Abstract
In vitro bone regeneration strategies that prime mesenchymal stem cells (MSCs) with chondrogenic factors, to mimic aspects of the endochondral ossification process, have been shown to promote mineralization and vascularization by MSCs both in vitro and when implanted in vivo. However, these approaches required the use of osteogenic supplements, namely dexamethasone, ascorbic acid, and β-glycerophosphate, none of which are endogenous mediators of bone formation in vivo. Rather MSCs, endothelial progenitor cells, and chondrocytes all reside in proximity within the cartilage template and might paracrineally regulate osteogenic differentiation. Thus, this study tests the hypothesis that an in vitro bone regeneration approach that mimics the cellular niche existing during endochondral ossification, through coculture of MSCs, endothelial cells, and chondrocytes, will obviate the need for extraneous osteogenic supplements and provide an alternative strategy to elicit osteogenic differentiation of MSCs and mineral production. The specific objectives of this study were to (1) mimic the cellular niche existing during endochondral ossification and (2) investigate whether osteogenic differentiation could be induced without the use of any external growth factors. To test the hypothesis, we evaluated the mineralization and vessel formation potential of (a) a novel methodology involving both chondrogenic priming and the coculture of human umbilical vein endothelial cells (HUVECs) and MSCs compared with (b) chondrogenic priming of MSCs alone, (c) addition of HUVECs to chondrogenically primed MSC aggregates, (d–f) the same experimental groups cultured in the presence of osteogenic supplements and (g) a noncoculture group cultured in the presence of osteogenic growth factors alone. Biochemical (DNA, alkaline phosphatase [ALP], calcium, CD31+, vascular endothelial growth factor [VEGF]), histological (alcian blue, alizarin red), and immunohistological (CD31+) analyses were conducted to investigate osteogenic differentiation and vascularization at various time points (1, 2, and 3 weeks). The coculture methodology enhanced both osteogenesis and vasculogenesis compared with osteogenic differentiation alone, whereas osteogenic supplements inhibited the osteogenesis and vascularization (ALP, calcium, and VEGF) induced through coculture alone. Taken together, these results suggest that chondrogenic and vascular priming can obviate the need for osteogenic supplements to induce osteogenesis of human MSCs in vitro, while allowing for the formation of rudimentary vessels.
Introduction
Bone tissue engineering is a promising strategy for treating bone diseases and reconstructing bone defects.1–3 It typically involves the culture of mesenchymal stem cells (MSCs), which are often grown on biomaterial scaffolds in an in vitro environment and in the presence of osteogenic growth factors and cell culture nutrients. However, these strategies have been associated with complications such as fibrous tissue encapsulation4–6 and degradation of the tissue-engineered constructs when implanted in vivo.5–8 Moreover, in vitro cultured mineralized tissue constructs lack a vascular supply, which may contribute to their poor performance after implantation.4–8
The standard procedure to induce osteogenic differentiation of MSCs in vitro is through the culture of the cells in the presence of a cocktail of dexamethasone, ascorbic acid, and β-glycerophosphate.9–17 Dexamethasone is a steroid that causes MSC differentiation into osteoblasts by activation of the WNT/β-catenin signaling pathway, which in turn activates Runx2 expression and induces the differentiation of MSCs into immature osteoblasts.18–20 Ascorbic acid acts as a cofactor for enzymes that hydroxylate proline and lysine in collagen21 and participates in collagen chain formation.22 It is also the predominant regulator of collagen type 1 secretion.18 β-Glycerophosphate is an inorganic phosphate needed to produce hydroxyapatite mineral and has been shown in many studies to play an important role in the osteogenic differentiation of MSCs.12,23–25 It also regulates expression of genes including osteopontin and BMP-2.26–28 Exposure of rat MSCs,12,14–17 human MSCs (hMSCs),9,11,13 or murine osteoblasts22,29 to dexamethasone, ascorbic acid, and β-glycerophosphate can significantly increase alkaline phosphatase (ALP) activity in vitro.
However, in vivo none of these in vitro supplements are present or regulate the physiological differentiation of osteoprogenitor cells. Instead, paracrine factors produced by various cell types, such as MSCs, endothelial progenitor cells, and chondrocytes, contribute to osteogenic differentiation. Recent studies have investigated the physical and chemical signaling that occurs because of the culture of MSCs with other cell types, including chondrocytes, endothelial cells, osteoblasts, and osteocytes. One such study confirmed for the first time the synergistic relationship between osteocytes and osteoblasts in stimulating osteogenic differentiation of MSCs.30 However, to date knowledge about MSC behavior, particularly the interactions between MSCs and endothelial cells within the stem cell niche in vivo, remains largely unknown.31–35
During endochondral ossification, MSCs, endothelial stem cells, and chondrocytes all reside in proximity within the cartilage template before the invasion by osteoblasts and bone formation. The anatomical location of MSCs and vascular endothelial cells suggests that these two cell types are in direct cell–cell interaction and experience juxtacrine or paracrine signaling within the stem cell niche during endochondral ossification. Previous in vitro studies have shown that direct coculture of MSCs or osteoblasts with endothelial cells can upregulate production of the early osteogenic marker ALP,36–39 without the presence of osteogenic supplements. Other studies have investigated whether coculture of MSCs and endothelial cells can increase ALP production in three-dimensional (3D) polymer scaffolds40,41 or 3D cellular aggregates,42–45 but the majority of these studies were conducted in the presence of osteogenic growth supplements.42–45 The osteogenic potential of MSC/chondrocyte or osteoblast/chondrocyte cocultures has been variable and inconclusive in both two-dimensional (2D) and 3D cultures.46–48
One study investigated the effect of coculture of hMSCs and chondrocytes, without the use of osteogenic supplements, and found that there was no ALP production/expression in 3D aggregate culture.47 However, direct 2D coculture of rat osteoblasts and bovine chondrocytes reported a significant increase in ALP production over a period of 6 weeks and there was significantly higher ALP activity in the coculture group than the osteoblast group alone.48 Coculture of MSCs and endothelial cells through transwell inserts induced MSCs to undergo both osteogenesis and chondrogenesis, through the endothelin-1 phosphatidylinositol 3-kinase/AKT (AKT) signaling pathway.34 Moreover, a novel coculture technique was investigated, which involved the coculture of human umbilical vein endothelial cells (HUVECs) and MSCs with MSCs that have been predifferentiated toward the chondrogenic lineage.49 This study showed that the HUVEC/MSC coculture group did not produce as much mineral as coculture with HUVECs alone. However, this approach involved the use of osteogenic supplements, which might interfere with the signaling occurring because of the direct coculture.
This study tests the hypothesis that an in vitro bone regeneration strategy that mimics the cellular niche of the endochondral template, through the coculture of MSCs, endothelial cells, and chondrocytes, will provide an alternative strategy for in vitro mineralization of MSCs, and thereby obviate the need for external osteogenic growth factors to induce osteogenesis in vitro in a 3D culture environment. The objective of this study was to evaluate the mineralization potential of (a) a novel methodology involving both chondrogenic priming and the coculture of HUVECs and MSCs without the use of osteogenic supplements compared with (b) chondrogenic priming of MSCs alone, (c) addition of human umbilical vein endothelial cells (HUVECs) to chondrogenically primed MSC aggregates, (d–f) the same experimental groups cultured in the presence of osteogenic supplements, and (g) a noncoculture group cultured in the presence of osteogenic growth factors alone. These studies involved the use of biochemical (DNA, ALP, calcium, CD31, and vascular endothelial growth factor [VEGF]) assays and histological (alcian blue and alizarin red) and immunohistological (CD31+) staining.
Materials and Methods
Cell culture
Human donor MSC: isolation and characterization
Bone marrow-derived hMSCs harvested from two male donors 20–25 years old, with established multipotency, were purchased from the Texas A&M University Health Science Centre (Temple, TX). The hMSCs were expanded in Minimum Essential Medium alpha (αMEM; Invitrogen, Carlsbad, CA) containing 16.7% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA) and 100 U/mL penicillin/100 μg/mL streptomycin/2 mM l-glutamine (PSL; Invitrogen) at 37°C and 5% CO2. For all cell cultures performed in this study, cell culture medium was changed twice weekly unless stated otherwise. At passage 2, cells from each donor were detached using 0.25% trypsin-EDTA (Invitrogen) and combined 1:1 after which they were further cultured to passages 3–4.
HUVECs culture
HUVECs were commercially available and purchased from Lonza (Walkersville, MD) and cultured in Clonetics endothelial growth medium (EGM) SingleQuots (Lonza). The medium was replaced every 3 days and, upon reaching 80–90% confluency, cells were passaged using 0.25% trypsin-EDTA (Invitrogen). HUVECs were further cultured to passage 3.
Aggregate formation
Once the hMSCs reached a confluency of ∼80%, the cells were trypsinized, counted, and centrifuged at 650 g at a temperature of 22°C for 5 min. The cells were then resuspended in expansion medium at a density of 0.25 × 106 cells/mL. This cell suspension was divided into 1.5 mL tubes so that there were 250,000 cells in each tube, and these were then centrifuged for 5 min (Eppendorf Centrifuge 5430R; Vashaw Scientific, Norcross, GA) at 400 g to create cellular aggregates. Carefully avoiding the newly formed aggregate, the medium was removed from each of the aggregates and 0.5 mL of chondrogenic medium was added. The chondrogenic medium consisted of a chemically defined medium, which contained high-glucose Dulbecco's modified Eagle medium (DMEM) GlutaMAXTM (Invitrogen), 10 ng/mL TGF-β3 (Invitrogen), 50 μg/mL ascorbic acid (Sigma-Aldrich), 4.7 μg/mL linoleic acid–oleic acid (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), and 1× insulin–transferrin–selenium (Invitrogen). For all experiments, aggregate cultures were fed twice per week by performing a 50% medium exchange. During each feed, the aggregates were agitated, so as to prevent them from adhering to the microtube. This was achieved through aspirating the medium beneath the aggregate with a micropipette.
After 21 days, the aggregates were separated into three different experimental conditions. (1) CP21-HUVECs: aggregates were chondrogenically primed for a period of 21 days and then cultured in EGM for a further 21 days [hereafter known as the CP21-HUVECs (-OM)]. (2) CP21+HUVECs: aggregates were chondrogenically primed for 21 days after which 250,000 suspended HUVECs in EGM were added to the cellular aggregate and cultured in EGM for further 21 days [hereafter known as the CP21+HUVECs (-OM)]. (3) CP21+HUVECs:MSCs: aggregates were chondrogenically primed for 21 days after which 250,000 suspended HUVECs and MSCs at a ratio of 1:1 (125,000:125,000 cells) in EGM were added and further cultured in EGM for 21 days [hereafter known as the CP21+HUVECs:MSCs (-OM)]. These experimental groups were then compared with the same groups cultured in the presence of osteogenic supplements after the chondrogenic priming period [CP21-HUVECs (+OM), CP21+HUVECs (+OM), and CP21+HUVECs:MSCs (+OM)], similar to those described previously.49 In brief, osteogenic growth factors (100 nM dexamethasone, 50 μg/mL ascorbic acid, and 10 mM β-glycerol phosphate; all Sigma-Aldrich) were added to the EGM for the remaining 21 days for each of experimental conditions already described (chondrogenic priming for 21 days and number of cells). These experimental groups were also compared with a control group of 250,000 MSCs cultured in osteogenic growth medium (DMEM supplemented with 10% FBS, growth factors already described) for 21 days (hereafter known as the Osteo alone).
HUVECs/MSCs were added to the relevant aggregates by suspending the cells depending on experimental conditions so that there were 0.5 × 106 cells/mL. In the case of the CP21+HUVECs:MSCs, the ratio of cells was 1:1 HUVECs:MSCs. The cells were suspended in EGM alone (no osteogenic supplements were added) and 20% methocel from a stock solution that was generated by dissolving 6 g of carboxymethylcellulose (Sigma-Aldrich) in 500 mL of DMEM as previously described.50 After 24 h, the medium that contained methocel was removed and was replaced with EGM alone and the aggregates were cultured for a further 20 days.
Histochemical and biochemical analyses
Aggregates were examined using histochemical and biochemical techniques at day 21 (preaddition of coculture cells), and also 1, 2, and 3 weeks after the addition of the cells. Aggregates were prepared for either histochemical analysis or biochemical analysis. At each of the time points, the culture medium from the aggregates was collected, frozen, and stored at −80°C until biochemical assays could be performed. The remaining aggregates were washed with phosphate-buffered saline (PBS) and then treated in one of the following two ways: (1) frozen and stored at −80°C for biochemical analysis or (2) fixed overnight in paraformaldehyde before being placed in PBS and refrigerated for histochemical analysis as previously described.49,51 Two independent experiments were carried out with at least two replicates in each experiment (n = 4 for histological analysis and n = 10 for biochemical analysis).
Quantitative biochemical analysis
DNA content
To assess DNA content, 500 μL of papain digest (100 mM sodium phosphate buffer containing 10 mM l-cysteine [Sigma-Aldrich], 125 μg/mL papain [Sigma-Aldrich], and 5 mM Na2EDTA [Sigma Aldrich] in ddH2O at pH 6.5) was added to the aggregates and the aggregates were placed in an oven at 60°C overnight, as previously described.52 Once the aggregates were digested, DNA content was performed using Picogreen DNA assay (BD Biosciences) with calf thymus DNA (Sigma-Aldrich) as a standard. In minimal light, 40 μL of papain digest of the sample/standard was added to a 96-well plate in triplicate. To this, 200 μL of working solution (1× Tris EDTA [TE] buffer and 5 μL/mL Picogreen solution [Bio-Sciences]) was added. The plate was incubated away from light for 5 min and then read on a microplate reader (Perkin Elmer HTS 7000 Microplate Reader) at an excitation wavelength of 485 nm and emission of 535 nm.
ALP production
Extracellular ALP production was determined using a colorimetric assay of enzyme activity, which uses p-nitrophenyl phosphate (pNPP) as a phosphatase substrate with ALP enzyme (Sigma-Aldrich) as a standard. Solutions of 2-amino-2 methyl-1-propanol (AMP; 1.5 M with a pH 10.25), pNPP (20 mM), and magnesium chloride (MgCl2; 10 mM) were prepared. Each of these solutions was combined in a ratio of 1:1:1 to make the substrate working solution (AMP–pNPP–MgCl2). Next, 50 μL of the medium was added to a 96-well plate in triplicate with 50 μL of substrate working solution. The samples were shielded from direct light at 37°C for 1 h. After this, 100 μL of stop solution (1 M NaOH) was added to the wells and the plate was read at 405 nm in a microplate reader.
Calcium content
Calcium deposition within the aggregates was measured using Arsenazo III calcium reagent (Sekisui Diagnostics) according to the manufacturer's protocol. In brief, aggregates were digested by adding 0.5 mL of 1 M acetic acid and the solution was rotated overnight in a cold room. If the samples were not fully digested, the samples were homogenized until fully digested. Next, 25 μL of each of the digested samples and assay standard was added to a 96-well plate and 300 μL of the calcium reagent was added. The plate was incubated for 30 s at room temperature and analyzed on a microplate reader at an absorbance of 650 nm.
Enzyme-linked immunosorbent assay for vascular growth factor and CD31+
An enzyme-linked immunosorbent assay (ELISA; R&D Systems) was used to quantify the levels of vascular endothelial growth factor (VEGF) and CD31+ expressed by the pellets. The cell culture medium was collected and analyzed at the specific time points. Assays were carried out according to the manufacturer's protocol (R&D Systems) and analyzed on a microplate reader at a wavelength of 450 nm.
Histology
Each sample was fixed overnight in 4% paraformaldehyde and then dehydrated and embedded in paraffin using an automatic tissue processor (Leica ASP300; Leica). All samples were sectioned with a thickness of 8 μm using a rotary microtome (Leica microtome; Leica). Sections were stained with 1% alcian blue 8GX solution for sGAG, as previously described,49,51,52 and finally with 2% alizarin red solution for mineralization (all Sigma-Aldrich) as previously described.49,51
CD31+ immunohistochemical analysis
Immunohistochemical analysis was used to detect CD31+ as previously described.49 In brief, sections were deparaffinized overnight before a series of rehydration steps. The samples were then treated with 40 μg/mL of proteinase K for 20 min at 37°C (Sigma-Aldrich), rinsed with PBS-Tween, and blocked with PBS with 1% w/v bovine serum albumin (BSA) and 3% w/v normal goat serum (NGS; Sigma-Aldrich) for 60 min. Sections were then incubated overnight at 4°C with rabbit polyclonal anti-CD31+ (ab28364, 1:50; Abcam). After three washing steps with PBS with 1% w/v BSA, the sections were incubated with Dylight488 goat antirabbit secondary antibody (115-485-209, 1/200; Jackson Immunoresearch) for 1 h at room temperature in the dark. Finally, samples were washed three times with PBS with 1% w/v BSA and sections were mounted using 4′,6-diamidino-2-phenylindole (DAPI) mounting medium (Sigma-Aldrich).
Statistical analysis
Results are expressed as mean ± standard deviation. For all the biochemical analyses, two-way analysis of variance with time and medium type as the independent factors followed by a pair-wise multiple comparison procedure (Tukey's HSD test), was used to test for significance. For quantitative analysis on vessel cross-sectional area, a standard student t-test was performed. All analyses were performed with Graphpad. For all comparisons, the level of significance was p ≤ 0.05.
Results
DNA content
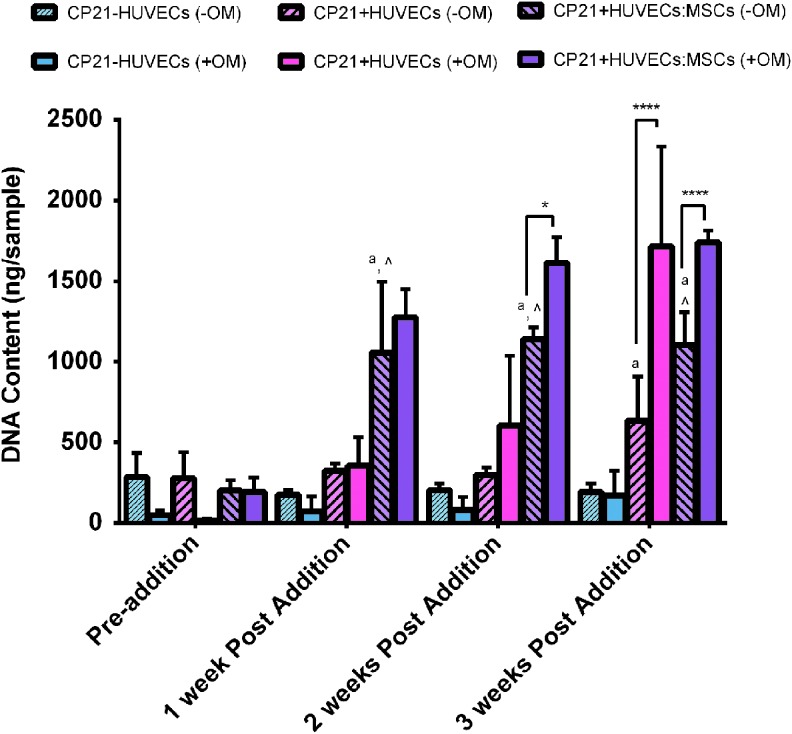
There was no statistical difference in number of cells for the CP21-HUVECs (-OM) group over the course of the experiment (Fig. 1). However, there was a significant increase (p < 0.05) in DNA content at the 3 week time point, in both the CP21+HUVECs (-OM) and the CP21+HUVECs:MSCs (-OM) groups, compared with the baseline condition before cells were added (preaddition of cells). The CP21+HUVECs:MSCs (-OM) group had significantly higher DNA content than both the CP21-HUVECs (-OM) (p < 0.001) and the CP21+HUVECs (-OM) groups (p < 0.001) at 1, 2, and 3 weeks postaddition of cells. The CP21+HUVECs (-OM) group had significantly higher DNA content than the CP21-HUVECs (-OM) group (p < 0.01) after 3 weeks.
FIG. 1.
DNA content/sample of all three experimental conditions cultured without osteogenic supplements (-OM) compared with the results from our previous study49 of the same groups cultured in the presence of osteogenic supplements (+OM). ^p < 0.05 versus CP21+HUVECs group, ap < 0.05 versus CP21-HUVECs.*p < 0.05, and ****p < 0.0001. (n = 6 samples per group per time point.) Error bars denote standard deviation. HUVECs, human umbilical vein endothelial cells. Color images available online at www.liebertpub.com/tea
There was no difference in DNA content in the CP21-HUVECs group cultured with osteogenic supplements compared with the same group cultured without relevant supplements. However, at both 2 and 3 weeks, the CP21+HUVECs:MSCs group cultured with osteogenic supplements (+OM) had significantly higher (p < 0.05, p < 0.0001, respectively) DNA content than the same group cultured without osteogenic supplements (-OM). Moreover, the CP21+HUVECs group cultured with osteogenic supplements (+OM) had significantly higher (p < 0.0001) DNA content than its counterpart cultured without osteogenic supplements (-OM) after 3 weeks of coculture.
Production of cartilage template
Alcian blue staining
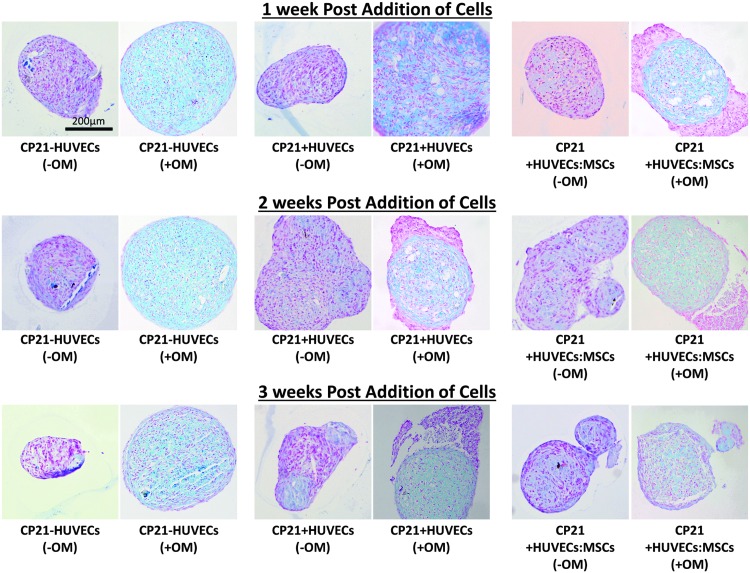
All three culture groups cultured without osteogenic factors stained positive blue for sGAG 1 week into culture and after 3 weeks of culture (Fig. 2).
FIG. 2.
Alcian blue staining of all three groups cultured with (+OM) and without osteogenic supplements (-OM) over the course of the experiment. Each of the images was imaged at a magnification of 20 × . Color images available online at www.liebertpub.com/tea
In the CP21+HUVECs group cultured in osteogenic medium (+OM), HUVECs were identified around the periphery of the aggregate throughout the course of the experiment (as indicated by pink staining around the periphery of the cartilage template in Fig. 2). However, in the CP21+HUVECs group cultured without osteogenic supplements (-OM), the HUVECs invaded the cartilage template by 2 weeks. The size of aggregates also differed; in general, the average cross-sectional area of the groups cultured without osteogenic growth factors was smaller than that of their corresponding groups cultured in the presence of the osteogenic growth factors. However, there was no significant difference in size between any of the groups at any time point.
Mineralization of the cartilage template
ALP production
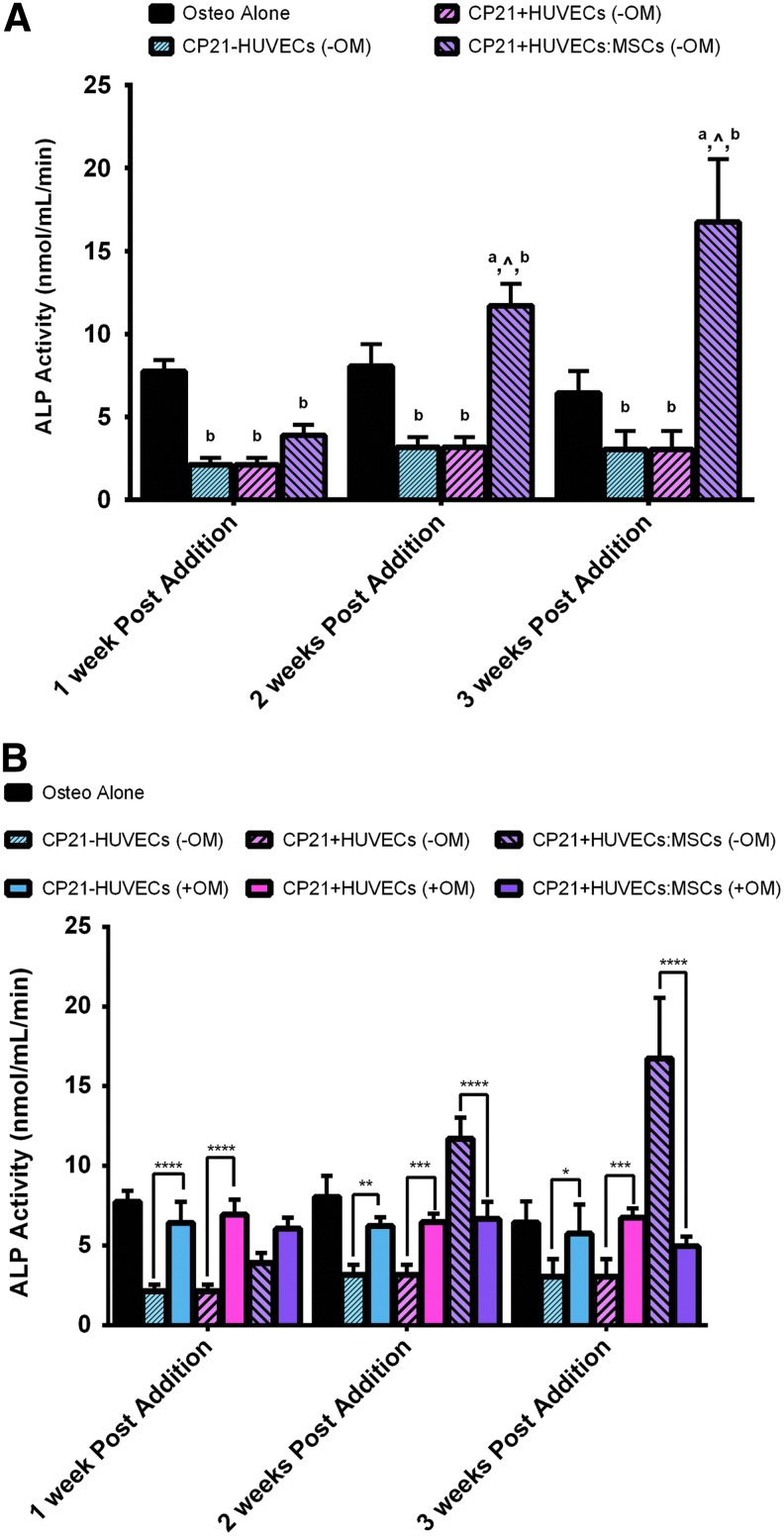
There was no significant increase in ALP expression in either the CP21-HUVECs (-OM) or the CP21+HUVECs (-OM) groups, without osteogenic supplements, over the course of the experiment (Fig. 3A). However, there was a significant increase in ALP expression in the CP21+HUVECs:MSCs (-OM) group from 1 to 2 weeks (p < 0.0001) and from 2 to 3 weeks (p < 0.0001) of coculture. Moreover, the CP21+HUVECs:MSCs (-OM) group had significantly higher ALP expression than the CP21+HUVECs (-OM) group after 2 weeks (p < 0.0001) and 3 weeks (p < 0.0001) of coculture and significantly higher ALP expression than the CP21-HUVECs (-OM) group at all three time points (p < 0.0001). The CP21+HUVECs:MSCs (-OM) group also had significantly higher ALP expression than the osteogenic group (MSCs cultured in osteogenic medium alone) at both 2 (p < 0.001) and 3 weeks (p < 0.0001) after the addition of cells.
FIG. 3.
(A) ALP activity of all of the experimental groups cultured without osteogenic supplements compared with osteogenic group alone at 1, 2, and 3 weeks after the addition of HUVECs/MSCs. ^p < 0.05 versus CP21+HUVECs group, ap < 0.05 versus CP21-HUVECs, and bp < 0.05 versus Osteo alone. (B) ALP activity of all three experimental groups cultured without osteogenic supplements compared with the same groups cultured in the presence of osteogenic supplements and the osteogenic group alone. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. (n = 6 samples per group per time point.) Error bars denote standard deviation. ALP, alkaline phosphatase; MSCs, mesenchymal stem cells. Color images available online at www.liebertpub.com/tea
At all three time points, the ALP expression in the CP21-HUVECs and CP21+HUVECs groups cultured in osteogenic medium (+OM) was significantly higher (p < 0.05) than the same groups cultured without (-OM) (Fig. 3B). However, the opposite was seen in the CP21+HUVECs:MSCs groups. There was significantly higher (p < 0.0001) ALP expression in the CP21+HUVECs:MSCs (-OM) group than in the same group cultured with osteogenic supplements as well as in all other groups at both 2 and 3 weeks into coculture.
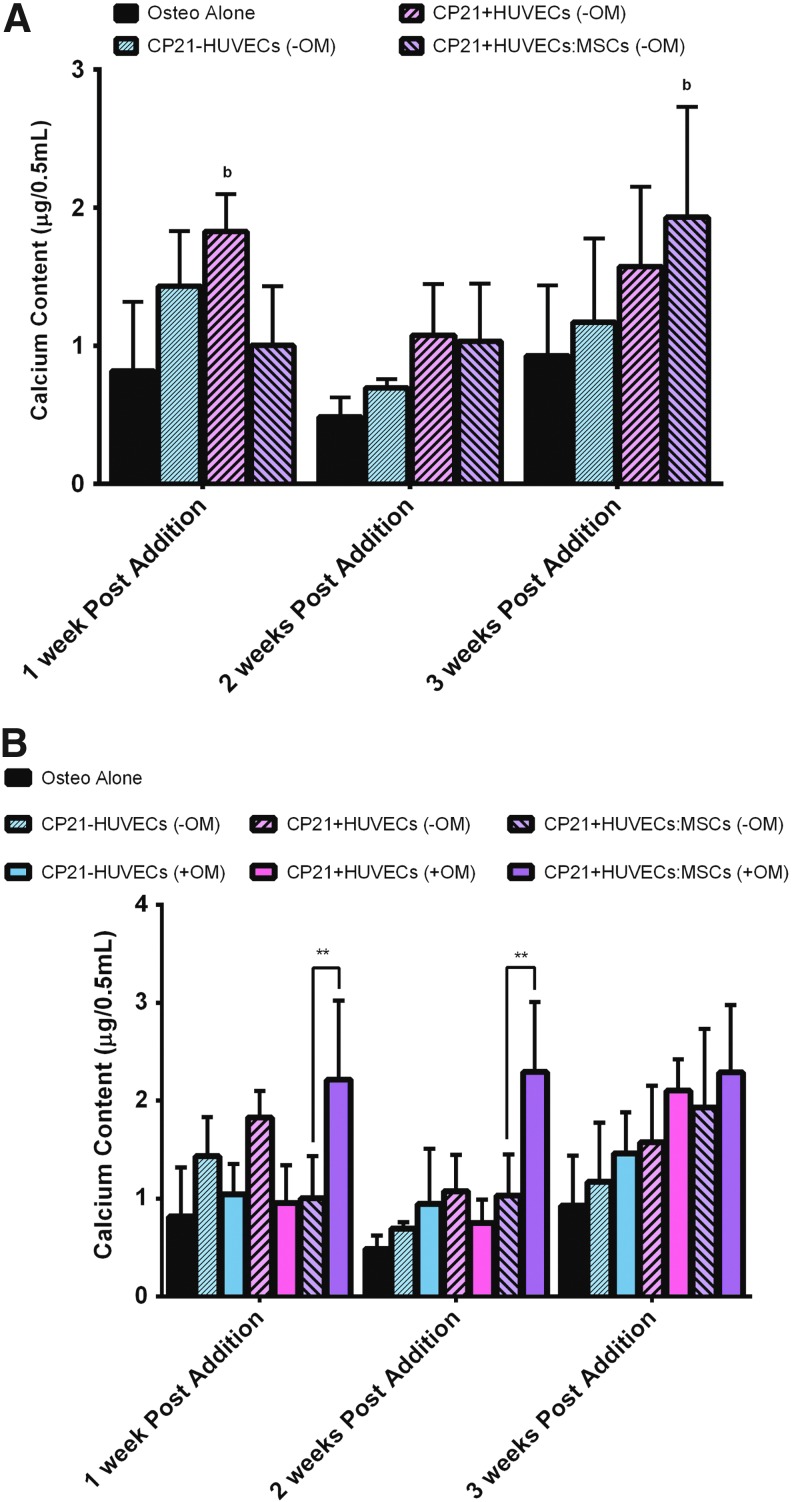
Calcium content
There was no significant difference in calcium content in both the CP21-HUVECs (-OM) and the CP21+HUVECs (-OM) groups over the course of the coculture period (Fig. 4A). However, in the CP21+HUVECs:MSCs (-OM) group, there was a significant increase in calcium content from 2 to 3 weeks into coculture. Moreover, there was significantly higher (p < 0.05) calcium content in the CP21+HUVECs:MSCs (-OM) group than in the osteogenic group alone after 3 weeks of coculture.
FIG. 4.
(A) Calcium content of all of the experimental groups cultured without osteogenic supplements (-OM) compared with osteogenic group alone at 1, 2, and 3 weeks after the addition of HUVECs/MSCs. bp < 0.05 versus Osteo alone. (B) Calcium content of all three experimental groups cultured without osteogenic supplements compared with the same groups cultured in the presence of osteogenic supplements (+OM) and the osteogenic group alone. **p < 0.01, n = 6 samples per group per time point. Error bars denote standard deviation. Color images available online at www.liebertpub.com/tea
Interestingly, there was no significant difference in calcium content in both the CP21-HUVECs (-OM) and the CP21+HUVECs (-OM) groups, compared with their counterparts cultured in the presence of osteogenic supplements (+OM) at all time points (Fig. 4B). There was, however, significantly higher calcium content in the CP21+HUVECs:MSCs group that was cultured in the presence of osteogenic supplements (+OM) than in its counterpart cultured without osteogenic supplements (-OM) at both 1 (p < 0.0001) and 2 weeks (p < 0.0001) into coculture. However, this significant difference was lost by 3 weeks into coculture.
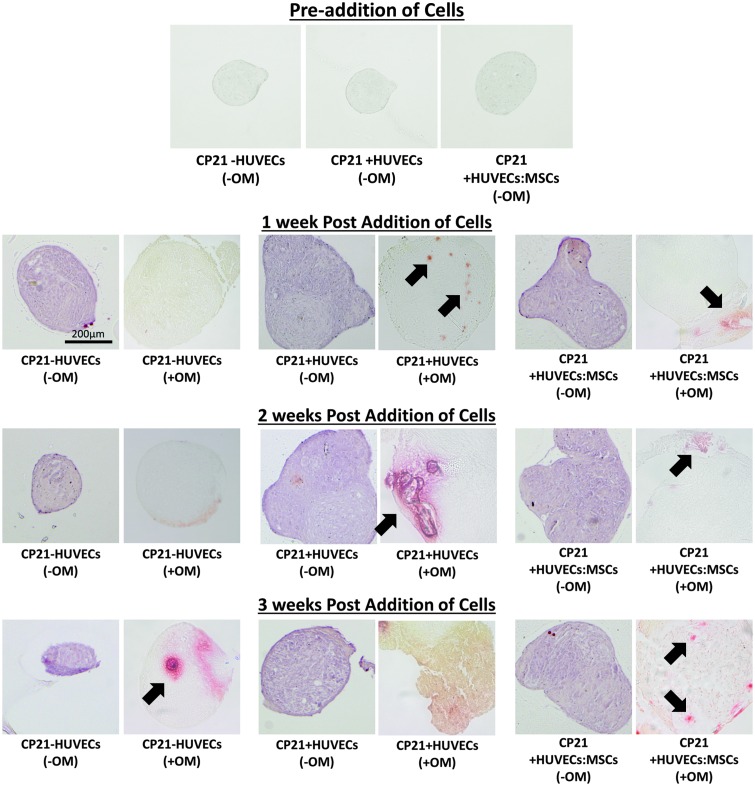
Alizarin red staining
There was no positive alizarin red staining in any of the groups cultured without osteogenic supplements before the coculture period (Fig. 5). Interestingly, in the groups cultured without osteogenic supplements (-OM), there was positive red staining throughout the aggregate after 1 week of coculture, (Fig. 5). No difference was seen between the coculture groups cultured without osteogenic supplements and the CP21-HUVECs (-OM) group at all time points as positive red staining was seen throughout all the aggregates. However, in the same groups cultured in the presence of osteogenic supplements, mineralization was only seen in the formation of nodules within the aggregate, and the only group to have mineralization throughout the aggregate was the CP21+HUVECs (+OM) group 3 weeks after the addition of cells (Fig. 5).
FIG. 5.
Alizarin red staining of the aggregates cultured without osteogenic supplements before addition of cells and all three groups cultured with (+OM) and without osteogenic supplements (-OM) at 1, 2, and 3 weeks after the addition of cells. Each of the images was imaged at a magnification of 20 × . Arrows denote mineralization nodules present within the cellular aggregates. Color images available online at www.liebertpub.com/tea
Vascularization of the cartilage template
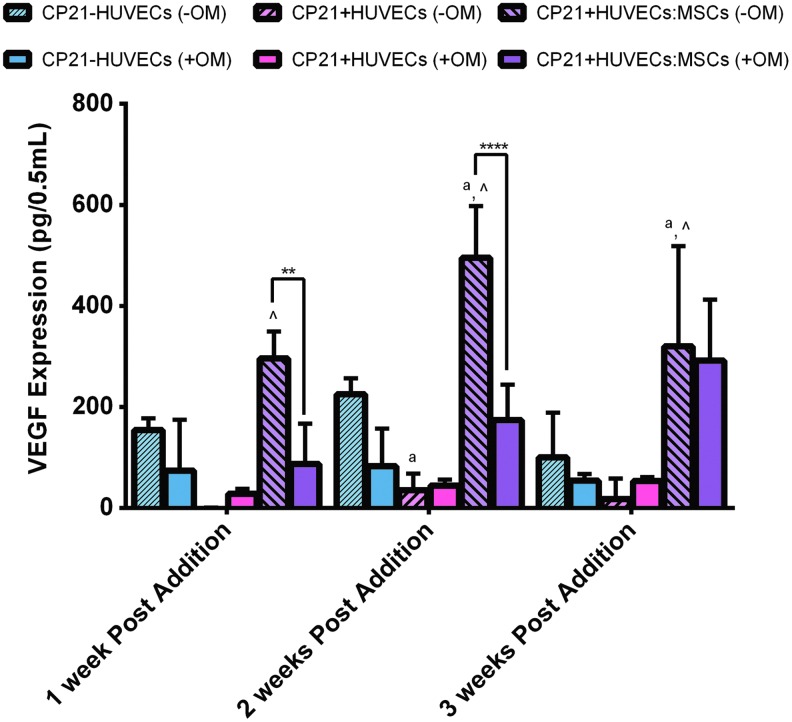
VEGF production
There was no significant difference in VEGF content between the CP21-HUVECs (-OM) and the CP21+HUVECs (-OM) groups over the course of the experiment (Fig. 6). However, there was a significant increase (p < 0.01) in VEGF content in the CP21+HUVECs:MSCs (-OM) group from 1 to 2 weeks into coculture and a significant decrease (p < 0.05) in VEGF content from 2 to 3 weeks into coculture. There was also significantly higher (p < 0.0001) VEGF content in the CP21+HUVECs:MSCs (-OM) group than in the CP21+HUVECs (-OM) group at all time points and significantly higher VEGF content (p < 0.001) than the CP21-HUVECs (-OM) group at both 2 and 3 weeks into coculture. Moreover, the CP21-HUVECs (-OM) group had significantly higher (p < 0.05) VEGF expression 2 weeks into coculture than the CP21+HUVECs group.
FIG. 6.
An ELISA of VEGF expression of all three experimental groups cultured without osteogenic supplements (-OM) compared with the same groups cultured in the presence of osteogenic supplements (+OM). ^p < 0.05 versus CP21+HUVECs group, ap < 0.05 versus CP21-HUVECs, **p < 0.01 and ****p < 0.0001, n = 6 samples per group per time point. Error bars denote standard deviation. ELISA, enzyme-linked immunosorbent assay; VEGF, vascular endothelial growth factor. Color images available online at www.liebertpub.com/tea
There was no significant difference in VEGF expression of both the CP21-HUVECs (-OM) and the CP21+HUVECs (-OM) groups compared with their counterparts cultured with osteogenic supplements (Fig. 6). However, at both 1 and 2 weeks into coculture, there was significantly higher (p < 0.01) VEGF content in the CP21+HUVECs:MSCs (-OM) group than in its counterpart cultured in the presence of osteogenic supplements. This significant difference was lost by 3 weeks into coculture.
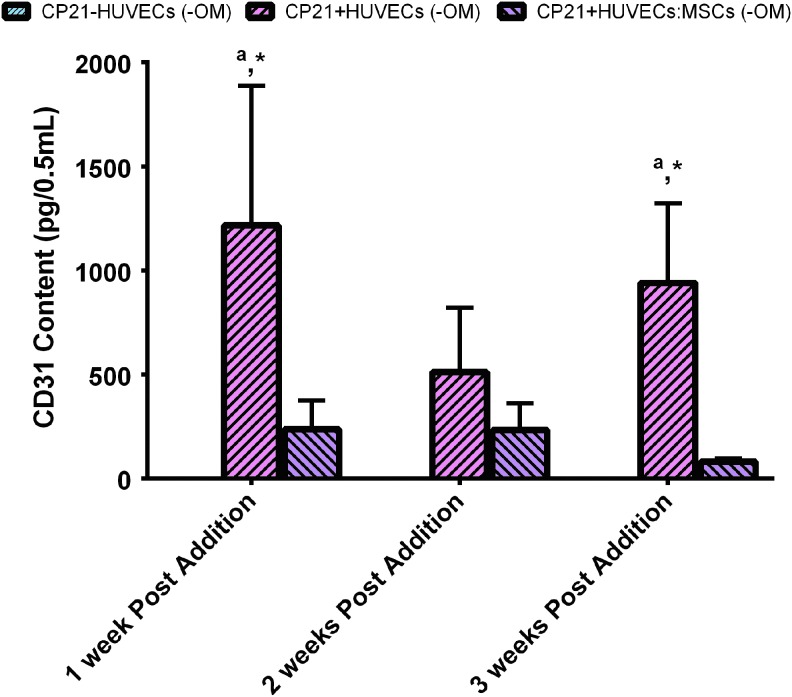
CD31+ production
At all time points, there was no detectable CD31+ content in the CP21-HUVECs (-OM) group, because of the lack of endothelial cells present (Fig. 7). However, there was CD31+ content in both the CP21+HUVECs (-OM) and the CP21+HUVECs:MSCs (-OM) groups. There was no significant increase or decrease in CD31+ content in either group over the course of the experiment. Moreover, 1 and 3 weeks after cells were added, there was significantly more CD31+ content in the CP21+HUVECs (-OM) group than in both the CP21-HUVECs (-OM) and the CP21+HUVECs:MSCs (-OM) groups.
FIG. 7.
An ELISA of CD31 content of all of the experimental groups cultured without osteogenic supplements at 1, 2, and 3 weeks after the addition of HUVECs/MSCs. ap < 0.05 versus CP21-HUVECs, and *p < CP21+HUVECs:MSCs. (n = 6 samples per group per time point.) Error bars denote standard deviation. Color images available online at www.liebertpub.com/tea
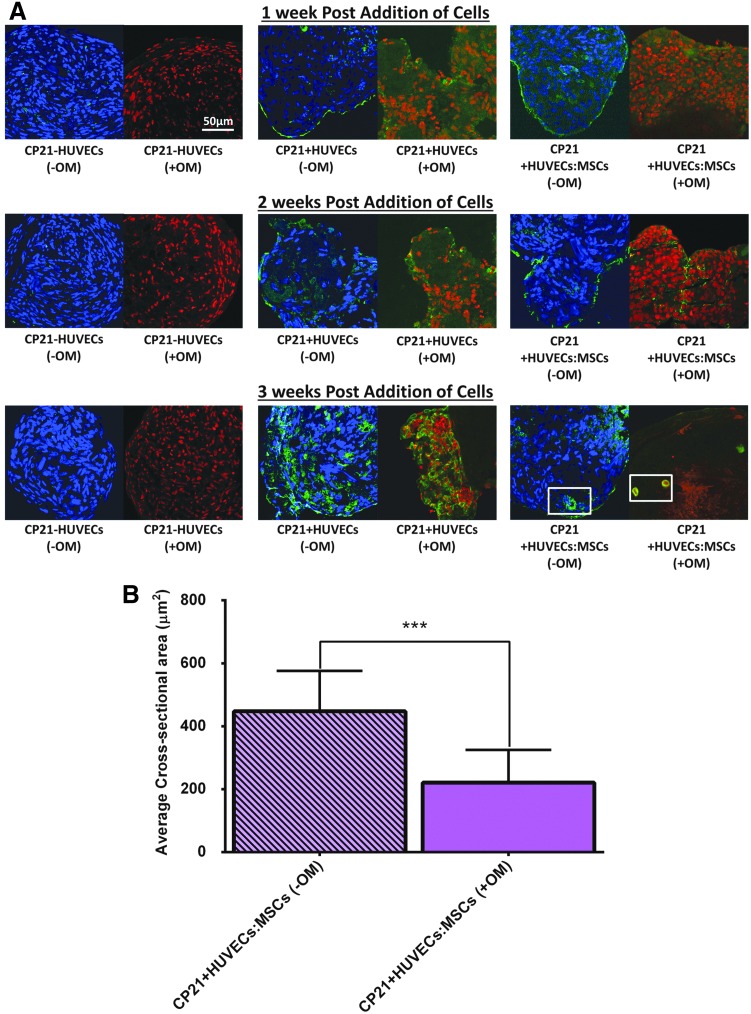
CD31+ staining
At all time points, there was a lack of positive (green) staining for CD31+ in the CP21-HUVECs (-OM) group cultured without osteogenic supplements, as there was no endothelial cells present (Fig. 8). However, for both the CP21+HUVECs (-OM) and the CP21+HUVECs:MSCs (-OM) groups, there was positive (green) staining seen around the periphery of both groups at 1 and 2 weeks into coculture. By 3 weeks into coculture, both groups had positive green staining present within the center of the aggregates; however, the CP21+HUVECs:MSCs (-OM) group was the only group to have rudimentary vessels beginning to form. The structure of these vessels was the same in all of the aggregates, characterized by a circular CD31+ positive wall with irregularly shaped nuclei present within the lumen (indicated by white box in Fig. 8A).
FIG. 8.
(A) CD31 staining (green) of all three experimental groups cultured with (+OM) and without (-OM) osteogenic supplements over the course of the experiment. Each of the images was imaged at a magnification of 40× with either a nuclei counterstain DAPI (blue) or propidium iodide (red). Boxes denote examples of the rudimentary vessels present within the aggregates. (B) Quantitative analysis of cross-sectional area of the rudimentary vessels present in the aggregates. ***p < 0.001 and error bars denote standard deviation (n = 9). DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
At all time points, there was positive green staining around the periphery of the aggregate in the CP21+HUVECs (+OM) group cultured in the presence of osteogenic supplements (Fig. 8). However, its counterpart cultured without osteogenic supplements had positive staining present both at the periphery and within the center of the aggregates. Moreover, by 3 weeks, preliminary vessels were present in the center of the aggregates in the CP21+HUVECs:MSCs group irrespective of whether it was cultured with or without osteogenic supplements. Interestingly, the cross-sectional area of the preliminary vessels present within the CP21+HUVECs:MSCs (-OM) group cultured without osteogenic supplements was significantly higher (p < 0.001) than the vessels present within its counterpart cultured with osteogenic supplements (Fig. 8B).
Discussion
This study investigated whether an in vitro bone regeneration strategy that mimics the cellular niche existing during endochondral ossification, through the coculture of MSCs, endothelial cells, and chondrocytes, could obviate the need for osteogenic supplements to induce osteogenesis in a 3D cellular aggregate in vitro. The results from this study showed that the application of both chondrogenic and vascular priming could significantly enhance the production of both early (ALP) and late (calcium) osteogenic markers of MSCs, in the coculture group with MSCs/HUVECs added, even without the use of osteogenic supplements. The increase in early osteogenic markers, seen with the MSCs/HUVECs added coculture group, was also found to be significantly higher than the same group cultured in the presence of osteogenic supplements. The results from this study also show that the presence of osteogenic supplements had a significant effect on the way mineralization is deposited within a construct. Without osteogenic supplements, mineral was deposited throughout the construct. However, when osteogenic supplements were added, mineralization was deposited through the formation of discrete mineralization nodules. Thus, the addition of osteogenic supplements inhibits the expression of early osteogenic markers (ALP), while promoting mineral deposition in the form of discrete mineralization nodules.
Various in vitro bone regeneration strategies have relied on the use of the osteogenic supplements (dexamethasone, ascorbic acid, and β-glycerophosphate) to induce mineralization in vitro,12,13,49,51,53–62 and each supplement has been shown to be imperative to induce mineralization of MSCs in vitro.9,11–17,22,29,63–67 The main rationale behind the use of these supplements is to provide signaling proteins or enzymes to activate mineral production by MSCs. Previous in vitro 2D studies have shown that the direct coculture of MSCs with endothelial cells36–39 or chondrocytes68 can provide the necessary factors to induce early markers of osteogenic differentiation (ALP expression) without the need for these supplements. A recent 2D study found that indirect coculture of MSCs with osteocytes led to increased calcium deposition compared with MSCs cocultured with osteoblasts.30 This study was conducted without the use of any osteogenic supplements and the results suggested that osteocytes are more influential than osteoblasts at inducing osteogenesis in MSCs in vitro.30 However, in vivo, endochondral ossification involves the direct interaction of cartilage cells with both MSCs and endothelial stem cells. We recently investigated a novel coculture technique, which involved not only the coculture of HUVECs and MSCs but also the coculture of HUVECs and MSCs with MSCs that had been predifferentiated toward the chondrogenic lineage.49 However, that previous study49 was conducted in the presence of osteogenic growth factors and as such whether mimicking the cellular niche existing during endochondral ossification alone (without the use of any external growth factors) could induce mineralization was still unknown.
The results from this study found that a combination of both chondrogenic priming and vascular priming obviated the need for osteogenic supplements, and enhanced both early (ALP) and late (calcium) osteogenic markers compared with MSCs cultured in osteogenic supplements alone. Moreover, similar to previous studies,37,40–44,46–49,69 the coculture group with both HUVECs and MSCs added had significantly higher ALP production than the control groups (osteogenic and noncoculture group) after 3 weeks of coculture. However, unlike previous studies, there was also an increase in late osteogenic markers (calcium) compared with the control group (osteogenic). Importantly, and in contrast to previous studies,37,40–44,46–49,69 this increase in ALP and calcium content was achieved in a 3D scaffoldless construct, without the use of any osteogenic supplements. When compared with the calcium content results,49 the increase in calcium content in the coculture group with added MSCs/HUVECs, but no osteogenic supplements, was significantly higher than in the same group cultured in osteogenic supplements as reported by Freeman et al.49 There was significantly higher calcium content in the group cultured with osteogenic supplements than in the group without osteogenic supplements 1 and 2 weeks after the addition of the cells. However, although calcium content was lower in the groups cultured without osteogenic supplements, the mineral was deposited throughout the aggregate. In contrast, mineral deposition in the aggregates cultured with osteogenic supplements was in the form of mineralization nodules within the aggregate (as seen in alizarin red staining). The reduction in size seen in all three groups cultured without osteogenic growth factors may also indicate that these aggregates are undergoing mineralization through the endochondral ossification process, as in vivo there is a reduction of matrix volume per tissue volume70 through the contraction of matrix before mineralization. However, our current study did not address this directly and thus future studies are required to investigate whether the mineralization seen through this study was initiated by an endochondral ossification-like process, by examining the expression of specific endochondral ossification markers including Ihh, osterix, and noggin.71,72
It has been widely documented that osteogenic supplements are needed to produce mineralization in vitro. However, the results from this study show that a coculture technique can actually provide all the necessary signaling to induce mineralization of a 3D construct in vitro, while allowing for the formation of rudimentary vessels. Dexamethasone can induce mineralization in MSCs by activating Runx2 expression through the (WNT/β-catenin) signaling pathway.18–20 However, in this coculture model, the Runx2 expression is likely produced by chondrocytes within the cellular aggregates, which undergo hypertrophy because of the addition of endothelial cells to the cartilage template,73 as seen in the collagen type X staining in our previous study.49 Moreover, the formation of mineralization nodules in the aggregates cultured in osteogenic supplements might be explained by the use of dexamethasone within the osteogenic medium, as similar results have been reported in costochondral chondrocyte cultures exposed to dexamethasone.74
Therefore, we proposed that if dexamethasone is not supplemented, MSCs are free to form mineral throughout the aggregates rather than in discrete nodules. Ascorbic acid has been shown to be the main regulator of collagen type X production18 and ALP expression. Studies have shown that coculture of endothelial cells with chondrocytes induces both collagen type X and ALP expression,75 as was also shown in the collagen type X staining presented in our previous study.49 Finally, β-glycerophosphate is the source of phosphate needed to produce hydroxyapatite,26–28 but we propose that in this coculture model, these phosphates are naturally provided by cartilage cells undergoing hypertrophy within the cartilage template,76 or from the serum contained in the expansion medium. The results suggest that a direct coculture technique can obviate the need for osteogenic supplements to induce osteogenesis in vitro. However, further studies are needed to elucidate the exact molecular mechanisms that underpin the changes in mineral production and deposition as a result of the coculture technique seen in this study. Future studies should determine the messenger RNA (mRNA) expression level for markers for different stages of osteogenic differentiation, including Runx-2, osteopontin, osteonectin, osteocalcin, and particularly osterix, a specific maker at early stages of endochondral ossification.
Current bone tissue engineered constructs have had limited success postimplantation, which has been attributed to poor nutrient delivery and waste removal arising from a lack of vasculature.4–8,53,77,78 Previous studies have investigated whether prevascularizing 3D constructs in vitro would eradicate this limitation, through the coculture of endothelial stem cells with MSCs40–43,79–83 or osteoblasts,45,84,85 and found that prevascular networks are formed both in vitro and in vivo. Our previous study previously found that rudimentary vessels were formed in vitro when both MSCs and HUVECs were added to the already formed cartilage template.49 The results from this study agreed with the results from our previous study as rudimentary vessels were only formed in the aggregates with added HUVECs/MSCs (as seen by CD31+ staining). However, unlike the results seen previously,49 the average vessel cross-sectional area was significantly higher in aggregates cultured without osteogenic supplements than in those seen in the aggregates cultured in their presence.
Furthermore, unlike the results seen previously,49 when HUVECs alone were added to an already formed cartilage template, in the absence of osteogenic supplements, they did not just attach and proliferate around the periphery, but also began to invade into the center of the construct (as identified by CD31+ and alcian blue staining). Moreover, the omission of osteogenic supplements not only influenced vessel formation but VEGF expression as well. VEGF is well documented as a stimulator of vascular cells to undergo the formation of early vessels.53,68,86–89 The results from both our previous study49 and this study corroborate this as the group with the highest VEGF content in both studies was also the only group to have rudimentary vessels present within the aggregate. However, in this study VEGF expression was seen to peak after 2 weeks of coculture without any osteogenic factors, whereas the group with osteogenic factors continued to have an increase in VEGF expression for up to 3 weeks of coculture. Moreover, even after 3 weeks of coculture, the VEGF expression in the coculture groups with osteogenic growth factors never reached the same levels as that of the coculture group without any osteogenic factors. This suggests that the addition of osteogenic supplements to the medium might delay the vascularization of in vitro bone constructs; however, further studies are needed to verify this.
In our previous study, we saw that vascularization was a precursor to mineralization49; therefore, the vascularization potential was analyzed, by CD31+ immunostaining and quantitative VEGF expression, to compare the results with our previous study. The goal of this study was not to derive an understanding of the effect of excreted VEGF on the maintenance and progress of vasculogenesis at different experimental conditions, but rather to observe whether vascularization occurred before mineralization. Our results demonstrated that vascularization did occur in our coculture group with added HUVECs and MSCs without the presence of any osteogenic growth factors. Future studies should use Western blotting or quantitative polymerase chain reaction to investigate how coculture without osteogenic growth factors enhances the vascularization potential of MSC aggregates.
Although little is known about the direct interaction between hMSCs and endothelial cells in vivo,31–35 because of their anatomical position these cells are either in direct cell–cell contact or communicate through paracrine signaling within the endochondral template. The results from this study show that the direct interaction between the two cell types has a significant effect on both the migration and the vascularization potential of the endothelial stem cells, as well as the osteogenic potential of hMSCs, even without the use of any osteogenic supplements. By replicating the cellular niche existing during endochondral ossification, we were able to obtain an insight into the symbiotic relationship between hMSCs and endothelial stem cells that might exist during endochondral ossification in vivo. We proposed that the MSCs undertake a perivascular role during vascularization within the cartilage template, as no vessels were formed unless both MSCs and HUVECs were added to the already formed cartilage template. Moreover, the addition of HUVECs to a cartilage template also seemed to increase the osteogenic potential of the MSCs, even without the use of any osteogenic supplements, compared with MSCs only cultured in osteogenic supplements. Taken together, this study elucidates that the direct coculture of MSCs and endothelial cells, which mimics the cellular niche existing during endochondral ossification in vivo, induces endothelial cells to form preliminary vessels within the cartilage template, and also induces MSCs to begin mineralizing the cartilage template. However, the exact signaling pathways that occur because of this direct cell–cell interaction need to be further investigated.
A possible limitation of the study is that MSCs from two male donors were pooled and we did not directly explore whether the hMSCs displayed a donor-dependent response to mineral formation. Previous studies have reported donor variability in the expression of osteogenic supplements in vitro.90 However, the control groups also contained pooled cells, so the differences observed between the groups cannot be explained by donor variability. Another limitation of this study is the use of ascorbic acid and dexamethasone in the chondrogenic medium, which might be expected to cause MSCs to differentiate toward the osteogenic lineage. However, the use of dexamethasone and ascorbic acid in the chondrogenic medium is well documented53,60,66,78,91–102 and has not been shown to cause mineralization. Moreover, alizarin red staining of the aggregates before the coculture showed no positive staining and the control groups were all exposed to the same chondrogenic medium, so any differences seen between the groups were not because of exposure to these growth factors. Finally, there was a significant increase in DNA content in the CP21+HUVECs and CP21+HUVECs:MSCs groups cultured in the presence of osteogenic supplements compared with the same groups cultured without. Dexamethasone has been shown to induce MSC proliferation18,103 and ascorbic acid has been shown to promote endothelial cell proliferation,104–106 which might account for this increase in DNA content. However, the additional cells did not appear to influence mineralization or vascularization potential.
Conclusions
The results of this study show that both chondrogenic priming (for 21 days) and subsequent vascular priming can induce osteogenesis of a 3D MSC aggregate (scaffoldless) without the use of any osteogenic supplements. Most interestingly, the inclusion of osteogenic supplements in vitro actually inhibits the promotion of ALP content, calcium content, and VEGF content produced through coculture alone. Moreover, the results showed that chondrogenic and vascular priming not only increased the expression of ALP, calcium content, and VEGF expression but also allowed for the formation of rudimentary vessels, seen within all of the aggregates in which both MSCs and HUVECs are added. These rudimentary vessels were larger in cross-sectional area than those seen in the same groups cultured in the presence of osteogenic growth factors. Taken together, these results indicate for the first time the beneficial effect that coculture of MSCs, endothelial cells, and chondrocytes has on both osteogenesis and vasculogenesis of 3D MSC aggregate (scaffoldless) constructs in vitro. We propose that the application of both chondrogenic and vascular priming of hMSCs can obviate the need for osteogenic supplements to induce osteogenesis by hMSCs, while allowing for the formation of rudimentary vessels in vitro in 3D bone tissue-engineered constructs.
Acknowledgments
Confocal imaging was performed at the Centre for Microscopy and Imaging, National University of Ireland, Galway, Ireland, with special thanks to P.O. for allowing the use of the microscope. This study was supported by the European Research Council Grant 258992-BONEMECHBIO and the NUI Travelling Scholarship 2013. This work was supported by the Army, Navy, NIH, Air Force, VA and Health Affairs to support the AFIRM II effort, under Award No. W81XWH-14-2-0003. The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014, is the awarding and administering acquisition office. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Disclosure Statement
No competing financial interests exist.
References
- 1.Cancedda R., Giannoni P., and Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 28, 4240, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Dawson J.I., and Oreffo R.O. Bridging the regeneration gap: stem cells, biomaterials and clinical translation in bone tissue engineering. Arch Biochem Biophys 473, 124, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Rose F.R., and Oreffo R.O. Bone tissue engineering: hope vs hype. Biochem Biophy Res Commun 292, 1, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Lyons F.G., Al-Munajjed A.A., Kieran S.M., Toner M.E., Murphy C.M., Duffy G.P., et al. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials 31, 9232, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Amini A.R., Laurencin C.T., and Nukavarapu S.P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng 40, 363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today 14, 88, 2011 [Google Scholar]
- 7.Phelps E.A., and Garcia A.J. Update on therapeutic vascularization strategies. Regen Med 4, 65, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ko H.C., Milthorpe B.K., and McFarland C.D. Engineering thick tissues—the vascularisation problem. Eur Cell Mater 14, 1; discussion 18–19, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Song I.H., Caplan A.I., and Dennis J.E. In vitro dexamethasone pretreatment enhances bone formation of human mesenchymal stem cells in vivo. J Orthop Res 27, 916, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Tenenbaum H.C., and Heersche J.N. Dexamethasone stimulates osteogenesis in chick periosteum in vitro. Endocrinology 117, 2211, 1985 [DOI] [PubMed] [Google Scholar]
- 11.Cheng S.L., Yang J.W., Rifas L., Zhang S.F., and Avioli L.V. Differentiation of human bone marrow osteogenic stromal cells in vitro: induction of the osteoblast phenotype by dexamethasone. Endocrinology 134, 277, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Maniatopoulos C., Sodek J., and Melcher A.H. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res 254, 317, 1988 [DOI] [PubMed] [Google Scholar]
- 13.Jaiswal N., Haynesworth S.E., Caplan A.I., and Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 64, 295, 1997 [PubMed] [Google Scholar]
- 14.Leboy P.S., Beresford J.N., Devlin C., and Owen M.E. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol 146, 370, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Kamalia N., McCulloch C.A., Tenebaum H.C., and Limeback H. Dexamethasone recruitment of self-renewing osteoprogenitor cells in chick bone marrow stromal cell cultures. Blood 79, 320, 1992 [PubMed] [Google Scholar]
- 16.Herbertson A., and Aubin J.E. Dexamethasone alters the subpopulation make-up of rat bone marrow stromal cell cultures. J Bone Miner Res 10, 285, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Peter S.J., Liang C.R., Kim D.J., Widmer M.S., and Mikos A.G. Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem 71, 55, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Langenbach F., and Handschel J. Effects of dexamethasone, ascorbic acid and beta-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 4, 117 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komori T. Regulation of osteoblast differentiation by Runx2. Adv Exp Med Biol 658, 43, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Hamidouche Z., Hay E., Vaudin P., Charbord P., Schule R., Marie P.J., et al. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/beta-catenin signaling-dependent Runx2 expression. FASEB J 22, 3813, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Vater C., Kasten P., and Stiehler M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater 7, 463, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Franceschi R.T., and Iyer B.S. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res 7, 235, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Tenenbaum H.C. Role of organic phosphate in mineralization of bone in vitro. J Dent Res 60, Spec No. C:1586, 1981 [DOI] [PubMed] [Google Scholar]
- 24.Tenenbaum H.C., and Heersche J.N.M. Differentiation of osteoblasts and formation of mineralized bone in vitro. Calcif Tissue Int 34, 76, 1982 [DOI] [PubMed] [Google Scholar]
- 25.Ecarot-Charrier B., Glorieux F.H., van der Rest M., and Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol 96, 639, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster B.L., Nociti F.H., Jr., Swanson E.C., Matsa-Dunn D., Berry J.E., Cupp C.J., et al. Regulation of cementoblast gene expression by inorganic phosphate in vitro. Calcif Tissue Int 78, 103, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Fatherazi S., Matsa-Dunn D., Foster B.L., Rutherford R.B., Somerman M.J., and Presland R.B. Phosphate regulates osteopontin gene transcription. J Dent Res 88, 39, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tada H., Nemoto E., Foster B.L., Somerman M.J., and Shimauchi H. Phosphate increases bone morphogenetic protein-2 expression through cAMP-dependent protein kinase and ERK1/2 pathways in human dental pulp cells. Bone 48, 1409, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Franceschi R.T., Iyer B.S., and Cui Y. Effects of ascorbic acid on collagen matrix formation and osteoblast differentiation in murine MC3T3-E1 cells. J Bone Miner Res 9, 843, 1994 [DOI] [PubMed] [Google Scholar]
- 30.Birmingham E., Niebur G.L., McHugh P.E., Shaw G., Barry F.P., and McNamara L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur Cell Mater 23, 13, 2012 [DOI] [PubMed] [Google Scholar]
- 31.da Silva Meirelles L., Caplan A.I., and Nardi N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 26, 2287, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Jones E., and McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford) 47, 126, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Augello A., Kurth T.B., and De Bari C. Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20, 121, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Tsai T.L., Wang B.W., Squire M.W., Guo L.W., and Li W.J. Endothelial cells direct human mesenchymal stem cells for osteo- and chondro-lineage differentiation through endothelin-1 and AKT signaling. Stem Cell Res Ther 6, 88, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan C.K.F., Chen C.C., Luppen C.A., Kim J.B., DeBoer A.T., Wei K., et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villars F., Bordenave L., Bareille R., and Amedee J. Effect of human endothelial cells on human bone marrow stromal cell phenotype: role of VEGF? J Cell Biochemy 79, 672, 2000 [DOI] [PubMed] [Google Scholar]
- 37.Villars F., Guillotin B., Amedee T., Dutoya S., Bordenave L., Bareille R., et al. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol 282, C775, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Guillotin B., Bareille R., Bourget C., Bordenave L., and Amédée J. Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone 42, 1080, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Zhao X., Liu L., Wang F-K., Zhao D-P., Dai X-M., and Han X-S. Coculture of vascular endothelial cells and adipose-derived stem cells as a source for bone engineering. Ann Plast Surg 69, 91, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Sun H., Qu Z., Guo Y., Zang G., and Yang B. In vitro and in vivo effects of rat kidney vascular endothelial cells on osteogenesis of rat bone marrow mesenchymal stem cells growing on polylactide-glycoli acid (PLGA) scaffolds. Biomed Eng Online 6, 41 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pedersen T.O., Blois A.L., Xing Z., Xue Y., Sun Y., Finne-Wistrand A., et al. Endothelial microvascular networks affect gene-expression profiles and osteogenic potential of tissue-engineered constructs. Stem Cell Res Ther 4, 52 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rouwkema J., de Boer J., and Van Blitterswijk C.A. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng 12, 2685, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Saleh F.A., Whyte M., and Genever P.G. Effects of endothelial cells on human mesenchymal stem cell activity in a three-dimensional in vitro model. Eur Cell Mater 22, 242; discussion 257, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Liao J., Hammerick K.E., Challen G.A., Goodell M.A., Kasper F.K., and Mikos A.G. Investigating the role of hematopoietic stem and progenitor cells in regulating the osteogenic differentiation of mesenchymal stem cells in vitro. J Orthop Res 29, 1544, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuchs S., Hofmann A., and Kirkpatrick C.J. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng 13, 2577, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Jiang J., Nicoll S.B., and Lu H.H. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochem Biophys Res Commun 338, 762, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Giovannini S., Diaz-Romero J., Aigner T., Heini P., Mainil-Varlet P., and Nesic D. Micromass co-culture of human articular chondrocytes and human bone marrow mesenchymal stem cells to investigate stable neocartilage tissue formation in vitro. Eur Cell Mater 20, 245, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Nakaoka R., Hsiong S.X., and Mooney D.J. Regulation of chondrocyte differentiation level via co-culture with osteoblasts. Tissue Eng 12, 2425, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Freeman F.E., Haugh M.G., and McNamara L. An in vitro bone tissue regeneration strategy combining chondrogenic and vascular priming enhances the mineralisation potential of MSCs in vitro whilst also allowing for vessel formation. Tissue Eng Part A 21, 1320, 2015 [DOI] [PubMed] [Google Scholar]
- 50.Korff T., and Augustin H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol 143, 1341, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Freeman F., Haugh M., and McNamara L. Investigation of the optimal timing for chondrogenic priming of MSCs to enhance osteogenic differentiation in vitro as a bone tissue engineering strategy. J Tissue Eng Regen Med 10, E250, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Haugh M.G., Meyer E.G., Thorpe S.D., Vinardell T., Duffy G.P., and Kelly D.J. Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Eng Part A 17, 3085, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Farrell E., van der Jagt O.P., Koevoet W., Kops N., van Manen C.J., Hellingman C.A., et al. Chondrogenic priming of human bone marrow stromal cells: a better route to bone repair? Tissue Eng Part C Methods 15, 285, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Ohgushi H., Dohi Y., Tamai S., and Tabata S. Osteogenic differentiation of marrow stromal stem cells in porous hydroxyapatite ceramics. J Biomed Mater Res 27, 1401, 1993 [DOI] [PubMed] [Google Scholar]
- 55.Ohgushi H., Dohi Y., Katuda T., Tamai S., Tabata S., and Suwa Y. In vitro bone formation by rat marrow cell culture. J Biomed Mater Res 32, 333, 1996 [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa T., Ohgushi H., Dohi Y., and Davies J.E. Viable bone formation in porous hydroxyapatite: marrow cell-derived in vitro bone on the surface of ceramics. Biomed Mater Eng 7, 49, 1997 [PubMed] [Google Scholar]
- 57.Kotobuki N., Ioku K., Kawagoe D., Fujimori H., Goto S., and Ohgushi H. Observation of osteogenic differentiation cascade of living mesenchymal stem cells on transparent hydroxyapatite ceramics. Biomaterials 26, 779, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Kotobuki N., Kawagoe D., Nomura D., Katou Y., Muraki K., Fujimori H., et al. Observation and quantitative analysis of rat bone marrow stromal cells cultured in vitro on newly formed transparent β-tricalcium phosphate. J Mater Sci Mater Med 17, 33, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Oreffo R.O.C., Driessens F.C.M., Planell J.A., and Triffitt J.T. Growth and differentiation of human bone marrow osteoprogenitors on novel calcium phosphate cements. Biomaterials 19, 1845, 1998 [DOI] [PubMed] [Google Scholar]
- 60.Dawson J.I., Wahl D.A., Lanham S.A., Kanczler J.M., Czernuszka J.T., and Oreffo R.O.C. Development of specific collagen scaffolds to support the osteogenic and chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 29, 3105, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Morgan S.M., Tilley S., Perera S., Ellis M.J., Kanczler J., Chaudhuri J.B., et al. Expansion of human bone marrow stromal cells on poly-(dl-lactide-co-glycolide) (PDLLGA) hollow fibres designed for use in skeletal tissue engineering. Biomaterials 28, 5332, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Bolland B.J.R.F., Kanczler J.M., Ginty P.J., Howdle S.M., Shakesheff K.M., Dunlop D.G., et al. The application of human bone marrow stromal cells and poly(dl-lactic acid) as a biological bone graft extender in impaction bone grafting. Biomaterials 29, 3221, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Hofmann S., Hagenmüller H., Koch A.M., Müller R., Vunjak-Novakovic G., Kaplan D.L., et al. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials 28, 1152, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Martin I., Padera R.F., Vunjak-Novakovic G., and Freed L.E. In vitro differentiation of chick embryo bone marrow stromal cells into cartilaginous and bone-like tissues. J Orthop Res 16, 181, 1998 [DOI] [PubMed] [Google Scholar]
- 65.Meinel L., Fajardo R., Hofmann S., Langer R., Chen J., Snyder B., et al. Silk implants for the healing of critical size bone defects. Bone 37, 688, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Meinel L., Karageorgiou V., Fajardo R., Snyder B., Shinde-Patil V., Zichner L., et al. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng 32, 112, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Meinel L., Karageorgiou V., Hofmann S., Fajardo R., Snyder B., Li C., et al. Engineering bone-like tissue in vitro using human bone marrow stem cells and silk scaffolds. J Biomed Mater Res A 71, 25, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Nakagawa M., Kaneda T., Arakawa T., Morita S., Sato T., Yomada T., et al. Vascular endothelial growth factor (VEGF) directly enhances osteoclastic bone resorption and survival of mature osteoclasts. FASEB J 473, 161, 2000 [DOI] [PubMed] [Google Scholar]
- 69.Grellier M., Ferreira-Tojais N., Bourget C., Bareille R., Guillemot F., and Amédée J. Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem 106, 390, 2009 [DOI] [PubMed] [Google Scholar]
- 70.Poole A.R. The growth plate: cellular physiology, cartilage assembly and mineralization. In: Hall B., and Newman S., eds. Cartilage: Molecular Aspects. London: CRC Press, 1991, p. 180 [Google Scholar]
- 71.Benedito R., Roca C., Sorensen I., Adams S., Gossler A., Fruttiger M., et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Ramasamy S.K., Kusumbe A.P., Wang L., and Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bittner K., Vischer P., Bartholmes P., and Bruckner P. Role of the subchondral vascular system in endochondral ossification: endothelial cells specifically derepress late differentiation in resting chondrocytesin vitro. Exp Cell Res 238, 491, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Schwartz Z., Hancock R.H., Dean D.D., Brooks B.P., Gomez R., Boskey A.L., et al. Dexamethasone promotes von kossa-positive nodule formation and increased alkaline phosphatase activity in costochondral chondrocyte cultures. Endocrine 3, 351, 1995 [DOI] [PubMed] [Google Scholar]
- 75.Leboy P.S., Vaias L., Uschmann B., Golub E., Adams S.L., and Pacifici M. Ascorbic acid induces alkaline phosphatase, type X collagen, and calcium deposition in cultured chick chondrocytes. J Biol Chem 264, 17281, 1989 [PubMed] [Google Scholar]
- 76.Bourne G.H. Phosphate and calcification. In: Bourne G.H., ed. Physiology and Pathology. 2nd ed. London: Academic Press Ltd., 1972, p. 89 [Google Scholar]
- 77.Krishnan L., Willett N., and Guldberg R. Vascularization strategies for bone regeneration. Ann Biomed Eng 42, 432, 2014 [DOI] [PubMed] [Google Scholar]
- 78.Farrell E., Both S.K., Odorfer K.I., Koevoet W., Kops N., O'Brien F.J., et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet Disord 12, 31 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Correia C., Grayson W.L., Park M., Hutton D., Zhou B., Guo X.E., et al. In vitro model of vascularized bone: synergizing vascular development and osteogenesis. PLoS One 6, 28352 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghanaati S., Fuchs S., Webber M.J., Orth C., Barbeck M., Gomes M.E., et al. Rapid vascularization of starch-poly(caprolactone) in vivo by outgrowth endothelial cells in co-culture with primary osteoblasts. J Tissue Eng Regen Med 5, 136, 2011 [DOI] [PubMed] [Google Scholar]
- 81.McFadden T.M., Duffy G.P., Allen A.B., Stevens H.Y., Schwarzmaier S.M., Plesnila N., et al. The delayed addition of human mesenchymal stem cells to pre-formed endothelial cell networks results in functional vascularization of a collagen–glycosaminoglycan scaffold in vivo. Acta Biomater 9, 9303, 2013 [DOI] [PubMed] [Google Scholar]
- 82.Duffy G.P., McFadden T.M., Byrne E.M., Gill S.L., Farrell E., and O'Brien F.J. Towards in vitro vascularisation of collagen-GAG scaffolds. Eur Cell Mater 21, 15, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Scherberich A., Galli R., Jaquiery C., Farhadi J., and Martin I. Three-dimensional perfusion culture of human adipose tissue-derived endothelial and osteoblastic progenitors generates osteogenic constructs with intrinsic vascularization capacity. Stem Cells 25, 1823, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Hofmann A., Ritz U., Verrier S., Eglin D., Alini M., Fuchs S., et al. The effect of human osteoblasts on proliferation and neo-vessel formation of human umbilical vein endothelial cells in a long-term 3D co-culture on polyurethane scaffolds. Biomaterials 29, 4217, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Kyriakidou K., Lucarini G., Zizzi A., Salvolini E., Belmonte M.M., Mollica F., et al. Dynamic co-seeding of osteoblast and endothelial cells on 3D polycaprolactone scaffolds for enhanced bone tissue engineering. J Bioact Compat Polym 23, 227, 2008 [Google Scholar]
- 86.Gerber H.P., and Ferrara N. Angiogenesis and bone growth. Trends Cardiovasc Med 10, 223, 2000 [DOI] [PubMed] [Google Scholar]
- 87.Hans-Peter G., Thiennu H.V., Anne M.R., Joe K., Zena W., and Napoleone F. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5, 623, 1999 [DOI] [PubMed] [Google Scholar]
- 88.Fiedler J., Leucht F., Waltenberger J., Dehio C., and Brenner R.E. VEGF-A and PlGF-1 stimulate chemotactic migration of human mesenchymal progenitor cells. Biochem Biophys Res Commun 334, 561, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Mayr-Wohlfart U., Waltenberger J., Hausser H., Kessler S., Gunther K.P., Dehio C., et al. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 30, 472, 2002 [DOI] [PubMed] [Google Scholar]
- 90.Mendes S.C., Tibbe J.M., Veenhof M., Both S., Oner F.C., van Blitterswijk C.A., et al. Relation between in vitro and in vivo osteogenic potential of cultured human bone marrow stromal cells. J Mater Sci Mater Med 15, 1123, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Thorpe S.D., Buckley C.T., Vinardell T., O'Brien F.J., Campbell V.A., and Kelly D.J. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-beta3 induced chondrogenic differentiation. Ann Biomed Eng 38, 2896, 2010 [DOI] [PubMed] [Google Scholar]
- 92.Erickson G.R., Gimble J.M., Franklin D.M., Rice H.E., Awad H., and Guilak F. Chondrogenic potential of adipose tissue-derived stromal cells in vitro and in vivo. Biochem Biophys Res Commun 290, 763, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Meyer E.G., Buckley C.T., Steward A.J., and Kelly D.J. The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J Mech Behav Biomed Mater 4, 1257, 2011 [DOI] [PubMed] [Google Scholar]
- 94.El-Serafi A.T., Wilson D.I., Roach H.I., and Oreffo R.O. Developmental plasticity of human foetal femur-derived cells in pellet culture: self assembly of an osteoid shell around a cartilaginous core. Eur Cell Mater 21, 558, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Sheehy E.J., Vinardell T., Buckley C.T., and Kelly D.J. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater 9, 5484, 2013 [DOI] [PubMed] [Google Scholar]
- 96.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4, 415, 1998 [DOI] [PubMed] [Google Scholar]
- 97.Jukes J.M., Both S.K., Leusink A., Sterk L.M.T., van Blitterswijk C.A., and de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A 105, 6840, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scotti C., Piccinini E., Takizawa H., Todorov A., Bourgine P., Papadimitropoulos A., et al. Engineering of a functional bone organ through endochondral ossification. Proc Natl Acad Sci U S A 110, 3997, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scotti C., Tonnarelli B., Papadimitropoulos A., Scherberich A., Schaeren S., Schauerte A., et al. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc Natl Acad Sci U S A 107, 7251, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Harada N., Watanabe Y., Sato K., Abe S., Yamanaka K., Sakai Y., et al. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 35, 7800, 2014 [DOI] [PubMed] [Google Scholar]
- 101.van der Stok J., Koolen M.K., Jahr H., Kops N., Waarsing J.H., Weinans H., et al. Chondrogenically differentiated mesenchymal stromal cell pellets stimulate endochondral bone regeneration in critical-sized bone defects. Eur Cell Mater 27, 137; discussion 48, 2014 [DOI] [PubMed] [Google Scholar]
- 102.Tare R.S., Howard D., Pound J.C., Roach H.I., and Oreffo R.O.C. Tissue engineering strategies for cartilage generation—micromass and three dimensional cultures using human chondrocytes and a continuous cell line. Biochem Biophys Res Commun 333, 609, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Wang H.Y., Pang B., Li Y., Zhu D.L., Pang T.X., and Liu Y.J. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy 14, 423, 2012 [DOI] [PubMed] [Google Scholar]
- 104.Ulrich-Merzenich G., Metzner C., Bhonde R.R., Malsch G., Schiermeyer B., and Vetter H. Simultaneous isolation of endothelial and smooth muscle cells from human umbilical artery or vein and their growth response to low-density lipoproteins. In Vitro Cell Dev Biol Anim 38, 265, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Ulrich-Merzenich G., Metzner C., Schiermeyer B., and Vetter H. Vitamin C and vitamin E antagonistically modulate human vascular endothelial and smooth muscle cell DNA synthesis and proliferation. Eur J Nutr 41, 27, 2002 [DOI] [PubMed] [Google Scholar]
- 106.Ulrich-Merzenich G., Zeitler H., Panek D., Bokemeyer D., and Vetter H. Vitamin C promotes human endothelial cell growth via the ERK-signaling pathway. Eur J Nutr 46, 87, 2007 [DOI] [PubMed] [Google Scholar]