Abstract
Regular cycles of exposure to light and dark control pineal melatonin production and temporally coordinate circadian rhythms of metabolism and physiology in mammals. Previously we demonstrated that the peak circadian amplitude of nocturnal blood melatonin levels of rats were more than 6-fold higher after exposure to cool white fluorescent (CWF) light through blue-tinted (compared with clear) rodent cages. Here, we evaluated the effects of light-phase exposure of rats to white light-emitting diodes (LED), which emit light rich in the blue-appearing portion of the visible spectrum (465–485 nm), compared with standard broad-spectrum CWF light, on melatonin levels during the subsequent dark phase and on plasma measures of metabolism and physiology. Compared with those in male rats under a 12:12-h light:dark cycle in CWF light, peak plasma melatonin levels at the middark phase (time, 2400) in rats under daytime LED light were over 7-fold higher, whereas midlight phase levels (1200) were low in both groups. Food and water intakes, body growth rate, and total fatty acid content of major metabolic tissues were markedly lower, whereas protein content was higher, in the LED group compared with CWF group. Circadian rhythms of arterial plasma levels of total fatty acids, glucose, lactic acid, pO2, pCO2, insulin, leptin, and corticosterone were generally lower in LED-exposed rats. Therefore, daytime exposure of rats to LED light with high blue emissions has a marked positive effect on the circadian regulation of neuroendocrine, metabolic, and physiologic parameters associated with the promotion of animal health and wellbeing and thus may influence scientific outcomes.
Abbreviations: CWF, cool white fluorescent; ipRGC, intrinsically photosensitive retinal ganglion cell; LED, light-emitting diodes; SCN, suprachiasmatic nuclei; TFA, total fatty acids
Since the advent of light-emitting diodes (LED), both the laboratory animal science community and the international lighting industry have given much consideration to the use of this emerging technology. Currently, the most common light source used in vivaria and offices around the world is broad-band fluorescent white light, referred to as cool white fluorescent (CWF) light. LED light has many advantages over both CWF and incandescent lighting, including higher efficiency (that is, illuminance per watt of power), lower heat production, and a significantly longer operating life.34 Furthermore, when its additional advantages of superior spectral control, sturdiness (solid state), size, and weight are considered, LED lighting offers the obvious long-term, inexpensive alternative to conventional lighting. Interestingly, although more animal supply companies and biomedical research institutions worldwide are embracing this new technology, little to no information is available regarding the long-term use of LED lighting and its potential effects on animal behavior, physiology and metabolism.
Light profoundly influences circadian, neuroendocrine, and neurobehavioral regulation in all mammals.2,3,13,38,54 The suprachiasmatic nucleus (SCN), the ‘master biologic clock’ located in the hypothalamus of the brain, is entrained by the daily light-dark cycle. Photobiologic responses that include circadian rhythms of metabolism and physiology, are mediated by chromophores, small organic molecules, contained within a small subset of retinal cells called intrinsically sensitive retinal ganglion cells (ipRGC)3,14,25,32, 47,50,55 Light quanta are detected by the chromophore melanopsin mostly in the blue-appearing portion of the visible spectrum (465 to 485 nm) in both humans and rodents, and the photic information is transmitted to the SCN via the retinohypothalamic tract. The SCN controls the daily pineal gland production of the circadian neurohormone melatonin (N-acetyl-5-methoxytryptamine), resulting in high blood levels at night and low levels during daytime.36,42,51 The daily, rhythmic melatonin signal provides temporal coordination of normal behavioral and physiologic functions associated with the promotion of animal health and wellbeing.2,7,8,12,16-20,41,51,57,58 Therefore, light in an intensity-, duration-, and wavelength- (spectral quality, or color) dependent manner at a given time of day is essential to regulation of the molecular clock of the SCN.1,3,7-12
Previous epidemiologic studies in nightshift workers reveal that light-at-night exposure23,30,43,52 acutely suppresses endogenous melatonin levels, as occurs in most mammalian species8,10,36,41,42,51,52 which may lead to various disease states, including obesity, metabolic syndrome5,59 and carcinogenesis.4-6,15,18 Our laboratory's earlier light-to-night studies in tumor-bearing rats provided experimental support for these studies.4-6,15,18,21,22 Recently we demonstrated that relatively small changes in the spectral transmittance (color) of light that rodents experience during the light phase markedly influences the normal nighttime melatonin signal, leading to alterations in the temporal coordination of metabolism and physiology.16,19,20,58 Most notable was the finding that, in both male and female pigmented nude rats exposed to primarily blue light during daytime, nighttime melatonin levels were nearly 6 times higher than those in animals exposed to full-spectrum CWF light. We also showed that the same daytime blue-light exposure that enhanced the nighttime melatonin levels so dramatically also significantly inhibited the metabolism, signal transduction activity, and growth of human prostate cancer xenografts in nude male rats.22 One way to expand the melatonin duration in the context of a 12-h dark phase is by using blue-enriched LED light to markedly increase the melatonin amplitude during the 12-h light phase. This possibility represents both hyperproduction and hypersecretion of melatonin, given that, following its synthesis, melatonin is released immediately into the bloodstream and cerebrospinal fluid.
A study in human subjects diagnosed with midwinter insomnia coupled with low nighttime melatonin levels demonstrated that daily exposure to intense morning bright polychromatic light therapy for up to one week resulted in a restoration of nocturnal melatonin levels to those of control subjects.31 In a second study, exposure to blue-tinted (470 nm) LED light (100 lx) for a period of about 20 min in the morning after 2 sleep-restricted (6 h) nights led to earlier onset of the melatonin surge at nighttime.26
The 8th edition of the Guide for the Care and Use of Laboratory Animals (2011),37 as well as the several prior editions, focuses primarily on retinopathy concerns in rodents relative to vivarium lighting. Little to no mention is made regarding circadian regulation by light and lighting protocols in the brief section devoted to animal facility lighting. In addition, there is no information pertaining to different types of lighting, such as LED lighting, and their potential influence on animal health and wellbeing. Currently, research in this area is minimal, and a consistent and adequate method for quantifying light is unavailable, thus complicating efforts to replicate experimental conditions and to compare results across investigations. Therefore, our laboratory aims to thoroughly report all light measurements necessary to facilitate the replication of experimental conditions, including comprehensive spectral power distribution measurements, which are currently the most widely accepted methodology available in the lighting industry, thereby enabling comparison of results across investigations.
With these issues in mind, the overall purpose of this study was to compare the influence of daytime LED lighting, which is high in emission of blue-appearing, short-wavelength light in visible spectrum with that of broad-spectrum CWF light on metabolic and physiologic measures of laboratory animal health and wellbeing. Specifically, we examined the hypothesis that, compared with those of standard CWF lighting, the spectral characteristics (color) of bright blue-enriched LED light during the light phase not only amplifies the normal circadian nocturnal melatonin signal but also alters the circadian regulation of plasma measures of metabolism and physiology in male albino rats. Ultimately the goal of this research is to optimize lighting for homeostatic regulation in laboratory animals, thus fostering improvement of their health and wellbeing.
Materials and Methods
Animals, housing conditions, and diet.
The male, nonpigmented, inbred Buffalo rats (Rattus norvegicus; BUF/CrCrl; age, 3 to 5 wk) used in this study were purchased from Charles River (Wilmington, MA) and maintained in an AAALAC-accredited facility in accordance with The Guide for the Care and Use of Laboratory Animals.37 All procedures for animal use were approved by the Tulane University IACUC.
Rats were maintained as described below in autoclaved cages by using hardwood maple bedding (catalog no. 7090, Teklad Maple Sanichips, Envigo, Madison, WI; 2 bedding changes weekly). To ensure that all rats remained free from both bacterial and viral agents, serum samples from sentinel animals were tested quarterly (Multiplex Fluorescent Immunoassay 2, IDEXX Research Animal Diagnostic Laboratory, Columbia, MO) and during the course of this study, as described previously.19-21,58 Throughout the experiment, rats were measured daily for dietary and water intake and body weight changes. Animals were given free access to diet (no. 5053 Irradiated Laboratory Rodent Diet, Purina, Richmond, IN) and acidified water. Quadruplicate determinations of this diet contained 4.75 g total fatty acid (TFA) per 100 g of diet composed of 0.70% myristic (C14:0), 13.75% palmitic (C16:0), 1.20% palmitoleic (C16:1n7), 3.60% stearic (C18:0), 24.10% oleic (C18:1n9), 50.20% linoleic (C18:2n6), 6.02% γ-linolenic, and 0.25% arachidonic (C20:4n6) acids. Minor amounts of other FA comprised 0.18%. Conjugated linoleic acids and trans FA were not found. More than 90% of the TFA were in the form of triglycerides; more than 5% was in the form of free fatty acids.
Caging, lighting regimens, and spectral transmittance measurements.
After a 1-wk acclimation period under control lighting conditions (that is, CWF), rats were randomized into 2 designated groups of 8 control (CWF) and 16 experimental (LED lighting) rats in standard translucent laboratory rodent cages (10.5 in. × 19.0 in. × 8.0 in.; wall thickness, 0.1 in.; 2 rats per cage). Standard rodent cages used in this study were purchased from Ancare (polycarbonate translucent clear, catalog no. R20PC; Bellmore, NY). The SPF animals were maintained in environmentally controlled rooms (25 °C; 50% to 55% humidity) with diurnal lighting (12:12 h light:dark; lights on, 0600). The CWF control animal room was lighted with a series of 2 overhead luminaires containing 4 Philips standard soft, cool-white (2700 lm; 4100 correlated color temperature) fluorescent lamps per ballast (F32T8TL841 [model no. 272484], Alto II Collection, 32 W, 48 in., series 800; Philips, Somerset, NJ). The experimental LED animal room was lighted with a series of 2 overhead luminaires containing 4 LED lamps, high in emission of blue-appearing portion of the visible spectrum (465 to 485 nm; 2650 lm; >5000 correlated color temperature), per ballast (12T8/AMB/48 [model no. 9290011242], T 8 12 W, 48 in., Philips). Study lamps are shown in Figure 1. Animal rooms were completely devoid of light contamination during the dark phase.4,5
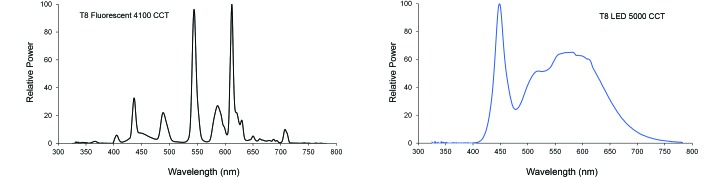
Figure 1.
Normalized spectral power distributions of the cool white fluorescent (CWF; left panel) and blue-enriched light emitting diode (LED; right panel) lamps. CCT, color-correlated temperature.
Daily during this experiment, the animal room was monitored for normal light-phase lighting intensity at 1 m above the floor in the center of the room (at rodent eye level) and outside, from within, and at the front of the animal cages. Irradiance measures were recorded by using an radiometer–photometer (IL-1400A, International Light Technologies, Peabody, MA) with a silicon-diode detector head (model no. SEL033) with a wide-angle input optic (no. W6849) and a filter (no. 23104) that provided a flat response across the visible spectrum. Illuminance measures used a silicon-diode detector head (model no. SEL033) with a wide angle input optic (no. W10069) and a filter (no. Y23104) to provide a photopic illuminance response. The meter and associated optics were calibrated annually, as described previously.5-11,15-22 Each day and at the same time (0800), prior to light intensity measurements for that day, all cages on the rack shelf were rotated one position to the right (placed at an identical, premeasured distance apart) in the same horizontal plane; the cage at position no. 4 (last position at far right on the shelf) was moved to position no. 1 (first position at far left on the shelf). Although differences in light intensity, as measured outside and from within the front of each cage at each of the 4 positions, did not differ significantly, the daily cage shift further insured uniformity of intensity of ocular light exposure and accounted for the effects of any unforeseen subtle differences due to position on the rack shelf.
Under current convention, when discussing human and laboratory animal environments, the term ‘lux’ (lx) is used to indicate the amount of light falling on a surface that stimulates the mammalian eye during daytime, that is, the perceived brightness to the eye (photometric values). Measures of lux are appropriate for human daytime vision but are not appropriate for quantifying light stimuli that regulate circadian, neuroendocrine, or neurobehavioral physiology in animals or humans.12,13 Consequently, radiometric values of irradiance (μW/cm2) in the cages were measured by using the described radiometric detector. Given these standards, the light stimuli in the investigation reported here are presented in terms of lx and μW/cm2 for ease of understanding.
Spectral power measurements.
The lamps were installed in an overhead T8 assembly in a light-proof room and then the spectral characteristics of each light source were taken separately by using a handheld spectroradiometer (FieldSpec, ASD, Boulder, CO) with a cosine receptor attachment. Spectral power distributions, a measure of the concentration (as a function of wavelength) of any radiometric quantity (that is, irradiance compared with wavelength), were recorded when the meter was pointing directly at the lighting source at a distance of 30 cm with an exposure time of 1 s.
Calculation of effective rod, cone and melanopsin photoreceptor illuminances.
To calculate the effective rodent rod, cone, and melanopsin photoreceptor illuminances, the light sources were entered into a Toolbox worksheet, a software model for rodent photoreception that is freely available online.48 The spectral power distributions for the experiments shown here were imported into the worksheet in 1-nm increments between 325 and 782 nm. Toolbox lists the rodent spectral range as extending to 298 nm, outside the range of the spectroradiometer used in this study; therefore, according to Toolbox instructions, values between 298 nm - 325 nm were manually changed to 0.
Arterial blood collection.
After 2 wk of the described lighting regimens, blood was drawn by cardiocentesis in a series of 6 low-volume (> 0.5 mL) collections to obtain left ventricular arterial blood, as described previously,4-6,15-22 over a period of 30 d. Briefly, blood collections were designated at 4-h intervals to include the 24-h feeding period; each animal was tested only once every 5 d to eliminate the effects on feeding, stress, and potential mortality. Whole-blood samples were measured for pH, pO2, pCO2, glucose and lactate levels, and Hct by using an iSTAT1 analyzer and CG4+ and CG8+ cartridges (Abbott Laboratories, East Windsor, NJ). Values for glucose and lactate are reported as mg/dL and mmol/L, and the limits of detection for pH, pO2, pCO2, glucose, and lactate were, respectively, 0.01, 0.1 mm Hg, 0.1 mm Hg, 0.2 mg/dL, and 0.01 mmol/L.
Tissue harvest.
Five days after the final arterial blood collection, all rats were euthanized by means of CO2 narcosis followed by exsanguination via the right carotid artery. Tissues (liver, fat muscle, heart, lungs, kidneys, adrenals, lower intestine [gut], and brain) were rapidly removed, snap-frozen under liquid nitrogen, and then stored at –85 °C until further analysis.
Melatonin analysis.
Arterial plasma melatonin levels were measured by radioimmunoassay using the melatonin rat 125I radioimmunoassay kit (catalog no. 01-RK-MEL2, Alpco, Salem, NH; lot no. 1429.18, prepared by Bühlmann Laboratories AG, Schönenbuch, Switzerland) and analyzed by using a automated gamma counter (Cobra 5005, Packard, Palo Alto, CA), as previously described.19-22,58 The minimal detection level for the assay was 1 to 2 pg/mL plasma.
Fatty acid extraction and analysis.
Arterial plasma and tissue TFA were extracted from 0.1 mL arterial and venous samples after the addition of heptadecanoic acid (C17:0), methylated, and analyzed by using gas chromatography as previously described.4-7,15-22,58 Values for TFA represent the sum of the 7 major fatty acids (myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acids) in the blood plasma. Tissue TFA and linoleic acid levels in control and experimental groups were extracted from 0.2 mL of 20% homogenates, as previously described.15,16,21,22 The minimal detectable limit for the assay was 0.05 μg/mL.
ELISA of corticosterone, insulin, and leptin.
Arterial plasma samples were prepared in duplicate for the measurement of corticosterone, insulin, and leptin levels by using corticosterone (catalog no. 55-CORMS-E01; mouse/rat; protocol version 4-09/11–ALPCO 9/13/11), insulin (catalog no. 80-INSRTH-E01; rat, high range; protocol version 2.0–12/2/11), and leptin (cat. #22-LEPMS-E01; mouse/rat; protocol version 030112 version10 –ALPCO 2/29/12) chemiluminescent ELISA diagnostic kits (ALPCO, Salem, NH). Samples were measured by using a microplate reader (VersaMax, Molecular Devices, Sunnyvale, CA) at 450 nM. Detection sensitivity for corticosterone, insulin, and leptin plasma analyses were, respectively, 4.5 ng/mL, 0.124 ng/mL, and 10 pg/mL; lower limits of the assays were 15 ng/mL, 0.15 ng/mL, and 10 pg/mL, respectively; and the coefficient of variation of all assays was less than 4.0%.
Statistical analysis.
Unless otherwise noted, all data are presented as mean ± 1 SD for 8 animals for the CWF control group and 16 animals for the LED experimental group. The nonparametric JTK_CYCLE algorithm,35 as implemented in scripts for the R software package (R version 3.1.0; http://openwetware.org/wiki/HughesLab:JTK Cycle), was used to determine statistical significance of 24-h cycling for each analyte, with adjustments for multiple comparisons. This algorithm also was used to estimate phase (time of peak levels) and amplitude of cycling. Statistical differences between mean values in group LED compared with the control CWF group at each circadian time point were assessed by using an unpaired Student t test. A P value of less than 0.05 was considered to indicate a significant difference compared with baseline values within each group.
Results
Animal-room illumination and T8 light spectral comparison.
Animal room illumination during the daytime at the center each room and at 1 m above the floor (with the detector facing upward toward the luminaires) varied minimally (n = 240 measurements). Illuminance and irradiance values were 500.68 ± 11.24 lx (200.27 ± 4.50 µW/cm2) in the control CWF room and 494.52 ± 12.24 lx (197.81 ± 4.88 µW/cm2) in the experimental LED room. Measurements of photometric illuminance (lx) and radiometric irradiance (μW/cm2) within the cages of each group are a result of a mean of the values taken at 6 locations within the cages with the detector facing forward (front, center, and rear on right and left sides of cage) at all cage positions to accurately account for actual ocular light levels within the cages independent of the animal location. Average interior ocular light levels showed little to no intercage or intergroup variability (n = 1296 measurements) and are reported here for the CWF and LED groups, respectively, at 168.13 ± 5.35 lx (67.25 ± 2.14 µW/cm2) and 165.25 ± 5.20 lx (66.25 ± 2.08 µW/cm2). Normalized spectral power distributions of the T8 lights used in this study are shown (Figure 1). The fluorescent lamp shows signature peaks in the appropriate wavelengths (545 nm and 612 nm) for this type of light. The LED lamp shows a standard blue-weighted phosphor spectral power distribution with a peak at 448 nm. In terms of total energy in the 465- to 485-nm range, the LED lamp had more than 50% greater emissions than did the CWF lamp. Table 1 provides the calculated photon flux, irradiances, and weighted rodent photopigment illuminances for both lights used in the study. The data illustrate that, although photon flux and irradiance are similar in the 2 lights, there are marked differences (P < 0.05) in stimulation of the rodent photoreceptors. In terms of the potential light stimulation to the melanopsin containing ipRGC, the LED lamp was calculated to have more than 30% greater emissions compared with the CWF lamp.
Table 1.
Light sources used in this study
| Radiometric and photometric values (380–780 nm, inclusive) |
Retinal photopigment-weighted illuminances (α-opic lux) |
|||||
| Photon flux (photons/cm2/s) | Irradiance (µW/cm2) | S cone | Melanopsin ipRGC | Rod | M cone | |
| White fluorescent (4100 CCT) | 2.39 × 1015 | 854 | 108 | 1424 | 1701 | 1887 |
| White LED (5000 CCT) | 2.33 × 1015 | 839 | 12 | 1853 | 1998 | 2084 |
CCT, correlated color temperature
Animal food and water intakes and growth measurements.
Food and water intakes and body growth rates differed significantly (P < 0.05) between the rats maintained under either the CWF or LED lighting regimen during the course of this study. Mean (n = 87 measurements) daily dietary intake for CWF and LED groups, respectively, were 15.9 ± 0.1 and 13.1 ± 0.5 g per 100 g of body weight daily, representing a 23.1% ± 2.5% increase in average daily dietary intake of CWF over LED animals. Water intake was 21.9 ± 0.9 and 17.8 ± 1.0 mL per 100 g of body weight daily for the CWF and LED groups, respectively. The mean (n = 87 measurements per group) body growth rate for groups CWF and LED, respectively, were 4.7 ± 0.6 and 2.5 ± 0.1 g daily.
Plasma melatonin levels.
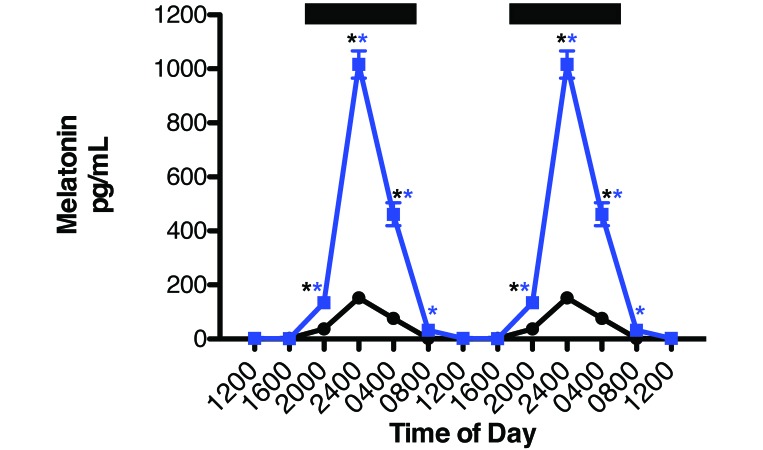
Circadian rhythms in concentrations of plasma melatonin for rats in CWF and LED lighting are shown in Figure 2. The overall pattern of daily plasma melatonin level rhythms was similar for both groups: low during daytime (less than 10 pg/mL) and significantly (P < 0.001) higher during the dark phase, with peak levels occurring between 2400 and 0400 and decreasing to a nadir between 1200 and 1600 (Table 2). However, either the peak height or phase duration of the nocturnal melatonin signal differed between the 2 groups of rats. Melatonin levels in the LED group began to rise rapidly after the onset of the dark phase, reaching the peak of those in CWF group (control) by 2000. However, the peak dark-phase melatonin level for rats in the LED group (that is, at 2400) was more than 7-fold higher (P < 0.0001) than that in control rats at the same time point. Arterial plasma melatonin levels remained more than 3-fold higher (P < 0.05) in LED compared with CWF, even at 2 h after the onset of light phase, and did not reach normal daytime levels (less than 10 pg/mL) until 1200. The integrated mean levels of melatonin over the 24-h period for LED rats (1652.1 pg/mL) were more than 7-fold higher than those of animals in CWF (228.6 pg/mL; P < 0.001).
Figure 2.
Circadian plasma melatonin levels (mean ± 1 SD) of male nonpigmented Buffalo rats (n = 12 per group) maintained for 6 wk in standard polycarbonate, translucent, clear cages under CWF (controls, solid black circles) or LED (experimental, solid blue squares) lighting with a 12:12-h light:dark photoperiod. Both groups were exposed similarly during the light phase (300 lx; 123 µW/cm2); during the 12-h dark phase lighting conditions from 1800 to 0600 (dark bars), animals were exposed to no light at night. Data are plotted twice in panels to better demonstrate rhythmicity and clarity of scale. Dark bars indicate the period of darkness. Rhythmicity analysis (Table 2) revealed robust and highly significant (P < 0.0001) rhythmic patterns under control lighting conditions for both groups, with 9.6-fold and 53.3-fold increases in nighttime amplitude compared with daytime amplitudes in CWF and LED rats, respectively, and a 5.6-fold increase in amplitude observed in LED-exposed rats compared with controls at 2400 (P < 0.001; Student t test). Concentrations with asterisks (black, CWF peaks; blue, LED peaks) differ (P < 0.05) from concentrations without asterisks.
Table 2.
Summary of JTK_CYCLE analysis for rats maintained in control CWF and experimental LED lighting environments
| Estimated peak phasea |
Amplitudea |
Q value for circadian cyclinga |
||||||
| CWF | LED | Phase shiftb (h) | CWF | LED | Fold changeb | CWF | LED | |
| Corticosterone | 2100 | 2100 | 0 | 26.25 | 7.88 | −3.33 | 7.08 × 10–4 | 3.22 × 10–2 |
| Glucose | 1400 | 1700 | 3 | 4.31 | 5.87 | 1.36 | 2.46 × 10–7 | 3.22 × 10–2 |
| Insulin | 0500 | 0400 | −1 | 0.13 | 0.08 | −1.63 | 2.48 × 10–4 | 3.27 × 10–3 |
| Lactate | 1400 | 1600 | 2 | 0.06 | 0.06 | 1.00 | 8.57 × 10–5 | 4.03 × 10–4 |
| Leptin | 2200 | 2100 | −1 | 0.05 | 0.06 | 1.20 | 4.61 × 10–4 | 3.84 × 10–1 |
| Linoleic acid | 0400 | 0400 | 0 | 382.54 | 383.25 | 1.00 | 9.07 × 10–16 | 3.52 × 10–20 |
| Melatonin | 0200 | 0200 | 0 | 14.90 | 107.61 | 7.22 | 1.22 × 10–14 | 9.56 × 10–20 |
| pCO2 | 1300 | 1300 | 0 | 1.56 | 1.27 | −1.22 | 2.64 × 10–1 | 8.74 × 10–2 |
| pO2 | 1600 | 1400 | −2 | 9.19 | 7.07 | −1.30 | 4.64 × 10–12 | 1.63 × 10–9 |
| Total fatty acids | 0400 | 0400 | 0 | 1123.59 | 1123.59 | 1.00 | 5.01 × 10–20 | 1.46 × 10–20 |
Phase-, amplitude-, and multiple-testing-adjusted P value (Q) estimated by JTK_CYCLE analysis with a fixed 24-h period and using original units as described in the text.
Phase difference and fold change are for the LED group relative to the CWF group. A decrease in LED amplitude with regard to CWF is a negative value (—); an increase is represented by a positive value.
Plasma measures of TFA and linoleic acid.
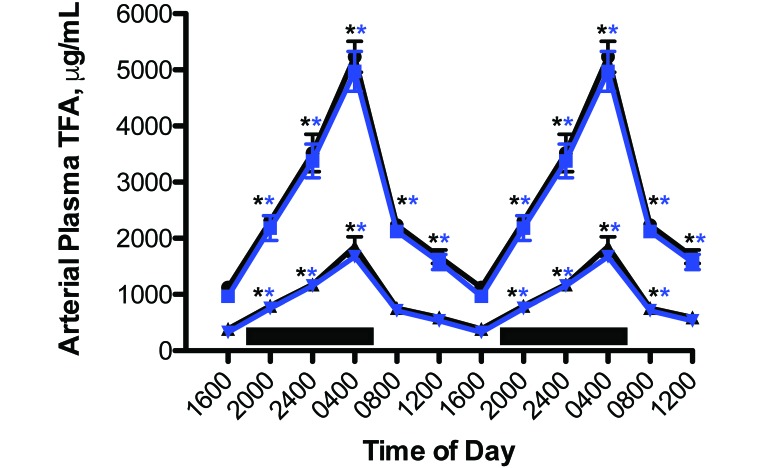
Circadian rhythms in the concentrations of arterial blood plasma TFA and linoleic acid were measured in rats with free access to the food (Figure 3). The plasma pattern of lipid levels in the experimental LED rats followed that of the control animals, which was reported earlier.4,5,15-18 Total TFA and linoleic acid areas assessed over the 24-h day for curves shown, however, differed significantly (P < 0.05) from one another at 16.0 and 5.6 mg/mL (CWF controls) and 15.2 and 5.2 mg/mL (experimental LED animals), respectively. Circadian cycling was evident for both groups, with a severely dampened amplitude during daytime (Table 2).
Figure 3.
Circadian changes in the blood plasma total fatty (TFA) and linoleic (LA) levels (mean ± 1 SD) of male nonpigmented rats (n = 12 per group) maintained fed normal chow ad libitum and maintained on either control CWF (TFA, solid black circles; LA, solid black triangles) or experimental LED (TFA, solid blue squares; LA solid inverted blue triangles) lighting conditions. Rats were exposed to dark-phase lighting conditions (see Methods) from 1800 to 0600 (dark bars). TFA values (mean ± 1 SD; n = 12 per group) are the sums of myristic, palmitic, palmitoleic, stearic, oleic, linoleic, and arachidonic acid concentrations collected at the various time points. Data are plotted twice to better demonstrate rhythmicity. Rhythmicity analysis (Table 2) revealed robust and highly significant (P < 0.0001) rhythmic patterns under baseline lighting conditions for both groups, with greater than 6-fold increases in nighttime amplitude in both groups. Concentrations with asterisks (black, CWF peaks; blue, LED peaks) differ (P < 0.05) from concentrations without asterisks.
Arterial blood glucose, lactate, acid-gas levels.
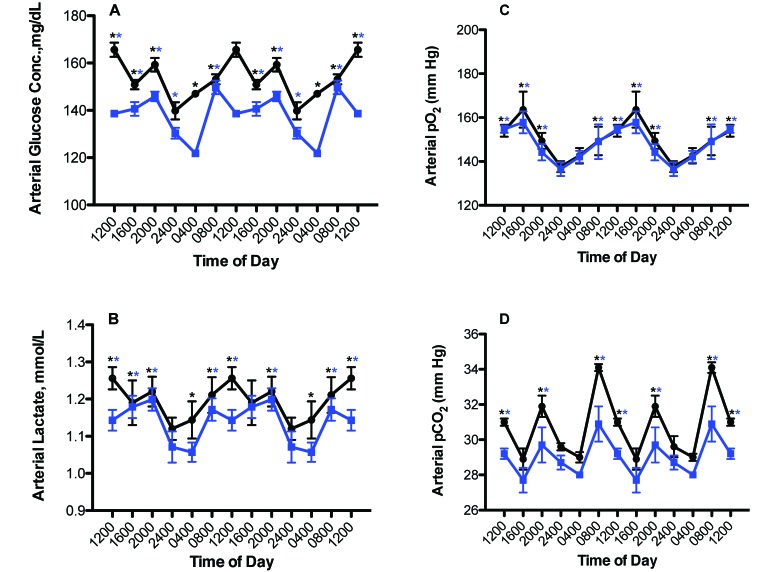
Figure 4 depicts the 24-h rhythms in levels of arterial blood glucose, lactate, pO2, and pCO2 levels in the male rats from both groups. Phase shifts were determined by comparing the peak values (acrophases) between the rats in LED (experimental) and CWF lighting regimens. A ‘phase advance’ was defined as a shift in a group peak level to an earlier time (for example, from 1600 to 1200), whereas a ‘phase delay’ was defined as a shift in a group to a later time (for example, from 0800 to 1200), as compared with control values. Daily rhythms for arterial glucose and lactate concentrations (Figure 4 A and B) were nearly similar between groups, with peaks for both constituents occurring at 1200 and 2000 for the control rats but phase-advanced 4 h at 0800 with a secondary minor peak at 2000 in the LED group (Table 2). However, values over the 24-h day were higher (P < 0.05) in control rats compared with those in the LED experimental group. The average mean blood glucose concentration calculated over the 24-h day was 152.6 ± 3.6 mg/dL for the control group and 137.8 ± 4.1 mg/dL for the experimental group (P < 0.05). The average mean blood lactate concentration calculated over the 24-h day for control CWF and experimental LED groups, respectively, were 1.2 ± 0.1 and 1.1 ± 0.1 mmol/L (P < 0.05).
Figure 4.
Circadian changes in arterial blood (A) glucose, (B) lactate, (C) pO2, and (D) pCO2 levels (mean ± 1 SD; n = 12 per group) of male rats maintained on either control (solid black circles), or experimental (solid blue squares) lighting conditions. Rats were exposed to dark-phase lighting conditions from 1800 to 0600 (dark bars). Data are plotted twice to better visualize rhythmicity. Rhythmicity analysis (Table 2) revealed robust and highly significant (P < 0.0001) rhythmic patterns for both control (CWF) and experimental (LED) groups but a significantly disrupted (P < 0.05) phase pattern for the LED group. *, P < 0.001 experimental (LED) with control (CWF) conditions (Student t test); concentrations with asterisks (black, CWF peaks; blue, LED peaks) differ (P < 0.05) from concentrations without asterisks.
Circadian rhythms in arterial pO2 and pCO2 (Figure 4 C and D) followed similar trends as the glucose and lactate rhythms. Peak values of arterial pO2 occurred at 1600 in control CWF and experimental LED rats, with a nadir at 2400. Values over the 24-h day were significantly (P < 0.05) higher in the CWF group compared with the LED group (Table 2). The calculated mean daily arterial pO2 assessed over the 24-h day (Figure 4 C) was 149.3 ± 3.1 mm Hg for the CWF rats and 146.1 ± 2.1 mm Hg in those in experimental LED (n = 72 measurements; P < 0.05). The calculated mean daily arterial pCO2 assessed over the 24-h day (Figure 5 D) was 30.9 ± 0.3 mm Hg for the CWF group and 29.1 ± 0.4 mm Hg for the LED group (P < 0.05; n = 72 measurements).
Figure 5.
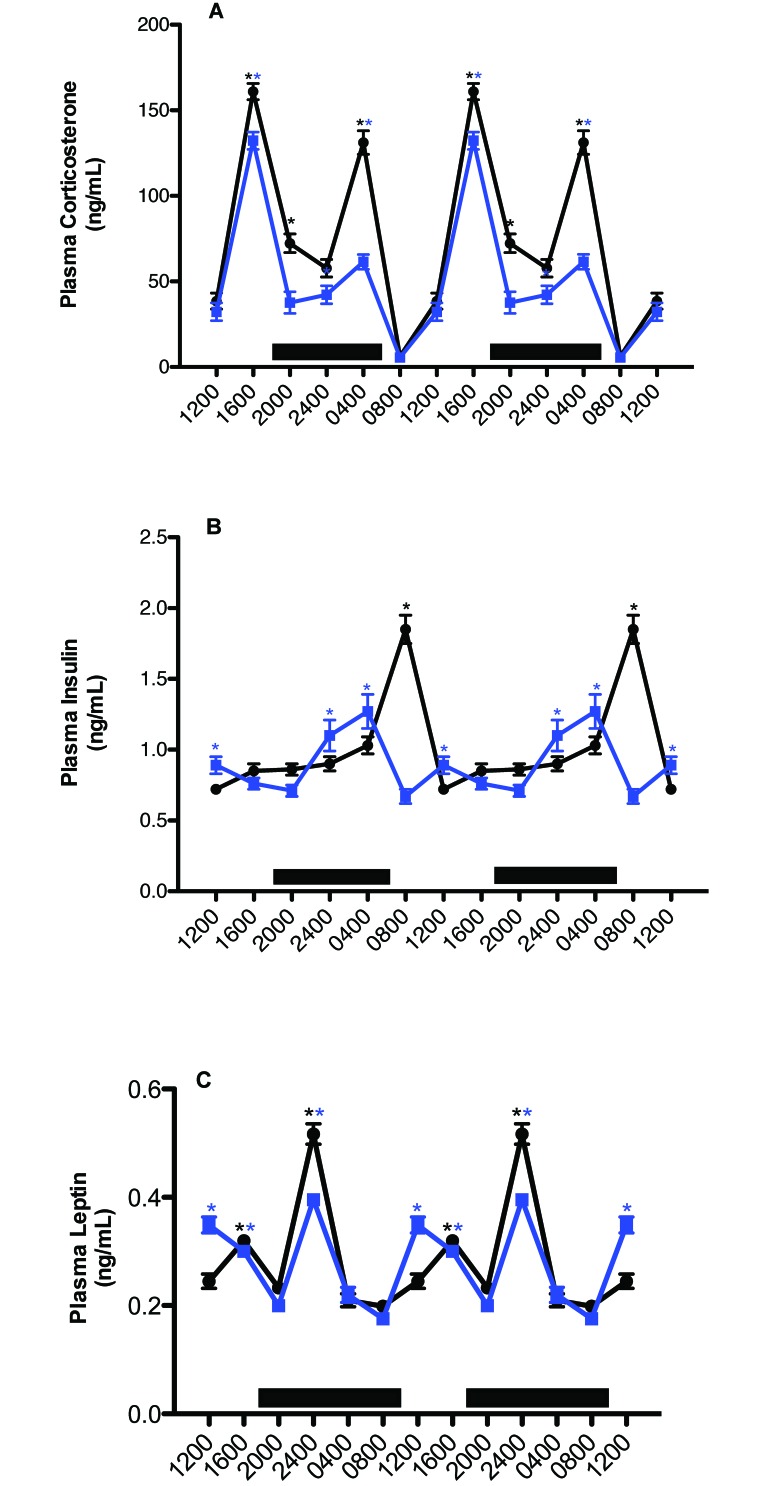
Circadian changes in plasma (A) corticosterone, (B) insulin, and (C) leptin concentrations (mean ± 1 SD; n = 12 per group) in the arterial blood of rats maintained on either CWF control (solid black circles) or LED, experimental (solid blue squares) lighting conditions. Data are plotted twice to better demonstrate rhythmicity. Rats were exposed to dark-phase lighting conditions from 1800 to 0600 (dark bars). Rhythmicity analysis (Table 2) revealed robust and highly significant (P < 0.0001) rhythmic patterns under control conditions, significant (P < 0.05) but disrupted rhythmic patterns under experimental conditions for corticosterone, insulin, and leptin, *, P < 0.001 LED compared with control CWF conditions. Concentrations with asterisks (black, CWF peaks; blue, LED peaks) differ (P < 0.05) from concentrations without asterisks.
Arterial blood pH, O2 saturation, and Hct remained relatively constant for both groups over the 24-h day, at 7.4 ± 0.1, 99.1% ± 0.02%, and 45.6% ± 0.07% (n = 72), respectively. These values are consistent with the carotid arterial values determined during previous cardiocentesis investigations at this time of day.5,15-18,19-22
Plasma measures of corticosterone, insulin, and leptin.
Figure 5 A depicts 24-h rhythms in concentrations of arterial blood plasma corticosterone. Plasma corticosterone levels revealed clear differences between the CWF and LED groups with regard to integrative concentrations, and the amplitude of the 2nd peak in the LED group was significantly lower (P < 0.05) that than of the CWF group (Table 2). Values for arterial plasma corticosterone in rats of both groups began to increase after 1200 (P < 0.05), with a major peak value occurring at 1600 in the experimental and control groups (secondary peak; P < 0.05), decreasing to a low value at 2400 (P < 0.05) for both groups. A second major peak occurred in both groups at 0400 (but was higher in control CWF rats), decreasing to a nadir at 0800 for both groups. Integrated plasma corticosterone concentrations calculated over the 24-h day were 520.4 ± 4.2 ng/mL in the CWF animals as compared with 311.4 ± 4.6 nmol/L in LED animals.
Plasma concentrations of insulin (Figure 5 B) showed clear intergroup differences with regard to 24-h rhythms (Table 2) and integrative levels. Values for arterial plasma insulin in CWF animals were at their highest levels at 2 h after onset of the light phase (0800), with a second, minor peak prior to onset of the dark phase (1600), and lowest levels occurred throughout the light phase (1200 to 1600). Rats in LED group showed peak insulin levels at 2 h prior to onset of the light phase (0400), with a second, minor peak occurring at 1200; in addition, these peaks were lower and phase-advanced 4 h compared with those in controls (Table 2). Whereas control rats experienced a rapid increase to peak insulin levels at 0800, followed by a rapid decline, this process was more protracted in the LED group, with insulin gradually increasing over an 8-h period after the onset of dark phase, rapidly declining over 4 h from their peak at 0400 to the lowest levels at 0800, and increasing once again to a secondary peak at 1200, when levels were lowest for control rats (P < 0.05). Integrated mean plasma insulin concentrations calculated over the 24-h day were significantly different (P < 0.001), at 6.2 ± 0.1 ng/mL for rats in CWF group and 5.3 ± 0.1 ng/mL for those in LED group.
Plasma concentrations of leptin (Figure 5 C) revealed clear intergroup differences with regard to 24-h rhythms (Table 2) and integrative levels. For both groups, values for arterial plasma leptin began to increase 2 h after the onset of the dark phase (P < 0.05), with peak levels in both groups occurring at 2400 and gradually decreasing to a nadir at 0400 (P < 0.05) in controls. This process was again more protracted in the experimental group, with the nadir occurring 8 h after the nighttime peak at 2400 and then rising again to a second, similar (albeit broader) peak at 1200, compared with that in control rats (P < 0.05); in addition, peaks in the experimental group were phase-advanced 4 h compared with those in controls. Nadirs in blood leptin concentrations were achieved at 0800 and 2000 in both groups of rats. Integrated plasma leptin concentrations calculated over the 24-h day were 1.7 ± 0.0 ng/mL in CWF control rats and 1.6 ± 0.0 ng/mL in rats in LED group.
Major metabolic tissue total fatty acid and protein content.
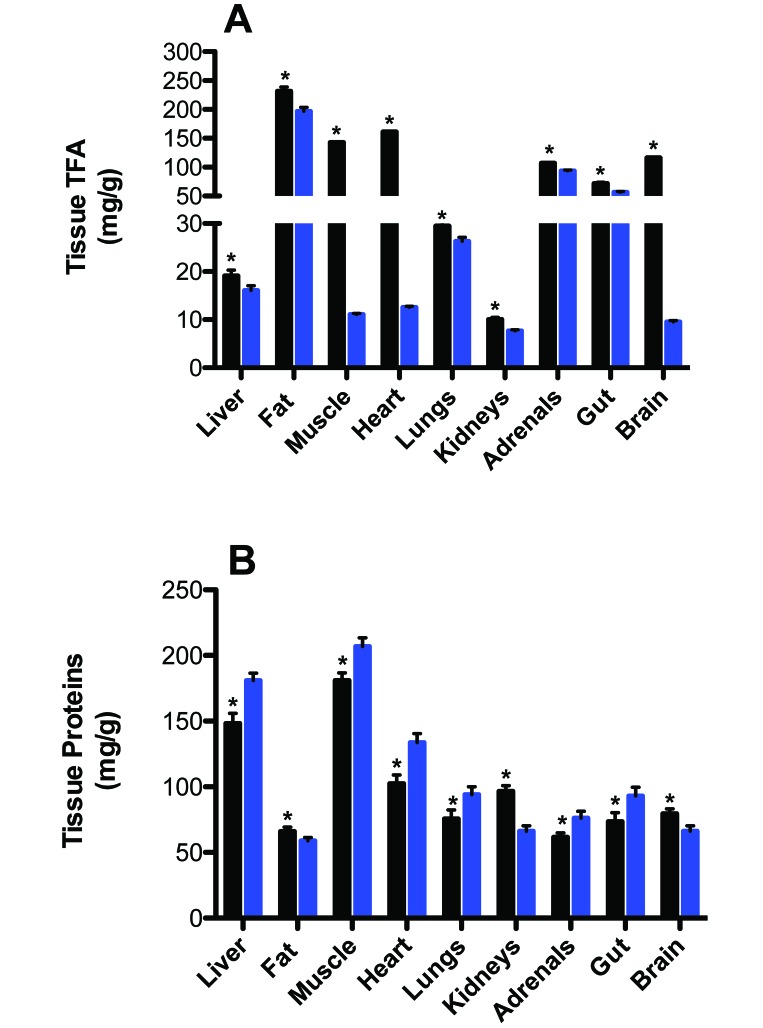
Figure 6 depicts the tissue TFA and protein contents of the major metabolic organs of rats in the control CWF and experimental LED groups. TFA levels (mg/g) in liver, adipose (fat), hind limb skeletal muscle, heart, lung, kidney, adrenal glands, small intestine (gut), and brain tissues were significantly higher (P < 0.05) in rats of the CWF group compared with those of the LED group, with adrenal glands revealing the lowest (13.1%) and muscle tissue the highest (29.3%) percentage decrease in TFA in LED compared with CWF groups. Overall, the mean of combined tissue TFA levels in the CWF control group was 21.9% ± 6.9% higher than that of the LED experimental group. Conversely, with the exception of adipose, kidney, and brain tissues, protein content (mg/g) was significantly higher (P < 0.05) in all other tissues of LED compared with CWF rats. Overall mean of combined protein content was 11.8% ± 2.9% higher in LED compared with CWF group.
Figure 6.
Tissue (A) total fatty acid (TFA) and (B) protein concentrations in CWF light (solid black bar) compared with LED (solid blue bar) groups in liver, adipose (epigastric fat depot), hindlimb skeletal muscle, heart, lung, kidneys, adrenal, small intestine [gut], and brain tissues. Each bar represents the mean ± 1 SD (n = 12 per group). Concentrations with asterisks differ (P < 0.05) from concentrations without asterisks.
Discussion
We previously reported that in male nude rats housed in blue-tinted cages, blue-appearing light during the daytime markedly amplified the nocturnal melatonin signal and markedly reduced human PC3 prostate xenograft metabolism, signaling activity, and growth compared with animals housed in clear cages.22 Given these observations, we tested the hypothesis that LED light, enriched in the blue-appearing portion of the visible spectrum, similarly enhances circadian nocturnal melatonin levels and alters the circadian patterns of plasma measures of physiology and metabolism. To test this hypothesis, LED luminaires with 30% greater emissions for stimulating melanopsin-containing ipRGC were compared with standard fluorescent luminaires used in laboratory animal housing facilities. This study is the first to show that long-term exposure to daytime blue-enhanced LED lighting, compared with standard CWF lighting, resulted in a markedly augmented amplitude and extended duration of the nocturnal melatonin signal that was associated with alterations in the circadian dynamics of important plasma indicators of neuroendocrine, metabolic, and physiologic homeostatic stability normally associated with optimal animal health and wellbeing.2,7,8,12,16-20,41,51,57,58
Globally, institutions are currently embracing new blue-enriched LED lighting technology over older lighting technologies, including incandescent lighting and the most commonly used standard broad-band CWF lighting. A number of factors have contributed to this change, including higher efficiency, lower heat production, and a significantly longer operating life than those of conventional luminaires.34 The standard CWF and LED T8 lamps used in the present study are some of the most commonly used lights in the laboratory animal science and biomedical research fields today. However, little is understood about their long-term effects on animal physiology and metabolism. The apparent anomaly of blue-enriched LED light during the day stimulating a 7-fold increase in the peak amplitude (approximately 1050 pg/mL) of the nocturnal plasma melatonin surge over the normal nocturnal melatonin peak (approximately 150 pg/mL) is not completely without precedent. One earlier study noted similar findings of significantly elevated nighttime pineal gland levels in male rats exposed to bright, daytime natural sunlight (enriched in blue-appearing, short-wavelength light) over 13-h compared with CWF light in animal rooms over the same period of time;44 the underlying mechanism was not determined, however. Although intensity and duration each may play a role, decreased red- and increased blue-appearing light, as shown in the spectral power distribution measurements (Figure 1), cannot be ruled out as a causative factor.
The elevated nocturnal levels of circulating melatonin observed in the experimental LED group are presumably due to a hyperstimulation of pineal melatonin production or secretion, perhaps resulting from hyperactivation of arylalkylamine-N-acetyl transferase, the rate-limiting enzyme in the melatonin synthetic pathway.42 However a concomitant inhibition of hepatic melatonin metabolism cannot be ruled out as a contributing factor. The high plasma concentrations achieved in the LED group early in the dark phase (2000) were nearly equivalent to the peak levels attained 4 h later in the control CWF group (2400). Furthermore, significantly elevated nocturnal melatonin concentrations persisted well beyond the dark phase into the light phase (0800) by more than 4 h, given that they were still 30-fold higher than those at the same circadian time point in control rats. In effect, the early and robust nighttime rise in melatonin with high levels extending well into the light phase effectively extended the ‘biological night’ into the daytime. The extension of elevated melatonin levels into the light phase could have been attributed to a reduced hepatic metabolism rather than elevated pineal melatonin production.22 Under normal circumstances, light has a gating effect on the synthesis of melatonin by the pineal gland; therefore melatonin levels would have been expected to decrease rapidly in response to the onset of light phase (0600) if the extended duration in melatonin levels had been solely due to persistently enhanced pineal melatonin production.
The circadian rhythm for all physiologic and metabolic rhythms in the experimental animals was changed to a lesser or greater degree in response to the altered spectral characteristics of blue-enriched LED light compared with broad-spectrum CWF light during the light phase. Depending on the circulating factor measured, the alterations included changes in rhythm amplitude, phasing, or duration, or combinations of these circadian rhythm characteristics. These altered rhythms appeared to be completely independent from the SCN-generated rhythms in dietary intake of TFA,4 which were nearly identical for both the control and experimental groups, indicating that the phasing of overall SCN rhythmicity was intact and not affected by short wavelengths during the light phase. This interpretation was further corroborated by the fact that although the melatonin amplitudes were markedly different, the acrophases of the SCN-driven melatonin rhythms for both groups were identical.
Circadian oscillations in arterial plasma glucose, lactate, pO2, and pCO2 levels were, in general, nearly identical, with the exception of the 0800 time point for glucose and lactate phase-advanced by 4 h in LED animals, whereas overall 24-h integrated levels were lower in rats exposed to LED compared with CWF lighting, as previously observed in female nude rats16 suggesting lower rates of basal metabolism in these animals as compared with the control CWF group. Similarly, circadian variations in the phasing, amplitude, and duration of corticosterone, insulin, and leptin, which all have critical effects on whole-animal metabolism, also were altered in response to exposure to daytime short wavelength-enriched LED light, again corroborating the same changes in these parameters demonstrated in our previous study in nude female rats.16
TFA concentrations in all the major metabolic tissues examined in the current study were significantly higher in the CWF controls compared with the LED-exposed experimental animals. This result follows a trend of increased dietary intake of the CWF animals compared with that of the LED animals. With minor exceptions (that is, adipose, kidney, and brain tissues), overall protein levels (mg per g of tissue) of most organs examined, particularly skeletal muscle (corresponding to lean body mass), was sustained and even slightly increased in LED compared with CWF rats as the animals aged. These trends, as previously shown in both humans52 and albino rats,56 strongly suggest that the rats in the LED experimental group may not have been aging as rapidly as those of the CWF group. A previous study56 demonstrated that daily melatonin supplementation to middle-aged rats inhibited body weight, reduced epigastric fat, and lowered both plasma leptin and insulin concentrations independent of food intake. Therefore, the markedly elevated and extended nocturnal melatonin levels may have been responsible for the more youthful metabolic phenotype observed in LED-exposed rats.
Melatonin exerts regulatory effects on glucose and lactate metabolism, as well as corticosterone, insulin, and leptin in humans9,40,47 and rats.16,21,24,25,40,47 Arguably, the marked circadian changes in melatonin levels in animals exposed to LED may have been responsible for some of the circadian changes in these constituents, as observed in the present study in male albino rats and our previous study in female nude rats.16 This contention is further supported by our previous observations in nude rats,16,20,21 in which the spectral transmittance of longer wavelength light through either amber- or red-tinted cages resulted in circadian melatonin as well as metabolic and hormonal profiles that markedly differed from the circadian profiles seen here and in our previous blue-light study.22 This observation suggests that each circadian response is dependent on exposure to a specific wavelength as conveyed to peripheral tissues by a corresponding wavelength-dependent melatonin signature. Because the exact mechanism by which this effect may occur is unknown, other wavelength-dependent but melatonin-independent factors that influence circadian hormonal and neural outputs from the SCN should also be considered.
As mentioned earlier, a standardized, single measurement unit is currently unavailable for quantifying light that regulates the circadian, neuroendocrine, and neurobehavioral effects of light. Recently, a consensus position statement was developed among many of the laboratories that have studied wavelength regulation of the biologic and behavioral effects of light in rodents, humans and other species for best practices for measuring and reporting experimental light stimuli.47 Included within that consensus statement is a freely available web-based Toolbox35 that permits calculation of the effective irradiance experienced by each of the rodent ipRGC, cone, and rod photoreceptors that is capable of driving circadian, neuroendocrine, and neurobehavioral effects.37 CWF luminaires emit slightly higher light irradiances to rats inside the cages but less effective stimulation to each of the retinal photoreceptors. Abundant data have illustrated that the melanopsin-containing ipRGC are anatomically and functionally interconnected with the rods and cones that support vision. Physiologic responses to CWF or LED light reflect input from all of the retinal photoreceptor classes, with the relative importance of each being labile within and between response types. Therefore, the spectral sensitivity of this photoreceptive system is fundamentally context-dependent.1,14,27-29,32,39,44,49,50,58 It is important (and essential to ‘best practices’) for different groups of investigators to use commonly accepted metrics for reporting spectral response functions to be able to pool results, such as those shown in Table 1. As data using this measurement system emerge from different laboratories, it will become possible to generate testable hypotheses that predict the spectral characteristics for a targeted physiologic response to light from various sources, including CWF and LED lighting.
Light intensity, spectral quality, and duration and timing are essential in the regulation of mammalian circadian rhythms, and variations in any of these parameters affect virtually every biologic process associated with animal physiology and metabolism. The nocturnal melatonin signal represents a critical internal zeitgeber, or synchronizer, that is responsible for the normal circadian rhythms of metabolism and physiology in experimental rodents and thus influences animal health and wellbeing and the outcome of scientific investigations. The present study provides compelling evidence for the hypothesis that supraphysiologic nocturnal levels of melatonin, as evinced in animals exposed to blue-enriched LED light during daytime, may contribute to the circadian reorganization of the metabolic and hormonal milieu. Additional studies in both rats and human subjects are warranted to better understand the potentially beneficial effects of daytime blue-enriched LED light exposure and high nocturnal melatonin levels in optimizing the homeostatic control of animal health and wellbeing.
Acknowledgments
This work was supported in part by a Tulane University School of Medicine and Louisiana Cancer Research Consortium Startup Grant (no. 631455 to DEB), NIH grant (National Cancer Institute no. 1R56CA193518-01 to SMH and DEB) the American Association for Laboratory Animal Science Grants for Laboratory Animal Science (GLAS) Award (to RTD and DEB). Additional support for the Jefferson co-investigators (JPH, BW, and GCB) was from The Institute for Integrative Health (Baltimore, MD) and the National Science Foundation (no. EEC-0812056 to GCB). The authors declare no potential conflicts of interest with respect to the research, authorship, or publication of this article. We acknowledge and are grateful for the technical support of Dr Georgina Dobek, Ms Lynell Dupepe, Mrs Lee Barton, Ms Joy Ettling, Mr Joshua Davis, Ms Katie Castillo, and Mr Michael Webb.
References
- 1.Altimus CM, Guler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. 2010. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci 13:1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschoff J. 1981. Handbook of behavioral neurobiology, biological rhythms, vol.4 New York (NY): Plenum Press. [Google Scholar]
- 3.Berson DM, Dunn FA, Takao M. 2002. Phototransduction by retinal ganglion cells that set the circadian clock. Science 295:1070–1073. [DOI] [PubMed] [Google Scholar]
- 4.Blask DE, Brainard GC, Dauchy RT, Hanifin JP, Davidson LK, Krause JA, Sauer LA, Rivera-Bermudez MA, Dubocovich ML, Jasser SA, Lynch DT, Rollag MD, Zalatan F. 2005. Melatonin-depleted blood from premenopausal women exposed to light at night stimulates growth of human breast cancer xenografts in nude rats. Cancer Res 65:11174–11184. [DOI] [PubMed] [Google Scholar]
- 5.Blask DE, Dauchy RT, Dauchy EM, Mao L, Hill SM, Greene MW, Belancio VP, Sauer LA, Davidson LK. 2014. Light exposure at night disrupts host–cancer circadian regulatory dynamics: impact on the Warburg effect, lipid signaling, and tumor growth prevention. PLoS One 9:e102776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blask DE, Dauchy RT, Sauer LA, Krause JA. 2004. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 25:951–960. [DOI] [PubMed] [Google Scholar]
- 7.Brainard GC. 1989. Illumination of laboratory animal quarters: participation of light irradiance and wavelength in the regulation of the neuroendocrine system, p 69–74. In: Guttman HN, Mench JA, Simmonds RC. Science and animals: addressing contemporary issues. Bethesda (MD): Scientists Center for Animal Welfare. [Google Scholar]
- 8.Brainard GC, Hanifin JP. 2005. Photons, clocks, and consciousness. J Biol Rhythms 20:314–325. [DOI] [PubMed] [Google Scholar]
- 9.Brainard GC, Hanifin JP, Rollag MD, Greeson JM, Byrne B, Glickman G, Gerner E, Sanford B. 2001. Human melatonin regulation is not mediated by the 3-cone photopic visual system. J Clin Endocrinol Metab 86:433–436. [DOI] [PubMed] [Google Scholar]
- 10.Brainard GC, Richardson BA, King TS, Matthews SA, Reiter RJ. 1983. The suppression of pineal melatonin content and N-acetyl-transferase activity by different light irradiances in the Syrian hamster: a dose–response relationship. Endocrinology 113:293–296. [DOI] [PubMed] [Google Scholar]
- 11.Brainard GC, Richardson BA, King TS, Reiter RJ. 1984. The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res 294:333–339. [DOI] [PubMed] [Google Scholar]
- 12.Brainard GC, Vaughan MK, Reiter RJ. 1986. Effect of light irradiance and wavelength on the Syrian hamster reproductive system. Endocrinology 119:648–654. [DOI] [PubMed] [Google Scholar]
- 13.Commission International de L'Eclairage 2004. Ocular lighting effects on human physiology and behavior. Technical Report 158. Vienna (Austria): Commission International de l'Eclairage. [Google Scholar]
- 14.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KY, Gamlin PD. 2005. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433:749–754. [DOI] [PubMed] [Google Scholar]
- 15.Dauchy RT, Blask DE, Sauer LA, Brainard GC, Krause JA. 1999. Dim light during darkness stimulated tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett 144:131–136. [DOI] [PubMed] [Google Scholar]
- 16.Dauchy RT, Dauchy EM, Hanifin JP, Gauthreaux SL, Mao L, Beleancio VP, Ooms TG, Dupepe LM, Jablonski MR, Warfield B, Wren MA, Brainard GC, Hill SM, Blask DE. 2013. Effects of spectral transmittance through standard laboratory cages on circadian metabolism and physiology in nude rats. J Am Assoc Lab Anim Sci 52:146–156. [PMC free article] [PubMed] [Google Scholar]
- 17.Dauchy RT, Dauchy EM, Tirrell RP, Hill CR, Davidson LK, Greene MW, Tirrell PC, Wu J, Sauer LA, Blask DE. 2010. Dark-phase light contamination disrupts circadian rhythms in plasma measures of physiology and metabolism. Comp Med 60:348–356. [PMC free article] [PubMed] [Google Scholar]
- 18.Dauchy RT, Dupepe LM, Ooms TG, Dauchy EM, Hill CR, Mao L, Belancio VP, Slakey LM, Hill SM, Blask DE. 2011. Eliminating animal facility light-at-night contamination and its effect on circadian regulation of rodent physiology, tumor growth and metabolism: a challenge in the relocation of a cancer research laboratory. J Am Assoc Lab Anim Sci 50:326–336. [PMC free article] [PubMed] [Google Scholar]
- 19.Dauchy RT, Sauer LA. 1986. Preparation of tissue-isolated rat tumors for perfusion: a new surgical technique that preserves continuous blood flow. Lab Anim Sci 36:678–681. [PubMed] [Google Scholar]
- 20.Dauchy RT, Wren MA, Dauchy EM, Hanifin JP, Jablonski MR, Warfield B, Brainard GC, Hill SM, Mao L, Ooms TG, Dupepe LM, Blask DE. 2013. Effects of spectral transmittance through red-tinted cages on circadian metabolism and physiology in nude rats. J Am Assoc Lab Anim Sci 52:745–755. [PMC free article] [PubMed] [Google Scholar]
- 21.Dauchy RT, Xiang S, Mao L, Brimmer S, Wren MA, Anbalagan M, Hauch A, Frasch T, Rowan BG, Blask DE, Hill SM. 2014. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res 74:4099–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dauchy RT, Hoffman AE, Wren-Dail MA, Hanifin JP, Warfield B, Brainard GC, Xiang S, Yuan L, Hill SM, Belancio VP, Dauchy EM, Smith K, Blask DE. 2015. Daytime blue light enhances the nighttime circadian melatonin inhibition of human prostate cancer growth. Comp Med 65:473–485. [PMC free article] [PubMed] [Google Scholar]
- 23.Davis S, Mirrick DK, Stevens RG. 2001. Night shift work, light at night, and the risk of breast cancer. J Natl Cancer Inst 93:1557–1562. [DOI] [PubMed] [Google Scholar]
- 24.Diaz B, Blazquez E. 1986. Effect of pinealectomy on plasma glucose, insulin, and glucagon levels in the rat. Horm Metab Res 18:225–229. [DOI] [PubMed] [Google Scholar]
- 25.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. 2010. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron 67:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabel V, Maire M, Reichert CF, Chellappa CF, Schmidt C, Hommes V, Viola AU, Cajochen C. 2013. Effects of artificial dawn and morning blue light on daytime cognitive performance, wellbeing, cortisol, and melatonin levels. Chronobiol Int 30:988–997. [DOI] [PubMed] [Google Scholar]
- 27.Gamlin PDR, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. 2007. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res 47: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge D, Dauchy RT, Liu S, Zhang Q, Mao L, Dauchy EM, Blask DE, Hill SM, Rowan BG, Brainard GC, Hanifin JP, Cecil KS, Xiong Z, Myers L, You Z. 2013. Insulin and IGF1 enhance IL17-induced chemokine expression through a GSK3B-dependent mechanism: a new target for melatonin's antiinflammatory action. J Pineal Res 55:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gooley JJ, Rajaratnam SM, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. 2010. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med 2:31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen J. 2001. Increased breast cancer risk among women who work predominantly at night. Epidemiology 12:74–77. [DOI] [PubMed] [Google Scholar]
- 31.Hansen T, Bratlid T, Lingjarde O, Brenn T. 1987. Midwinter insomnia in the subarctic region: evening levels of serum melatonin and cortisol before and after treatment with bright artificial light. Acta Psychiatr Scand 75:428–434. [DOI] [PubMed] [Google Scholar]
- 32.Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. 2003. Melanopsin and rod–cone photoreceptive systems account for all major accessory visual functions in mice. Nature 424:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He Q, Heshka S, Albu J, Boxt L, Krasnow N, Elia M, Gallagher D. 2009. Smaller organ mass with greater age, except for heart. J Appl Physiol (1985) 106:1780–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heeke DS, White MP, Mele GD, Hanifin JP, Brainard GC, Rollage MD, Winget CM, Holley DC. 1999. Light-emitting diodes and cool white fluorescent light similarly suppress pineal gland melatonin and maintain retinal function and morphology in the rat. Lab Anim Sci 49:297–304. [PubMed] [Google Scholar]
- 35.Hughes ME, Hogenesch JB, Kornacker K. 2010. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. J Biol Rhythms 25:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Illnerova H, Vanecek J, Hoffman K. 1983. Regulation of the pineal melatonin concentration in the rat (Rattus norvegicus) and the Djungarian hamster (Phodopus sungorus). Comp Biochem Physiol A Comp Physiol 74:155–159. [DOI] [PubMed] [Google Scholar]
- 37.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 38.Illuminating Engineering Society of North America 2008. Light and human health: an overview of the impact of optical radiation on visual, circadian, neuroendocrine, and neurobehavioral responses. New York (NY): Illuminating Engineering Society. [Google Scholar]
- 39.Jasser SA, Hanifin JP, Rollag MD, Brainard GC. 2006. Dim light adaptation attenuates acute melatonin suppression in humans. J Biol Rhythms 21:394–404. [DOI] [PubMed] [Google Scholar]
- 40.Kalsbeek A, Strubbe JH. 1998. Circadian control of insulin secretion is independent of the temporal distribution of feeding. Physiol Behav 63:553–560. [DOI] [PubMed] [Google Scholar]
- 41.Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. 2002. Melatonin in mice: rhythms, response to light, adrenergic stimulation, and metabolism. Am J Physiol Regul Integr Comp Physiol 282:R358–R365. [DOI] [PubMed] [Google Scholar]
- 42.Klein DC, Weller JL. 1972. Rapid light-induced decrease in pineal serotonin N-acetyltransferase activity. Science 177:532–533. [DOI] [PubMed] [Google Scholar]
- 43.Kubo T, Ozasa K, Mikami K, Wakai K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, Mori M, Washio M, Sakauchi F, Ito Y, Yoshimura T, Tamakoshi A. 2006 Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol 164:549–555. [DOI] [PubMed] [Google Scholar]
- 44.Laakso ML, Porkka-Heiskanen T, Alila A, Peder M, Johansson G. 1988. Twenty-four-hour patterns of pineal melatonin and pituitary and plasma prolactin in male rats under ‘natural’ and artificial lighting conditions. Neuroendocrinology 48:308–313. [DOI] [PubMed] [Google Scholar]
- 45.Lima FB, Machado UF, Bartol I, Seraphim PM, Sumida DH, Moraes SMF, Hell NS, Okamoto MM, Saad MJ, Carvalho CR, Cipolla-Neto J. 1998. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol 275:E934–E941. [DOI] [PubMed] [Google Scholar]
- 46.Lucas RJ, Peirson SN, Berson DM, Brown TM, Cooper HM, Czeisler CA, Figueiro MG, Gamlin PD, Lockely SW, O'Hagan JB, Price LLA, Provencio I, Skene DJ, Brainard GC. 2014. Measuring and using light in the melanopsin age. Trends Neurosci 37:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson DL, Cox MM. 2005. Hormonal regulation of food metabolism, p 881–992. In: Lehninger AL, Nelson DL, Cox MM. Lehninger principles of biochemistry. New York (NY): WH Freeman. [Google Scholar]
- 48.Neufield Department of Clinical Neurosciences Medical Sciences Division. [Internet] 2016. Rodent Toolbox version 1.xlsx. [Cited 24 February 2016]. Available at: https://www.ndcn.ox.ac.uk/team/stuart-peirson.
- 49.Panda S, Provencio I, Tu DC, Pires SS, Rollag MD, Castrucci AM, Pletcher MT, Sato TK, Wiltshire T, Andahazy M, Kay SA, Van Gelder RN, Hogenesch JB. 2003. Melanopsin is required for nonimage-forming photic responses in blind mice. Science 301:525–527. [DOI] [PubMed] [Google Scholar]
- 50.Reiter RJ. 1991. Pineal gland: interface between photoperiodic environment and the endocrine system. Trends Endocrinol Metab 2:13–19. [DOI] [PubMed] [Google Scholar]
- 51.Rollag MD, Niswender GD. 1976. Radioimmunoassay of serum concentrations of melatonin in sheep exposed to different lighting regimens. Endocrinology 98:482–489. [DOI] [PubMed] [Google Scholar]
- 52.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Colditz GA. 2001. Rotating night shifts and risk of breast cancer in women participating in the Nurses’ Health study. J Natl Cancer Inst 93:1563–1568. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz WJ. 2005. Human circadian rhythms: regulation and impact.[Special issue]. J Biol Rhythms 20:279–386.16077148 [Google Scholar]
- 54.Weng S, Estevez ME, Berson DM. 2013. Mouse ganglion-cell photoreceptors are driven by the most sensitive rod pathway and by both types of cones. PLoS One 8:e66480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wolden-Hanson T. 2010. Changes in body composition in response to challenges during aging in rats. Interdiscip Top Gerontol 37:64–83 [DOI] [PubMed] [Google Scholar]
- 56.Wolden-Hanson T, Mitton DR, McCants RL, Yellon SM, Wilkinson CW, Matsumoto AM, Rasmussen DD. 2000. Daily melatonin administration to middle-aged rats suppresses body weight, intraabdominal adiposity, plasma leptin, and insulin independent of food intake and total body fat. Endocrinology 141:487–497. [DOI] [PubMed] [Google Scholar]
- 57.Wren MA, Dauchy RT, Hanifin JP, Jablonski MR, Warfield B, Brainard GC, Blask DE, Hill SM, Ooms TG, Bohm RP., Jr 2014. Effect of different spectral transmittances through tinted animal cages on circadian metabolism and physiology in Sprague-Dawley rats. J Am Assoc Lab Anim Sci 53:44–51. [PMC free article] [PubMed] [Google Scholar]
- 58.Wu J, Dauchy RT, Tirrell PC, Wu SS, Lynch DT, Jitawatanarat P, Burrington CM, Dauchy EM, Blask DE, Greene MW. 2011. Light at night activates IGF1R–PDK1 signaling and accelerates tumor growth in human breast cancer xenografts. Cancer Res 71:2622–2631. [DOI] [PubMed] [Google Scholar]