Abstract
Recent research on podocytes has proposed B7-1 as an important player in podocyte biology and as a potential new therapeutic target. B7-1 was upregulated in injured podocytes and described as a biomarker to identify patients who may benefit from abatacept, a B7-1 blocker. However, after this initial enthusiasm, several reports have not confirmed the efficiency of abatacept at inducing proteinuria remission in patients. In order to resolve these discrepancies, we explored the role of B7-1 in the injured podocyte. Both primary cultured and immortalized podocytes were exposed to lipopolysaccharides, but this failed to induce B7-1 expression at the mRNA and protein levels. Importantly, TLR-4 engagement confirmed lipopolysaccharide efficacy. We then evaluated B7-1 expression in several mouse models of podocyte injury including treatment with lipopolysaccharide or Adriamycin, a lupus prone model (NZB/W F1) and subtotal nephrectomy. Using 3 commercially available anti-B7-1 antibodies and appropriate controls, we could not find B7-1 expression in podocytes, whereas some infiltrating cells were positive. Thus, our findings do not support a role for B7-1 in podocyte biology. Hence, further studies are mandatory before treating proteinuric patients with B7-1 blockers.
Keywords: albuminuria, B7-1, cytoskeleton, podocytes
The renal glomerulus functions as a barrier between blood and urinary spaces. It permits the passage of small molecules and water freely into the urine while preventing the loss of large serum proteins such as albumin. The barrier is composed of podocytes and fenestrated endothelial cells separated by the glomerular basement membrane. Podocytes are differentiated cells that function as vasculature-supporting cells producing basement membrane and vascular growth factors.1 Podocyte injuries lead to protein redistribution in the slit diaphragm, the loss of nephrin and podocin, and the effacement of the foot processes.2 Foot process effacement represents a reversible step that becomes irreversible if the injury is sustained. The failure of repair mechanisms promotes podocyte detachment, apoptosis, podocyte depletion, and the development of focal and segmental glomerulosclerosis (FSGS).3
Recently, B7-1, a costimulatory receptor on antigen presenting cells, was found to be induced in podocytes during injury.4 B7-1 engagement in vitro seems to lead to cytoskeleton modification through β1 integrin.4 In vivo, podocyte B7-1 recruitment seems to participate to foot process effacement and proteinuria occurrence in a variety of conditions, including activation of innate immune signaling via Toll-like receptor 4 (TLR-4) by bacterial endotoxin (LPS).4 In humans, B7-1 was upregulated in several proteinuric states, including FSGS.4, 5 Indeed, B7-1 expression in podocytes was proposed as both a biomarker that can identify proteinuric patients who might benefit from treatment with abatacept, a B7-1 blocker,6 and as a new therapeutic target.5 These findings recently led to the use of abatacept in the treatment of 4 patients with a resistant form of recurrent FSGS after transplantation.5 This treatment pushed patients into remission with only 1 or 2 abatacept infusions.5
However, although this first report was exciting, further work has not confirmed the beneficial effect observed with abatacept at inducing FSGS remission after transplantation.7, 8 Furthermore, B7-1 immunostaining on kidney biopsies has been largely controversial,9, 10 leading to the hypothetical theory of epitope instability.11
To understand these discrepancies, we decided to explore B7-1 expression in mouse podocytes using several injury models and in cultured podocytes.
Results
B7-1 is not induced in cultured podocytes after injury
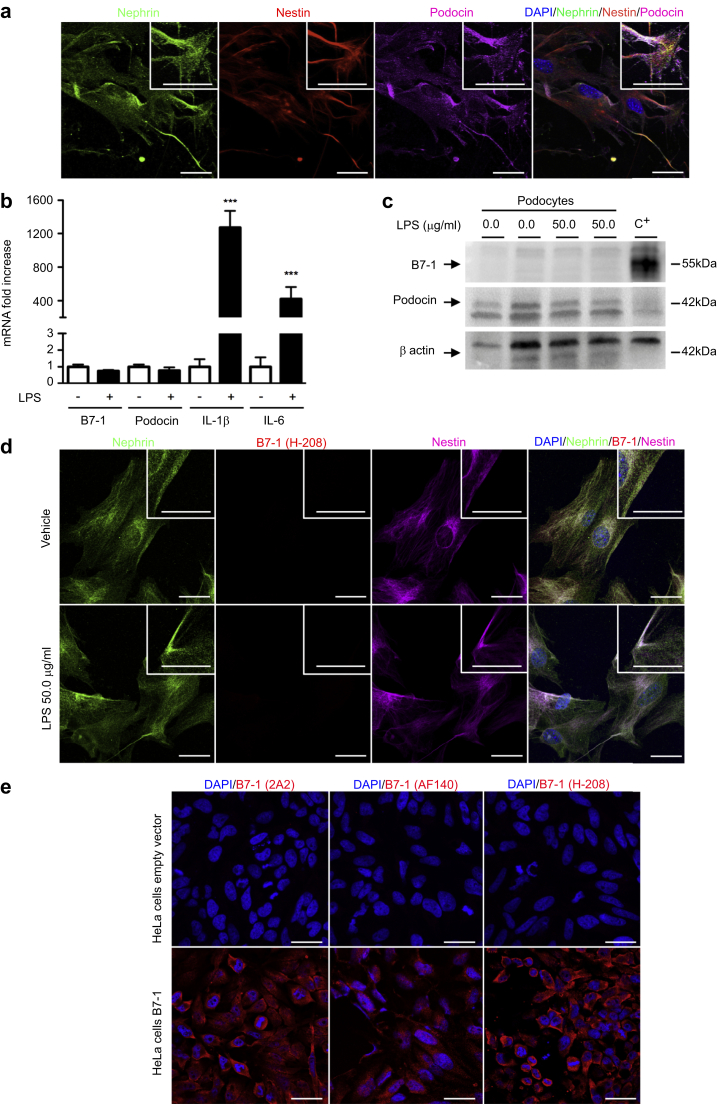
We first decided to investigate B7-1 expression in vitro. Toward this aim, we isolated mouse podocytes. We confirmed that after 14 days in culture, the podocytes were still in a differentiated state with suggestive podocyte morphology and that they expressed typical podocyte markers, such as nephrin, podocin, and nestin (Figure 1a). Then, we exposed the podocytes to LPS, a molecule reported to induce B7-1 mRNA expression in podocytes.4, 5 At baseline, we observed a very small amount of B7-1 mRNA in our primary culture of podocytes. After LPS treatment, we did not see B7-1 mRNA induction (Figure 1b), whereas we could detect IL-6 and IL-1β mRNA induction confirming TLR-4 engagement in primary cultured cells (Figure 1b). Next, we explored whether B7-1 was detectable at the protein level in our differentiated podocytes. A Western blot analysis revealed that B7-1 was not present at the protein level in podocytes, neither at baseline nor after LPS treatment (Figure 1c). The costaining experiments did not confirm B7-1 expression in primary culture of podocytes either at baseline or after LPS treatment (Figure 1d). To ensure that the anti-B7-1 antibody was able to detect the murine form of B7-1, we transfected HeLa cells with a vector encoding mouse B7-1 cDNA as positive controls. Additionally, we tested 2 other anti-B7-1 antibodies that were commercially available. We confirmed that the antibodies were able to detect the B7-1 protein in HeLa transfected cells (Figure 1e) but failed to detect B7-1 in podocytes exposed either to vehicle or LPS.
Figure 1.
B7-1 is not induced in primary cultured podocytes after injury. (a) Coimmunostaining for nephrin (green), nestin (red), and podocin (purple) in mouse podocytes isolated from primary culture. (b) B7-1 mRNA is barely detectable in podocytes at baseline and is not induced after lipopolysaccharide (LPS) stimulation. IL-6 and IL-1β mRNA confirmed Toll-like receptor 4 engagement after LPS treatment. (c) Western blot of B7-1 (SC-9091 from Santa Cruz Biotechnology Inc., Heidelberg, Germany) in primary cultures of mouse podocytes exposed to either vehicle or 50.0 μg/ml LPS for 24 hours. C+: positive control, HeLa cells transfected with a vector containing mouse B7-1 DNA. Western blot of podocin (upper band) confirms podocyte enrichment. (d) Coimmunostaining for nephrin (green), B7-1 (red, SC-9091 from Santa Cruz Biotechnology Inc.), and podocin (purple) in mouse podocytes 24 hours after exposure to either vehicle (upper panel) or 50.0 μg/ml LPS (lower panel). (e) B7-1 immunostaining using 3 anti-B7-1 antibodies (2A2: ab86473 from Abcam; AF140: from R&D Systems; and H-208: SC-9091 from Santa Cruz Biotechnology Inc.) in HeLa cells transfected with plasmids containing either an empty vector or mouse B7-1 DNA. Bar = 10 μm. The data are represented as the means ± SEM. Analysis of variance followed by a Tukey-Kramer test; podocytes treated with vehicle versus LPS: *** P < 0.001. DAPI, 4′,6-diamidino-2-phenylindole.
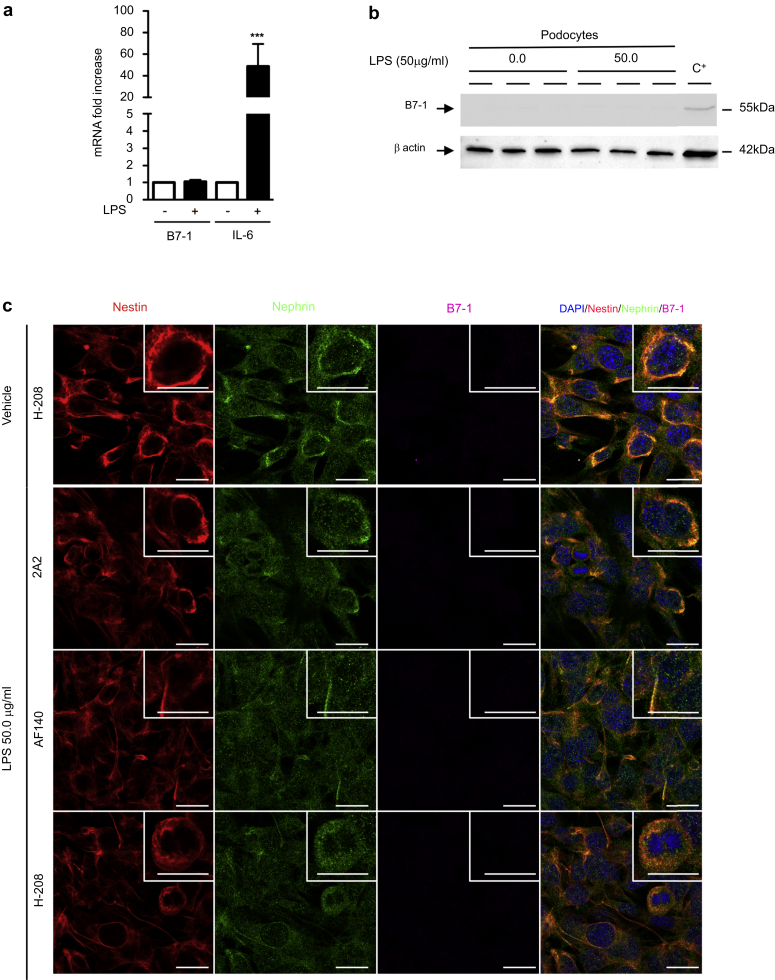
Because a primary culture of glomerular cells contain podocytes but also a few endothelial and mesangial cells, we explored B7-1 expression in immortalized mouse podocytes.12 At baseline, we observed a very small amount of B7-1 mRNA in cultured podocytes, but we did not detect B7-1 mRNA induction (Figure 2a) or B7-1 protein after LPS treatment (Figure 2b). IL-6 mRNA induction was detected, confirming TLR-4 engagement in podocytes (Figure 2a). Costaining experiments using 3 commercially available anti-B7-1 antibodies confirmed the absence of B7-1 expression in immortalized mouse podocytes at baseline or after LPS treatment (Figure 2c).
Figure 2.
B7-1 is not induced in immortalized podocytes after injury. (a) B7-1 mRNA is barely detectable in immortalized podocytes at baseline and is not induced after lipopolysaccharide (LPS) stimulation. IL-6 mRNA confirmed Toll-like receptor 4 engagement after LPS treatment. (b) Western blot of B7-1 (SC-9091 from Santa Cruz Biotechnology Inc.) in immortalized podocytes exposed to either vehicle or 50.0 μg/ml LPS for 24 hours. C+: positive control, HeLa cells transfected with a vector containing mouse B7-1 DNA. (c) Coimmunostaining for nestin (red), nephrin (green), and 3 different anti-B7-1 antibodies (purple, 2A2: ab86473 from Abcam [Paris, France]; AF140: from R&D Systems [Lille, France]; and H-208: SC-9091 from Santa Cruz Biotechnology Inc.) in immortalized podocytes 24 hours after exposure to either vehicle (upper panel) or 50.0 μg/ml LPS (lower panel). Bar = 10 μm. The data are represented as the means ± SEM. Analysis of variance followed by a Tukey-Kramer test; podocytes treated with vehicle versus LPS: *** P < 0.001. DAPI, 4′,6-diamidino-2-phenylindole.
Indeed, in our hands, we did not confirm B7-1 expression in stressed podocytes.
B7-1 is not induced in mouse models of podocyte injuries
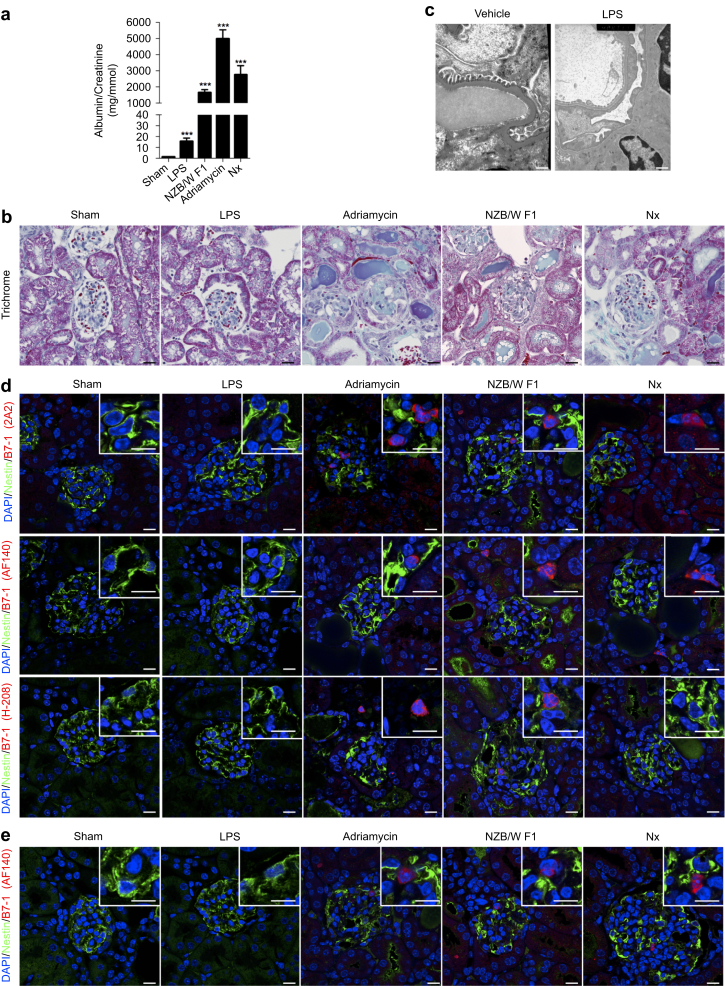
B7-1 has been upregulated in several mouse models of proteinuria.4 We next investigated B7-1 expression in different mouse models of podocyte injury, including LPS, Adriamycin (Sigma Aldrich, St. Quentin Fallavier, France) nephropathy, lupus prone mice (NZB/W F1), and subtotal nephrectomy. We first validated that the LPS (200 μg), Adriamycin, lupus prone mouse, and nephrectomy models produced albuminuria (Figure 3a). As expected, almost all the models lead to albuminuria. However, LPS-treated mice showed only minor albuminuria induction. We decided to test 2 additional dosages of LPS (300 and 400 μg) and failed to induce more proteinuria. Using 300 and 400 μg, the mice died within 24 hours of injection due to sepsis shock condition (not shown). Next, we performed a morphological analysis of the kidney sections in each condition. Histological analyses revealed that glomeruli appeared grossly normal in LPS-injected mice, whereas glomeruli were severely injured in mice injected with Adriamycin, in lupus prone mice, and after nephrectomy (Figure 3b). However, electron microscopy revealed that LPS induced foot process effacement with cytoskeleton reorganization (Figure 3c). We then performed colocalization studies in paraffin-embedded kidneys between nestin, a podocyte marker, and B7-1 in all models of injuries. The colocalization studies were performed with the 3 commercially available B7-1 antibodies. Careful examination of glomeruli showed that B7-1 was not detectable in podocytes (Figure 3d). Importantly, we could detect B7-1 expression in some infiltrating cells, confirming that anti-B7-1 antibodies were efficient in detecting the B7-1 epitope in our sections. Lastly, we performed costaining between anti-B7-1 antibody and nestin on frozen kidney sections. Similarly, using the 3 commercially available anti-B7-1 antibodies, we could not find B7-1 expression in podocytes, whereas we could find B7-1 expression in some infiltrating cells (Figure 3e). Of note, because the anti-B7-1 antibodies gave similar results, only the AF140, goat antibody from R&D Systems Europe (Lille, France) is shown in Figure 3e. We therefore conclude that B7-1 is not detectable in these proteinuric mouse models.
Figure 3.
B7-1 is not induced in murine models of podocyte injury. (a) The albumin-creatinine ratio in the different models of podocytes injuries. (b) Glomerular morphology in sham-operated mice, in mice injected with lipopolysaccharide (LPS) (day 1 post injection), in mice injected with Adriamycin (day 7 post injection), in NZB/W F1 mice (age 46 weeks), and in mice 2 months after nephrectomy (Nx). (c) Transmission electron microscopy of the glomerular basement membrane in kidneys of vehicle- or LPS-treated mice 1 day after injection. Bar = 0.5 μm. (d) Coimmunostaining for nestin (green) and 3 different anti-B7-1 antibodies (red, 2A2: ab86473 from Abcam; AF140: from R&D Systems; and H-208: SC-9091 from Santa Cruz Biotechnology Inc.) in the paraffin-embedded kidneys of all models of podocyte injuries. (e) Coimmunostaining for nestin (green) and anti-B7-1 antibody (red, AF140: from R&D Systems) in the frozen sections of kidney of all models of podocyte injuries. Bar = 10 μm. Analysis of variance followed by a Tukey-Kramer test; all models versus sham: *** P < 0.001. DAPI, 4′,6-diamidino-2-phenylindole.
Discussion
In this study, we did not observe B7-1 expression in mouse podocytes at the protein level at baseline or after injury. We could detect only a very small amount of mRNA, which did not increase after LPS stimulation, a condition that was reported to induce its expression.4 Our in vivo data using several mouse models of proteinuric diseases corroborate our in vitro findings. Importantly, our experimental findings are in line with accumulating evidence from independent groups showing that B7-1 blockers are not associated with albuminuria improvement in patients.7, 8, 13
B7-1, a costimulatory molecule, was unexpectedly observed in injured podocytes.4 Indeed, it was shown that injured podocytes expressed B7-1 leading to cytoskeletal modification in vitro. Furthermore, these in vitro findings were also observed in several models of proteinuria including activation of innate immune signaling via TLR-4 by bacterial endotoxin (LPS).4 More strikingly, mice with severe combined immunodeficiency who were exposed to LPS rapidly upregulate B7-1 in podocytes and develop proteinuria, whereas mice lacking B7-1 were protected from LPS-induced nephrotic syndrome, suggesting a link between podocyte B7-1 expression and proteinuria.4 Importantly, the mice used in the study were knockouts for B7-1−/− but not for podocyte-specific B7-1 knockouts, meaning that the beneficial effect that was observed could be related to an effect on immune cells rather than a direct effect on podocytes. Yu et al.5 then reported the successful treatment of 4 patients with recurrent FSGS on the allografted kidney using a B7-1 blocker. However, others did not confirm these clinical results,7, 8, 13 and B7-1 expression in podocytes in vivo has been controversial.14, 15
Using appropriate controls, we could not confirm that B7-1 is expressed at the protein level or induced at the mRNA level in injured murine podocytes. We observed that LPS engaged the TLR-4 pathway in podocytes as assessed by the mRNA induction of IL-6 and IL-1β but did not induce B7-1 expression. These results were obtained in primary cultured podocytes and then confirmed in immortalized podocytes. We also failed to detect B7-1 in different mouse models of proteinuria. In all these models, we could not detect B7-1 expression in podocytes. Importantly, we could detect infiltrating cells stained for B7-1, an internal control of the validity of the different antibodies used. All sample kidneys were fixed, processed in the same way, and stained in the same time to avoid any bias.
We must be cautious in the interpretation of the results. In fact, it is possible that in human kidney diseases, B7-1 plays a role in podocytes, a condition that was not explored here. Additionally, abatacept was efficient at inducing proteinuria remission in a few patients.5 However, it is possible that the effect that was observed with abatacept in FSGS patients was not directly related to a podocyte effect but rather, was due to an action on immune cells or an off-target effect of the drug. Many reports did not observe such an effect of abatacept on proteinuria in recurrent FSGS after transplantation, but we know that the disease is extremely heterogeneous, and not all patients will benefit from abatacept treatment. The identification of such responders will remain an important challenge.
In conclusion, using the appropriate tools, we did not confirm that B7-1 was expressed in murine podocytes under pathological conditions, and further studies are recommended before using B7-1 blockers in patients with proteinuric diseases.
Materials and Methods
Animal experiments
The mouse strains that were used in these studies included FVB/N, C57BL/6, and BALB/c (Charles River Laboratories, Wilmington, MA). The animals were fed ad libitum and housed at a constant ambient temperature under a 12-hour light cycle. All animal procedures were approved by the Departmental Director of “Services Vétérinaires de la Préfecture de Police de Paris” and by the ethical committee of Paris Descartes University. Several groups of mice were investigated in complementary studies.
Lipopolysaccharide injections
Eight-week-old male C57BL/6 mice were i.p. administered either 200 μg of lipopolysaccharide (Ultrapure LPS; 1 mg/ml in sterile LPS-free phosphate-buffered saline, n = 6) in a total volume of 200 μl or an equal volume of sterile LPS-free phosphate-buffered saline (n = 6) (Ultrapure LPS from E. coli O111:B4, InvivoGen, Toulouse, France). Urine samples were collected and analyzed 24 hours post injection and immediately prior to sacrifice. Kidneys and spleens were then removed for morphological and protein studies.
Adriamycin nephropathy
Eight-week-old male BALB/c mice (n = 12) were administered either 17 μg/g body weight Adriamycin (Sigma Aldrich) diluted in 0.9% saline or an equal volume of 0.9% saline (n = 12) into the retrobulbar plexus. Twenty-four hours before sacrifice, urine was collected for determination of proteinuria. The mice were sacrificed 7 days after injection, and their kidneys were removed for morphological and protein studies.
Subtotal nephrectomy
Eight-week-old female FVB/N mice were subjected to 75% nephrectomies (n = 10) or sham-operations (n = 10) and were sacrificed 2 months after surgery. The subtotal nephrectomies were performed as previously described.3 After surgery, the mice were fed a defined diet containing 30% casein and 0.5% sodium. Twenty-four hours before sacrifice, urine samples were collected for determination of proteinuria. At the time of sacrifice, kidneys were removed for morphological and protein studies.
NZB/W F1
Forty-six-week-old mice (n = 6) from Jackson Laboratories (Bar Harbor, ME) were used as a model of lupus nephritis. Twenty-four hours before they were killed, urine samples were collected for determination of proteinuria. At the time they were killed, their kidneys were removed for morphological and protein studies.
Urine analyses
For urine samples, albumin and creatinine concentrations were measured using an Olympus multiparametric analyzer (Instrumentation Laboratory, Bedford, MA).
Morphological analysis
Mouse kidneys were fixed in 4% paraformaldehyde and embedded with paraffin, and 4-μm sections were stained with Masson trichrome.
Electron microscopy studies were performed as previously described.16
Immunofluorescence
Four-micrometer, paraffin-embedded sections were incubated with 3 different anti-B7-1 antibodies: (i) anti-B7-1 antibody from Santa Cruz Biotechnology Inc. (Heidelberg, Germany; (ii) anti-B7-1 antibody (mouse, 2A2) from Abcam (Paris, France); (iii) anti-B7-1 antibody (AF140, goat) from R&D Systems Europe. We also used anti-Nephrin (Progen Biotechnik GmbH, Heidelberg, Deutschland), anti-Podocin (H208, rabbit; Sigma Aldrich), and anti-Nestin (Rat-401; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) antibodies after appropriate antigen retrieval. The sections were deparaffinized and rehydrated, then the slides were brought to boil in Tris–ethylenediamine tetraacetic acid buffer (10 mM Tris base, 1 mM ethylenediamine tetraacetic acid solution, 0.05% Tween 20, pH 9.0) and maintained at a subboiling temperature (95 °C) for 40 minutes. This step was followed by cooling time on the bench for 30 minutes. Then, each section was blocked with Tris-buffered saline Tween-20/5% normal goat serum for 30 minutes. Anti-B7-1 antibodies were used at a dilution of 1:50 and incubated on the sections overnight at 4 °C. The primary antibodies were revealed with the appropriate Alexa 488-, 555-, or 647-conjugated secondary antibodies (Molecular Probes, San Diego, CA) used at a dilution of 1:200 for 2 hours at room temperature. Immunofluorescence staining was visualized using a Zeiss LSM 700 confocal microscope (Jena, Germany). For each sample, we evaluated all glomeruli per section (a minimum of 50 glomeruli, 2 to 4 sections per mouse, were evaluated per animal).
Kidneys frozen in optimal cutting temperature compound were cut in 4-μm sections, fixed in acetone, and then incubated with the anti-B7-1 antibody from Santa Cruz Biotechnology the anti-B7-1 antibody (mouse, 2A2) from Abcam, or the anti-B7-1 antibody (AF140, goat) from R&D Systems Europe, and anti-Nestin (Rat-401, Developmental Studies Hybridoma Bank) antibodies.
Cell culture and cell experiments
Primary podocyte cell culture
Mouse podocytes were isolated and cultured as previously described.17 Briefly, primary cultures of podocytes were maintained in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and insulin-transferrin-sodium selenite media (Sigma Aldrich) for 14 days. For LPS experiments, the cells were treated with 50 μg/ml of Ultrapure E. coli (LPS from E. coli O111:B4; InvivoGen) for 24 hours before performing immunofluorescence, quantitative polymerase chain reaction, and a Western blot analysis. Each experiment was performed in triplicate and repeated ≥3 times.
Immortalized podocyte cell culture
Mouse immortalized podocytes were cultured as previously described.18 Briefly, cells were maintained in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. To propagate podocytes, cells were cultivated at 33 °C on type I collagen (permissive conditions) in culture medium supplemented with 10 UI/ml recombinant interferon-γ. To induce differentiation, podocytes were maintained on type I collagen at 37 °C without supplementation with interferon-γ (nonpermissive conditions). For LPS experiments, the cells were treated with 50 μg/ml of Ultrapure E. coli (LPS from E. coli O111:B4, InvivoGen) for 24 hours before performing immunofluorescence, quantitative polymerase chain reaction, and a Western blot analysis. Each experiment was performed in triplicate and repeated ≥3times.
B7-1 transfection
HeLa cells were transfected with pcDNA3.1-C-(k)DYK (NM_009855,ORF Sequence; GenScript HK Limited, Hong Kong, China) plasmids using Lipofectamine 2000 according to the supplier’s instructions (Invitrogen, Carlsbad, CA).
Cell immunofluorescence studies
Primary cultures of podocytes and differentiated podocytes were fixed in 4% paraformaldehyde for 20 minutes, permeabilized in phosphate-buffered saline containing 0.2% Triton X-100, and then incubated with anti-Nestin (Rat-401, Developmental Studies Hybridoma Bank), anti-Nephrin (Progen Biotechnik), anti-Podocin (Sigma Aldrich), and the 3 different anti-B7-1 (H-208, rabbit from Santa Cruz Biotechnology Inc.; 2A2, mouse from Abcam; AF140, goat from R&D Systems Europe) antibodies. The primary antibodies were revealed with the appropriate Alexa 488-, 555-, or 647-conjugated secondary antibodies (Molecular Probes). Immunofluorescence staining was visualized using a Zeiss LSM 700 confocal microscope.
Real-time reverse transcriptase polymerase chain reaction
B7-1 mRNA was detected in podocytes by real-time reverse transcriptase polymerase chain reaction using an ABI PRISM 7700 Sequence Detection system (Applied Biosystems, Foster City, CA). The primers (Eurogentec, Liège, Belgium) used were as follows: B7-1 forward 5′-TGTATGCCCAGGAAACAGGT-3′ and reverse 5′-AGCCCGATCACCAC-TGATTA-3′, podocin forward 5′-GCT-GTC-TGC-TAC-TAC-CGC-AT-3′ and reverse 5′-CTC-TCC-ACT-TTG-ATG-CCC-CA-3′, IL-6 forward 5′-CCA-GAG-TCC-TTC-AGA-GAG-ATA-CA-3′ and reverse 5′-CCT-TCT-GTG-ACT-CCA-GCT-TAT-C-3′ and IL-1β forward 5′-TCA-TTG-TGG-CTG-TGG-AGA-AG-3′ and reverse 5′-GCC-TGT-AGT-GCA-GTT-GTC-TAA-3′.
RPL13 and Hprt were used as a normalization control.
Western blot
Western blots were performed as previously described.19 Briefly, protein extracts from podocytes were resolved by sodium dodecylsulfate–polyacrylamide gel electrophoresis before being transferred onto the appropriate membranes and incubated with the 3 different anti-B7-1 (H-208, rabbit from Santa Cruz Biotechnology; 2A2, mouse from Abcam; AF140, goat from R&D Systems Europe), anti-Podocin (Sigma Aldrich); and anti-β actin (Sigma Aldrich) antibodies, followed by the appropriate peroxidase-conjugated secondary antibodies. Chemiluminescence was acquired using a Fusion FX7 camera (Vilber Lourmat, Eberhardzell, Germany), and densitometry was performed using ImageJ software (National Institutes of Health, Bethesda, MD).
Data analysis and statistics
The data are expressed as the means ± SEM. Differences between the experimental groups were evaluated using analyses of variance followed by the Tukey-Kramer test when significant (P < 0.05). Statistical analyses were performed using GraphPad Prism Software (San Diego, CA).
Disclosure
All the authors declared no competing interests.
Acknowledgments
This work was supported by the Emmanuel Boussard Foundation (London, UK), the Day Solvay Foundation (Paris, France), Institut National de la Santé et de la Recherche Médicale, Assistance Publique-Hôpitaux de Paris, and University Paris Descartes. This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation program grant 679254.
Author Contributions
EB, MG, and MD designed and performed the experiments and analyzed the data. CL and FT were involved in data analysis and the conceptual framework. GC provided the conceptual framework, designed the study, supervised the project, and wrote the paper.
References
- 1.D'Agati V.D., Kaskel F.J., Falk R.J. Focal segmental glomerulosclerosis. N Engl J Med. 2011;365:2398–2411. doi: 10.1056/NEJMra1106556. [DOI] [PubMed] [Google Scholar]
- 2.Canaud G., Dion D., Zuber J. Recurrence of nephrotic syndrome after transplantation in a mixed population of children and adults: course of glomerular lesions and value of the Columbia classification of histological variants of focal and segmental glomerulosclerosis (FSGS) Nephrol Dial Transplant. 2010;25:1321–1328. doi: 10.1093/ndt/gfp500. [DOI] [PubMed] [Google Scholar]
- 3.Canaud G., Bienaime F., Viau A. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med. 2013;19:1288–1296. doi: 10.1038/nm.3313. [DOI] [PubMed] [Google Scholar]
- 4.Reiser J., von Gersdorff G., Loos M. Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–1397. doi: 10.1172/JCI20402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu C.C., Fornoni A., Weins A. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416–2423. doi: 10.1056/NEJMoa1304572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreland L., Bate G., Kirkpatrick P. Abatacept. Nat Rev Drug Discov. 2006;5:185–186. doi: 10.1038/nrd1989. [DOI] [PubMed] [Google Scholar]
- 7.Delville M, Baye E, Durrbach A, et al. B7-1 blockade does not improve post-transplant nephrotic syndrome caused by recurrent FSGS [e-pub ahead of print]. J Am Soc Nephrol. pii: ASN.2015091102, accessed December 23, 2015. [DOI] [PMC free article] [PubMed]
- 8.Grellier J., Del Bello A., Milongo D. Belatacept in recurrent focal segmental glomerulosclerosis after kidney transplantation. Transpl Int. 2015;28:1109–1110. doi: 10.1111/tri.12574. [DOI] [PubMed] [Google Scholar]
- 9.Benigni A., Gagliardini E., Remuzzi G. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1261–1263. doi: 10.1056/NEJMc1400502. [DOI] [PubMed] [Google Scholar]
- 10.Larsen C.P., Messias N.C., Walker P.D. B7-1 immunostaining in proteinuric kidney disease. Am J Kidney Dis. 2014;64:1001–1003. doi: 10.1053/j.ajkd.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 11.Mundel P., Greka A. Developing therapeutic 'arrows' with the precision of William Tell: the time has come for targeted therapies in kidney disease. Curr Opin Nephrol Hypertens. 2015;24:388–392. doi: 10.1097/MNH.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mundel P., Reiser J., Zuniga Mejia Borja A. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 13.Alachkar N., Carter-Monroe N., Reiser J. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1263–1264. doi: 10.1056/NEJMc1400502. [DOI] [PubMed] [Google Scholar]
- 14.Novelli R., Gagliardini E., Ruggiero B. Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am J Physiol Renal Physiol. 2016;310:F335–F341. doi: 10.1152/ajprenal.00510.2015. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardini E., Novelli R., Corna D. B7-1 is not induced in podocytes of human and experimental diabetic nephropathy. J Am Soc Nephrol. 2016;27:999–1005. doi: 10.1681/ASN.2015030266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mollet G., Ratelade J., Boyer O. Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol. 2009;20:2181–2189. doi: 10.1681/ASN.2009040379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takemoto M., Asker N., Gerhardt H. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankland S.J., Pippin J.W., Reiser J., Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- 19.Canaud G., Bienaime F., Tabarin F. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med. 2014;371:303–312. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]