Abstract
Borrelia burgdorferi is the causative agent of Lyme disease in the U.S., with at least 25,000 cases reported to the CDC each year. B. burgdorferi is thought to enter and exit the bloodstream to achieve rapid dissemination to distal tissue sites during infection. Travel through the bloodstream requires evasion of immune surveillance and pathogen clearance in the host, a process at which B. burgdorferi is adept. B. burgdorferi encodes greater than 19 adhesive outer surface proteins many of which have been found to bind to host cells or components of the extracellular matrix. Several others bind to host complement regulatory factors, in vitro. Production of many of these adhesive proteins is tightly regulated by environmental cues, and some have been shown to aid in vascular interactions and tissue colonization, as well as survival in the blood, in vivo. Recent work has described multifaceted and redundant roles of B. burgdorferi outer surface proteins in complement component interactions and tissue targeted adhesion and colonization, distinct from their previously identified in vitro binding capabilities. Recent insights into the multifunctional roles of previously well-characterized outer surface proteins such as BBK32, DbpA, CspA, and OspC have changed the way we think about the surface proteome of these organisms during the tick–mammal life cycle. With the combination of new and old in vivo models and in vitro techniques, the field has identified distinct ligand binding domains on BBK32 and DbpA that afford tissue colonization or blood survival to B. burgdorferi. In this review, we describe the multifunctional and redundant roles of many adhesive outer surface proteins of B. burgdorferi in tissue adhesion, colonization, and bloodstream survival that, together, promote the survival of Borrelia spp. throughout maintenance in their multi-host lifestyle.
Keywords: Lyme disease, adhesion, Borrelia, colonization, complement, pathogenicity, virulence
Introduction
Borrelia burgdorferi, a diderm motile spirochete bacterium, is the causative agent of Lyme disease in the U.S. Each year greater than 25,000 confirmed cases of Lyme disease are reported to the United States Centers for Disease Control and Prevention with about 96% of those cases reported from only 14 states in the Midwest and East (1). Lyme disease is also a significant health problem in parts of Europe and Asia where it is more commonly caused by Borrelia afzelii and Borrelia garinii than B. burgdorferi.
Borrelia spp. are maintained in nature in a tick–mammal life cycle. B. burgdorferi is carried by several species of the Ixodes genus of tick and is transmitted to mammals through tick saliva (2). The spirochetes are maintained in the tick midgut as the tick progresses through its life stages, but the bacteria are not passed transovarially to its offspring (2). The primary mammalian reservoir for B. burgdorferi is the white-footed mouse, Peromyscus leucopus (2). This reservoir is not known to be physically affected by the infection (3). Additional small animals and birds can also serve as reservoirs, whereas large animals and humans can be accidental hosts for tick feeding and subsequent infection.
Human infection with Borrelia spp. often results in a number of generic symptoms including headache, fatigue, and general malaise, and for this reason, the infection is often misdiagnosed or goes untreated. A large percentage of individuals infected with B. burgdorferi will display a rash, termed erythema migrans, at the site of a tick bite (4). An untreated infection with B. burgdorferi can result in late stage symptoms including arthritis, carditis, and neurologic issues (5–7). The CDC reported from 2001 to 2010 that 31% of confirmed Lyme disease cases presented with Lyme arthritis, 14% with neurologic symptoms, and 1% with cardiac involvement (1). The results of a late stage Borrelia infection vary depending on the infecting species, with B. garinii most often associated with neurologic symptoms and B. afzelii infection commonly associated with a skin rash called acrodermatitis chronica atrophicans (8–10).
Outer Surface Proteins of Borrelia burgdorferi
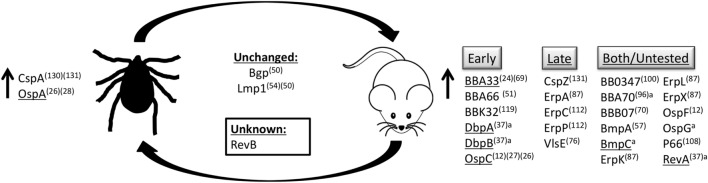
Borrelia spp. are able to exist in the tick–mammal life cycle due to their ability to adapt to the environment in which they reside. In in vitro studies, Borrelia spp. are able to respond to changes in pH and temperature of the environment, as well as cell density of spirochetes, to differentially regulate the production of many of their outer surface proteins (Figure 1) (11–14).
Figure 1.
Outer surface protein regulation. B. burgdorferi senses changes in temperature, pH, and cell density, as well as unknown stimuli to modulate production of proteins on the bacterial surface. Proteins listed are upregulated in their respective environments, in the tick vector or the mammalian host, or are produced at similar levels in both environments. Proteins produced during mammalian infection are grouped based on temporal expression pattern, expressed early during infection (early), during persistent infection (late), or those that have been detected both early and late during infection or have not been experimentally determined (both/untested). aBased on in vivo qRT-PCR and microarray data (15). (#)Reference. Underline indicates genes regulated by the RpoS regulon.
One way in which B. burgdorferi is able to respond to changes in these environmental conditions is through the RpoN–RpoS signaling system (14, 16). RpoS, RpoN, Rrp2, and BosR are considered the master regulators of virulence gene expression in B. burgdorferi (17–23). RpoS and Rrp2 have been shown to be required for mouse infectivity (18, 24). One example of such control is the reciprocal expression of outer surface protein A (ospA) and outer surface protein C (ospC) tightly regulated by RpoN, RpoS, and Rrp2 (19–21, 25, 26).
Borrelia burgdorferi produces OspA on its surface while in the unfed tick (11). Upon the uptake of blood into the midgut of the tick, ospA expression is maintained until transmission into the mammal when expression is decreased and ospC expression is increased in conjunction with many other genes that encode outer surface proteins, to aid in survival within the mammal (15, 27, 28). Interestingly, OspC production is not necessary and is, in fact, detrimental to survival of the bacteria, likely due to the high immunogenicity of the OspC protein (29). In addition to regulation of surface protein production by RpoN, RpoS, Rrp2, or BosR, B. burgdorferi also utilizes other mechanisms to rapidly change the epitopes available on the surface inside the mammalian host, but not within the tick (30). For example, B. burgdorferi encodes a variable membrane protein-like sequence (Vls) antigenic variation system that enables evasion of recognition by the host-adaptive immune system by continual recombination of silent vls gene segments encoding different vlsE sequences into the expression site (31–34).
Outer Surface Proteins and Virulence
For years, a focus of the Borrelia field, as with any pathogen field, has been to identify bacterial proteins that could contribute to virulence. Due to the cumbersome nature of Borrelia genetics, the roles of very few proteins have been described in mammalian infection. Using traditional cloning methods, glycosaminoglycan (GAG) and fibronectin (Fn)-binding protein, BBK32, and GAG and decorin-binding proteins, DbpA and DbpB, were all identified as being important for the establishment or persistence of mammalian infection (35–44). Additionally, the Fn-binding protein, RevA, was also found to have an effect on bacterial virulence (45, 46), though the affinity of the interaction between RevA and Fn was found to be less than that of BBK32 and Fn (41). Likewise, deletion of the ospC gene from B. burgdorferi strain B31 also has a negative impact on the establishment of infection in mice (29, 38, 47). This may be due to the antiphagocytic properties of OspC on Borrelia, though the importance of this activity has not yet been elucidated in vivo (48). Recent headway has been made in identifying Borrelia genes involved in mammalian infection by the generation and utilization of a transposon library in B. burgdorferi (49). By inoculating mice with the signature-tagged transposon mutagenesis (STM) library, the list of virulence determinants of B. burgdorferi was expanded to include additional outer surface proteins, many with undescribed function including BBB07, Bgp, BmpC, ErpA, RevB, and VlsE (Table 1) (49, 50). Through the use of traditional cloning, it was shown that the lipoprotein, BBA66, is also required for mammalian infection (51, 52).
Table 1.
Adhesive outer surface proteins of B. burgdorferi.
| Adhesin | Genetic locusa | In vitro binding | Reference | In vivo function | Reference |
|---|---|---|---|---|---|
| Adhesins with a role in mammalian infection | |||||
| Lmp1 | bb0210 | Chondroitin-6-sulfate | (53) | Not determined | (53–55) |
| BmpA | bb0383 | Laminin | (56) | Joint persistence | (57) |
| BmpC | bb0384 | Not determined | Not determined | (49) | |
| Bgp | bb0588 | Heparin, dermatan sulfate, GAGs, and aggrecan | (58–60) | Not determined | (49, 58) |
| P66 | bb0603 | Integrins αIIbβ3 and αvβ3 | (61) | Heart and skin adhesion, dissemination, and vascular transmigration (integrin-binding domain) | (62–64) |
| DbpA | bba24 | Decorin, GAGs | (65–68) | Joint colonization | (29, 35–44, 47, 49) |
| DbpB | bba25 | Decorin, GAGs | (65–68) | Joint colonization | (36, 43, 44, 49) |
| BBA33 | bba33 | Collagen | (69) | Not determined | (69) |
| BBB07 | bbb07 | Integrin α3β1 | (70) | Not determined | (49) |
| OspC | bbb19 | Plasminogen, Salp15 (in tick saliva) | (71, 72) | Bloodstream survival | (29, 38, 47, 62) |
| RevB | bbc10 | Fibronectin | (41, 45) | Not determined | (49) |
| VlsE | bbf32 | Not determined | Not determined | (49, 73–76) | |
| BBK32 | bbk32 | Fibronectin, GAGs, and complement component C1r | (77–80) | Vascular adhesion and joint colonization (GAG-binding domain) | (35, 39–42, 49, 62) |
| RevA | bbm27, bbp27 | Fibronectin, laminin | (41, 45) | Heart colonization | (46, 49, 62) |
| ErpA (CRASP-5) | bbp38 | Factor H, Factor H-related proteins 1, 2, and 5, and plasminogen | (81–86) | Not determined | (49, 87) |
| Adhesins with no role in mammalian infection | |||||
| OspA | bba15 | TROSPA (in tick) | (88) | N/A | (89) |
| CspZ (CRASP-2) | bbh06 | Factor H, Factor H-like 1 | (82, 90) | N/A | (91) |
| Adhesins with an undetermined role in mammalian infection | |||||
| CspA (CRASP-1) | bba68 | Factor H, Factor H-like 1, and complement components C7 and C9 | (86, 92–95) | Not determined | |
| BBA70 | bba70 | Plasminogen | (96) | Not determined | |
| ErpC (CRASP-4) | bbl39 | Factor H, Factor H-related protein 1, and plasminogen | (82, 83, 85) | Not determined | |
| ErpK | bbm38 | Heparan sulfate | (97) | Not determined | |
| ErpP (CRASP-3) | bbn38 | Factor H, Factor H-related proteins 1, 2, and 5, and plasminogen | (81–85, 98) | Not determined | |
| ErpL | bbo39 | Heparan sulfate | (97) | Not determined | |
| ErpX | bbq47 | Laminin | (85) | Not determined | |
| OspF | bbr42 | Heparan sulfate | (97) | Not determined | |
| ErpG (OspG) | bbs41 | Heparan sulfate, heparin | (97) | Not determined | |
N/A, not applicable.
aAll gene designations are based on B. burgdorferi strain B31.
Ligand Binding Mediated by Borrelia burgdorferi Outer Surface Proteins
Borrelia burgdorferi is known to produce at least 19 adhesive proteins on its surface (Table 1) (35). Previous work has focused on describing the binding capability of three B. burgdorferi outer surface proteins to the ECM component, Fn, which is expressed by a variety of cells types and has been shown to be important in neural and vascular development (99): RevA, BBK32, and BB0347. RevA is a 17 kDa outer surface lipoprotein of B. burgdorferi produced within the mammalian environment, which has been shown to bind to Fn in vitro, though reports on the affinity of this interaction are conflicting (41, 45). Recently, it was found that production of RevA is required for the colonization of heart tissue 1 month p.i. (46). In addition, B. burgdorferi produces BB0347 during mammalian infection (100), which has also been shown to bind to Fn with low affinity by surface plasmon resonance (41). RevA and BB0347 were both found to have a minimal role in vascular binding in mouse flank skin in vivo 1-h post infection (h.p.i.) (41). Much work has been done to decipher the role of the surface protein BBK32, produced during mammalian infection, and its high affinity interactions with Fn and GAGs, which are evenly distributed throughout the ECM of all mammalian tissues (41, 77, 101, 102). Tissue distribution and function of GAGs was expertly reviewed by Jinno and Park (86). Early work with BBK32 identified distinct regions of the protein required for binding to GAGs (residues 45–68) and Fn (residues 158–182) (41, 103). In contrast to other Fn-binding adhesins on B. burgdorferi, the region of Fn that interacts with BBK32 has been precisely defined (41). Interactions of BBK32 on the surface of B. burgdorferi with GAGs and Fn are important for tethering and dragging interactions with endothelial cells of the vasculature in vivo, respectively (33, 39, 98). This was demonstrated by a restoration of vascular adhesion to non-adhesive mutant B. burgdorferi upon BBK32 production as determined by intravital microscopy in mouse flank skin (35, 41, 104). Another component of the ECM, collagen, which is a structural component of bone, tendon, and ligaments [as reviewed in Ref. (99)] also acts as a ligand for B. burgdorferi. BBA33, was shown to bind to collagen type VI in vitro, and is required for mammalian infection (69). Additionally, the basement membrane glycoprotein, laminin, found in the epithelium [as reviewed in Ref. (99)], has been shown to be a ligand for B. burgdorferi adhesins BmpA, ErpX, and RevA (41, 56, 105). Studies using BmpA-deficient B. burgdorferi showed a role for this protein in bacterial persistence in the joints of mice (57).
Adhesive surface proteins of B. burgdorferi have also been identified that bind to integrins, integral membrane proteins found on the surface of all nucleated mammalian cells that function to bind to several ECM components [as reviewed in Ref. (106)]. One such protein is the 66 kDa putative porin, P66, which has been shown to be important for the establishment of mammalian infection (63, 107, 108). P66 was shown to bind to β3-chain integrins and is involved in bacterial dissemination from the site of inoculation in the skin (64, 109). B. burgdorferi encodes another integrin-binding protein, BBB07, which is produced during mammalian infection as evidenced by the presence of a specific antibody response in the serum of infected individuals (70). This protein has been shown to have a role in signaling through integrin α3β1 to induce the production of pro-inflammatory cytokines in vitro, though this activity has not yet been described in vivo (70).
Additional outer surface proteins (Osp) have been described on B. burgdorferi that are involved in binding to other host proteins, including the 22 kDa outer surface protein, OspC. OspC production is induced upon bacterial entry into mammalian tissue (Figure 1) (19) and has been shown to bind plasminogen in vitro (71). Plasminogen is a mammalian protein important for the degradation of the ECM to facilitate cellular migration. OspE protein family members, ErpA, ErpC, and ErpP, were all found to bind to plasminogen in vitro, as is seen with a number of other Borrelia proteins (85, 96, 110–112). However, the presence or role of plasminogen binding by these proteins in vivo has not yet been described. A function for plasminogen binding has been recently described for the adhesive B. burgdorferi protein, BBA70. Through in vitro experiments, it was shown that BBA70 binds to plasminogen, which cleaves and inactivates complement component C5, ultimately inhibiting membrane attack complex formation (96).
Along with producing proteins that bind to Fn, integrins, and plasminogen, Borrelia spp. also produce proteins, which have been shown to bind to a variety of GAGs. Just as was seen with BBK32, outer surface proteins DbpA and B, produced during mammalian infection (37), have been shown to bind to decorin, heparin, dermatan sulfate, and heparan sulfate in vitro (66–68, 113–115). OspF-related family members, ErpG, ErpK, and ErpL, were all found to bind to heparan sulfate, in addition to plasminogen, with varying affinities as determined by a series of in vitro assays (97). To add a layer of complexity, DbpA from different Borrelia species was found to bind to dermatan sulfate with differing affinities, which may contribute to the differences seen in clinical manifestations of disease caused by B. garinii, B. burgdorferi, and B. afzelii (114).
Tissue Colonization by Borrelia burgdorferi
In order to colonize tissue sites distal to the site of tick bite in the mammal, it is thought that Borrelia travel within the vascular system of the host. Specific tissue colonization by the spirochetes is thought to occur by targeted exit from the vasculature dictated by the binding specificity of the outer surface proteins of Borrelia spp. This hypothesis is plausible, as characteristics of vascular beds differ depending on the tissue they are associated with as well as the size and type of vessel (116, 117). Tissue bed-specific endothelial cell surface receptors have been documented including VCAM1 in liver, CD36 in lung and heart, L-selectin (CD62L/SELL) in the spleen, and CD133 in the skin, brain, eye, and testicular microvasculature (116). Vascular beds can also vary in their production of proteoglycans, GAGs, and ECM components [as reviewed in Ref. (118)]. The ability of B. burgdorferi to bind to GAGs and ECM components has been specifically associated with effects on bacterial virulence and tissue colonization for Borrelia outer surface proteins such as DbpA and BBK32 (36, 39, 42–44, 51, 71, 77, 113, 119).
It is possible that B. burgdorferi are able to interact with differentially available endothelial cell surface proteins and utilize them to preferentially bind different tissues. Endothelial cell binding by B. burgdorferi has been observed to occur in vitro through interaction with cell surface proteoglycans such as dermatan sulfate, fibronectin, and heparin (120, 121). The binding of B. burgdorferi to the vasculature has been observed, in vivo, to occur in three stages (35, 104). Using intravital microscopy techniques on mouse flank skin, Norman et al. observed both tethering and dragging interactions to occur during the first stage of B. burgdorferi interactions with the vasculature (35). Using a flow chamber, tethering and dragging interactions with the endothelium were confirmed to occur both at the cell surface and at endothelial cell junctions (121). Stationary interactions, which occur after tethering and dragging, may be controlled by B. burgdorferi integrin-binding proteins such as P66 and BBB07. After the spirochete is tightly associated with the endothelial cell junction, it is able to transmigrate into the tissue where it is presumed to colonize and replicate.
By inoculating mice with a library of filamentous phage-expressing B. burgdorferi N40 D10/E9 gene fragments, a number of outer surface proteins of B. burgdorferi that have a tropism for particular mouse tissues were identified (122). Several of the identified proteins in these experiments have been shown to bind to a small number of host proteins, as described in Table 1. This redundancy in protein function suggests a high importance for tissue tropic binding by the bacteria during a mammalian infection. The tissue targeting effects of the different outer surface proteins of Borrelia may be due to the presence of nutrients required for survival of the bacteria at those particular sites. The cumulative data suggest an association between in vitro ligand affinity and the ability to bind to particular tissues in vivo. For example, the GAG-binding proteins BBK32 and DbpA from B. burgdorferi have been shown to be tropic for joint tissue in mouse models of Borrelia infection (40, 114). Detailed work with BBK32 has shown the joint targeting effects of the protein to be mediated specifically by the GAG-binding domain (40). This association between ligand affinity and tissue targeting does not hold true with Fn-binding adhesins. Fn-binding proteins, BB0347 and BBK32, have been shown to preferentially bind joint tissue (40, 62), whereas the Fn-binding protein, RevA, was found to have a tropism for heart tissue in vivo (62). These data highlight the inherent difficulties in using in vitro binding data between two proteins to infer in vivo function of a protein. It is becoming increasingly clear that the outer surface proteome of Borrelia spp. during mammalian infection functions collaboratively to direct the bacteria to specific tissue sites. Further experiments using in vivo models will need to be performed to determine functions for each outer surface protein of Lyme disease Borrelia.
Interestingly, tissue tropism of Borrelia spp. appears not only to be determined by different bacterial proteins but also by allelic differences in a gene encoding a given protein taken from different Borrelia species. For example, dbpA gene sequences are variable between strains as well as species of Borrelia, and this variation not only affects ligand binding, as mentioned earlier, but also tissue tropism (114). It was shown that DbpA from B. burgdorferi strains B31-A3 and N40 D10/E9 is tropic for joint tissue adhesion and colonization, while DbpA from B. afzelii and B. garinii is not (114). DbpA from B. afzelii strain VS461 showed a tropism for skin, while DbpA from B. garinii strain PBr was uniquely tropic for heart tissue colonization among the dbpA alleles tested (75). These differences in tissue tropism of allelic gene variants from different strains and species of Borrelia could explain the differences observed in the symptoms of a late stage infection with Borrelia spp. DbpA may contribute to these symptoms as B. burgdorferi infection often results in arthritis symptoms, while late stage B. afzelii infection commonly presents with skin lesions.
Complement Component Binding
Rapid dissemination of B. burgdorferi during an infection is thought to be facilitated by the spirochetes traveling in the bloodstream of the mammalian host. During this dissemination process, the bacteria are exposed to the innate immune system of the host, designed to detect and clear invading pathogens. B. burgdorferi is also exposed to these blood products inside the midgut of a feeding tick during a blood meal. Resistance to killing by innate immune mechanisms in the blood and host tissues is, therefore, essential for maintenance of the enzoonotic lifestyle of B. burgdorferi.
One of the major innate immune components in the host blood and tissues is the complement cascade. The complement cascade is a series of proteolytic cleavage events in which inactive precursors are converted into active enzymes in the host serum and tissues. These proteolytic cleavage events are activated by three distinct pathogen-recognition mechanisms. The first is termed the “classical” complement cascade, mediated by antibodies that recognize the surface of the pathogen and recruit complement component C1q molecules, which then activate the cascade of enzymatic cleavage events. Mannose-binding lectin molecules present in the serum can bind to sugar moieties on the surface of an invading pathogen, activating the complement cascade by the “lectin” pathway. A third pathway known as the “alternative” pathway activates the complement cascade by random deposition of complement component C3 molecules onto the surface of the pathogen, activating the cascade. All three of the complement pathways converge on the activation of complement component C3, ultimately resulting in formation of the membrane attack complex pore in the pathogen membrane, and pathogen lysis.
The host has adapted many different regulatory mechanisms to control the activation of this pathway to minimize harm to host tissues. One such mechanism is the C1 inhibitor protein (C1-INH), which acts on the C1s and C1r molecules of the first step of the classical pathway (123). Additionally, the host produces C4-binding protein (C4BP), which inhibits the classical and lectin pathways by binding to C4b and inducing its proteolytic cleavage and subsequent inactivation [reviewed in Ref. (124)]. The host also has a number of different mechanisms that it employs for regulating the alternative complement cascade. The host produces Factor H, Factor H-like protein 1 (FHL-1), and complement Factor H-related (CFHR) proteins, which all inhibit the alternative complement cascade by acting on C3b. Factor H can act to sterically hinder the interactions of C3b with Factor B, compete with Factor Bb for binding to C3b, and induce its proteolytic degradation and inactivation into iC3b [reviewed in Ref. (125)]. Regulation of the complement cascade can occur at the end stages of the cascade by employing factors such as clusterin (ApoJ), vitronectin, and CD59. Vitronectin is known to inhibit the binding of complement factor complex C5–C7 to the pathogen membrane, as well as inhibit C9 oligomerization (126). CD59 functions in a similar fashion to vitronectin, intercalating into the membrane attack complex, and inhibiting its polymerization. Clusterin is a complement regulatory glycoprotein associated with apolipoprotein AI, a protein component of high-density lipoprotein (HDL) cholesterol molecules (125). Clusterin inhibits insertion of the membrane attack complex into the pathogen membrane by forming attack complex aggregates away from the pathogen surface (125).
Borrelia spp. produce several proteins on their surfaces, which are proposed to allow them to evade clearing by the complement cascade. Many of the proteins act by recruiting complement regulatory factors to the surface of B. burgdorferi, such as the surface proteins CspA (86, 92–95), CspZ (82, 90, 91), ErpP (94), ErpA (81, 98), and ErpC (127–129) as determined in vitro. Full length CspA and ErpP were found to be required for binding to purified human Factor H and FHL-1 in vitro using a series of C-terminal truncation proteins in a solid phase binding assay (92). The binding of Factor H and FHL-1 by CspA on the surface of B. burgdorferi was confirmed by far western blot and immunofluorescence assays (95) and contributes to cleavage and inactivation of complement component C3b (93). CspA has also been shown, in vitro, to interact with complement components C7 and C9, at a distinct location from the site of Factor H binding on CspA (92, 93, 95). Binding of CspA to C7 and C9 inhibits assembly of the membrane attack complex at the spirochete surface when incubated in active human serum as determined by immunofluorescence microscopy, contributing to bacterial resistance to lysis by human serum proteins in vitro (92, 93, 95). Interestingly, cspA was found to be expressed only in an unfed tick and not during mammalian infection, suggesting a necessary role for complement resistance of Borrelia within the tick (130, 131). Similar to CspA, another outer membrane protein of B. burgdorferi, CD59-like protein, also binds complement component C9 as well as C8β and inhibits the insertion of the membrane attack complex in vitro (132).
Borrelia burgdorferi also produces several OspE-related protein family members including ErpA, ErpP, and ErpC, which have been shown to recruit Factor H and CFHR proteins to the bacterial surface in vitro (81–83). By solid phase-binding assay and immunoblot, ErpA, ErpP, and ErpC were found to bind to CFHR-1, unlike CspA and CspZ, which showed no binding to CFHR-1 in active human serum in vitro (82). Additionally, recombinant ErpP and ErpA were found to bind full length recombinant Factor H with a low dissociation constant, suggesting a potential role for this interaction in vivo, though this has not yet been established (81, 83).
CspZ, a gene encoding another Factor H and FHL-1-binding protein on the surface of B. burgdorferi, has a reciprocal expression pattern to cspA (90). CspZ was found to be expressed primarily during mammalian infection but was not found to be required for establishment of mammalian infection (91). Consistent with this result, CspA and CspZ on the surface of B. burgdorferi were found to interact with human Factor H and FHL-1 in vitro, although production of CspZ was not found to be required for survival in active human serum in vitro (82, 91).
Recently, BBK32 was found to interact with a component of the classical complement cascade in vitro (80). BBK32 was shown to bind C1r, a member of the first protein complex in the classical complement cascade, whereby inhibiting its zymogen activity and effectively blocking attack complex formation in vitro (80). A C-terminal portion of BBK32 (residues 206–354) outside of the Fn- and GAG-binding domains, was found to be necessary for the interaction with C1 in vitro (80). We would predict based on the in vitro binding capabilities of BBK32 to C1, that BBK32 cooperates in vivo with additional Borrelia surface proteins to ensure successful inhibition of multiple branches of the complement cascade.
Experiments have been performed to discern the role of complement in the clearance of Borrelia during mammalian infection using C5-deficient mice (133). Complement component C5 is a point of convergence of all three pathways of the complement cascade. The activation of C5 ultimately results in the formation of the membrane attack complex pore in the pathogen membrane. B. burgdorferi recovery by culture from tissues of several infected strains of mice naturally deficient in C5, including A/J, AKR/J, B10.D2/oSnJ, DBA/2J, and SWR/J, was not found to be different than recovery from infected C5 sufficient C3H/HeJ mouse tissues at 2, 4, and 12 weeks p.i. (133). The authors concluded that complement activity is not required for clearance of B. burgdorferi during a mouse infection. Similar results were seen in experimentally infected C3-deficient mice where complement sensitive B. garinii was not recovered from complement-deficient mice (134). Additionally, it was shown that recruitment of Factor H to the surface of B. burgdorferi in vivo is not necessary for serum resistance of the bacteria as evidenced by similarities in bacterial burdens of WT and Factor H-deficient mice (135). Given what we now know about the ability of B. burgdorferi to interact with several host complement regulatory factors upstream of C3 and C5 in vitro, one would not predict to see a difference in WT bacteria survival in these experiments.
The evasion of the alternative complement cascade is not unique to B. burgdorferi and has been observed in Borrelia hermsii, a causative agent of relapsing fever (136, 137), Borrelia bavariensis (138), as well as other bacterial pathogens such as Streptococcus pyogenes (139), Bordetella pertussis (140, 141), Neisseria gonorrhoeae (142–145), Escherichia coli (146–148), Leptospira interrogans (149), Yersinia pseudotuberculosis (150, 151), Salmonella enterica serovar Typhimurium (152), and Moraxella catarrhalis (153). Many of these organisms obtain resistance to host complement by coating themselves in C4-binding protein (C4BP) and Factor H, similar to B. burgdorferi [reviewed in Ref. (124)].
Summary
Borrelia spp. are highly adept at preventing clearance by the innate immune system of the host while adhering to and colonizing host tissues. The success of this mammalian pathogen can, so far, be attributed to the presences of a few bacterial proteins produced on the pathogen surface though there are many more adhesins whose in vivo functions have yet to be determined. For many years, researchers in the Borrelia field have identified and studied outer surface proteins of Borrelia spp. that may contribute to the efficiency of Borrelia as a pathogen. The host proteins that are specifically bound by different outer surface proteins are beginning to be revealed. Borrelia outer surface proteins BBK32, DbpA, RevA, CspA and Erps A, C, and P, have all been shown to bind to multiple macromolecules from the host cell surface and ECM. In addition to binding to multiple host cell surface proteins, Borrelia proteins BBK32, CspA, and Erps A, C, and P have all been found to bind to host complement components. The multifunctional and redundant capabilities of many of the proteins on the surface of Borrelia provide the bacteria with a higher probability of successfully infecting the arthropod and mammalian hosts. As research of Borrelia outer surface proteins progresses, it becomes increasingly clear that redundancy in ECM and host protein-binding specificity acts to guarantee that the bacteria will successfully colonize host tissues while evading detection by the host immune system.
As suggested in this review, there is a strong correlation between ligand specificity of different bacterial outer surface proteins and the tissue tropism of those proteins. When examining the literature it becomes apparent that the binding affinity for host macromolecules such as GAGs by BBK32 and DbpA may be the necessary interactions required for joint tropism and colonization (40, 114). This is further evidenced by the documented species to species differences in GAG-binding capacity and joint colonization seen with the production of DbpA from B. afzelli and B. garinii, Borrelia species, which do not commonly cause arthritis in humans (114).
As discussed in this review, complement protein resistance is achieved by Borrelia spp. through the action of a few outer surface proteins with multiple levels of redundancy. One aspect of redundancy is the ability of multiple Borrelia proteins to bind to and recruit the same complement inhibitory protein. This functional redundancy ensures the successful inhibition of the complement cascade at different stages of the pathway by multiple Borrelia proteins. Redundancy in protein function is a common theme for both the tissue binding and complement protein recruitment aspects of Borrelia pathogenesis, and likely contribute largely to the pathogenic success of this bacterial genus.
Author Contributions
JAC and JC both contributed to the writing of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was supported by National Institute of Allergy and Infectious Diseases (R01 AI093104 and R01 AI121401).
References
- 1.Centers for Disease Control and Prevention. Lyme Disease – Data and Statistics. (2015). Available from: http://www.cdc.gov/lyme/stats/
- 2.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol (2012) 10(2):87–99. 10.1038/nrmicro2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver JH, Lin T, Gao L, Clark KL, Banks CW, Durden LA, et al. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc Natl Acad Sci U S A (2003) 100(20):11642–5. 10.1073/pnas.1434553100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med (1983) 308(13):733–40. 10.1056/NEJM198303313081301 [DOI] [PubMed] [Google Scholar]
- 5.Steere AC. Musculoskeletal manifestations of Lyme disease. Am J Med (1995) 98(4A):44S–51S. 10.1016/S0002-9343(99)80043-6 [DOI] [PubMed] [Google Scholar]
- 6.Steere AC. Diagnosis and treatment of Lyme arthritis. Med Clin North Am (1997) 81(1):179–94. 10.1016/S0025-7125(05)70510-1 [DOI] [PubMed] [Google Scholar]
- 7.Steere AC. Lyme disease. N Engl J Med (2001) 345(2):115–25. 10.1056/NEJM200107123450207 [DOI] [PubMed] [Google Scholar]
- 8.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J, Assous M, et al. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol (1992) 42(3):378–83. 10.1099/00207713-42-3-378 [DOI] [PubMed] [Google Scholar]
- 9.van Dam AP, Kuiper H, Vos K, Widjojokusumo A, de Jongh BM, Spanjaard L, et al. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis (1993) 17(4):708–17. 10.1093/clinids/17.4.708 [DOI] [PubMed] [Google Scholar]
- 10.Strle F, Ružić-Sabljić E, Cimperman J, Lotrič-Furlan S, Maraspin V. Comparison of findings for patients with Borrelia garinii and Borrelia afzelii isolated from cerebrospinal fluid. Clin Infect Dis (2006) 43(6):704–10. 10.1086/506936 [DOI] [PubMed] [Google Scholar]
- 11.Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci U S A (1995) 92(7):2909–13. 10.1073/pnas.92.7.2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun (1995) 63(11):4535–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Indest KJ, Ramamoorthy R, Solé M, Gilmore RD, Johnson BJ, Philipp MT. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun (1997) 65(4):1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, et al. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol (2000) 37(6):1470–9. 10.1046/j.1365-2958.2000.02104.x [DOI] [PubMed] [Google Scholar]
- 15.Iyer R, Caimano MJ, Luthra A, Axline D, Corona A, Iacobas DA, et al. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host-adaptation. Mol Microbiol (2015) 95(3):509–38. 10.1111/mmi.12882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hyde JA, Trzeciakowski JP, Skare JT. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol (2007) 189(2):437–45. 10.1128/JB.01109-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN). J Bacteriol (2007) 189(5):2139–44. 10.1128/JB.01653-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun (2008) 76(9):3844–53. 10.1128/IAI.00467-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN–RpoS regulatory pathway. Proc Natl Acad Sci U S A (2001) 98(22):12724–9. 10.1073/pnas.231442498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Dadhwal P, Li X, Liang FT. BosR functions as a repressor of the ospAB operon in Borrelia burgdorferi. PLoS One (2014) 9(10):e109307. 10.1371/journal.pone.0109307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ouyang Z, Zhou J, Norgard MV. Synthesis of RpoS is dependent on a putative enhancer binding protein Rrp2 in Borrelia burgdorferi. PLoS One (2014) 9(5):e96917. 10.1371/journal.pone.0096917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyde JA, Shaw DK, Smith R, III, Trzeciakowski JP, Skare JT. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol Microbiol (2009) 74(6):1344–55. 10.1111/j.1365-2958.2009.06951.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouyang Z, Kumar M, Kariu T, Haq S, Goldberg M, Pal U, et al. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol Microbiol (2009) 74(6):1331–43. 10.1111/j.1365-2958.2009.06945.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol (2007) 65(5):1193–217. 10.1111/j.1365-2958.2007.05860.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srivastava SY, de Silva AM. Reciprocal expression of ospA and ospC in single cells of Borrelia burgdorferi. J Bacteriol (2008) 190(10):3429–33. 10.1128/JB.00085-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hefty PS, Jolliff SE, Caimano MJ, Wikel SK, Radolf JD, Akins DR. Regulation of OspE-related, OspF-related, and Elp lipoproteins of Borrelia burgdorferi strain 297 by mammalian host-specific signals. Infect Immun (2001) 69(6):3618–27. 10.1128/IAI.69.6.3618-3627.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol (2000) 38(1):382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun (2003) 71(9):5042–55. 10.1128/IAI.71.9.5042-5055.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun (2006) 74(6):3554–64. 10.1128/IAI.01950-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Indest KJ, Howell JK, Jacobs MB, Scholl-Meeker D, Norris SJ, Philipp MT. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect Immun (2001) 69(11):7083–90. 10.1128/IAI.69.11.7083-7090.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, et al. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun (2004) 72(10):5759–67. 10.1128/IAI.72.10.5759-5767.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J-R, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease Borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell (1997) 89(2):275–85. 10.1016/S0092-8674(00)80206-8 [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Brisson D. Potentially conflicting selective forces that shape the vls antigenic variation system in Borrelia burgdorferi. Infect Genet Evol (2014) 27(0):559–65. 10.1016/j.meegid.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawrenz MB, Hardham JM, Owens RT, Nowakowski J, Steere AC, Wormser GP, et al. Human antibody responses to vlse antigenic variation protein of Borrelia burgdorferi. J Clin Microbiol (1999) 37(12):3997–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog (2008) 4(10):e1000169. 10.1371/journal.ppat.1000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blevins JS, Hagman KE, Norgard MV. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol (2008) 8(1):82. 10.1186/1471-2180-8-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carroll JA, Cordova RM, Garon CF. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect Immun (2000) 68(12):6677–84. 10.1128/IAI.68.12.6677-6684.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A (2004) 101(9):3142–7. 10.1073/pnas.0306845101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyde JA, Weening EH, Chang M, Trzeciakowski JP, Höök M, Cirillo JD, et al. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol Microbiol (2011) 82(1):99–113. 10.1111/j.1365-2958.2011.07801.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin Y-P, Chen Q, Ritchie JA, Dufour NP, Fischer JR, Coburn J, et al. Glycosaminoglycan binding by Borrelia burgdorferi adhesin BBK32 specifically and uniquely promotes joint colonization. Cell Microbiol (2015) 17(6):860–75. 10.1111/cmi.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moriarty TJ, Shi M, Lin Y-P, Ebady R, Zhou H, Odisho T, et al. Vascular binding of a pathogen under shear force through mechanistically distinct sequential interactions with host macromolecules. Mol Microbiol (2012) 86(5):1116–31. 10.1111/mmi.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, Höök M, et al. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol (2006) 59(5):1591–601. 10.1111/j.1365-2958.2005.05042.x [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Xu Q, McShan K, Liang FT. Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun (2008) 76(3):1239–46. 10.1128/IAI.00897-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Höök M, Skare JT. Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun (2008) 76(12):5694–705. 10.1128/IAI.00690-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect Immun (2009) 77(7):2802–12. 10.1128/IAI.00227-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Byram R, Gaultney RA, Floden AM, Hellekson C, Stone BL, Bowman A, et al. Borrelia burgdorferi RevA significantly affects pathogenicity and host response in the mouse model of Lyme disease. Infect Immun (2015) 83(9):3675–83. 10.1128/IAI.00530-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tilly K, Bestor A, Jewett MW, Rosa P. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect Immun (2007) 75(3):1517–9. 10.1128/IAI.01725-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carrasco SE, Troxell B, Yang Y, Brandt SL, Li H, Sandusky GE, et al. Outer surface protein OspC is an antiphagocytic factor that protects Borrelia burgdorferi from phagocytosis by macrophages. Infect Immun (2015) 83(12):4848–60. 10.1128/IAI.01215-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, et al. Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One (2012) 7(10):e47532. 10.1371/journal.pone.0047532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kenedy MR, Akins DR. The OspE-related proteins inhibit complement deposition and enhance serum resistance of Borrelia burgdorferi, the Lyme disease spirochete. Infect Immun (2011) 79(4):1451–7. 10.1128/IAI.01274-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patton TG, Brandt KS, Nolder C, Clifton DR, Carroll JA, Gilmore RD. Borrelia burgdorferi bba66 gene inactivation results in attenuated mouse infection by tick transmission. Infect Immun (2013) 81(7):2488–98. 10.1128/IAI.00140-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gilmore RD, Jr, Howison RR, Schmit VL, Carroll JA. Borrelia burgdorferi expression of the bba64, bba65, bba66, and bba73 genes in tissues during persistent infection in mice. Microb Pathog (2008) 45(5–6):355–60. 10.1016/j.micpath.2008.08.006 [DOI] [PubMed] [Google Scholar]
- 53.Yang X, Lin Y-P, Heselpoth RD, Buyuktanir O, Qin J, Kung F, et al. Middle region of the Borrelia burgdorferi surface-located protein 1 (Lmp1) interacts with host chondroitin-6-sulfate and independently facilitates infection. Cell Microbiol (2016) 18(1):97–110. 10.1111/cmi.12487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang X, Lenhart TR, Kariu T, Anguita J, Akins DR, Pal U. Characterization of unique regions of Borrelia burgdorferi surface-located membrane protein 1. Infect Immun (2010) 78(11):4477–87. 10.1128/IAI.00501-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X, Coleman AS, Anguita J, Pal UA. Chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog (2009) 5(3):e1000326. 10.1371/journal.ppat.1000326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verma A, Brissette CA, Bowman A, Stevenson B. Borrelia burgdorferi BmpA is a laminin-binding protein. Infect Immun (2009) 77(11):4940–6. 10.1128/IAI.01420-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pal U, Wang P, Bao F, Yang X, Samanta S, Schoen R, et al. Borrelia burgdorferi basic membrane proteins A and B participate in the genesis of Lyme arthritis. J Exp Med (2008) 205(1):133–41. 10.1084/jem.20070962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parveen N, Cornell KA, Bono JL, Chamberland C, Rosa P, Leong JM. Bgp, a secreted glycosaminoglycan-binding protein of Borrelia burgdorferi strain N40, displays nucleosidase activity and is not essential for infection of immunodeficient mice. Infect Immun (2006) 74(5):3016–20. 10.1128/IAI.74.5.3016-3020.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saidac DS, Marras SA, Parveen N. Detection and quantification of Lyme spirochetes using sensitive and specific molecular beacon probes. BMC Microbiol (2009) 9(1):43. 10.1186/1471-2180-9-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell TM, Johnson BJB. Lyme disease spirochaetes possess an aggrecan-binding protease with aggrecanase activity. Mol Microbiol (2013) 90(2):228–40. 10.1111/mmi.12276 [DOI] [PubMed] [Google Scholar]
- 61.Coburn J, Chege W, Magoun L, Bodary SC, Leong JM. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol Microbiol (1999) 34(5):926–40. 10.1046/j.1365-2958.1999.01654.x [DOI] [PubMed] [Google Scholar]
- 62.Caine JA, Coburn JA. Short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect Immun (2015) 83(8):3184–94. 10.1128/IAI.00349-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ristow LC, Miller HE, Padmore LJ, Chettri R, Salzman N, Caimano MJ, et al. The β3-integrin ligand of Borrelia burgdorferi is critical for infection of mice but not ticks. Mol Microbiol (2012) 85(6):1105–18. 10.1111/j.1365-2958.2012.08160.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ristow LC, Bonde M, Lin Y-P, Sato H, Curtis M, Geissler E, et al. Integrin binding by Borrelia burgdorferi P66 facilitates dissemination but is not required for infectivity. Cell Microbiol (2015) 17(7):1021–36. 10.1111/cmi.12418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer JR, Parveen N, Magoun L, Leong JM. Decorin-binding proteins A and B confer distinct mammalian cell type-specific attachment by Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A (2003) 100(12):7307–12. 10.1073/pnas.1231043100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo BP, Brown EL, Dorward DW, Rosenberg LC, Höök M. Decorin-binding adhesins from Borrelia burgdorferi. Mol Microbiol (1998) 30(4):711–23. 10.1046/j.1365-2958.1998.01103.x [DOI] [PubMed] [Google Scholar]
- 67.Brown EL, Guo BP, O’Neal P, Höök M. Adherence of Borrelia burgdorferi: identification of critical lysine residues in DbpA required for decorin binding. J Biol Chem (1999) 274(37):26272–8. 10.1074/jbc.274.37.26272 [DOI] [PubMed] [Google Scholar]
- 68.Guo BP, Norris SJ, Rosenberg LC, Höök M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun (1995) 63(9):3467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhi H, Weening EH, Barbu EM, Hyde JA, Höök M, Skare JT. The BBA33 lipoprotein binds collagen and impacts Borrelia burgdorferi pathogenesis. Mol Microbiol (2015) 96(1):68–83. 10.1111/mmi.12921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Behera AK, Durand E, Cugini C, Antonara S, Bourassa L, Hildebrand E, et al. Borrelia burgdorferi BBB07 interaction with integrin α3β1 stimulates production of pro-inflammatory mediators in primary human chondrocytes. Cell Microbiol (2008) 10(2):320–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Önder Ö, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, et al. OspC is potent plasminogen receptor on surface of Borrelia burgdorferi. J Biol Chem (2012) 287(20):16860–8. 10.1074/jbc.M111.290775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature (2005) 436(7050):573–7. 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dresser AR, Hardy P-O, Chaconas G. Investigation of the genes involved in antigenic switching at the vlsE locus in Borrelia burgdorferi: an essential role for the RuvAB branch migrase. PLoS Pathog (2009) 5(12):e1000680. 10.1371/journal.ppat.1000680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bankhead T, Chaconas G. The role of VlsE antigenic variation in the Lyme disease spirochete: persistence through a mechanism that differs from other pathogens. Mol Microbiol (2007) 65(6):1547–58. 10.1111/j.1365-2958.2007.05895.x [DOI] [PubMed] [Google Scholar]
- 75.Lin T, Gao L, Edmondson DG, Jacobs MB, Philipp MT, Norris SJ. Central role of the holliday junction helicase RuvAB in vlsE recombination and infectivity of Borrelia burgdorferi. PLoS Pathog (2009) 5(12):e1000679. 10.1371/journal.ppat.1000679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crother TR, Champion CI, Wu X-Y, Blanco DR, Miller JN, Lovett MA. Antigenic composition of Borrelia burgdorferi during infection of SCID mice. Infect Immun (2003) 71(6):3419–28. 10.1128/IAI.71.6.3419-3428.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fischer JR, LeBlanc KT, Leong JM. Fibronectin binding protein BBK32 of the Lyme disease spirochete promotes bacterial attachment to glycosaminoglycans. Infect Immun (2006) 74(1):435–41. 10.1128/IAI.74.1.435-441.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Probert WS, Johnson BJB. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol (1998) 30(5):1003–15. 10.1046/j.1365-2958.1998.01127.x [DOI] [PubMed] [Google Scholar]
- 79.Harris G, Ma W, Maurer LM, Potts JR, Mosher DF. Borrelia burgdorferi protein BBK32 binds to soluble fibronectin via the N-terminal 70-kDa region, causing fibronectin to undergo conformational extension. J Biol Chem (2014) 289(32):22490–9. 10.1074/jbc.M114.578419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia BL, Zhi H, Wager B, Höök M, Skare JT. Borrelia burgdorferi BBK32 inhibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog (2016) 12(1):e1005404. 10.1371/journal.ppat.1005404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alitalo A, Meri T, Lankinen H, Seppälä I, Lahdenne P, Hefty P, et al. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J Immunol (2002) 169(7):3847–53. 10.4049/jimmunol.169.7.3847 [DOI] [PubMed] [Google Scholar]
- 82.Haupt K, Kraiczy P, Wallich R, Brade V, Skerka C, Zipfel PF. Binding of human factor H-related protein 1 to serum-resistant Borrelia burgdorferi is mediated by borrelial complement regulator-acquiring surface proteins. J Infect Dis (2007) 196(1):124–33. 10.1086/518509 [DOI] [PubMed] [Google Scholar]
- 83.Metts MS, McDowell JV, Theisen M, Hansen PR, Marconi RT. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect Immun (2003) 71(6):3587–96. 10.1128/IAI.71.6.3587-3596.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siegel C, Hallström T, Skerka C, Eberhardt H, Uzonyi B, Beckhaus T, et al. Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS One (2010) 5(10):e13519. 10.1371/journal.pone.0013519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brissette CA, Haupt K, Barthel D, Cooley AE, Bowman A, Skerka C, et al. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect Immun (2009) 77(1):300–6. 10.1128/IAI.01133-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kraiczy P, Rossmann E, Brade V, Simon MM, Skerka C, Zipfel PF, et al. Binding of human complement regulators FHL-1 and factor H to CRASP-1 orthologs of Borrelia burgdorferi. Wien Klin Wochenschr (2006) 118(21–22):669–76. 10.1007/s00508-006-0691-1 [DOI] [PubMed] [Google Scholar]
- 87.Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun (1998) 66(6):2648–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest (2004) 113(2):220–30. 10.1172/JCI200419894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med (2004) 199(5):641–8. 10.1084/jem.20031960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, et al. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol (2006) 61(5):1220–36. 10.1111/j.1365-2958.2006.05318.x [DOI] [PubMed] [Google Scholar]
- 91.Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, et al. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One (2008) 3(8):3010e. 10.1371/journal.pone.0003010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hallström T, Siegel C, Mörgelin M, Kraiczy P, Skerka C, Zipfel PF. CspA from Borrelia burgdorferi inhibits the terminal complement pathway. MBio (2013) 4(4):e004891–13. 10.1128/mBio.00481-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammerschmidt C, Koenigs A, Siegel C, Hallström T, Skerka C, Wallich R, et al. Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect Immun (2014) 82(1):380–92. 10.1128/IAI.01094-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kraiczy P, Hellwage J, Skerka C, Becker H, Kirschfink M, Simon MM, et al. Complement resistance of Borrelia burgdorferi correlates with the expression of BbCRASP-1, a novel linear plasmid-encoded surface protein that interacts with human factor H and FHL-1 and is unrelated to Erp proteins. J Biol Chem (2004) 279(4):2421–9. 10.1074/jbc.M308343200 [DOI] [PubMed] [Google Scholar]
- 95.Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, Akins DR. CspA-mediated binding of human factor h inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun (2009) 77(7):2773–82. 10.1128/IAI.00318-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koenigs A, Hammerschmidt C, Jutras BL, Pogoryelov D, Barthel D, Skerka C, et al. BBA70 of Borrelia burgdorferi is a novel plasminogen-binding protein. J Biol Chem (2013) 288(35):25229–43. 10.1074/jbc.M112.413872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin Y-P, Bhowmick R, Coburn J, Leong JM. Host cell heparan sulfate glycosaminoglycans are ligands for OspF-related proteins of the Lyme disease spirochete. Cell Microbiol (2015) 17(10):1464–76. 10.1111/cmi.12448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kraiczy P, Hartmann K, Hellwage J, Skerka C, Kirschfink M, Brade V, et al. Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int J Med Microbiol (2004) 293(37):152–7. 10.1016/S1433-1128(04)80029-9 [DOI] [PubMed] [Google Scholar]
- 99.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development (1993) 119(4):1079–91. [DOI] [PubMed] [Google Scholar]
- 100.Kern A, Schnell G, Bernard Q, Boeuf A, Jaulhac B, Collin E, et al. Heterogeneity of Borrelia burgdorferi sensu stricto population and its involvement in Borrelia pathogenicity: study on murine model with specific emphasis on the skin interface. PLoS One (2015) 10(7):e0133195. 10.1371/journal.pone.0133195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.He M, Boardman BK, Yan D, Yang XF. Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J Bacteriol (2007) 189(22):8377–80. 10.1128/JB.01199-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jinno A, Park PW. Role of glycosaminoglycans in infectious disease. In: Balagurunathan K, Nakato H, Desai UR, editors. Glycosaminoglycans. 1229. Methods in Molecular Biology. Clifton, NJ: Humana Press; (2015). p. 567–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Prabhakaran S, Liang X, Skare JT, Potts JR, Höök M. A novel fibronectin binding motif in MSCRAMMs targets F3 modules. PLoS One (2009) 4(4):e5412. 10.1371/journal.pone.0005412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, Chaconas G. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog (2008) 4(6):e1000090. 10.1371/journal.ppat.1000090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology (2009) 155(3):863–72. 10.1099/mic.0.024604-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alberts B, Johnson A, Lewis J. Integrins. Molecular Biology of the Cell. 4th ed New York: Garland Science; (2002). [Google Scholar]
- 107.Kenedy MR, Luthra A, Anand A, Dunn JP, Radolf JD, Akins DR. Structural modeling and physicochemical characterization provide evidence that P66 forms a β-barrel in the Borrelia burgdorferi outer membrane. J Bacteriol (2014) 196(4):859–72. 10.1128/JB.01236-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cugini C, Medrano M, Schwan TG, Coburn J. Regulation of expression of the Borrelia burgdorferi β3-chain integrin ligand, P66, in ticks and in culture. Infect Immun (2003) 71(2):1001–7. 10.1128/IAI.71.2.1001-1007.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coburn J, Cugini C. Targeted mutation of the outer membrane protein P66 disrupts attachment of the Lyme disease agent, Borrelia burgdorferi, to integrin α(v)β(3). Proc Natl Acad Sci U S A (2003) 100(12):7301–6. 10.1073/pnas.1131117100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hallström T, Haupt K, Kraiczy P, Hortschansky P, Wallich R, Skerka C, et al. Complement regulator – acquiring surface protein 1 of Borrelia burgdorferi binds to human bone morphogenic protein 2, several extracellular matrix proteins, and plasminogen. J Infect Dis (2010) 202(3):490–8. 10.1086/653825 [DOI] [PubMed] [Google Scholar]
- 111.Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci U S A (1994) 91(26):12594–8. 10.1073/pnas.91.26.12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kraiczy P, Stevenson B. Complement regulator-acquiring surface proteins of Borrelia burgdorferi: structure, function and regulation of gene expression. Ticks Tick Borne Dis (2013) 4(1–2):26–34. 10.1016/j.ttbdis.2012.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Benoit VM, Fischer JR, Lin Y-P, Parveen N, Leong JM. Allelic variation of the lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect Immun (2011) 79(9):3501–9. 10.1128/IAI.00163-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lin Y-P, Benoit V, Yang X, Martínez-Herranz R, Pal U, Leong JM. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS Pathog (2014) 10(7):e1004238. 10.1371/journal.ppat.1004238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng W, Wang X. Structure of decorin binding protein B from Borrelia burgdorferi and its interactions with glycosaminoglycans. Biochim Biophys Acta (2015) 1854(12):1823–32. 10.1016/j.bbapap.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell (2013) 26(2):204–19. 10.1016/j.devcel.2013.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yano K, Gale D, Massberg S, Cheruvu PK, Monahan-Earley R, Morgan ES, et al. Phenotypic heterogeneity is an evolutionarily conserved feature of the endothelium. Blood (2007) 109(2):613–5. [DOI] [PubMed] [Google Scholar]
- 118.Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans. 2nd ed In: Varki A, Cummings R, Esko J, editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; (2009). [PubMed] [Google Scholar]
- 119.Fikrig E, Feng W, Barthold SW, Telford SR, Flavell RA. Arthropod- and host-specific Borrelia burgdorferi BBK32 expression and the inhibition of spirochete transmission. J Immunol (2000) 164(10):5344–51. 10.4049/jimmunol.164.10.5344 [DOI] [PubMed] [Google Scholar]
- 120.Leong JM, Wang H, Magoun L, Field JA, Morrissey PE, Robbins D, et al. Different classes of proteoglycans contribute to the attachment of Borrelia burgdorferi to cultured endothelial and brain cells. Infect Immun (1998) 66(3):994–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ebady R, Niddam Alexandra F, Boczula Anna E, Kim Yae R, Gupta N, Tang Tian T, et al. Biomechanics of Borrelia burgdorferi vascular interactions. Cell Rep (2016) 16(10):2593–604. 10.1016/j.celrep.2016.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Antonara S, Chafel RM, LaFrance M, Coburn J. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol Microbiol (2007) 66(1):262–76. 10.1111/j.1365-2958.2007.05924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ratnoff OD, Pensky J, Ogston D, Naff GB. The inhibition of plasmin, plasma kallikrein, plasma permeability factor, and the C1r subcomponent of the first componenet of complement by serum C1 esterase inhibitor. J Exp Med (1969) 129(2):315–31. 10.1084/jem.129.2.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blom AM, Villoutreix BO, Dahlbäck B. Complement inhibitor C4b-binding protein – friend or foe in the innate immune system? Mol Immunol (2004) 40(18):1333–46. 10.1016/j.molimm.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 125.Meri S, Jarva H. Complement regulation. Vox Sang (1998) 74(S2):291–302. 10.1111/j.1423-0410.1998.tb05434.x [DOI] [PubMed] [Google Scholar]
- 126.Sheehan M, Morris CA, Pussell BA, Charlesworth JA. Complement inhibition by human vitronectin involves non-heparin binding domains. Clin Exp Immunol (1995) 101(1):136–41. 10.1111/j.1365-2249.1995.tb02289.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Brangulis K, Petrovskis I, Kazaks A, Akopjana I, Tars K. Crystal structures of the Erp protein family members ErpP and ErpC from Borrelia burgdorferi reveal the reason for different affinities for complement regulator factor H. Biochim Biophys Acta (2014) 1854(5):349–55. [DOI] [PubMed] [Google Scholar]
- 128.Caesar JJE, Johnson S, Kraiczy P, Lea SM. ErpC, a member of the complement regulator-acquiring family of surface proteins from Borrelia burgdorferi, possesses an architecture previously unseen in this protein family. Acta Crystallogr Sect F Struct Biol Cryst Commun (2013) 69(6):624–8. 10.1107/S1744309113013249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hammerschmidt C, Hallström T, Skerka C, Wallich R, Stevenson B, Zipfel PF, et al. Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin Dev Immunol (2012) 2012:349657. 10.1155/2012/349657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun (2003) 71(6):3371–83. 10.1128/IAI.71.6.3371-3383.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, et al. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the lyme disease spirochete’s mammal-tick infection cycle. Infect Immun (2007) 75(9):4227–36. 10.1128/IAI.00604-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pausa M, Pellis V, Cinco M, Giulianini PG, Presani G, Perticarari S, et al. Serum-resistant strains of Borrelia burgdorferi evade complement-mediated killing by expressing a CD59-like complement inhibitory molecule. J Immunol (2003) 170(6):3214–22. 10.4049/jimmunol.170.6.3214 [DOI] [PubMed] [Google Scholar]
- 133.Bockenstedt LK, Barthold S, Deponte K, Marcantonio N, Kantor FS. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect Immun (1993) 61(5):2104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.van Burgel ND, Balmus NCM, Fikrig E, van Dam AP. Infectivity of Borrelia burgdorferi sensu lato is unaltered in C3-deficient mice. Ticks Tick Borne Dis (2011) 2(1):20–6. 10.1016/j.ttbdis.2010.10.003 [DOI] [PubMed] [Google Scholar]
- 135.Woodman ME, Cooley AE, Miller JC, Lazarus JJ, Tucker K, Bykowski T, et al. Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect Immun (2007) 75(6):3131–9. 10.1128/IAI.01923-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Colombo MJ, Alugupalli KR. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol (2008) 180(7):4858–64. 10.4049/jimmunol.180.7.4858 [DOI] [PubMed] [Google Scholar]
- 137.Meri T, Amdahl H, Lehtinen MJ, Hyvärinen S, McDowell JV, Bhattacharjee A, et al. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog (2013) 9(4):e1003308. 10.1371/journal.ppat.1003308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hammerschmidt C, Klevenhaus Y, Koenigs A, Hallström T, Fingerle V, Skerka C, et al. BGA66 and BGA71 facilitate complement resistance of Borrelia bavariensis by inhibiting assembly of the membrane attack complex. Mol Microbiol (2016) 99(2):407–24. 10.1111/mmi.13239 [DOI] [PubMed] [Google Scholar]
- 139.Carlsson F, Berggård K, Stålhammar-Carlemalm M, Lindahl G. Evasion of phagocytosis through cooperation between two ligand-binding regions in Streptococcus pyogenes M protein. J Exp Med (2003) 198(7):1057–68. 10.1084/jem.20030543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Berggård K, Johnsson E, Mooi FR, Lindahl G. Bordetella pertussis binds the human complement regulator C4BP: role of filamentous hemagglutinin. Infect Immun (1997) 65(9):3638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berggård K, Lindahl G, Dahlbäck B, Blom AM. Bordetella pertussis binds to human C4b-binding protein (C4BP) at a site similar to that used by the natural ligand C4b. Eur J Immunol (2001) 31(9):2771–80. [DOI] [PubMed] [Google Scholar]
- 142.Jarva H, Ngampasutadol J, Ram S, Rice PA, Villoutreix BO, Blom AM. Molecular characterization of the interaction between porins of Neisseria gonorrhoeae and C4b-binding protein. J Immunol (2007) 179(1):540–7. 10.4049/jimmunol.179.1.540 [DOI] [PubMed] [Google Scholar]
- 143.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, et al. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med (2001) 193(3):281–96. 10.1084/jem.193.3.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blom AM, Rytkönen A, Vasquez P, Lindahl G, Dahlbäck B, Jonsson A. A novel interaction between type IV pili of Neisseria gonorrhoeae and the human complement regulator C4b-binding protein. J Immunol (2001) 166(11):6764–70. 10.4049/jimmunol.166.11.6764 [DOI] [PubMed] [Google Scholar]
- 145.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Boden R, et al. C4bp binding to porin mediates stable serum resistance of Neisseria gonorrhoeae. Int Immunopharmacol (2001) 1(3):423–32. 10.1016/S1567-5769(00)00037-0 [DOI] [PubMed] [Google Scholar]
- 146.Prasadarao NV, Blom AM, Villoutreix BO, Linsangan LC. A novel interaction of outer membrane protein A with C4b binding protein mediates serum resistance of Escherichia coli K1. J Immunol (2002) 169(11):6352–60. 10.4049/jimmunol.169.11.6352 [DOI] [PubMed] [Google Scholar]
- 147.Tseng Y, Wang S-W, Kim KS, Wang Y-H, Yao Y, Chen C, et al. NlpI facilitates deposition of C4bp on Escherichia coli by blocking classical complement-mediated killing, which results in high-level bacteremia. Infect Immun (2012) 80(10):3669–78. 10.1128/IAI.00320-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wooster DG, Maruvada R, Blom AM, Prasadarao NV. Logarithmic phase Escherichia coli K1 efficiently avoids serum killing by promoting C4bp-mediated C3b and C4b degradation. Immunology (2006) 117(4):482–93. 10.1111/j.1365-2567.2006.02323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Souza NM, Vieira ML, Alves IJ, de Morais ZM, Vasconcellos SA, Nascimento ALTO. Lsa30, a novel adhesin of Leptospira interrogans binds human plasminogen and the complement regulator C4bp. Microb Pathog (2012) 53(3–4):125–34. 10.1016/j.micpath.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 150.Ho DK, Riva R, Skurnik M, Meri S. The Yersinia pseudotuberculosis outer membrane protein ail recruits the human complement regulatory protein factor H. J Immunol (2012) 189(7):3593–9. 10.4049/jimmunol.1201145 [DOI] [PubMed] [Google Scholar]
- 151.Ho DK, Riva R, Kirjavainen V, Jarva H, Ginström E, Blom AM, et al. Functional recruitment of the human complement inhibitor C4BP to Yersinia pseudotuberculosis outer membrane protein Ail. J Immunol (2012) 188(9):4450–9. 10.4049/jimmunol.1103149 [DOI] [PubMed] [Google Scholar]
- 152.Ho DK, Tissari J, Järvinen HM, Blom AM, Meri S, Jarva H. Functional recruitment of human complement inhibitor C4b-binding protein to outer membrane protein Rck of Salmonella. PLoS One (2011) 6(11):e27546. 10.1371/journal.pone.0027546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nordström T, Blom AM, Forsgren A, Riesbeck K. The emerging pathogen Moraxella catarrhalis interacts with complement inhibitor C4b binding protein through ubiquitous surface proteins A1 and A2. J Immunol (2004) 173(7):4598–606. 10.4049/jimmunol.173.7.4598 [DOI] [PubMed] [Google Scholar]