation of microglia/astrocytes and cytokine/chemokine levels in different mice strains. J Neuroimmunol. 2008;204:43–51. doi: 10.1016/j.jneuroim.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Shinagawa R, Yamada M, Mori T, Tateishi N, Fujita S. Long-lasting reactive changes observed in microglia in the striatal and substantia nigral of mice after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2007;1138:196–202. doi: 10.1016/j.brainres.2006.12.054. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ. Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J Nucl Med. 2013;54:1320–1322. doi: 10.2967/jnumed.112.118885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang J, Zhang Y, Yuan Y, Guan W, Jin C, Chen H, Wang X, Yang X, He F. The scaffold protein TANK/I-TRAF inhibits NF-kappaB activation by recruiting polo-like kinase 1. Mol Biol Cell. 2010;21:2500–2513. doi: 10.1091/mbc.E09-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]