Abstract
We characterized extended-spectrum beta-lactamases (ESBLs) and plasmid-mediated quinolone resistance (PMQR) in 32 Escherichia coli extended spectrum cephalosporin (ESC)-resistant clinical isolates from UK companion animals from several clinics. In addition, to investigate the possible dissemination of ESBL clinical isolates within a veterinary hospital, two ESBL-producing E. coli isolates from a dog with septic peritonitis and a cluster of environmental ESC-resistant E. coli isolates obtained from the same clinic and during the same time period, as these two particular ESBL-positive clinical isolates, were also included in the study. Molecular characterization identified blaCTX-M to be the most prevalent gene in ESC-resistant isolates, where 66% and 27% of clinical isolates carried blaCTX-M-15 and blaCTX-M-14, respectively. The only PMQR gene detected was aac(6')-Ib-cr, being found in 34% of the ESC E. coli isolates and was associated with the carriage of blaCTX-M-15. The clinical and environmental isolates investigated for hospital dissemination had a common ESBL/AmpC phenotype, carried blaCTX-M-15, and co-harbored blaOXA-1, blaTEM-1, blaCMY-2, and aac(6')-Ib-cr. Multilocus sequence typing identified them all as ST410, while pulse-field gel electrophoresis demonstrated 100% homology of clinical and environmental isolates, suggesting hospital environmental dissemination of CTX-M-15–producing E. coli ST410.
Keywords: : E. coli, ESBL, surveillance, veterinary, infection control
Introduction
Escherichia coli are opportunistic pathogens in humans and companion animals and can be associated with a variety of extraintestinal infections, which may require antimicrobial therapy.1 Increased use of antimicrobials in companion animals may select for antimicrobial-resistant bacteria, and concerns have been raised that these host species may act as potential reservoirs for human infections.2 Particularly concerning for both human and veterinary health is the increasing resistance to extended spectrum cephalosporins (ESCs) through the production of extended-spectrum beta-lactamases (ESBLs).3–6 There is increasing evidence that E. coli-producing ESBL and/or AmpC beta-lactamases are emerging in companion animals,7,8 setting new challenges for veterinary practitioners due to therapeutic and infection control implications.
Several studies have described the prevalence of ESBL resistance in bacteria from companion animals,3,4,9–11 but the role that these animals may play in the spread of such resistant bacteria or determinants, is not yet fully determined. Previous molecular studies have shown that, in human hospital settings, ESBL genetic determinants have the potential to spread either through clonal dissemination or plasmid transfer, posing a serious threat to patient care and safety.6,12 The development of large veterinary hospitals with intensive care facilities has created similar conditions for the emergence of animal hospital acquired infections and a few studies have shown the association of multidrug resistant (MDR) organisms, such as E. coli, Acinetobacter baumannii, Enterobacter spp., and Enterococcus spp., with animal nosocomial infections.13–19 However, with the exception of a few recent studies showing the hospital acquisition and/or dissemination of beta-lactamases or ESBL-producing Klebsiella pneumoniae and A. baumannii20–23 and compared with the wealth of data from human medicine, there is a paucity of studies investigating the potential of ESBL-producing E. coli to spread and cause nosocomial infections in veterinary clinics or hospital settings.
The aim of this study was dual; first, to characterize ESBL and plasmid-mediated quinolone resistance (PMQR) genes in E. coli from clinical specimens submitted for routine bacterial culture to the Veterinary Microbiology Diagnostics laboratory in the Liverpool School of Veterinary Science. Second, to analyze and compare a cluster of clinical and environmental ESBL-producing E. coli obtained from a UK Veterinary Hospital to identify the potential of clinical isolates to spread within such environments.
Materials and Methods
Bacterial isolates
All E. coli isolates were obtained from companion animal clinical specimens submitted from a veterinary hospital and a number of small veterinary clinics and collected for this study between January 2010 and November 2011. Clinical specimens were plated out aerobically on 5% sheep blood agar (Oxoid) and incubated for 24 hours at 37°C. Clinical isolates presumptively identified as E. coli based on a positive reaction on Eosin Methylene Blue Agar (EMBA; Oxoid, Basingstoke, UK) and which showed reduced susceptibility to cefpodoxime (10 μg) and/or cefoxitin (30 μg), used as indicators for ESBL and AmpC production, were selected for this study. In addition, samples from active bacterial environmental surveillance, which is also offered for veterinary hospitals as part of the Diagnostic Service, are also processed by the laboratory, and a cluster of hospital environmental E. coli isolates was also included in this study. Detection of resistance to ESC in environmental E. coli isolates followed the same protocols as for clinical isolates. The identification of clinical and environmental isolates was performed using API 20E Identification Kits (bioMerieux, France) and also by PCR detection of the uidA gene for confirmation of E. coli.24
Antimicrobial susceptibility testing
Susceptibility testing was performed by disc diffusion to representatives of beta-lactam and non-beta-lactam antimicrobial classes on ISO-Sensitest agar (Oxoid, Basingstoke, UK) and results were interpreted according to the BSAC (British Society for Antimicrobial Chemotherapy) interpretative criteria.25 E. coli ATCC 25922 was used as control strain. All isolates were tested for ESBL production by the double disc synergy test (DDST).26
Characterization of ESBL and other resistance genes
Cell lysates obtained from all investigated isolates were screened by PCR and DNA sequencing for the presence of blaCTX-M, blaSHV, blaTEM, blaOXA, plasmid-mediated blaAmpC variants, PMQR genes qnrA, B, S, as well as the cr variant of aac(6')-Ib, as previously described.27–31 Specific PCR assays were performed to identify the possible association of blaCTX-M-15 with ISEcp1 or IS26 insertion elements, which have been shown to be involved in the mobilization and expression of blaCTX-M genes.32,33
Resistance transfer and PCR-based replicon typing
To determine the transferability of the ESBL and PMQR genes, conjugation by plate mating was performed with streptomycin-resistant E. coli HB101as the recipient and seven selected donors harboring blaCTX-M-15 (n = 6) or blaCTX-M-14 (n = 1). Plasmid replicons involved in the transfer of the resistance genes were analyzed by PCR-based plasmid replicon typing (PBRT) as described by Carattoli et al.34
Molecular characterization of isolates
A multiplex PCR described by Clemont et al.,35 was used to assign the E. coli isolates to a phylogenetic group. Genetic relatedness of isolates identified to carry blaCTX-M genes was analyzed by macrorestriction pulsed-field gel electrophoresis (PFGE) (www.cdc.gov/pulsenet/pathogens/). Data were analyzed using BioNumerics software version 5.1 (Applied Maths). A tolerance of 1.00% was selected and cluster analysis of PFGE pulsotypes was performed by the unweighted pair group method with average linkages (UPGMA), using the Dice coefficient to analyze similarities and define pulsotypes. PFGE pulsotypes were identified as isolates with ≥90% similarity. Multilocus sequence typing (MLST) was performed as previously described36 for at least one isolate from each identified PFGE cluster.
Results
Bacterial isolates and antimicrobial susceptibility testing
Four hundred and forty five E. coli isolates (n = 445) were obtained from companion animal clinical specimens between January 2010 and November 2011, of which 32 (7%) cefpodoxime and/or cefoxitin-resistant nonduplicate isolates (30 canine and two feline) were characterized in this study. The selected isolates were both from normally sterile sites [urine (n = 6), liver/bile (n = 4), abdominal fluid (n = 3), bronchoalveolar lavage (n = 1), lymph-node biopsy (n = 1)] and also from sites colonized with normal flora and where cultures yielded mixed bacterial growth [colon biopsies (n = 3), wounds (n = 4), skin/ear swabs (n = 5), fecal samples (n = 5)]. In addition, a cluster of environmental ESC-resistant E. coli isolates (n = 6) obtained from the same clinic and during the same time period as two particular ESBL-positive clinical isolates, was also included in the study and characterized by the same methods as the clinical isolates. These two ESBL-positive clinical isolates were from the same dog, which had been admitted with septic peritonitis following duodenal ulceration; one isolate was obtained from abdominal fluid (12L-0659) and one from a surgical site wound swab (12L-0671) following surgery. The environmental ESC-resistant E. coli isolates were obtained from the ultrasound table (EBM-111) where the dog was examined and also from various areas of the ward where the dog was hospitalized; these included the kennel area, the drip pump attached to the kennel, the ward door handle, the ward fridge handle, and the ward computer keyboard (EBM-114, EBM-115, EBM-116, EBM-118, and EBM-119).
All isolates characterized in this study showed resistance to ampicillin, amoxicillin–clavulanic acid (CV), cefotaxime, cefpodoxime, ceftazidime, and tetracycline. In addition, 71% of isolates exhibited resistance to cefoxitin, 65% to ciprofloxacin, and 59% to trimethoprim/sulfamethoxazole (Table 1). Interestingly, 29% of isolates showed resistance to amoxicillin-clavulanic acid, but susceptibility to cefoxitin. This may indicate that mechanisms such as those which involve combinations blaCTX-M-15 and blaOXA-1 genes (as seen in isolates 10L-4543, 11L-1298, 10L-2646) may give rise to this phenotype. Other mechanisms responsible for amoxicillin-clavulanic acid resistance, but which do not normally confer resistance to the cephamycins includes hyperproduction of TEM-1 or SHV-1 beta-lactamases. Although we did not attempt to determine whether this was the case, a number of the tested isolates carried blaTEM-1b only and were fully susceptible to cefoxitin (10L-1747, 10L-2253, 11L-2520, Table 1).
Table 1.
Phenotypic and Genotypic Characteristics of ESC-Resistant Feline and Canine Escherichia coli Clinical and Environmental Isolates (Groupings Based on Common Gene Combinations, PG, and ST, Were Determined)
| Number of isolates (n = 38) | Species | Isolate ID | Source | Common antimicrobial resistance profile | IS type | Beta-lactamase genes | PMQR genes | PG | ST | PT |
|---|---|---|---|---|---|---|---|---|---|---|
| n = 9 | Dog | 11L-2603 | Colon biopsy | AMP, AMC, CPD, CTX, CAZ, FOX, CIP, NA, CN, TE, STX | ISEcp1 disrupted by IS26 | blaCTX-M-15, OXA-1, TEM-1, CMY-2 | aac-6Ib-cr | A | 410 | 4 |
| 12L-0659 | Wound | 4 | ||||||||
| 12L-0671 | Abdominal fluid | 4 | ||||||||
| EBM (111, 114, 115, 116, 118, 119) | Hospital environment | 4 (x6) | ||||||||
| n = 1 | Dog | 11L-1050A | Liver biopsy | AMP, AMC, CPD, CTX, CAZ, FOX, CIP, NA, CN, TE, S, STX | ISEcp1 | blaCTX-M-15, OXA-1, TEM-1 | aac-6Ib-cr | D | 2348 | 7 |
| n = 2 | Dog | 10L-3852 | Feces | AMP, AMC, CPD, CTX, CAZ, FOX, NA, CIP, CN, TE, S, STX | ISEcp1 | blaCTX-M-15, OXA-1 | aac-6Ib-cr | A | 5 | |
| 10L-3690 | Skin swab | 4 | ||||||||
| n = 3 | Dog | 10L-4543 | Skin swab | AMP, AMC, CPD, CTX, CAZ, CIP, NA, CN, TE, S, STX | IS26 (400bp) | blaCTX-M-15, OXA-1 | aac-6Ib-cr | B2 | 131 | 6 |
| 11L-1298 | Bile | 6 | ||||||||
| 10L-2646 | Colon biopsy | 6 | ||||||||
| n = 1 | Dog | 11L-0348 | Ear swab | AMP, AMC, CPD, CTX, CAZ, FOX, CIP, NA, CN, TE, S, STX | ISEcp1 | blaCTX-M-15, OXA | aac-6Ib-cr | D | 2348 | 8 |
| n = 1 | Dog | 11L-4755 | Feces | AMP, AMC, CPD, CTX, CAZ, FOX, CIP, NA, CN, TE, STX | ISEcp1 | blaCTX-M-15, OXA | aac-6Ib-cr | A | 1284 | 9 |
| n = 1 | Dog | 10L-1340 | Feces | AMP, AMC, CPD, CTX, CAZ, CIP, NA, TE, S, STX | ISEcp1 | blaCTX-M-15 | – | A | 4184 | 1 |
| n = 1 | Dog | 10L-0827 | Abdominal fluid | AMP, CPD, NA, CIP, TE, S, STX | – | blaCTX-M-27 | B2 | 131 | 7 | |
| n = 4 | Dog | 10L-0405/ | LN biopsy | AMP, AMC, CPD, CTX, CAZ, FOX, TE, S, STX | – | blaCTX-M-14, TEM-1 | – | A | 617 | 3 |
| 10L-0652 | Urine | 2 | ||||||||
| 10L-0784(A) | Bile | 2 | ||||||||
| 10L-0784(B) | Bile | |||||||||
| n = 1 | Dog | 11L-2596 | Colon biopsy | AMP, AMC, CPD, CTX, FOX, CIP, NA, TE, S | ISEcp1 | blaCTX-M-14, TEM-1 | A | 617 | 1 | |
| n = 3 | Dog | 10L-1747/ | Urine | AMP, AMC, CPD, CIP, NA, TE, S | – | blaTEM-1b | A | |||
| 10L-2253/ | Urine | |||||||||
| 11L-2520 | Skin swab | |||||||||
| n = 1 | Dog | 11L-1050B | BAL | AMP, AMC, CPD, CTX, CAZ, FOX, CIP, NA, CN, TE, S, STX | – | blaTEM-1, CMY-2 | D | |||
| n = 2 | Dog | 11L-1345/ | Abdominal fluid | AMP, AMC, CPD, CTX, CAZ, FOX, CIP, NA, TE | – | blaTEM-1, CMY-2 | A | |||
| 10L-4304 | Urine | |||||||||
| n = 1 | Dog | 12L-0098 | Urine | AMP, AMC, CPD, CTX, CAZ, FOX, TE, S, STX | – | blaTEM-1b, CMY-2 | B2 | |||
| n = 1 | Dog | 10L-4532 | Feces | AMP, AMC, CPD, CTX, CAZ, FOX, TE, S | – | blaOXA, CMY-2 | A | |||
| n = 3 | Dog | 10L-4885/ | Urine | AMP, AMC, CPD, CTX, CAZ, FOX, NA, TE | – | blaCMY-2 | D | |||
| 11L-0024 | Wound infection | |||||||||
| 12L-0372 | Feces | |||||||||
| n = 1 | Dog | 10L-3142 | Swab | AMP, AMC, CPD, CAZ, FOX, TE | – | blaCMY-2 | B2 | |||
| n = 2 | Feline | 11L-0677/ | Wound infection | AMP, AMC, CPD, FOX, TE, S | – | – | B2 | |||
| 10L-2129 | Wound infection |
The numbers of isolates with a common phenotype and genotype are shown in column 1.
EBM, environmental bacterial monitoring; BAL, bronchoalveolar lavage; LN, lymph node; PMQR, plasmid-mediated quinolone resistance; PG, phylogenetic group; ST, sequence type; PT, pulsotype; AMP, ampicillin; AMC, amoxicillin–clavulanic acid; CFP, cefpodoxime; CTX, cefotaxime; CAZ, ceftazidime, FOX, cefoxitin; CIP, ciprofloxacin; NA, nalidixic acid; CN, gentamicin; TE, tetracycline; S, streptomycin, STX, trimethoprim/sulfamethoxazole.
The eight E. coli isolates included in this study for comparison (two clinical and six environmental) were processed in the diagnostic laboratory simultaneously and the identical susceptibility phenotypes identified in this group of isolates triggered closer investigation. In the DDST, they showed no synergy for ceftazidime and cefotaxime with CV combinations, while only a small zone of inhibition (less than 4 mm) appeared for the cefpodoxime/CV combination. All isolates were resistant to cefoxitin and this raised the possibility of the ESBL phenotype being masked by the additional presence of AmpC cephalosporinase, which is not inhibited by CV; additional testing with a cefepime/CV combination revealed the presence of ESBL phenotypes in these eight clinical/environmental E. coli isolates.
Characterization of ESBL and other resistance genes
Among the ESC-resistant clinical E. coli isolates, CTX-M type ESBL was the most prevalent, found in 56% (18/32) of isolates, of which 66% (12/18) of isolates harbored blaCTX-M-15, with blaCTX-M-14 being found in five isolates (27%) and blaCTX-M-27 identified in one isolate. With the exception of one isolate, which carried blaCTX-M-15 alone, the remaining ESC-resistant clinical E. coli isolates also carried blaTEM-1, blaCMY-2, and/or blaOXA-1 in various combinations (Table 1). The aac(6')-Ib-cr gene was the only PMQR gene detected, although at high prevalence (34.3%), and was associated with the carriage of blaCTX-M-15. The eight clinical and environmental isolates that showed the common ESBL/AmpC phenotype, carried blaCTX-M-15 and also coharbored blaOXA-1, blaTEM-1, blaCMY-2, and aac(6')-Ib-cr. Specific PCR assays revealed that ISEcp1 or IS26, or in some isolates ISEcp1 disrupted by IS26, was associated with blaCTX-M-15 (Table 1). In addition, ISEcp1was associated with blaCTX-M-14 in one of the five isolates and was not found to be associated with blaCTX-M-27, findings which support the diversity of the CTX-M genetic arrangements in E. coli isolates resulting from various mobilization events.
Resistance transfer and PBRT
Five transconjugants (four blaCTX-M-15 and one blaCTX-M-14), for which the ESBL phenotype and the presence of blaCTX-M-14/15 was confirmed, were generated on nutrient agar supplemented with cefotaxime (1 mg/L) and streptomycin (50 mg/L). PBRT showed that the transfer of blaCTX-M-15 was mainly associated with the FIA (n = 4), FIB (n = 4), IncI1 (n = 3), Y (n = 2), and B/O (n = 1) replicon types, while IncY type replicon was associated with the transfer of blaCTX-M-14. PCR also showed that blaTEM-1, blaCMY-2, blaOXA-1, as well as aac(6')-Ib-cr, had cotransferred with the blaCTX-M-14/15 in all transconjugants, indicating that they are located on conjugative plasmids.
Molecular typing of isolates
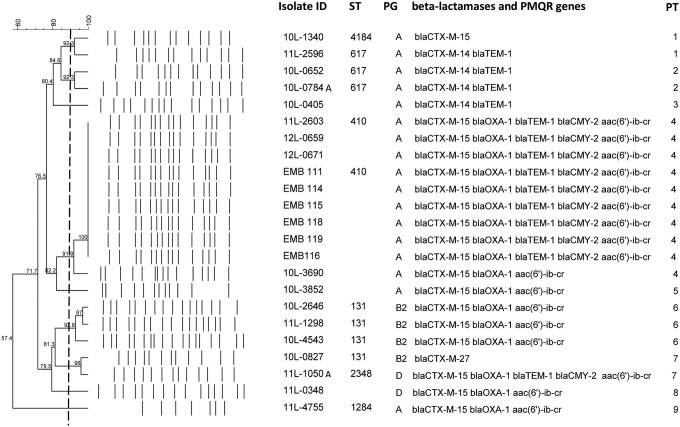
Phylogenetic typing identified that 63% of E. coli isolates belonged to phylogenetic group A and the remaining isolates were typed to the more potentially pathogenic groups, B2 (21%) or group D (15%). PFGE showed clonal diversity of the CTX-M-positive isolates and five pulsotypes (PT 1, 2, 4, 6, and 7) were identified with similarity of isolates greater than 90% (Fig. 1). The main group (PT 4, n = 9) included the eight clinical and environmental isolates with the common ESBL/AmpC phenotype and interestingly, another clinical isolate from a colon biopsy obtained from a dog (11L-2603), which was admitted with diarrhea in the same clinic 7 months previously. The second main cluster (PT 6) was formed by three isolates identified to belong to the human pandemic ST131 by MLST. Interestingly, a fourth member of this clone, which carried blaCTX-M-27, showed only a 73% similarity with the ST131 group. MLST also showed that the clinical and environmental isolates with the common ESBL/AmpC phenotype belonged to ST410, while the next most common ST identified in our blaCTX-M isolates was ST617 (Fig. 1).
FIG. 1.
Dendrogram showing cluster analysis of XbaI PFGE patterns of CTX-M–producing clinical and environmental (EBM) Escherichia coli isolates. The columns to the right of the PFGE pattern indicate the ID, ST, PG, and identified beta-lactamases and PMQR genes. PFGE pulsotypes (PT 1–9) were identified as isolates with ≥90% similarity (represented as a vertical dotted line). ID, isolate identification; ST, sequence type; PG, phylogenetic group; PFGE, pulsed-field gel electrophoresis; PMQR, plasmid-mediated quinolone resistance.
Discussion
This study characterized a collection of ESC-resistant E. coli and identified a high prevalence (7%) of ESC-resistant E. coli in clinical specimens from companion animals in the UK, which is considerably higher than that found in similar studies from pets in France (3.7%) or The Netherlands (2%).3,10 We also found a high prevalence (56%) of CTX-M type ESBL-producing E. coli from clinical specimens where 66% of clinical ESBL-producing E. coli carried blaCTX-M-15, which is among the highest reported rates in companion animals. To the best of our knowledge, higher carriage rates of blaCTX-M-15 in clinical animal isolates have only been reported in the United States where 78% of the ESBL-producing E. coli clinical isolates from companion animals were found to carry blaCTX-M-15.11 In Europe, 46% and 36% of canine ESBL-producing E. coli isolates (from Germany and France, respectively) carried blaCTX-M-15.10,37 In The Netherlands, Dierikx et al.,3 found that of 29 E. coli isolates with an ESBL/AmpC phenotype from diseased dogs, cats, and horses, only five isolates (from dogs) (17%) carried blaCTX-M-15. In addition, a lower prevalence of blaCTX-M-15 was found in Switzerland, where eight of the 107 E. coli isolates obtained from canine urine samples (7.4%) were ESBLs and all carried blaCTX-M-15.4 Furthermore, only one E. coli isolate carried this gene in a similar study in Italy and no blaCTX-M was identified in a study characterizing multidrug-resistant canine urinary E coli isolates from Scotland.38,39
E. coli carrying blaCTX-M-15 is the most common ESBL type associated with infections in humans in the United Kingdom and Europe.6 On this basis, the high prevalence of veterinary clinical isolates carrying blaCTX-M-15 identified in this study is worrying both in the context of likely interspecies transfer (man to animals), as well as previous studies identifying animals as a potential reservoir of ESBL-producing E. coli for human infection.40,41 In addition, the clinical and environmental isolates investigated for hospital dissemination had a common ESBL/AmpC phenotype and genotype and MLST showed that they all belonged to ST410. PCR analysis also demonstrated that all isolates had an identical genetic environment of blaCTX-M-15, where an IS26 element was inserted in between blaCTX-M-15 gene and its promoter found in ISEcp1. PFGE showed 100% homology for the two ESBL/AmpC E. coli clinical isolates from the dog with septic peritonitis and the environmental isolates obtained from hospital areas with which this patient came in direct contact (ultrasound table and kennel), or were likely to have spread through staff contact (door handle, the ward fridge handle, and the ward computer keyboard).
This study demonstrated veterinary hospital dissemination of clinical E. coli ST410 isolates co-harboring blaCTX-M-15, blaTEM-1, OXA-1 or CMY-2, and acc(6`)-Ib-cr, a genotype conferring MDR and often associated with human clinical isolates.42–44 Following the confirmation of the ESBL/AmpC phenotype in these isolates, the laboratory contacted the veterinary hospital's infection control team, which took action by cleaning and disinfection of the areas/surfaces identified as sources of these organisms and reinforced hand hygiene policy. The environmental sampling was repeated after reinforcing cleaning and disinfection protocols and no E. coli isolates with an ESBL/AmpC phenotype were identified in the subsequent bacterial environmental surveillance specimens. This study demonstrates the role that the microbiology laboratory can play in the early detection and prevention of MDR isolate dissemination in veterinary hospitals. The presence of multiple β-lactamases in Gram-negative bacteria may interfere with the ESBL phenotypic confirmatory tests45,46 and it is therefore important that veterinary diagnostic microbiology laboratories are continuously updating their detection methods to recognize ESBL, AmpC, or other emerging resistance phenotypes and to translate the therapeutic or epidemiological significance of these findings to veterinary clinicians. This study also highlights the importance of infection control programs and the benefits of environmental surveillance in the veterinary hospitals for limiting the spread of nosocomial pathogens. Furthermore, the dissemination of the ESBL/AmpC E. coli ST410 isolates from veterinary patients (probably from surgical wounds) to the environment, as shown in this study, may indicate a pattern of spread that can occur in the community, especially in the owners home, highlighting the associated human health risk. Therefore, accurate laboratory detection of ESBL/AmpC phenotypes can support the veterinary hospitals in the process of implementing policies for owner's information and infection control advice for limiting the owner's exposure and associated transmission risks.
Recent EUCAST and CLSI guidelines recommend that when using the new interpretative breakpoints, routine ESBL testing is no longer necessary and reporting of susceptibility results to penicillins and cephalosporins for ESBL-producing Enterobacteriaceae should be ‘as found’.47,48 However, these new guidelines indicate that ESBL screening may still be useful for epidemiological reasons.47,48 Our findings, demonstrating a high prevalence of CTX-M-15 ESBL–producing E. coli in clinical specimens from companion animals, as well as the dissemination of E. coli ST410 through the hospital environment, support the need for veterinary laboratories to continue ESBL screening and to continuously upgrade their expertise in detection of complex antimicrobial resistance phenotypes, to benefit both human and animal health.
Disclosure Statement
All authors declare that they have no conflicts of interest or any other competing financial interests to disclose.
References
- 1.Thompson M.F., Litster A.L., Platell J.L., and Trott D.J. 2011. Canine bacterial urinary tract infections: new developments in old pathogens. Vet. J. 190:22–27 [DOI] [PubMed] [Google Scholar]
- 2.Guardabassi L., Schwarz S., and Lloyd D.H. 2004. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 54:321–332 [DOI] [PubMed] [Google Scholar]
- 3.Dierikx C.M., van Duijkeren E., Schoormans A.H.W., van Essen-Zandbergen A., Veldman K., Kant A., Huijsdens X.W., van der Zwaluw K., Wagenaar J.A., and Mevius D.J. 2012. Occurrence and characteristics of extended-spectrum—lactamase- and AmpC-producing clinical isolates derived from companion animals and horses. J. Antimicrob. Chemother. 67:1368–1374 [DOI] [PubMed] [Google Scholar]
- 4.Huber H., Zweifel C., Wittenbrink M.M., and Stephan R. 2013. ESBL-producing uropathogenic Escherichia coli isolated from dogs and cats in Switzerland. Vet. Microbiol. 162:992–996 [DOI] [PubMed] [Google Scholar]
- 5.Carattoli A., Lovari S., Franco A., Cordaro G., Di Matteo P., and Battisti A. 2005. Extended-spectrum beta-lactamases in Escherichia coli isolated from dogs and cats in Rome, Italy, from 2001 to 2003. Antimicrob. Agents Chemother. 49:833–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodford N. 2008. Successful, multiresistant bacterial clones. J. Antimicrob. Chemother. 61:233–234 [DOI] [PubMed] [Google Scholar]
- 7.Ewers C., Grobbel M., Bethe A., Wieler L.H., and Guenther S. 2011. Extended-spectrum beta-lactamases-producing Gram-negative bacteria in companion animals: action is clearly warranted! Berl. Munch. Tierarztl. Wochenschr. 124:94–101 [PubMed] [Google Scholar]
- 8.Shaheen B.W., Nayak R., Foley S.L., Kweon O., Deck J., Park M., Rafii F., and Boothe D.M. 2011. Molecular characterization of resistance to extended-spectrum cephalosporins in clinical Escherichia coli isolates from companion animals in the United States. Antimicrob. Agents Chemother. 55:5666–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamang M.D., Nam H.-M., Jang G.-C., Kim S.-R., Chae M.H., Jung S.-C., Byun J.-W., Park Y.H., and Lim S.-K. 2012. Molecular characterization of extended-spectrum-beta-lactamase-producing and plasmid-mediated AmpC beta-lactamase-producing Escherichia coli isolated from stray dogs in South Korea. Antimicrob. Agents Chemother. 56:2705–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahmen S., Haenni M., Chatre P., and Madec J.-Y. 2013. Characterization of bla(CTX-M) IncFII plasmids and clones of Escherichia coli from pets in France. J. Antimicrob. Chemother. 68:2797–2801 [DOI] [PubMed] [Google Scholar]
- 11.Shaheen B.W., Nayak R., Foley S.L., and Boothe D.M. 2013. Chromosomal and plasmid-mediated fluoroquinolone resistance mechanisms among broad-spectrum-cephalosporin-resistant Escherichia coli isolates recovered from companion animals in the USA. J. Antimicrob. Chemother. 68:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hilty M., Betsch B.Y., Boegli-Stuber K., Heiniger N., Stadler M., Kueffer M., Kronenberg A., Rohrer C., Aebi S., Endimiani A., Droz S., and Muehlemann K. 2012. Transmission dynamics of extended-spectrum beta-lactamase-producing Enterobacteriaceae in the Tertiary Care Hospital and the Household Setting. Clin. Infect. Dis. 55:967–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boerlin P., Eugster S., Gaschen F., Straub R., and Schawalder P. 2001. Transmission of opportunistic pathogens in a veterinary teaching hospital. Vet. Microbiol. 82:347–359 [DOI] [PubMed] [Google Scholar]
- 14.Sidjabat H.E., Townsend K.M., Lorentzen M., Gobius K.S., Fegan N., Chin J.J.C., Bettelheim K.A., Hanson N.D., Bensink J.C., and Trott D.J. 2006. Emergence and spread of two distinct clonal groups of multidrug-resistant Escherichia coli in a veterinary teaching hospital in Australia. J. Med. Microbiol. 55:1125–1134 [DOI] [PubMed] [Google Scholar]
- 15.Sanchez S., Stevenson M.A.M., Hudson C.R., Maier M., Buffington T., Dam Q., and Maurer J.J. 2002. Characterization of multidrug-resistant Escherichia coli isolates associated with nosocomial infections in dogs. J. Clin. Microbiol. 40:3586–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidjabat H.E., Hanson N.D., Smith-Moland E., Bell J.M., Gibson J.S., Filippich L.J., and Trott D.J. 2007. Identification of plasmid-mediated extended-spectrum and AmpC beta-lactamases in Enterobacter spp. isolated from dogs. J. Med. Microbiol. 56:426–434 [DOI] [PubMed] [Google Scholar]
- 17.Umber J.K., and Bender J.B. 2009. Pets and antimicrobial resistance. Vet. Clin. North Am. Small Anim. Pract. 39:279–292 [DOI] [PubMed] [Google Scholar]
- 18.Stolle I., Prenger-Berninghoff E., Stamm I., Scheufen S., Hassdenteufel E., Guenther S., Bethe A., Pfeifer Y., and Ewers C. 2013. Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J. Antimicrob. Chemother. 68:2802–2808 [DOI] [PubMed] [Google Scholar]
- 19.Walther B., Luebke-Becker A., Stamm I., Gehlen H., Barton A.K., Janssen T., Wieler L.H., and Guenther S. 2014. Suspected nosocomial infections with multi-drug resistant E. coli, including extended-spectrum beta-lactamase (ESBL)-producing strains, in an equine clinic. Berl. Munch. Tierarztl. Wochenschr. 127:421–427 [PubMed] [Google Scholar]
- 20.Haenni M., Ponsin C., Metayer V., Medaille C., and Madec J.-Y. 2012. Veterinary hospital-acquired infections in pets with a ciprofloxacin-resistant CTX-M-15-producing Klebsiella pneumoniae ST15 clone. J. Antimicrob. Chemother. 67:770–771 [DOI] [PubMed] [Google Scholar]
- 21.Ewers C., Stamm I., Pfeifer Y., Wieler L.H., Kopp P.A., Schonning K., Prenger-Berninghoff E., Scheufen S., Stolle I., Guenther S., and Bethe A. 2014. Clonal spread of highly successful ST15-CTX-M-15 Klebsiella pneumoniae in companion animals and horses. J. Antimicrob. Chemother. 69:2676–2680 [DOI] [PubMed] [Google Scholar]
- 22.Endimiani A., Hujer K.M., Hujer A.M., Bertschy I., Rossano A., Koch C., Gerber V., Francey T., Bonomo R.A., and Perreten V. 2011. Acinetobacter baumannii isolates from pets and horses in Switzerland: molecular characterization and clinical data. J. Antimicrob. Chemother. 66:2248–2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohlwend N., Endimiani A., Francey T., and Perreten V. 2015. Third-Generation-Cephalosporin-Resistant Klebsiella pneumoniae Isolates from Humans and Companion Animals in Switzerland: spread of a DHA-Producing Sequence Type 11 Clone in a Veterinary Setting. Antimicrob. Agents Chemother. 59:2949–2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDaniels A.E., Rice E.W., Reyes A.L., Johnson C.H., Haugland R.A., and Stelma G.N. 1998. Confirmational identification of Escherichia coli, a comparison of genotypic and phenotypic assays for glutamate decarboxylase and beta-D-glucuronidase. Appl. Environ. Microbiol. 64:4113–4113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews J.M., and Howe R.A. 2011. BSAC standardized disc susceptibility testing method (version 10). J. Antimicrob. Chemother. 66:2726–2757 [DOI] [PubMed] [Google Scholar]
- 26.M'Zali F.H., Chanawong A., Kerr K.G., Birkenhead D., and Hawkey P.M. 2000. Detection of extended-spectrum beta-lactamases in members of the family Enterobacteriaceae: comparison of the MAST DD test, the double disc and the Etest ESBL. J. Antimicrob. Chemother. 45:881–885 [DOI] [PubMed] [Google Scholar]
- 27.Dallenne C., Da Costa A., Decre D., Favier C., and Arlet G. 2010. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 65:490–495 [DOI] [PubMed] [Google Scholar]
- 28.Perez-Perez F.J., and Hanson N.D. 2002. Detection of plasmid-mediated AmpC beta-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park C.H., Robicsek A., Jacoby G.A., Sahm D., and Hooper D.C. 2006. Prevalence in the United States of aac(6′)-Ib-cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 50:3953–3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essack S.Y., Hall L.M.C., Pillay D.G., McFadyen M.L., and Livermore D.M. 2001. Complexity and diversity of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases isolated in 1994 and 1996 at a teaching hospital in Durban, South Africa. Antimicrob. Agents Chemother. 45:88–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robicsek A., Strahilevitz J., Sahm D.F., Jacoby G.A., and Hooper D.C. 2006. qnr Prevalence in ceftazidime-resistant Enterobacteriaceae isolates from the United States. Antimicrob. Agents Chemother. 50:2872–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirel L., Gniadkowski M., and Nordmann P. 2002. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum beta-lactamase CTX-M-15 and of its structurally related beta-lactamase CTX-M-3. J. Antimicrob. Chemother. 50:1031–1034 [DOI] [PubMed] [Google Scholar]
- 33.Woodford N., Ward M.E., Kaufmann M.E., Turton J., Fagan E.J., James D., Johnson A.P., Pike R., Warner M., Cheasty T., Pearson A., Harry S., Leach J.B., Loughrey A., Lowes J.A., Warren R.E., and Livermore D.M. 2004. Community and hospital spread of Escherichia coli producing CTX-M extended-spectrum beta-lactamases in the UK. J. Antimicrob. Chemother. 54:735–743 [DOI] [PubMed] [Google Scholar]
- 34.Carattoli A., Bertini A., Villa L., Falbo V., Hopkins K.L., and Threlfall E.J. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 35.Clermont O., Bonacorsi S., and Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wirth T., Falush D., Lan R.T., Colles F., Mensa P., Wieler L.H., Karch H., Reeves P.R., Maiden M.C.J., Ochman H., and Achtman M. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60:1136–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmiedel J., Falgenhauer L., Domann E., Bauerfeind R., Prenger-Berninghoff E., Imirzalioglu C., and Chakraborty T. 2014. Multiresistant extended-spectrum beta-lactamase-producing Enterobacteriaceae from humans, companion animals and horses in central Hesse, Germany. BMC Microbiol. 14:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nebbia P., Tramuta C., Odore R., Nucera D., Zanatta R., and Robino P. 2014. Genetic and phenotypic characterisation of Escherichia coli producing cefotaximase-type extended-spectrum beta-lactamases: first evidence of the ST131 clone in cats with urinary infections in Italy. J. Feline Med. Surg. 16:966–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner S., Gally D.L., and Argyle S.A. 2014. Multidrug-resistant Escherichia coli from canine urinary tract infections tend to have commensal phylotypes, lower prevalence of virulence determinants and ampC-replicons. Vet. Microbiol. 169:171–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naseer U., and Sundsfjord A. 2011. The CTX-M Conundrum: dissemination of plasmids and Escherichia coli clones. Microb. Drug Resist. 17:83–97 [DOI] [PubMed] [Google Scholar]
- 41.Moodley A., and Guardabassi L. 2009. Transmission of IncN Plasmids Carrying bla(CTX-M-1) between Commensal Escherichia coli in Pigs and Farm Workers. Antimicrob. Agents Chemother. 53:1709–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavollay M., Mamlouk K., Frank T., Akpabie A., Burghoffer B., Ben Redjeb S., Bercion R., Gautier V., and Arlet G. 2006. Clonal dissemination of a CTX-M-15 beta-lactamase-producing Escherichia coli strain in the Paris Area, Tunis, and Bangui. Antimicrob. Agents Chemother. 50:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maciuca I.E., Williams N.J., Tuchilus C., Dorneanu O., Guguianu E., Carp-Carare C., Rimbu C., and Timofte D. 2015. High prevalence of Escherichia coli-producing CTX-M-15 extended-spectrum beta-lactamases in poultry and human clinical isolates in Romania. Microb. Drug Resist. 21:651–662 [DOI] [PubMed] [Google Scholar]
- 44.Coque T.M., Baquero F., and Canton R. 2008. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro. Surveill. 13:47. [PubMed] [Google Scholar]
- 45.Poulou A., Grivakou E., Vrioni G., Koumaki V., Pittaras T., Pournaras S., and Tsakris A. 2014. Modified CLSI extended-spectrum beta-lactamase (ESBL) confirmatory test for phenotypic detection of ESBLs among Enterobacteriaceae producing various beta-lactamases. J. Clin. Microbiol. 52:1483–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willems E., Verhaegen J., Magerman K., Nys S., and Cartuyvels R. 2013. Towards a phenotypic screening strategy for emerging beta-lactamases in Gram-negative bacilli. Int. J. Antimicrob. Agents 41:99–109 [DOI] [PubMed] [Google Scholar]
- 47.Leclercq R., Canton R., Brown D.F.J., Giske C.G., Heisig P., MacGowan A.P., Mouton J.W., Nordmann P., Rodloff A.C., Rossolini G.M., Soussy C.J., Steinbakk M., Winstanley T.G., and Kahlmeter G. 2013. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 19:141–160 [DOI] [PubMed] [Google Scholar]
- 48.Clinical and Laboratory Standards Institute. 2011. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-first Informational Supplement. 31. CLSI, Wayne, PA [Google Scholar]