Abstract
The amygdala plays a key role in emotion processing. Its functional connectivity with other brain regions has been extensively demonstrated to be associated with extraversion and neuroticism. However, how the amygdala affects other regions and is affected by others within these connectivity patterns associated with extraversion and neuroticism remains unclear. To address this issue, we investigated the effective connectivity of the amygdala using Granger causality analysis on the resting-state functional magnetic resonance imaging data of 70 participants. Results showed that extraversion was positively correlated with the influence from the right inferior occipital gyrus (IOG) to the left amygdala, and from the bilateral IOG to the right amygdala; such result may represent the neural correlates of social interactions in extraverts. Conversely, neuroticism was associated with an increased influence from right amygdala to right middle frontal gyrus and a decreased influence from right precuneus to right amygdala. This influence might affect the modulations of cognitive regulation function and self-referential processes in neurotic individuals. These findings highlight the importance of the causal influences of amygdala in explaining the individual differences in extraversion and neuroticism, and offer further insights into the specific neural networks underlying personality.
Personality describes the integrated pattern of thoughts, feelings and behaviors that varies among individuals but remains stable within each individual across time1. Eysenck’s biological approach is perhaps the most influential model of human personality, suggesting that individual variations in emotional arousal can be described along three dimensions: extraversion, neuroticism and psychoticism2. Among these traits, extraversion and neuroticism are of particular importance in individuals because their brain correlates may contribute to a predisposition toward socio-emotional functioning and psychopathology3,4. Differences in these dimensions are known to influence emotional and cognitive processing.
Extraversion is linked to the tendency to experience positive emotions and social engagement5, which is typically stem from the tendency to respond positively to socio-emotional stimuli6. Conversely, neuroticism is related to increased susceptibility to negative emotion7, which is considered to originate from self-generated thoughts8. Moreover, individuals with high levels of neuroticism frequently experience difficulties in emotion regulation9 and demonstrate a negative self-referential information processing style10.
As a region critical to the organization of a complex series of emotional and physiological responses11, the amygdala has been centrally implicated in extraversion and neuroticism12,13. The afferents to and efferents from the amygdala have extensive connections with cortical and subcortical regions, and these connections allow them to participate in critical functions relevant to extraversion and neuroticism. These functions include the modulation of sensory information, such as the delivery of “gut feelings” about things that are good and bad [through the integration of afferents from all senses (e.g., visual, auditory, and somatosensory) as well as visceral inputs]14, emotion motivation and responses (through projections to cortical, hypothalamic and brainstem regions)15,16, and emotion regulation (through reciprocal connections to prefrontal and insular areas)13,17,18. Given the importance of the amygdala in emotion processing and its implication in extraversion and neuroticism, the information flow of the amygdala is expected to be fundamental to stable individual differences in these two key dimensions.
Correlation-based functional connectivity (FC) studies have revealed that functional coupling between the amygdala and distinct brain regions plays a crucial role in the individual differences in extraversion and neuroticism. Neuroticism was associated with amygdala-prefrontal [i.e., anterior cingulate cortex (ACC), ventromedial prefrontal cortex, and dorsolateral prefrontal cortex (dlPFC)]19,20,21, amygdala-hippocampus21, and amygdala-visual cortex connectivity20 during threat-related stimuli, possibly reflecting a general proneness to anxiety-related disorders in highly neurotic participants20,21. Extraversion was related to the neural pathways of the amygdala with ACC, insula, and thalamus regions in response to reward-related stimuli13. Resting-state functional connectivity (RSFC) studies also revealed that neuroticism is related to connections between the amygdala and brain regions involved in the processing of self-referential cues and emotion regulation [i.e., precuneus (PCu), prefrontal cortex (PFC), and visual cortex], whereas extraversion is related to the connections between the amygdala and brain regions implicated in socio-emotional function (i.e., insula, and occipital cortex)12,22. Individual differences in these amygdala connectivity patterns subserving cognitive-emotional functions are likely to be associated specifically with extraversion and neuroticism. A particularly interesting question is raised: What role does the amygdala play? To date, the manner in which the amygdala affects other regions and is affected by others within these connectivity patterns associated with extraversion and neuroticism has been unclear. The current study aimed to evaluate whether extraversion and neuroticism are associated with the causal influences between the amygdala and other brain regions at rest in a large sample of healthy subjects.
Effective connectivity (EC) referes to the influence that one brain region exerts over another23 and is evaluated through Granger causality analysis (GCA). This technique has been widely used for time-directed prediction among different blood oxygen level-dependent functional magnetic resonance imaging (fMRI) time series, and has revealed the pattern of causal influence among resting-state networks24,25, and among brain regions within the cortical-subcortical circuit related to affective disorders26,27,28. Thereafter, multiple regression analysis was implemented to determine the resting-state EC patterns of the amygdala which are associated with extraversion, and neuroticism. On the basis of neuroimaging literature, we hypothesized that extraversion would predict the process that the visual cortex affects the amygdala, which may partly explain the described bias towards social engagement29,30. Neuroticism would predict the interactions between the amygdala and the PFC and PCu regions, which may modulate the cognitive-emotional process in neurotic individuals31,32.
Results
EC of the amygdala is associated with extraversion
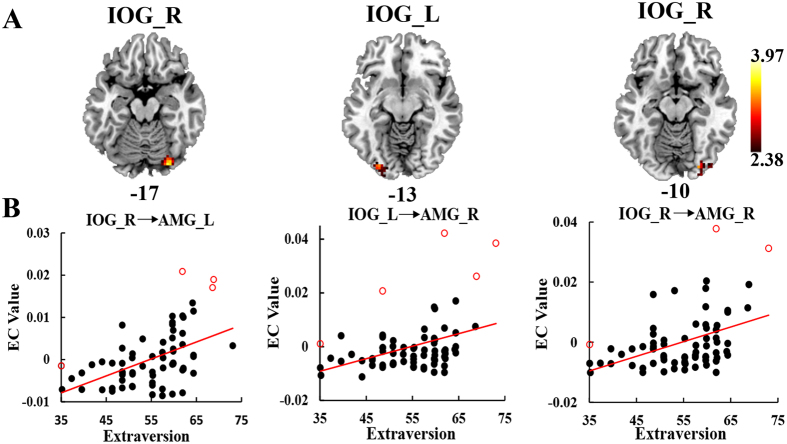
Extraversion was positively correlated with the EC from the right inferior occipital gyrus (IOG) to the left amygdala, and from the bilateral IOG to the right amygdala (p < 0.05, AlphaSim corrected, Fig. 1, Table 1). Extraversion showed no significant correlation with the EC from the left and right amygdala to other brain regions.
Figure 1. Regions of EC showing correlation with extraversion.
(A) Extraversion was positively associated with the EC from the right IOG to the left AMG, the EC from the left IOG to the right AMG, and the EC from the right IOG to the right AMG. The hot color indicates the EC of the amygdala that show positive associated with extraversion. The color scale represents t values. (B) The scatter plots represent individual mean EC estimates from extraversion after regressing age, gender, FD, and neuroticism scores. The red circles are the outliers detected by bootstrapping the Mahalanobis distance. EC, effective connectivity; AMG, amygdala; IOG, inferior occipital gyrus.
Table 1. The relationship between extraversion and the EC of the amygdala.
| Brain Regions | Brodmann Area | MNI | Cluster size (voxels) | Peak t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| other regions→amygdala_L | ||||||
| IOG_R | 18 | 33 | −96 | −12 | 103 | 4.34 |
| other regions→amygdala_R | ||||||
| IOG_L | 18 | −51 | −63 | −18 | 145 | 3.59 |
| IOG_R | 18 | 30 | −87 | −12 | 138 | 3.37 |
Regions show the EC of the amygdala. EC, effective connectivity; IOG, Inferior occipital gyrus; L, left; R, right. Positive and negative t-values indicate positive and negative correlations between extraversion and the EC of the amygdala, respectively.
EC of the amygdala is associated with neuroticism
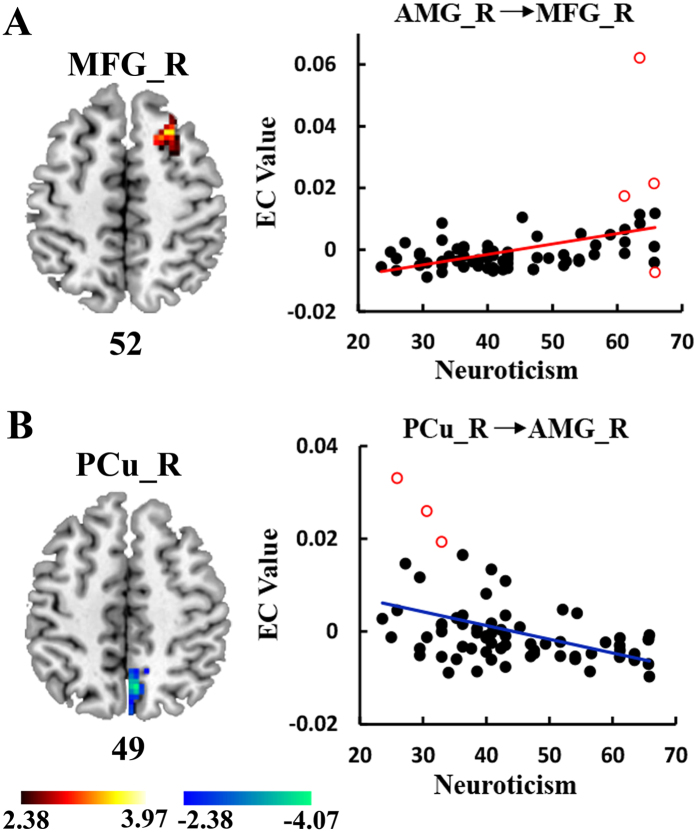
A positive correlation was observed between neuroticism and the EC from the right amygdala to the right middle frontal gyrus (MFG). Conversely, the EC from the right PCu to the right amygdala was negatively correlated with neuroticism (p < 0.05, AlphaSim corrected; Fig. 2, Table 2). Neuroticism showed no significant correlation with the EC of the left amygdala.
Figure 2. Regions of EC showing correlation with neuroticism.
(A) Positive association between neuroticism and the EC from the right AMG to the right MFG. (B) Negative association between neuroticism and the EC from the right PCu to the right AMG. The hot and cold colors indicate the EC of amygdala that show positive and negative associated with neuroticism, respectively. The color scale represents t values. Right scatter plots represent individual mean EC estimates from neuroticism after regressing age, gender, FD, and extraversion scores. The red circles are the outliers detected by bootstrapping the Mahalanobis distance. EC, effective connectivity; AMG, amygdala; MFG, middle frontal gyrus; PCu, precuneus.
Table 2. The relationship between neuroticism and the EC of the amygdala.
| Brain Regions | Brodmann Area | MNI | Cluster size (voxels) | Peak t-value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| amygdala_R→other regions | ||||||
| MFG_R | 8 | 30 | 9 | 66 | 139 | 3.91 |
| other regions→amygdala_R | ||||||
| PCu_R | 7 | 6 | −63 | 48 | 119 | −4.07 |
Regions show the EC of the amygdala. EC, effective connectivity; MFG, Middle frontal gyrus; PCu, Precuneus; L, left; R, right. Positive and negative t-values indicate positive and negative correlations between neuroticism and the EC of the amygdala, respectively.
Discussion
This study is the first to examine the relationship between extraversion, neuroticism, and the resting-state EC of the amygdala. Extraversion was found to be associated with an increased influence from visual processing streams to the amygdala. Neuroticism was associated with an increased influence from the amygdala to the MFG and a decreased influence from the PCu to the amygdala. These results excluded the influence of the hippocampus region, age, and gender. Moreover, the average EC maps of the amygdala across the sample revealed that the amygdala was connected to the PFC, occipital cortex, default mode network, and limbic regions (see Supplementary Figure S1); this finding was consistent with those of previous studies. The present findings indicate that individual differences in extraversion and neuroticism are associated with potential causal interactions between the amygdala and other distinct regions related to cognitive-emotional functions at rest.
EC of amygdala is associated with extraversion
Our analysis showed that extraversion was positively correlated with the influences from the right IOG to the left amygdala, and from the bilateral IOG to the right amygdala. Extraverts who spend more time on interpersonal interaction are deemed to be better at processing faces than introverts30. The occipital face area pertains to the “core face-processing system” that mediates the visual analysis of faces22,33. The amygdala also belongs to the “extended” system that acts in concert with the core system to extract the meaning of information gleaned from faces34. The heightened activation of these regions was related to extraversion in response to positive social stimuli, such as happy faces3,35,36. The amygdala-visual cortex FC was implicated in attentional set37 and face recognition22, with the functions specially associated with the extraversion trait30. The amygdala receives the perceptual information from visual regions in processing socio-emotional stimuli and is involved in extracting emotional meaning to make appropriate responses, such as social judgments29, and attentional biases toward positive stimuli38. In this study, the increased connectivity between visual regions and the amygdala in extraverts might provide further neural pathway evidence of the relation between face recognition ability and social communication. Extraverts who are thought to be better at detecting and decoding the meaning of social cues have a better ability to navigate the dynamics of social interactions in social contexts than introverts11.
EC of amygdala is associated with neuroticism
In the current study, a positive correlation between neuroticism and the influence from the right amygdala to the right MFG (BA8, dlPFC) was found. The amygdala-prefrontal network was shown to be associated with individual differences in the cognitive-emotional process31,32,39. The dlPFC mainly suppresses or promotes emotional responses controlled by the amygdala in complex scenes40,41. Alternatively, the amygdala conveys the emotional signal to the dlPFC region and is involved in handling the challenges to the organism by using different cognitive strategies32. According to these findings, people can modulate negative emotional responses through different cognitive strategies (i.e., suppression and reappraisal); in turn, the negative emotion can affect cognition function to determine behavioral outcomes in social surroundings42,43. These cognitive-emotional processes are strongly related to inter-individual variability in neuroticism44, which is a trait geared toward the emotional and cognitive processing of negatively affective stimuli8. Recent functional and structural connectivity studies have confirmed that the amygdala-PFC connectivity subserving emotion regulation processes is related to neuroticism19,20,21,45,46. With the support of the abovementioned findings, we suggest that the increased influence from the amygdala to the dlPFC might represent the neurobiological counterpart of the suboptimal cognition function associated with the internal negative emotion processes and characteristic of individuals with high neuroticism scores.
A negative association was found between neuroticism and the influence from the right PCu to the right amygdala. The PCu is implicated in self-related mental representations during rest47,48,49, especially among individuals high in neuroticism50,51, who tend to be more socially dysfunctional52. As shown in literature, the connectivity between this region and amygdala is important for different aspects of self-processing and for the modulation of the physiological response to emotion, and it contributes to the “disproportionate emotional coloring of self-referential processing”12,52. The amygdala-PCu pathway in the context of self-referential processing contributes to neuroticism12. Previous FC studies have likewise revealed that a decreased amygdala-PCu connectivity pattern is associated with high anxiety symptoms53,54. Thus, the current finding complements this notion and shows that neuroticism is associated with a decreased coupling between the amygdala and the PCu probably involving negative emotional coloring bias during states of spontaneous thought. This finding may imply that high levels of neuroticism are related to suboptimal self-relevant information processing.
Potential confounding factors
To test the potential influence of the hippocampus, we calculated the EC between the hippocampus and other brain regions (see Supplementary S1). The results showed that only the influence from the PCu to the hippocampus positively associated with neuroticism was overlapped with the influence from the PCu to the amygdala negatively associated with neuroticism (see Supplementary Table S1). Therefore, the EC value was regressed out from the PCu to the hippocampus, and the result of neuroticism associated with a decreased influence from the PCu to the amygdala still remained (see Supplementary S3). These findings confirm the reliability of our results. Moreover, the effects of age and gender were assessed. We found no correlation between age and extraversion and neuroticism or between age and EC value (see Supplementary Figure S2). Thereafter, a consistent correlation was found between males and females (see Supplementary Figure S3). These pieces of evidence confirm that our findings are not confounded by age and gender.
Many inferenced studies on extraversion and neuroticism used the NEO questionnaire is derived from the Costa and McCrae five-factor model (Extraversion, Neuroticism, Agreeableness, Conscientiousness, and Openness)55. This model and the Eysenck three-factor model are the most influential and widely used models in the field of personality structure56. Despite differences between the two models, the results of factor analysis suggested that extraversion and neuroticism were highly convergent with the corresponding trait measures in both systems56,57. The relationship between Psychoticism from the Eysenck model and Agreeableness, Conscientiousness, and Openness factors from the Costa and McCrae model had no clear conclusion57,58. Previous neuroscience studies have suggested that the neural mechanisms underlining extraversion and neuroticism across the two models were similar59,60,61. According to literature, these two systems measure the same or at least highly overlapped traits, namely, extraversion and neuroticism.
Limitation
The interpretation of our causal influence between the amygdala and other brain regions is restricted by the inherent limitations of GCA measures. Although GCA is widely used to model the causal influence that one neural time series exerts on another, it has some drawbacks in fMRI applications. It should be noted that systematic differences across brain regions in hemodynamic lag can potentially lead to spurious estimations of causality62,63. Until now, arguments are still existing on whether this disturbance will affect the studies at the group level64,65,66. This factor is an important limitation in considering, for example, the argued influence of the amygdala over dlPFC. In addressing these issues, the analyses of data collected through short TRs may help. To some extent, our findings hold potential for the causal connectivity of the amygdala related to extraversion and neuroticism. Future neuroimaging studies based on specific tasks and some interventions are needed to test the precise associations between the amygdala and other regions underlining the individual differences in extraversion and neuroticism.
Conclusion
In summary, we provide evidence on the association of individual differences in extraversion and neuroticism and the EC pattern of the amygdala. In particular, extraversion was positively correlated with the influence from the visual cortex to the amygdala, which may represent the neural correlates of social interactions in extraverts. Conversely, neuroticism was positively associated with the influence from the amygdala to the MFG and negatively correlated with the influence from the PCu to the amygdala. This finding may underline the mechanism of suboptimal cognitive regulation function and self-referential processes in neurotic individuals. These findings indicate that the causal interactions between the amygdala and other brain regions related to cognition-emotional function are associated with individual differences in extraversion and neuroticism. The implementation of the resting-state EC of the amygdala in this study may help advance our understanding of the complex neural mechanisms of personality.
Methods
Participants
Seventy young, healthy, right-handed volunteers (34 males; age range: 19–26 years, mean age: 22.3 years, SD = 1.5) participated in this study. The participants had no history of neurological disorders or head injury and were not receiving any medications. The study was approved by the local Medical Ethics Committee of Jinling Hospital, Nanjing University School of Medicine, and was carried out in accordance with the approved guidelines. All participants provided written informed consent before any study procedure was initiated.
Personality questionnaires
Personality dimensions were assessed using the Eysenck Personality Questionnaire-Revised Short Scale for Chinese (EPQ-RSC)4,67. The EPQ-RSC is a self-reported questionnaire that includes four dimensions of extraversion (E), neuroticism (N), psychoticism (P), and lie (L), with each dimension measured by 12 true-or-false questions. Each dichotomous item was scored with 1 or 0, and each dimension had a maximum possible score of 12 and a minimum of 0. As two basic and significant dimensions, extraversion and neuroticism were used for measuring correlations with EC values in this study. In the present study, raw extraversion scores ranged from 3 to 12 (M = 8.97, SD = 2.24), and raw neuroticism scores ranged from 0 to 9 (M = 3.33, SD = 3.10). Then, the initial extraversion and neuroticism scores were converted into T scores through the formula67:
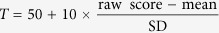 |
where mean represents the mean value of the personality scores over the normative sample; SD is the standard deviation of the personality scores over the normative sample. Subjects yielded mean values of M = 54.61 (SD = 8.44; score range: 35.07–73.05) on the extraversion dimension and M = 44.31 (SD = 12; score range: 23.57–65.82) on the neuroticism dimension. Furthermore, the distribution of extraversion scores was skewed negatively (skew = −0.51; kurtosis = −0.09), and the distribution of neuroticism scores was skewed positively (skew = 0.35; kurtosis = −0.92). A significantly negative correlation was found between extraversion and neuroticism through Pearson's correlation (r = −0.40, p < 0.0001).
Image acquisition
Resting-state fMRI data were acquired using a 3T Siemens Trio scanner (Jinling Hospital, Nanjing, China). Foam pads and headphones were used to minimize head movement and scanner noise for all subjects. During data acquisition, the participants were instructed to simply rest with their eyes closed, relax their minds, not fall asleep, and not move if possible. Functional images were collected transversely using a gradient-recalled echo-planar imaging sequence with the following parameters: repetition time (TR)/echo time (TE) = 2000/30 ms, flip angle (FA) = 90°, field of view (FOV) = 24 cm, slices = 30, in-plane matrix = 64 × 64, and voxel size = 3.75 × 3.75 × 4.40 mm3. For each subject, a total of 250 volumes were acquired.
Data preprocessing
Data preprocessing was performed using the SPM8 software (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/software/spm8). The first ten volumes were discarded due to the instability of the initial MRI signal and the adaptation of participants to the experimental procedure. The remaining 240 volumes were first corrected by the acquisition time delay among different slices and then realigned to correct for head-motion. No subject was excluded under the head motion criterion of ±1.5 mm and ±1.5°. Subsequently, the corrected images were spatially normalized to the Montreal Neurological Institute template and resampled to 3 × 3 × 3 mm3 cubic voxels. Filtering (0.01 Hz–0.08 Hz) and linear detrend removal were implemented to remove low-frequency drift and minimize high-frequency physiological noises68. As an additional motion correction, we calculated the frame-wise displacement (FD)69 to express instantaneous head motion, and the threshold of 0.5 was suggested. The maximum FD value was 0.33, and no subjects’ FD values were beyond 0.5. The M ± SD of FD over subjects was 0.12 ± 0.0630. The correlation analysis showed no significant correlation between extraversion and FD (r = −0.21, p = 0.09) and a positive correlation between neuroticism and FD (r = 0.24, p = 0.04).
EC analysis
Recently, GCA and dynamic causal modeling (DCM) have become two state-of-the-art approaches for estimating EC from fMRI data70,71. DCM requires a prior specification of an anatomical network model and then tests the best possible networks72. By contrast, GCA only requires the predefined of regions of interest (ROIs) and can be applied directly to detect the coupling between ROI and other regions, without prior knowledge of the connections model between regions73. Accordingly, GCA was used with the left and right amygdala as ROIs to conduct the EC analysis. ROIs derived from the automated anatomical labeling template implemented in the MarsBaR toolbox (http://marsbar.sourceforge.net) were created. For each subject, the time series in the ROIs and the rest of the voxels of the whole brain were extracted. Several nuisance covariates including six rigid body head motion parameters, white matter, and cerebrospinal fluid signals, were regressed through linear regressions to remove the source of spurious variances74,75,76.
Residual-based voxel-wise GCA on the was performed through the in-house MATLAB toolbox (http://guorongwu.weebly.com/software.html)77,78 to describe the EC between the seed regions (left and right amygdala) and all other brain regions in each subject. In this study, the preprocessed mean time course of the seed region was defined as the time series x, and y denotes the time series of all other brain regions. The bivariate residual GCA was conducted to calculate the liner directional influence from x to y (Fx→y) and from y to x (Fy→x)79,80,81,82. Thus, two Granger causality maps (i.e., Fx→y and Fy→x maps) were generated for each subject and for each seed region. The order of the autoregressive model was set to 1 according to the Schwartz criterion. The coefficients of the models were calculated using a standard least squares optimization. Finally, the Granger causality maps were spatially smoothed by convolution with an isotropic Gaussian kernel (FWHM = 4 mm).
Statistical analysis
A voxel-based multiple regression analysis (based on the general linear model) was implemented to map the effect of personality dimensions on amygdala EC, with EC value (i.e., the mean value of the left amygdala with Fx→y and Fy→x and the right amygdala with Fx→y and Fy→x) as the dependent variable, and extraversion and neuroticism scores of the personality test as the covariates of interest. In addition, age, gender, and FD were used as external regressions to control their effects on the association between extraversion, neuroticism and brain EC59,83.
Given the theoretical and statistical anticorrelation between extraversion and neuroticism, the variance specific to each dimension should be partial out to obtain effects that were uniquely driven by each personality dimension. Therefore, we added extraversion (or neuroticism) scores as covariate of no interest in the model when calculating the relationship between neuroticism (or extraversion) and the EC of the amygdala. The correction for multiple comparisons used a combined height-extent threshold calculated through Alphasim Monte-Carlo simulation with 1,000 iterations. The corrected statistical threshold (p < 0.05) was accomplished as follows84. The regression analysis result was corrected (p < 0.05/8) [2 (extraversion/neuroticism) × 2 (left/right amygdala) × 2 (direction)] by the AlphaSim program in the REST 1.8 software (http://www.restfmri.net/forum/REST_V1.8), with each multiple regression analysis (with a voxel-wise threshold of uncorrected p < 0.01 with a minimum cluster size of 95 connected voxels for the relationship between extraversion and the left amygdala with Fx→y, 98 voxels for the relationship between extraversion and the left amygdala with Fy→x, 105 voxels for the relationship between extraversion and the right amygdala with Fx→y, 126 voxels for the relationship between extraversion and the right amygdala with Fy→x, 84 voxels for the relationship between neuroticism and the left amygdala with Fx→y, 89 voxels for the relationship between neuroticism and the left amygdala with Fy→x, 110 voxels for the relationship between neuroticism and the right amygdala with Fx→y, and 100 voxels for the relationship between neuroticism and the right amygdala with Fy→x).
Additional Information
How to cite this article: Pang, Y. et al. Extraversion and neuroticism related to the resting-state effective connectivity of amygdala. Sci. Rep. 6, 35484; doi: 10.1038/srep35484 (2016).
Supplementary Material
Acknowledgments
Authors thank Guorong Wu, Lixia Zhu and XiaoWang for their assistance with this study. This work was supported by the 863 project (2015AA020505), the Natural Science Foundation of China (61533006, 61125304, 31400901), the Science Foundation of Ministry of Education of China (14XJC190003), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20120185110028) and the Fundamental Research Funds for the Central Universities (ZYGX2013Z004, ZYGX2014J104).
Footnotes
Author Contributions Y.P. and H.C. conceived and designed the experiments. Z.Z. and G.L. prepared the samples. Y.P., X.W. and S.H. analyzed the data. Y.P., Q.C. and Y.W. participated in the interpretation of data. Y.P., Q.C. and Y.C. wrote the paper.
References
- Suslow T. et al. Automatic brain response to facial emotion as a function of implicitly and explicitly measured extraversion. Neuroscience 167, 111–123 (2010). [DOI] [PubMed] [Google Scholar]
- Eysenck H. J. The biological basis of personality. Vol. 689 (Transaction publishers, 1967). [Google Scholar]
- Canli T. Functional brain mapping of extraversion and neuroticism: learning from individual differences in emotion processing. Journal of personality 72, 1105–1132 (2004). [DOI] [PubMed] [Google Scholar]
- Eysenck H. J. (London: Hodder & Stoughton, 1991).
- Clark L. A. & Watson D. Temperament: An organizing paradigm for trait psychology. Handbook of personality: Theory and Research, 3rd ed., 265–286 (2008). [Google Scholar]
- Fishman I., Ng R. & Bellugi U. Do extraverts process social stimuli differently from introverts? Cognitive neuroscience 2, 67–73 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen R. J. & Ketelaar T. Personality and susceptibility to positive and negative emotional states. Journal of personality and social psychology 61, 132, 10.1037/0022-3514.61.1.132 (1991). [DOI] [PubMed] [Google Scholar]
- Perkins A. M., Arnone D., Smallwood J. & Mobbs D. Thinking too much: self-generated thought as the engine of neuroticism. Trends in cognitive sciences 19, 492–498 (2015). [DOI] [PubMed] [Google Scholar]
- Ng W. & Diener E. Personality differences in emotions: Does emotion regulation play a role? Journal of Individual Differences 30, 100–106 (2009). [Google Scholar]
- Trapnell P. D. & Campbell J. D. Private self-consciousness and the five-factor model of personality: distinguishing rumination from reflection. Journal of personality and social psychology 76, 284 (1999). [DOI] [PubMed] [Google Scholar]
- Bickart K. C., Hollenbeck M. C., Barrett L. F. & Dickerson B. C. Intrinsic amygdala–cortical functional connectivity predicts social network size in humans. The Journal of Neuroscience 32, 14729–14741 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajani M. et al. Neuroticism and extraversion are associated with amygdala resting-state functional connectivity. Cognitive, Affective, & Behavioral Neuroscience 83, 836–848, 10.3758/s13415-013-0224-0 (2014). [DOI] [PubMed] [Google Scholar]
- Schweckendiek J., Stark R. & Klucken T. Neuroticism and extraversion moderate neural responses and effective connectivity during appetitive conditioning. Hum. Brain Mapp. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J. L. Comparative aspects of amygdala connectivity. Annals of the New York Academy of Sciences 985, 50–58 (2003). [DOI] [PubMed] [Google Scholar]
- Suyama S., Takano E., Iwasaki Y., Nakata M. & Yada T. Roles and functional interplay of the gut, brain stem, hypothalamus and limbic system in regulation of feeding. Nihon rinsho. Japanese journal of clinical medicine 67, 277–286 (2009). [PubMed] [Google Scholar]
- LeDoux J. E., Iwata J., Cicchetti P. & Reis D. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience 8, 2517–2529 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei H., Hilgetag C. & Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 34, 905–923 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S. J., Eddy K. T., Angstadt M., Nathan P. J. & Phan K. L. Amygdala–frontal connectivity during emotion regulation. Social cognitive and affective neuroscience 2, 303–312, 10.1093/scan/nsm029 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers H. R. et al. Neuroticism modulates amygdala—prefrontal connectivity in response to negative emotional facial expressions. NeuroImage 49, 963–970 (2010). [DOI] [PubMed] [Google Scholar]
- Madsen M. K. et al. Threat-related amygdala functional connectivity is associated with 5-HTTLPR genotype and neuroticism. Social Cognitive and Affective Neuroscience, nsv098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschoppe J. et al. Aversive Learning in Adolescents: Modulation by Amygdala–Prefrontal and Amygdala–Hippocampal Connectivity and Neuroticism. Neuropsychopharmacology 39, 875–884 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz J. et al. 5-HTTLPR/rs25531 polymorphism and neuroticism are linked by resting state functional connectivity of amygdala and fusiform gyrus. Brain Structure and Function 220, 2373–2385 (2015). [DOI] [PubMed] [Google Scholar]
- Friston K., Frith C., Liddle P. & Frackowiak R. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of cerebral blood flow and metabolism 13, 5–14 (1993). [DOI] [PubMed] [Google Scholar]
- Liao W. et al. Evaluating the effective connectivity of resting state networks using conditional Granger causality. Biological cybernetics 102, 57–69 (2010). [DOI] [PubMed] [Google Scholar]
- Uddin L. Q., Clare Kelly A., Biswal B. B., Xavier Castellanos F. & Milham M. P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 30, 625–637 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W. et al. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS One 5, e15238 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Zou K., He Z., Sun X & Chen , H. Causal connectivity alterations of cortical-subcortical circuit anchored on reduced hemodynamic response brain regions in first-episode drug-naïve major depressive disorder. Sci. Rep. 6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M. et al. Association of resting-state network dysfunction with their dynamics of inter-network interactions in depression. Journal of affective disorders 174, 527–534 (2015). [DOI] [PubMed] [Google Scholar]
- Miyahara M., Harada T., Ruffman T., Sadato N. & Iidaka T. Functional connectivity between amygdala and facial regions involved in recognition of facial threat. Social cognitive and affective neuroscience 8, 181–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. et al. Extraversion predicts individual differences in face recognition. Commun. Integr. Biol. 3, 295–298 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr C. et al. Individual differences in common factors of emotional traits and executive functions predict functional connectivity of the amygdala. NeuroImage 120, 154–163 (2015). [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in cognitive sciences 13, 160–166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwind M., Pourtois G., Schwartz S., Van De Ville D. & Vuilleumier P. White-matter connectivity between face-responsive regions in the human brain. Cerebral cortex 22, 1564–1576 (2012). [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Hoffman E. A. & Gobbini M. I. The distributed human neural system for face perception. Trends in cognitive sciences 4, 223–233 (2000). [DOI] [PubMed] [Google Scholar]
- Canli T., Sivers H., Whitfield S. L., Gotlib I. H. & Gabrieli J. D. Amygdala response to happy faces as a function of extraversion. Science 296, 2191–2191 (2002). [DOI] [PubMed] [Google Scholar]
- Amin Z., Constable R. T. & Canli T. Attentional bias for valenced stimuli as a function of personality in the dot-probe task. Journal of Research in Personality 38, 15–23 (2004). [Google Scholar]
- Pessoa L., Kastner S. & Ungerleider L. G. Attentional control of the processing of neutral and emotional stimuli. Cognitive Brain Research 15, 31–45 (2002). [DOI] [PubMed] [Google Scholar]
- Derryberry D. & Reed M. A. Temperament and attention: orienting toward and away from positive and negative signals. Journal of personality and social psychology 66, 1128 (1994). [DOI] [PubMed] [Google Scholar]
- Salzman C. D. & Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annual review of neuroscience 33, 173 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Y. J., Bullock D., Zikopoulos B. & Barbas H. Anatomy and computational modeling of networks underlying cognitive-emotional interaction. Frontiers in human neuroscience 7, 10.3389/fnhum.2013.00101 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. A. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53 (2006). [DOI] [PubMed] [Google Scholar]
- Sladky R. et al. Disrupted effective connectivity between the amygdala and orbitofrontal cortex in social anxiety disorder during emotion discrimination revealed by dynamic causal modeling for fMRI. Cerebral Cortex 25, 895–903 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K. N. & Gross J. J. The cognitive control of emotion. Trends in cognitive sciences 9, 242–249 (2005). [DOI] [PubMed] [Google Scholar]
- Ormel J. et al. The biological and psychological basis of neuroticism: current status and future directions. Neuroscience & Biobehavioral Reviews 37, 59–72 (2013). [DOI] [PubMed] [Google Scholar]
- Bjørnebekk A. et al. Neuronal correlates of the five factor model (FFM) of human personality: Multimodal imaging in a large healthy sample. NeuroImage 65, 194–208 (2013). [DOI] [PubMed] [Google Scholar]
- Xu J. & Potenza M. N. White matter integrity and five-factor personality measures in healthy adults. NeuroImage 59, 800–807 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer T. W., Nowak M. & Lou H. C. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage 17, 1080–1086 (2002). [PubMed] [Google Scholar]
- Cavanna A. E. & Trimble M. R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006). [DOI] [PubMed] [Google Scholar]
- Buckner R. L. & Carroll D. C. Self-projection and the brain. Trends in cognitive sciences 11, 49–57 (2007). [DOI] [PubMed] [Google Scholar]
- Jimura K., Konishi S., Asari T. & Miyashita Y. Temporal pole activity during understanding other persons’ mental states correlates with neuroticism trait. Brain Res 1328, 104–112 (2010). [DOI] [PubMed] [Google Scholar]
- Adelstein J. S. et al. Personality is reflected in the brain’s intrinsic functional architecture. PloS one 6, e27633, 10.1371/journal.pone.0027633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K. R. et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA psychiatry 71, 1138–1147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A. et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. NeuroImage 56, 881–889 (2011). [DOI] [PubMed] [Google Scholar]
- Lui S. et al. Resting-state functional connectivity in treatment-resistant depression. American Journal of Psychiatry (2011). [DOI] [PubMed] [Google Scholar]
- Costa P. T. & McCrae R. R. (Odessa, FL: Psychological Assessment Resources, 1992).
- Aluja A., Garcia O. & Garcia L. F. A comparative study of Zuckerman’s three structural models for personality through the NEO-PI-R, ZKPQ-III-R, EPQ-RS and Goldberg’s 50-bipolar adjectives. Pers. Individ. Dif. 33(5), 713–726 (2002). [Google Scholar]
- Zuckerman M., Kuhlman D. M., Joireman J., Teta P. & Kraft M. A comparison of three structural models for personality: The Big Three, the Big Five, and the Alternative Five. Journal of personality and social psychology 65, 757 (1993). [Google Scholar]
- Heaven P. C., Ciarrochi J., Leeson P. & Barkus E. Agreeableness, conscientiousness, and psychoticism: Distinctive influences of three personality dimensions in adolescence. Br. J. Psychol. 104, 481–494 (2013). [DOI] [PubMed] [Google Scholar]
- Pang Y. et al. Extraversion modulates functional connectivity hubs of resting‐state brain networks. Journal of neuropsychology (2015). [DOI] [PubMed] [Google Scholar]
- Lu F. et al. Relationship between personality and gray matter volume in healthy young adults: a voxel-based morphometric study. PloS one 9, e88763, 10.1371/jouranl.pone.0088763 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaas M. N. et al. Connectomics and neuroticism: An altered functional network organization. Neuropsychopharmacology 40, 296–304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M. et al. Network modelling methods for FMRI. NeuroImage 54, 875–891 (2011). [DOI] [PubMed] [Google Scholar]
- Webb J. T., Ferguson M. A., Nielsen J. A. & Anderson J. S. BOLD Granger causality reflects vascular anatomy. PloS one 8, e84279 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A., Formisano E. & Goebel R. The identification of interacting networks in the brain using fMRI: Model selection, causality and deconvolution. NeuroImage 58, 296–302 (2011). [DOI] [PubMed] [Google Scholar]
- Schippers M. B., Renken R. & Keysers C. The effect of intra- and inter-subject variability of hemodynamic responses on group level Granger causality analyses. NeuroImage 57, 22–36 (2011). [DOI] [PubMed] [Google Scholar]
- Ryali S., Supekar K., Chen T. & Menon V. Multivariate dynamical systems models for estimating causal interactions in fMRI. NeuroImage 54, 807–823 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian M., Wu G., Zhu R. & Zhang S. Development of the Revised Eysenck Personality Questionnaire Short Scale for Chinese (EPQ-RSC)(Article written in Chinese). Acta Psychologica Sinica 32, 317–323 (2000). [Google Scholar]
- Liu F. et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: A resting-state fMRI study. Progress in Neuro-Psychopharmacology and Biological Psychiatry 39, 326–331 (2012). [DOI] [PubMed] [Google Scholar]
- Power J. D., Barnes K. A., Snyder A. Z., Schlaggar B. L. & Petersen S. E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage 59, 2142–2154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- David O. et al. Identifying neural drivers with functional MRI: an electrophysiological validation. PLoS Biol. 6, e315 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. PLoS Biol. 7, e1000033 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh I. E. H. fMRI resting state time series causality: comparison of Granger causality and phase slope index. (2014).
- Deshpande G., Sathian K. & Hu X. Effect of hemodynamic variability on Granger causality analysis of fMRI. NeuroImage 52, 884–896 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K. R. et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology 103, 297–321 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. et al. Multivariate classification of social anxiety disorder using whole brain functional connectivity. Brain Structure & Function 220, 1–15 (2013). [DOI] [PubMed] [Google Scholar]
- Liu F. et al. Disrupted cortical hubs in functional brain networks in social anxiety disorder. Clinical Neurophysiology Official Journal of the International Federation of Clinical Neurophysiology 126, 1711–1716 (2015). [DOI] [PubMed] [Google Scholar]
- Wu G.-R., Stramaglia S., Chen H., Liao W. & Marinazzo D. Mapping the voxel-wise effective connectome in resting state fMRI. PloS one 8, e73670 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.-R. et al. A blind deconvolution approach to recover effective connectivity brain networks from resting state fMRI data. Medical image analysis 17, 365–374 (2013). [DOI] [PubMed] [Google Scholar]
- Goebel R., Roebroeck A., Kim D.-S. & Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magnetic resonance imaging 21, 1251–1261 (2003). [DOI] [PubMed] [Google Scholar]
- Roebroeck A., Formisano E. & Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. NeuroImage 25, 230–242 (2005). [DOI] [PubMed] [Google Scholar]
- Gao Q., Chen H. & Gong Q. Evaluation of the effective connectivity of the dominant primary motor cortex during bimanual movement using Granger causality. Neuroscience letters 443, 1–6 (2008). [DOI] [PubMed] [Google Scholar]
- Chen H., Yang Q., Liao W., Gong Q. & Shen S. Evaluation of the effective connectivity of supplementary motor areas during motor imagery using Granger causality mapping. NeuroImage 47, 1844–1853 (2009). [DOI] [PubMed] [Google Scholar]
- DeYoung C. G. et al. Testing predictions from personality neuroscience brain structure and the big five. Psychological science 21, 820–828, 10.1177/0956797610370159 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji G. J. et al. Identifying Corticothalamic Network Epicenters in Patients with Idiopathic Generalized Epilepsy. American Journal of Neuroradiology 36 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.