Abstract
Drugs of abuse induce sensitization, which is defined as enhanced response to additional drug following a period of withdrawal. Sensitization occurs in both humans and animal models of drug reinforcement and contributes substantially to the addictive nature of drugs of abuse, because it is thought to represent enhanced motivational wanting for drug. The ventral pallidum, a key member of the reward pathway, contributes to behaviors associated with reward, such as sensitization. Dopamine inputs to the ventral pallidum have not been directly characterized. Here we provide anatomical, neurochemical, and behavioral evidence demonstrating that dopamine terminals in the ventral pallidum contribute to reward in mice. We report subregional differences in dopamine release, measured by ex vivo fast-scan cyclic voltammetry: rostral ventral pallidum exhibits increased dopamine release and uptake compared with caudal ventral pallidum, which is correlated with tissue expression of dopaminergic proteins. We then subjected mice to a methamphetamine-sensitization protocol to investigate the contribution of dopaminergic projections to the region in reward related behavior. Methamphetamine-sensitized animals displayed a 508% and 307% increase in baseline dopamine release in the rostral and caudal ventral pallidum, respectively. Augmented dopamine release in the rostral ventral pallidum was significantly correlated with sensitized locomotor activity. Moreover, this presynaptic dopaminergic plasticity occurred only in the ventral pallidum and not in the ventral or dorsal striatum, suggesting that dopamine release in the ventral pallidum may be integrally important to drug-induced sensitization.
Keywords: Dopamine, ventral pallidum, voltammetry, sensitization, methamphetamine
Psychostimulant (e.g., cocaine and methamphetamine) abuse is a major public health concern. In 2013, an estimated 2.15 million Americans were recent psychostimulant users, contributing significantly to the estimated $712 billion societal cost of substance abuse.1−4 One of the most pernicious characteristics of addiction is its persistence: 40–60% of drug users relapse within one year of abstinence.5 Chronic drug use alters brain neurochemistry, and these changes do not quickly normalize after drug cessation.6−8 Understanding the long lasting neurobiological changes induced by chronic drug use is critical for both the treatment of addiction and the prevention of relapse.
One such persistent neurobiological change caused by drugs of abuse is sensitization, defined by heightened response to additional drug following a period of withdrawal. Sensitization occurs in human9,10 and animal11,12 models following chronic drug exposure. The incentive sensitization hypothesis of addiction posits that sensitized behavior (typically measured in rodents as augmented locomotor behavior) stems from hypersensitization of mesocorticolimbic circuits, resulting in enhanced salience, or motivational wanting, to drugs and drug-related cues.13 Noncontingent dosing regimens, such as sensitization and conditioned place preference, recapitulate many neurocircuitry alterations induced by response-contingent dosing regimens, such as self-administration and reinstatement (reviewed by Steketee and Kalivas14 and Vezina15). Given the similarity in circuitry changes, it has been proposed that these models share similar construct validity, and both recapitulate important aspects of the human condition.14 Recent studies have identified the ventral pallidum, the major output of the nucleus accumbens, as a mediator of sensitization.16−24
The ventral pallidum was originally described as the ventral extension of the globus pallidus; while this description partially defines the anatomy of the ventral pallidum, the subcommisural structure extends far more rostral than its globus counterpart, reaching to the most rostral portions of the striatum (Figures 1 and 2). The ventral pallidum forms reciprocal feedback loops with the major structures involved in reward signaling, including the nucleus accumbens (NAc), ventral tegmental area (VTA), substantia nigra, lateral hypothalamus, thalamus, amygdala, and others.25−27 In turn, the ventral pallidum projects strongly to the brain stem, including the pedunculopontine tegmentum, acting as a central convergence point for translation of limbic stimuli into motor output (reviewed by Smith et al.28). Additionally, stimulation of the ventral pallidum is capable of directly initiating reward signaling.29−31
Figure 1.
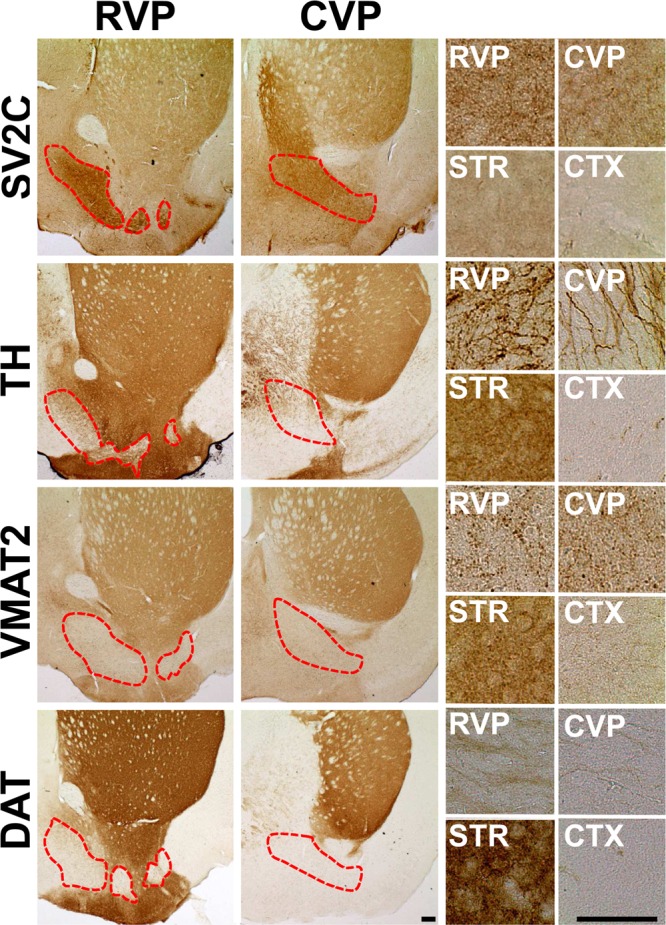
Dopamine neuroanatomy in the ventral pallidum. Immunohistochemistry in coronal (field view) and sagittal (magnified) slices revealed the expression of dopaminergic proteins in the ventral pallidum (dotted red lines). SV2C expression was used to delineate the ventral pallidum. TH, VMAT2, and DAT expression are shown in the RVP, CVP, dorsal striatum (DSTR), and cortex (CTX). Scale bar = 200 μm.
Figure 2.
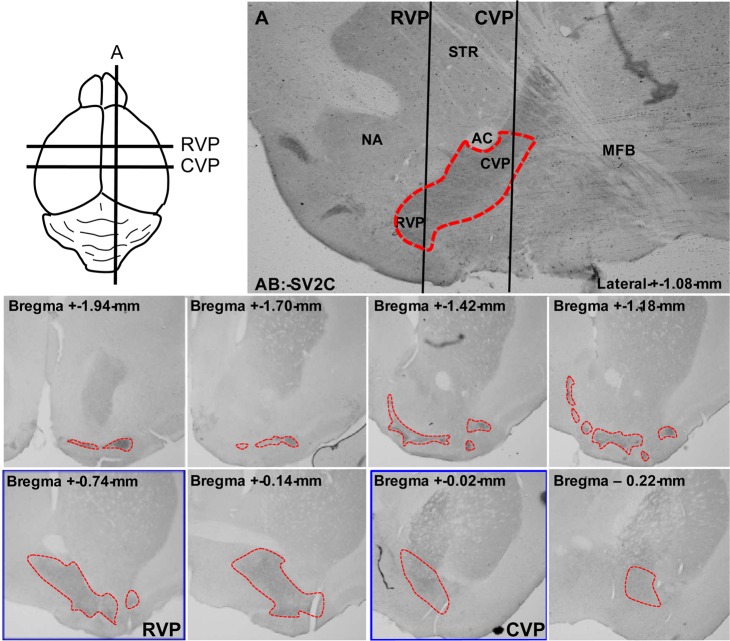
Identification of ventral pallidal slices. RVP and CVP slices (blue boxes) were chosen as shown in the diagram, using the anterior commissure for reference. The most rostral slices were not measured, given the technical difficulty of targeting ventral pallidum islands in unstained tissue. Additionally, the breadth of the ventral pallidum is shown by synaptic vesicle glycoprotein 2C (SV2C) immunohistochemistry. Dotted lines delineate the border of the ventral pallidum.
Electrophysiological, neuroanatomical, and behavioral data suggest differential roles for rostral versus caudal ventral pallidum (RVP and CVP, respectively). The CVP, defined here as the ventral pallidum caudal to the fused anterior commissure (+0.14 bregma),32 contains neurons electrophysiologically similar to neurons in the globus pallidus. In contrast, RVP neurons, defined as rostral to the fused anterior commissure,32 are more akin to their neighboring neurons in the NAc.33−35 Further, CVP stimulation is considerably more rewarding than RVP stimulation, though rats will self-administer direct electrical stimulation to both regions.29 Ablation of RVP signaling attenuates cue-induced cocaine reinstatement, whereas CVP inhibition blocks drug-primed reinstatement.32
Several pieces of evidence identify the ventral pallidum as an important participant in drug-induced sensitization. The ventral pallidum is integrally involved in induction of morphine sensitization; pharmacological inhibition of μ opioid receptors by microinjection into the ventral pallidum completely abolishes both induction and expression of sensitized behavior.16,20 For stimulants, methamphetamine (METH) sensitization alters pCREB and ΔFosB expression in the ventral pallidum and NAc of sensitized rats at 3 days post-drug withdrawal, indicative of increased postsynaptic activity. At 14 days postwithdrawal rats remain sensitized to METH, and activity-dependent changes (upregulation of pCREB and ΔFosB expression) persist only in the ventral pallidum,18 suggesting that activity in the ventral pallidum may drive sensitized behavioral response to stimulants.
The ventral pallidum receives input from VTA dopaminergic neurons. The role of dopaminergic innervation in subregions of the ventral pallidum has not been fully described, though our research builds on several key experiments suggesting that dopamine in the region plays a key role in reward behavior. First, microinjection of stimulants or dopamine agonists or antagonists into the ventral pallidum elicits a motor response and can induce sensitization and place preference.30,31,36,37 Further, 6-OHDA lesioning of the ventral pallidum, which preferentially lesions dopamine terminals, blocks cocaine place preference acquisition.38 Additionally, amphetamine sensitization in rats increases production of dopamine metabolites 3,4-dihydroxyphenoylacetic acid and homovanillic acid in the ventral pallidum.23 Though the mechanism of this augmentation has not been established, one likely explanation is increased dopamine release. Given these data, we hypothesized that stimulant sensitization induces presynaptic dopamine plasticity and that such enhanced dopamine release contributes to the long-term behavioral alterations associated with stimulants. To test this hypothesis, we used fast-scan cyclic voltammetry to measure dopamine release in the RVP and the CVP and demonstrate a substantial and selective enhancement of dopamine transmission in the ventral pallidum of METH-sensitized mice.
Results and Discussion
The contribution of dopaminergic inputs in the ventral pallidum to reward behavior is not well established. Here, we present the first recording of dopamine release in the ventral pallidum by FSCV. We utilized the technique to assess differential dopamine neurotransmission in rostral versus caudal ventral pallidum. Additionally, we provide evidence of selective augmentation of baseline dopamine transmission in the ventral pallidum of sensitized mice.
Dopaminergic Neuroanatomy of the Ventral Pallidum
We performed immunohistochemistry to define dopaminergic neuroanatomy in the ventral pallidum (Figure 1). The synaptic vesicle glycoprotein 2C (SV2C) is robustly expressed throughout the entire ventral pallidum,39,40 irrespective of subregion, and was used to define the structure. Dopamine terminal markers tyrosine hydroxylase (TH), the dopamine transporter (DAT), and the vesicular monoamine transporter 2 (VMAT2) are expressed in the ventral pallidum. TH expression is robust in the region, particularly in the RVP, though less than in canonically dopamine-rich regions such as the striatum. VMAT2 is sparsely but consistently expressed throughout the ventral pallidum. Of the three dopamine terminal markers, DAT is expressed at the lowest levels, with slightly more expression in RVP than CVP. Given the relative abundance of TH compared with DAT, these terminals likely rely on synthesis of new dopamine for release rather than recycling of neurotransmitter via plasmalemmal uptake.
These results are supported by immunogold electron microscopy studies conducted by Mengual and Pickel, which show that TH and DAT are both expressed in terminal regions and small, unmyelinated axons within the ventral pallidum, though DAT expression is significantly lower than TH expression.41,42 Interestingly, this work also identified graded expression of the proteins in medial and lateral subregions of the ventral palidum, with DAT expressed most strongly in lateral versus medial ventral pallidum.41 Additionally, TH expression in the medial region does not strongly colocalize with presynaptic dopamine D2 autoreceptors.42 These three observations, low DAT, high TH, and no autoreceptor expression, are the hallmark identifiers of a recently identified subpopulation of atypically fast-firing VTA dopamine neurons that project to the medial prefrontal cortex.43 In line with the tissue expression of DAT, voltammetry recordings of dopamine release in the prefrontal cortex reveal substantially decreased dopamine clearance compared with that in striatal regions.44 Low DAT/TH ratio and reduced D2 autoreceptor expression are also hallmarks of dopamine neuron terminals within the ventral pallidum, and as described below, these neurons also have substantially decreased clearance kinetics. Additionally, it has been demonstrated that animals that lack DAT have profound neuronal plasticity due to reduced clearance rate, which potentiates the signal.45 Extrapolation of this data implies that regions that express very little DAT but moderate amounts of TH, such as the prefrontal cortex or ventral pallidum, may be at enhanced risk for pathogenic alterations due to augmented signaling produced by drugs of abuse.
Dopamine Release in Subregions of the Ventral Pallidum by Fast-Scan Cyclic Voltammetry (FSCV)
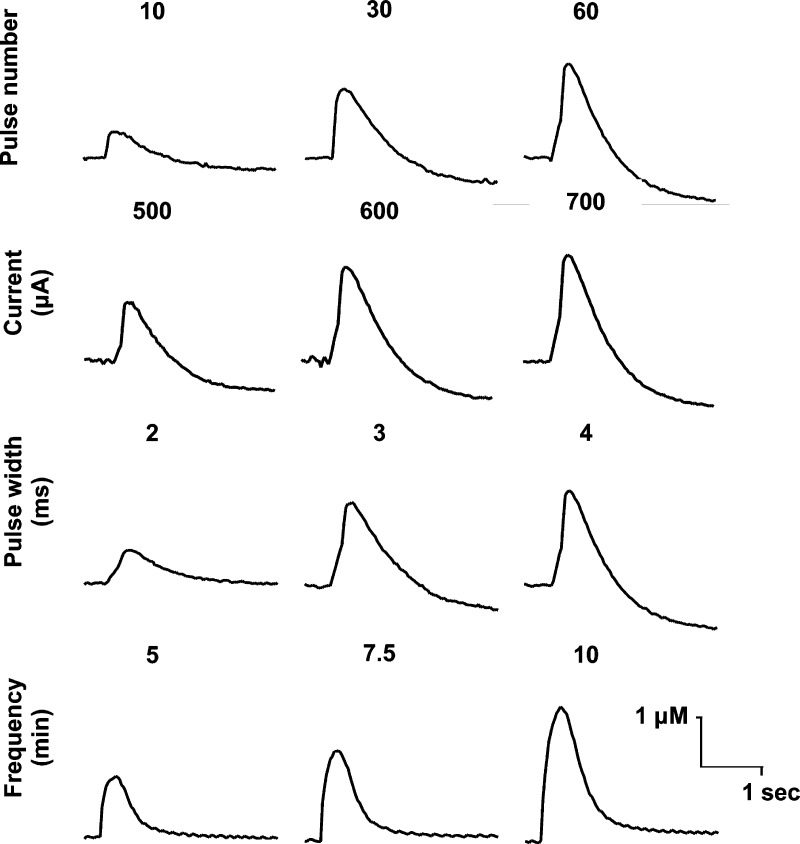
Dopamine release in the ventral pallidum was detected by FSCV. Initial experiments to identify optimal stimulation parameters were conducted in sagittal brain slices, irrespective of subregion. The optimal parameters identified were 60 pulses, 60 Hz, 600 μA, 2 ms, at 10 min intervals (Figure 3). Though larger current and pulse width stimulation resulted in larger dopamine overflow, these settings resulted in electrolytic lesioning in a number of brain slices, necessitating reduction of these parameters.
Figure 3.
Optimization of stimulation parameters. Pulse number (10–60), current (500–700 μA), pulse width (2–4 ms), and collection frequency (5–10 min) were varied systematically, and resultant dopamine release was measured. Optimal stimulation parameters of 60 pulses, 60 Hz, 600 μA, and 2 ms pulse width at 10 min intervals produced the most consistent release without lesioning the slice.
To investigate subregional differences within the ventral pallidum, coronal slices containing the RVP and CVP were carefully chosen to ensure correct identification of the appropriate region (Figures 2 and 4A–D). Stimulation elicited an average of 1.04 and 0.38 μM dopamine release in the RVP and CVP, respectively (p = 0.011, RVP n = 19, CVP n = 12, Figure 5C–D, G). Additionally, dopamine clearance, as measured by the rate constant tau, was faster in RVP than CVP (3.19 versus 8.25 s, respectively, RVP n = 19, CVP n = 12, Figure 5H), though significantly slower than in dorsal or ventral striatum (Supplemental Figure 1, 0.59 and 0.75 s, respectively, p = 0.002, one-way ANOVA with Newman–Keuls multiple comparison test). Nomifensine (10 μM), a dopamine and norepinephrine transporter inhibitor, increased release 260.2% in the RVP (p = 0.003, n = 3, Figure 6I, one-way ANOVA with Newman–Keuls multiple comparison test) and 23.9% in the CVP (p = 0.003, n = 3, Figure 6J, one-way ANOVA with Newman–Keuls multiple comparison test). These data further confirm enhanced DAT expression in RVP compared with CVP, given the 10-fold increase in augmentation in RVP. Neither region demonstrated substantial or significant enhancement of signal in response to α2-adrenergic autoreceptor idazoxan (Figure 6 I,J), which augments norepinephrine release, further confirming that the measured analyte was dopamine.
Figure 4.
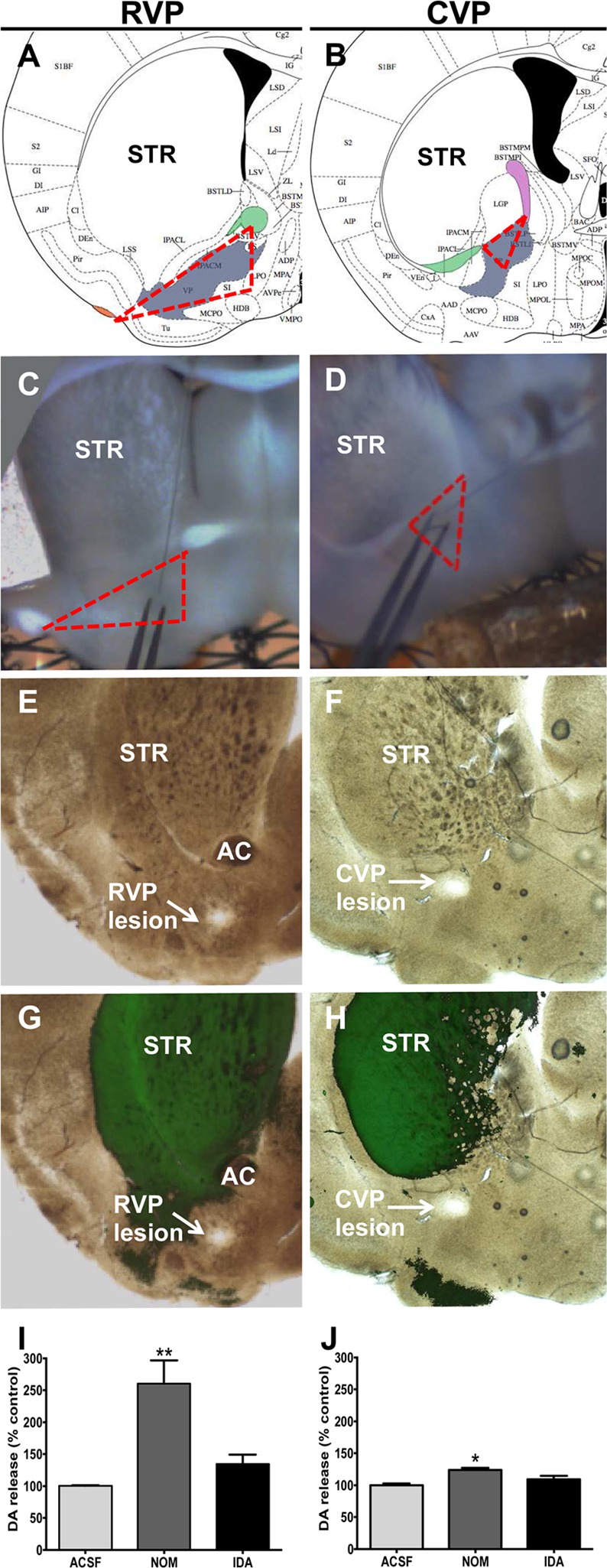
Confirmation of recording site. Slices were carefully chosen by visualization of structures in accordance with the Allen Brain Atlas (A–D, VP shown in gray). Representative recording sites demonstrate identification of ventral pallidum boundaries (dotted red lines) using the anterior commissure (green, A, B), lateral olfactory tubercle (orange, A), and the internal capside (fuschia, B). To further confirm, several slices were electrolytically lesioned at the recording site, and the tissue was cleared via CLARITY. RVP and CVP lesions in transmitted light (E, F) and DAT immunolabeled (green, G, H) representative slices are shown. Lack of significant colocalization with DAT expression is indicative of correct electrode placement. NET and DAT inhibitor nomifensine (10 μM) augmented release in RVP (I, p = 0.003, n = 3, one-way ANOVA with Newman–Keuls multiple comparison test) and CVP (J, p = 0.003, n = 3, one-way ANOVA with Newman–Keuls multiple comparison test). Selective NET inhibition with idazoxan (10 μM) did not significantly increase release in either region (I, J).
Figure 5.
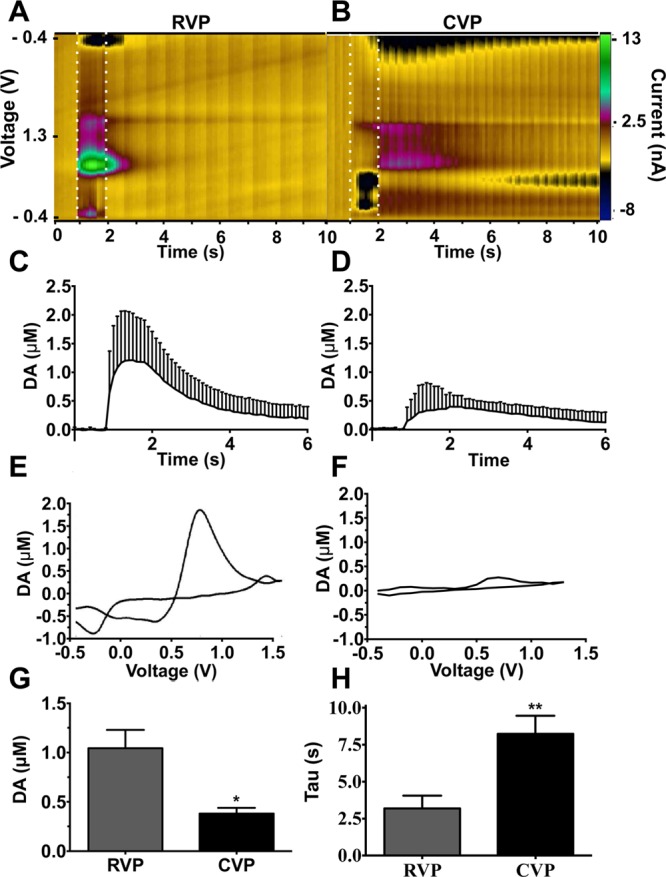
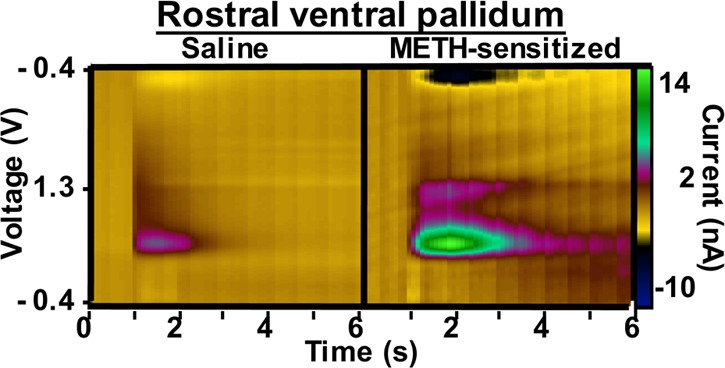
Dopamine release in RVP and CVP. Stimulation of RVP produced significantly greater dopamine release compared with stimulation of CVP (A–G, 1.04 μM vs 0.38 μM, respectively; p = 0.011, RVP n = 19, CVP, n = 12, two-tailed t test). Dopamine clearance is slower in CVP than RVP (H, p = 0.002, RVP n = 19, CVP n = 12, two-tailed t test). Representative color plots (A, B) and voltammagrams (E, F) are shown. Current time traces (C, D) are cumulative. Dotted lines indicate stimulation boundaries.
Figure 6.
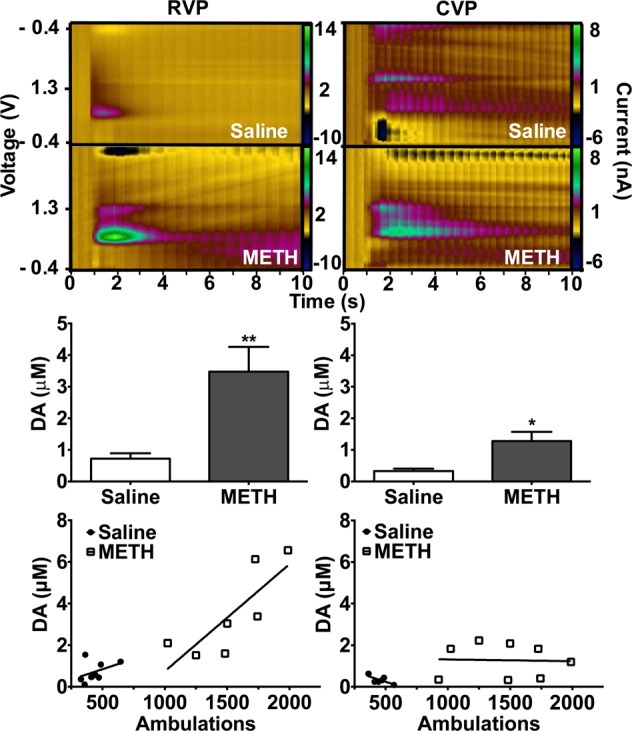
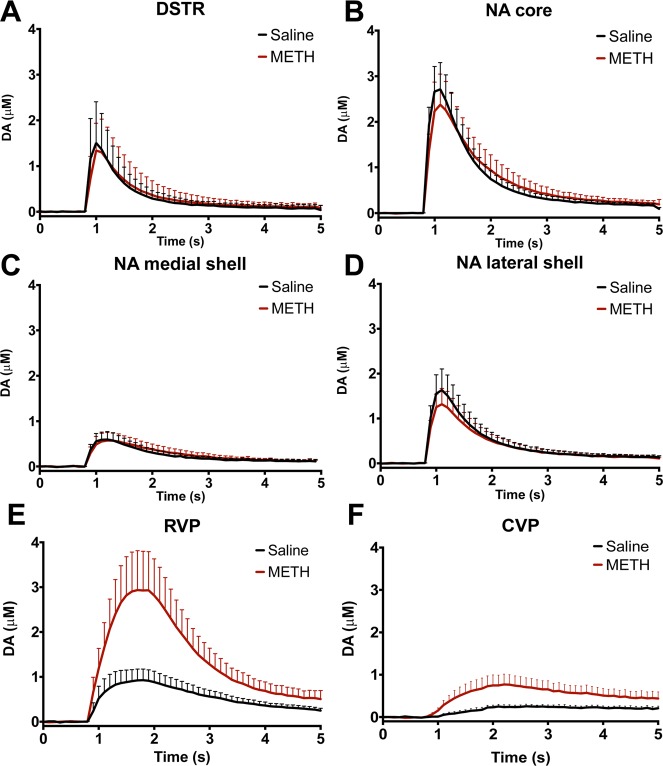
Chronic METH treatment enhances baseline dopamine release in the ventral pallidum. Mice were sensitized to METH or saline (2 mg/kg, IP, 7 days). METH pretreated animals have enhanced baseline DA release. In RVP slices, METH pretreatment augmented baseline dopamine release by 508% (A,C, p = 0.003, n = 6, two-tailed t test). In CVP slices, METH pretreatment increased dopamine release by 307% (B,D, p = 0.017, n = 8, two-tailed t test). RVP release is correlated with motor behavior (E, r2 = 0.6449, p = 0.0296, n = 7, linear regression analysis), whereas there is no correlation between baseline dopamine release and motor behavior in the CVP (F, r2 = 0.001, p = 0.931, n = 8, linear regression analysis).
Because this is a novel region for FSCV experiments, recording site validity was carefully assessed. One key advantage of slice voltammetry is visualization of the recording site, which ameliorates many of the concerns of electrode placement. Slices were carefully chosen as described in Figure 5A–D, using the anterior commissure, internal capsule, and lateral olfactory tract (which are readily visible in slices) as reference. Additionally, the recording site in a number of brain slices was electrolytically lesioned using a new electrode (using the stimulation electrode as a reference to maintain site location, n = 6). These brain slices were then embedded in a hydrogel solution followed by passive lipid clearing, generating an optically clear and antibody-permeable slice.46,47 In both RVP and CVP brain slices, recording sites did not colocalize strongly with DAT compared with neighboring characteristically dopaminergic structures, NAc and dorsal striatum (STR, Figure 4H). DAT expression was chosen due to the incredibly high specificity of the antibody for dopaminergic regions. Strong and specific antibodies are vital to effective CLARITY staining. Other antibodies that specifically label the ventral pallidum, including SV2C and substance P, generated insufficient resolution to be viable for assessing electrode placement.
The ventral pallidum is a heterogeneous structure; several groups have identified key differences in the neurochemical,28 electrical,35,48 and anatomical25,26,32,35,48 properties of the RVP versus the CVP. Here, we add to the evidence of dichotomy between the structures by characterizing differential dopamine release within the ventral pallidum, with highest dopamine release and uptake in the rostral regions (Figure 5). Increased dopamine tone in the RVP versus the CVP is coupled with enhanced dopamine clearance, evidenced by decreased rate constant, tau (Figure 5H), enhanced effect of plasmalemmal transporter inhibition (Figure 4I,J), and increased tissue expression of DAT (Figure 1).
The potential behavioral importance of differential dopamine release within the ventral pallidum is of particular interest. Microiontophoretic injection of dopamine or dopamine receptor agonists into the ventral pallidum alters firing in about 50% of tested neurons, both increasing and decreasing postsynaptic activity.49,50 Additionally, coadministration of dopamine with GABA or glutamate reduces neuronal firing rate, though potentiation was observed in a subset of recordings.51 Interestingly, this work displayed a rostrocaudal distribution in the neuromodulatory effect of exogenous dopamine administration, with less alteration of GABA and glutamate activity with coapplication of dopamine in the rostral subregion. This is not surprising, given the reduction in tau and dopamine transporter level in the region. Assuming an equivalent administration in both regions, dopamine injection in the CVP should have a greater effect, because it persists in the synaptic space substantially longer than in the RVP. Recent work identified that in a rat reinstatement model, RVP modulates cue response,32 whereas CVP is more attuned to modulation of hedonic response.29,52−55 Given the enhanced efficacy of dopamine in the CVP, the potential importance of these terminals for modulation of plasticity in response to hedonic stimuli is profound. Likewise, plasticity induced by dopamine release in the RVP may be integral to cue-dependent behavior. Though clearance is faster in rostral than caudal subregions of the ventral pallidum, it is still substantially slower than striatal clearance. Additionally, the RVP releases significantly more dopamine than the CVP; thus dopamine-induced synaptic modulation may be quite profound in the RVP. Finally, the lack of association with presynaptic autoreceptors makes these neurons prime candidates for presynaptic plasticity, since activation of D2 autoreceptors is thought to reduce dopamine production by inhibition of TH,56−58 alter VMAT2 expression,59 and augment DAT function,60−62 thereby inhibiting dopamine signaling. Further, D2 knockout mice have substantially augmented dopamine release compared with WT controls.63 Thus, dopamine release in the ventral pallidum, which persists for many seconds within the synaptic space and is not regulated by D2-dependent feedback mechanisms, may make the region uniquely vulnerable to modulation by drugs of abuse. Further investigation into how dopamine inputs in the ventral pallidum modulate both pre- and postsynaptic plasticity is key to more fully understanding the mechanistic importance of these projections.
Chronic METH Treatment Enhances Baseline Dopamine Release in the Ventral Pallidum
To determine whether sensitization to METH persistently enhances dopamine release in the ventral pallidum, we performed sensitization experiments.64 Mice received 2 mg/kg METH or saline intraperitoneally for 7 days. Following a 7-day washout (on day 14), we challenged all animals with 1 mg/kg METH and measured locomotor response. Mice pretreated with METH exhibited marked sensitization (Supplementary Figure 2, 224% increase compared with saline pretreated animals, p < 0.0001, n = 21, two-tailed t test). METH-induced behavior,65 dopamine overflow,66 and direct measurement of METH concentration in brain tissue67 return to baseline within 4 h of drug administration. Because METH is no longer present within the brains of challenged animals, baseline neurochemical changes were assessed the following day. On day 15, we extracted brains and performed FSCV. In this drug-free state, sensitized mice displayed a 507% increase in dopamine release in RVP (p = 0.003, n = 6, Figure 6A, two-tailed t test) and a 308% increase in CVP (p = 0.017, n = 8, Figure 6B, two-tailed t test) compared with saline controls. This effect was selective to the ventral pallidum: no augmented release was observed in DSTR or NAc core or shell (Figure 7, Table 1). Interestingly, elevated locomotor activity is directly correlated with the magnitude of dopamine release in the RVP (r2 = 0.645, p = 0.03, n = 7, Figure 6, linear regression analysis). No correlation exists between baseline dopamine release and motor behavior in the CVP (r2 = 0.001, p = 0.931, n = 8, Figure 6, linear regression analysis). Though it is not likely that residual drug is present during FSCV experiments, it is possible that drug challenge produces an acute augmentation of dopamine release in sensitized animals that we are capturing by assessing 24 h after testing. Because all animals (METH sensitized and saline controls) receive the 1 mg/kg METH challenge, this effect would still be due to sensitization. Though beyond the scope of this initial work, additional experiments to assess the time course of augmented release could reveal interesting insights into the role of the ventral pallidum in sensitization.
Figure 7.
METH-sensitization preferentially augments dopamine release in the ventral pallidum. FSCV of dorsal and ventral striatal regions revealed no increase in baseline dopamine release of sensitized animals (A–D). Augmented dopamine release is restricted to the ventral pallidum (E, F). Concentration time traces are averaged for 3–8 mice; dotted lines represent standard error of the mean.
Table 1. Peak Dopamine Release in Sensitized Animalsa.
| DA release (μM) |
|||
|---|---|---|---|
| region | saline | METH | relative change |
| DSTR | 1.38(0.40) | 1.54(0.23) | 111.5 (p = 0.62, n = 7) |
| NA, core | 2.85(0.60) | 3.26(1.01) | 114.3 (p = 0.73, n = 8) |
| NA, med shell | 0.62(0.19) | 0.75(0.23) | 121.1 (p = 0.70, n = 3) |
| NA, lat shell | 1.43(0.39) | 1.08(0.14) | 75.5 (p = 0.40, n = 7) |
| RVP | 0.75(0.16) | 3.8(0.85) | 506.7 (p = 0.002, n = 6) |
| CVP | 0.38(0.06) | 1.17(0.35) | 307.9 (p = 0.004, n = 5) |
Animals sensitized to METH have significantly augmented peak release in RVP and CVP. No significant enhancement was observed in dorsal striatum or nucleus accumbens (two-tailed t-test). Parentheticals are standard error of the mean.
The subregional difference in motor response is of particular interest, given the heterogeneity of signaling in the two subregions with respect to behavior. The CVP modulates hedonic response.53 Direct electrical stimulation of the CVP is highly rewarding, with threshold frequency (a mathematical calculation indicative of the reinforcing efficacy of stimulation) similar to those observed in the regions of highest reward, VTA and dorsal raphe.29 Ablation of CVP signaling produces sucrose aversion28,54 and blocks drug primed reinstatement of cocaine seeking.32 This proposed hedonic hotspot led to the theory that the CVP plays a major role in drug “liking”. Less is known about the RVP, but it may be more involved in modulation of drug “wanting” since ablation of the region abolishes cue-induced cocaine reinstatement.28,32 Interestingly, expression of locomotor sensitization is cue dependent: animals moved to a novel environment following sensitization induction do not express heightened locomotor response on test day.68−71 Thus, it is logical that augmented dopamine release in the RVP is strongly associated with a cue-dependent behavior like locomotor sensitization.
Mechanistic Considerations
The mechanism of augmented dopamine release in the ventral pallidum of sensitized animals is not clear, though long-term plasticity in the region is apparent. In general, discussions of plasticity normally address augmented postsynaptic response to a given stimulus. Postsynaptic plasticity can have two possible causes: enhanced sensitivity of postsynaptic receptors or enhanced presynaptic release. One of the key advantages of voltammetry is the ability to directly examine presynaptic release. Here we show that chronic METH administration enhances presynaptic release in the absence of exogenous drug (Figure 6), suggesting that METH induces long-term modulation and enhancement of baseline dopamine signaling. Further, dopamine plasticity only occurs in the ventral pallidum (Figure 7, Table 1) and is tightly correlated with sensitized behavior in rostral subregions (Figure 6). Plasticity in reward circuits underlies addiction, particularly relapse.72 A major addiction hypothesis posits that addiction occurs when normally innocuous cues become linked with drug consumption.13 Reintroduction to such cues, even after years of abstinence, can elicit relapse.72 It is thought that these cue associations are encoded in the brain via plasticity in reward circuits.72 Thus, long-term dopamine plasticity in the ventral pallidum, particularly in the RVP, which modulates cue associations,32 likely plays an integral role in addiction.
Additional mechanistic contributions may include neurocircuitry changes to reward pathways in sensitized animals. Chronic stimulant administration induces long-term depression of GABAergic NAc projections to the ventral pallidum.17,73 Additionally, pharmacological GABA receptor inhibition in the ventral pallidum increases locomotor behavior.74 It follows that depression of GABAergic inputs to the ventral pallidum would contribute to enhanced dopamine tone in the structure, which may contribute to the augmented motor response observed in sensitization. Additionally, the NAc shell is thought to be particularly important in sensitized behavior.75 Interestingly, the shell projects primarily to the medial subcommissural ventral pallidum,76 which is encompassed in our RVP slice. This may explain both the disparity in augmentation of dopamine tone between the CVP and RVP and the correlation between dopamine release and motor behavior in the RVP of sensitized mice (Figure 6).
The ventral pallidum is a heterogeneous structure, and the behaviors it contributes to are complex. Here, we define subregional differences in dopamine release, dopamine clearance, and dopaminergic protein expression in the ventral pallidum. Additionally, we identify subregional differences in response to METH sensitization in the ventral pallidum. To our knowledge, this is the first report of enhanced baseline dopamine release in any brain region of behaviorally sensitized mice. These data demonstrate that METH induces presynaptic dopaminergic plasticity and suggest that augmented dopamine release, selectively in the ventral pallidum, mediates locomotor sensitization and may initiate drug seeking motor behavior.
Methods
Mice
All procedures were carried out in accordance with NIH guidelines and those of the Institutional Animal Care and Use Committee at Emory University. Male C57BL/6 mice were purchased from Charles River Laboratories. Mice were group housed in a 12-h light cycled room with food and water ad libitum. Behavioral and neurochemical experiments were conducted at 3–6 months of age. Most of the literature cited within the introductory section was conducted in rats. These experiments were conducted in mice due to the enhanced genetic tools available in mice, since subsequent research will build upon these preliminary studies, analyzing dopamine release within the ventral pallidum of genetically manipulated mice.
Immunohistochemistry
Mice were perfused transcardially with 4% paraformaldehyde. Brains were removed and processed for frozen sectioning. Slices (40 μm) underwent hot citrate buffer antigen retrieval (Biogenex) and were blocked in 10% normal goat serum or 3% normal horse serum. Polyclonal anti-SV2C serum was isolated from rabbits injected with an N-terminal peptide (amino acids 97–114: STNQGKDSIVSVGQPKG) conjugated to Imject maleimide activated mcKLH (Thermo Scientific), and sera were generated for our laboratory using Covance Custom Immunology Services. Sections were incubated with polyclonal rabbit anti-SV2C serum (Covance, 1:2500), rat anti-DAT (Millipore MAB369, 1:1000), mouse anti-TH (Millipore MAB318, 1:1000), or rabbit anti-VMAT2 (generated in our laboratory,77 1:10000) followed by biotinylated secondary antibody (Jackson ImmunoResearch: goat anti-rabbit biotin 111-065-144, goat anti-mouse biotin 115-065-003, goat anti-rat 112-065-003). Visualization was performed with a 3,3-diaminobenzidine (DAB) reaction (Vector Laboratories) for biotinylated secondary antibodies. Images were acquired with a NeuroLucida epifluorescent microscope (MicroBrightField).
Fast-Scan Cyclic Voltammetry
FSCV was performed in sagittal and coronal slices as previously described.77 In brief, animals were rapidly decapitated, and the brain was sliced at 300 μM in oxygenated, ice-cold artificial cerebral spinal fluid (aCSF [in mM]: NaCl [126], KCl [2.5], NaH2PO4 [1.2], CaCl2 [2.4], MgCl2 [1.2], NaHCO3 [25], and glucose [11], pH 7.4) with added 194 mM sucrose using a vibrating tissue slicer. Slices containing the ventral pallidum were identified visually primarily by the shape of the anterior commissure (AC), which is a key advantage of slice voltammetry. The slice rostral of the fully fused AC (Figure 2) was chosen as the RVP slice (Figures 2 and 4A,C), and the slice immediately caudal, where the AC begins to break up, was chosen as the CVP slice (Figures 2 and 4B,D). Slice orientation was maintained throughout the experiment to ensure that the slice surface recorded from was nearest the fused AC in either the rostral or caudal direction. Brain slices were placed in a slice perfusion chamber and incubated at room temperature in oxygenated aCSF for 30 min. The appropriate slice was then transferred to a recording chamber where it was perfused with oxygenated aCSF at 32 °C. Following a 30 min incubation, a carbon fiber microelectrode was inserted 50–70 μM below the surface of the brain slice and the stimulating electrode was placed approximately 250 μM away. DA release was elicited by electrical stimulation (1–60 pulses, 30–60 Hz, 300–700 μA). Optimal stimulation parameters identified for the ventral pallidum were 60 Hz, 60 pulses, 600 μA, and 2 ms pulse width with 10 min intervals between stimuli (Figure 3). Stimulation parameters for all other regions were 1 pulse, 700 μA, and 4 ms pulse width at 5 min intervals. A cyclic voltage ramp (−0.4 to 1.3 V) was applied to the carbon fiber microelectrode, and resultant background-subtracted current was measured. All reported regions were surveyed with 4 recording replicates at 3 independent sites, which were averaged for each animal. Experiments were conducted and analyzed using Demon software (Wake Forest University). Following experimentation, a number of slices were electrolytically lesioned with a new electrode to further confirm recording site. The experimental recording electrodes were calibrated to known dopamine standards using a flow cell.
Electrophoretic Tissue Clearing
Following FSCV experiments, a number of brain slices underwent CLARITY preparation, as described by Chung and Diesseroth.46,47 Slices were incubated at 4 °C in hydrogel monomer solution (40% acrylamide, 0.25% VA-044, 4% PFA in PBS) for several days. Slices were polymerized at 37 °C following nitrogen degassing. Polymerized slices were passively cleared for 1 week in clearing buffer (200 mM boric acid, 4% SDS) at 37 °C. Slices were then rinsed for 2 days in 0.1% Triton-PBS. For imaging, slices were incubated in buffer (0.1% Triton X-100, 1 M sodium borate, pH 8.5) plus the antibody of interest (1:500) for 2 days at 37 °C, rinsed for 1 day in PBST, incubated with secondary antibody (1:100) in PBST + 1 M sodium borate for 2 days then rinsed for 1 day. The slices were transferred to 80% glycerol in water, which matches the optical density of the clarified slice, and incubated for 1 day. The slices were imaged with a NeuroLucida epifluorescent microscope (MicroBrightField).
Locomotor Sensitization
The day prior to the first day of drug administration, animals were habituated to the locomotor recording chamber. Male mice, 8–10 weeks old, were injected intraperitoneally with either 2 mg/kg METH or an equivalent volume of saline control for 7 days.64 On day 14, all animals received 1 mg/kg METH intraperitoneally. Consecutive beam breaks were recorded for 30 min following drug injection in a chamber that measures infrared beam breaks (Photobeam Activity System, San Diego Instruments). On day 15, mice were rapidly decapitated, and FSCV recordings were taken to measure dopamine release in the VP. Because voltammetry experiments are rate limiting, with a maximum of recording from two animals in a day, drug administration was staggered such that all animals received identical dosing regimens.
Drugs
Free-base corrected methamphetamine hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was prepared immediately before injections. Nomifensine (Sigma-Aldrich, St. Louis, MO, USA) and idazoxan (Sigma-Aldrich, St. Louis, MO, USA) were made in DMSO at 10 mM then serially diluted in aCSF to designated concentrations.
Statistical Analysis
Unless otherwise noted, all data are represented as means with standard error of the mean. Data were analyzed by two-tailed t tests or ANOVA to determine statistical significance. Statistical analysis was conducted using GraphPad Prism 6 and significance defined as p < 0.05.
Acknowledgments
We thank Dr. Sara Jones and her laboratory, in particular James Melchior, for their considerable technical assistance, wisdom, and encouragement. We also thank Dr. Donita Robinson for suggesting the presentation of the correlation between enhanced locomotion and dopamine release in the ventral pallidum. Finally, we acknowledge the expert technical assistance of Minagi Ozawa.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acschemneuro.6b00131.
Dopamine clearance in striatum and ventral pallidum and locomotor sensitization (PDF)
This work was supported by NIH Grants R01 ES023839, P30ES019776, T32ES012870, F31DA037652, and F31NS089242, and the Lewis Dickey Memorial Fund.
The authors declare no competing financial interest.
Supplementary Material
References
- Substance Abuse and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14–4863. Substance Abuse and Mental Health Services Administration, Rockville, MD, 2014.
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA, 2014.
- Bouchery E. E.; Harwood H. J.; Sacks J. J.; Simon C. J.; Brewer R. D. (2011) Economic costs of excessive alcohol consumption in the U.S., 2006. Am. J. Prev. Med. 41 (5), 516–524. 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Drug Enforcement Agency Strategic Intelligence Section. National Drug Threat Assessment Summary. DEA-DCT-DIR-008-16. U.S. Department of Justice, Washington, DC, 2015.
- McLellan A. T.; Lewis D. C.; O’Brien C. P.; Kleber H. D. (2000) Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284, 1689–1695. 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- Nestler E. J. (2005) The neurobiology of cocaine addiction. Sci. Pract Perspect 3, 4–10. 10.1151/spp05314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J. (2001) Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2, 119–128. 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W.; Volkow N. D. (2005) The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413. 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Boileau I.; Dagher A.; Leyton M.; Gunn R. N.; Baker G. B.; Diksic M.; Benkelfat C. (2006) Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry 63, 1386–1395. 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Cox S. M.; Benkelfat C.; Dagher A.; Delaney J. S.; Durand F.; McKenzie S. A.; Kolivakis T.; Casey K. F.; Leyton M. (2009) Striatal dopamine responses to intranasal cocaine self-administration in humans. Biol. Psychiatry 65, 846–850. 10.1016/j.biopsych.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Shuster L.; Webster G. W.; Yu G. (1975) Increased running response to morphine in morphine-pretreated mice. J. Pharmacol Exp Ther 192, 64–67. [PubMed] [Google Scholar]
- Wallach M. B.; Gershon S. (1971) Sensitization to amphetamines. Psychopharmacol. Bull. 7, 30–31. [PubMed] [Google Scholar]
- Robinson T. E.; Berridge K. C. (2008) Review. The incentive sensitization theory of addiction: some current issues. Philos. Trans. R. Soc., B 363, 3137–3146. 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee J. D.; Kalivas P. W. (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 63, 348–365. 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. (2004) Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 27, 827–839. 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Johnson P. I.; Napier T. C. (2000) Ventral pallidal injections of a mu antagonist block the development of behavioral sensitization to systemic morphine. Synapse 38, 61–70. . [DOI] [PubMed] [Google Scholar]
- McDaid J.; Dallimore J. E.; Mackie A. R.; Mickiewicz A. L.; Napier T. C. (2005) Cross-sensitization to morphine in cocaine-sensitized rats: behavioral assessments correlate with enhanced responding of ventral pallidal neurons to morphine and glutamate, with diminished effects of GABA. J. Pharmacol. Exp. Ther. 313, 1182–1193. 10.1124/jpet.105.084038. [DOI] [PubMed] [Google Scholar]
- McDaid J.; Dallimore J. E.; Mackie A. R.; Napier T. C. (2006) Changes in accumbal and pallidal pCREB and deltaFosB in morphine-sensitized rats: correlations with receptor-evoked electrophysiological measures in the ventral pallidum. Neuropsychopharmacology 31, 1212–1226. 10.1038/sj.npp.1300854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J.; Tedford C. E.; Mackie A. R.; Dallimore J. E.; Mickiewicz A. L.; Shen F.; Angle J. M.; Napier T. C. (2007) Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 86, 55–66. 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Mickiewicz A. L.; Dallimore J. E.; Napier T. C. (2009) The ventral pallidum is critically involved in the development and expression of morphine-induced sensitization. Neuropsychopharmacology 34, 874–886. 10.1038/npp.2008.111. [DOI] [PubMed] [Google Scholar]
- Napier T. C.; Istre E. D. (2008) Methamphetamine-induced sensitization includes a functional upregulation of ventral pallidal 5-HT2A/2C receptors. Synapse 62, 14–21. 10.1002/syn.20460. [DOI] [PubMed] [Google Scholar]
- Rokosik S. L.; Persons A. L.; Napier T. C. (2013) Sensitization by ventral pallidal DAMGO: lack of cross-sensitization to morphine. NeuroReport 24, 152–158. 10.1097/WNR.0b013e32835e11a2. [DOI] [PubMed] [Google Scholar]
- Chen J. C.; Liang K. W.; Huang Y. K.; Liang C. S.; Chiang Y. C. (2001) Significance of glutamate and dopamine neurons in the ventral pallidum in the expression of behavioral sensitization to amphetamine. Life Sci. 68, 973–983. 10.1016/S0024-3205(00)00995-4. [DOI] [PubMed] [Google Scholar]
- McDaid J.; Graham M. P.; Napier T. C. (2006) Methamphetamine-induced sensitization differentially alters pCREB and DeltaFosB throughout the limbic circuit of the mammalian brain. Mol. Pharmacol. 70, 2064–2074. 10.1124/mol.106.023051. [DOI] [PubMed] [Google Scholar]
- Groenewegen H. J.; Berendse H. W.; Haber S. N. (1993) Organization of the output of the ventral striatopallidal system in the rat: ventral pallidal efferents. Neuroscience 57, 113–142. 10.1016/0306-4522(93)90115-V. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W.; Churchill L.; Klitenick M. A. (1993) GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 57, 1047–1060. 10.1016/0306-4522(93)90048-K. [DOI] [PubMed] [Google Scholar]
- Lavin A.; Grace A. A. (1998) Response of the ventral pallidal/mediodorsal thalamic system to antipsychotic drug administration: involvement of the prefrontal cortex. Neuropsychopharmacology 18, 352–363. 10.1016/S0893-133X(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Smith K. S.; Tindell A. J.; Aldridge J. W.; Berridge K. C. (2009) Ventral pallidum roles in reward and motivation. Behav. Brain Res. 196, 155–167. 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagis G.; Miliaressis E.; Anagnostakis Y.; Spyraki C. (1995) Ventral pallidum self-stimulation: a moveable electrode mapping study. Behav. Brain Res. 68, 165–172. 10.1016/0166-4328(94)00169-G. [DOI] [PubMed] [Google Scholar]
- Gong W.; Neill D.; Justice J. B. Jr. (1996) Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 707, 64–74. 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Gong W.; Neill D. B.; Lynn M.; Justice J. B. Jr. (1999) Dopamine D1/D2 agonists injected into nucleus accumbens and ventral pallidum differentially affect locomotor activity depending on site. Neuroscience 93, 1349–1358. 10.1016/S0306-4522(99)00235-3. [DOI] [PubMed] [Google Scholar]
- Mahler S. V.; Vazey E. M.; Beckley J. T.; Keistler C. R.; McGlinchey E. M.; Kaufling J.; Wilson S. P.; Deisseroth K.; Woodward J. J.; Aston-Jones G. (2014) Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat. Neurosci. 17, 577–585. 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogenson G. J.; Swanson L. W.; Wu M. (1983) Neural projections from nucleus accumbens to globus pallidus, substantia innominata, and lateral preoptic-lateral hypothalamic area: an anatomical and electrophysiological investigation in the rat. J. Neurosci. 3, 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier T. C.; Simson P. E.; Givens B. S. (1991) Dopamine electrophysiology of ventral pallidal/substantia innominata neurons: comparison with the dorsal globus pallidus. J. Pharmacol. Exp. Ther. 258, 249–262. [PubMed] [Google Scholar]
- Kupchik Y. M.; Kalivas P. W. (2013) The rostral subcommissural ventral pallidum is a mix of ventral pallidal neurons and neurons from adjacent areas: an electrophysiological study. Brain Struct. Funct. 218, 1487–1500. 10.1007/s00429-012-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier T. C.; Chrobak J. J. (1992) Evaluations of ventral pallidal dopamine receptor activation in behaving rats. NeuroReport 3, 609–611. 10.1097/00001756-199207000-00016. [DOI] [PubMed] [Google Scholar]
- Fletcher P. J.; Korth K. M.; Sabijan M. S.; DeSousa N. J. (1998) Injections of D-amphetamine into the ventral pallidum increase locomotor activity and responding for conditioned reward: a comparison with injections into the nucleus accumbens. Brain Res. 805, 29–40. 10.1016/S0006-8993(98)00633-7. [DOI] [PubMed] [Google Scholar]
- Gong W.; Neill D.; Justice J. B. Jr. (1997) 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 754, 103–112. 10.1016/S0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Janz R.; Sudhof T. C. (1999) SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 94, 1279–1290. 10.1016/S0306-4522(99)00370-X. [DOI] [PubMed] [Google Scholar]
- Dardou D.; Monlezun S.; Foerch P.; Courade J. P.; Cuvelier L.; De Ryck M.; Schiffmann S. N. (2013) A role for Sv2c in basal ganglia functions. Brain Res. 1507, 61–73. 10.1016/j.brainres.2013.02.041. [DOI] [PubMed] [Google Scholar]
- Mengual E.; Pickel V. M. (2004) Regional and subcellular compartmentation of the dopamine transporter and tyrosine hydroxylase in the rat ventral pallidum. J. Comp. Neurol. 468, 395–409. 10.1002/cne.10979. [DOI] [PubMed] [Google Scholar]
- Mengual E.; Pickel V. M. (2002) Ultrastructural immunocytochemical localization of the dopamine D2 receptor and tyrosine hydroxylase in the rat ventral pallidum. Synapse 43, 151–162. 10.1002/syn.10033. [DOI] [PubMed] [Google Scholar]
- Lammel S.; Hetzel A.; Hackel O.; Jones I.; Liss B.; Roeper J. (2008) Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57, 760–773. 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Garris P. A.; Collins L. B.; Jones S. R.; Wightman R. M. (1993) Evoked extracellular dopamine in vivo in the medial prefrontal cortex. J. Neurochem. 61, 637–647. 10.1111/j.1471-4159.1993.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Jones S. R.; Gainetdinov R. R.; Jaber M.; Giros B.; Wightman R. M.; Caron M. G. (1998) Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. U. S. A. 95, 4029–4034. 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K.; Deisseroth K. (2013) CLARITY for mapping the nervous system. Nat. Methods 10, 508–513. 10.1038/nmeth.2481. [DOI] [PubMed] [Google Scholar]
- Chung K.; Wallace J.; Kim S. Y.; Kalyanasundaram S.; Andalman A. S.; Davidson T. J.; Mirzabekov J. J.; Zalocusky K. A.; Mattis J.; Denisin A. K.; Pak S.; Bernstein H.; Ramakrishnan C.; Grosenick L.; Gradinaru V.; Deisseroth K. (2013) Structural and molecular interrogation of intact biological systems. Nature 497, 332–337. 10.1038/nature12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root D. H.; Melendez R. I.; Zaborszky L.; Napier T. C. (2015) The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Prog. Neurobiol. 130, 29. 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier T. C.; Potter P. E. (1989) Dopamine in the rat ventral pallidum/substantia innominata: biochemical and electrophysiological studies. Neuropharmacology 28, 757–760. 10.1016/0028-3908(89)90163-9. [DOI] [PubMed] [Google Scholar]
- Napier T. C.; Maslowski-Cobuzzi R. J. (1994) Electrophysiological verification of the presence of D1 and D2 dopamine receptors within the ventral pallidum. Synapse 17, 160–166. 10.1002/syn.890170304. [DOI] [PubMed] [Google Scholar]
- Johnson P. I.; Napier T. C. (1997) GABA- and glutamate-evoked responses in the rat ventral pallidum are modulated by dopamine. Eur. J. Neurosci 9, 1397–1406. 10.1111/j.1460-9568.1997.tb01494.x. [DOI] [PubMed] [Google Scholar]
- Smith K. S.; Berridge K. C. (2005) The ventral pallidum and hedonic reward: neurochemical maps of sucrose ″liking″ and food intake. J. Neurosci. 25, 8637–8649. 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C. Y.; Berridge K. C. (2013) An orexin hotspot in ventral pallidum amplifies hedonic ’liking’ for sweetness. Neuropsychopharmacology 38, 1655–1664. 10.1038/npp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromwell H. C.; Berridge K. C. (1993) Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus?. Brain Res. 624, 1–10. 10.1016/0006-8993(93)90053-P. [DOI] [PubMed] [Google Scholar]
- Tindell A. J.; Berridge K. C.; Zhang J.; Pecina S.; Aldridge J. W. (2005) Ventral pallidal neurons code incentive motivation: amplification by mesolimbic sensitization and amphetamine. Eur. J. Neurosci 22, 2617–2634. 10.1111/j.1460-9568.2005.04411.x. [DOI] [PubMed] [Google Scholar]
- Pothos E. N.; Przedborski S.; Davila V.; Schmitz Y.; Sulzer D. (1998) D2-Like dopamine autoreceptor activation reduces quantal size in PC12 cells. J. Neurosci. 18, 5575–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara C. M.; Uhland-Smith A.; O’Malley K. L.; Todd R. D. (1996) Inhibition of dopamine synthesis by dopamine D2 and D3 but not D4 receptors. J. Pharmacol. Exp. Ther. 277, 186–192. [PubMed] [Google Scholar]
- Lindgren N.; Xu Z. Q.; Herrera-Marschitz M.; Haycock J.; Hokfelt T.; Fisone G. (2001) Dopamine D(2) receptors regulate tyrosine hydroxylase activity and phosphorylation at Ser40 in rat striatum. Eur. J. Neurosci 13, 773–780. 10.1046/j.0953-816x.2000.01443.x. [DOI] [PubMed] [Google Scholar]
- Brown J. M.; Hanson G. R.; Fleckenstein A. E. (2001) Cocaine-induced increases in vesicular dopamine uptake: role of dopamine receptors. J. Pharmacol. Exp. Ther. 298, 1150–1153. [PubMed] [Google Scholar]
- Meiergerd S. M.; Patterson T. A.; Schenk J. O. (1993) D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J. Neurochem. 61, 764–767. 10.1111/j.1471-4159.1993.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Cass W. A.; Gerhardt G. A. (1994) Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci. Lett. 176, 259–263. 10.1016/0304-3940(94)90096-5. [DOI] [PubMed] [Google Scholar]
- Benoit-Marand M.; Borrelli E.; Gonon F. (2001) Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 21, 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz Y.; Schmauss C.; Sulzer D. (2002) Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J. Neurosc. 22, 8002–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L.; Zhang M.; Li J. X.; Huang P.; Liu Q.; Li Y. L.; Liang H.; Liang J. H. (2014) Comparison of single versus repeated methamphetamine injection induced behavioral sensitization in mice. Neurosci. Lett. 560, 103–106. 10.1016/j.neulet.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T.; Akiyama M.; Moriya T.; Shibata S. (2001) Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol. Pharmacol. 59, 894–900. [DOI] [PubMed] [Google Scholar]
- Chen R.; Zhang M.; Park S.; Gnegy M. E. (2007) C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacol., Biochem. Behav. 87, 158–163. 10.1016/j.pbb.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck J. A.; Gupta T.; Rhodes J. S. (2009) Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology (Berl) 201, 589–599. 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]
- Mead A. N.; Crombag H. S.; Rocha B. A. (2004) Sensitization of psychomotor stimulation and conditioned reward in mice: differential modulation by contextual learning. Neuropsychopharmacology 29, 249–258. 10.1038/sj.npp.1300294. [DOI] [PubMed] [Google Scholar]
- Cabib S. (1993) Strain-dependent behavioural sensitization to amphetamine: role of environmental influences. Behav. Pharmacol. 4, 367–374. 10.1097/00008877-199308000-00010. [DOI] [PubMed] [Google Scholar]
- Tirelli E.; Terry P. (1998) Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav. Pharmacol. 9, 409–419. 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Battisti J. J.; Uretsky N. J.; Wallace L. J. (1999) Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology (Berl) 146, 42–48. 10.1007/s002130051086. [DOI] [PubMed] [Google Scholar]
- Luscher C. (2013) Drug-evoked synaptic plasticity causing addictive behavior. J. Neurosci. 33, 17641–17646. 10.1523/JNEUROSCI.3406-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. J.; Beurrier C.; Bonci A.; Malenka R. C. (2001) Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 4, 1217–1223. 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Kalivas P. W.; Duffy P. (1990) Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse 5, 48–58. 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Pierce R. C.; Kalivas P. W. (1995) Amphetamine produces sensitized increases in locomotion and extracellular dopamine preferentially in the nucleus accumbens shell of rats administered repeated cocaine. J. Pharmacol. Exp. Ther. 275, 1019–1029. [PubMed] [Google Scholar]
- Heimer L.; Zahm D. S.; Churchill L.; Kalivas P. W.; Wohltmann C. (1991) Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41, 89–125. 10.1016/0306-4522(91)90202-Y. [DOI] [PubMed] [Google Scholar]
- Lohr K. M.; Bernstein A. I.; Stout K. A.; Dunn A. R.; Lazo C. R.; Alter S. P.; Wang M.; Li Y.; Fan X.; Hess E. J.; Yi H.; Vecchio L. M.; Goldstein D. S.; Guillot T. S.; Salahpour A.; Miller G. W. (2014) Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc. Natl. Acad. Sci. U. S. A. 111, 9977–9982. 10.1073/pnas.1402134111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.