Abstract
Type 2 Diabetes mellitus (DM2) includes a continuum of metabolic disorders characterized by hyperglycemia that causes several chronic long-term complications such as coronary artery disease, peripheral arterial disease, nephropathy, and neuropathy. The hair follicle could reveal signs of early vascular impairment, yet its relationship to early metabolic injuries has been largely ignored. We propose that in earlier stages of the continuum of DM2-related metabolic disorders, a group of susceptible patients who do not yet meet the diagnostic criteria to be considered as persons with DM2 may present chronic vascular impairment and end organ damage, including hair follicle damage, which can be evaluated to identify an early risk marker. This hypothesis is based in the association found between insulin resistance and alopecia in non-diabetic persons, and the hair loss on the lower limbs as a manifestation of long-term peripheral arterial disease among subjects with DM2. In order to test this hypothesis, studies are required to evaluate if hair follicle characteristics are related to and can predict hyperglycemic complications, and if they do so, which feature of the hair follicle, such as hair growth, best characterizes such DM2-related conditions. If this hypothesis were proven to be true, significant advances towards a personalized approach for early prevention strategies and management of DM2 would be made. By focusing on the hair follicles, early stages of metabolic-related organ damage could be identified using non-invasive low-cost techniques. In so doing, this approach could provide early identification of DM2-susceptible individuals and lead to the early initiation of adequate primary prevention strategies to reduce or avoid the onset of large internal organ damage.
INTRODUCTION
Type 2 Diabetes Mellitus (DM2) is a continuum of metabolic disorders characterized by hyperglycemia resulting from defects in insulin action (1). Today, DM2 is the most common endocrine disorder and one of the major causes of worldwide mortality (2) and disability adjusted life-years (3).
As the disease progresses, hyperglycemia injures target cells through multiple pathways, including the effects of aldose reductase, advanced glycation end products, polyol accumulation, oxidative stress, protein kinase C isoforms, growth factors, and atherosclerosis. Endothelial cell damage leads to vascular impairment, which in turn decreases oxygen and nutrients in virtually all organs (1), causing a variety of chronic complications that are responsible for the majority of DM2-related morbidity and mortality (4, 5). These are classified by the diameter of the impaired arteries in macrovascular complications —including coronary artery disease, peripheral arterial disease, and stroke— and microvascular complications —including diabetic nephropathy, neuropathy, and retinopathy (6, 7).
The current DM2 diagnosis is based on epidemiological studies that showed a higher risk of the development of retinopathy up to eight years after certain biological thresholds are exceeded (8). The current American Diabetes Association (ADA) thresholds are A1C ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dL, 2-h plasma glucose ≥ 200 mg/dL during an oral glucose tolerance test, and random plasma glucose ≥ 200 mg/dL in patients with classic symptoms of hyperglycemia or hyperglycemic crisis (9).
Currently, an important goal in DM2 care is to minimize organ damage by preventing DM2 onset, so a precursor condition called prediabetes was created for people with greater risk of developing DM2 in the next few years, yet this approach is not free from criticism (10). The ADA thresholds for prediabetes are: A1C from 5.7% to 6.4%, fasting plasma glucose from 100 mg/dL to 125 mg/dL, and 2-h plasma glucose from 140 mg/dL to 199 mg/dL during an oral glucose tolerance test (9). In general, there are important discrepancies among common tests to diagnose diabetes (11). In addition, the diagnosis may differ depending on the guidelines followed, e.g. ADA or World Health Organization guidelines (12).
DM2 diagnosis criteria using cut-offs for various glucose-related markers are currently based on thresholds obtained from population data. Yet, DM2 is a continuous process that starts long before these thresholds are surpassed (13–15). For example, alterations in fasting glucose, 2-h glucose, HOMA insulin sensitivity and HOMA β-cell function are present at least 13 years before DM2 diagnosis (16). This suggests a long compensation period in which the insulin resistance is decreasing beta cell function (17, 18). This compensation period is not equal for all people: complications can start their progression to various end-organ damage and complications earlier or later depending on individual susceptibility (19, 20). For example, it has been reported that hyperglycemic complications are already present in some newly diagnosed DM2 patients (21). Also, many complications such as diabetic nephropathy, chronic kidney disease, neuropathies, retinopathy, stroke, and myocardial infarction are already present in the prediabetic phase (13, 22, 23).
To improve the sensitivity of DM2 diagnosis, it has been proposed to lower the diagnostic thresholds (24), but that would increase the rates of false positives, leading to the unnecessary treatment of and associated risks to healthy people. Also, it has been proposed to use a personalized glucose profile that could identify early deterioration in glucose metabolism on an individual level. This is based on the assumption that some people will have a lower baseline glucose level, and increments in this glucose level that are exceptional for those persons are regarded as normal on a population basis (25, 26). Therefore, in order to minimize organ damage, there is a need for an early risk marker that takes into consideration the patient’s actual level of vascular impairment or organ damage.
As seen, current glucose thresholds used for DM2 diagnosis give a false sense of security to a susceptible group that could develop complications of lower glucose levels. These persons are not treated early, which allows the development of serious complications that could become irreversible when they finally meet the diagnostic criteria (24). This is described by the metabolic memory theory, which postulates that early hyperglycemia is remembered in end organs, increasing the rate of complications in late interventions, despite adequate subsequent control (27).
The hair follicle, like many other organs, is susceptible to hyperglycemia damage. This can be noted in the relation between hyperglycemia and androgenetic alopecia, and the hair loss of DM2 patients. Therefore, focusing on the hair follicles may provide an entry point to evaluate hyperglycemia organ damage.
HYPOTHESIS
We propose that dysglycemic states produce vascular impairment resulting in early manifestations of injury in the hair follicle even before a DM2/prediabetes diagnosis can be made, reflected in changes in the hair characteristics: either the number of hairs/cm2, linear hair growth, percentage of anagen bulbs, hair diameter, or time to hair regrowth after teloptosis. Thus, these hair follicle manifestations can be translated to an objective, simple, low-cost, minimally- or non-invasive evaluation of hyperglycemia damage providing a novel, cheap and easy-to-use tool to evaluate glycemic susceptibility and organ damage in patients before the DM2/prediabetes diagnosis can be made.
EVALUATION OF THE HYPOTHESIS
Organ damage in DM2
Hyperglycemia damages many organs during the DM2 continuum. In order to detect early manifestations of this damage, physicians could evaluate retinopathy (28), nephropathy, neuropathy, endothelial dysfunction (29), artery damage (30), or hair follicle characteristics (Figure).
Figure.
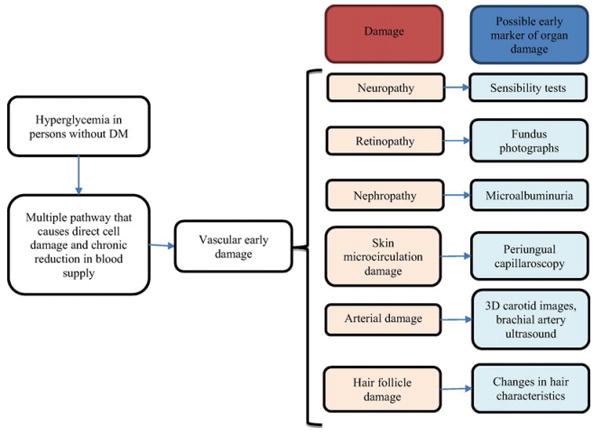
Hair follicle as an early manifestation of hyperglycemia
The hair follicle could be considered an end organ, with its own complex microenvironment (31). There are three types of hair follicles: terminal follicles, characterized by thick hair, found on the scalp and in the axillary and genital areas; sebaceous follicles, characterized by their production of large amounts of sebum, found on the face, upper back and chest; and vellus follicles, characterized by small thin vellus hairs, found in the remaining hairy surfaces of the body (32, 33).
Hair growth takes place in a cyclical model that has three stages: anagen (growth phase), catagen (regression phase), and telogen (resting phase). The exogen (hair shredding) does not occur at every cycle, and the kenogen refers to a brief interval in which the hair follicle remains empty after the hair loss (34, 35). The hair cycle regulation is complex, and includes various cytokines, hormones, neurotransmitters, growth factors, and also changes in tissue biology and underlying epigenetic controls (36).
The hair follicle, as a highly active organ, needs a special regulatory microenviroment with adequate supply of oxygen and nutrients (37–39). A chronic decrease in the oxygen and nutrient supply caused by hyperglycemic vascular impairment can cause follicle damage, resulting in hair alterations like hair thinning, hair fragility, sparseness of hair, or decreased hair growth speed (40–42). These alterations may be subtle, so patients do not always notice them, and they must be detected with an active approach using dermatologic techniques.
Whilst skin damage has been described in advanced stages of DM2 (43), the relationship between DM2 and hair follicle is not yet fully understood. The current literature has addressed this relationship focusing on the following topics.
Androgenetic alopecia
Some studies have found an association between androgenetic alopecia and insulin resistance. Although the pathways involved are not clear and there is a lot of controversy about the real meaning of this association (44), it has been proposed that insulin resistance could produce microvascular impairment, thus playing a role in the pathophysiology of androgenic alopecia (45, 46).
Hair loss in the inferior limbs
An Indian cohort found that 26.6% of persons with DM2 present hair loss beneath the knees (47), which was attributed to peripheral arterial disease (43). It is also proposed that lower leg hair loss could be an early predictor of diabetic foot through vascular impairment (48–50).
Circulating amino acids
Insulin not only intervenes in glucose metabolism, but also decreases the breakdown of protein and helps cells absorb circulating amino acids from the blood. Since the hair follicle uses circulating amino acids as building blocks of keratin, hair characteristics may also be influenced by this pathway, as the lack or decrease in function of insulin increases circulating amino acids (51–54). This is a novel field of study, and new diagnostic techniques have been proposed based on the concentration of certain amino acids in the hair shaft (52).
Alopecia areata
Type 1 diabetes is an autoimmune disease, and is associated with other autoimmune conditions like alopecia areata, which causes variable patterns of hair loss (55, 56). Because of the autoimmunity component, our proposed hypothesis of alterations in the hair follicle and related hair growth would not be applicable in type 1 diabetes. Our current hypothesis is therefore specific for DM2.
Other
Other studied links between DM2 and hair follicles include reports of Demodex folliculorum infestation in DM2 patients that can affect some hair characteristics (57). Arrector pili muscle fat degeneration in DM2 patients has also been described to be involved in hair loss (58, 59). Reduced circulating levels and resistance of insulin-like growth factor-I (60, 61) has been shown to cause early catagen, which is a cease in cell proliferation towards detention of hair growth (62).
How to test this hypothesis
In order to test this hypothesis, with an emphasis on DM2, we propose some potential measurable objective characteristics of the hair follicle and hair growth that are amenable to be studied, some study designs, and various other considerations that may play a role in the link proposed for our hypothesis. With the advancement of technology and communications, including the expansion of Internet connectivity and mobile devices, many features of the hair follicle, especially hair tropism and hair characteristics, can be ascertained with ease. Thus, we are focusing on the above-the-skin observations while acknowledging that some subcutaneous changes could also be occurring in the follicle. Such subcutaneous evaluations would require some sort of invasive techniques to penetrate the skin, e.g. biopsies, to be followed by pathological or microscopic evaluations of the samples.
Hair follicle and hair characteristics
The evaluation of the hair and its follicle can be made by many non-invasive tests and include the number of hairs/cm2, linear hair growth, percentage of anagen bulbs, hair diameter, and time to hair regrowth after teloptosis. Current available techniques to measure hair characteristics include trichoscan, trichogram, phototrichogram, trichoscopy, folliscope, global photography, hair pull test, sonography, among others. Each utilizes different methods of measuring hair characteristics, and some can evaluate more characteristics than others (63, 64). Alternatively, a new technique ad hoc could be created to evaluate hair characteristics.
Study designs
Cross-sectional studies comparing index cases, i.e. newly diagnosed or long-established DM2, with those without the condition could compare the patterns of hair characteristics and various glucose-related markers including oral glucose tolerance tests, currently the gold standard technique for diagnosing DM2. Longitudinal studies would be required to evaluate if hair follicle characteristics can predict, over time, the appearance or development of certain conditions, be it the transition from a non-DM2 status into an established diagnosis of DM2, or the progression towards hyperglycemic complications.
In the short term, from a primary prevention point of view, a case-control design nested within future expanding longitudinal cohorts would compare metabolic markers with hair characteristics in offspring of DM2 parents versus offspring without DM2 ancestry. This would provide preliminary data in support of or against the utility of continuing the longitudinal study. Continuing with a longitudinal study will allow exploring hair follicle’s damage later in the continuum of DM2 complications, more in the remit of secondary prevention. This will also enable the evaluation of the effects of active management, including pharmacological therapies, and control of DM2 on hair characteristics.
Another design, in the remit of secondary prevention, could look at people with established DM2 and correlate hair growth characteristics to internal end-organ damage while accounting for length of disease and drug treatment. Further into the spectrum of disease complications, possibly as tertiary prevention and once end-organ damage has already been established, particularly in cases of diabetic feet, hair growth could serve as an early warning signal to avoid ulcers and amputations in the diabetic feet.
Other determinants that will influence study designs
To test this hypothesis, we also have to understand that there are other major determinants that play a role in influencing hair characteristics. These should be taken into consideration in the research design stages.
Sex
Hair characteristics are influenced by sex hormones, which cause large differences between males and females (65). Moreover, hair depilation is more common in females (66).
Age
Hair characteristics change with age. Hair growth increases during puberty under hormonal influence, and eventually decreases because of complex systemic signals (67). Also, age is independently associated with macrovascular events (68).
Race
Hair characteristics differ among races in the scalp (69, 70), while these differences may be difficult to ascertain in the rest of the body (71). Therefore, to test this hypothesis, some sort of within-subject comparisons would be required, e.g. exploring hair growth characteristics in upper versus lower extremities.
Hormones
Hair cycling is influenced by various hormones (72). Within this group, androgenic hormones are probably the most important and most researched. The most relevant, and recently known, feature of androgens is “the androgen paradox” by which they enhance beard hair growth but suppress hair growth in androgenetic alopecia (73, 74). This could be explained by the differing amounts and characteristics of some dermal papillae cell components as 5a-reductase (75, 76), androgen receptor (77, 78), and androgen receptor coactivators (79, 80). Thus, to decrease the hormonal effect in the test of our hypothesis, it is important to avoid the study of places where androgens have the greatest effect, such as beard or frontal hair follicles (33, 81, 82), as well as to evaluate androgenic status and look for hormonal alterations such as polycystic ovary, hirsutism, and corticoid intake, among others.
Genetics and normal biologic variations
Signaling during hair follicle cycling relies on some key molecules such as Wnt, Bmp, Shh, and Notch (83, 84). However, regardless of these processes common to all hair follicles, normal biologic variations of hair characteristics occur, related to age, gender, race, and ethnicity (85–87), even within a single phenotypic group (88, 89). Unfortunately, there is no genetic test to control for this possible confounder.
Seasonal variation
Seasonal variation of hair characteristics has also been reported with anagen percentage decreased during the summer (90, 91). Thus, follow up evaluations have to be made ideally in the same season or month.
Autoimmune Diseases
Systemic autoimmune diseases can lead to hair loss, such as lupus, which may affect the scalp, eyebrows, eyelashes, beard hair, or body hair and is associated with active disease and medication used to treat lupus; it can be scarring or nonscarring. Dermatomyositis, scleroderma, and pemphigus can also affect hair characteristics (92, 93). Alopecia areata is a chronic multifactorial disease that targets anagen hair follicles and has genetic and autoimmune components related to type 1 diabetes (94, 95). These and other autoimmune diseases would be exclusion criteria for the evaluation of the present hypothesis.
Also, there are other situations to take in consideration such as eating disorders (96), concomitant illness, and work activity, among others. This calls for careful planning during the research design phase.
CONSEQUENCES OF THE HYPOTHESIS
If hair characteristics were demonstrated to have a solid link with early phases of hyperglycemia as well as early damage, we would be able to develop various tools to support primary prevention strategies for DM2 and its related complications. This approach could complement an ongoing array of behavioral activities recommended for DM2 such as calorie restriction, diet, weight loss, and regular exercise.
Operationalizing this hypothesis could yield a simple, non-invasive, low-cost technique that can be self-performed without the need of standardized laboratory tests. In addition, if this tool is based on skin photographs, it opens the possibility of the implementation of a teledermatology system that analyzes the photographs or sends them to a specialist. This approach is especially important in geographically or economically disadvantaged populations with limited access to health services.
Acknowledgments
The authors are indebted to various colleagues who provided valuable comments and feedback to earlier versions of this manuscript. Special thanks to Kasia Lipska, Dean S Morrell, María Lazo Porras, and Katie Sacksteder for their valuable input in earlier versions of the manuscript.
FUNDING SOURCES
There was no specific funding for this study. JJM is supported by Fogarty International Centre (R21TW009982), Grand Challenges Canada (0335-04), International Development Research Center Canada (106887-001), Inter-American Institute for Global Change Research (IAI CRN3036), Medical Research Council UK (M007405), National Heart, Lung and Blood Institute (U01HL114180), National Institutes of Mental Health (U19MH098780). JJM, AT-R, JCT, MGG-G, and RR-C are with the CRONICAS Centre of Excellence in Chronic Diseases at Universidad Peruana Cayetano Heredia which was supported by Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under contract No. HHSN268200900033C. The funders had no role in decision to publish, or preparation of the manuscript.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(Supplement 1):S81–S90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. WHO | Global status report on noncommunicable diseases 2014. WHO; 2015. [DOI] [PubMed] [Google Scholar]
- 3.Vos T, Barber RM, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015 doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-Year Natural History of Type 1 Diabetes Complications The Pittsburgh Epidemiology of Diabetes Complications Study Experience. Diabetes. 2006;55(5):1463–9. doi: 10.2337/db05-1423. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson J, Fuller JH, Group EICS. Microvascular and acute complications in IDDM patients: the EURODIAB IDDM Complications Study. Diabetologia. 1994;37(3):278–85. doi: 10.1007/BF00398055. [DOI] [PubMed] [Google Scholar]
- 6.Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical diabetes. 2008;26(2):77–82. [Google Scholar]
- 7.Stratton IM, Adler AI, Neil HAW, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Bmj. 2000;321(7258):405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gavin III, JR, Alberti K, Davidson MB, DeFronzo RA. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care. 1997;20(7):1183. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes care. 2014;37:S14. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 10.Yudkin JS, Montori VM. The epidemic of pre-diabetes: the medicine and the politics. Bmj. 2014;349:g4485. doi: 10.1136/bmj.g4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.NCD Risk Factor Collaboration. Effects of diabetes definition on global surveillance of diabetes prevalence and diagnosis: a pooled analysis of 96 population-based studies with 331 288 participants. The Lancet Diabetes & Endocrinology. 2015;3(8):624–37. doi: 10.1016/S2213-8587(15)00129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heianza Y, Arase Y, Fujihara K, Tsuji H, Saito K, Hsieh S, et al. Screening for pre-diabetes to predict future diabetes using various cut-off points for HbA1c and impaired fasting glucose: the Toranomon Hospital Health Management Center Study 4 (TOPICS 4) Diabetic Medicine. 2012;29(9):e279–e85. doi: 10.1111/j.1464-5491.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 13.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: A high-risk state for developing diabetes. Lancet. 2012;379:2279–90. doi: 10.1016/S0140-6736(12)60283-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergman M. Inadequacies of current approaches to prediabetes and diabetes prevention. Endocrine. 2013;44(3):623–33. doi: 10.1007/s12020-013-0017-9. [DOI] [PubMed] [Google Scholar]
- 15.Bergman M, Dankner R, Roth J, Narayan KV. Are current diagnostic guidelines delaying early detection of dysglycemic states? Time for new approaches. Endocrine. 2013;44(1):66–9. doi: 10.1007/s12020-013-9873-6. [DOI] [PubMed] [Google Scholar]
- 16.Tabák AG, Jokela M, Akbaraly TN, Brunner EJ, Kivimäki M, Witte DR. Trajectories of glycaemia, insulin sensitivity, and insulin secretion before diagnosis of type 2 diabetes: an analysis from the Whitehall II study. The Lancet. 2009;373(9682):2215–21. doi: 10.1016/S0140-6736(09)60619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(suppl 3):S16–S21. doi: 10.2337/diabetes.53.suppl_3.s16. [DOI] [PubMed] [Google Scholar]
- 18.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes care. 2006;29(5):1130–9. doi: 10.2337/diacare.2951130. [DOI] [PubMed] [Google Scholar]
- 19.Brownlee M. The pathobiology of diabetic complications a unifying mechanism. Diabetes. 2005;54(6):1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 20.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes. 2005;54(11):3274–81. doi: 10.2337/diabetes.54.11.3274. [DOI] [PubMed] [Google Scholar]
- 21.Khan KA, Kamran SM, Qureshi MN, Jamal Y. Frequency of retinopathy in newly diagnosed patients of type 2 diabetes mellitus (DM) Pakistan Armed Forces Medical Journal. 2015;(1) [Google Scholar]
- 22.Park C, Guallar E, Linton JA, Lee D-C, Jang Y, Son DK, et al. Fasting glucose level and the risk of incident atherosclerotic cardiovascular diseases. Diabetes care. 2013;36(7):1988–93. doi: 10.2337/dc12-1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DECODE Study Group. Is the current definition for diabetes relevant to mortality risk from all causes and cardiovascular and noncardiovascular diseases? Diabetes Care. 2003;26(3):688–96. doi: 10.2337/diacare.26.3.688. [DOI] [PubMed] [Google Scholar]
- 24.Bergman M. Inadequacies of absolute threshold levels for diagnosing prediabetes. Diabetes/metabolism research and reviews. 2010;26(1):3–6. doi: 10.1002/dmrr.1013. [DOI] [PubMed] [Google Scholar]
- 25.Dankner R, Danoff A, Roth J. Can ‘personalized diagnostics’ promote earlier intervention for dysglycaemia? Hypothesis ready for testing. Diabetes/metabolism research and reviews. 2010;26(1):7–9. doi: 10.1002/dmrr.1039. [DOI] [PubMed] [Google Scholar]
- 26.Dankner R, Bergman M, Danoff A, Qureshi S, Whitford I, Kaviani N, et al. The metabolic deterioration that antedates diabetes: personal trajectories of HbA1c and fasting glucose as early indicators and possible triggers for intervention. Diabetes/metabolism research and reviews. 2013;29(1):1–7. doi: 10.1002/dmrr.2373. [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, Ihnat MA, Thorpe JE. The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? The Journal of Clinical Endocrinology & Metabolism. 2009;94(2):410–5. doi: 10.1210/jc.2008-1824. [DOI] [PubMed] [Google Scholar]
- 28.Garg S, Davis RM. Diabetic retinopathy screening update. Clinical Diabetes. 2009;27(4):140. [Google Scholar]
- 29.Rao G. Non-Traditional Approaches to Diagnosis and Management of Type-2 Diabetes Mellitus: Point of View. J Diabetes Metab. 2015;6(489):2. [Google Scholar]
- 30.Polak JF, Backlund J-YC, Cleary PA, Harrington AP, O’Leary DH, Lachin JM, et al. Progression of carotid artery intima-media thickness during 12 years in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes. 2011;60(2):607–13. doi: 10.2337/db10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rishikaysh P, Dev K, Diaz D, Qureshi WMS, Filip S, Mokry J. Signaling involved in hair follicle morphogenesis and development. International journal of molecular sciences. 2014;15(1):1647–70. doi: 10.3390/ijms15011647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperling LC. Hair anatomy for the clinician. Journal of the American Academy of Dermatology. 1991;25(1):1–17. doi: 10.1016/0190-9622(91)70167-z. [DOI] [PubMed] [Google Scholar]
- 33.Pierard G, Pierard-Franchimont C, Marks R, Elsner P. EEMCO guidance for the assessment of hair shedding and alopecia. Skin pharmacology and physiology. 2004;17(2):98–110. doi: 10.1159/000076020. [DOI] [PubMed] [Google Scholar]
- 34.Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131(10):2257–68. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 35.Paus R, Christoph T, Müller-Röver S, editors. Journal of Investigative Dermatology Symposium Proceedings. Nature Publishing Group; 1999. Immunology of the hair follicle: a short journey into terra incognita. [DOI] [PubMed] [Google Scholar]
- 36.Brajac I, Vičić M, Periša D, Kaštelan M. Human Hair Follicle: An Update on Biology and Perspectives in Hair Growth Disorders Treatment. Hair Ther Transplant. 2014;4(115) 2167-0951.1000115. [Google Scholar]
- 37.Xiao Y, Woo W-M, Nagao K, Li W, Terunuma A, Mukouyama Y-s, et al. Perivascular hair follicle stem cells associate with a venule annulus. Journal of Investigative Dermatology. 2013;133(10):2324–31. doi: 10.1038/jid.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finner AM. Nutrition and hair: deficiencies and supplements. Dermatologic clinics. 2013;31(1):167–72. doi: 10.1016/j.det.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Maeda S, Ueda K, Yamana H, Tashiro-Yamaji J, Ibata M, Mikura A, et al. Blood Supply--Susceptible Formation of Melanin Pigment in Hair Bulb Melanocytes of Mice. Plastic and Reconstructive Surgery Global Open. 2015;3(3) doi: 10.1097/GOX.0000000000000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi S, Miyamoto I, Takeda K. Measurement of human hair growth by optical microscopy and image analysis. British Journal of Dermatology. 1991;125(2):123–9. doi: 10.1111/j.1365-2133.1991.tb06058.x. [DOI] [PubMed] [Google Scholar]
- 41.Plikus MV, Van Spyk EN, Pham K, Geyfman M, Kumar V, Takahashi JS, et al. The Circadian Clock in Skin Implications for Adult Stem Cells, Tissue Regeneration, Cancer, Aging, and Immunity. Journal of biological rhythms. 2015;30(3):163–82. doi: 10.1177/0748730414563537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres EB, Torres-Pradilla M. Cutaneous manifestations in children with diabetes mellitus and obesity. Actas Dermo-Sifiliográficas (English Edition) 2014;105(6):546–57. doi: 10.1016/j.ad.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Piérard GE, Seité S, Hermanns-Lê T, Delvenne P, Scheen A, Piérard-Franchimont C. The skin landscape in diabetes mellitus. Focus on dermocosmetic management. Clinical, cosmetic and investigational dermatology. 2013;6:127. doi: 10.2147/CCID.S43141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nabaie L, Kavand S, Robati R, Sarrafi-rad N, Shahgholi L, Meshkat-Razavi G. Androgenic alopecia and insulin resistance: are they really related? Clinical and experimental dermatology. 2009;34(6):694–7. doi: 10.1111/j.1365-2230.2008.03118.x. [DOI] [PubMed] [Google Scholar]
- 45.Matilainen V, Koskela P, Keinänen-Kiukaanniemi S. Early androgenetic alopecia as a marker of insulin resistance. The Lancet. 2000;356(9236):1165–6. doi: 10.1016/S0140-6736(00)02763-X. [DOI] [PubMed] [Google Scholar]
- 46.Goldman BE, Fisher DM, Ringler SL. Transcutaneous PO2 of the scalp in male pattern baldness: a new piece to the puzzle. Plastic and reconstructive surgery. 1996;97(6):1109–16. doi: 10.1097/00006534-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Chatterjee N, Chattopadhyay C, Sengupta N, Das C, Sarma N, Pal SK. An observational study of cutaneous manifestations in diabetes mellitus in a tertiary care Hospital of Eastern India. Indian journal of endocrinology and metabolism. 2014;18(2):217. doi: 10.4103/2230-8210.129115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brueseke TJ, Macrino S, Miller JJ. Lack of lower extremity hair not a predictor for peripheral arterial disease. Archives of dermatology. 2009;145(12):1456–7. doi: 10.1001/archdermatol.2009.310. [DOI] [PubMed] [Google Scholar]
- 49.Parfrey N, Ryan J, Shanahan L, Brady M. Hairless lower limbs and occlusive arterial disease. The Lancet. 1979;313(8110):276. doi: 10.1016/s0140-6736(79)90807-9. [DOI] [PubMed] [Google Scholar]
- 50.Sontheimer DL. Peripheral vascular disease: diagnosis and treatment. American family physician. 2006;73(11):1971–6. [PubMed] [Google Scholar]
- 51.Anuradha CV. Aminoacid support in the prevention of diabetes and diabetic complications. Current protein and peptide science. 2009;10(1):8–17. doi: 10.2174/138920309787315194. [DOI] [PubMed] [Google Scholar]
- 52.Rashaid AH, Harrington PdB, Jackson GP. Profiling Amino Acids of Jordanian Scalp Hair as a Tool for Diabetes Mellitus Diagnosis: A Pilot Study. Analytical chemistry. 2015;87(14):7078–84. doi: 10.1021/acs.analchem.5b00460. [DOI] [PubMed] [Google Scholar]
- 53.Jackson GP, An Y, Konstantynova KI, Rashaid AH. Biometrics from the carbon isotope ratio analysis of amino acids in human hair. Science & Justice. 2015;55(1):43–50. doi: 10.1016/j.scijus.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Zubair S, Mujtaba G. Hair-A mirror of diabetes. J Pak Assoc Dermatol. 2009;19:31–3. [Google Scholar]
- 55.Jabbari A, Petukhova L, Cabral RM, Clynes R, Christiano AM. Genetic basis of alopecia areata: a roadmap for translational research. Dermatologic clinics. 2013;31(1):109–17. doi: 10.1016/j.det.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Šipetić S, Vlajinac H, Kocev N, Marinković J, Radmanović S, Denić L. Family history and risk of type 1 diabetes mellitus. Acta diabetologica. 2002;39(3):111–5. doi: 10.1007/s005920200028. [DOI] [PubMed] [Google Scholar]
- 57.Gökçe C, Aycan-Kaya Ö, Yula E, Üstün İ, Yengil E, Sefil F, et al. The effect of blood glucose regulation on the presence of opportunistic Demodex folliculorum mites in patients with type 2 diabetes mellitus. Journal of International Medical Research. 2013;41(5):1752–8. doi: 10.1177/0300060513494730. [DOI] [PubMed] [Google Scholar]
- 58.Torkamani N, Rufaut NW, Jones L, Sinclair RD. Beyond goosebumps: Does the arrector pili muscle have a role in hair loss? International journal of trichology. 2014;6(3):88. doi: 10.4103/0974-7753.139077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatric diabetes. 2004;5(4):219–26. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 60.Thrailkill KM. Insulin-like growth factor-I in diabetes mellitus: its physiology, metabolic effects, and potential clinical utility. Diabetes technology & therapeutics. 2000;2(1):69–80. doi: 10.1089/152091599316775. [DOI] [PubMed] [Google Scholar]
- 61.Zemva J, Schubert M. Central insulin and insulin-like growth factor-1 signaling-implications for diabetes associated dementia. Current Diabetes Reviews. 2011;7(5):356–66. doi: 10.2174/157339911797415594. [DOI] [PubMed] [Google Scholar]
- 62.Panchaprateep R, Asawanonda P. Insulin-like growth factor-1: roles in androgenetic alopecia. Experimental dermatology. 2014;23(3):216–8. doi: 10.1111/exd.12339. [DOI] [PubMed] [Google Scholar]
- 63.Wortsman X, Wortsman J, Matsuoka L, Saavedra T, Mardones F, Saavedra D, et al. Sonography in pathologies of scalp and hair. The British journal of radiology. 2014 doi: 10.1259/bjr/22636640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mubki T, Shamsaldeen O, McElwee KJ, Shapiro J. An update on diagnosis and treatment of female pattern hair loss. 2013 [Google Scholar]
- 65.Toerien M, Wilkinson S, editors. Women’s Studies International Forum. Elsevier; 2003. Gender and body hair: Constructing the feminine woman. [Google Scholar]
- 66.Giacomoni PU, Mammone T, Teri M. Gender-linked differences in human skin. Journal of dermatological science. 2009;55(3):144–9. doi: 10.1016/j.jdermsci.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 67.Mirmirani P. Age-related hair changes in men: Mechanisms and management of alopecia and graying. Maturitas. 2015;80(1):58–62. doi: 10.1016/j.maturitas.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, et al. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia. 2014;57(12):2465–74. doi: 10.1007/s00125-014-3369-7. [DOI] [PubMed] [Google Scholar]
- 69.Sperling LC. Hair density in African Americans. Archives of dermatology. 1999;135(6):656–8. doi: 10.1001/archderm.135.6.656. [DOI] [PubMed] [Google Scholar]
- 70.Bernstein RM, Rassman WR. The aesthetics of follicular transplantation. Dermatologic Surgery. 1997;23(9):785–99. doi: 10.1111/j.1524-4725.1997.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhao X, Ni R, Li L, Mo Y, Huang J, Huang M, et al. Defining hirsutism in Chinese women: a cross-sectional study. Fertility and sterility. 2011;96(3):792–6. doi: 10.1016/j.fertnstert.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 72.Al-Nuaimi Y, Baier G, Watson RE, Chuong CM, Paus R. The cycling hair follicle as an ideal systems biology research model. Experimental dermatology. 2010;19(8):707–13. doi: 10.1111/j.1600-0625.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Randall VA, editor. Seminars in cell & developmental biology. Elsevier; 2007. Hormonal regulation of hair follicles exhibits a biological paradox. [DOI] [PubMed] [Google Scholar]
- 74.Randall VA, Hibberts NA, Thornton MJ, Hamada K, Merrick AE, Kato S, et al. The hair follicle: a paradoxical androgen target organ. Hormone Research in Paediatrics. 2000;54(5–6):243–50. doi: 10.1159/000053266. [DOI] [PubMed] [Google Scholar]
- 75.Sawaya ME, Price VH. Different Levels of 5α-Reductase Type I and II, Aromatase, and Androgen Receptor in Hair Follicles of Women and Men with Androgenetic Alopecia. Journal of Investigative Dermatology. 1997;109(3):296–300. doi: 10.1111/1523-1747.ep12335779. [DOI] [PubMed] [Google Scholar]
- 76.Gerst C, Dalko M, Pichaud P, Galey J, Buan B, Bernard B. Type-1 steroid 5α-reductase is functionally active in the hair follicle as evidenced by new selective inhibitors of either type-1 or type-2 human steroid 5α-reductase. Experimental dermatology. 2002;11(1):52–8. doi: 10.1034/j.1600-0625.2002.110106.x. [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi S, Itami S, Adachi K, Takayasu S. Hair Research for the Next Millennium. Amsterdam: Elsevier; 1996. Expression of androgen receptor, type I and type II 5α-reductase in human dermal papilla cells; pp. 333–7. [Google Scholar]
- 78.Thornton M, Taylor AH, Mulligan K, Al-Azzawi F, Lyon CC, O’Driscoll J, et al., editors. The distribution of estrogen receptor β is distinct to that of estrogen receptor α and the androgen receptor in human skin and the pilosebaceous unit. Journal of Investigative Dermatology Symposium Proceedings; Nature Publishing Group; 2003. [DOI] [PubMed] [Google Scholar]
- 79.Inui S, Fukuzato Y, Nakajima T, Kurata S, Itami S. Androgen receptor co-activator Hic-5/ARA55 as a molecular regulator of androgen sensitivity in dermal papilla cells of human hair follicles. Journal of Investigative Dermatology. 2007;127(10):2302–6. doi: 10.1038/sj.jid.5700883. [DOI] [PubMed] [Google Scholar]
- 80.Lee P, Zhu CC, Sadick N, Diwan AH, Zhang PS, Liu JS, et al. Expression of androgen receptor coactivator ARA70/ELE1 in androgenic alopecia. Journal of cutaneous pathology. 2005;32(8):567–71. doi: 10.1111/j.0303-6987.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- 81.Randall VA. Androgens: the main regulator of human hair growth. Hair and its disorders Biology, pathology and management. 2000:69–82. [Google Scholar]
- 82.Sawaya ME. Differences in the mechanisms of androgen action in hair follicles from women and men with androgenetic alopecia. Hair and Its Disorders: Biology, Pathology, and Management. 2000:153–58. [Google Scholar]
- 83.Duverger O, Morasso MI, editors. Seminars in cell & developmental biology. Elsevier; 2014. To grow or not to grow: hair morphogenesis and human genetic hair disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Tumbar T, editors. Seminars in cell & developmental biology. Elsevier; 2012. Hairy tale of signaling in hair follicle development and cycling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Javorsky E, Perkins AC, Hillebrand G, Miyamoto K, Kimball AB. Race, Rather than Skin Pigmentation, Predicts Facial Hair Growth in Women. The Journal of clinical and aesthetic dermatology. 2014;7(5):24. [PMC free article] [PubMed] [Google Scholar]
- 86.Harkins DK, Susten AS. Hair analysis: exploring the state of the science. Environmental health perspectives. 2003;111(4):576. doi: 10.1289/ehp.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loussouarn G. African hair growth parameters. British Journal of Dermatology. 2001;145(2):294–7. doi: 10.1046/j.1365-2133.2001.04350.x. [DOI] [PubMed] [Google Scholar]
- 88.Sinclair RD, editor. Healthy hair: what is it?. Journal of Investigative Dermatology Symposium Proceedings; 2007; Nature Publishing Group; [DOI] [PubMed] [Google Scholar]
- 89.LeBeau MA, Montgomery MA, Brewer JD. The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic science international. 2011;210(1):110–6. doi: 10.1016/j.forsciint.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Kahn CM, Guerrero A, Césped M. Seasonal variation of trichogram in Chilean subjects. Revista medica de Chile. 2009;137(11):1437–40. [PubMed] [Google Scholar]
- 91.Liu C, Yang J, Qu L, Gu M, Liu Y, Gao J, et al. Changes in Chinese hair growth along a full year. International journal of cosmetic science. 2014;36(6):531–6. doi: 10.1111/ics.12151. [DOI] [PubMed] [Google Scholar]
- 92.Moghadam-Kia S, Franks AG. Autoimmune disease and hair loss. Dermatologic clinics. 2013;31(1):75–91. doi: 10.1016/j.det.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 93.Parodi A, Cozzani E. Hair loss in autoimmune systemic diseases. Giornale italiano di dermatologia e venereologia: organo ufficiale, Societa italiana di dermatologia e sifilografia. 2014;149(1):79–81. [PubMed] [Google Scholar]
- 94.Estefan J, Ribeiro M, Abad E, Saintive S, Ramos-e-Silva M. Alopecia areata--Part I: Background. Skinmed. 2014;13(1):42–53. [PubMed] [Google Scholar]
- 95.Hordinsky MK, editor. Overview of alopecia areata. Journal of Investigative Dermatology Symposium Proceedings; 2013; Nature Publishing Group; [DOI] [PubMed] [Google Scholar]
- 96.Zabielinski M, Tosti A. Eating Disorders and the Skin. Springer; 2013. General characteristics of hair in eating disorders; pp. 71–7. [Google Scholar]


