Abstract
It has been difficult to translate promising results from DNA vaccination in mice to larger animals and humans. Previously, DNA vaccines encoding proteins that target Ag to MHC class II (MHC-II) molecules on APCs have been shown to induce rapid, enhanced, and long-lasting Ag-specific Ab titers in mice. In this study, we describe two novel DNA vaccines that as proteins target HLA class II (HLA-II) molecules. These vaccine proteins cross-react with MHC-II molecules in several species of larger mammals. When tested in ferrets and pigs, a single DNA delivery with low doses of the HLA-II–targeted vaccines resulted in rapid and increased Ab responses. Importantly, painless intradermal jet delivery of DNA was as effective as delivery by needle injection followed by electroporation. As an indication that the vaccines could also be useful for human application, HLA-II–targeted vaccine proteins were found to increase human CD4+ T cell responses by a factor of ×103 in vitro. Thus, targeting of Ag to MHC-II molecules may represent an attractive strategy for increasing efficacy of DNA vaccines in larger animals and humans.
Introduction
Infectious diseases continuously pose significant threats to the human population. Thus, there is a great need to develop novel subunit vaccines against pathogens that display considerable variability, and that often have evolved complex mechanisms to evade immune recognition (1). DNA vaccines are promising in this respect, because they represent a highly versatile format that allows easy construction and rapid manufacture. However, encouraging results with DNA vaccination in mice have been difficult to translate to larger animals and humans (2), even though exceptions exist in the form of a melanoma vaccine for dogs (3) and a West Nile virus vaccine for horses (4).
During the second half of the 1980s, targeting of Ag to surface molecules on APCs was shown to substantially increase immune responses after immunization (5–7). This basic finding was confirmed by later studies (8–13). Initially, these experiments were performed with Ag that had been chemically conjugated to APC-specific Abs (5–10). Later, genetic fusion of an APC-specific targeting unit and Ag has been a preferred method (11–17). Several different versions of APC-specific targeting units have been reported, including Ig-based formats (11, 13), single-chain variable fragment (scFv) formats (14, 15), and chemokines (12, 16, 17). Although the APC-targeted vaccines have mostly been delivered as proteins (5–13), the principle has also been demonstrated for DNA vaccines (12, 14–17).
Recent work has indicated that the types of immune responses elicited by targeted vaccines are influenced by which surface molecules they bind to on APCs (16, 18). This issue is relevant because most successful vaccines owe their efficiency to the induction of protective Abs (19). A promising APC target for induction of Abs appears to be MHC class II (MHC-II) molecules (6, 14, 18, 20–22), which are extensively expressed on professional APCs such as macrophages, dendritic cells, and B cells.
An effective large-scale production of APC-targeted proteins is challenging because it is both time-consuming and costly. Circumventing this problem, it has previously been demonstrated that APC-targeted fusion proteins may be delivered as DNA plasmids that can be rapidly and cheaply constructed and produced (14, 22). Upon injection of plasmids in combination with electroporation, transfected cells secrete DNA-encoded fusion protein that delivers Ag to MHC-II on APCs, resulting in rapid and enhanced Ab and T cell responses that protect against tumors (14) and influenza virus infection (22).
Based on these encouraging results, our aim has been to develop novel DNA-based vaccines that improve the weak immune responses otherwise elicited by DNA vaccines in larger animals and humans. To this end, in this study we have used a DNA vaccine format that encodes secreted bivalent vaccine proteins composed of targeting units, dimerization units, and full-length Ags (14, 16, 17, 22, 23). Because human MHC-II (HLA class II [HLA-II]) molecules are highly polymorphic, and because a vaccine should be deployable in all humans, we generated two different scFv targeting units that should bind HLA-II molecules in humans. Fortuitously, these scFvs cross-react with MHC-II of several large species, allowing testing of DNA vaccines against influenza in ferrets and pigs. The results show that the DNA vaccines could be delivered in low doses by pain-free jet delivery, and that a single delivery of influenza hemagglutinin (HA) targeted to MHC-II molecules rapidly induced HA-specific Abs in these larger species. The induced immune responses could be further increased by a booster vaccination.
Materials and Methods
Cells and Abs
Human embryonic kidney 293E cells and NS0 cells were purchased from the American Type Culture Collection (Manassas, VA). T18 is a human HLA-DR4 (DRA1, DRB1*0401)–restricted T cell clone specific for aa 40–48 of mouse Ig Cκ (24). The murine hybridoma producing the anti–HLA-II mAb (HKB1, IgM) (25) was a gift from Dr. Steinar Funderud (Oslo University Hospital). The anti–HLA-DR mAb (L243, IgG2a) (26) was purchased from the American Type Culture Collection. The use of human PBMCs was approved by the Norwegian Regional Committee for Medical and Health Research Ethics (REC, 2014/1505). The cells were purified from whole blood by LymphoPrep density gradient centrifugation (Nycomed, Oslo, Norway).
Construction of vaccine molecules
mRNA was isolated from the hybridomas HKB1 and L243 by use of a Dynabeads mRNA Direct kit (Dynal, Oslo, Norway), and cDNA was synthesized using a cDNA Synthesis Kit (Amersham Biosciences, Oslo, Norway). The V(D)J of HKB1 and L243 H and L chain genes were PCR amplified from the cDNA with degenerate primers complementary to the leader sequence of VL and VH and IgM, IgG1, and Cκ. The PCR products for the L chain and H chain were ligated into individual pGEM-T easy vectors (Promega, Madison, WI). To obtain scFvs specific for HKB1 and L243, PCR reactions were run to reamplify the VH and VL domains from the pGEM-T easy vectors with specific primers, including linkers and restriction enzyme sites (BsmI/BsiWI) for subcloning. Primers included 5′-VH HKB1/L243: ggc gga ggt ggc tct ggc ggt ggc gga tcg CAG ATC CAG TTG GTG CAG TCT and 3′-VH HKB1/L243: ga c gtacg a ctc acc TGA GGA GAC TGT GAG AGT GG. Next, the various scFvs were BsmI/BsiWI digested and cloned into the BsmI/BsiWI cassette of pLNOH2 (27) or pUMVC (28) (gift from Bob Weinberg, Addgene plasmid no. 8449) expression vectors to generate vaccine constructs with specificities for NIP, pan anti–HLA-II and anti–HLA-DR. The vaccine constructs were equipped with antigenic units by subcloning either the scFv-like homodimer CκCκ, HA from influenza A/Puerto Rico/8/1934 (H1N1) (PR8), or HA from influenza A/California/07/2009 (H1N1) (Cal07) (22) on SfiI sites.
Western blot
Vaccine proteins containing the scFv CκCκ as Ag, affinity purified (187.1 mAb column) from supernatants of stably transfected NS0 cells, were run on a Novex 4–12% Tris-glycine gel (Invitrogen Life Technologies, Carlsbad, CA) together with a SeeBlue Plus2 prestained standard (LC5925; Invitrogen Life Technologies), blotted (Immun-Blot polyvinylidene difluoride membrane, 162-0177; Bio-Rad Laboratories, Hercules, CA), and incubated with biotinylated HP6017 (anti-human IgG CH3; BioLegend, San Diego, CA) and streptavidin-HRP (RPN1231V; GE Healthcare, Buckinghamshire, U.K.). The membrane was developed with the ECL Western blotting analysis system (RPN2109; GE Healthcare) and analyzed on a Kodak Image Station 200R (LabX Canada) with Molecular Imaging Software v4.0.5.
One microgram of DNA plasmids encoding vaccine constructs with HA as Ag was transiently transfected into 293E cells (1 × 105/well) by addition of Lipofectamine 2000 (11668-019; Invitrogen Life Technologies). Supernatants were collected after 48 h, and Western blot was performed as described above. Proteins were detected with biotinylated H36-4-52 anti-HA mAb (gift from Siegfried Weiss, Braunschweig, Germany) (29) and developed as described above.
Sandwich ELISA
ELISA plates (Costar 3590; Sigma-Aldrich, St. Louis, MO) were coated with either 1 μg/ml mAb 187.1 (anti-mouse Cκ) (30), MCA878 (anti-human CH3) (AbD Serotec, Oxford, U.K.), or NIP-BSA, blocked, and incubated with supernatants from transiently transfected 293E cells (as above). Next, plates were incubated (1 μg/ml) with either biotinylated 187.1, HP6017 (BioLegend), or H36-4-52 and streptavidin–alkaline phosphatase (1:5000) (GE Healthcare). Plates were developed using phosphatase substrate (P4744-10G; Sigma-Aldrich) dissolved in substrate buffer and read with a Tecan reader (Tecan, Mannedorf, Switzerland) using the Magellan v5.03 program.
Flow cytometry
Vaccine protein staining of PBMCs.
Freshly isolated human PBMCs were stained sequentially with purified vaccine proteins, biotinylated HP6017, and streptavidin-allophycocyanin (BD Pharmingen, San Diego, CA). Cells were run on a BD FACSCalibur system (BD Biosciences, Franklin Lakes, NJ) and analyzed with FlowJo software (version 7.6) (FlowJo, Ashland, OR).
Vaccine protein staining of cells from DQ2 transgene mice.
Splenocytes from three DQ2 transgenic mice (31) were FcγR blocked by incubation with 30% heat-aggregated rat serum and 0.1 mg/ml 2.4G2 mAb (Fisher Scientific, Waltham, MA) and then stained sequentially with vaccine proteins (10 μg/ml), biotinylated HP6017 (1:2000) (042M4810; Sigma-Aldrich), and streptavidin-PE (2 μg/ml) (554061; BD Pharmingen). The staining solution also contained either FITC-conjugated mAb against CD19 (4 μg/ml) (35-0193; Tonbo Biosciences, San Diego, CA), PerCP-Cy5.5–conjugated mAb against CD11b (3 μg/ml) (66-0112-U100; Tonbo Biosciences), or allophycocyanin-conjugated mAb against CD11c (20-0114-U100; Tonbo Biosciences). Samples were run on an LSR II (BD Biosciences), and data were analyzed as above.
Vaccine protein staining of cells from larger animals.
Blood collected from horses, cows, sheep, ferrets, and pigs (n = 3 for all species) on EDTA were added to Lympholyte (CL5120; Cedarlane Laboratories, Burlington, Canada) (1:1) and centrifuged for 40 min at 1500 rpm. The lymphocyte layer was collected, washed with PBS, and plated out in 96-well plates (1 × 106 cells/well). Cells were blocked as above and stained sequentially with vaccine proteins (10 μg/ml), biotinylated mAb against HA (H36-4-52), and allophycocyanin-conjugated streptavidin (554067; BD Biosciences). Samples were run on an LSRII, and data were analyzed with FlowJo software (version 10.0.5). For staining cells from ferrets, PE-conjugated mAbs against CD11b (clone M1/70) (553311; BD Pharmingen) were also included in the staining solution. For staining of cells from pigs, the vaccine proteins were detected with PE-conjugated mAbs against human IgG (2043-09; SouthernBiotech, Birmingham, AL). FITC-conjugated mAb against CD3ε (559582; BD Biosciences) and mAb against CD11R3 (MCA2309; AbD Serotec) (detected with allophycocyanin-conjugated mAb against IgG1 [550874; BD Pharmingen]) were included in the staining.
T cell proliferation assays
APCs were freshly isolated from PBMCs (7 × 104/well) of HLA-DR4 (DRA1, DRB1*0401) donors. Experiments were approved by the Norwegian Regional Committee for Medical and Health Research Ethics (REC, 2014/1505). Irradiated APCs (20 Gy) and mouse Cκ40–48-specific, DR4-restricted human CD4+ T cells (4 × 104/well) (24) were cultured in 96-well plates with titrated amounts of the various bivalent vaccines expressing CκCκ. After 48 h, the cultures were pulsed for 16–24 h with 1 μCi of [3H]thymidine, harvested, and [3H]thymidine incorporated into DNA of proliferating cells was measured using a TopCount NXT scintillation counter (Packard, Meriden, CT).
Specificity of vaccine proteins and HKB1 mAb for HLA-II
One microgram of vaccine proteins expressing CκCκ (purified on column with 187.1 mAb linked to Sepharose) (GE Healthcare) was mixed with HLA-II–coated xMAP microbeads (LS2A01; One Lambda, Canoga Park, CA) in 96-well plates and incubated for 30 min at 22°C. Next, plates were washed three times and samples were incubated with PE-conjugated Abs directed against the CH3 domain dimerization unit (409304; BioLegend) for 30 min at 22°C. Samples containing HKB1 were detected with PE-conjugated anti-mouse IgM (553517; BD Pharmingen). Plates were washed twice and samples were resuspended in PBS before being read on a Luminex 100 flow analyzer (Luminex, Austin, TX) and analyzed with HLA Visual Software (One Lambda). The obtained HLA reactivity profile was then used to evaluate shared regions of the otherwise polymorphic HLA β-chains. Briefly, the complete HLA directory was downloaded (http://www.ebi.ac.uk/ipd/imgt/hla/), aligned with ClustalX 2.1 (32), and manually edited and annotated in GenDoc (33) to identify the likely HKB epitope. Representative Protein Data Bank entries were contoured by APBS (34) and visualized using the PyMOL molecular graphics system (Schrödinger).
Animals
Six- to eight-week-old BALB/c and DQ2 transgene mice (31) (on a BALB/c background) were used. Mice were housed under minimal disease conditions at Oslo University Hospital (Oslo, Norway). Six- to eight-week-old pigs (Noroc) of both sexes were used (Noroc: 50% Norwegian landgris, 25% Norwegian Yorkshire, and 25% Duroc). Weights at the start of the experiments ranged from 16 to 30 kg, whereas weights at the termination of experiments ranged from 34 to 48 kg. Pigs were housed at the Animal Production Experimental Center, Norwegian University of Life Science (Ås, Norway). Experiments on mice and pigs were approved by the Norwegian Animal Research Authority (Oslo, Norway). Ferret yearlings (female and male) were used, with weights ranging from 2.3 to 7 kg. Ferrets were housed at the Laboratory Animal Facility, University of Copenhagen (Copenhagen, Denmark). Experiments in ferrets were approved by the Danish Society for the Protection of Laboratory Animals (Copenhagen, Denmark).
Virus
Influenza A/Puerto Rico/8/1934 (H1N1) (PR8) was provided by Dr. Anna Germundsson (National Veterinary Institute, Oslo, Norway). The virus was propagated by an inoculation into the allantoic cavity of 10-d-old embryonated chicken eggs. Allantoic fluid was harvested, confirmed negative for bacterial contaminations, and 50% tissue culture–infective dose (TCID50) was determined.
Vaccination and viral challenge
Mice.
Mice were anesthetized by s.c. injection of fluniasone (Hypnorm) and midazolam (Dormicum) (0.05 ml working solution/10 g) and vaccinated as previously described (22). Briefly, 25 μl of vaccine solution (0.5 mg/ml DNA) was injected intradermally (i.d.) on each flank of the mouse, immediately followed by skin electroporation with Agile Pulse (Harvard Apparatus/BTX, Holliston, MA). For viral challenge, LD50 was determined as previously described (22), and anesthetized mice were intranasally infected with 5 × LD50 of PR8 (2.0 × 104 TCID) in 20 μl (10 μl per nostril). Mice were monitored for weight loss (n = 6 per group), with an endpoint of 20% weight reduction, as required by the National Committee for Animal Experiments.
Ferrets.
Prior to immunization, ferrets were confirmed negative for influenza-reactive Abs (<23) in a hemagglutination inhibition (HI) assay. Ferrets were immunized i.d. with 100 μg of DNA (pLNOH2-vector) in total, delivered by two separate injections of 100 μl (0.5 mg/ml DNA) at each side of the lower back region. Injection was immediately followed by skin electroporation (Agile Pulse; Harvard Apparatus/BTX).
Pigs.
Pigs were confirmed negative for influenza-reactive Abs (<23) in an HI assay, and DNA (pUMVC-vector) was immunized either by i.d. needle injection followed by skin electroporation (Agile Pulse; Harvard Apparatus/BTX) or by i.d. jet delivery (Tropis; PharmaJet, Golden, CO).
Serum ELISA
Sera were isolated from blood by two successive centrifugations for 5 min at 13,000 rpm. Ninety-six–well plates were coated with either inactivated PR8 (Charles River Laboratories, Wilmington, MA) (1:1600 in PBS) or Pandemrix (Ag suspension with A/California/7/2009 [H1N1]v-like strain [X-179A], GlaxoSmithKline) (1:100 in PBS), blocked with 0.1% BSA in PBS, and incubated overnight at 4°C with titrated amounts of sera. Abs in mouse sera were detected with biotinylated anti-IgG (A2429; Sigma-Aldrich), anti-IgG1a (553599; BD Pharmingen), or anti-IgG2aa (553502; BD Pharmingen), followed by streptavidin–alkaline phosphatase (GE Healthcare) and development with phosphatase substrate (P4744-10G; Sigma-Aldrich) dissolved in substrate buffer. The plates were read as above. Titers are given, defined as the last serum dilution giving an absorbance above background (mean absorbance for NaCl-vaccinated mice plus 5 × SEM). In ferrets and pigs, HA-specific IgG Abs in sera were detected with alkaline phosphatase–conjugated anti-ferret IgG (LS-C61240; LSBio, Seattle, WA) and biotinylated anti-pig IgG (ab112747; Abcam, Cambridge, U.K.), respectively. Plates were developed and analyzed as above. For detection of Abs elicited by the pHLA-II–specific scFvs encoded by the targeted DNA vaccine, plates were coated with either HKB1 or TIB211 (1 μg/ml in PBS). Abs in sera were then detected with biotinylated anti-pig IgG as above.
Microneutralization assay for influenza virus–specific Abs
The microneutralization assay was performed as previously described (22). Briefly, sera were treated with receptor-destroying enzyme (II) (Denka Seiken, Tokyo, Japan), and 2-fold duplicate dilutions were set up in triplicates. Fifty microliters of 100 × TCID50 virus (PR8 or Cal07) was added to each well, and plates were incubated for 2 h at 37°C in a 5% CO2 humidified atmosphere. Madin–Darby canine kidney cells (2 × 105) were added to each well, and plates were incubated for 20 h at 37°C and 5% CO2. Monolayers were washed with PBS and fixed in cold 80% acetone for 10 min, and viral proteins were detected by an ELISA using biotinylated mAb against the influenza nucleoprotein (HB65; American Type Culture Collection) and streptavidin–alkaline phosphatase (GE Healthcare). Plates were read as described above, and the percentage neutralization of each serum sample was calculated with the following equation: x = [(average OD of virus control wells − OD of sample)/(average OD of virus control wells − average OD of cell control wells)] × 100.
HI assay
Sera from individual animals were treated with receptor-destroying enzyme (II) (Denka Seiken) at 37°C for 20 h, and the enzyme was deactivated by incubation for 40 min at 56°C. Titrated sera were added in triplicates to 96-well plates, and PR8 or Cal07 virus (4HAU) was added prior to a 40-min incubation at room temperature. Turkey RBCs (1%) were then added to the wells, and the plates were read for hemagglutination inhibition 45 min later. HI was scored as the highest dilution of antiserum giving a complete inhibition of hemagglutination. Validity of results were confirmed by a positive control serum (influenza PR8 antiserum; Charles River Laboratories) reaching its predicted titer and a negative control serum giving a titer of <23.
Statistical analysis
Statistical analyses were performed using one-way ANOVA and a Bonferroni multiple comparison test with GraphPad Prism software version 5 (GraphPad Software).
Results
Cloning of scFv targeting units with specificity for human HLA-II molecules
We have previously described efficient immune responses in mice elicited by a DNA vaccine format that encodes bivalent homodimers with N-terminal scFv targeting units specific for mouse MHC-II molecules, a human IgG3-derived dimerization unit (shortened hinge plus CH3), and with C-terminal antigenic units (14, 22) (Fig. 1A). To test the efficacy of the vaccine format in larger animals and humans, in this study we have developed novel scFv targeting units directed against human HLA-II molecules. The scFvs were derived from two different anti–HLA-II–specific mAbs. The first mAb, L243 (mouse IgG2a), is well characterized and is specific for HLA-DR molecules (26, 35). The second mAb, HKB1 (mouse IgM), reacts more generally with HLA-II molecules and is denoted pan (p)HLA-II–specific (25). The V regions of the two mAbs were cloned from B cell hybridomas and sequenced. scFvs were constructed and inserted into cassette vectors (14) that already contained the dimerization unit and an antigenic unit [either two mouse Ig Cκ domains linked in an scFv-like format (36) or influenza HA (22)]. The resulting HLA-II–targeted vaccines are designated αHLADR-CκCκ, αHLADR-HA, αpHLAII-CκCκ, and αpHLAII-HA, respectively. As nontargeted controls, we also prepared a vaccine where the HLA-II–specific targeting unit was replaced with an scFv specific for the hapten NIP (αNIP-CκCκ and αNIP-HA, respectively) (14, 22). Additionally, a plasmid expressing HA Ag alone was used. (Note that HA in all the vaccine constructs had been truncated [aa 18–541], with the cytosolic tail of HA and part of the transmembrane region being removed to prevent retention in the cell membrane of transfected cells) (22).
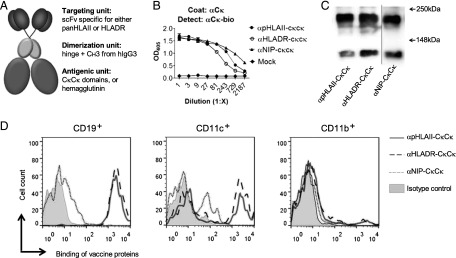
FIGURE 1.
Characterization of vaccine proteins. (A) Schematic structure of a DNA-delivered dimeric vaccine protein. The vaccine protein consists of two N-terminal HLA-II–specific targeting units in an scFv format that are linked to an Ig-based dimerization unit and two C-terminal antigenic units. (B) Presence of vaccine proteins in supernatants of transiently transfected 293E cells detected by sandwich ELISA. (C) Western blot of affinity-purified vaccine proteins detected with mAb against human CH3 dimerization unit. The vaccine proteins are indicated below lanes; molecular mass is indicated by arrows. The vertical line indicates joining of two parts from the same blot. (D) FACS analyses of human PBMCs stained with the indicated vaccine proteins. Binding to gated CD19+, CD11c+, and CD11b+ cells is shown.
Vaccine proteins were secreted by cells transiently transfected with the vaccine-encoding plasmids and had expected sizes and reactivity with mAbs, as evaluated by ELISAs and Western blotting (Fig. 1B, 1C). Apparently, not all vaccine molecules were disulfide bonded because a significant proportion was monomeric by SDS-PAGE analysis (Fig. 1C). Furthermore, HLA-II–targeted vaccine proteins bound all CD19+ cells, ∼37–47% of CD11c+ cells, and 5–6% of CD11b+ cells among human PBMCs (Fig. 1D). The nontargeted control, αNIP-CκCκ, failed to significantly bind any of these cell populations, although some unspecific binding was observed.
Specificity of vaccine proteins for human HLA-II molecules
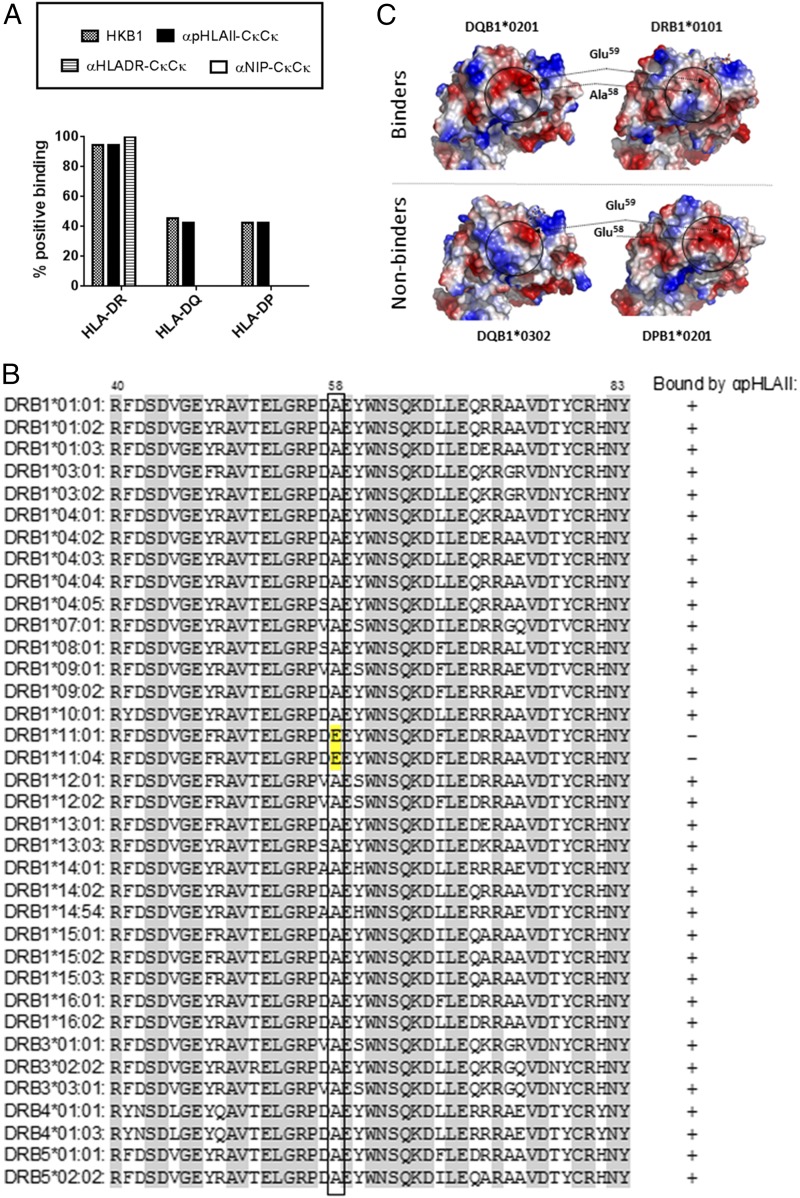
The HLA-II loci are among the most polymorphic genes described in the human genome, with some HLA-II genes having 100 identified alleles (37, 38). We therefore assessed the novel vaccine proteins for their ability to bind 91 different HLA-II molecules displayed on the surface of xMAP microbeads. αpHLAII-CκCκ bound 57 of 91 of the HLA-II molecules tested (Fig. 2A, Supplemental Fig. 1), including all but 2 HLA-DR molecules (34 of 36). Binding to HLA-DQ (12 of 29) and HLA-DP (11 of 26) allelic variants was less frequent. As expected, αHLADR-CκCκ bound all HLA-DR molecules tested (36 of 36), but none of the HLA-DQ and HLA-DP molecules. The negative control, αNIP-CκCκ, failed to bind any HLA-II molecules at all. The binding profile of αpHLAII-CκCκ was compared with IgM mAb HKB1, from which the scFvs in the targeting units of αpHLAII-CκCκ were derived (25). The binding profiles of the HKB1 IgM and the vaccine proteins were overlapping, except that the original Ab weakly bound one more HLA-DQ molecule (DQA1*06:01/DQB1*03:01) than did the vaccine protein (Supplemental Fig. 1). These results demonstrate that the specificity of a pentameric IgM molecule may be transplanted with scFv to a dimeric vaccine format without significantly changing the specificity.
FIGURE 2.
Specificity of vaccine proteins for HLA-II molecules. (A) The indicated vaccine proteins and HKB1 IgM mAb (donor of scFv for αpHLAII vaccine proteins) were assayed for binding to 91 beads coated with either HLA-DR (36 different types), HLA-DQ (29 types), or HLA-DP (26 types) molecules. Shown is the percentage of binding to each series of HLA-II molecules. αNIP-CκCκ did not bind any of the HLA-II molecules tested. Information on specific beads employed, and binding, is provided in Supplemental Fig. 1. (B) Sequence alignment of selected vaccine-interacting and noninteracting β1 domains with the critical residue 58 boxed. The relevant HLA sequences were downloaded from the IMGT/HLA database, aligned using ClustalX, and annotated using GenDoc. HLA-DP and HLA-DQ sequences are provided in Supplemental Fig. 2. (C) Structural and topological comparison of the postulated HKB1 epitope centered on the critical residue position 58 of the β1 chain (side view onto the β1 domain). Position 59 is also solvent exposed and is likely to represent an anchor residue for binding to the postulated epitope. The solvent-exposed surface electrostatic potentials were generated using APBS and contoured onto the molecular surfaces using PyMOL. Positively charged amino acids are colored in blue, whereas negatively charged amino acids are colored in red. Protein Data Bank ID codes: 1V9S (DQB1*02:01), 2NNA (DQB1*03:02), 2IAN (DRB1*01:01), and 3LQZ (DPB1*02:01).
Given the ability of αpHLAII-CκCκ to bind members of all three series of HLA-II molecules, a hitherto unappreciated shared structural feature between DP, DR, and DQ may comprise the epitope bound by HKB1 mAb. Most of the genetic variation in HLA-II molecules is located to the more distal parts of the ectodomains. A highly peculiar exception here is the monomorphic α-chain of HLA-DR, with only one functional allele (39). The inability of HKB1 to bind all HLA-DR molecules made us exclude the α-chain from the analysis, and rather focus on the β-chain. A global alignment of the IMGT/HLA database entries (PMID: 25414341) readily singled out a small region in the DR β1 domain that is unique for the two nonreactive DRB1*11:01 and DRB1*11:04 alleles (Fig. 2B). The key feature of this region appears to be an Ala-to-Glu substitution in the otherwise conserved position 58 of the β-chain. In line with this, the β1 domains of HLA-DP molecules that bound HKB1 have an Ala in position 58, whereas the nonbinding domains have Glu (Supplemental Fig. 2A). However, whereas all HKB1 binding HLA-DQ molecules have the Ala in position 58, so do several of the nonreactive HLA-DQ molecules (Supplemental Fig. 2B). Thus, binding is most likely influenced by a more complex epitope architecture, where topologically neighboring residues to position 58 in the folded HLA-II molecule also influence the HKB1 epitope (Fig. 2C).
Vaccine proteins that target Ag to HLA-II molecules enhance proliferation of human T cells
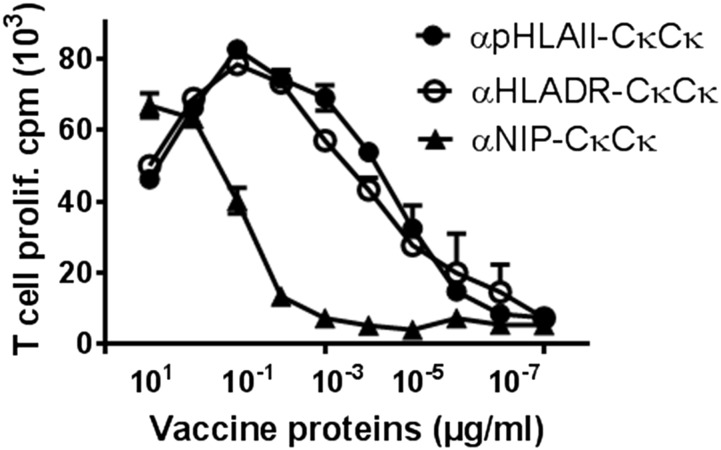
The two Cκ domains paired in an scFv-like format in the antigenic unit contain an immunogenic T cell epitope (aa 40–48), for which we have previously established a DR4-restricted (DRA1/DRB1*0401) human CD4+ T cell clone (36, 40). We could therefore test in vitro whether the different vaccine constructs could efficiently stimulate Cκ-specific human CD4+ T cells (24). Irradiated PBMCs from DR4+ donors were pulsed with titrated amounts of vaccine proteins and CD4+ T cell proliferation was assessed. Strikingly, αHLAII-CκCκ and αHLADR-CκCκ were 1000- to 10,000-fold more efficient at stimulating the Cκ-specific T cells compared with αNIP-CκCκ (Fig. 3).
FIGURE 3.
HLA-II–specific vaccine proteins enhance proliferation of Ag-specific CD4+ T cells. Irradiated human PBMCs (HLA-DR4+ [DRA1, B1*0401]) were incubated with cloned mouse Cκ-specific DR4-restricted CD4+ T cells in the presence of titrated amounts of various affinity-purified vaccine proteins expressing mouse Cκ Ag in a CκCκ scFv format. The cultures were pulsed with [3H]thymidine, and incorporation into DNA was assessed as an indicator of T cell proliferation.
Targeted DNA vaccines induce protection against challenge with influenza virus in DQ2-transgenic mice
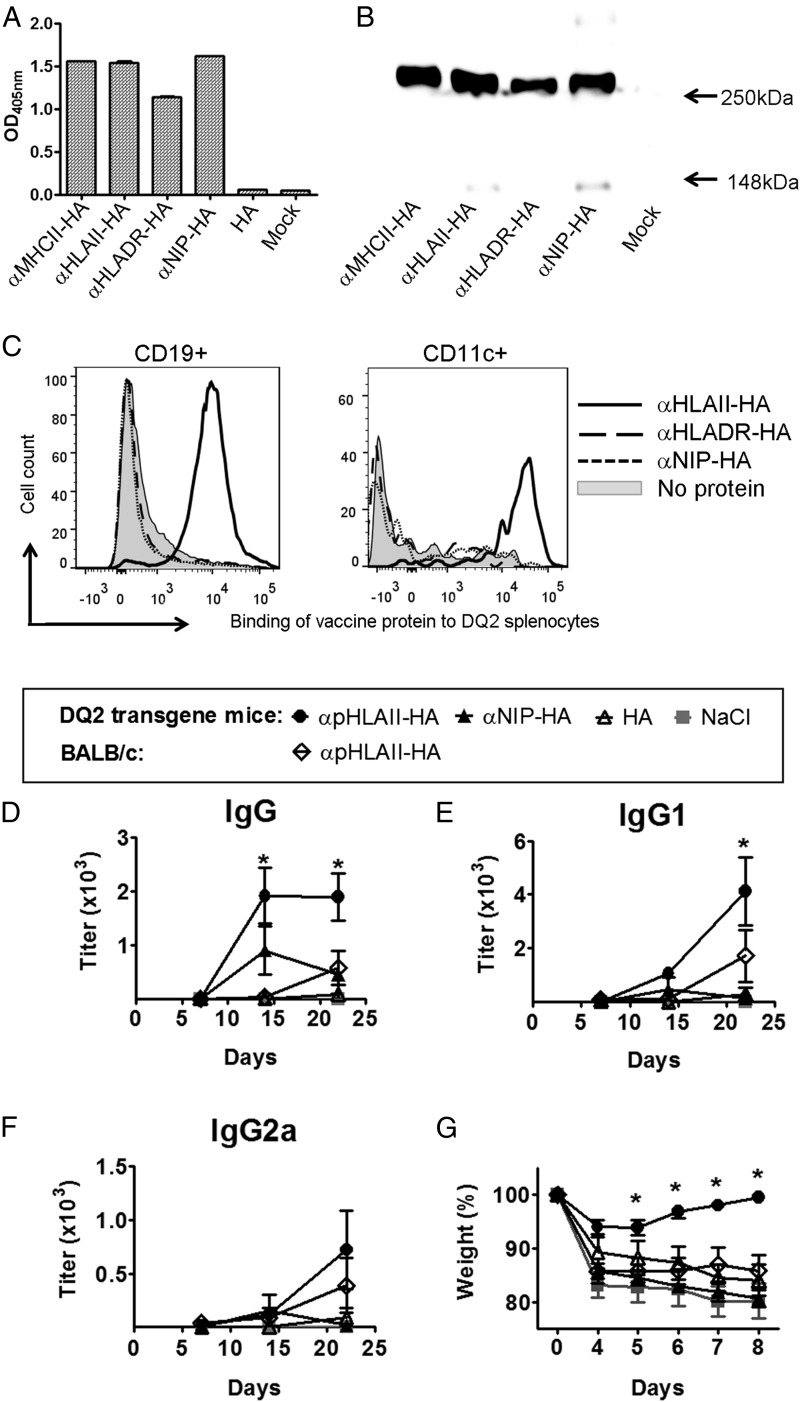
For active immunization of animals, we generated HLA-II–targeted vaccine molecules that expressed HA from influenza PR8 (H1N1). αpHLAII-HA and αHLADR-HA vaccine plasmids or nontargeted controls (αNIP-HA and HA alone) were transiently transfected into 293E cells. The secreted vaccine protein dimers displayed the expected sizes and reactivities with mAbs, as evaluated by ELISAs (Fig. 4A) and Western blotting (Fig. 4B). In agreement with results from the microbead assay (Supplemental Fig. 1), αpHLAII-targeted vaccine proteins bound cells from DQ2 transgenic mice (DQA1*05:01/DQB1*02:01). Consistent with results obtained with human PBMCs, the vaccine proteins bound most CD19+ cells and a substantial fraction of CD11c+ splenocytes from DQ2-transgenic mice, whereas αHLADR-HA and αNIP-HA failed to bind (Fig. 4C).
FIGURE 4.
DNA vaccination with αpHLAII-HA confers protection against influenza challenge in DQ2-transgenic mice. (A) Supernatants from 293E cells transiently transfected with DNA expressing the indicated vaccine proteins were examined for binding to mAb against the CH3 dimerization unit (MCA878) in sandwich ELISA, followed by detection with biotinylated mAb (H36-4-52) directed against PR8 HA in the antigenic unit. (B) Western blot of unreduced supernatants from transfected 293E cells, detected with anti-HA mAb specific for PR8 HA expressed in the vaccine proteins. Constructs are indicated below lanes; molecular mass is indicated by arrows. (C) Binding of indicated vaccine proteins to splenocytes from DQ2-transgenic mice on a BALB/c background. (D–G) Mice were vaccinated once with 25 μg of the indicated DNA plasmids i.d. combined with electroporation. Sera obtained on days 7, 14, and 21 were tested for presence of (D) IgG, (E) IgG1, and (F) IgG2a anti-HA Abs in ELISA. (G) At day 22, mice were challenged i.n. with a lethal dose of PR8 virus and monitored for weight. In (D), (E), and (G), mean ± SEM is given (n = 6 per group). *p < 0.05 for αpHLAII-HA compared with all other controls (two-way ANOVA and Bonferroni posttest).
Next, DQ2-transgenic mice were DNA vaccinated once i.d., immediately followed by skin electroporation to enhance cellular plasmid uptake. A single vaccination with αpHLAII-HA significantly increased the levels of anti-HA IgG in sera, compared with the nontargeted controls (αHLADR-HA, αNIP-HA, HA) (Fig. 4D). The strong increases in Ab levels were observed already 14 d after a single DNA immunization and were particularly pronounced for IgG1 (Fig. 4E), although IgG2a was also increased (Fig. 4F).
At day 22 after vaccination, the mice were intranasally inoculated with a lethal dose of influenza virus (PR8). Weight monitoring demonstrated that DQ2-transgenic mice vaccinated with αpHLAII-HA were protected against influenza (Fig. 4G). In contrast, DQ2-transgenic mice vaccinated with αNIP-HA or HA alone succumbed to the infection. Protection required expression of DQ2 because nontransgenic BALB/c mice vaccinated with αpHLAII-HA were not protected. These results demonstrate that the pan HLA-specific HA DNA flu vaccine elicited a protection against viral infection that was contingent upon targeting to HLA-II (DQ2) molecules expressed in the mouse.
αHLAII-targeted vaccines bind MHC-II molecules expressed in several larger mammals
Because the αpHLAII– and αHLADR-targeted vaccine proteins bound a number of different human HLA-II molecules, we tested whether the vaccine proteins could cross-react with MHC-II molecules of larger animals. The αpHLAII-HA bound PBMCs from horses, cows, sheep, ferrets, and pigs (Fig. 5A, 5B, Supplemental Fig. 3), whereas αHLADR-HA bound the same species except cows (Supplemental Fig. 3). The vaccine proteins failed to bind rabbit and rat PBMCs and BALB/c mouse splenocytes (not shown). A caveat is that limited numbers of animals of each species were tested (n = 3 per species); therefore, given the polymorphic nature of MHC-II molecules, no conclusion about frequencies of binding can be made for the different species. In summary, the vaccine proteins are likely to bind a region of MHC-II molecules that appears to be conserved between several larger mammals, but that is absent in rodents.
FIGURE 5.
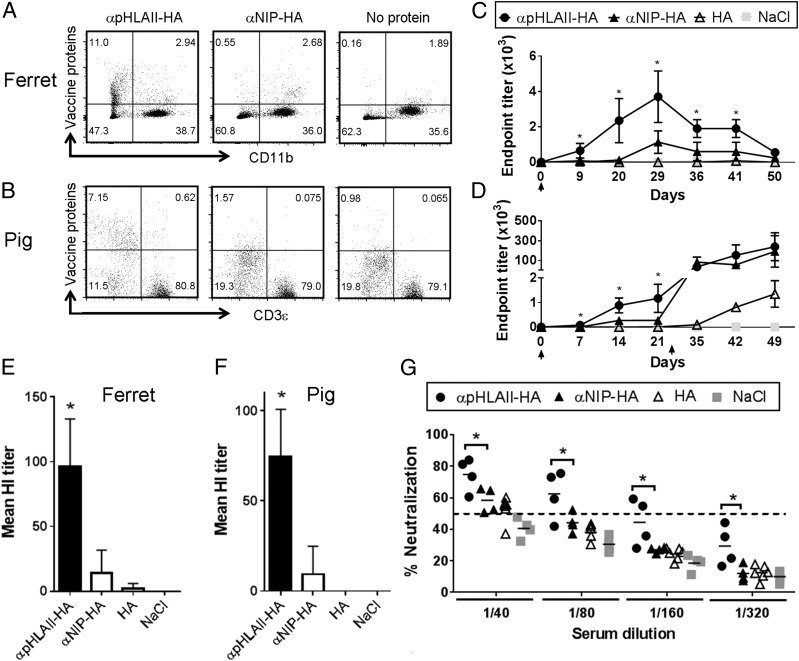
αpHLAII-HA DNA vaccine delivered by injection and electroporation increases immune responses in ferrets and pigs. (A and B) The indicated vaccine proteins were examined for specific binding to PBMCs from ferrets (A) and pigs (B). Selection gates for live cells were used on ferret PBMCs, whereas an additional gate for CD11R− cells was used for pig PBMCs. Representative images are shown (n = 3 for both species). (C) Ferrets were immunized once on day 0 (↑) with 100 μg of DNA i.d./electroporation. Vaccine constructs are indicated; HA was from A/California/07/2009 (H1N1) (Cal07). Serum samples were tested for IgG against inactivated Cal07 influenza in ELISA (mean ± SEM, n = 6 per group) (*p < 0.05, two-way ANOVA). (D) Norwegian farm pigs were immunized on day 0 (↑) and boosted on day 21 (↑) with 400 μg of DNA i.d./electroporation. Vaccine constructs are indicated, with plasmids encoding HA from Cal07. Sera were assayed for IgG in ELISA (mean ± SEM, n = 4 per group) (*p < 0.05, two-way ANOVA). (E) Ferret sera from animals with negative prevaccination titers (<23) were collected at day 29 after vaccination and tested in an HI assay (mean ± SEM, n = 6 per group). (F) Pig sera from animals with negative prevaccination titers (<23) were collected at day 21 after vaccination and tested in an HI assay (mean ± SEM, n = 4 per group). (G) Sera harvested at day 21 after the first immunization of pigs were assayed in a microneutralization assay against influenza Cal07 (n = 4 per group). Individual values are depicted, and the dotted line represents the 50% neutralization threshold used for positive scoring (*p < 0.05, two-way ANOVA).
Strong Ab responses induced after MHC-II–targeted DNA vaccination of ferrets and pigs
Ferrets are susceptible to infection with human influenza viruses and do develop some of the symptoms associated with human influenza disease. To assess vaccine efficacy in this relevant influenza model, ferrets were first confirmed negative for pre-existing HA-specific Abs in an HI assay (titer <23) and then immunized once with 100 μg of plasmids encoding αpHLAII-HA or nontargeted controls (αNIP-HA and HA) (Fig. 5C). The DNA was injected i.d., immediately followed by skin electroporation to increase DNA uptake. Ferrets immunized with αpHLAII-HA had serum anti-HA IgG levels that were significantly increased above those of the control groups (Fig. 5C). The elevated Ab levels were evident from day 9, increased until day 29, and then gradually declined. An HI assay performed on sera obtained on day 29 further confirmed a significant increase of neutralizing Abs after vaccination with αpHLAII-HA (Fig. 5E).
The weight of a ferret typically is 2–7 kg. To assess the vaccine efficacy in animals approaching human sizes, and that represents a natural host for influenza, vaccinations were performed in Norwegian farm pigs. Vaccines for DNA immunization of pigs were prepared in the Food and Drug Administration–approved pUMVC vector (28) (rather than the pLNOH2 vector used in the previous experiments). Pigs were confirmed negative for pre-existing Abs against HA (HI titer < 23) and immunized with DNA by i.d. injection, immediately followed by skin electroporation. A single DNA immunization with 400 μg of αpHLAII-HA was found to significantly increase levels of anti-HA IgG in sera, compared with nontargeted controls (αNIP-HA and HA) (Fig. 5D). The enhancement was evident already at day 7 and continued to increase during the next 2 wk. The pigs received a second immunization with DNA/electroporation 3 wk after the first vaccination. The boost greatly enhanced anti-HA IgG levels. Interestingly, whereas the first immunization with αNIP-HA failed to induce significant levels of Abs in sera, the boost resulted in increased anti-HA Ab levels. For the HA alone group, only low anti-HA responses were observed even after the boost.
For a more qualitative assessment of the induced Abs, an HI assay was performed with sera collected at day 21 after a single immunization. Importantly, DNA immunization with αpHLAII-HA significantly increased the amount of Abs able to block binding of HA virus to erythrocytes, compared with the nontargeted controls αNIP-HA and HA (Fig. 5F). This result was also confirmed by a microneutralization assay, where a single immunization with αpHLAII-HA induced significantly more neutralizing Abs than did αNIP-HA or HA (Fig. 5G). All animals (four of four) vaccinated with αpHLAII-HA had sera with neutralizing activity above the positive threshold (50% neutralization) with titers ranging from 40 to 160 (Fig. 5G).
Comparison of electroporation and jet delivery for enhancement of DNA vaccination in pigs
The device (Agile Pulse; Harvard Apparatus/BTX) that we used for skin electroporation is approved for human use and represents a well-tolerated system for human vaccination. However, a drawback is that DNA needs to be injected i.d. with a needle and syringe prior to electroporation, and as such presents an obstacle to prophylactic mass vaccination. We therefore decided to explore jet delivery of DNA. By jet delivery, drugs are propelled into the skin by high pressure with a hand-held mechanical device (41), and the procedure is essentially pain-free.
Pigs were immunized with titrated amounts of αHLAII-HA plasmids, delivered i.d. by either jet delivery (Tropis; PharmaJet) or injection/electroporation (Agile Pulse; Harvard Apparatus/BTX) (Fig. 6). Animals were boosted 3 wk after the first injection with the same amount of DNA per method, and immune responses were monitored by longitudinal measurements of anti-HA IgG in sera (Fig. 6A). The results show that a single vaccination with 100 μg of αpHLAII-HA DNA induced significant levels of anti-HA Abs after a single vaccination delivered with either electroporation or jet. The two delivery methods yielded similar results. A DNA boost with either of the two delivery methods equally enhanced the anti-HA Ab levels measured by ELISA. The boost with jet delivery appeared slightly more efficient for induction of neutralizing Abs (Fig. 6B, 6C), but this difference was not significant. These results suggest that painless and needle-free jet delivery of plasmids may substitute for needle-dependent injection of DNA combined with electroporation.
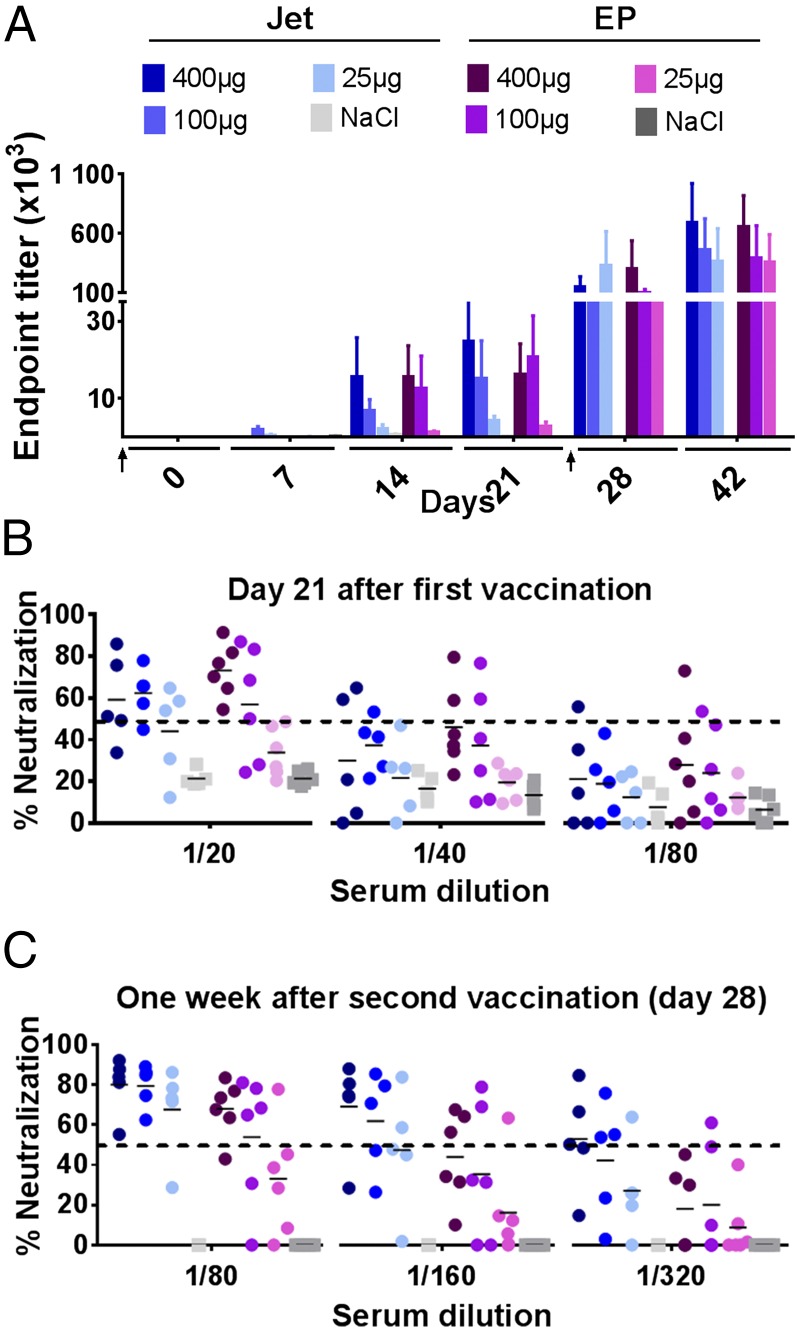
FIGURE 6.
Painless jet delivery of DNA vaccine in pigs with maintenance of efficiency. (A) Norwegian farm pigs were DNA immunized twice (days 0 and 28, ↑) with titrated amounts of αpHLAII-HA (encoding HA from influenza virus PR8). Plasmids were delivered i.d. either with needle injection/electroporation (EP) (n = 6 per group) or by needle-free jet delivery (Jet) (n = 5 per group). Sera were harvested at the indicated time points and assayed for IgG against inactivated influenza PR8 by ELISA (mean ± SEM). (B and C) Sera collected either 21 d after the first vaccination (B) or 1 wk after the second vaccination (C) were assayed in microneutralization assays against influenza PR8. Individual values are given, and the dotted lines indicate the threshold for positive neutralization.
Both for electroporation and jet delivery, there was no significant difference in Ab levels elicited by either 400 or 100 μg of DNA. A single delivery of 25 μg of DNA resulted in small but detectable IgG levels. However, a boost increased the anti-HA Ab similar to that obtained with either 100 or 400 μg of DNA (Fig. 6A). A more qualitative assessment of the induced Abs by microneutralization assays demonstrated that a single immunization with 100 or 400 μg of αHLAII-HA (but not 25 μg) was sufficient for induction of neutralizing Abs (Fig. 6B). Titers of neutralizing Abs were enhanced after the boost, and even 25 μg of plasmids encoding αHLAII-HA induced Abs capable of neutralizing virus in four of five animals with titers ranging from 80 to 160 (Fig. 6C).
MHC-II targeting reduces the amount of DNA needed for efficient vaccination of pigs
Because the above experiment did not contain nontargeted controls, we performed new experiments where targeted DNA vaccines (αpHLAII-HA and αHLADR-HA) were compared with nontargeted vaccines (αNIP-HA and HA alone), this time employing only jet delivery (Fig. 7). A single immunization with 75 μg of DNA of either αpHLAII-HA or αHLADR-HA significantly enhanced anti-HA IgG levels to above those observed for the nontargeted controls (Fig. 7A). In contrast, neither the nontargeted control αNIP-HA nor HA alone induced any anti-HA Abs. A boost with 75 μg of plasmid per jet delivery on day 28 significantly increased responses to both targeted αpHLAII-HA and αHLADR-HA. Surprisingly, a boost with nontargeted αNIP-HA induced high levels of anti-HA Abs even though Abs were not detected prior to the boost. A boost with HA alone failed to significantly raise any anti-HA Ab responses.
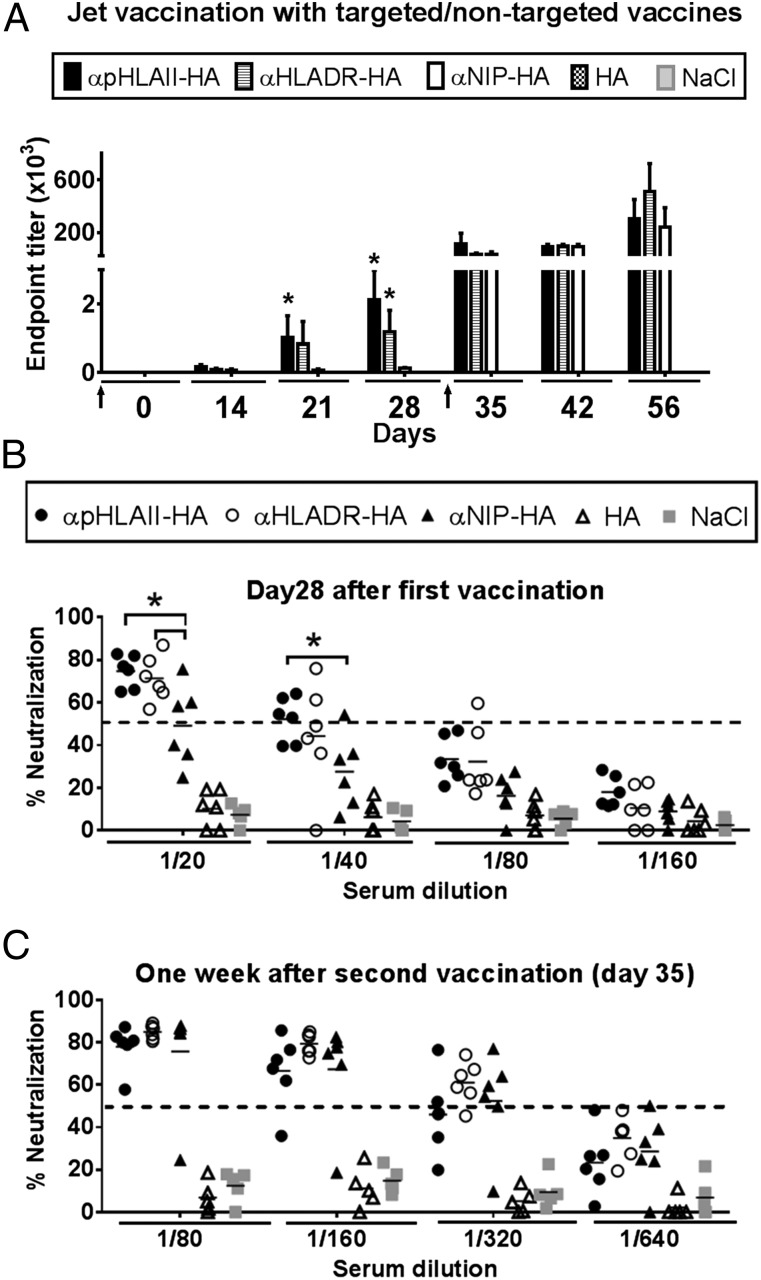
FIGURE 7.
Painless jet delivery of DNA vaccine in pigs with maintenance of targeting effect. (A) Norwegian farm pigs (n = 6 per group) were immunized twice (days 0 and 28, ↑) with 75 μg of DNA i.d. by jet delivery. The indicated constructs expressed HA from PR8 influenza. Sera obtained at the indicated time points were tested for IgG Abs binding recombinant HA (PR8) (mean ± SEM). (B and C) Sera of (A) were collected either 28 d after a single vaccination (B) or 1 wk after the second vaccination (C) and assayed in microneutralization assays against influenza PR8. Individual values are given, and the dotted lines indicate threshold for positive neutralization. *p < 0.05 compared with αNIP-HA, HA, and NaCl by two-way ANOVA.
The results obtained with ELISA were corroborated by qualitative measurements of neutralizing Abs in sera (Fig. 7B, 7C). Importantly, a single vaccination with 75 μg/jet delivery of targeted αpHLAII-HA or αHLADR-HA, but not αNIP-HA or HA, was sufficient for induction of anti-HA Abs that could neutralize virus (Fig. 7B). In groups receiving αpHLAII-HA or αHLADR-HA, the induced Abs were efficient (>50% positive threshold) in six of six animals with titers ranging from 20 to 80 (Fig. 7B). The boost on day 28 increased titers of neutralizing Abs, including for the nontargeted control (Fig. 7C). However, HA alone failed to induce neutralizing Abs even after two vaccinations. These results indicate that plasmid DNA encoding MHC-II–targeted vaccine proteins can induce clinically relevant Ab responses in 16–30 kg animals, with relatively low amounts (75 μg) and volumes (100 μl) of DNA, even after a single immunization with jet delivery.
The scFv representing the targeting structure of the αpHLAII-HA vaccine is of mouse origin and could be immunogenic in pigs. To investigate this, sera from days 28 to 35 of immunized pigs were evaluated for the presence of Abs against HKB1 (the mouse IgM mAb from which the αpHLAII-targeted scFv was derived) and TIB211 (control IgM). The results demonstrate that whereas the first immunization with αpHLAII-HA did not induce detectable levels of HKB1-specific Abs, a boost significantly increased levels of HKB1-specific IgG (Supplemental Fig. 4). These Abs did not bind an isotype-matched control IgM, an thus Abs were most likely directed against the V regions of the HKB1 mAb. These results indicate that there may possibly be some restrictions in vaccine reuse for the different HLA-II–targeted constructs.
Discussion
DNA vaccination is generally hampered by low immunogenicity, and several injections of large DNA doses are typically required for induction of protective immune responses in larger animals. In this study, we present a strategy that might remedy this shortcoming. DNA vaccines encoding bivalent fusion proteins, which target Ag to MHC-II molecules on APCs, enhanced Ab responses in larger animals such as ferrets and pigs. Importantly, a single immunization with comparatively low amounts of DNA was sufficient for induction of anti-HA Ab levels surpassing the protective threshold for neutralizing Abs.
The DNA vaccine format can rapidly be tailor-made to accommodate modified or novel Ags from newly emerged viruses. Production of plasmid DNA can easily be scaled up and does not depend on the availability of hen eggs, thus potentially overcoming a bottleneck for influenza vaccine production. Targeting of HA to MHC-II molecules strongly increases DNA vaccine efficacy in larger animals compared with vaccination with Ag alone. Although more data would be needed to infer a similar efficacy for MHC-II–targeted DNA vaccination in humans, APC-targeted DNA vaccines could represent a useful format for mass vaccination against pandemic influenza. Relevant to this, in the wake of the 2009 pandemic, an MHC-II–targeted DNA vaccine was produced and successfully tested in mice within 4 wk (22).
The efficacy of DNA vaccines, including APC-targeted versions, has previously been shown to be enhanced by electroporation (42, 43). Electroporation increases the number of transfected cells and hence the levels of secreted APC-targeted vaccine proteins. Additionally, local inflammation induced by electroporation may contribute to enhanced immune responses (44, 45). In this study, we have compared DNA delivery by i.d. injection/electroporation to that of jet delivery, by which plasmids are delivered with a hand-held mechanical device that uses high pressure for skin penetration. Interestingly, we observed comparable Ab titers after delivery by both methods. This finding may be relevant to prophylactic DNA vaccinations, because jet delivery does not require needle injection and allows for essentially painless vaccine delivery. Also, the relatively high resistance of plasmids to degradation, and thus a relative independence of a cold chain, may further add to the attractiveness of DNA/jet delivery as a platform for rapid and worldwide mass deployment. Naked DNA, enhanced delivery methods, and APC-targeted protein represent a three-pronged approach that should be further pursued also for use in humans.
Why were the DNA vaccines designed to target MHC-II molecules? Previous experiments in mice (6, 7, 9, 14, 22) and rats (8) have demonstrated that MHC-II targeting of Ags enhances Ab responses. Furthermore, when MHC-II has been compared with a number of different targets on APCs, MHC-II has been the most potent vaccine target identified so far for induction of Abs (Braathen and B. Bogen, unpublished observations). The present data support these observations and expand them to larger animals. The efficiency of MHC-II as a target may be related to the relatively high number of MHC-II molecules on professional APCs, thus enabling display of aggregated vaccine Ag to B cells in a putative APC–B cell synapse (46). In addition to improved Ab responses, it has been shown that targeting of vaccine proteins to MHC-II results in increased CD4+ T cell responses to Ag (7, 14, 18, 22). Consistent with this, in this study we observed that HLA-II–targeted vaccine proteins were 103-fold more potent at eliciting human CD4+ T cell responses compared with nontargeted controls. Enhanced CD4+ T cell responses may contribute to enhanced B cell/Ab responses via increased T cell–B cell collaboration.
The efficacy of the various DNA vaccine constructs for induction of Abs in ferrets and pigs was in the following order: MHC-II targeted (αpHLAII-HA and αHLADR-HA) > nontargeted (αNIP-HA) > Ag alone (HA). Thus, the effectiveness was mainly influenced by targeting of secreted protein to APCs. This hierarchy was especially pronounced after a single immunization. After a boost, responses to the nontargeted version increased, approaching that seen with the targeted versions. However, the responses to HA alone remained low even after a boost. This hierarchy observed in larger animals is reminiscent of previous results in the mouse (22). It is possible that bivalency of Ag, conferred by the dimerization unit of the vaccine format, could add to the immunogenicity. As an explanation, bivalent vaccine proteins could more efficiently cross-link BCRs of Ag-specific B cells than do monovalent versions, thus increasing B cell responses. In addition to bivalency, our data clearly point to MHC-II targeting as an important factor for early and strong induction of immune responses after a single immunization. It may be speculated that MHC-II targeting increases APC–B cell synapses, perhaps resulting in increased germinal center reactions (46).
A long-term goal is to develop HLA-II–targeted DNA vaccines for human application. To this end, it is important that a vaccine binds one or more HLA-II molecules expressed in all humans. Given the extensive polymorphisms of HLA-II molecules, this is a challenge. However, αHLADR-targeted vaccine proteins bound all HLA-DR molecules tested (S1) and are thus likely to function in most if not all humans. αpHLAII-targeted vaccine proteins bound to all but two HLA-DR molecules (DR1104 and DR1101). DR1104 has a frequency of 0.01780 in the population and is almost exclusively found in the region around Southeastern Europe. DRB1*11:01 has a frequency of 0.05945 and is typically found in Southern Africa or Western Asia (47). However, individuals that express these two nonbound DR alleles will most often be heterozygous, expressing another DR molecule to which anti–pHLA-II–targeted vaccine proteins bind. Additionally, individuals homozygous for nonbound DR molecules will most often display HLA-DQ and HLA-DP molecules that are bound by αpHLAII-targeted vaccine proteins. In conclusion, the binding profiles of the pHLA-II– and HLA-DR–targeted bivalent proteins suggest that these vaccines could be globally effective for vaccinations of humans. It remains to be demonstrated whether the APC density of HLA-II molecules targeted by the vaccine proteins will influence magnitude of B and T cell responses. If there is a positive correlation, αpHLAII-targeted vaccines may be more potent than αHLADR-targeted in a sizeable fraction of individuals.
The cross-reactivity of αpHLAII- and αHLADR-targeted vaccine proteins to several species of large mammals is important because it has allowed testing of the HLA-II–targeted vaccine constructs in ferrets and pigs. Of note, we have not tested reactivity toward a broad variety of MHC-II molecules in larger animals, but we restricted the analysis to three randomly selected individuals from each species. The obtained binding profiles were corroborated by in silico assessments of sequence homologies, but it cannot conclusively be ruled out that the cross-reactivity of the HLA-II–targeted vaccines does not extend to all allotypes. However, the in vivo data in ferrets and pigs, demonstrating in all animals significant increases in immune responses after HLA-II–targeted vaccination, indicate that the HLA-II–reactive vaccine proteins bind to MHC-II in most individual animals. Thus, the DNA vaccines described in the present study could be useful for veterinary purposes.
Supplementary Material
Acknowledgments
We thank Harvard Apparatus/BTX for providing the electroporator, and PharmaJet Inc. for providing the apparatus for jet injections. The technical help of Tore Jensen at the Section for Transplantation Immunology, Oslo University Hospital (Oslo, Norway), is gratefully acknowledged, as well as the contribution of Lise Torp Jensen in generation of the DQ2-transgenic mice. Furthermore, we gratefully acknowledge the technical help of Linda Andreassen and the other staff at the Animal Production Experimental Centre, Norwegian University of Life Science (Ås, Norway), and that of Thomas Jakob Olsen and the other staff at the Laboratory Animal Facility, University of Copenhagen (Copenhagen, Denmark).
This work was supported by the K.G. Jebsen Foundation and Helse Sør-Øst (Regional Health Authority), Norway.
The online version of this article contains supplemental material.
- HA
- hemagglutinin
- HI
- hemagglutination inhibition
- HLA-II
- HLA class II
- i.d.
- intradermal(ly)
- MHC-II
- MHC class II
- p
- pan
- scFv
- single-chain variable fragment
- TCID
- tissue culture–infective dose.
Disclosures
B.B., A.B.F., and G.G. are inventors on patent applications filed on the vaccine molecules by the Technology Transfer Offices of the University of Oslo and Oslo University Hospital, according to institutional rules. B.B. is head of the scientific panel, and A.B.F. is Chief Science Officer of Vaccibody AS. They both hold shares in the company. The other authors have no financial conflicts of interest.
References
- 1.Koff W. C., Burton D. R., Johnson P. R., Walker B. D., King C. R., Nabel G. J., Ahmed R., Bhan M. K., Plotkin S. A. 2013. Accelerating next-generation vaccine development for global disease prevention. Science 340: 1232910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li L., Saade F., Petrovsky N. 2012. The future of human DNA vaccines. J. Biotechnol. 162: 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergman P. J., Camps-Palau M. A., McKnight J. A., Leibman N. F., Craft D. M., Leung C., Liao J., Riviere I., Sadelain M., Hohenhaus A. E., et al. 2006. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 24: 4582–4585. [DOI] [PubMed] [Google Scholar]
- 4.Davis B. S., Chang G. J., Cropp B., Roehrig J. T., Martin D. A., Mitchell C. J., Bowen R., Bunning M. L. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75: 4040–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawamura H., Berzofsky J. A. 1986. Enhancement of antigenic potency in vitro and immunogenicity in vivo by coupling the antigen to anti-immunoglobulin. J. Immunol. 136: 58–65. [PubMed] [Google Scholar]
- 6.Carayanniotis G., Barber B. H. 1987. Adjuvant-free IgG responses induced with antigen coupled to antibodies against class II MHC. Nature 327: 59–61. [DOI] [PubMed] [Google Scholar]
- 7.Snider D. P., Segal D. M. 1987. Targeted antigen presentation using crosslinked antibody heteroaggregates. J. Immunol. 139: 1609–1616. [PubMed] [Google Scholar]
- 8.Mjaaland S., Fossum S. 1990. Modulation of immune responses with monoclonal antibodies. I. Effects on regional lymph node morphology and on anti-hapten responses to haptenized monoclonal antibodies. Eur. J. Immunol. 20: 1457–1461. [DOI] [PubMed] [Google Scholar]
- 9.Skea D. L., Douglas A. R., Skehel J. J., Barber B. H. 1993. The immunotargeting approach to adjuvant-independent immunization with influenza haemagglutinin. Vaccine 11: 994–1002. [DOI] [PubMed] [Google Scholar]
- 10.Caminschi I., Proietto A. I., Ahmet F., Kitsoulis S., Shin Teh J., Lo J. C., Rizzitelli A., Wu L., Vremec D., van Dommelen S. L., et al. 2008. The dendritic cell subtype-restricted C-type lectin Clec9A is a target for vaccine enhancement. Blood 112: 3264–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunde E., Munthe L. A., Vabø A., Sandlie I., Bogen B. 1999. Antibodies engineered with IgD specificity efficiently deliver integrated T-cell epitopes for antigen presentation by B cells. Nat. Biotechnol. 17: 670–675. [DOI] [PubMed] [Google Scholar]
- 12.Biragyn A., Tani K., Grimm M. C., Weeks S., Kwak L. W. 1999. Genetic fusion of chemokines to a self tumor antigen induces protective, T-cell dependent antitumor immunity. Nat. Biotechnol. 17: 253–258. [DOI] [PubMed] [Google Scholar]
- 13.Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M. C., Steinman R. M. 2002. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 196: 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fredriksen A. B., Sandlie I., Bogen B. 2006. DNA vaccines increase immunogenicity of idiotypic tumor antigen by targeting novel fusion proteins to antigen-presenting cells. Mol. Ther. 13: 776–785. [DOI] [PubMed] [Google Scholar]
- 15.Schjetne K. W., Fredriksen A. B., Bogen B. 2007. Delivery of antigen to CD40 induces protective immune responses against tumors. J. Immunol. 178: 4169–4176. [DOI] [PubMed] [Google Scholar]
- 16.Fossum E., Grødeland G., Terhorst D., Tveita A. A., Vikse E., Mjaaland S., Henri S., Malissen B., Bogen B. 2015. Vaccine molecules targeting Xcr1 on cross-presenting DCs induce protective CD8+ T-cell responses against influenza virus. Eur. J. Immunol. 45: 624–635. [DOI] [PubMed] [Google Scholar]
- 17.Fredriksen A. B., Bogen B. 2007. Chemokine-idiotype fusion DNA vaccines are potentiated by bivalency and xenogeneic sequences. Blood 110: 1797–1805. [DOI] [PubMed] [Google Scholar]
- 18.Grødeland G., Mjaaland S., Tunheim G., Fredriksen A. B., Bogen B. 2013. The specificity of targeted vaccines for APC surface molecules influences the immune response phenotype. PLoS One 8: e80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambert P. H., Liu M., Siegrist C. A. 2005. Can successful vaccines teach us how to induce efficient protective immune responses? Nat. Med. 11(Suppl.): S54–S62. [DOI] [PubMed] [Google Scholar]
- 20.Baier G., Baier-Bitterlich G., Looney D. J., Altman A. 1995. Immunogenic targeting of recombinant peptide vaccines to human antigen-presenting cells by chimeric anti-HLA-DR and anti-surface immunoglobulin D antibody Fab fragments in vitro. J. Virol. 69: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estrada A., McDermott M. R., Underdown B. J., Snider D. P. 1995. Intestinal immunization of mice with antigen conjugated to anti-MHC class II antibodies. Vaccine 13: 901–907. [DOI] [PubMed] [Google Scholar]
- 22.Grodeland G., Mjaaland S., Roux K. H., Fredriksen A. B., Bogen B. 2013. DNA vaccine that targets hemagglutinin to MHC class II molecules rapidly induces antibody-mediated protection against influenza. J. Immunol. 191: 3221–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grødeland G., Bogen B. 2015. Efficient vaccine against pandemic influenza: combining DNA vaccination and targeted delivery to MHC class II molecules. Expert Rev. Vaccines 14: 805–814. [DOI] [PubMed] [Google Scholar]
- 24.Schjetne K. W., Thompson K. M., Aarvak T., Fleckenstein B., Sollid L. M., Bogen B. 2002. A mouse Cκ-specific T cell clone indicates that DC-SIGN is an efficient target for antibody-mediated delivery of T cell epitopes for MHC class II presentation. Int. Immunol. 14: 1423–1430. [DOI] [PubMed] [Google Scholar]
- 25.Holte H., Blomhoff H. K., Beiske K., Funderud S., Torjesen P., Gaudernack G., Stokke T., Smeland E. B. 1989. Intracellular events associated with inhibition of B cell activation by monoclonal antibodies to HLA class II antigens. Eur. J. Immunol. 19: 1221–1225. [DOI] [PubMed] [Google Scholar]
- 26.Lampson L. A., Levy R. 1980. Two populations of Ia-like molecules on a human B cell line. J. Immunol. 125: 293–299. [PubMed] [Google Scholar]
- 27.Norderhaug L., Olafsen T., Michaelsen T. E., Sandlie I. 1997. Versatile vectors for transient and stable expression of recombinant antibody molecules in mammalian cells. J. Immunol. Methods 204: 77–87. [DOI] [PubMed] [Google Scholar]
- 28.Stewart S. A., Dykxhoorn D. M., Palliser D., Mizuno H., Yu E. Y., An D. S., Sabatini D. M., Chen I. S., Hahn W. C., Sharp P. A., et al. 2003. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 9: 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staudt L. M., Gerhard W. 1983. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. I. Significant variation in repertoire expression between individual mice. J. Exp. Med. 157: 687–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yelton D. E., Desaymard C., Scharff M. D. 1981. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma 1: 5–11. [DOI] [PubMed] [Google Scholar]
- 31.Du Pré M. F., Kozijn A. E., van Berkel L. A., ter Borg M. N., Lindenbergh-Kortleve D., Jensen L. T., Kooy-Winkelaar Y., Koning F., Boon L., Nieuwenhuis E. E., et al. 2011. Tolerance to ingested deamidated gliadin in mice is maintained by splenic, type 1 regulatory T cells. Gastroenterology 141: 610–620, 620.e1. [DOI] [PubMed] [Google Scholar]
- 32.Larkin M. A., Blackshields G., Brown N. P., Chenna R., McGettigan P. A., McWilliam H., Valentin F., Wallace I. M., Wilm A., Lopez R., et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- 33.Nicholas, K. B., H. B. Nicholas, Jr., and D. W. Deerfield II. 1997. GeneDoc: analysis and visualization of genetic variation. EMBNEW News 4. Available at: http://www.nrbsc.org/gfx/genedoc/ebinet.htm.
- 34.Baker N. A., Sept D., Joseph S., Holst M. J., McCammon J. A. 2001. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 98: 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nepom B. S., Nepom G. T., Coleman M., Kwok W. W. 1996. Critical contribution of β chain residue 57 in peptide binding ability of both HLA-DR and -DQ molecules. Proc. Natl. Acad. Sci. USA 93: 7202–7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tunheim G., Thompson K. M., Fredriksen A. B., Espevik T., Schjetne K. W., Bogen B. 2007. Human receptors of innate immunity (CD14, TLR2) are promising targets for novel recombinant immunoglobulin-based vaccine candidates. Vaccine 25: 4723–4734. [DOI] [PubMed] [Google Scholar]
- 37.Robinson J., Halliwell J. A., Hayhurst J. D., Flicek P., Parham P., Marsh S. G. 2015. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 43: D423–D431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maiers M., Gragert L., Klitz W. 2007. High-resolution HLA alleles and haplotypes in the United States population. Hum. Immunol. 68: 779–788. [DOI] [PubMed] [Google Scholar]
- 39.Silver J., Ferrone S. 1979. Structural polymorphism of human DR antigens. Nature 279: 436–437. [DOI] [PubMed] [Google Scholar]
- 40.Schjetne K. W., Thompson K. M., Nilsen N., Flo T. H., Fleckenstein B., Iversen J. G., Espevik T., Bogen B. 2003. Cutting edge: link between innate and adaptive immunity: Toll-like receptor 2 internalizes antigen for presentation to CD4+ T cells and could be an efficient vaccine target. J. Immunol. 171: 32–36. [DOI] [PubMed] [Google Scholar]
- 41.Logomasini M. A., Stout R. R., Marcinkoski R. 2013. Jet injection devices for the needle-free administration of compounds, vaccines, and other agents. Int. J. Pharm. Compd. 17: 270–280. [PubMed] [Google Scholar]
- 42.Roos A. K., Eriksson F., Timmons J. A., Gerhardt J., Nyman U., Gudmundsdotter L., Bråve A., Wahren B., Pisa P. 2009. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One 4: e7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roos A. K., Eriksson F., Walters D. C., Pisa P., King A. D. 2009. Optimization of skin electroporation in mice to increase tolerability of DNA vaccine delivery to patients. Mol. Ther. 17: 1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Løvås T. O., Bruusgaard J. C., Øynebråten I., Gundersen K., Bogen B. 2014. DNA vaccines: MHC II-targeted vaccine protein produced by transfected muscle fibres induces a local inflammatory cell infiltrate in mice. PLoS One 9: e108069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng B., Zhao Y., Xu L., Xu Y. 2007. Electric pulses applied prior to intramuscular DNA vaccination greatly improve the vaccine immunogenicity. Vaccine 25: 2064–2073. [DOI] [PubMed] [Google Scholar]
- 46.Fredriksen A. B., Sandlie I., Bogen B. 2012. Targeted DNA vaccines for enhanced induction of idiotype-specific B and T cells. Front. Oncol. 2: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solberg O. D., Mack S. J., Lancaster A. K., Single R. M., Tsai Y., Sanchez-Mazas A., Thomson G. 2008. Balancing selection and heterogeneity across the classical human leukocyte antigen loci: a meta-analytic review of 497 population studies. Hum. Immunol. 69: 443–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.