Abstract
Background: This study was conducted to confirm the 24-hour bronchodilator efficacy and pharmacokinetic profile of once-daily tiotropium Respimat® 5 μg add-on to inhaled corticosteroids (ICS) in adults with symptomatic asthma. It used a trial protocol designed to minimize the risk of pharmacokinetic sample contamination resulting from improper sampling procedure, sample handling, or device handling during priming and subsequent inhalation procedure.
Methods: A Phase II, randomized, double-blind, two-way crossover study (NCT01696071) comparing two daily dosing regimens of tiotropium for 4 weeks, once-daily 5 μg (evening dosing) or twice-daily 2.5 μg (morning and evening dosing), as add-on to maintenance therapy with ICS (400–800 μg budesonide or equivalent) as controller medication. There was no washout between treatment periods.
Results: An increase in the area under the curve of the 24-hour forced expiratory volume in 1 second profile from study baseline was observed following once-daily tiotropium 5 μg (217 mL) and twice-daily 2.5 μg (219 mL), with no difference between the two regimens (−2 mL [95% confidence interval: −38, 34]). In a subset of the study population, total tiotropium exposure, expressed as area under the plasma concentration versus time curve over 24 hours, was comparable between dosing regimens. Unexpected tiotropium plasma levels were observed in two patients, possibly because of contamination.
Conclusions: The observed bronchodilator efficacy over 24 hours was similar with once-daily tiotropium 5 μg and twice-daily 2.5 μg as add-on to ICS therapy, supporting the suitability of once-daily dosing to provide sustained improvements in lung function in adults with symptomatic asthma.
Keywords: : asthma, blood-sampling contamination, pharmacodynamics, pharmacokinetics, tiotropium, Respimat®
Introduction
Despite current treatment recommendations with inhaled corticosteroids (ICS) or ICS with long-acting β2-agonists, about half of asthma patients are classified as having poorly controlled disease.(1–3) Poorly controlled asthma places a burden on healthcare systems, with healthcare costs rising with increasing asthma severity and the occurrence of asthma exacerbations.(4,5) Patients with uncontrolled asthma also suffer from poorer health-related quality of life, with decreased work productivity(6) and increased risk of anxiety and depression.(7)
Tiotropium, a long-acting anticholinergic bronchodilator, has recently undergone investigation as an add-on maintenance therapy in patients with symptomatic asthma. Kerstjens and colleagues have shown that once-daily tiotropium 5 μg, delivered via the Respimat® Soft Mist™ inhaler (Boehringer Ingelheim, Ingelheim am Rhein, Germany) as add-on to ICS and long-acting β2-agonists, significantly increased the time to the first severe exacerbation and provided modest sustained bronchodilation in adult patients with severe symptomatic asthma.(8) Similarly, once-daily tiotropium 5 μg and 2.5 μg, as add-on to ICS, both provided improvements in lung function and asthma control, comparable with twice-daily salmeterol 50 μg, in patients with moderate symptomatic asthma.(9)
The potential effect of the daily dosing regimen of tiotropium on improvements in lung function was addressed in a Phase II study evaluating and comparing the 24-hour forced expiratory volume in 1 second (FEV1) profiles following two different dosing regimens in patients with symptomatic asthma.(10) In each treatment period of the two-way crossover study, patients received either once-daily tiotropium 5 μg administered in the evening with matching placebo Respimat® inhaler in the morning, or tiotropium 2.5 μg administered in the morning and evening. Improvements in lung function were sustained and similar for both daily dosing regimens, supporting tiotropium as a once-daily bronchodilator in patients with symptomatic asthma.
However, the pharmacokinetic (PK) assessments revealed tiotropium plasma levels in some samples, which was not expected taking into account the dosing regimen of the study drug, and was therefore considered to be caused by contamination. Contamination itself can be caused by a variety of factors, including laboratory errors during the PK blood-sampling procedure and sample handling, or improper device handling during priming and the subsequent inhalation procedure.(11) While it was concluded that the contamination did not affect the robustness of the PK or efficacy outcomes, it cannot be ruled out that unexpected tiotropium levels in plasma samples could be caused by randomization errors with, for example, twice-daily inhalation from the same evening device delivering a 5 μg dose instead of one inhalation of tiotropium 5 μg in the evening and one inhalation from the placebo inhaler in the morning.
Although contamination of PK blood and urine samples with inhaled drugs has not been widely studied, it has been reported in a number of trials in other therapy areas, with sources of contamination including personal contamination from the research staff (contamination from human hair)(12) or because of certain laboratory practices (ethylenediaminetetraacetic acid contamination of blood sample)(13) or clinical practices (contamination of blood samples with testosterone-containing gels).(14) Particles of some inhaled therapeutic agents, such as salbutamol sulfate, ipratropium bromide, and fluticasone propionate, are known for their tendency to agglomerate and adhere to many surfaces(15–17) and could represent a source of sample contamination in laboratory settings. It is therefore important that preventative steps are taken at all stages of a study that includes PK assessments, from drug administration to blood sampling and analysis,(18) to avoid the detection of a study drug in placebo samples which would jeopardize the validity of subsequent analyses.
The objective of the present study was to confirm the 24-hour FEV1 and PK profiles of once-daily tiotropium 5 μg and twice-daily 2.5 μg observed in a previous Phase II, randomized, double-blind, placebo-controlled, three-way crossover study,(10) using a trial protocol designed to reduce errors caused by improper use of the inhaler (i.e., switching between morning and evening inhalers) and introducing precautionary steps to minimize the risk of contamination.
Materials and Methods
Study design
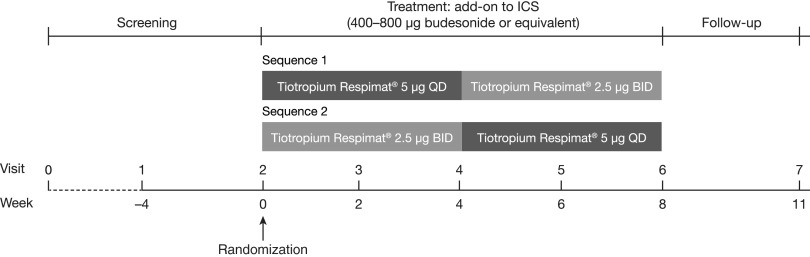
This was a Phase II, randomized, double-blind, two-way crossover study (ClinicalTrials.gov identifier: NCT01696071) conducted in 22 sites in Austria, Germany, Hungary, and Slovenia. Patients entered a 28-day screening period, after which eligible patients were randomized to two 4-week treatment periods, with no washout between treatments, and with a 21-day follow-up period (Fig. 1). The study was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation for Good Clinical Practice Guidelines. The patient information sheet and consent form were reviewed and approved by each participating institution's review board. Before participation in the study, written, informed consent was obtained from all individual participants included in the study.
FIG. 1.
Study design. Sequence 1: tiotropium 5 μg administered once daily in the evening and placebo in the morning for 4 weeks, followed by tiotropium 2.5 μg administered in the morning and evening for 4 weeks. Sequence 2: tiotropium 2.5 μg administered twice daily for 4 weeks followed by tiotropium 5 μg administered once daily for 4 weeks. Patients (n = 98) were randomized 1:1 to sequence 1 or sequence 2. BID, twice daily; ICS, inhaled corticosteroids; QD, once daily.
Study treatment
Tiotropium and placebo were both delivered using the Respimat® Soft Mist™ inhaler; each treatment was delivered via two inhalations, irrespective of dose. There were two treatment sequences, and each patient was randomly assigned to sequence 1 (tiotropium 5 μg administered once daily in the evening and placebo in the morning for 4 weeks, followed by tiotropium 2.5 μg administered in the morning and evening for 4 weeks) or sequence 2 (tiotropium 2.5 μg administered twice daily for 4 weeks, followed by tiotropium 5 μg administered once daily for 4 weeks). Patients continued maintenance treatment with ICS (a daily dose of 400–800 μg budesonide or equivalent) throughout the study.
To reduce the potential risks of switching inhalers between morning and evening administrations, simplified labeling for the medication kit was introduced so that inhalers intended for morning use were labeled with a sun symbol and a yellow label, while those for evening use were labeled with a moon symbol and a blue label.
Medications permitted for the treatment of acute asthma exacerbations during the study included salbutamol (albuterol) metered-dose inhaler as needed (with ≥8-hour washout before all visits, including lung function tests), a temporary increase in the dose of ICS or the addition of a systemic steroid, and a short-acting theophylline.
Non-permitted maintenance medications after screening (Visit 1) included systemic steroids, long-acting β2-agonists, anticholinergics other than the study drug, methylxanthines, anti-immunoglobulin E treatment, leukotriene receptor antagonists, and cromones.
Study population
The study population included patients with similar baseline characteristics, as previously described,(10) but using a different cohort of patients. Inclusion criteria included male and female patients aged 18–75 years who had at least a 3-month documented history of asthma, with an initial confirmed diagnosis before the age of 40 years, and treated with stable-dose ICS (400–800 μg budesonide or equivalent) for at least 4 weeks before screening. Patients had to have a pre-bronchodilator FEV1 of 60%–90% of predicted normal at screening, and bronchodilator reversibility defined as an FEV1 increase of ≥12% and ≥200 mL 10 min before and 15–30 min after inhalation of 400 μg salbutamol. Patients were required never to have smoked, or had to be ex-smokers with a history of <10 pack-years who stopped smoking ≥1 year before enrollment. All patients had to be symptomatic at screening and randomization, as defined by a seven-question Asthma Control Questionnaire mean score of ≥1.5. Major exclusion criteria included significant disease other than asthma (e.g., chronic obstructive pulmonary disease).
Study end points
All FEV1-based end points were analyzed as responses, defined as the change from study baseline at randomization before inhalation of the first dose of study drug.
The primary efficacy end point was FEV1 area under the curve (AUC) from 0 to 24 hours (FEV1 AUC(0–24h)), measured after inhalation of the last evening dose at the end of each 4-week period of randomized treatment. FEV1 AUC(0–24h) was defined as the mean FEV1 over the 24-hour observation period (0–24 hours) normalized for time after inhalation of the last evening dose of study drug and calculated using the trapezoidal rule divided by the corresponding duration (i.e., 24 hours) to provide results expressed in liters. FEV1 AUC(0–24h) response was defined as the change in FEV1 AUC(0–24h) from the common study baseline FEV1 value, which was the measurement obtained before the first evening dose of randomized study drug.
Secondary lung function end points included peak FEV1 measured within 24 hours of the last evening inhalation at the end of each 4-week period, FEV1 AUC from 0 to 12 hours (FEV1 AUC(0–12h)), FEV1 AUC from 12 to 24 hours (FEV1 AUC(12–24h)), and trough FEV1, measured 10 min before the last evening inhalation at the end of each 4-week period. Pre-dose morning and evening peak expiratory flow was evaluated based on the weekly mean of the last week of each 4-week treatment period and was measured by patients at home using the AM3® device (eResearch Technology GmbH, Estenfeld, Germany).
In a subset of 35 patients who had been randomized to both dosing regimens, PK end points included maximum concentration at steady state in plasma (morning and evening separately for twice-daily administration), area under the tiotropium plasma concentration–time curve at steady state, time from dosing to maximum plasma concentration, and amount of drug eliminated unchanged in urine over a 24-hour period.
Study assessments
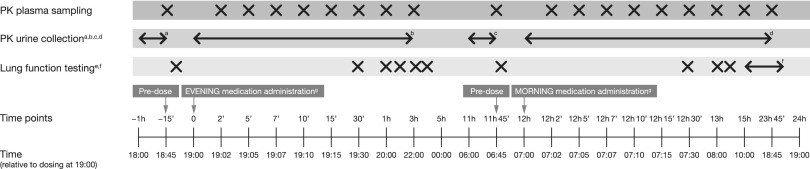
Spirometry (MasterScope® CT; eResearch Technology GmbH) was conducted at all clinic visits. Qualifying lung function testing was conducted at screening, and FEV1, forced vital capacity, and peak expiratory flow were performed at randomization before inhalation of the first evening dose of study drug. At Visits 4 and 6 (Fig. 2), lung function testing started at approximately the same time of day (± 30 min), with visits scheduled to enable the start of the evening pre-dose tests between the hours of 17:50 and 19:50. At each time point, spirometric maneuvers were conducted in triplicate. The highest FEV1 from an acceptable maneuver was recorded, along with the time.
FIG. 2.
Pharmacokinetic procedures and lung function testing at Visits 4 and 6. Pharmacokinetic subset (n = 35). aPre-dose urine sample obtained from urine collected in the last hour before study drug administration. bAll urine voided during the 0- to 6-hour post-dose interval. cAll urine voided during the 6- to 12-hour post-dose interval. dAll urine voided during the 12- to 24-hour post-dose interval. eForced expiratory volume in 1 second, forced vital capacity, and peak expiratory flow completed using the MasterScope® CT. fLung function testing performed at 10:00, 11:00, 13:00, 15:00, 17:00, 18:00, and 18:50. gAdministration of a patient's own inhaled corticosteroids according to his/her regular dosing. PK, pharmacokinetics.
Lung function tests started 10 min before evening dosing of the study drug at the end of each 4-week treatment period (Visits 4 and 6) (Fig. 1). Subsequently, lung function tests were performed at 0.5, 1, 2, 3, 4, and 11.8 hours after inhalation of the last evening dose of study drug. Morning dosing was performed 12 hours after evening dosing, and lung function assessments were continued at 12.5, 13, 14, 15, 16, 18, 20, 22, 23, and 23.8 hours after evening dosing. At Visits 3 and 5 (Weeks 2 and 6, respectively) (Fig. 1), patients were followed up to record study drug adherence, concomitant medications, and adverse events.
Patients performed twice-daily peak expiratory flow measurements at home at approximately the same time of the day using the AM3® device. Morning and evening peak expiratory flow measurements were performed before inhalation of the maintenance ICS dose and study drug. Patients performed three maneuvers with the AM3® device while standing, and the highest value was used for evaluation.
For PK analysis, blood samples were collected at Visits 4 and 6 (steady state) 15 min before administration of the evening dose of study drug (self-administered in the inhalation room between the hours of 18:00 and 20:00) and after 2, 5, 7, 10, 15, and 30 min, and then 1, 3, and 11.75 hours. This was followed by administration of the morning dose (self-administered in the inhalation room between 06:00 and 08:00, 12 h after administration of the evening dose ±5 min), with subsequent blood sampling carried out after 12 h + 2, 5, 7, 10, 15, and 30 min, and after 13, 15, and 23.75 h relative to the evening dose. Urine collected pre-dosing (−1 hour to 0) and all urine voided post-dosing were collected over 24 h post-dose (Fig. 2). A protocol detailing the preventive measures taken to avoid contamination of plasma and urine samples was followed (see Supplementary Table 1; supplementary material is available online at www.liebertpub.com/jamp).
Plasma and urine concentrations of tiotropium were determined by a validated assay using high-performance liquid chromatography coupled to tandem mass spectrometry using electrospray ionization in the positive-ion mode, performed at NUVISAN GmbH, Neu-Ulm, Germany. Safety and tolerability were assessed by the occurrence of adverse events.
Pharmacokinetic analyses
Non-compartmental PK analyses were carried out using a validated software program, WinNonlin™ (Professional Version 5.2; Pharsight Corporation, Mountain View, CA, USA). AUC values were calculated using the linear up/log down algorithm.
Statistical analyses
Efficacy data are reported for the full analysis set, defined as all treated patients who had baseline data and at least one on-treatment efficacy measurement after 4 weeks of treatment within a treatment period. The PK subset was defined as all patients who provided samples for the evaluation of tiotropium PK in blood and urine. Safety data are reported for the treated set, defined as all randomized patients who received at least one dose of study drug.
Assuming a standard deviation of 225 mL for within-patient difference of FEV1 AUC(0–24h), a sample size of 92 randomized patients to obtain 82 completed patients was needed for a full crossover design to detect a mean difference between the two active treatments within −50 mL, 50 mL with a probability of 95%. AUC was calculated by using the trapezoidal rule divided by the observation time (24 hours). AUC values were also calculated relative to the evening dosing, with AUC(0–12h) referring to the night-time and AUC(12–24h) referring to the daytime.
No hypothesis testing was planned, and the treatment comparison was exploratory only. The statistical model included ‘treatment’ and ‘period’ as fixed effects and ‘patient’ as a random effect. ‘Study baseline’ for FEV1, defined as pre-treatment values measured at the randomization visit in the evening, was included as a covariate, which was accomplished by using compound symmetry covariance structure for within-patient variation.
For morning and evening peak expiratory flow, only the assessments of the last week of each 4-week treatment period were evaluated. Baseline was defined as the mean of the values obtained during the last week of the 28-day screening period before randomization.
Descriptive statistics were calculated for tiotropium concentrations in plasma and urine as well as PK parameters. Mean PK profiles are presented as geometric means, using the planned blood-sampling time.
Safety and tolerability, assessed by an analysis of adverse events, were descriptive only.
Results
Baseline demographics, disease characteristics, and disposition
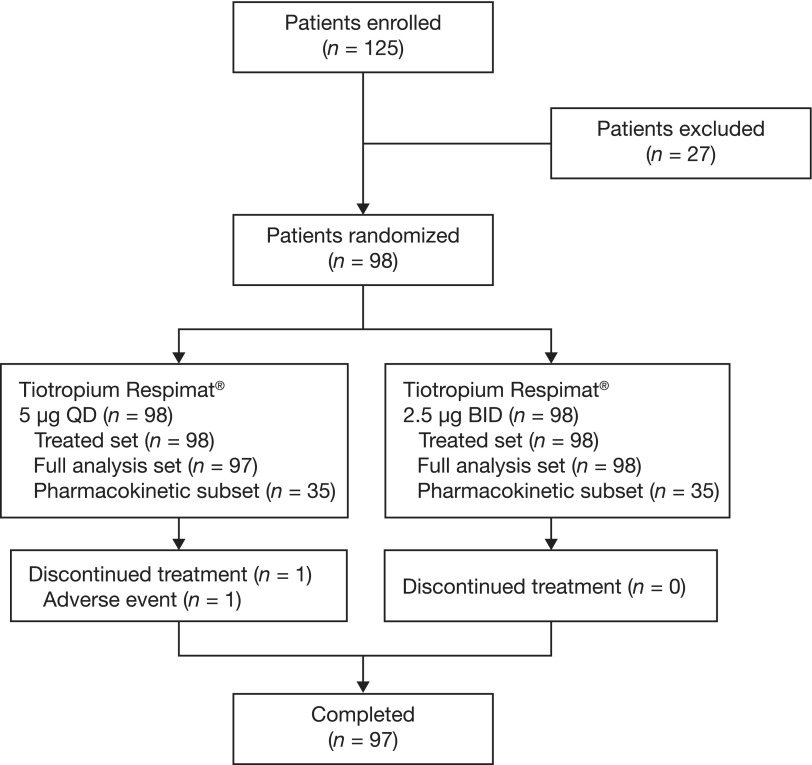
A total of 98 patients were randomized, and all patients were included in the full analysis set. One patient was withdrawn from the study before entering the second 4-week period (once-daily 5 μg period) due to cervical carcinoma, meaning no data were available (Fig. 3). A subset of 35 patients was included in the PK analyses.
FIG. 3.
CONSORT diagram. BID, twice daily; QD, once daily.
Overall, the majority of patients were male (55.1%), with a mean age of 43.8 years and a mean duration of asthma of 20.2 years, and 69.4% of patients had never smoked (Table 1). In the 30.6% of patients who were ex-smokers, the mean number of pack-years was 4.8. During the treatment period, all patients continued their ICS medication: budesonide (48.0% of the patients) and fluticasone (28.6% of the patients) were the most frequently used (mean budesonide or equivalent dose ± standard deviation at randomization: 661 ± 252 μg).
Table 1.
Baseline Patient Demographics and Disease Characteristics
| Total (N = 98) | |
|---|---|
| Age, yearsa | 43.8 ± 12.1 |
| Sex, n (%) | |
| Male | 54 (55.1) |
| Female | 44 (44.9) |
| Body mass index, kg/m2 a | 27.7 ± 4.8 |
| Smoking status | |
| Never smoked, n (%) | 68 (69.4) |
| Ex-smoker, n (%) | 30 (30.6) |
| Pack-yearsa | 4.8 ± 2.7 |
| Duration of asthma, yearsa | 20.2 ± 12.6 |
| Pre-bronchodilator FEV1 at screeninga,b | |
| Actual (mL) | 2518 ± 660 |
| % of predicted | 73.7 ± 8.6 |
| Pre-bronchodilator FEV1 at randomization (study baseline)a,c | |
| Actual (mL) | 2634 ± 773 |
| % of predicted | 76.7 ± 11.7 |
| Pre-bronchodilator FVC at randomization (study baseline)a,c | |
| Actual (mL) | 3964 ± 1027 |
| % of predicted | 97.0 ± 12.3 |
| ACQ-7 scorea,d | 2.5 ± 0.7 |
| ICS dose of stable maintenance treatment, μga,e | 661 ± 252 |
Treated set.
Values are mean ± standard deviation. bVisit 1 (screening), measured 10–15 min after inhalation of four puffs of salbutamol (100 μg per actuation). cVisit 2 (randomization), measured 10 min before inhalation of the first dose of study drug. dACQ-7 questions 1–6 were self-administered by patients and preceded any discussion with a healthcare professional. ACQ-7 question 7 was completed after pre-dose MasterScope® spirometry. eBudesonide or equivalent dose.
ACQ-7, seven-question Asthma Control Questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroids.
Efficacy
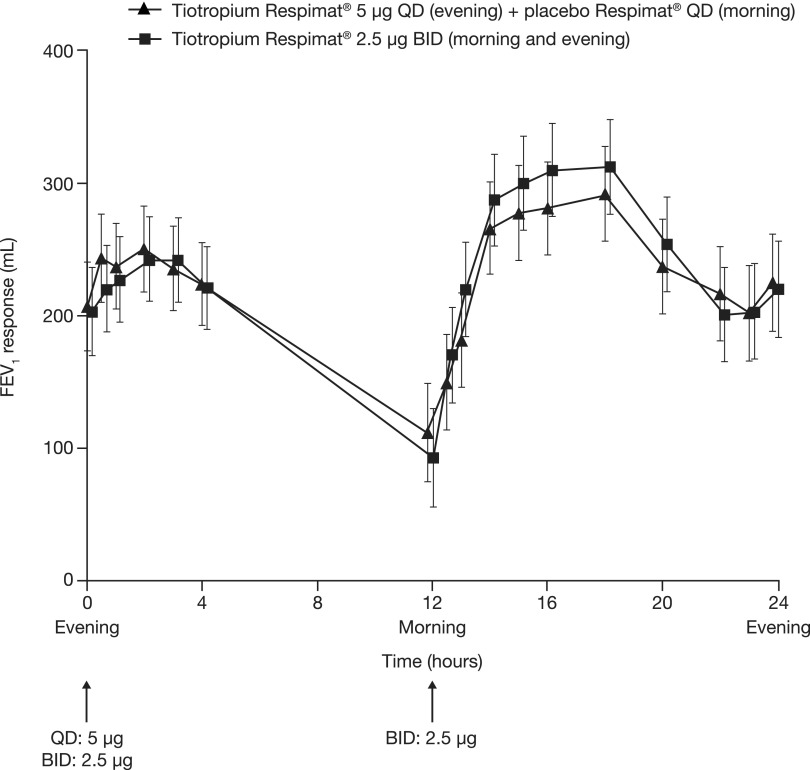
Both daily tiotropium dosing regimens provided an improvement in FEV1 from study baseline. The adjusted mean (standard error) FEV1 AUC(0–24h) response was 217 (31) mL following once-daily tiotropium 5 μg and 219 (31) mL following twice-daily 2.5 μg. No difference was found between the two daily dosing regimens: mean (standard error) was −2 (18) mL (95% confidence interval: −38, 34) (Table 2, Fig. 4).
Table 2.
Fev1 Auc(0–24h) Response, Secondary Fev1-based End Point Responses, and Mean Morning and Evening PEF Responses (Last Week) at the End of the Two 4-week Treatment Periods
| Adjusted differenceabetween treatments | |||
|---|---|---|---|
| Treatment and parameter | Adjusted mean responsea ± SE | Mean ± SE | 95% CI |
| FEV1 AUC(0–24h), mL | |||
| Tiotropium Respimat® 5 μg QD (n = 97) | 217 ± 31 | −2 ± 18 | −38, 34 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 219 ± 31 | ||
| FEV1 AUC(0–12h), mL | |||
| Tiotropium Respimat® 5 μg QD (n = 97) | 192 ± 31 | 10 ± 21 | −32, 52 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 182 ± 31 | ||
| FEV1 AUC(12–24h), mL | |||
| Tiotropium Respimat® 5 μg QD (n = 97) | 243 ± 33 | −14 ± 18 | −50, 22 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 256 ± 33 | ||
| Peak FEV1(0–24h), mL | |||
| Tiotropium Respimat® 5 μg QD (n = 97) | 451 ± 31 | −14 ± 18 | −51, 23 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 465 ± 31 | ||
| Trough FEV1, mL | |||
| Tiotropium Respimat® 5 μg QD (n = 97) | 207 ± 34 | 4 ± 32 | −60, 68 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 203 ± 33 | ||
| Morning PEFb, L/min | |||
| Tiotropium Respimat® 5 μg QD (n = 96) | 33 ± 6 | 2 ± 4 | −6, 11 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 31 ± 6 | ||
| Evening PEFb, L/min | |||
| Tiotropium Respimat® 5 μg QD (n = 96) | 34 ± 6 | −0.3 ± 4 | −8, 8 |
| Tiotropium Respimat® 2.5 μg BID (n = 98) | 34 ± 6 | ||
Full analysis set.
Adjusted for treatment, period, patient, and baseline. bBased on the last week before randomization.
Common baseline mean ± standard deviation: FEV1 = 2634 ± 773 mL. Common baseline mean ± standard deviation: morning PEF = 389 ± 121 L/min; evening PEF = 402 ± 123 L/min.
BID, twice daily; CI, confidence interval; FEV1, forced expiratory volume in 1 second; FEV1 AUC(0–12h), forced expiratory volume in 1 second area under the curve from 0 to 12 hours (night-time period); FEV1 AUC(0–24h), forced expiratory volume in 1 second area under the curve from 0 to 24 hours; FEV1 AUC(12–24h), forced expiratory volume in 1 second area under the curve from 12 to 24 hours (daytime period); PEF, peak exploratory flow; QD, once daily; SE, standard error.
FIG. 4.
FEV1 response over 24 hours following the final once-daily and twice-daily doses at the end of each 4-week treatment period (full analysis set). BID, twice daily; FEV1, forced expiratory volume in 1 second; QD, once daily.
For all secondary FEV1-based end points, the adjusted means for once-daily tiotropium 5 μg and twice-daily 2.5 μg were comparable, with no differences between the two daily dosing regimens (Table 2). A diurnal variation in lung function was observed, with FEV1 AUC(12–24h) responses of 243 mL and 256 mL for once-daily tiotropium 5 μg and twice-daily 2.5 μg, compared with 192 mL and 182 mL, respectively, for FEV1 AUC(0–12h) responses.
Morning and evening peak expiratory flow responses at the end of each treatment period (calculated as weekly mean) were comparable between the two tiotropium daily dosing regimens, with no difference observed (Table 2).
Pharmacokinetics
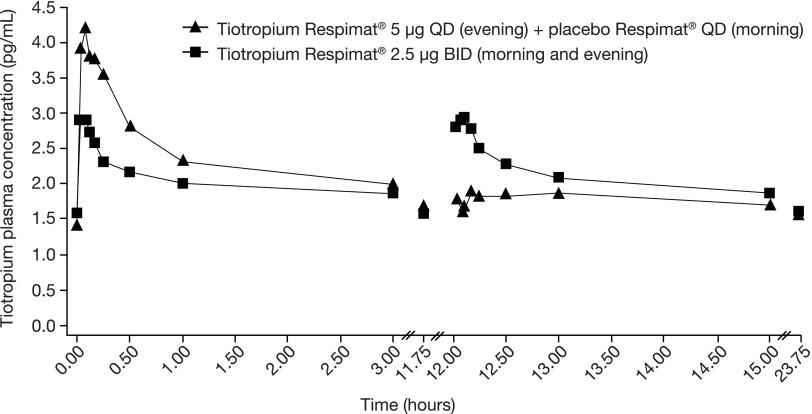
PK parameters were assessed at steady state and are shown in Table 3 and Figure 5. Geometric mean values of pre-dose plasma concentrations of tiotropium at steady state ranged between 1.43 pg/mL (5 μg once daily) and 1.59 pg/mL (2.5 μg twice daily). By contrast, tiotropium could not be detected in pre-dose plasma samples, before administration of the first dose of study treatment. Total exposures measured over the first 12 hours after dosing and over 24 hours were comparable between the two tiotropium dosing regimens. As expected, maximum plasma concentration values following morning and evening dosing were lower (35% to 37%, respectively) for twice-daily tiotropium 2.5 μg than for once-daily 5 μg evening dosing. The cumulative urinary excretion of tiotropium over 24 hours was similar for both dosing regimens at steady state.
Table 3.
Tiotropium Respimat® Pharmacokinetic Parameters at Steady State
| Tiotropium Respimat®5 μg QD | Tiotropium Respimat®2.5 μg BID | |||||
|---|---|---|---|---|---|---|
| Parameter | n | Geometric mean | Geometric CV (%) | n | Geometric mean | Geometric CV (%) |
| AUC(0–24h)ss, pg·h/mL | 24 | 43.8 | 28.9 | 23 | 45.3 | 20.6 |
| AUC(0–12h)ss, pg·h/mL | 31 | 23.4 | 33.5 | 26 | 21.5 | 25.3 |
| Cmax,ss, pg/mL | 34 | 4.95 | 106 | 33 | 3.10 | 40.4 |
| Cmax,ss,2, pg/mL | – | – | – | 34 | 3.23 | 50.8 |
| Ae(0–24h)ss, ng | 32 | 677 | 54.2 | 34 | 722 | 49.4 |
Pharmacokinetic subset (n = 35).
Ae(0–24h)ss, urinary excretion over 24 hours at steady state; AUC(0–12h)ss, area under the curve from 0 to 12 hours at steady state; AUC(0–24h)ss, area under the curve over 24 hours at steady state; BID, twice daily; Cmax,ss, steady-state maximum plasma concentration after the evening dose; Cmax,ss,2, steady-state maximum plasma concentration after the morning dose; CV, coefficient of variation; QD, once daily.
FIG. 5.
Geometric mean tiotropium plasma concentration–time profiles following multiple inhalations to steady state of 5 μg via the Respimat® inhaler by patients with moderate persistent asthma. PK measurements performed 15 min before evening administration, and after 2, 5, 7, 10, 15, and 30 min and 1, 3, and 11.75 h (morning administration 12 hours following the evening dose), 12 hours +2, 5, 7, 10, 15, and 30 min, and 13, 15, and 23.75 h relative to the evening dose. Pharmacokinetic subset (n = 35). BID, twice daily; QD, once daily.
Tiotropium was rapidly absorbed, with a median time to observed maximum plasma concentration value of 5 minutes post-dosing following once- or twice-daily dosing, and a long half-life of around 30 hours. There was no difference in the median time to observed maximum plasma concentration following morning and evening dosing of tiotropium 2.5 μg; the maximum tiotropium plasma concentrations were also comparable (Table 3).
Unexpected tiotropium plasma levels were observed in six blood samples (0.9%) from two patients. These two patients exhibited a second peak plasma concentration 12 h 10 min and 13 h post-dosing following once-daily tiotropium 5 μg (at the time of scheduled placebo administration). In one of the patients, tiotropium plasma concentrations remained elevated for the subsequent blood samples collected after this second maximum plasma concentration value following morning dosing. Comparison of data with and without the inclusion of these two patients in the analyses with suspected sample contamination did not influence the PK outcome of the trial (data not shown).
Safety and tolerability
The overall occurrence of adverse events was comparable between the two tiotropium regimens (Table 4). A total of 18 patients (18.4%) reported adverse events during treatment with once-daily tiotropium 5 μg, and 19 patients (19.4%) during treatment with twice-daily tiotropium 2.5 μg. The most frequently reported adverse events were nasopharyngitis (both treatment periods, 4.1%) and headache (once-daily tiotropium 5 μg, 3.1%; twice-daily tiotropium 2.5 μg, 2.0%). Drug-related events were infrequent and occurred in two patients (2.0%) during treatment with twice-daily tiotropium 2.5 μg (dry mouth), and one patient (1.0%) during treatment with once-daily tiotropium 5 μg (headache). Serious adverse events were reported in one patient (1.0%) while on treatment with twice-daily tiotropium 2.5 μg (cervical dysplasia and cervical carcinoma in situ), which led to premature discontinuation; this patient completed the 2.5 μg treatment period and was withdrawn before switching to tiotropium 5 μg. No fatal events were reported.
Table 4.
Overview of All Reported Adverse Eventsa
| n (%) | Tiotropium Respimat®5 μg QD(n = 98) | Tiotropium Respimat®2.5 μg BID(n = 98) |
|---|---|---|
| Any adverse event | 18 (18.4) | 19 (19.4) |
| Nasopharyngitis | 4 (4.1) | 4 (4.1) |
| Headache | 3 (3.1) | 2 (2.0) |
| Dysphonia | 1 (1.0) | 2 (2.0) |
| Rhinitis | 1 (1.0) | 2 (2.0) |
| Asthma worsening | 2 (2.0) | 0 |
| Contusion | 2 (2.0) | 0 |
| Cough | 0 | 2 (2.0) |
| Dry mouth | 0 | 2 (2.0) |
| Rash | 1 (1.0) | 1 (1.0) |
| Urticaria | 1 (1.0) | 1 (1.0) |
| Investigator-defined drug-related adverse events | 1 (1.0) | 2 (2.0) |
| Adverse events leading to discontinuation | 0 | 1 (1.0) |
| Serious adverse events | 0 | 1 (1.0) |
Treated set.
Frequency of adverse events that occurred in more than one patient in any treatment group, sorted by preferred term and system organ class.
BID, twice daily; QD, once daily.
Discussion
The results of this study show that once-daily tiotropium 5 μg and twice-daily tiotropium 2.5 μg, both as add-on to ICS, provide comparable bronchodilation over 24 hours, in line with previous findings and with a comparable magnitude of the effect size.(10) For all end points, the responses were comparable between tiotropium daily regimens, with no numerical difference observed between dosing regimens. Tiotropium 5 μg was administered once daily in the evening during this study; however, similar results would be expected if the 5 μg dose was administered in the morning. The diurnal variation observed in the 24-hour FEV1 profile in both treatment groups has been reported previously(10,19) and is attributed to a night-time increase, and subsequent morning decrease, in parasympathetic activity with associated changes in bronchodilation, rather than a consequence of tiotropium dosing.(20)
PK parameters are consistent with fast absorption and long elimination time of tiotropium, with maximum plasma concentrations reached within 5 min, and half-life elimination time in the range of 30 h. The PK profile following both dosing regimens was also similar and of the same magnitude as previously reported,(10) with comparable AUC values over 24 h observed in both studies, following once-daily 5 μg and twice-daily 2.5 μg administrations. Similarly, maximum plasma concentrations values for morning and evening dosing were lower (35% to 37%, respectively) for twice-daily tiotropium 2.5 μg than for once-daily 5 μg evening dosing, similar to the values noted in the previous PK study.(10) Cumulative urinary excretion and area under the plasma concentration curve both demonstrated comparable total tiotropium exposure following once-daily tiotropium 5 μg and twice-daily tiotropium 2.5 μg dosing.
Unexpected plasma concentrations in some samples were observed for two of the 35 patients in the once-daily tiotropium 5 μg subset reported here. Most sources of sample contamination in respiratory drug analysis are thought to derive from sample handling or during device priming,(11) which may have been the case for one unexpected tiotropium plasma level in our study (a second increase in plasma concentration was observed 13 h post-dosing). Other possible sources include: swapping of multiple samples during processing at the site; tiotropium inhalation from the evening instead of the morning Respimat® inhaler; or inhalation from other tiotropium sources. The observed increase in tiotropium concentration in subsequent plasma samples from one patient following scheduled inhalation from the placebo Respimat® inhaler indicates that the incorrect inhaler was used, in this case the one containing tiotropium. Therefore, there is a need to thoroughly instruct patients and educate clinical site personnel to follow protocols rigorously when using two different inhalers on the same day. In this study, the introduction of color-coded labeling to increase differentiation between morning and evening inhalers, as well as detailed steps to prevent contamination such as using separate rooms for priming of the device and inhalation of the study drug, wearing gloves when handling the Respimat® inhaler, and thorough washing of hands (Supplementary Table 1), resulted in a low number of unexpected PK results.
The extensive preventative steps applied at all stages of the device-priming procedure and drug administration, sample collection, and sample handling in this study, to avoid contamination of blood and urine samples with tiotropium, highlight the difficulties in ensuring there is no contamination, especially in multisite, multinational trials in Phase II or Phase III settings. However, the approaches taken in the present study indicate that contamination can be reduced to a minimum when evaluating the PK of inhaled drugs.
To date, results from the current study and those reported by Timmer et al.(10) are the only data from two large Phase II clinical trials, conducted across multiple sites, to describe the PK profile of tiotropium in patients with asthma. The strength of both studies lies in the large number of different patients involved in two similarly designed studies, which enabled us to assess the impact of two dosing regimens on the 24-hour bronchodilation efficacy of tiotropium. In addition, the current study demonstrates the importance of using a trial protocol designed to both reduce errors caused by improper use of the inhaler and minimize the risk of contamination, in large clinical trials conducted across multiple sites.
Careful consideration should therefore be given when performing clinical dosing studies of inhalation drugs. Providing initial training to both clinical and laboratory staff on best practices across trial centers could prove a useful way of keeping the level of sample contamination low, as well as ensuring consistency of practice. Similarly, clearly labeled devices with a combination of simple shape and color-coding could help patients ensure they use the correct device at the correct time.
In this reported study, total tiotropium exposure over 24 hours was comparable between the two dosing regimens. The observed bronchodilation efficacy over 24 hours was similar with the once-daily 5 μg and twice-daily 2.5 μg dosing regimens of tiotropium, as add-on to ICS therapy, supporting the suitability of once-daily dosing to improve and sustain lung function in adults with symptomatic asthma.
Supplementary Material
Acknowledgments
This work was supported by Boehringer Ingelheim. Medical writing assistance was provided by Angelique Stalmach, PhD, of Complete HealthVizion, which was contracted and compensated by Boehringer Ingelheim.
Funding: This study was funded by Boehringer Ingelheim.
Author Disclosure Statement
K-MB is employed by insaf Respiratory Research Institute GmbH and has received compensation from various pharmaceutical companies for organizing or participating in advisory board and scientific meetings, and for the design, performance, or participation in single- or multicenter clinical trials. RD has received personal fees from Boehringer Ingelheim, Novartis, GlaxoSmithKline, and Almirall, outside the submitted work. DD has received consulting fees, lecturing fees, and payment from Boehringer Ingelheim for the development of educational activities. A-MK is employed by Pulmonary Research Institute at LungClinic Grosshansdorf, which has been compensated for the conduct of the study, and received compensation for scientific meetings or lectures from various pharmaceutical companies, including Boehringer Ingelheim. AS, RS, and PMZ are employees of, and PC is a former employee of, Boehringer Ingelheim.
References
- 1.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJH, Pauwels RA, Pedersen SE, and for the GOAL Investigators Group: Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844 [DOI] [PubMed] [Google Scholar]
- 2.Demoly P, Paggiaro P, Plaza V, Bolge SC, Kannan H, Sohier B, and Adamek L: Prevalence of asthma control among adults in France, Germany, Italy, Spain and the UK. Eur Respir Rev. 2009;18:105–112 [DOI] [PubMed] [Google Scholar]
- 3.Partridge MR, Dal Negro RW, and Olivieri D: Understanding patients with asthma and COPD: Insights from a European study. Prim Care Respir J. 2011;20:315–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, and McLaurin K: Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129:1229–1235 [DOI] [PubMed] [Google Scholar]
- 5.Braman SS: The global burden of asthma. Chest. 2006;130:4S–12S [DOI] [PubMed] [Google Scholar]
- 6.Dean BB, Calimlim BM, Kindermann SL, Khandker RK, and Tinkelman D: The impact of uncontrolled asthma on absenteeism and health-related quality of life. J Asthma. 2009;46:861–866 [DOI] [PubMed] [Google Scholar]
- 7.Urrutia I, Aguirre U, Pascual S, Esteban C, Ballaz A, Arrizubieta I, and Larrea I: Impact of anxiety and depression on disease control and quality of life in asthma patients. J Asthma. 2012;49:201–208 [DOI] [PubMed] [Google Scholar]
- 8.Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, Sigmund R, Seibold W, Moroni-Zentgraf P, and Bateman ED: Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207 [DOI] [PubMed] [Google Scholar]
- 9.Kerstjens HAM, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, Engel M, Bour L, Verkleij CB, Moroni-Zentgraf P, and Bateman ED: Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: Two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomized trials. Lancet Respir Med. 2015;3:367–376 [DOI] [PubMed] [Google Scholar]
- 10.Timmer W, Moroni-Zentgraf P, Cornelissen P, Unseld A, Pizzichini E, and Buhl R: Once-daily tiotropium Respimat® 5 μg is an efficacious 24-hour bronchodilator in adults with symptomatic asthma. Respir Med. 2015;109:329–338 [DOI] [PubMed] [Google Scholar]
- 11.Adcock N: Bioanalysis for the development of respiratory drugs: What are the challenges? Bioanalysis. 2014;6:1143–1145 [DOI] [PubMed] [Google Scholar]
- 12.Karlsson AS, and Renström A: Human hair is a potential source of cat allergen contamination of ambient air. Allergy. 2005;60:961–964 [DOI] [PubMed] [Google Scholar]
- 13.Cornes MP, Davidson F, Darwin L, Gay C, Redpath M, Waldron JL, Ford C, and Gama R: Multi-centre observational study of spurious hyperkalaemia due to EDTA contamination. Clin Lab. 2010;56:597–599 [PubMed] [Google Scholar]
- 14.Kirk D, and Misita C: Spuriously elevated testosterone measurements caused by application of testosterone gel at or near the phlebotomy site. Ann Pharmacother. 2013;47:e5. [DOI] [PubMed] [Google Scholar]
- 15.Elajnaf A, Carter P, and Rowley G: Electrostatic characterisation of inhaled powders: Effect of contact surface and relative humidity. Eur J Pharm Sci. 2006;29:375–384 [DOI] [PubMed] [Google Scholar]
- 16.Carter PA, Rowley G, Fletcher EJ, and Stylianopoulos V: Measurement of electrostatic charge decay in pharmaceutical powders and polymer materials used in dry powder inhaler devices. Drug Dev Ind Pharm. 1998;24:1083–1088 [DOI] [PubMed] [Google Scholar]
- 17.Le VNP, Hoang Thi TH, Robins E, and Flament MP: Dry powder inhalers: Study of the parameters influencing adhesion and dispersion of fluticasone propionate. AAPS PharmSciTech. 2012;13:477–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Noord JA, Cornelissen PJG, Aumann J-L, Platz J, Mueller A, and Fogarty C: The efficacy of tiotropium administered via Respimat® Soft Mist™ Inhaler or HandiHaler® in COPD patients. Respir Med. 2009;103:22–29 [DOI] [PubMed] [Google Scholar]
- 19.Sterling R, Lim J, Frith L, Snowise NG, Jacques L, and Haumann B: Efficacy and optimal dosing interval of the long-acting beta2 agonist, vilanterol, in persistent asthma: A randomized trial. Respir Med. 2012;106:1110–1115 [DOI] [PubMed] [Google Scholar]
- 20.Postma DS, Keyzer JJ, Koëter GH, Sluiter HJ, and De Vries K: Influence of the parasympathetic and sympathetic nervous system on nocturnal bronchial obstruction. Clin Sci (Lond). 1985;69:251–258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.