Abstract
Summary
The objective of NRG Oncology RTOG 0123 was to test the ability of the angiotensin converting enzyme inhibitor (ACEI) captopril to reduce pulmonary damage after radiation for lung cancer. Despite significant effort, the trial did not analyze a sufficient number of patients to test the hypothesis due to early study closure. However, it did show the safety of the ACEI mitigation approach and the use of newer ACEIs, started during radiotherapy, may solve the accrual problems.
Objectives
The primary objective of NRG Oncology RTOG 0123 was to test the ability of the angiotensin converting enzyme inhibitor (ACEI) captopril to alter the incidence of pulmonary damage after radiation therapy for lung cancer; secondary objectives included analyzing pulmonary cytokine expression, quality of life, and the long-term effects of captopril.
Methods
Eligible patients included Stage II-IIIB non-small cell lung cancer, Stage I central NSCLC, or limited-stage small-cell. Patients who met eligibility for randomization at the end of radiotherapy received either captopril or standard care for one year. The captopril was to be escalated to 50 mg TID. Primary endpoint was incidence of Grade 2+ radiation-induced pulmonary toxicity in the first year.
Results
81 patients were accrued between 6/2003 and 8/2007. Given the low accrual rate, the study was closed early. No significant safety issues were encountered. Eight patients were ineligible for registration or withdrew consent prior to randomization and 40 patients were not randomized post-radiation. Major reasons for non-randomization included patients' refusal and physician preference. Of the 33 randomized patients, 20 were analyzable (13 observation, 7 captopril). The incidence of Grade 2+ pulmonary toxicity attributable to radiation therapy was 23% (3/13) in the observation arm and 14% (1/7) in the captopril arm.
Conclusion
Despite significant resources and multiple amendments, NRG Oncology RTOG 0123 was unable to test the hypothesis that captopril mitigates radiation-induced pulmonary toxicity. It did show the safety of such an approach and the use of newer ACEIs started during radiotherapy may solve the accrual problems.
Keywords: Lung Cancer, Radiotherapy, Captopril, Pneumonitis
Introduction
The majority of patients with lung cancer present with locally advanced, unresectable disease; this is managed with primary thoracic irradiation often delivered with or without neo-adjuvant or concurrent chemotherapy with continued overall poor survival rates (1). Radiation pneumonitis and radiation-induced pulmonary fibrosis are refractive to management. As a result, lung tolerance is a major dose-limiting factor in the radiotherapeutic management of thoracic tumors (2).
During the 1990's, there was work suggesting that captopril might mitigate radiation-induced pulmonary injury. Ward and colleagues (3,4) had shown that captopril administration after hemi-thorax irradiation decreased pulmonary arterial pressure in rats, reduced radiation-induced endothelial dysfunction, reduced pulmonary fibrosis, and delayed the onset of radiation-induced pulmonary arterial hypoperfusion. In addition, rats given chemotherapy and total body irradiation were noted to have significantly less lung parenchymal damage with the addition of captopril (5), and rats given total body irradiation alone had a decreased incidence of radiation nephropathy when treated with captopril (6). In most of these studies, captopril was administered after the completion of radiation, in what we would now term mitigation regimens (7). Captopril was typically effective at dose regimens 12-25 times higher than the standard anti-hypertensive dose in humans, based on equivalent body weight, although on an equivalent body surface area basis the dose is well within the range used in humans (8). This work suggested a novel application for captopril in the management of radiation injury.
Subsequent work has noted that the mitigation effect was a property of many different ACEIs (9-11). In addition, some recent clinical studies provide even stronger support for the concept behind the study, as both prospective (9) and retrospective (10-12) studies have shown evidence that ACEIs can decrease radiation-induced pulmonary injury.
NRG Oncology RTOG 0123 was also designed to investigate the pulmonary expression of CCL3, TNFα, IL-1 and IL-6 at specific time intervals after irradiation. RTOG 91-03 found that elevated serum IL-6 after 10 Gy of lung irradiation was associated with grade 2+ acute lung toxicity (13). The role of these cytokines required further study.
Methods
Study objectives
NRG Oncology RTOG 0123 had four study objectives: 1) Test the ability of captopril to alter the incidence of pulmonary damage at 12 months after completion of radiotherapy (primary endpoint); 2) Investigate the pulmonary expression of CCL3, TNFα, IL-1 and IL-6 at specific time intervals; 3) Prospectively analyze whether the European Organization for Research and Treatment for Cancer (EORTC) Quality of Life Questionnaire (QLQ-C30) and the lung cancer module (QLQ-L13) scales are consistent in their measurement of pre-therapy symptoms and ensuing changes after therapy for patients with primary lung cancer, including effects related to the addition of captopril; 4) Determine if captopril's effect on pulmonary toxicity persists after completion of drug delivery.
Eligibility Criteria
Study patients had Stage II-IIIB non-small cell lung cancer, Stage I central NSCLC, or limited-stage small-cell lung cancer (nonmetastatic disease that would be receiving radiotherapy confined to a single treatment area and chemotherapy). Patients were ≥18 years of age and had a Zubrod Performance Status of 0-1. The planned total dose of radiotherapy, ≥45 Gy, was delivered to the target volume, with >25% of total lung volume receiving >20 Gy if receiving radiotherapy alone. Patients with < 25% of the lung receiving >20 Gy were only eligible if receiving concurrent chemoradiotherapy. Lobectomy or segmentectomy was allowed, as was induction or concomitant chemotherapy (either during radiotherapy or during therapy with captopril).
Patients had to meet the following pretreatment evaluation criteria: systolic BP >110, diastolic BP >60, absolute granulocyte count ≥1000/mm3, platelets ≥75000/mm3, Hg >9.0 g/dl, BUN <25 mg/dl, serum creatinine <1.6 mg/dl, serum bilirubin <1.5 mg/dl, SGOT <2X normal, serum Na and K within institutional normal, urine protein <10 mg/dl, urine glucose negative, and negative pregnancy test for women of childbearing potential.
Patients were ineligible if they were currently pregnant, receiving IMRT, required ACE inhibitor or ACE therapy for hypertension or congestive heart failure, had collagen vascular disease, rheumatoid arthritis, or a known hypersensitivity to ACE inhibitors, or if they were on lithium, methotrexate, or procainamide.
Trial Design
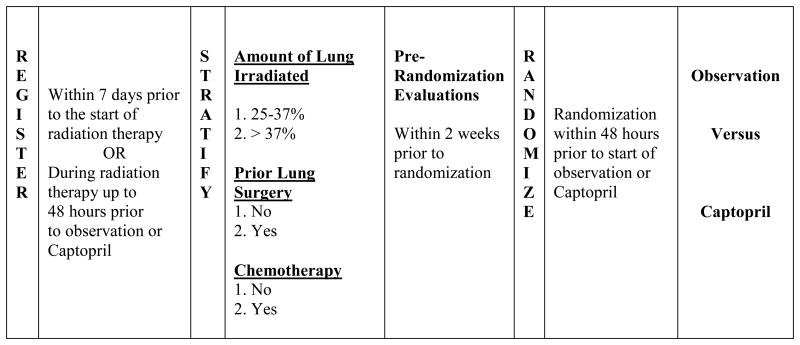
NRG Oncology RTOG 0123 was a multi-institution randomized phase II trial in which the observational control arm was incorporated to determine pulmonary toxicity rates in the current patient population. Patients registered to the trial during radiation therapy. Patients meeting pretreatment evaluation criteria at the completion of radiation therapy were eligible to continue to the randomization portion of the trial. Specifically, patients with a resting blood pressure lower than 110/60 were not permitted to continue. Patients were stratified according to amount of lung irradiated to > 20 Gy [25%-37%, >37%], prior lung surgery [no vs. yes], and chemotherapy [no vs. yes]. Patients were then randomized to the captopril treatment or observation arm within 48 hours prior to completion of radiation therapy, and captopril therapy was to begin the first business day after completing radiotherapy. Patients randomized to captopril were administered a 6.25 mg test dose pill before beginning treatment. Patients were scheduled to receive 12.5 mg three times a day (TID), one hour before meals for weeks 1-2, 25 mg TID for weeks 3-4, and 50 mg TID for weeks 5-52. Patients with a resting blood pressure lower than 110/60 did not escalate to the next dose level and remained on the current dose level. Randomization was performed using the Zelen (14) treatment allocation scheme to balance patient factors and institution. Patients were evaluated weekly for adverse events using the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0 (CTCAE v3.0) [Figure 1]. Quality of Life was assessed using the EORTC QLQ-C30 and the LC-13 module (v3).
Figure 1. Registration, stratification, and randomization schema for NRG Oncology RTOG 0123.
Statistical Methods
The primary endpoint of the study was incidence of Grade 2+ radiation-induced pulmonary toxicity within 1 year after completion of radiation. Assuming that the incidence of pulmonary toxicity would be 50%, based on Fisher's exact test with a significance level of 0.05 (one-sided), 168 randomized patients would be required to have 80% statistical power to detect a 40% relative reduction (from 50% to 30%) in the number of patients experiencing pulmonary toxicity while receiving captopril. Assuming that 15% of cases would not continue to the randomization stage and 5% of patients would be found retrospectively ineligible, the target sample size was set at 205 patients. Patients that received no captopril or just the test dose, or were lost to follow-up or died within the first year prior to experiencing a radiation-induced pulmonary toxicity, were excluded from analysis of the primary endpoint.
Results
NRG Oncology RTOG 0123 accrued 81 patients between June-2003 and August-2007 for an average monthly accrual of 1.6 patients. The trial closed early due to slow accrual. Patients were followed for a minimum of 12 months. Three patients did not meet the eligibility criteria at registration. Of the 78 eligible patients, 17 (22%) were randomized to the observation arm, 16 (21%) were randomized to receive captopril, and 45 (58%) did not reach the randomization phase. Of these 45 patients 5 withdrew consent. Forty patients were not randomized post-radiation due to patients' refusal (11), physician preference (6), ineligibility for randomization (5), adverse event during RT (3), disease progression/death (4), or not specified (11). There were four patient deaths due to lung cancer in the observation arm; there was one patient death due to lung cancer and two patients lost to follow-up in the captopril arm. Six patients randomized to receive captopril did not begin study drug treatment due to either an adverse drug reaction to the test dose (in their case, hypotension) or patient refusal to begin the study drug, and thus were excluded. Thus, of the 33 randomized patients, 20 were analyzable (13 observation, 7 captopril) [Figure 2]. Patient characteristics were well balanced between the observation and captopril treatment arms (Table 1).
Figure 2. CONSORT Diagram.
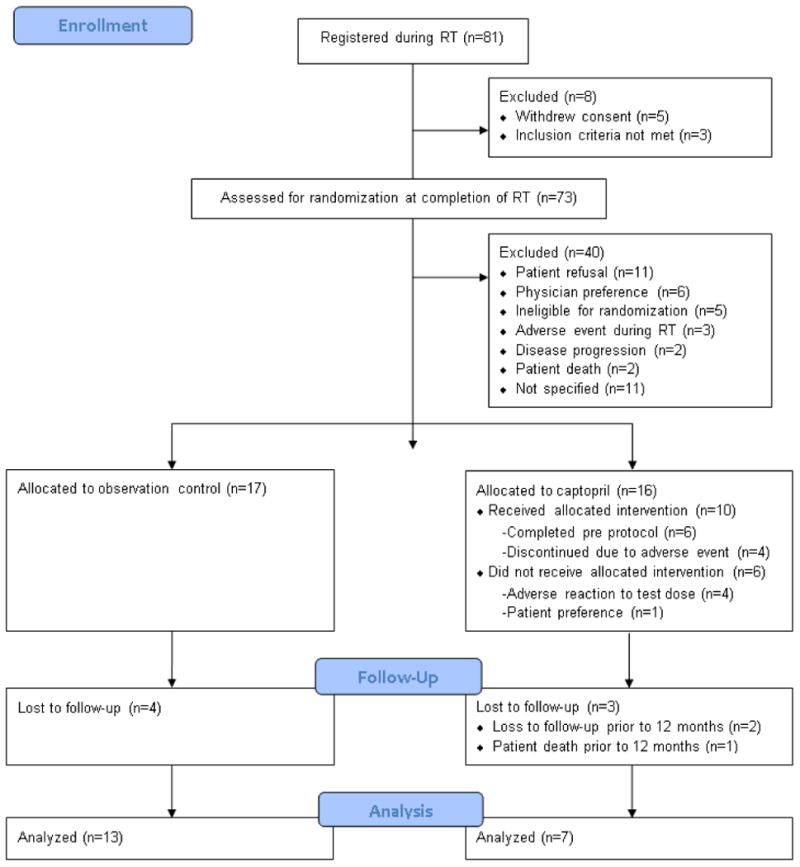
Table 1. Pretreatment Characteristics.
| Observation (n=17) | Captopril (n=10) | |||
|---|---|---|---|---|
| Age | ||||
| Median | 67 | 64 | ||
| Min-Max | 42-87 | 46-75 | ||
| n | % | n | % | |
|
|
||||
| Gender | ||||
| Male | 8 | 47 | 5 | 50 |
| Female | 9 | 53 | 5 | 50 |
| Zubrod Performance Status | ||||
| 0 | 8 | 47 | 5 | 50 |
| 1 | 9 | 53 | 5 | 50 |
| Amount of Lung Irradiated | ||||
| 25-37% | 11 | 65 | 8 | 80 |
| >37% | 6 | 35 | 2 | 20 |
| Prior Lung Surgery | ||||
| No | 14 | 82 | 8 | 80 |
| Yes | 3 | 18 | 2 | 20 |
| Planned Systemic Chemotherapy | ||||
| No | 1 | 6 | 0 | 0 |
| Yes | 16 | 94 | 10 | 100 |
Treatment compliance was reported for the first 5 weeks of study drug treatment during the dose escalation phase. Treatment compliance beyond 5 weeks was monitored by institution and not reported centrally. Of the 10 patients that started captopril treatment, six (60%) completed treatment per protocol and escalated to the 50 mg regimen and four (40%) discontinued treatment due to side effects. Of the four cases discontinuing treatment prior to 5 weeks, two cases completed 2 weeks of treatment with a maximum dose of 12.5 mg, one case completed 4 weeks of treatment with a maximum dose of 12.5 mg, and one case completed 4 weeks of treatment with a maximum dose of 50 mg.
Since the number of patients reaching the randomization phase (33) was much less than anticipated (168), the study did not have sufficient power to detect the projected 40% reduction in pulmonary toxicity due to captopril. Given the actual sample size, the power would have been 25%. P-values are not reported as the following is a descriptive analysis of the study results.
The median follow-up time for the 20 analyzable patients included was 16.5 months (range 3.4 – 30.0 months). The incidence of grade 2+ pulmonary toxicity attributed to radiation was 14% (1/7) and 23% (3/13) in the captopril and observation treatment arms, respectively. The incidence of all grade 2+ pulmonary toxicities was 57% (4/7) and 39% (5/13) in the captopril and observation treatment arms, respectively (Table 2). Table 3 lists all nine grade 2+ pulmonary toxicities along with treatment and modality attributions. Due to the small number of patients accrued and low compliance on the EORTC QLQ-C30 and EORTC QLQ-LC13 assessments, QOL and the cytokines were not analyzed.
Table 2. Grade 2+ Pulmonary Adverse Events within 12 months.
| Observation<br>(n=13) | Captopril<br>(n=7) | |||
|---|---|---|---|---|
| Follow-up Time (months) | ||||
| Median | 16.3 | 17.8 | ||
| Range | 3.4 – 30.0 | 4.0 – 27.2 | ||
| Time to Grade 2+ Pulmonary adverse event(Radiation) | ||||
| Median | 2.93 | 3.29 | ||
| Range | 1.9 – 7.9 | 3.29 – 3.29 | ||
| Time to Grade 2+ Pulmonary adverse event(Any modality) | ||||
| Median | 2.73 | 4.77 | ||
| Range | 1.9 – 7.9 | 3.3 – 6.7 | ||
| Events | % | Events | % | |
|
|
||||
| Grade 2+ Pulmonary adverse event<br>(Radiation) | 3 | 23 | 1 | 14 |
| Grade 2+ Pulmonary adverse event<br>(Any modality) | 5 | 39 | 4 | 57 |
CTCAE v3.0
Table 3. All Grade 2+ Pulmonary Adverse Events.
| RX | CN | Treatment Attribution | Modality Attribution | Months from Randomization | Grade |
|---|---|---|---|---|---|
| Observation | 1A | Unrelated/Unlikely | Chemotherapy | 2.5 | 3 |
|
| |||||
| 1B | Unrelated/Unlikely | Radiotherapy | 7.9 | 2 | |
|
| |||||
| 1C | Unrelated/Unlikely | Chemotherapy | 2.7 | 4 | |
|
| |||||
| 1D | Possible/Probable/Definite | Radiotherapy | 1.9 | 3 | |
| Unrelated/Unlikely | Radiotherapy | 3.6 | 2 | ||
|
| |||||
| 1E | Possible/Probable/Definite | Radiotherapy | 2.9 | 3 | |
|
| |||||
| Captopril | 2A | Possible/Probable/Definite | Chemotherapy | 6.7 | 2 |
|
| |||||
| 2B | Possible/Probable/Definite | Captopril | 5.9 | 3 | |
| Unrelated/Unlikely | Chemotherapy | 5.9 | 2 | ||
|
| |||||
| 2C | Possible/Probable/Definite | Radiotherapy | 3.3 | 3 | |
| Unrelated/Unlikely | Thoracentesis | 3.7 | 3 | ||
|
| |||||
| 2D | Possible/Probable/Definite | Thoracentesis | 3.7 | 3 | |
| Unrelated/Unlikely | Thoracentesis | 4.0 | 2 | ||
CTCAE v3.0
Discussion
The clinical syndromes of pneumonitis and fibrosis are associated with radiation therapy and several cytotoxic drugs, including bleomycin and methotrexate. The disease course is biphasic and is dependent upon the dose and volume of lung exposed. Lower doses of lung irradiation produce subclinical pathologic effects that can be expressed by added insult such as infection or drugs. Clinical manifestations typically occur 1-3 months after completion of radiation or drug therapy; occasionally, an accelerated phase of the syndrome results within days after an offending drug is administered. There may be a low-grade fever along with respiratory symptoms such as congestion and cough. In more severe cases, dyspnea and pleuritic chest pain may be present. When tolerance doses are exceeded, pneumonitis can be very severe and produce acute respiratory distress along with spiking fever, acute cor pulmonale, or death (15). Patients who survive this period experience a protracted period of pneumonitis that can last from days to months.
After the acute symptoms, there is an intermediate phase during which histologic changes continue, but in which the symptoms are not as marked. This eventually progresses to a fibrotic phase. Chronic effects of cytotoxic therapy are observed from months to years following treatment. Pulmonary fibrosis develops insidiously in the previously irradiated field, but stabilizes after 1-2 years. Most patients with radiation pulmonary fibrosis are asymptomatic. In a few patients, chronic respiratory failure may be present with symptoms such as dyspnea on exertion, cyanosis, or chronic cor pulmonale. Corresponding changes in pulmonary function tests are measured as a reduction in tidal volume and minute ventilation, and an increase in respiratory rate. Improvement in pulmonary function resulting from radiation response of lung tumor may compensate for losses caused by radiation fibrosis (16).
The incidence of radiation pneumonitis has been correlated with the percentage of total lung receiving greater than 20 Gy. In one series, when the percentage of total lung volume receiving >20 Gy was less than 25%, the incidence of pneumonitis was 0-4%. When the total lung volume receiving >20 Gy was 25-37%, the incidence of pneumonitis was 2-12%. When the total lung volume receiving >20 Gy was >37%, the incidence of pneumonitis rose to 19-30% (17).
Several randomized trials have shown survival advantages in patients receiving higher dosages of radiation therapy. In the Radiation Therapy Oncology Group (RTOG) 73-01 study, 376 patients were randomized between a radiation dose of 40 Gy split course and a continuous fractionation schedule of 40, 50, or 60 Gy; all patients were treated with two-dimensional treatment techniques. The local-regional failure rates at 3 years in the different arms were 44%, 52%, 42% and 33% respectively (18). Strategies to increase pulmonary tolerance to radiation might enable the oncologist to treat local-regional lung cancer more aggressively. NRG Oncology RTOG 0123 sought to investigate the utility of the angiotensin converting enzyme inhibitor (ACEI) captopril in this regard.
Interest in the concept of mitigating radiation induced pulmonary injury with ACEIs remains strong despite the failure of this study to accrue an adequate number of patients. The basic concept now has the active support of the National Cancer Institute (19), and there is a growing base of experimental (9-11) and clinical (9-12) evidence that supports the efficacy of this approach.
This trial failed for two reasons, low accrual and a surprising large number of accrued patients who were not analyzable (>75% loss rather than the anticipated 20%). It has been suggested that a major problem was “the difficulty lung cancer patients have taking a blood pressure medication”; however, since a large number of lung cancer patients (>40% in a recent retrospective study (14)) are already taking such drugs, this is unlikely to be the critical issue. Informal discussions with some of the investigators and data managers suggest that there were at least two other factors: the extra patient visits required for the 5-week drug escalation period, and a TID drug schedule. There are potential solutions to both issues. First, the newer generation of ACEIs are taken only once per day, and some of these (e.g., enalapril) have mitigation efficacy in experimental models at least as good as that found for captopril (5, 9,11). Second, as there is now strong experimental (20) and clinical (10-12) evidence that the use of ACEIs during cancer therapy is safe, ACEI dose escalation could be done before the end of radiotherapy. In fact, a pilot study at Mayo Clinic in preparation for a larger Alliance for Clinical Trials in Oncology study is looking at the safety of lisinopril vs placebo starting within 7 days of thoracic radiation therapy to try to mitigate radiation pneumonitis (ClinicalTrials.gov Identifier: NCT01880528). As expected, the biggest problem with these types of trials is the large number of lung cancer patients who are already on ACEIs and other antihypertensives (10-12) and who would hence be ineligible for the trial.
Conclusions
In summary, while NRG Oncology RTOG 0123 did not accrue enough patients to assess efficacy, it did show the safety of such an approach and suggests that the use of newer ACEIs (e.g., enalapril or lisinopril) started during radiotherapy should solve many of the accrual problems faced by this trial.
Acknowledgments
Source of funding: This project was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI) Cancer Institute.
Footnotes
Abstract presented at the American Society for Radiation Oncology (ASTRO) Annual Meeting, Chicago, IL, 2009.
Conflicts of Interest: Dr. Moore reports a current consultancy for BMS. Dr. Fintel reports past grants to institution for work under consideration for publication. Dr. Lutz reports royalties from Wiley Publishers. Dr. Movsas reports current grant to institution from ACR/RTOG for work under consideration for publication, current support for travel from ACR/RTOG for role of QOL Chair for work under consideration for publication, and current and pending grants from Varian and Philips for research unrelated to this study. Dr. Moulder reports past grants to institution from RTOG for work under consideration for publication, past grants to Dr. Moulder and his institution from NIH outside the submitted work, and pending grant to institution from the VA outside the submitted work. Stephanie Pugh reports a past grant from the PA Department of Health.
References
- 1.Siegel R, Naishadham, D, Jemal, A Cancer statistics, 2013. CA CANCER J CLIN. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76(Suppl. 1):S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward WF, Kim YT, Molteni A, et al. Radiation-induced pulmonary endothelial dysfunction in rats: Modification by an inhibitor of angiotensin converting enzyme. Int J Radiat Oncol Biol Phys. 1988;15:135–140. doi: 10.1016/0360-3016(88)90357-4. [DOI] [PubMed] [Google Scholar]
- 4.Ward WF, Lin PJ, Wong PS, et al. Radiation pneumonitis in rats and its modification by the angiotensin-converting enzyme inhibitor captopril evaluated by high-resolution computed tomography. Radiat Res. 1993;135:81–87. [PubMed] [Google Scholar]
- 5.Molteni A, Moulder JE, Cohen EP, et al. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76:523–532. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 6.Cohen EP, Molteni A, Hill P, et al. Captopril preserves function and ultrastructure in experimental radiation nephropathy. Lab Invest. 1996;75:349–360. [PubMed] [Google Scholar]
- 7.Stone HB, Moulder JE, Coleman CN, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI workshop, December 3-4, 2003 Radiat Res. 2004;162:711–728. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 8.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen EP, Bedi M, Irving AA, R E, et al. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83(1):292–296. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharofa J, Cohen EP, Tomic R, et al. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84(1):238–243. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Bezjak A, Soyfer V, Yi Q, et al. Radiation pneumonitis in lung cancer patients - The neglected patient-related variables. Int J Radiat Oncol Biol Phys. 2005;63(Suppl. 1):S229–S229. [Google Scholar]
- 12.Jenkins P, Watts J. An improved model for predicting radiation pneumonitis incorporating clinical and dosimetric variables. Int J Radiat Oncol Biol Phys. 2011;80:1023–1029. doi: 10.1016/j.ijrobp.2010.03.058. [DOI] [PubMed] [Google Scholar]
- 13.Hartsell WF, Scott CB, Dundas GS, et al. Can serum markers be used to predict acute and late toxicity in patients with lung cancer? Analysis of RTOG 91-03 Am J Clin Oncol. 2007;30:368–376. doi: 10.1097/01.coc.0000260950.44761.74. [DOI] [PubMed] [Google Scholar]
- 14.Zelen M. The randomization and stratification of patients to clinical trials. J Chron Dis. 1974;27:365–375. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 15.Rubin P, Casarett GW. Clinical Radiation Pathology. Philadelphia: W. B. Saunders Co; Respiratory system; pp. 1968pp. 423–470. [Google Scholar]
- 16.Evans RF, Sagerman RH, Ringrose TL, et al. Pulmonary function following mantle-field irradiation for Hodgkin's disease. Radiology. 1974;111:729–731. doi: 10.1148/111.3.729. [DOI] [PubMed] [Google Scholar]
- 17.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 18.Perez CA, Bauer M, Edelstein S, et al. Impact of tumor control on survival in carcinoma of the lung treated with irradiation. Int J Radiat Oncol Biol Phys. 1986;12:539–547. doi: 10.1016/0360-3016(86)90061-1. [DOI] [PubMed] [Google Scholar]
- 19.Movsas B, Vikram B, Hauer-Jensen M, et al. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clini Cancer Res. 2010;17:222–228. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 20.Kohl RR, Kolozsvary A, Brown SL, et al. Differential radiation effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440–445. doi: 10.1667/RR0707.1. [DOI] [PubMed] [Google Scholar]