Abstract
Filensin (BFSP1) and CP49 (BFSP2) represent two members of the IF protein superfamily that are thus far exclusively expressed in the eye lens. Mutations in both proteins cause lens cataract and careful consideration of the detail of these cataract phenotypes alerts us to several interesting features concerning the function of filensin (BFSP1) and CP49 (BFSP2) in the lens. With the first filensin (BFSP1) mutation now having been reported to cause a recessive cataract phenotype, there is the suggestion that the mutation could predispose heterozygote carriers to the early onset of age-related nuclear cataract. In the case of CP49 (BFSP2), there are now three unrelated families who have been identified with a common E233Δ mutation. Very interestingly this is linked to myopia in one family. Despite the apparent phenotypic differences of the filensin (BFSP1) and CP49 (BFSP2) mutations, the data are still consistent with the beaded filament proteins being essential for lens function and specifically contributing to the optical properties of the lens. The fact that none of the mutations thus far reported affect either the conserved LNDR or TYRKLLEGE motifs that flank the central rod domain supports the view that this pair of IF proteins have unusual structural features and a distinctive assembly mechanism. The multiple sequence divergences suggest these proteins have been adapted to the specific functional requirements of lens fibre cells, a function that can be traced from squid to man.
Introduction
The eye lens is a transparent tissue comprising highly elongated lens fibre cells, which are derived from the differentiation of a single layer of polarised epithelial cells that underlie the anterior surface of the lens (Fig. 1). The process of differentiation is accompanied by characteristic changes in the intracellular architecture of lens fibre cells. These include cellular elongation, the synthesis of certain crystallins [1, 2] and the loss of all the membrane-bound organelles including nuclei [3]. Despite these alterations, the differentiated lens fibre cells maintain a well-organised lenticular cytoskeleton comprising actin-containing microfilaments, microtubules and at least two different IF networks, one based on vimentin and the other based on a copolymer of lens-specific IF proteins [4].
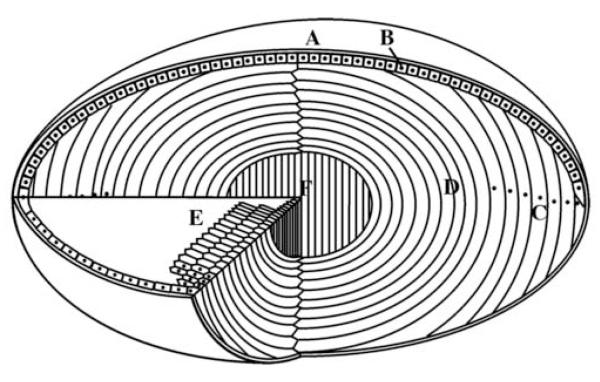
Figure 1.
Schematic view of the eye lens showing the main features and regions. The lens of the eye is enclosed by lens capsule (A) and the anterior surface of the lens is covered with a single layer of epithelial cells (B). The lens fibre cells are formed from epithelial cells at the lens equator (C) as part of their differentiation pathway. The epithelial elongate until their ends reach the two lens poles and are joined at the lens sutures. One of the striking features of the lens differentiation process at this stage is the removal of membrane-bound organelles (D) including nuclei, as indicated here as black dots. A cross section of the lens reveals the elongated fibre cells with characteristic hexagonal shape (E). The bulk of the lens thus consists of long, ribbon-like fibre cells arranged as concentric layer with the primary fibres cells at the centre of the lens (F).
The lenticular IF cytoskeleton
The first identified IF protein in the eye lens is vimentin, a type III IF protein found in both the undifferentiated lens epithelium as well as the differentiated lens fibre cells [5, 6]. The other IF proteins found exclusively in the lens fibre cells [4, 7] comprise a unique cytoskeletal structure called the beaded filament [8]. Based on SDS-PAGE of the beaded filament fraction enriched from the chicken lens, the two structural proteins of the beaded filament were called cytoskeletal protein 49 (CP49) and cytoskeletal protein 95 (CP95) [9, 10]. The CP95 orthologues in the cow and rat lenses were called CP115 [11] and CP94 [12] respectively. To avoid confusion due to these different apparent molecular weights in the different species, this protein was renamed filensin [13, 14] or beaded filament structural protein 1 (BFSP1). CP49 has also been called phakinin [15] or beaded filament structural protein 2 (BFSP2), but it does not exhibit as much electrophoretic mobility variability between species. Unlike vimentin that is expressed in many cell types, filensin (BFSP1) and CP49 (BFSP2) are exclusively expressed in the fibre cells of the eye lens [4, 7].
Unique structural details of lens-specific IF proteins
Analysis of primary and secondary sequence of filensin (BFSP1) and CP49 (BFSP2) from several species confirmed that both proteins are members of IF protein family [14-19]. These two proteins, however, revealed several unique sequence characteristics, which do not fit easily into any of the currently established classes of IF proteins [20, 21].
Filensin (BFSP1) has a number of distinguishing features, such as a shortened rod domain resulting from a truncation of 29 amino acids within the helix 2 immediate after the fourth heptad repeat [14, 18]. This truncation means that filensin has the shortest rod domain of all cytoplasmic IF proteins. Filensin (BFSP1) also exhibits sequence divergence in both the highly conserved motifs at ends of the rod domain. Whilst the TYRKLLEGEE motif at the end of the rod domain has the modified sequence RYHRIIE(I/N)EG, the LNDR motif at the beginning of the rod domain is altered to LGER in mammalian filensin (BFSP1) [22].
CP49 (BFSP2) is usually a tailless IF protein with a stop codon located immediately after the final amino acid residue of helix 2B of the rod domain [15, 16]. The fish provide an exception to this rule. For example Zebra Fish, stickleback and both the Japanese and spotted-green puffer fish all have predicted tail domains [22], as observed for another fish, the trout [20]. The highly conserved TYRKLLEGE sequence at the end of the rod domain is characteristically conserved amongst the mammalian CP49 (BFSP2), with the sequence SYHALLDREE [22]. In contrast, CP49 (BFSP2) showed exceptional sequence divergence at the highly conserved helix initiation LNDR motif, resulting in the consistent substitution of arginine for cysteine [22]. The LNDR motif is usually critical for IF assembly [23, 24]. In other IF proteins, this very substitution is the genetic basis of many inherited human diseases, compromising IF assembly and its function [25]. There is a splice variant called CP49ins, containing an insertion of 49 amino acids within helix 1B of the rod domain [17, 26]. An extended rod domain is one of the hallmarks of ancestral IF proteins [25, 27], from which vertebrate cytoplasmic IFs are evolved. Indeed the IF proteins present in Squid and Octopus lenses bear such familiar hallmarks [28].
Both filensin (BFSP1) and CP49 (BFSP2) have a number of quite distinctive sequence features that distinguish them from the other cytoplasmic IFs. Interestingly, this sequence divergence continues between orthologues. Although one might expect a high degree of sequence homology (>85%) between orthologues from different species [29], this is neither the case for filensin (BFSP1) nor CP49 (BFSP2) [22]. For instance, the tail domain of filensin (BFSP1) is amongst the most variable regions between orthologues for all IF proteins [22]. The radical sequence changes observed for the filensin (BFSP1) and CP49 (BFSP2) sequences may reflect different functional requirements of the different lenses. For instance, eye accommodation differs between species requiring lens distortion in some species, but not in others [30]. It remains to be seen whether specific functions can be attributed to individual features in the sequences of these two proteins.
The assembly properties of the filensin (BFSP1) and CP49 (BFSP2)
With such distinctive sequence characteristics, it is perhaps not surprising that filensin (BFSP1) and CP49 (BFSP2) are also distinct in their assembly behaviour and the polymers that form, at least in vivo. In vitro assembly studies with purified filensin (BFSP1) and CP49 (BFSP2) have shown that each individual protein is incapable of forming conventional 10-nm filaments [31]. CP49 (BFSP2) can self-assemble into thin filament-like structure that associates laterally to form thicker bundles. Such thicker filamentous structures can also form from a variety of tail-truncated or tail-mutagenised IF proteins [32-36], suggesting that the tail domain is indeed important in controlling lateral associations between IFs [25] and highlighting the need for coassembly of CP49 (BFSP2) with filensin (BFSP1). On its own, filensin (BFSP1) can only form short, kinked fibrils [37, 38], which are reminiscent of those formed by NF-M and NF-H [39, 40]. When co-assembled in vitro, filensin (BFSP1) and CP49 (BFSP2) readily form heteropolymeric filaments with 10-nm morphology [31, 41].
This clearly demonstrates that filensin (BFSP1) and CP49 (BFSP2) require each other for filament assembly in vitro. The coassembly between filensin (BFSP1) and CP49 (BFSP2) is specific as neither filensin (BFSP1) nor CP49 (BFSP2) is able to co-assemble with vimentin in vitro, although recent studies suggested that filensin (BFSP1), or its proteolytic fragment(s), can interact with vimentin both in vitro [13] and in vivo [42]. In addition, CP49 (BFSP2) is unable to co-assemble with keratin, despite its relatedness to type I keratins [16]. The optimal molar stoichiometric of filensin (BFSP1) and CP49 (BFSP2) required for filament formation is reported to vary between 2:1 [31] and 3:1 [15], which is reminiscent of those formed by the neurofilament proteins.
Although filensin (BFSP1) and CP49 (BFSP2) coassemble into 10 nm IFs in vitro, they form a unique cytoskeletal structure called the beaded filament (Fig. 2A) in all vertebrate lenses as well as in squid [43]. The beaded filament has two distinct morphological features: a 6-8 nm filament backbone and a 12-15 nm bead that decorates the filaments at regular intervals. Some have reported that beaded filaments can be reconstituted from filensin (BFSP1) and CP49 (BFSP2) alone in vitro [38]. It is proposed that there is a core comprising four homotypic CP49 protofilaments that is then surrounded by the peripheral association of up to four heterotypic filensin/CP49 protofilaments [44]. It is thought that the long C-terminal tail projections of filensin (BFSP1) contribute to the regularly spaced beads [44]. The fact, however, that α-crystallins are a major component of the beaded filament fraction (Fig. 2B) and they associate very readily with filensin/CP49 filaments with similar bead spacing and dimensions of native beaded filaments [21] suggests that a complex with these protein chaperones is also very likely as previously proposed [45].
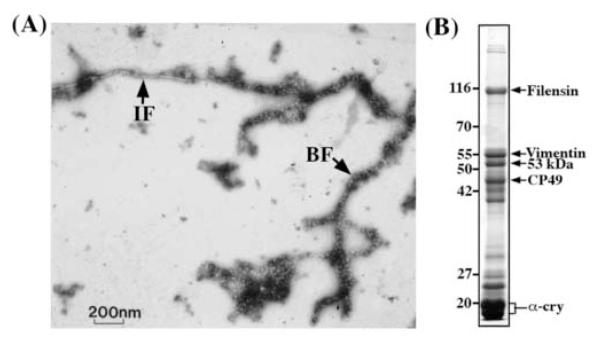
Figure 2.
(A) Transmission electron micrograph of native intermediate filament and beaded filament isolated from bovine lens. Samples were visualized by negative staining with uranyl acetate and a representative field is shown. The arrows denote intermediate filament (IF) with10-nm morphology and the typical beaded filament (BF) consisting of a filament backbone decorated with beads. Bar, 200 nm. (B) SDS-PAGE analysis of the cytoskeletal components prepare from the bovine lens cortex. The sample of final lens membrane pellet was analysed by 12% polyacrylamide gel and proteins were visualized by Coomassie blue staining. The proteins of interest include filensin (BFSP1), vimentin, 53 kDa filensin proteolytic fragment (53 kDa), CP49 (BFSP2) and α-crystallin are indicated by arrows. Molecular weight markers (Mr × 10−3) are shown and labeled on the left.
Functional studies of filensin (BFSP1) and CP49 (BFSP2)
Mouse knockout studies have shown that beaded filaments are essential for the optical properties of the lens by maintaining the three dimensional architecture of lens fibre cells [42]. Given the unusual sequence characteristics and their selective co-assembly properties, it has been suggested that filensin (BFSP1) and CP49 (BFSP2) play a functional role that is unique to the lens fibre cell biology. This hypothesis is tested by generating knockout animals with targeted deletion of the lens-specific IF gene BFSP1 and BFSP2 encoding filensin and CP49. Knockout of filensin (BFSP1) [46] and CP49 (BFSP2) [47, 48] each destabilised the other coassembly partner, resulting in the loss of beaded filaments. Although lenses from both knockouts show increased light scatter that progressively worsened with age, there appeared to be subtle differences between filensin (BFSP1) and CP49 (BFSP2) knockouts. The degree of light scatter in the filensin (BFSP1) knockout appeared to be greater, and at an earlier age [46], than CP49 (BFSP2) knockouts. In addition, the filensin heterozygotes also showed a slight increase in light scatter. The most important discovery, however, was the loss of optical function for the lenses of the BFSP2 knockout mice [48]. Ultrastructural studies of the CP49 (BFSP2) lens revealed substantial changes in the fibre cell shape and plasma membrane organisation [48] as well as the loss of the beaded filament. Interestingly, the remaining filensin (BFSP1) in the CP49 (BFSP2) knockout appeared to associate with the vimentin filaments, dramatically altering the morphology of these filaments [42]. Table 1 summarises the phenotypes of filensin (BFSP1) and CP49 (BFSP2) knockout mice. These observations from BFSP1 and BFSP2 knockouts suggest that the filensin/CP49 filament network is required to maintain cell morphology and correct three-dimensional membrane architecture. Several studies have demonstrated the localisation of filensin (BFSP1) and CP49 (BFSP2) at the fibre cell membranes [4, 13, 49] and recent studies suggested that an aspect of this localisation is the association with the lens plasma membrane proteins, such as tropomodulin [50] and aquaporin 0 (AQP0) [51]. Of course such protein interactions have important functional implications, especially for such a major plasma membrane protein such as AQP0 and relates perhaps to the very tight association and specific binding of filensin (BFSP1) to lens plasma membranes [52].
Table 1.
Summary of the phenotypes of BFSP1 (filensin) and BFSP2 (CP49) knockouts
| Knockouts | BFSP1 (filensin) | BFSP2 (CP49) |
|---|---|---|
| Lens phenotypes | No obvious change in lens morphology. Normal lens development and fibre cell differentiation |
No obvious change in lens morphology. Substantial change in fibre cell membrane architecture |
| Optical property | Light scatter starts at 2 months, worsened with age |
Loss of optical function that worsened with age |
| Cataract | No | No |
| Levels of assembly partner | Reduced | Reduced |
| Beaded filament | Lost | Lost, but vimentin filaments now have modified morphology |
| Heterozygous mice | Exhibited intermediate phenotype |
Similar to WT litter mates |
A splice site mutation in BFSP2 mimics CP49 (BFSP2) knockouts
A natural mutation in BFSP2 that mimics CP49 (BFSP2) knockouts has been reported in a wide range of mouse species, including CBA, 101, several 129 strains of mice [42, 53] and the FBV/N strain [54], which are popular for transgenesis. This mutation introduces a premature stop codon and will result in a severely truncated CP49 (BFSP2) protein if the mRNA is not destroyed by nonsense-mediated decay. Detailed analysis of the lens from 129 strains of mice has demonstrated that these mice lack CP49 (BFSP2). Filensin (BFSP1) levels were greatly reduced as seen in the knockout, but vimentin levels were unaffected. Ultrastructural analyses of the lens fibre cell cytoskeleton revealed the loss of beaded filaments, which were replaced by poorly defined filament-like materials where vimentin was confirmed as one of the major components [42]. Indeed, this deletion mutation generates a natural CP49 (BFSP2) knockout because of the many features in common with the targeted deletion of BFSP2 [47, 48].
Mutations in filensin (BFSP1) and CP49 (BFSP2) cause inherited cataract
There have now been two different mutations reported for CP49 (BFSP2), one in exon 3 (E233Δ; [55-57]) and the other in exon 4 (R287W; [58]). It is interesting to note that both residues are well conserved (Table 2), albeit the R287 residue is absolutely preserved in all animal sequences determined to date. Clearly a tryptophan substitution for arginine would be expected to have quite considerable impact given that the position of tryptophans is usually highly conserved in coiled coil proteins (eg [59]). The fact that the mutation introduces a second tryptophan just 8 residues away from the first (Table 2; W279) would be expected to compound the deleterious effects upon the coiled coil and the assembly of beaded filament. The mutation, however, appeared to be not fully penetrant as one carrier (IV:5; see [58]) certainly had good vision (corrected visual acuity 20:40 and 20:25) and no cataract. The age of cataract surgery for the other affected family members varied from 6-50 years and it was proposed that allelic heterogeneity might explain the variation.
Table 2.
Comparison of the amino acid sequences of residues 225-234 and 279-289 in the CP49 (BFSP2) from various species
| ANIMAL | E233 | R287 |
|---|---|---|
| Mouse | EHQIESLKEE | WERDVEKNRAE |
| Rat | EHQIESLKEE | WERDVEKNRAE |
| Rabbit | WERDVEKNRVE | |
| Cow | ESQIESLKEE | WERDVEKNRLQ |
| Elephant | WERDVEKSRVE | |
| Dog | ESQIESLKEE | WERDVEKNRAE |
| Man | ESQIESLKEE | WERDVEKNRVE |
| Chimp | ESQIESLKEE | WERNVEKNRVE |
| Macaque* | ESQIESLKEE | WEEEEEKLREE |
| Hedgehog | ESQIEGLKEE | WERDVEKKRME |
| Opossum | ESQIESMKEE | WEKDIEKNRAE |
| Platypus | ESQIESMKEE | |
| Chick | ESQIESMKEE | WERDIEKNRAE |
| Puffer-SpottedGreen | EEHMEDLRAE | WEKVSEKNRAE |
| Puffer-Japanese | EEQMEDLRAE | WEKVTEKNRAE |
| Trout | ESQMEDLRAE | WERVIEKNRAE |
| Zebrafish* | ESMKTENVEQ | WERVVEKNRAE |
| Stickleback | EEQMENMRAD | WEKVMERNRAE |
| Medaka | EEQMEMMRQE | WEKVTEKNRVE |
The identified mutations (E233 and R287) are highlighted. Sequences were extracted from the ENSEMBL database release 42 using the human gene BFSP2 (ENSG00000170819) for reference and using the orthologue prediction section to trace sequences in the other animals. The asterisk indicates sequences that have been deduced from ESTs and database mining to address obvious database errors. For example the entry ENSBTAP00000024638 for the bovine Bfsp2 contains an error in exon assignment and translation when compared to the NCBI entry NM_174248 which affects the sequence around E233.
The E233Δ mutation is the more interesting considering the fact that this has now occurred in three unrelated families across two continents [55-57]. Deletion of the residue E233 would be expected to completely alter the heptad repeat for CP49 (BFSP2) and be equally disruptive for beaded filament assembly. Given such a prediction, it is therefore perhaps a little surprising to find differences in the cataract phenotypes reported for the different families.
In the first family [55], there were phenotypic variations, but the light scattering properties of these cataracts meant that all affected individuals had surgery early in their life and the mutation appeared fully penetrant. The range of cataract phenotypes varied from nuclear to stellate or spoke-like cortical cataracts and also, sutural cataract, but this was the only eye phenotype reported. Subsequently there have been two Chinese family pedigrees reported. In one family from northern China, the E233Δ mutation caused a characteristic Y-shaped cataract at the lens sutures [56]. Loss in visual acuity required surgery for the affected individuals usually at an early age. Some phenotypic variability was noted, as one of the E233Δ carriers has not yet required surgery, but this is likely due in part to restricted location of the cataract.
In the other unrelated family from Southern China, the distinctive Y-shaped cataract at the lens sutures was again reported (Fig. 3), but this time it coincided with myopia [57]. The cataract usually started later in life after the first decade and progressed slowly resulting in the further development of punctuated cortical opacities that increased light scatter to the point that required surgery. Cataract developed more slowly in this family than the others that have been reported. One family member was found to have the E233Δ mutation (patient 31; [57]) but no loss in acuity despite presenting with a mild form of the Y-sutural cataract. Although three of the families examined here share the same E233 deletion mutation, there are differences in terms of age of onset and severity and corresponding loss of visual acuity. It is clear that as with mutations in other IF proteins, some phenotypic variation will occur as seen in other IF-based diseases [60]. For instance, the same R94C mutation in keratin 17 causes typical Pachyonychia Congenita type-2 (PC-2) nail dystrophy in one family, but steatocystoma multiplex is the only phenotype in affected members of another family [61]. The I451M mutation in desmin provides another example, where this mutation was identified in two families with different phenotypes. Patients in the family reported by Li et al. [62] had cardiomyopathy with no sign of skeletal myopathy, whereas patients reported in another family [63] had progressive skeletal myopathy without cardiac involvement. These observations suggest that both the environment and epigenetic factors will likely contribute to the phenotypes observed and disease progression [24].
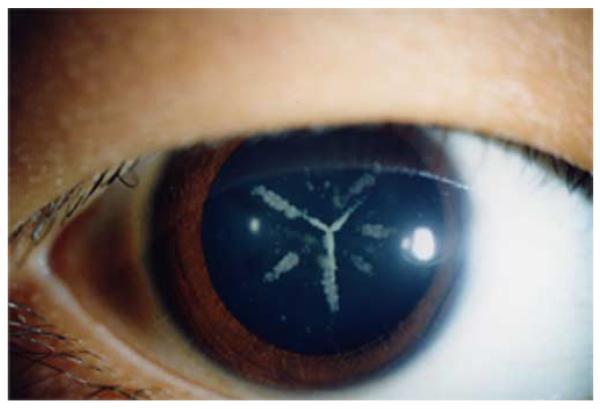
Figure 3.
Photograph showing the cataract found in a family with the E233Δ mutation. The photograph shows the typical Y appearance of the cataract that corresponds to the lens sutures – those places where the ends of individual lens fibre cells meet at the anterior and posterior parts of the lens (see [87] for an extensive review). This figure is reprinted from [57].
The first mutation in filensin (BFSP1) has now been reported and it is a recessive mutation caused by the deletion of exon 6 [64]. This introduces a premature stop codon after the addition of a novel hexapeptide sequence, but the introduction of this stop codon is expected to trigger nonsense-mediated decay of the mRNA, therefore effectively creating a filensin (BFSP1) knockout. In this family, homozygotes indeed presented with cortical cataracts in the first decade of life, but two heterozygote carriers (10, 16; [64]) presented with nuclear cataract at the age of 50. Whilst IF-related diseases caused by recessive mutation is considered to be rare, there have been a few cases of recessive keratin disease caused by homozygous mutations in keratin14 gene [65]. In one case, a homozygous deletion mutation led to a premature termination codon and complete loss of keratin14 expression presumably as a result of nonsense-mediated mRNA decay.
When these data are interpreted in conjunction with the mouse knockout studies, several observations emerge. The first is that filensin (BFSP1) and CP49 (BFSP2) are essential for lens function as described above. The second is that mutations in both genes can predispose individuals to cataract or other eye problems [58] such as age related (nuclear) cataract or even myopia. For instance, the E233 deletion mutation induced myopia in one family [57] concurs with the conclusion from one of the mouse CP49 (BFSP2) knockout studies [48]. Measurements of the back focal length of eyes from the CP49 (BFSP2) knockout animals indicated significant variation across the lens, which will likely distort the retinal image. The blurring of the image is not necessarily important; rather it is the relative spatial frequency composition of the image signal that reaches the retina that triggers myopia [66]. Cataract has more effect upon the shorter wavelength, higher energy light ([67] and discussion therein) and therefore mutations in either filensin (BFSP1) and CP49 (BFSP2) that interfere with the transmission of the visual signal through the lens by the deterioration of its optical properties, could potentially lead to myopia.
Filensin (BFSP1) and CP49 (BFSP2) mutations may also predispose individuals to nuclear cataract. For instance, two heterozygotic carriers of the filensin (BFSP1) exon 6 deletion presented with nuclear cataract later in life [64]. It is clearly worth following the other heterozygotes in this family to see if there is increased incidence of nuclear cataract in these individuals. Although there is a higher incidence (~3%) of nuclear cataract recorded for this part of India [68], it is still significant that two of the 19 family members presented with this type of cataract at a relatively early age [64]. By analogy, the lenses of the heterozygote filensin (BFSP1) knockout mice had more light scatter than lenses from wild type litter mates [46], indicating a deterioration in lens function for these heterozygotes. A similar observation was not made for the heterozygote CP49 (BFSP2) knockout mice, suggesting that the influence of filensin (BFSP1) and CP49 (BFSP2) is not equivalent in the lens. What accounts for this difference is unclear, but could be related to the levels of unpartnered filensin (BFSP1) and CP49 (BFSP2). Studies on the keratin pair 8 and 18 demonstrate how disparity in protein levels of one IF protein (e.g. K8) in a partnership can cause pathology [69-71], and perhaps the same is true for filensin (BFSP1).
Previous studies using mouse models of cataractogenesis suggest the level of IF expression plays an important role in normal lens development and differentiation. For instance, over-expression of vimentin in the lens of transgenic mice results in abnormal fibre cell elongation and extensive fibre cell degeneration, eventually leading to lens cataract [72, 73]. The level of expressed vimentin is proportional to the severity of the lens opacity and the age of onset. These observations raise the possibility that vimentin could be a candidate gene for lens cataract. Indeed, it has been reported that expression of mutant vimentin in mice induces cataract formation [74]. Other IF proteins such as keratins [75], nestin [76] and synemin [77] are also present during lens development. They are, therefore, also potential candidate genes for new cases of inherited cataract.
Then another feature of both the human [55-58, 64] and the murine [78] studies is that the lens phenotypes for both point mutations, the deletion mutation and the targeted knockouts in mice, were progressive. This illustrates that ageing of the lens is a contributory factor in the phenotype attributed to the filensin (BFSP1) and CP49 (BFSP2) mutations and gene knockouts and is an important feature.
Inherited human cataract caused by IF-associated proteins
Cataract is not only caused by mutations in the lens IF proteins filensin (BFSP1) and CP49 (BFSP2), but it is also caused by mutations in genes encoding IF-associated proteins (Table 3). For instance, mutations in the small heat shock protein chaperones, αA-and αB-crystallin also cause inherited human cataract. As molecular chaperones, they can prevent stress-induced protein aggregation [79] and in the lens, both proteins are intimately associated with the IF cytoskeleton [80, 81] in the form of α-crystallin, a natural complex of both αA- and αB-crystallin. These chaperones help maintain the individuality of IFs by controlling filament-filament interactions [82] and assist in the formation of IF networks in cells [83]. The functional importance of αB-crystallin in the lens has been demonstrated by the discovery of a number of mutations causing inherited human cataract (Table 3). The R120G mutation alters αB-crystallin-IF interactions, promoting inter-filament interactions [83], but it should be noted that not all mutations in αB-crystallin cause cataract. Outside the lens, αB-crystallin mutations also cause human muscular disorders, including myofibrillary myopathies [84], desmin-related myopathies [85] and dilated cardiomyopathy [86]. It remains to be shown that αA- and αB-crystallin mutations cause human cataract by inducing beaded filament aggregation and a loss of function in the lens.
Table 3.
Inherited human cataract caused by mutations in IF-associated proteins
| Disease | Mutation | Ref. |
|---|---|---|
| Autosomal dominant cataract | αA-Cry (R49C) | [88] |
| Congenital cataract | αA-Cry (G98R) | [89] |
| Autosomal dominant congenital cataract | αA-Cry (R116C) | [90] |
| Autosomal dominant posterior polar cataract | αB-Cry (P20S) | [91] |
| Cataract and desmin-related myopathy | αB-Cry (R120G) | [92] |
| Autosomal dominant congenital lamellar cataract | αB-Cry (D140N) | [93] |
| Dominant congenital posterior polar cataract | αB-Cry (450ΔA) | [94] |
| Dominant congenital cataract | Aquaporin 0 (missense) | [95] |
| Autosomal dominant congenital cataract | Aquaporin 0 (deletion) | [96] |
Conclusions and perspectives
The two lens-specific IF proteins filensin (BFSP1) and CP49 (BFSP2) have acquired a string of unique structural features that coincide with distinct assembly characteristics. Recent studies confirmed that filensin (BFSP1) and CP49 (BFSP2 are two key structural elements required for lens function (transparency, optical properties). The future elucidation of the precise functional roles that these proteins may play in the lens and the identification of interacting partners in the cytoplasm and at the plasma membrane are of fundamental importance to the understanding of lens fibre cell differentiation and the potential role that filensin (BFSP1) and CP49 (BFSP2) play in eye development and in cataractogenesis.
Acknowledgments
The financial support of the Welcome Trust and National Institute of Health is gratefully acknowledged.
Abbreviations
- IF
intermediate filament
- BFSP1 and 2
beaded filament structural protein 1 and 2
- CP49
cytoskeletal protein 49
- CP115
cytoskeletal protein 115
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graw J. The crystallins: genes, proteins and diseases. Biol Chem. 1997;378:1331–48. [PubMed] [Google Scholar]
- 2.Cvekl A, Piatigorsky J. Lens development and crystallin gene expression: many roles for Pax-6. Bioessays. 1996;18:621–30. doi: 10.1002/bies.950180805. [DOI] [PubMed] [Google Scholar]
- 3.Piatigorsky J. Lens differentiation in vertebrates. A review of cellular and molecular features. Differentiation. 1981;19:134–53. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 4.Sandilands A, Prescott AR, Carter JM, Hutcheson AM, Quinlan RA, Richards J, FitzGerald PG. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J Cell Sci. 1995;108(Pt 4):1397–406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]
- 5.Ramaekers FC, Dunia I, Dodemont HJ, Benedetti EL, Bloemendal H. Lenticular intermediate-sized filaments: biosynthesis and interaction with plasma membrane. Proc Natl Acad Sci U S A. 1982;79:3208–12. doi: 10.1073/pnas.79.10.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramaekers FC, Osborn M, Schimid E, Weber K, Bloemendal H, Franke WW. Identification of the cytoskeletal proteins in lens-forming cells, a special epitheloid cell type. Exp Cell Res. 1980;127:309–27. doi: 10.1016/0014-4827(80)90437-1. [DOI] [PubMed] [Google Scholar]
- 7.Ireland ME, Wallace P, Sandilands A, Poosch M, Kasper M, Graw J, Liu A, Maisel H, Prescott AR, Hutcheson AM, Goebel D, Quinlan RA. Up-regulation of novel intermediate filament proteins in primary fiber cells: an indicator of all vertebrate lens fiber differentiation? Anat Rec. 2000;258:25–33. doi: 10.1002/(SICI)1097-0185(20000101)258:1<25::AID-AR3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Maisel H, Perry MM. Electron microscope observations on some structural proteins of the chick lens. Exp Eye Res. 1972;14:7–12. doi: 10.1016/0014-4835(72)90136-4. [DOI] [PubMed] [Google Scholar]
- 9.Ireland M, Maisel H. Identification of native actin filaments in chick lens fiber cells. Exp Eye Res. 1983;36:531–6. doi: 10.1016/0014-4835(83)90046-5. [DOI] [PubMed] [Google Scholar]
- 10.Ireland M, Maisel H. A cytoskeletal protein unique to lens fiber cell differentiation. Exp Eye Res. 1984;38:637–45. doi: 10.1016/0014-4835(84)90182-9. [DOI] [PubMed] [Google Scholar]
- 11.FitzGerald PG, Gottlieb W. The Mr 115 kd fiber cell-specific protein is a component of the lens cytoskeleton. Curr Eye Res. 1989;8:801–11. doi: 10.3109/02713688909000870. [DOI] [PubMed] [Google Scholar]
- 12.Masaki S, Watanabe T. cDNA sequence analysis of CP94: rat lens fiber cell beaded-filament structural protein shows homology to cytokeratins. Biochem Biophys Res Commun. 1992;186:190–8. doi: 10.1016/s0006-291x(05)80792-2. [DOI] [PubMed] [Google Scholar]
- 13.Merdes A, Brunkener M, Horstmann H, Georgatos SD. Filensin: a new vimentin-binding, polymerization-competent, and membrane-associated protein of the lens fiber cell. J Cell Biol. 1991;115:397–410. doi: 10.1083/jcb.115.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gounari F, Merdes A, Quinlan R, Hess J, FitzGerald PG, Ouzounis CA, Georgatos SD. Bovine filensin possesses primary and secondary structure similarity to intermediate filament proteins. J Cell Biol. 1993;121:847–53. doi: 10.1083/jcb.121.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merdes A, Gounari F, Georgatos SD. The 47-kD lens-specific protein phakinin is a tailless intermediate filament protein and an assembly partner of filensin. J Cell Biol. 1993;123:1507–16. doi: 10.1083/jcb.123.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess JF, Casselman JT, FitzGerald PG. Gene structure and cDNA sequence identify the beaded filament protein CP49 as a highly divergent type I intermediate filament protein. J Biol Chem. 1996;271:6729–35. doi: 10.1074/jbc.271.12.6729. [DOI] [PubMed] [Google Scholar]
- 17.Sawada K, Agata J, Eguchi G, Quinlan R, Maisel H. The predicted structure of chick lens CP49 and a variant thereof, CP49ins, the first vertebrate cytoplasmic intermediate filament protein with a lamin-like insertion in helix 1B. Curr Eye Res. 1995;14:545–53. doi: 10.3109/02713689508998401. [DOI] [PubMed] [Google Scholar]
- 18.Remington SG. Chicken filensin: a lens fiber cell protein that exhibits sequence similarity to intermediate filament proteins. J Cell Sci. 1993;105(Pt 4):1057–68. doi: 10.1242/jcs.105.4.1057. [DOI] [PubMed] [Google Scholar]
- 19.Hess JF, Casselman JT, Kong AP, FitzGerald PG. Primary sequence, secondary structure, gene structure, and assembly properties suggests that the lens-specific cytoskeletal protein filensin represents a novel class of intermediate filament protein. Exp Eye Res. 1998;66:625–44. doi: 10.1006/exer.1998.0478. [DOI] [PubMed] [Google Scholar]
- 20.Binkley PA, Hess J, Casselman J, FitzGerald P. Unexpected variation in unique features of the lens-specific type I cytokeratin CP49. Invest Ophthalmol Vis Sci. 2002;43:225–35. [PubMed] [Google Scholar]
- 21.Quinlan RA, Carter JM, Sandilands A, Prescott AR. The beaded filament of the eye lens: an unexpected key to intermediate filament structure and function. Trends Cell Biol. 1996;6:123–6. doi: 10.1016/0962-8924(96)20001-7. [DOI] [PubMed] [Google Scholar]
- 22.Perng MD, Quinlan RA. Seeing is believing! The optical properties of the eye lens are dependent upon a functional intermediate filament cytoskeleton. Exp Cell Res. 2005;305:1–9. doi: 10.1016/j.yexcr.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Strelkov SV, Herrmann H, Aebi U. Molecular architecture of intermediate filaments. Bioessays. 2003;25:243–51. doi: 10.1002/bies.10246. [DOI] [PubMed] [Google Scholar]
- 24.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem. 2004;73:749–89. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- 26.Wallace P, Signer E, Paton IR, Burt D, Quinlan R. The chicken CP49 gene contains an extra exon compared to the human CP49 gene which identifies an important step in the evolution of the eye lens intermediate filament proteins. Gene. 1998;211:19–27. doi: 10.1016/s0378-1119(98)00117-6. [DOI] [PubMed] [Google Scholar]
- 27.Schaffeld M, Schultess J. Genes coding for intermediate filament proteins closely related to the hagfish “thread keratins (TK)” alpha and gamma also exist in lamprey, teleosts and amphibians. Exp Cell Res. 2006;312:1447–62. doi: 10.1016/j.yexcr.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Tomarev SI, Zinovieva RD, Piatigorsky J. Primary structure and lens-specific expression of genes for an intermediate filament protein and a beta-tubulin in cephalopods. Biochim Biophys Acta. 1993;1216:245–54. doi: 10.1016/0167-4781(93)90151-3. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–82. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- 30.Ott M. Visual accommodation in vertebrates: mechanisms, physiological response and stimuli. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:97–111. doi: 10.1007/s00359-005-0049-6. [DOI] [PubMed] [Google Scholar]
- 31.Carter JM, Hutcheson AM, Quinlan RA. In vitro studies on the assembly properties of the lens proteins CP49, CP115: coassembly with alpha-crystallin but not with vimentin. Exp Eye Res. 1995;60:181–92. doi: 10.1016/s0014-4835(95)80009-3. [DOI] [PubMed] [Google Scholar]
- 32.Quinlan RA, Moir RD, Stewart M. Expression in Escherichia coli of fragments of glial fibrillary acidic protein: characterization, assembly properties and paracrystal formation. J Cell Sci. 1989;93(Pt 1):71–83. doi: 10.1242/jcs.93.1.71. [DOI] [PubMed] [Google Scholar]
- 33.Kaufmann E, Weber K, Geisler N. Intermediate filament forming ability of desmin derivatives lacking either the amino-terminal 67 or the carboxy-terminal 27 residues. J Mol Biol. 1985;185:733–42. doi: 10.1016/0022-2836(85)90058-0. [DOI] [PubMed] [Google Scholar]
- 34.Coulombe PA, Chan YM, Albers K, Fuchs E. Deletions in epidermal keratins leading to alterations in filament organization in vivo and in intermediate filament assembly in vitro. J Cell Biol. 1990;111:3049–64. doi: 10.1083/jcb.111.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kouklis PD, Traub P, Georgatos SD. Involvement of the consensus sequence motif at coil 2b in the assembly and stability of vimentin filaments. J Cell Sci. 1992;102(Pt 1):31–41. doi: 10.1242/jcs.102.1.31. [DOI] [PubMed] [Google Scholar]
- 36.Rogers KR, Eckelt A, Nimmrich V, Janssen KP, Schliwa M, Herrmann H, Franke WW. Truncation mutagenesis of the non-alpha-helical carboxyterminal tail domain of vimentin reveals contributions to cellular localization but not to filament assembly. Eur J Cell Biol. 1995;66:136–50. [PubMed] [Google Scholar]
- 37.Quinlan RA. The soluble plasma membrane-cytoskeleton complexes and ageing in the lens. In: Vrensen GFJM, Clauwaert J, editors. Eye Lens Membrane and Ageing. Eurage; Leiden: 1991. [Google Scholar]
- 38.Goulielmos G, Remington S, Schwesinger F, Georgatos SD, Gounari F. Contributions of the structural domains of filensin in polymer formation and filament distribution. J Cell Sci. 1996;109(Pt 2):447–56. doi: 10.1242/jcs.109.2.447. [DOI] [PubMed] [Google Scholar]
- 39.Aebi U, Haner M, Troncoso J, Eichner R, Engel A. Unifying principles in intermediate filament (IF) structure and assembly. Protoplasma. 1988;145:73–81. [Google Scholar]
- 40.Troncoso JC, Haner M, March JL, Reichelt R, Engel A, Aebi U. Structure and assembly of specific NF combinations. In: Aebi U, Engel J, editors. Springer series in Biophysics. Springer-Verlag; Heildelberg: 1989. [Google Scholar]
- 41.Goulielmos G, Gounari F, Remington S, Muller S, Haner M, Aebi U, Georgatos SD. Filensin and phakinin form a novel type of beaded intermediate filaments and coassemble de novo in cultured cells. J Cell Biol. 1996;132:643–55. doi: 10.1083/jcb.132.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandilands A, Wang X, Hutcheson AM, James J, Prescott AR, Wegener A, Pekny M, Gong X, Quinlan RA. BFSP2 mutation found in mouse 129 strains causes the loss of CP49′ and induces vimentin-dependent changes in the lens fibre cell cytoskeleton. Exp Eye Res. 2004;78:875–89. doi: 10.1016/j.exer.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 43.FitzGerald PG, Casselman J. Immunologic conservation of the fiber cell beaded filament. Curr Eye Res. 1991;10:471–8. doi: 10.3109/02713689109001754. [DOI] [PubMed] [Google Scholar]
- 44.Georgatos SD, Gounari F, Goulielmos G, Aebi U. To bead or not to bead? Lens-specific intermediate filaments revisited. J Cell Sci. 1997;110(Pt 21):2629–34. doi: 10.1242/jcs.110.21.2629. [DOI] [PubMed] [Google Scholar]
- 45.Benedetti EL, Dunia I, Bentzel CJ, Vermorken AJ, Kibbelaar M, Bloemendal H. A portrait of plasma membrane specializations in eye lens epithelium and fibers. Biochim Biophys Acta. 1976;457:353–84. doi: 10.1016/0304-4157(76)90004-6. [DOI] [PubMed] [Google Scholar]
- 46.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Targeted deletion of the lens fiber cell-specific intermediate filament protein filensin. Invest Ophthalmol Vis Sci. 2003;44:5252–8. doi: 10.1167/iovs.03-0224. [DOI] [PubMed] [Google Scholar]
- 47.Alizadeh A, Clark JI, Seeberger T, Hess J, Blankenship T, Spicer A, FitzGerald PG. Targeted genomic deletion of the lens-specific intermediate filament protein CP49. Invest Ophthalmol Vis Sci. 2002;43:3722–7. [PubMed] [Google Scholar]
- 48.Sandilands A, Prescott AR, Wegener A, Zoltoski RK, Hutcheson AM, Masaki S, Kuszak JR, Quinlan RA. Knockout of the intermediate filament protein CP49 destabilises the lens fibre cell cytoskeleton and decreases lens optical quality, but does not induce cataract. Exp Eye Res. 2003;76:385–91. doi: 10.1016/s0014-4835(02)00330-5. [DOI] [PubMed] [Google Scholar]
- 49.Sandilands A, Prescott AR, Hutcheson AM, Quinlan RA, Casselman JT, FitzGerald PG. Filensin is proteolytically processed during lens fiber cell differentiation by multiple independent pathways. Eur J Cell Biol. 1995;67:238–53. [PubMed] [Google Scholar]
- 50.Fischer RS, Quinlan RA, Fowler VM. Tropomodulin binds to filensin intermediate filaments. FEBS Lett. 2003;547:228–32. doi: 10.1016/s0014-5793(03)00711-7. [DOI] [PubMed] [Google Scholar]
- 51.Rose K. M. Lindsey, Gourdie RG, Prescott AR, Quinlan RA, Crouch RK, Schey KL. The C terminus of lens aquaporin 0 interacts with the cytoskeletal proteins filensin and CP49. Invest Ophthalmol Vis Sci. 2006;47:1562–70. doi: 10.1167/iovs.05-1313. [DOI] [PubMed] [Google Scholar]
- 52.Brunkener M, Georgatos SD. Membrane-binding properties of filensin, a cytoskeletal protein of the lens fiber cells. J Cell Sci. 1992;103(Pt 3):709–18. doi: 10.1242/jcs.103.3.709. [DOI] [PubMed] [Google Scholar]
- 53.Alizadeh A, Clark J, Seeberger T, Hess J, Blankenship T, FitzGerald PG. Characterization of a mutation in the lens-specific CP49 in the 129 strain of mouse. Invest Ophthalmol Vis Sci. 2004;45:884–91. doi: 10.1167/iovs.03-0677. [DOI] [PubMed] [Google Scholar]
- 54.Simirskii VN, Lee RS, Wawrousek EF, Duncan MK. Inbred FVB/N mice are mutant at the cp49/BFSP2 locus and lack beaded filament proteins in the lens. Invest Ophthalmol Vis Sci. 2006;47:4931–4. doi: 10.1167/iovs.06-0423. [DOI] [PubMed] [Google Scholar]
- 55.Jakobs PM, Hess JF, FitzGerald PG, Kramer P, Weleber RG, Litt M. Autosomal-dominant congenital cataract associated with a deletion mutation in the human beaded filament protein gene BFSP2. Am J Hum Genet. 2000;66:1432–6. doi: 10.1086/302872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang L, Gao L, Li Z, Qin W, Gao W, Cui X, Feng G, Fu S, He L, Liu P. Progressive sutural cataract associated with a BFSP2 mutation in a Chinese family. Mol Vis. 2006;12:1626–31. [PubMed] [Google Scholar]
- 57.Zhang Q, Guo X, Xiao X, Yi J, Jia X, Hejtmancik JF. Clinical description and genome wide linkage study of Y-sutural cataract and myopia in a Chinese family. Mol Vis. 2004;10:890–900. [PubMed] [Google Scholar]
- 58.Conley YP, Erturk D, Keverline A, Mah TS, Keravala A, Barnes LR, Bruchis A, Hess JF, FitzGerald PG, Weeks DE, Ferrell RE, Gorin MB. A juvenile-onset, progressive cataract locus on chromosome 3q21-q22 is associated with a missense mutation in the beaded filament structural protein-2. Am J Hum Genet. 2000;66:1426–31. doi: 10.1086/302871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dibb NJ, Maruyama IN, Krause M, Karn J. Sequence analysis of the complete Caenorhabditis elegans myosin heavy chain gene family. J Mol Biol. 1989;205:603–13. doi: 10.1016/0022-2836(89)90229-5. [DOI] [PubMed] [Google Scholar]
- 60.Corden LD, McLean WH. Human keratin diseases: hereditary fragility of specific epithelial tissues. Exp Dermatol. 1996;5:297–307. doi: 10.1111/j.1600-0625.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 61.Covello SP, Smith FJ, Smitt J. H. Sillevis, Paller AS, Munro CS, Jonkman MF, Uitto J, McLean WH. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475–80. doi: 10.1046/j.1365-2133.1998.02413.x. [DOI] [PubMed] [Google Scholar]
- 62.Li D, Tapscoft T, Gonzalez O, Burch PE, Quinones MA, Zoghbi WA, Hill R, Bachinski LL, Mann DL, Roberts R. Desmin mutation responsible for idiopathic dilated cardiomyopathy. Circulation. 1999;100:461–4. doi: 10.1161/01.cir.100.5.461. [DOI] [PubMed] [Google Scholar]
- 63.Dalakas MC, Dagvadorj A, Goudeau B, Park KY, Takeda K, Simon-Casteras M, Vasconcelos O, Sambuughin N, Shatunov A, Nagle JW, Sivakumar K, Vicart P, Goldfarb LG. Progressive skeletal myopathy, a phenotypic variant of desmin myopathy associated with desmin mutations. Neuromuscul Disord. 2003;13:252–8. doi: 10.1016/s0960-8966(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 64.Ramachandran RD, Perumalsamy V, Hejtmancik JF. Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet. 2007 doi: 10.1007/s00439-006-0319-6. [DOI] [PubMed] [Google Scholar]
- 65.Lanschuetzer CM, Klausegger A, Pohla-Gubo G, Hametner R, Richard G, Uitto J, Hintner H, Bauer JW. A novel homozygous nonsense deletion/insertion mutation in the keratin 14 gene (Y248X; 744delC/insAG) causes recessive epidermolysis bullosa simplex type Kobner. Clin Exp Dermatol. 2003;28:77–9. doi: 10.1046/j.1365-2230.2003.01218.x. [DOI] [PubMed] [Google Scholar]
- 66.Hess RF, Schmid KL, Dumoulin SO, Field DJ, Brinkworth DR. What image properties regulate eye growth? Curr Biol. 2006;16:687–91. doi: 10.1016/j.cub.2006.02.065. [DOI] [PubMed] [Google Scholar]
- 67.Pigaga V, Quinlan RA. Lenticular chaperones suppress the aggregation of the cataract-causing mutant T5P gamma C-crystallin. Exp Cell Res. 2006;312:51–62. doi: 10.1016/j.yexcr.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 68.Nirmalan PK, Krishnadas R, Ramakrishnan R, Thulasiraj RD, Katz J, Tielsch JM, Robin AL. Lens opacities in a rural population of southern India: the Aravind Comprehensive Eye Study. Invest Ophthalmol Vis Sci. 2003;44:4639–43. doi: 10.1167/iovs.03-0011. [DOI] [PubMed] [Google Scholar]
- 69.Nakamichi I, Toivola DM, Strnad P, Michie SA, Oshima RG, Baribault H, Omary MB. Keratin 8 overexpression promotes mouse Mallory body formation. J Cell Biol. 2005;171:931–7. doi: 10.1083/jcb.200507093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamichi I, Hatakeyama S, Nakayama KI. Formation of Mallory body-like inclusions and cell death induced by deregulated expression of keratin 18. Mol Biol Cell. 2002;13:3441–51. doi: 10.1091/mbc.01-10-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magin TM. Lessons from keratin transgenic and knockout mice. Subcell Biochem. 1998;31:141–72. [PubMed] [Google Scholar]
- 72.Capetanaki Y, Smith S, Heath JP. Overexpression of the vimentin gene in transgenic mice inhibits normal lens cell differentiation. J Cell Biol. 1989;109:1653–64. doi: 10.1083/jcb.109.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Capetanaki Y, Starnes S, Smith S. Expression of the chicken vimentin gene in transgenic mice: efficient assembly of the avian protein into the cytoskeleton. Proc Natl Acad Sci U S A. 1989;86:4882–6. doi: 10.1073/pnas.86.13.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meier-Borheim R, Bussow H, Brohl D, Loch S, Magin TM. Cataract formation upon expression of mutant vimentin in mice. Eur J Cell Biol. 2005;84:100–101. (meeting abstract) [Google Scholar]
- 75.Kasper M, Viebahn C. Cytokeratin expression and early lens development. Anat Embryol (Berl) 1992;186:285–90. doi: 10.1007/BF00174151. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Bian W, Gao X, Chen L, Jing N. Nestin expression during mouse eye and lens development. Mech Dev. 2000;94:287–91. doi: 10.1016/s0925-4773(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 77.Tawk M, Titeux M, Fallet C, Li Z, Daumas-Duport C, Cavalcante LA, Paulin D, Moura-Neto V. Synemin expression in developing normal and pathological human retina and lens. Exp Neurol. 2003;183:499–507. doi: 10.1016/s0014-4886(03)00240-1. [DOI] [PubMed] [Google Scholar]
- 78.Crum AV, Seeberger TM, Alizadeh A, Fitzgerald PG, Clark JI. Structural Proteins, Mouse Models and Human Aging Cataract. Invest. Ophthalmol. Vis. Sci. 2004;45:2661. (abstract) [Google Scholar]
- 79.Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992;89:10449–53. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. Embo J. 1994;13:945–53. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.FitzGerald PG, Graham D. Ultrastructural localization of alpha A-crystallin to the bovine lens fiber cell cytoskeleton. Curr Eye Res. 1991;10:417–36. doi: 10.3109/02713689109001750. [DOI] [PubMed] [Google Scholar]
- 82.Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- 83.Perng MD, Wen SF, van den IP, Prescott AR, Quinlan RA. Desmin aggregate formation by R120G alphaB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol Biol Cell. 2004;15:2335–46. doi: 10.1091/mbc.E03-12-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54:804–10. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- 85.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–5. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 86.Inagaki N, Hayashi T, Arimura T, Koga Y, Takahashi M, Shibata H, Teraoka K, Chikamori T, Yamashina A, Kimura A. Alpha B-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;342:379–86. doi: 10.1016/j.bbrc.2006.01.154. [DOI] [PubMed] [Google Scholar]
- 87.Kuszak JR, Zoltoski RK, Tiedemann CE. Development of lens sutures. Int J Dev Biol. 2004;48:889–902. doi: 10.1387/ijdb.041880jk. [DOI] [PubMed] [Google Scholar]
- 88.Mackay DS, Andley UP, Shiels A. Cell death triggered by a novel mutation in the alphaA-crystallin gene underlies autosomal dominant cataract linked to chromosome 21q. Eur J Hum Genet. 2003;11:784–93. doi: 10.1038/sj.ejhg.5201046. [DOI] [PubMed] [Google Scholar]
- 89.Santhiya ST, Soker T, Klopp N, Illig T, Prakash MV, Selvaraj B, Gopinath PM, Graw J. Identification of a novel, putative cataract-causing allele in CRYAA (G98R) in an Indian family. Mol Vis. 2006;12:768–73. [PubMed] [Google Scholar]
- 90.Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7:471–4. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- 91.Liu M, Ke T, Wang Z, Yang Q, Chang W, Jiang F, Tang Z, Li H, Ren X, Wang X, Wang T, Li Q, Yang J, Liu J, Wang QK. Identification of a CRYAB mutation associated with autosomal dominant posterior polar cataract in a Chinese family. Invest Ophthalmol Vis Sci. 2006;47:3461–6. doi: 10.1167/iovs.05-1438. [DOI] [PubMed] [Google Scholar]
- 92.Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20:92–5. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- 93.Liu Y, Zhang X, Luo L, Wu M, Zeng R, Cheng G, Hu B, Liu B, Liang JJ, Shang F. A novel alphaB-crystallin mutation associated with autosomal dominant congenital lamellar cataract. Invest Ophthalmol Vis Sci. 2006;47:1069–75. doi: 10.1167/iovs.05-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berry V, Francis P, Reddy MA, Collyer D, Vithana E, MacKay I, Dawson G, Carey AH, Moore A, Bhattacharya SS, Quinlan RA. Alpha-B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet. 2001;69:1141–5. doi: 10.1086/324158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Francis P, Chung JJ, Yasui M, Berry V, Moore A, Wyatt MK, Wistow G, Bhattacharya SS, Agre P. Functional impairment of lens aquaporin in two families with dominantly inherited cataracts. Hum Mol Genet. 2000;9:2329–34. doi: 10.1093/oxfordjournals.hmg.a018925. [DOI] [PubMed] [Google Scholar]
- 96.Geyer DD, Spence MA, Johannes M, Flodman P, Clancy KP, Berry R, Sparkes RS, Jonsen MD, Isenberg SJ, Bateman JB. Novel single-base deletional mutation in major intrinsic protein (MIP) in autosomal dominant cataract. Am J Ophthalmol. 2006;141:761–3. doi: 10.1016/j.ajo.2005.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]