Publisher's Note: There is an Inside Blood Commentary on this article in this issue.
Key Points
C1 domain antibodies with low inhibitor titers by the Bethesda assay are pathogenic in mice due to increased fVIII clearance.
Monoclonal and patient-derived polyclonal anti-fVIII C1 domain antibodies recognize similar B-cell epitopes.
Abstract
Inhibitor formation in hemophilia A is the most feared treatment-related complication of factor VIII (fVIII) therapy. Most inhibitor patients with hemophilia A develop antibodies against the fVIII A2 and C2 domains. Recent evidence demonstrates that the C1 domain contributes to the inhibitor response. Inhibitory anti-C1 monoclonal antibodies (mAbs) have been identified that bind to putative phospholipid and von Willebrand factor (VWF) binding epitopes and block endocytosis of fVIII by antigen presenting cells. We now demonstrate by competitive enzyme-linked immunosorbent assay and hydrogen-deuterium exchange mass spectrometry that 7 of 9 anti-human C1 mAbs tested recognize an epitope distinct from the C1 phospholipid binding site. These mAbs, designated group A, display high binding affinities for fVIII, weakly inhibit fVIII procoagulant activity, poorly inhibit fVIII binding to phospholipid, and exhibit heterogeneity with respect to blocking fVIII binding to VWF. Another mAb, designated group B, inhibits fVIII procoagulant activity, fVIII binding to VWF and phospholipid, fVIIIa incorporation into the intrinsic Xase complex, thrombin generation in plasma, and fVIII uptake by dendritic cells. Group A and B epitopes are distinct from the epitope recognized by the canonical, human-derived inhibitory anti-C1 mAb, KM33, whose epitope overlaps both groups A and B. Antibodies recognizing group A and B epitopes are present in inhibitor plasmas from patients with hemophilia A. Additionally, group A and B mAbs increase fVIII clearance and are pathogenic in a hemophilia A mouse tail snip bleeding model. Group A anti-C1 mAbs represent the first identification of pathogenic, weakly inhibitory antibodies that increase fVIII clearance.
Introduction
Hemophilia A is an X-linked bleeding disorder characterized by a deficiency of blood coagulation factor VIII (fVIII). Approximately 30% of individuals with severe hemophilia A and 5% of individuals with mild or moderate hemophilia A will develop inhibitors, defined as neutralizing alloantibodies against fVIII.1,2 Inhibitors significantly impact hemophilia treatment by rendering fVIII infusions ineffective and increasing morbidity and mortality of disease.3-6
The fVIII protein consists of 6 domains in a sequence designated as A1-A2-B-ap-A3-C1-C2.7 Most inhibitor patients with congenital hemophilia A develop antibodies against the A2 and C2 domains.8-10 Healey et al11 demonstrated a predominance of anti-fVIII antibodies to the A2 and C2 domains of fVIII in a murine hemophilia A model, but antibodies to the other domains were also detected. Characterization of the structural and functional properties of anti-A2 and anti-C2 domain monoclonal antibodies (mAbs) has advanced our understanding of the epitope spectrum of anti-fVIII antibodies12-15 and their mechanisms of inhibition.16-19 However, there is increasing evidence of the C1 domain’s contribution to fVIII function and immunogenicity.
Three major functions of the C1 domain have been described: binding to von Willebrand factor (VWF) and phospholipid membranes and mediating the uptake of fVIII by antigen presenting cells (APCs). Several studies have described the involvement of the C1 domain in binding to VWF and phospholipid membranes.20,21 Recently negative-stain electron microscopy and hydrogen-deuterium exchange mass spectrometry (HDX MS) studies have revealed that VWF primarily interacts with the C1 domain of fVIII.22,23 VWF binding protects fVIII from endocytosis24 and the human-derived anti-C1 domain antibody KM33 inhibits fVIII uptake by human monocyte-derived dendritic cells (MDDCs).25 Moreover, B-domain deleted (BDD) fVIII mutant constructs containing alanine substitutions at exposed C1 residues Arg2090, Lys2092, and Phe2093 alone or in combination showed reduced fVIII uptake by human MDDCs and macrophages and immunogenicity in fVIII knockout mice compared with wild-type BDD fVIII.26
C1 domain antibodies have been reported in patients with hemophilia A. An anti-fVIII C1 domain antibody derived from a patient with mild hemophilia A and inhibitors showed competitive inhibition of fVIII binding to VWF and incomplete (type 2) inhibition of fVIII activity at saturating concentrations of antibody.27 Additionally, inhibitor plasmas from 2 unrelated patients with mild hemophilia A due to a missense mutation in the C1 domain (Arg2150His) demonstrated type 2 inhibition and recognition of wild type but not autologous fVIII.28
Given the complexity of the inhibitor response to fVIII, in-depth investigation of the immunologic response to all fVIII domains is warranted. In this study, we characterized the humoral response to the C1 domain using anti-human fVIII antibodies developed in a murine hemophilia A model. Pathogenic group A mAbs were identified that increase the clearance of fVIII, despite having weak, type 2 inhibitory activity. A mAb that recognizes a distinct nonoverlapping epitope, designated a group B mAb, is similar to the canonical mAb, KM33, and inhibits fVIII binding to VWF and phospholipids, fVIIIa incorporation in the intrinsic Xase complex, and thrombin generation. Epitopes recognized by group A and B mAbs are shared by anti-fVIII antibodies found in plasmas from patients with severe hemophilia A.
Methods
Anti-fVIII C1 domain antibody purification
Murine anti-human anti-C1 domain antibodies were purified from anti-fVIII hybridomas as previously described.11,29 The supernatants of hybridomas producing anti-C1 domain antibodies were collected and antibodies purified using SP Sepharose Fast Flow or Protein G agarose chromatography columns. The immunoglobulin G (IgG) isotype and subclass were determined by enzyme-linked immunosorbent assay (ELISA) using alkaline-phosphatase isotype and subclass-specific antibodies. The purity of IgG was assessed to be >95% by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Antibodies were biotinylated as previously described.11
Competitive ELISA for mapping of overlapping epitopes
High-binding ELISA plates were coated with a primary anti-C1 mAb at 6 µg/mL in phosphate-buffered saline/0.05% sodium azide (NaN3) and incubated at 4°C overnight. BDD fVIII at 100 ng/mL was incubated on the plate for 1 hour after blocking ELISA plates for 2 hours at room temperature with 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/0.15 M NaCl (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid buffered saline)/2 mM CaCl2/0.05% Tween-20/0.05% NaN3/2% bovine serum albumin, also referred to as blocking buffer, pH 7.4. A secondary biotinylated anti-C1 mAb was incubated on the plate for 1 hour, detected with alkaline phosphate–conjugated streptavidin, and developed with p-nitrophenyl-phosphate. Absorbance was measured at 405 nm. All anti-C1 mAbs were competed in the nonbiotinylated (primary) and biotinylated (secondary) form.
HDX MS
HDX-MS experiments were performed on a recombinant human serum albumin fVIII C1 domain fusion protein (HSA-C1) in the presence and absence of anti-C1 mAbs in replicates of 3 using a Waters UPLC HDX system coupled with a Q-Tof Premier mass spectrometer (Waters Corp, Milford, MA). Preparation of the HSA-C1 fusion protein is detailed in supplemental Data, available on the Blood Web site. The samples of antigen or antigen/mAb mixtures (1:1.1 molar ratio) were prepared separately in phosphate-buffered saline buffer to the final antigen and mAb concentration of 0.2 and 0.3 µg/µL, respectively. An autosampler was programmed to mix proteins 1:7 (v:v) with D2O-containing buffer (10 mM phosphate buffer, pD 7.0) at 20°C for a variable time period between 0 and 120 seconds before quenching the exchange reaction with an equal volume of precooled quenching buffer [100 mM phosphate, 0.5 M tris(2-carboxyethyl)phosphine, 0.8% formic acid, and 2% acetonitrile, pH 2.5] at 1°C. The quenched sample was passed through a Waters Enzymate BEH Pepsin Column (2.1 × 30 mm). Peptic peptides were separated in-line on a Waters ACQUITY UPLC BEH C18 column (1.7 µm, 1.0 × 100 mm) at a flow of 40 μL/min for 12 minutes (8-40% linear gradient, mobile phase: 0.1% formic acid in acetonitrile) at 1°C. The mass spectrometer was operated with the electrospray ionization source in positive ion mode, and the data were acquired in elevated-energy mass spectrometry mode. For internal calibration, a reference lock-mass of Glu-Fibrinopeptide (Sigma-Aldrich, St Louis, MO) was acquired along with each sample data collection. Peptides were sequenced and identified through database searching of the human fVIII-C1 sequence (2020-2172) in ProteinLynx Global SERVER (ver. 3.02), and the HDX-MS data were processed in DynamX (ver. 3.0). Mass assignment for each peptide at 0 seconds of exchange was checked manually; any assignment with a mass deviation >0.2 Da was removed. H/D exchange protection was quantitated by comparison of hydrogen exchange profiles at different time points as previously described.14
fVIII inhibitor assay
The inhibitor titer and inhibition type were determined by a modification of the Bethesda assay using citrated pooled normal human plasma as the source of fVIII as previously described.30 Specific inhibitory activities were converted to Bethesda Units (BUs)/mg IgG using the known concentration of the anti-C1 mAb.
Inhibition of fVIII binding to phospholipid and VWF
Phospholipid and VWF binding competitive ELISAs were performed as previously described and detailed in supplemental Data.16,31
Determination of binding affinities of anti-C1 mAbs for fVIII
The binding affinities of anti-C1 mAbs for BDD fVIII were determined by surface plasmon resonance (SPR) spectroscopy using a Biacore X100 instrument (GE Healthcare BioSciences, Pittsburgh, PA) as previously described32 and are detailed in supplemental Data. Single-cycle kinetic sensorgrams were assessed using a Langmuir binding model and fitted globally to estimate the association and dissociation rate constants, binding affinities, and errors in parameter estimates using the BIAevaluation software.
Intrinsic Xase assays
The inhibition of activated fVIII (fVIIIa) in the intrinsic Xase assay by anti-C1 mAbs was analyzed using a chromogenic substrate assay for factor X activation as previously described16 and detailed in supplemental Data. The initial velocity of factor X activation, which is proportional to fVIIIa activity, and subsequent first-order rate of decay of fVIIIa, along with the standard deviations (SDs) of these estimates, were determined by linear regression estimates of the slope and y-intercept of semi-log plots of factor Xa generation vs time.33
Thrombin generation
Thrombin generation was performed as previously described.34 Peak thrombin concentration, endogenous thrombin potential (ETP), defined as the area under the thrombin generation curve, and lag time were analyzed.
fVIII uptake by dendritic cells
Human MDDCs were generated from healthy donors as previously described25 and detailed in supplemental Data. MDDCs were treated with DyLight 650–conjugated full-length fVIII in the presence and absence of anti-C1 mAbs. Untreated MDDCs were used to account for the intrinsic fluorescence of MDDCs in the data analysis. Anti–factor IX antibody GMA-138 (Green Mountain Antibodies, Burlington, VT) was used as a control. The percentages of fVIII uptake, and median fluorescence intensities of treated MDDCs, normalized to untreated MDDCs, were analyzed using an LSRII flow cytometer (BD Biosciences, San Jose, CA).
Competitive ELISA of anti-C1 mAbs with patient inhibitor plasmas
The ability of plasma samples from patients with congenital hemophilia A and inhibitors to block the binding of anti-C1 mAbs was tested by competitive ELISA as previously described, with a few modifications detailed in supplemental Data.35
Hemophilia A mouse tail snip bleeding model
An in vivo tail snip assay was used to determine bleeding phenotype of hemophilia A mice in the presence and absence of anti-C1 mAbs as previously described.36,37
fVIII clearance in vivo
To determine fVIII clearance, mice were killed 2 hours following BDD fVIII injections and plasma samples were collected by cardiac puncture. fVIII antigen levels were determined by sandwich ELISA using anti-A1 domain mAb 2-116 and anti-A2 domain mAb 2-76 as capture and detection antibodies, respectively, as described in supplemental Data. fVIII coagulant activity was measured using the activated partial thromboplastin reagent-based 1-stage coagulation assay as previously described38 and pooled mouse plasma spiked with BDD fVIII to 1 U/mL for the standard curve.
Approval
After approval by the institutional review board and written informed consent from subjects, samples were obtained in accordance with Emory Institutional Review Board Protocol IRB00006290. Approval for the use of animals in this study and approval of study methods was granted by the Emory University Institutional Animal Care and Use Committee. The Emory University School of Medicine Division of Animal Resources provided training for the proper handling and euthanasia of animals.
Statistical Analysis
Data are presented as means ± SD unless otherwise specified. Significance testing of differences in blood loss in the tail snip assay, fVIII antigen, and fVIII activity were determined by the nonparametric Mann-Whitney U test using Prism 6.0 (GraphPad Software, La Jolla, CA). A value of P < .05 was considered statistically significant.
Results
Immunodominant B-cell epitope is present in the C1 domain of fVIII
To elucidate the overlapping and nonoverlapping B-cell epitopes within the C1 domain, a competitive ELISA between 8 murine and 1 human-derived anti-human C1 mAbs was used. A matrix of the competition pattern between pairs of mAbs revealed 2 distinct B-cell epitopes designated groups A and B within the C1 domain (supplemental Figure 1A). Group A contained 7 of the 9 mAbs from 5 different mice, 2A9, B153, F156, I41, I84, I88, and M6143, that competed for binding to fVIII. B136 was the only group B mAb. The human-derived mAb KM33 competed with group A mAbs B153, I84, I88, and M6143 and group B mAb B136 and is designated group AB (supplemental Figure 1B).
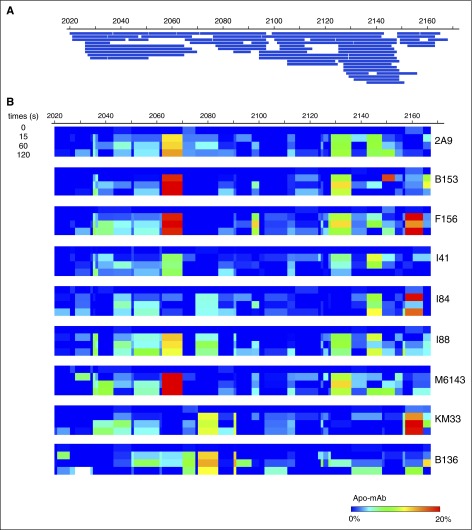
Epitope mapping of anti-C1 mAbs by HDX-MS
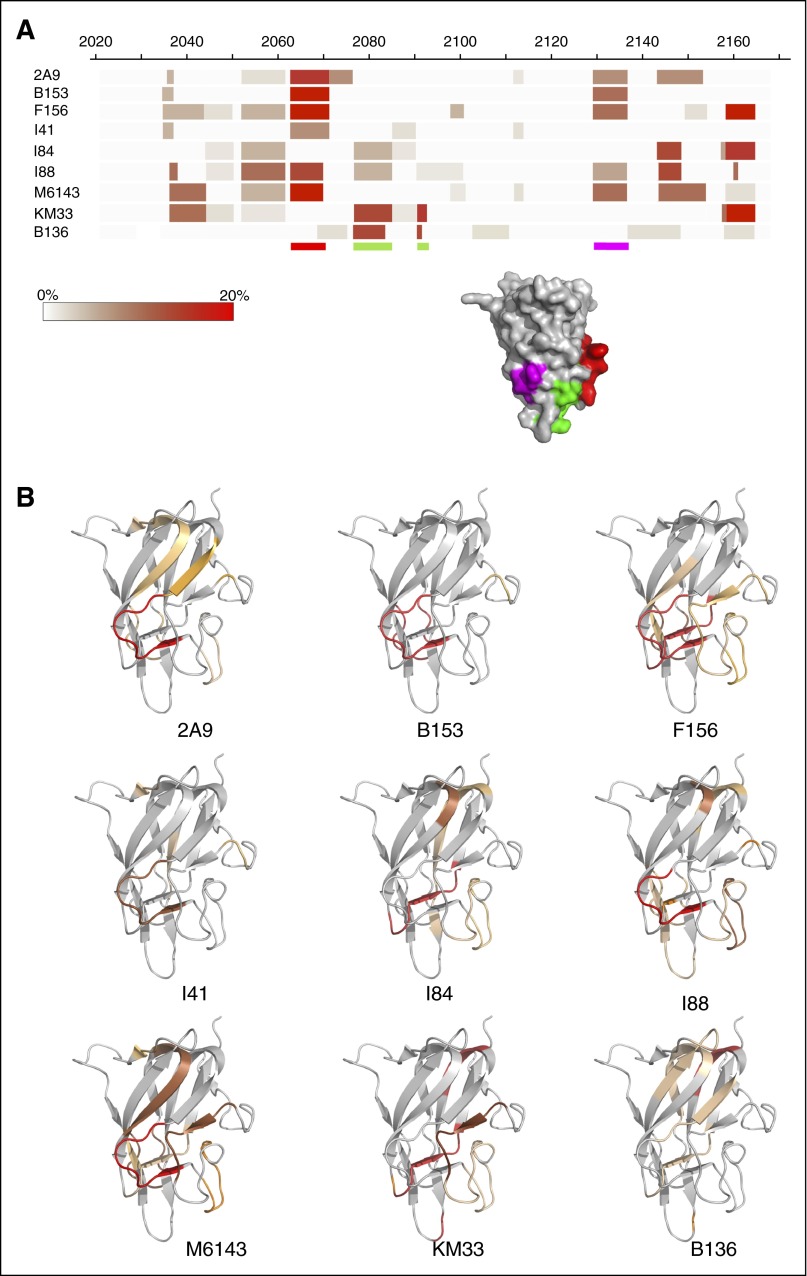
Mapping of 60 peptic peptides of HSA-C1 by HDX-MS achieved 97% sequence coverage of the C1 domain at an average 7.76-fold redundancy/residue (Figure 1A). Comparison of the C1 domain with and without bound mAbs identified sequences with altered HDX profiles, most of which exhibited reduced deuterium exchange with bound mAb (Figure 1B). Spectra demonstrating H/D exchange protection of HSA-C1 sequences by group A mAb 2A9 as a representative mAb are shown in supplemental Figure 2. Group A mAbs showed reduced deuterium exchange for C1 sequences 2063-2071 and 2129-2136 (Figures 1B and 2A-B). In contrast, group AB mAb KM33 demonstrated reduced deuterium exchange of residues 2157-2164 and 2091-2092 similar to previously described results39 with additional H/D exchange protection identified at sequences 2036-2044 and 2077-2085. Group B mAb B136 demonstrated decreased deuterium exchange at residues 2077-2084, similar to group AB mAb KM33, and consistent with the competitive ELISA findings indicating an overlapping epitope between KM33 and B136 (supplemental Figure 1A). Overall, the HDX-MS results identify distinct C1 domain epitopes recognized by group A and group AB/B mAbs and are consistent with the competitive ELISA results.
Figure 1.
Epitope mapping of mAbs within the C1 domain. (A) Peptide map of HSA-C1 protein showing 97% sequence coverage of the C1 domain. (B) Heat map of differences in deuterium exchange per residue at 0, 15, 60, and 120 seconds for the C1 domain demonstrating regions of H/D exchange protection in the presence of anti-C1 mAbs. Color scale indicates percentage of H/D exchange protection determined by the difference in percentage of deuterium exchange per residue between the antigen alone and antigen/mAb mixtures at each individual time (antigen/mAb, also referred to “Apo-mAb,” subtracted from antigen). Red indicates regions of increased H/D exchange protection and blue indicates no protection.
Figure 2.
Anti-C1 domain antibodies epitope mapping by HDX MS. (A) Heat map demonstrating percentage of H/D exchange protection of HSA-C1 fVIII protein in the presence of the anti-C1 mAbs. The color scale represents the difference in percentage of deuterium exchange per residue between antigen alone and antigen/mAb mixtures at 120 seconds (antigen/mAb subtracted from antigen) in which the darker shades represent decreased deuterium exchange in the presence of anti-C1 mAbs. The red and purple shaded regions on the C1 domain corresponding to the red and purple bars below the heat map adapted from the crystal structure of fVIII (Protein Data Bank ID code 4BDV) represents regions of reduced deuterium exchange rate by group A mAbs. The red shaded region and bar corresponds to the proposed primary binding epitope for group A mAbs. The green bars below the heat map and green shaded region on the fVIII C1 domain crystal structure corresponds to the proposed phospholipid binding site for group AB mAb KM33 and group B mAb B136. (B) Binding regions for each anti-C1 mAb imposed on the C1 domain. Shaded regions represent sequences with decreased deuterium exchange in the presence of the anti-C1 mAb. Regions shaded red correspond to sequences with increased H/D exchange protection and orange shaded regions correspond to sequences with a lesser degree of protection in HDX-MS.
Effect of anti-C1 mAbs on fVIII procoagulant activity and binding to phospholipids and VWF
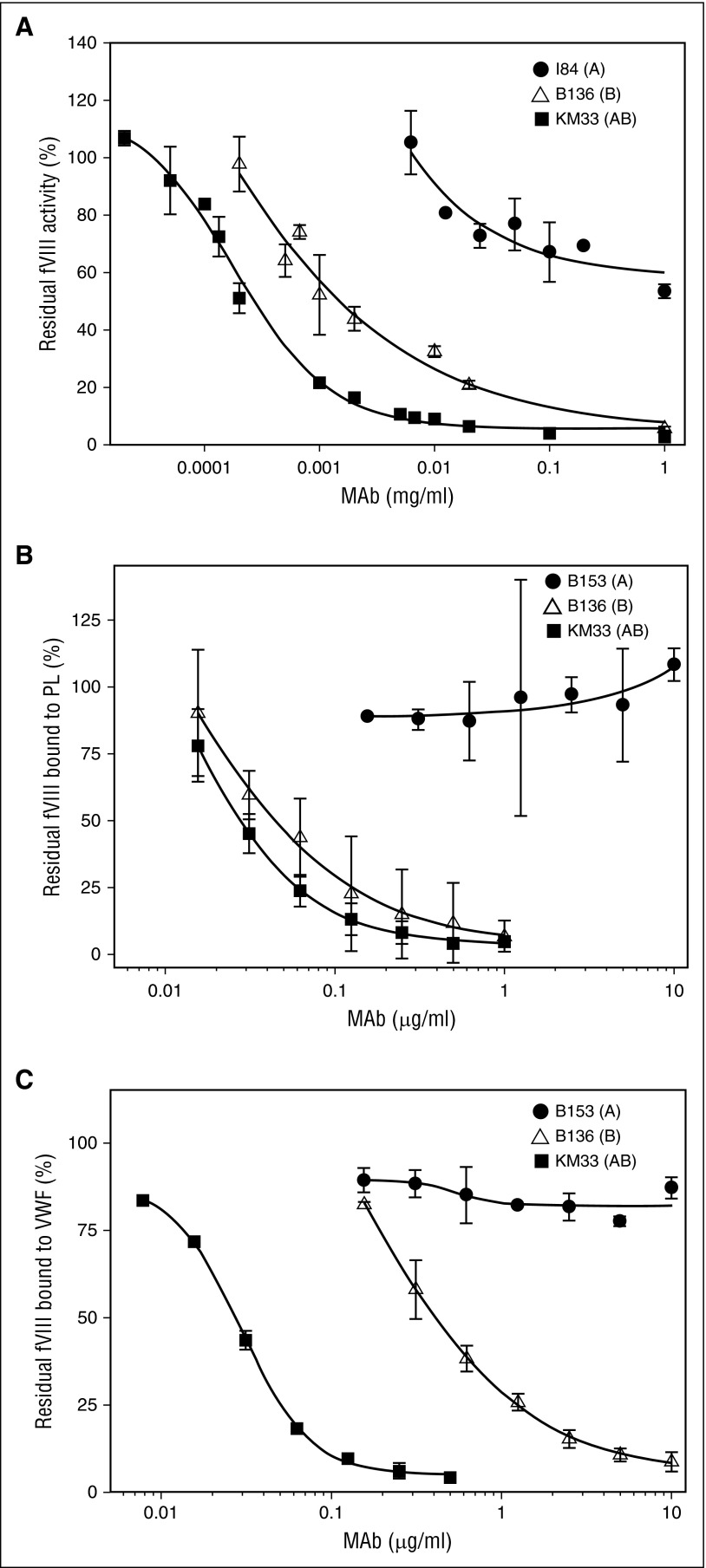
Group A mAbs demonstrated weak inhibition of fVIII activity with inhibitor titers ranging from indeterminate (50% fVIII inhibition was not reached) to 180 BU/mg IgG in the Bethesda assay (Figure 3A; Table 1). The group A mAbs demonstrated type 2 inhibition, defined as incomplete inhibition of fVIII activity in the presence of saturating concentrations of antibody. In contrast, group B mAb B136 and group AB mAb KM33 demonstrated higher inhibitor titers of 700 and 3700 BU/mg IgG, respectively. mAbs B136 and KM33 demonstrated complete, type 1 inhibition of fVIII activity at saturating concentrations.
Figure 3.
fVIII functional assays in the presence of group A, AB, and B anti-C1 mAbs. (A) Inhibition of fVIII procoagulant activity by type 2 group A mAb I84 (●), type 1 group AB mAb KM33 (▪), and type 1 group B mAb B136 (△) by Bethesda assay. (B) Inhibition of fVIII binding to PCPS and (C) fVIII binding to VWF in the presence of anti-C1 mAbs group A B153 (●), group AB KM33 (▪), and group B B136 (△) determined by competitive ELISAs.
Table 1.
Summary of anti-C1 mAb characteristics
| mAb no. | Name | Isotype | Group | Spleen prigin no. | BU/mg IgG | Inhibitor type | VWF binding IC50 (µg/mL) | Phospholipid binding IC50 (µg/mL) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2A9 | IgG2aκ | A | 1 | 23 | II | 1.1 | 0.9 |
| 2 | B153 | IgG2aκ | A | 2 | 3 | II | >10 | >10 |
| 3 | F156 | IgG1κ | A | 3 | 7 | II | >10 | >10 |
| 4 | I41 | IgG1κ | A | 4 | 15 | II | 2.7 | >10 |
| 5 | I84 | IgG2aκ | A | 4 | IND | II | 3.3 | >10 |
| 6 | I88 | IgG2aκ | A | 4 | 3 | II | 1.7 | >10 |
| 7 | M6143 | IgG1κ | A | 5 | 180 | II | 0.6 | >10 |
| 8 | KM33 | IgG1κ* | AB | † | 3700 | I | 0.03 | 0.03 |
| 9 | B136 | IgG2aκ | B | 2 | 700 | I | 0.4 | 0.04 |
IND, indeterminate.
Human-derived mAb KM33 was donated as a full-length human IgG1K converted from the original scFv.
KM33 is a human-derived mAb.
Compared with group AB mAb KM33, group A mAbs demonstrated weak or no inhibition of fVIII binding to phospholipids (Figure 3B; Table 1). In contrast, group A mAbs 2A9, I41, I84, I88, and M6143 inhibited the binding of fVIII to VWF, albeit with higher IC50s than KM33. Group A mAbs B153 and F156 did not inhibit the binding of fVIII to VWF at the highest concentrations tested, indicating functional heterogeneity within group A. Like KM33, group B mAb B136 was a potent inhibitor of fVIII binding to phospholipid and VWF (Figure 3B-C).
High-affinity binding of anti-C1 mAbs to fVIII
All of the mAbs exhibited equilibrium dissociation constants (KD) between 0.1 and 10 nM determined by SPR spectroscopy, which is consistent with high affinity for fVIII (Table 2). Despite high-affinity binding, group A mAbs with inhibitor titers of ∼10 BU/mg (Table 1) required a concentration of ∼600 nM to produce 50% inhibition of fVIII in the Bethesda assay. Thus, fVIII bound to group A mAbs is weakly inhibited. In contrast, group B mAb B136, which potently inhibits fVIII binding to VWF and phospholipids and displays type 1 inhibition of fVIII, revealed the highest affinity for fVIII and is similar to the previously reported KD for group AB mAb KM33 of 0.1 nM.40 B136 requires a concentration of 9.5 nM to produce 50% inhibition. Representative SPR sensorgrams are shown in supplemental Figure 3. These results indicate that the anticoagulant property of anti-C1 mAbs is a function of their ability to inhibit fVIII binding to phospholipid membranes.
Table 2.
Binding affinities of anti-C1 mAbs for fVIII determined by SPR spectroscopy
| mAb | Group | ka (M-1s−1) | kd (s−1) | KD (nM) | Rmax (RU) | χ2 (RU2) |
|---|---|---|---|---|---|---|
| 2A9 | A | (0.3 ± 0.1) × 106 | (2.2 ± 0.2) × 10−4 | 0.9 ± 0.2 | 97.8 ± 1.1 | 0.3 ± 0.2 |
| B153 | A | (0.3 ± 0.03) × 106 | (28 ± 10.8) × 10−4 | 10 ± 2.9 | 92.5 ± 22.8 | 1 ± 0.8 |
| F156 | A | (0.3 ± 0.1) × 106 | (8.3 ± 0.6) × 10−4 | 2.9 ± 1.5 | 74.0 ± 21.1 | 0.9 ± 1.2 |
| I41 | A | (0.3 ± 0.1) × 106 | (6.5 ± 0.4) × 10−4 | 1.9 ± 0.2 | 85.8 ± 3.3 | 0.1 ± 0.2 |
| I84 | A | (0.2 ± 0.03) × 106 | (3.5 ± 0.3) × 10−4 | 1.9 ± 0.5 | 108.9 ± 6.6 | 0.1 ± 0.04 |
| I88 | A | (0.4 ± 0.2) × 106 | (4.3 ± 1.1) × 10−4 | 1 ± 0.1 | 124.6 ± 11.2 | 0.2 ± 0.03 |
| M6143 | A | (0.5 ± 0.2) × 106 | (0.9 ± 0.1) × 10−4 | 0.2 ± 0.1 | 132.8 ± 25.3 | 0.1 ± 0.004 |
| B136 | B | (3.1 ± 1.2) × 106 | (3.2 ± 0.4) × 10−4 | 0.1 ± 0.03 | 301.9 ± 26.5 | 1.3 ± 0.04 |
Inhibition of intrinsic Xase activity and thrombin generation by anti-C1 mAbs
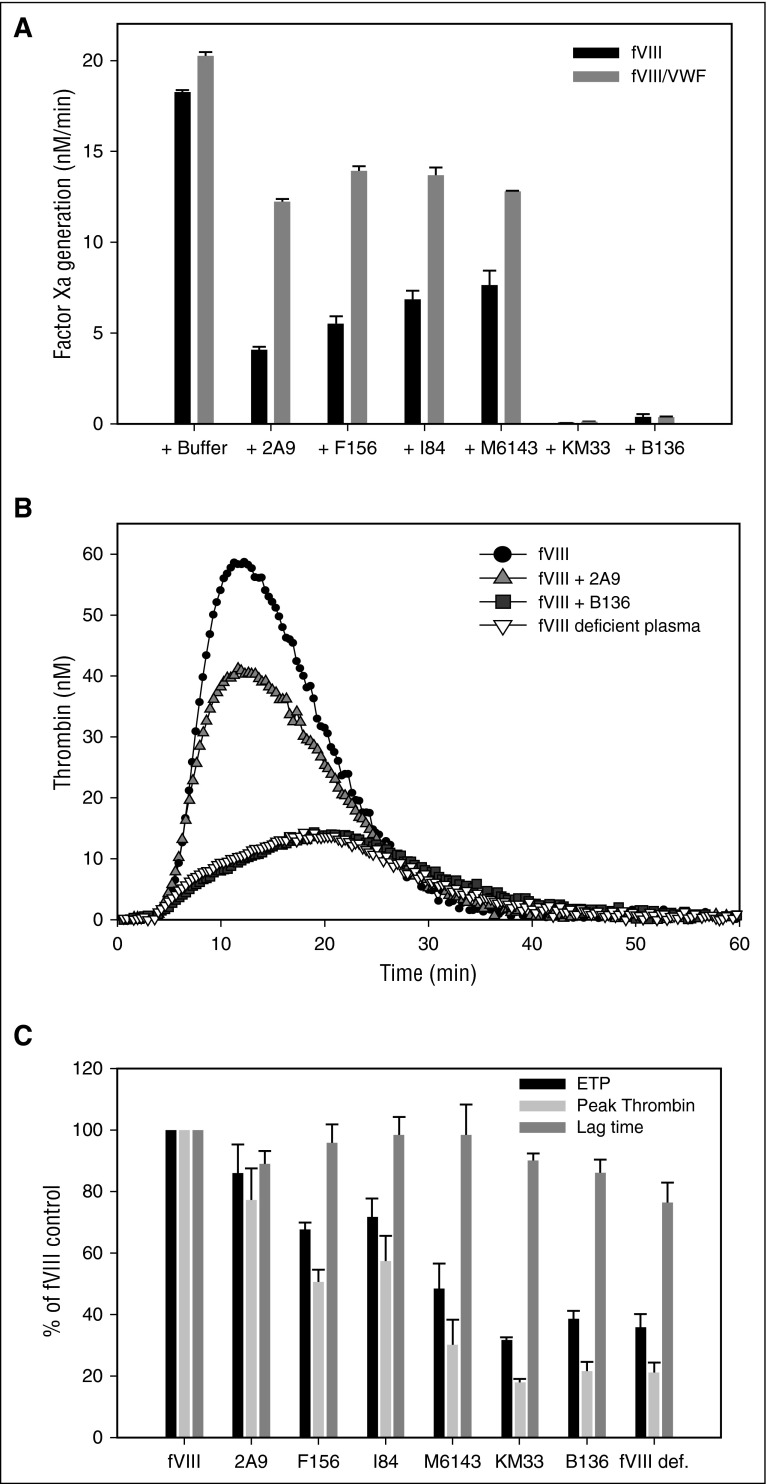
Preliminary experiments were done to determine that the potent fVIII inhibitor, group B mAb B136, inhibits fVIIIa function and not the activation of fVIII. fVIII was activated with thrombin followed by addition of B136 and dilution of this mixture into a solution containing factor IXa and PCPS. Initial fVIIIa activity was inhibited by B136 in a concentration-dependent manner (supplemental Figure 4). fVIIIa activity exhibited first-order decay over time due to the dissociation of the fVIII A2 subunit,41 which was not affected by B136. In subsequent experiments, anti-C1 mAbs were preincubated with fVIII, followed by rapid activation by thrombin and assembly of the intrinsic Xase complex. Extrapolation of the decay curves to zero time provided an estimate of the effect of anti-C1 mAbs on fVIIIa activity (Figure 4A). Group A mAbs 2A9, F156, I84, and M6143 showed weak inhibition of fVIIIa activity compared with group AB and B mAbs KM33 and B136, respectively. None of the mAbs affected the decay rate of fVIIIa (data not shown). The addition of VWF provided some degree of protection of fVIIIa activity for the group A mAbs. mAbs B136 and KM33 inhibited fVIIIa similarly in the absence or presence of VWF. Similar to the intrinsic Xase assays findings, group A mAbs showed weak inhibition of peak thrombin generation and ETP compared with mAbs B136 and KM33 (Figure 4B-C). Lag time was not significantly altered by anti-C1 mAbs.
Figure 4.
Inhibition of intrinsic Xase complex and thrombin generation by anti-C1 mAbs. (A) BDD fVIII at a limiting concentration of 1 nM was preincubated for 2 hours at 37°C in the presence of 20 nM anti-C1 mAbs or buffer control in the absence (black bars) or presence (gray bars) of 10 µg/mL VWF. fVIII was activated by 100 nM thrombin for 30 seconds followed by inhibition of thrombin by 150 nM hirudin. fVIII is completely activated within 10 seconds at this concentration. The intrinsic Xase complex was assembled by the addition of the fVIIIa sample to factor IXa (2 nM), PCPS vesicles (20 µM), and factor X (300 nM). fVIIIa activity was determined by measurement of factor Xa generation in the intrinsic fXase assay as described in “Methods.” (B) Representative thrombin generation in fVIII-deficient plasma spiked with 1 U/mL recombinant full-length fVIII in the presence of 5 µg/mL group A mAb 2A9 and group B mAb B136. Control curves of fVIII-deficient plasma with and without fVIII are also shown. (C) ETP (black bars), peak thrombin generated (light gray bars), and lag time (dark gray bars) of fVIII-deficient plasma spiked with fVIII and anti-C1 mAbs as a percentage of control fVIII-deficient plasma spiked with fVIII. ETP, peak thrombin, and lag time of fVIII-deficient plasma alone are shown as a negative control.
Anti-C1 mAbs binding epitopes play a role in fVIII uptake by dendritic cells
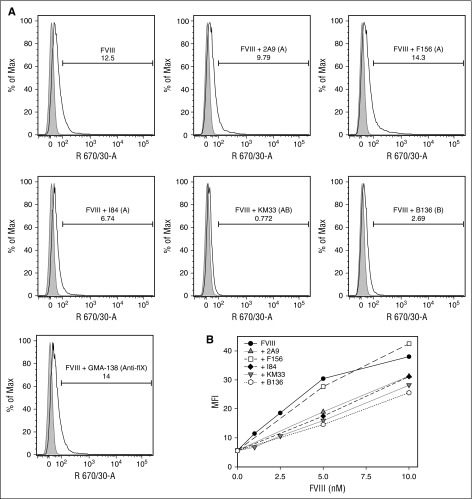
The intracellular uptake of fVIII by MDDCs in the presence of the group A mAbs 2A9, F156, and I84, group AB mAb KM33, and group B mAb B136 was compared with the uptake of fVIII alone or in the presence of control anti–factor IX antibody GMA-138 by flow cytometry. Cells treated with fluorescently labeled fVIII in the presence of KM33 and B136 inhibited fVIII uptake by 95% and 69%, respectively. In contrast, mAbs 2A9 and I84 reduced fVIII uptake by only 21% and 43%, respectively (Figure 5A). The noninhibitory mAb F156 increased fVIII uptake by 55%. GMA-138 did not alter fVIII uptake. fVIII uptake was also studied as a function of fVIII concentration and observed down to a physiologic concentration of 1 nM fVIII (Figure 5B). The increased inhibition of fVIII uptake by group AB and B mAbs compared with group A mAbs was observed at all concentrations tested.
Figure 5.
Inhibition of uptake of fVIII by dendritic cells by anti-C1 mAbs. (A) Flow cytometric histograms of 10 nM DyLight 650-conjugated recombinant full-length fVIII uptake by human MDDCs in the absence and presence of 80 nM group A anti-C1 mAbs 2A9, F156, and I84, group AB KM33, and group B B136. Anti–factor IX antibody GMA-138 is shown as a control. Representative histograms of untreated (gray) and treated (white) MDDCs displaying the percentage of internalized fVIII in the absence or presence of the anti-C1 mAbs tested are shown. Percentages of fVIII uptake shown in the insets were estimated by the integrated fluorescence beyond the control histogram. (B) Median fluorescence intensities of human MDDCs incubated with increasing concentrations of DyLight 650-conjugated fVIII alone (control) and in the presence of mAbs 2A9, F156, I84, KM33, and B136.
Anti-C1 mAbs are present in patients with severe hemophilia A and inhibitors
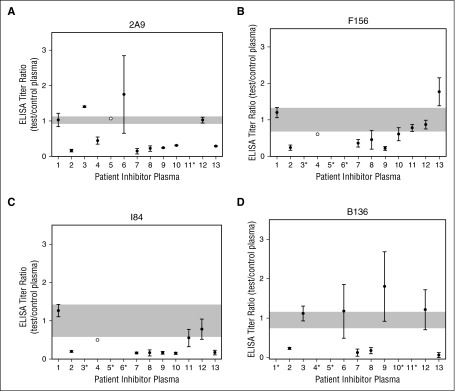
The ability of patient plasmas with inhibitor titers ranging from 1 to 3337 BU/mL (mean, 54 BU/mL; median, 308 BU/mL) to block the binding of anti-C1 mAbs to fVIII was assessed. Significant inhibition of binding by mAbs 2A9, F156, I84, and B136 was present in 58% (7 of 12), 30% (3 of 10), 60% (6 of 10), and 50% (4 of 8) of inhibitor plasmas, respectively (Figure 6). These results indicate that the epitopes bound by the anti-C1 mAbs in this study are recognized by antibodies present in patient inhibitor plasmas.
Figure 6.
Inhibition of anti-C1 mAb binding to fVIII by antibodies in plasmas from patients with severe hemophilia A and inhibitors. Inhibition of binding of biotinylated anti-C1 mAbs (A) 2A9, (B) F156, (C) I84, and (D) B136 to BDD fVIII in the presence of 13 inhibitor patient plasmas or fVIII-deficient plasma (severe hemophilia A noninhibitor) were determined by competitive ELISA. The corresponding normal range of control plasma ELISA titers for each mAb tested defined using EC0.3 values within 2 SDs of the mean is represented by the gray shaded area. A test-to-control ELISA titer ratio of less than 2 SDs from the mean of the control plasma for the anti-C1 mAb tested was considered significant. Inhibitor plasmas were tested in 2 independent experiments (black dots) or 1 experiment (white dot) based on available plasma volume. Due to limited plasma volume, the ELISA titer ratio of inhibitor patient plasmas was not available for some of the anti-C1 mAbs tested as denoted by an asterisk following the inhibitor patient plasma number.
Pathogenicity of anti-C1 mAbs in an in vivo murine tail snip bleeding model
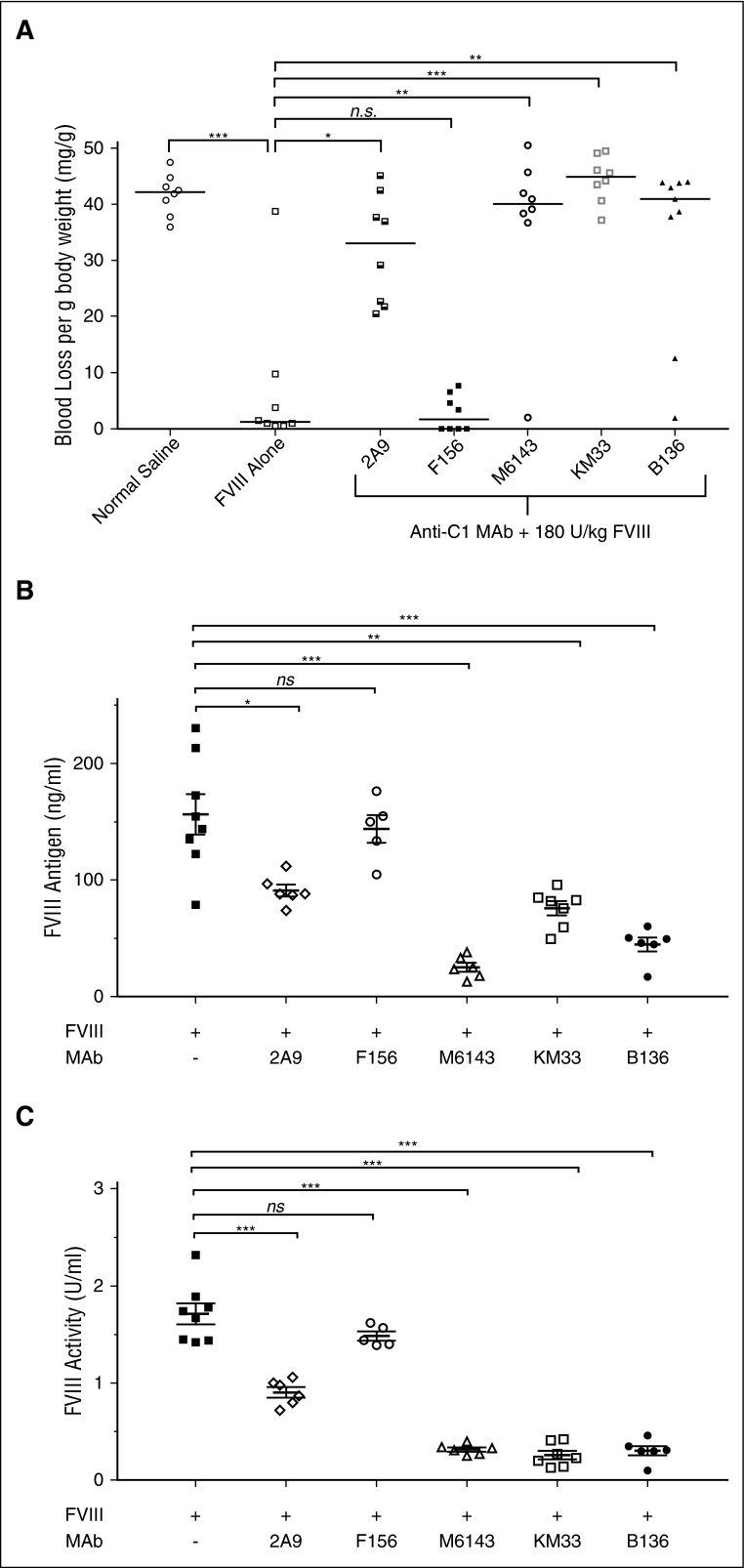
Whereas the inhibitory properties of C1 domain antibodies on key fVIII functions have been described and now expanded in this study, the effect of anti-C1 mAbs on bleeding phenotype in vivo has yet to be fully explored. Anti-C1 mAbs 2A9, M6143, KM33, and B136 all caused significant bleeding in mice producing median (mean ± SD) blood losses per gram of mouse body weight of 33 (32 ± 9.8), 40.1 (36.9 ± 14.8), 44.9 (44.5 ± 4.1), and 41 (34.1 ± 15.6) mg/g, respectively, compared with 1.2 (7.1 ± 3.2) mg/g in mice that received fVIII alone (P < .05; Figure 7A). Mice that received mAbs 2A9, M6143, KM33, and B136 were estimated to have plasma inhibitor titers of <1, 2.3, 46.3, and 8.8 BU/mL, respectively. mAb F156 did not produce significant bleeding in mice with a median (mean ± SD) blood loss of 1.7 (2.8 ± 3.2) mg/g (P = .36, Mann-Whitney U test) and has an estimated plasma inhibitor titer of <1 BU/mL. These findings demonstrate that in addition to the inhibitory mAbs B136 and KM33, the weakly inhibitory group A mAbs 2A9 and M6143 are pathogenic.
Figure 7.
Pathogenicity of anti-C1 mAbs in a murine hemophilia A bleeding model. (A) E16 hemophilia A mice received injections of 0.5 mg/kg (65 nM) anti-C1 mAbs or normal saline as controls followed by 180 U/kg (2.5 nM) BDD fVIII 15 minutes later. Tails snips were performed 2 hours after BDD fVIII injections. Median blood loss per gram of mouse body weight compared with fVIII alone is represented (n = 8-9 mice per group; *P < .05, **P < .01, ***P < .001, ns not significant, Mann-Whitney U test). (B) fVIII antigen and (C) fVIII activity at 2 hours following BDD fVIII injection in the presence or absence of anti-C1 mAbs is shown. Data are presented as mean ± standard error of the mean for fVIII antigen and mean ± SD for fVIII activity (n = 5-8 mice per group; *P < .05, **P < .01, ***P < .001, ns, not significant, Mann-Whitney U test). fVIII activity data are normalized to normal saline only control cohort due to background fVIII activity of 0.38 U/mL in mouse plasmas (data not shown).
Anti-C1 mAbs increase fVIII clearance in hemophilia A mice
The clearance of fVIII is increased in the absence of VWF. Because some of the weakly inhibitory group A mAbs inhibit the binding of fVIII to VWF, we hypothesized that increased fVIII clearance was responsible for pathogenic bleeding in the presence of these mAbs. Group A mAbs M6143 and 2A9 significantly reduced circulating fVIII antigen (Figure 7B) and activity (Figure 7C) compared with fVIII in the absence of mAb, consistent with this hypothesis. In contrast, group A mAb F156, which does not inhibit the binding of fVIII to VWF, did not reduce fVIII clearance. mAbs KM33 and B136 also reduced the level of circulating fVIII antigen. fVIII procoagulant activity was reduced even further, presumably due to carryover of these mAbs into the coagulation assay. This suggests both inhibition of fVIII procoagulant activity and increased fVIII clearance as contributing mechanisms for pathogenicity by mAbs KM33 and B136.
Discussion
We characterized epitope recognition and the functional properties of 8 murine-derived anti-human anti-C1 domain mAbs and a previously described human-derived mAb, KM33.25,26,39,40,42,43 We identified 2 novel antibody groups, designated A and B, by competitive ELISA and HDX-MS (Figures 1 and 2). KM33 binds to partially overlapping epitopes recognized by both group A and group B mAbs and is designated a group AB mAb.
Despite their recognition of overlapping epitopes, there is notable heterogeneity in the residues bound by group A anti-C1 mAbs as judged by differential inhibition in VWF and phospholipid binding assays (Table 1). Five of the 7 group A mAbs inhibited, although weakly, fVIII binding to VWF with IC50s ranging from 0.6 to 3.3 µg/mL. This is in contrast to an IC50 of 0.03 µg/mL observed for group AB mAb KM33 that is comparable to classical anti-C2 mAbs.16 Additionally, only mAb 2A9 inhibited fVIII binding to both VWF and phospholipids. mAbs F156 and B153 inhibited neither VWF nor phospholipid binding. Generally, the group A anti-C1 mAbs demonstrated weak and incomplete inhibition of fVIII by Bethesda assay at concentrations up to 1 mg/mL (∼7 µM). SPR experiments revealed that all of the anti-C1 mAbs display high-affinity, nanomolar or subnanomolar binding to fVIII. Thus, the functional differences between the mAbs are not due to the inability of some mAbs to bind fVIII at the concentrations used in the assays.
The weak anticoagulant activity of group A antibodies is consistent with the observation that they inhibit the binding of fVIII to phospholipid weakly or not at all (Table 1). Site-directed mutagenesis of C1 residues in fVIII indicates that residues 2042-2043, 2090-2093, and 2159 contribute to phospholipid membrane binding.21 Additionally, residues 2042-2043 and 2159, which are contiguous in the fVIII X-ray structure, interact with VWF. Consistent with this, HDX-MS results indicate that binding of group A mAbs protect sequences 2063-2071 and 2129-2136 (Figure 2A), which are remote from the phospholipid and VWF binding sites. Given the distance between these two sequences, it is unlikely that the footprints of an IgG antibody would mask both regions simultaneously. We hypothesize that the sequence 2063-2071 contains the primary group A binding epitope due to the unaltered deuterium exchange rates at 2129-2136 by mAb I41, which has high affinity binding for fVIII (KD, 1.9 nM) and similar characteristics to the other group A mAbs. One possibility to explain the reduced deuterium exchange observed at 2129-2136 is an allosteric effect on fVIII in which the C1 domain is stabilized on binding to the 2063-2071 sequence by group A mAbs, leading to protection from H/D exchange at residues 2129-2136 in HDX-MS.
Epitope mapping of the human-derived C1 antibody LE2E9 suggests binding to residues 2150 and 2153, which is in contrast to our proposed group A binding site; however, these studies were limited to the evaluation of 9 scattered residues in the C1 domain and were not confirmed by another mechanism.27 Additionally hemophilia A mice infused with LE2E9 demonstrated increased bleeding, decreased survival, and reduced fVIII activity over time compared with wild-type mice in tail snip studies.44 Given its properties, we hypothesize that LE2E9 would accelerate fVIII clearance similar to 2A9. mAb TB-402, LE2E9’s deglycosylated counterpart, behaves differently from LE2E9 in that it shows reduced inhibition of fVIII activity compared with LE2E9 and does not appear to inhibit fVIII binding to VWF.45
Three of the sequences identified by HDX-MS that interact with mAb KM33, 2036-2044, 2157-2164, and 2091-2092, include phospholipid and/or VWF binding sites, consistent with potent inhibition by KM33 of these interactions. mAb B136 is a potent inhibitor of fVIII binding to phospholipids (Table 1), and like KM33, demonstrates decreased deuterium exchange at residues 2077-2084 (Figure 2A). However, no evidence was found by HDX-MS for the interaction of this sequence with previously identified phospholipid or VWF binding sites. Thus, residues within 2077-2084 may represent a previously unrecognized functionally important region. This is in contrast to HDX-MS findings reported by Bloem et al39 that excluded partially overlapping peptide 2076-2090 as a protected site by KM33 due to unaltered time-dependent deuterium incorporation. Although an explanation for this observed difference is unclear, differences in experimental procedures, C1 domain sequence coverage following pepsin digestion, and the fVIII proteins used between the current study and published data may have contributed to sequence variances with HDX-MS.
Patients with congenital and acquired hemophilia A produce a polyclonal antibody response to fVIII that has been attributed primarily to epitopes in the A2 and C2 domains.8-10 However, in the current study, most of the inhibitor plasmas tested competed with anti-C1 mAbs for binding to fVIII. Combined with the pathogenicity of a subset of minimally inhibitory anti-C1 antibodies (Table 1; Figure 7A), this suggests that the clinical importance of anti-C1 antibodies in the humoral response to fVIII has been underestimated. In the polyclonal fVIII inhibitor environment, anti-C1 antibodies could be masked in the presence of A2 or C2 domain antibodies that exhibit potent inhibition of fVIII procoagulant activity.
fVIII residues within the C1 domain recognized by mAb KM33 have been implicated in mediating fVIII uptake by APCs.25 All of the anti-C1 mAbs tested, with the exception of F156, inhibited the endocytosis of fVIII by MDDCs (Figure 5). Potent fVIII inhibitors KM33 and B136 were the strongest inhibitors of fVIII uptake by MDDCs. These mAbs not only recognize the group B epitope but abrogate fVIII binding to VWF and phospholipids, indicating that the group B epitope contributes to fVIII presentation to the immune system and regulation of critical fVIII functions. Better understanding of the specific residues recognized by APCs could provide a mechanism for production of a less immunogenic fVIII protein. For instance, Wroblewska et al26 showed that hemophilia A mice that received 5 weekly injections of BDD fVIII with combined alanine substitutions at residues 2090/2092/2093, residues previously implicated in KM33 binding, had significantly reduced anti-fVIII ELISA titers, Bethesda titers, and splenic CD4+ T-cell proliferation on restimulation with fVIII compared with mice that received wild-type BDD fVIII.
The weakly inhibitory group A mAbs 2A9 and M6143 were surprisingly pathogenic in a hemophilia A mouse tail snip bleeding model (Figure 7A) despite having only mild/moderate inhibition of fVIII activity in multiple in vitro coagulation assays. Interestingly, there have been reports of patients in the literature and anecdotally with undetectable inhibitor titers that have significant bleeding episodes despite infusions of high doses of fVIII.46 Given that 1 potential source of in vivo pathogenicity not measured by in vitro assays is clearance of antibody/antigen complexes, we proposed increased fVIII clearance due to inhibition of VWF binding by 2A9 and M6143 as a possible pathogenic mechanism. To test this, we measured fVIII antigen and activity levels at the time of tail snips and found that both 2A9 and M6143 produced a reduction of fVIII activity levels that corresponded to the loss of the fVIII antigen (Figure 7B-C). In addition, there was increased clearance of fVIII antigen in the presence of the strongly inhibitory mAbs KM33 and B136, which also contributes to their pathogenicity.
In summary, anti-C1 mAbs have been identified that recognize 2 novel B cell epitopes and have differential effects on fVIII function. Weakly inhibitory group A mAbs are pathogenic due to their effect on fVIII clearance and are present in a significant portion of inhibitor patient plasmas. This antibody group represents the first documentation of minimally inhibitory, pathogenic anti-fVIII antibodies. Additionally, the identification of immunodominant C1 domain sequences by HDX-MS may guide development of a less immunogenic fVIII protein.
Acknowledgments
The authors thank Jan Voorberg for providing the human-derived anti-fVIII C1 domain antibody KM33 and John Kulman for helpful advice on performing SPR experiments.
This research was supported by the National Institutes of Health (NIH) National Institute of Child Health and Human Development Atlanta Pediatric Scholars Program K12 HD072245 (G.B.), NIH National Heart, Lung, and Blood Institute grant U54 HL112309 (P.L. and S.L.M.), Hemophilia of Georgia (P.L. and S.L.M.), Aspire Hemophilia Award (S.L.M. and R.L.), and NIH National Heart, Lung, and Blood Institute grant K08 HL102262 (S.L.M.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.B. designed and performed research, analyzed data, and cowrote the paper; W.D. performed research and cowrote the paper; J.F.H., E.T.P., and W.H.B. designed and performed research; C.C. and B.N. performed research; C.K. and J.K. contributed the plasmid encoding the HSA-C1 fusion protein and edited the manuscript; R.L. designed research, analyzed data, and edited the manuscript; and S.L.M. and P.L. designed and performed research, analyzed data, and cowrote the paper.
Conflict-of-interest disclosure: P.L. is an inventor on patents owned by Emory University claiming compositions of matter that include modified fVIII proteins with reduced reactivity with anti-fVIII antibodies. All other authors declare no competing financial interests.
Correspondence: Shannon L. Meeks, Emory Children’s Center, 2015 Uppergate Dr, Room 442, Atlanta, GA 30322; e-mail: shannon.meeks@choa.org.
References
- 1.Dimichele D. Inhibitors: resolving diagnostic and therapeutic dilemmas. Haemophilia. 2002;8(3):280–287. doi: 10.1046/j.1365-2516.2002.00626.x. [DOI] [PubMed] [Google Scholar]
- 2.Eckhardt CL, van Velzen AS, Peters M, et al. INSIGHT Study Group. Factor VIII gene (F8) mutation and risk of inhibitor development in nonsevere hemophilia A. Blood. 2013;122(11):1954–1962. doi: 10.1182/blood-2013-02-483263. [DOI] [PubMed] [Google Scholar]
- 3.Walsh CE, Soucie JM, Miller CH United States Hemophilia Treatment Center Network. Impact of inhibitors on hemophilia A mortality in the United States. Am J Hematol. 2015;90(5):400–405. doi: 10.1002/ajh.23957. [DOI] [PubMed] [Google Scholar]
- 4.Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood Rev. 2013;27(4):179–184. doi: 10.1016/j.blre.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Earnshaw SR, Graham CN, McDade CL, Spears JB, Kessler CM. Factor VIII alloantibody inhibitors: cost analysis of immune tolerance induction vs. prophylaxis and on-demand with bypass treatment. Haemophilia. 2015;21(3):310–319. doi: 10.1111/hae.12621. [DOI] [PubMed] [Google Scholar]
- 6.Gringeri A, Mantovani LG, Scalone L, Mannucci PM, Group CS COCIS Study Group. Cost of care and quality of life for patients with hemophilia complicated by inhibitors: the COCIS Study Group. Blood. 2003;102(7):2358–2363. doi: 10.1182/blood-2003-03-0941. [DOI] [PubMed] [Google Scholar]
- 7.Thompson AR. Structure and function of the factor VIII gene and protein. Semin Thromb Hemost. 2003;29(1):11–22. doi: 10.1055/s-2003-37935. [DOI] [PubMed] [Google Scholar]
- 8.Astermark J. Basic aspects of inhibitors to factors VIII and IX and the influence of non-genetic risk factors. Haemophilia. 2006;12(Suppl 6):8–13, discussion 13-14. doi: 10.1111/j.1365-2516.2006.01360.x. [DOI] [PubMed] [Google Scholar]
- 9.Prescott R, Nakai H, Saenko EL, et al. Recombinate and Kogenate Study Groups. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Blood. 1997;89(10):3663–3671. [PubMed] [Google Scholar]
- 10.Scandella DH, Nakai H, Felch M, et al. In hemophilia A and autoantibody inhibitor patients: the factor VIII A2 domain and light chain are most immunogenic. Thromb Res. 2001;101(5):377–385. doi: 10.1016/s0049-3848(00)00418-7. [DOI] [PubMed] [Google Scholar]
- 11.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The humoral response to human factor VIII in hemophilia A mice. J Thromb Haemost. 2007;5(3):512–519. doi: 10.1111/j.1538-7836.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin JC, Ettinger RA, Schuman JT, et al. Six amino acid residues in a 1200 Å2 interface mediate binding of factor VIII to an IgG4κ inhibitory antibody. PLoS One. 2015;10(1):e0116577. doi: 10.1371/journal.pone.0116577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen PC, Lewis KB, Ettinger RA, et al. High-resolution mapping of epitopes on the C2 domain of factor VIII by analysis of point mutants using surface plasmon resonance. Blood. 2014;123(17):2732–2739. doi: 10.1182/blood-2013-09-527275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevy AM, Healey JF, Deng W, Spiegel PC, Meeks SL, Li R. Epitope mapping of inhibitory antibodies targeting the C2 domain of coagulation factor VIII by hydrogen-deuterium exchange mass spectrometry. J Thromb Haemost. 2013;11(12):2128–2136. doi: 10.1111/jth.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter JD, Werther RA, Brison CM, et al. Structure of the factor VIII C2 domain in a ternary complex with 2 inhibitor antibodies reveals classical and nonclassical epitopes. Blood. 2013;122(26):4270–4278. doi: 10.1182/blood-2013-08-519124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007;110(13):4234–4242. doi: 10.1182/blood-2007-06-096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saenko EL, Shima M, Gilbert GE, Scandella D. Slowed release of thrombin-cleaved factor VIII from von Willebrand factor by a monoclonal and a human antibody is a novel mechanism for factor VIII inhibition. J Biol Chem. 1996;271(44):27424–27431. doi: 10.1074/jbc.271.44.27424. [DOI] [PubMed] [Google Scholar]
- 18.Markovitz RC, Healey JF, Parker ET, Meeks SL, Lollar P. The diversity of the immune response to the A2 domain of human factor VIII. Blood. 2013;121(14):2785–2795. doi: 10.1182/blood-2012-09-456582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lollar P, Parker ET, Curtis JE, et al. Inhibition of human factor VIIIa by anti-A2 subunit antibodies. J Clin Invest. 1994;93(6):2497–2504. doi: 10.1172/JCI117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu TC, Pratt KP, Thompson AR. The factor VIII C1 domain contributes to platelet binding. Blood. 2008;111(1):200–208. doi: 10.1182/blood-2007-01-068957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lü J, Pipe SW, Miao H, Jacquemin M, Gilbert GE. A membrane-interactive surface on the factor VIII C1 domain cooperates with the C2 domain for cofactor function. Blood. 2011;117(11):3181–3189. doi: 10.1182/blood-2010-08-301663. [DOI] [PubMed] [Google Scholar]
- 22.Chiu PL, Bou-Assaf GM, Chhabra ES, et al. Mapping the interaction between factor VIII and von Willebrand factor by electron microscopy and mass spectrometry. Blood. 2015;126(8):935–938. doi: 10.1182/blood-2015-04-641688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yee A, Oleskie AN, Dosey AM, et al. Visualization of an N-terminal fragment of von Willebrand factor in complex with factor VIII. Blood. 2015;126(8):939–942. doi: 10.1182/blood-2015-04-641696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dasgupta S, Repessé Y, Bayry J, et al. VWF protects FVIII from endocytosis by dendritic cells and subsequent presentation to immune effectors. Blood. 2007;109(2):610–612. doi: 10.1182/blood-2006-05-022756. [DOI] [PubMed] [Google Scholar]
- 25.Herczenik E, van Haren SD, Wroblewska A, et al. Uptake of blood coagulation factor VIII by dendritic cells is mediated via its C1 domain. J Allergy Clin Immunol. 2012;129(2):501-509. [DOI] [PubMed]
- 26.Wroblewska A, van Haren SD, Herczenik E, et al. Modification of an exposed loop in the C1 domain reduces immune responses to factor VIII in hemophilia A mice. Blood. 2012;119(22):5294–5300. doi: 10.1182/blood-2011-11-391680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacquemin M, Benhida A, Peerlinck K, et al. A human antibody directed to the factor VIII C1 domain inhibits factor VIII cofactor activity and binding to von Willebrand factor. Blood. 2000;95(1):156–163. [PubMed] [Google Scholar]
- 28.Peerlinck K, Jacquemin MG, Arnout J, et al. Antifactor VIII antibody inhibiting allogeneic but not autologous factor VIII in patients with mild hemophilia A. Blood. 1999;93(7):2267–2273. [PubMed] [Google Scholar]
- 29.Köhler G, Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- 30.Barrow RT, Lollar P. Neutralization of antifactor VIII inhibitors by recombinant porcine factor VIII. J Thromb Haemost. 2006;4(10):2223–2229. doi: 10.1111/j.1538-7836.2006.02135.x. [DOI] [PubMed] [Google Scholar]
- 31.Lagacé J, Arsenault S, Cohen EA. Alcian blue-treated polystyrene microtitre plates for use in an ELISA to measure antibodies against synthetic peptides. J Immunol Methods. 1994;175(1):131–135. doi: 10.1016/0022-1759(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson R, Katsamba PS, Nordin H, Pol E, Myszka DG. Analyzing a kinetic titration series using affinity biosensors. Anal Biochem. 2006;349(1):136–147. doi: 10.1016/j.ab.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 33.Bevington PR. New York, NY: McGraw Hill; 1969. Least-squares fit to a straight line. In: Data Reduction and Error Analysis for the Physical Sciences. pp. 92–118. [Google Scholar]
- 34.Doshi BS, Gangadharan B, Doering CB, Meeks SL. Potentiation of thrombin generation in hemophilia A plasma by coagulation factor VIII and characterization of antibody-specific inhibition. PLoS One. 2012;7(10):e48172. doi: 10.1371/journal.pone.0048172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Nonclassical anti-C2 domain antibodies are present in patients with factor VIII inhibitors. Blood. 2008;112(4):1151–1153. doi: 10.1182/blood-2008-01-132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Non-classical anti-factor VIII C2 domain antibodies are pathogenic in a murine in vivo bleeding model. J Thromb Haemost. 2009;7(4):658–664. doi: 10.1111/j.1538-7836.2009.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eubanks J, Baldwin WH, Markovitz R, et al. A subset of high-titer anti-factor VIII A2 domain antibodies is responsive to treatment with factor VIII. Blood. 2016;127(16):2028–2034. doi: 10.1182/blood-2015-09-670034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meeks SL, Cox CL, Healey JF, et al. A major determinant of the immunogenicity of factor VIII in a murine model is independent of its procoagulant function. Blood. 2012;120(12):2512–2520. doi: 10.1182/blood-2012-02-412361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bloem E, van den Biggelaar M, Wroblewska A, et al. Factor VIII C1 domain spikes 2092-2093 and 2158-2159 comprise regions that modulate cofactor function and cellular uptake. J Biol Chem. 2013;288(41):29670–29679. doi: 10.1074/jbc.M113.473116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Brink EN, Turenhout EA, Bovenschen N, et al. Multiple VH genes are used to assemble human antibodies directed toward the A3-C1 domains of factor VIII. Blood. 2001;97(4):966–972. doi: 10.1182/blood.v97.4.966. [DOI] [PubMed] [Google Scholar]
- 41.Parker ET, Doering CB, Lollar P. A1 subunit-mediated regulation of thrombin-activated factor VIII A2 subunit dissociation. J Biol Chem. 2006;281(20):13922–13930. doi: 10.1074/jbc.M513124200. [DOI] [PubMed] [Google Scholar]
- 42.Meems H, Meijer AB, Cullinan DB, Mertens K, Gilbert GE. Factor VIII C1 domain residues Lys 2092 and Phe 2093 contribute to membrane binding and cofactor activity. Blood. 2009;114(18):3938–3946. doi: 10.1182/blood-2009-01-197707. [DOI] [PubMed] [Google Scholar]
- 43.Ananyeva NM, Makogonenko YM, Kouiavskaia DV, et al. The binding sites for the very low density lipoprotein receptor and low-density lipoprotein receptor-related protein are shared within coagulation factor VIII. Blood Coagul Fibrinolysis. 2008;19(2):166–177. doi: 10.1097/MBC.0b013e3282f5457b. [DOI] [PubMed] [Google Scholar]
- 44.Singh I, Smith A, Vanzieleghem B, et al. Antithrombotic effects of controlled inhibition of factor VIII with a partially inhibitory human monoclonal antibody in a murine vena cava thrombosis model. Blood. 2002;99(9):3235–3240. doi: 10.1182/blood.v99.9.3235. [DOI] [PubMed] [Google Scholar]
- 45.Jacquemin M, Radcliffe CM, Lavend’homme R, et al. Variable region heavy chain glycosylation determines the anticoagulant activity of a factor VIII antibody. J Thromb Haemost. 2006;4(5):1047–1055. doi: 10.1111/j.1538-7836.2006.01900.x. [DOI] [PubMed] [Google Scholar]
- 46.Kempton CL, Meeks SL, Donald Harvey R, III, Abshire TC. Evaluation of factor VIII pharmacokinetics and anti-factor VIII antibodies in four boys with haemophilia A and a poor clinical response to factor VIII. Haemophilia. 2011;17(1):155–156. doi: 10.1111/j.1365-2516.2010.02345.x. [DOI] [PubMed] [Google Scholar]