Abstract
Purpose
X-linked inhibitor of apoptosis protein (XIAP) is an inhibitor of caspases 3 and 9 which are overexpressed in acute myeloid leukemia (AML) and may contribute to chemoresistance. We report on a phase I/II trial of the XIAP antisense oligonucleotide AEG35156 in combination with reinduction chemotherapy.
Patients and Methods
Twenty-four patients with rapidly relapsed or refractory AML were treated with escalating doses of AEG35156 (12 to 250 mg/m2) as an intravenous solution over 2 hours and 32 patients were treated with the highest planned dose of 350 mg/m2 in combination with idarubicin and high-dose cytarabine reinduction chemotherapy. Correlative studies were conducted to determine the effects of AEG35156 on levels of XIAP mRNA.
Results
Knockdown of XIAP mRNA during treatment increased with the dose of the antisense. All patients who received 350 mg/m2 of AEG35156 had higher than 30% target knockdown with a median maximal knockdown of 90% (range, 48% to 100%). The overall response rate was higher among the patients receiving the highest dose of AEG35156. In this group, 15 (47%) of 32 patients achieved complete response (CR)/CR with incomplete platelet count recovery (CRp) compared with only one (4%) of 24 receiving 12 to 250 mg/m2 AEG35156. Among the patients receiving 350 mg/m2 of AEG35156 in combination with chemotherapy, 10 (91%) of 11 who were refractory to a single induction chemotherapy regimen achieved CR/CRp after reinduction with AEG35156 and chemotherapy. AEG35156 was well tolerated save for two cases of peripheral neuropathy in patients receiving multiple doses of AEG35156.
Conclusion
At the highest dose tested, AEG35156 knocks down its target and appears very effective when combined with chemotherapy in patients with AML refractory to a single induction regimen.
INTRODUCTION
X-linked inhibitor of apoptosis protein (XIAP) is a member of the inhibitor of apoptosis protein family that inhibits apoptosis primarily by binding and inhibiting active caspases 3/7 and −9.1–4 Overexpression of XIAP confers chemoresistance in leukemia cell lines and its chemical5,6 or genetic inhibition7 induces cell death and sensitizes cells to chemotherapy. Therefore, molecules that inhibit XIAP could be useful therapeutic agents for the treatment of patients with leukemia and related malignancies.
AEG35156 is an antisense oligonucleotide that targets XIAP. It is a 19-mer oligonucleotide with a mixed backbone of 11 DNA bases flanked on each end by four 2′-O-methyl-modified RNA bases. The sequence of AEG35156 was designed to achieve maximal stability and potency. In addition, to minimize immunostimulation, AEG35156 does not contain cytosine-phosphate-guanine residues.8 AEG35156 and similar sequence AEG35146 knocked down their target mRNA and protein both in vitro and after systemic administration to mice and such knockdown sensitized malignant cells to chemotherapy.9–11 Recent phase I studies in patients with refractory malignancies also established safety.12,13 These results motivated a phase I/II study of AEG35156 in combination with idarubicin and high-dose cytarabine in patients with relapsed or refractory AML.
PATIENTS AND METHODS
Eligibility
Patients age older than 18 years with Eastern Cooperative Oncology Group performance status 0 to 2 and with relapsed or primary refractory acute myeloid leukemia (AML) except acute promyelocytic leukemia were eligible if they relapsed 6 months or fewer after their initial complete response (CR) or if they were refractory to at least one induction chemotherapy regimen. Additional details of the eligibility criteria are included in the Appendix (online only).
Study Drugs and Trial Design
Patients received escalating doses of AEG35156 from 12 mg/m2 to 350 mg/m2. AEG35156 was dissolved in sterile isotonic saline and administered as an intravenous solution over 2 hours on days 1 through 3 and 8 and then weekly thereafter until CR/CR with incomplete platelet count recovery (CRp), disease progression, or day 35, whichever came first. The starting dose was less than one tenth of the maximal dose tested in the phase I study of AEG35156 as a single agent.12,13 The two-hour infusion schedule was selected to reduce the incidence of elevated liver enzymes that was previously seen with prolonged continuous antisense infusions,12,13 as cynomolgus monkeys receiving a 2-hour infusion of AEG35156 had less accumulation of AEG35156 in the liver compared to monkeys receiving a 24-hour continuous infusion (data not shown). Initially, AEG35156 was continued weekly beyond day 8 in order to maintain target knockdown given the potential role of XIAP in leukemic stem cells.5,6,14 However, due to two cases of peripheral neuropathy in the phase II portion of the study, the protocol was modified so that the last eight patients received AEG35156 only on days 1 to 3 and 8 during the induction with no additional cases of peripheral neuropathy noted.
Patients received idarubicin 12 mg/m2 by intravenous infusion over 30 minutes on days 4, 5, and 6 and high-dose cytarabine 1.5 g/m2 by continuous infusion over 24 hours for 4 days on days 4 to 7 (patients < 65 years) or for 3 days on days 4 to 6 (patients ≥ 65 years). Patients achieving CR/CRp as previously described15 could receive up to four courses of consolidation chemotherapy with weekly AEG35156 and cytarabine at a daily dose of 0.75 g/m2 using the induction schedule. Patients experiencing improvement or CR could proceed to allogeneic hematopoietic stem-cell transplantation at the discretion of the individual institution.
The standard 3 + 3 escalation rule was used for the dose escalation portion of the trial and a Simon two-stage minimax design was used in the phase II portion of the trial which investigated the highest planned dose of 350 mg/m2. Details of the dose escalation and statistical design are presented in the Appendix.
Adverse events were graded based on the National Cancer Institute Common Toxicity Criteria, version 2.0. Criteria for responses for AML followed those defined by the WHO.15,16
The trial was sponsored by Aegera Therapeutics and the roles of the sponsor and academic investigators are described in the Appendix.
Pharmacokinetics
Peripheral blood samples were collected on day 1 immediately before the end of the infusion of AEG35136 and 30 minutes, 2 hours, and 4 hours after the end of the infusion. Plasma concentrations of AEG35156 were measured using capillary gel electrophoresis with ultraviolet detection. Curves of concentration of AEG35156 versus time in plasma were constructed for each patient and analyzed by a noncompartmental analysis technique. Cmax was determined and terminal elimination half-life calculated.
Pharmacodynamics
Peripheral blood samples were collected on days 1, 2, and 3 before the start of the AEG35156 infusion and on day 4 before the start of the idarubicin and cytarabine and enriched for leukemia cells as described in the Appendix. Total RNA was extracted from the leukemic blasts and XIAP mRNA levels were determined using real-time quantitative reverse transcription polymerase chain reaction (Q-RT-PCR) as previously described.10 XIAP levels were normalized to human glyceraldehyde 3-phosphate dehydrogenase or β2 microglobulin mRNA as previously described.10 Normalized XIAP levels were compared to levels before the first dose of AEG35156.
RESULTS
Demographics
Demographics of the patients enrolled in the study are outlined in Table 1. Twenty-four patients with relapsed or primary refractory AML were treated with increasing doses of AEG35156 from 12 to 250 mg/m2 and 32 were treated with the highest planned dose level of 350 mg/m2. Patients receiving 12 to 250 mg/m2 of AEG35156 differed from patients receiving 350 mg/m2 of AEG35156 in their disease status at the time of enrollment (P = .01 by χ2 analysis). Thirteen (54%) of 24 patients who received 12 to 250 mg/m2 of AEG35156 had relapsed ≤ 6 months before reinduction, 10 (42%) were refractory to ≥ 2 induction regimens and one (4%) was refractory to a single induction attempt. Fifteen (63%) of 24 patients who received 12 to 250 mg/m2 of AEG35156 had received prior therapy with high-dose cytarabine during induction or consolidation. In contrast, eight (25%) of 32 receiving 350 mg/m2 of AEG35156 had relapsed ≤ 6 months before reinduction, 13 (41%) were refractory to ≥ 2 induction regimens and 11 (34%) were refractory to one induction regimen.
Table 1.
Patient Demographics and Clinical Characteristics
| Parameter |
AEG35156 (dosage by mg/m2) |
|||
|---|---|---|---|---|
| 12-250 |
350 |
|||
| No. | % | No. | % | |
| No. of patients | 24 | 32 | ||
| Median age, years | 53 | 58 | ||
| Range | 25-71 | 32-72 | ||
| Cytogenetics | ||||
| Good | 1 | 4 | 1 | 3 |
| Intermediate | 19 | 80 | 28 | 72 |
| Poor | 4 | 16 | 8 | 25 |
| Disease status | ||||
| Relapse ≤ 6 months* | 13 | 54 | 8 | 25 |
| 1 refractory† | 1 | 4 | 11 | 34 |
| ≥ 2 refractory‡ | 10 | 42 | 13 | 41 |
| Prior high-dose cytarabine | 15 | 62 | 14 | 44 |
Relapse ≤ 6 months after first complete response.
Refractory to a single induction chemotherapy regimen.
Refractory to ≥ 2 induction chemotherapy regimens.
Fifteen (62%) of 24 and 14 (44%) of 32 patients who received 12 to 250 mg/m2 and 350 mg/m2 of AEG35156, respectively, had received prior therapy with high-dose cytarabine alone (n = 4) or in combination with an anthracycline (daunorubicin, idarubicin, mitoxantrone) with or without etoposide as an initial induction, reinduction, or consolidation regimen (P = .2 by χ2 analysis). Median age and cytogenetics did not differ significantly between the two groups.
Pharmacokinetics
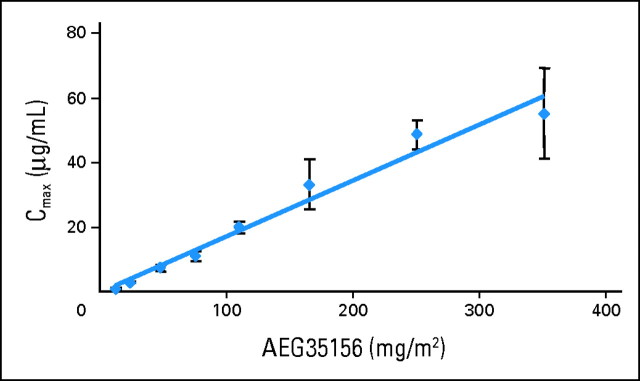
The pharmacokinetics of AEG35156 were investigated in patients after the first infusion of the drug at doses ranging from 12 to 350 mg/m2 (Fig 1). Mean end-of-infusion plasma concentrations of AEG35156 increased proportionally to dose. The initial terminal elimination half-life after discontinuation of infusion was 0.5 to 1.5 hours and appeared to be dose independent.
Fig 1.
Plasma levels of AEG35156 are proportional to the administered dose. Peripheral blood samples were collected on day 1 of AEG35156 infusion immediately before the end of the infusion of AEG35156 and 30 minutes, 2 hours, and 4 hours after the end of the infusion. Plasma concentrations of AEG35156 were measured using capillary gel electrophoresis with ultraviolet detection. Peak concentration (Cmax) was calculated for each patient. Data represent the mean with or without standard deviation Cmax for patients at each dose level.
Pharmacodynamics
Correlative studies were conducted to determine the effects of AEG35156 on its target, XIAP. Blood samples were obtained from 22 patients before the initiation of AEG35156 on day 1 and then daily on days 2 through 4. Leukemic blasts were isolated by density centrifugation and the blasts were enriched from the population of mononuclear cells by CD3+ and CD19+ depletion. mRNA was extracted from the leukemic blasts and levels of XIAP were measured by Q-RT-PCR. For each patient, the percent XIAP knockdown compared to the pretreatment sample was calculated.
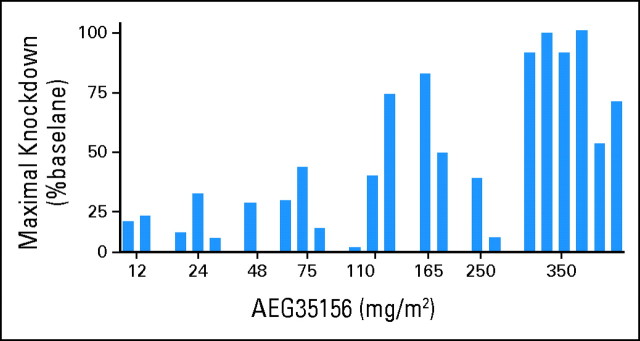
Maximum XIAP mRNA knockdown in the first 3 days of treatment increased with the dose of AEG35156 (Fig 2). Eleven of 13 patients who received ≥ 110 mg/m2 of AEG35156 had higher than 30% target knockdown on days 2 through 4. In contrast, only one (11%) of nine patients receiving lower than 110 mg/m2 of AEG35156 had higher than 30% knockdown on any of these days. Patients who received the highest dose of AEG35156 (350 mg/m2) achieved the greatest amount of target knockdown with a median maximal knockdown of 90% (range, 48% to 100%). Similar trends to target knockdown were observed when only target knockdown on day 4 before reinduction chemotherapy was considered. On day 4, five of six patients who received 350 mg/m2 AEG35156 had higher than 30% knockdown and the median knockdown on day 4 for these six patients was 75%. Evaluations of mean and median target knockdown over day 2 through 4 also produced similar results.
Fig 2.
AEG35156 decreases levels of X-linked inhibitor of apoptosis protein (XIAP) mRNA. Peripheral blood samples were collected on days 1, 2, and 3 before the start of the AEG35156 infusion and on day 4 before the start of the idarubicin and cytarabine. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation and enriched for leukemic blasts by CD19+ and CD3+ depletion. mRNA was extracted from the leukemic blasts and levels of XIAP measured by quantitative reverse transcription polymerase chain reaction. XIAP levels were normalized to housekeeping RNA. Data represent the maximal decrease in XIAP on days 2 through 4 expressed as a percentage of XIAP expression on day 1 before AEG35156 infusion.
Disease Response
One (4%) of 24 patients who received 12 to 250 mg/m2 of AEG35156 in combination with idarubicin and cytarabine chemotherapy achieved a CR after one course of therapy, reflecting the substantial prior therapy in this group of patients. The patient who achieved CR had normal cytogenetics and had failed to respond to 1 cycle of cloretazine. The response rate was higher among the patients receiving the 350 mg/m2 of AEG35156. In this group, 15 (47%) of 32 patients achieved CR/CRp. In particular, 10 (91%) of 11 patients who were refractory to a single induction chemotherapy regimen achieved CR (six patients) or CRp (four patients) after 350 mg/m2 AEG35156. Three of these 10 had failed to achieve remission after noncytarabine-containing chemotherapy (decitabine two patients and clofarabine one patient). However, CR/CRp was still achieved in seven of eight patients (88%; 95% CI, 47% to 100%) who did not respond to a conventional induction with cytarabine (100 to 200 mg/m2 × 7 days) in combination with daunorubicin (45 to 60 mg/m2 × 3days) or idarubicin (12 mg/m2 × 3 days). Median age of these eight patients was 60 years (range, 38 to 68 years) and 25% had poor risk cytogenetics. Median percentage of blasts in their bone marrow at the time of reinduction was 20% (range, 10% to 50%).
Only one of the 11 primary refractory patients who achieved CR after AEG35156 treatment relapsed at 2 months after CR with a median follow-up of 9.5 months (range, 2.3 to 13.7 months). Of these 10 patients who remain in CR/CRp, all proceeded to allogeneic stem cell transplantation and nine of 10 are alive and in remission except for one transplant-related death. Their actuarial progression-free survival and overall survival were both 90% (95% CI, 88% to 92%). Similarly high rates of progression-free and overall survival were noted for the seven patients who achieved CR/CRp after failing a single induction regimen containing standard dose cytarabine (data not shown).
Four of eight patients (50%; 95% CI, 16% to 84%) who had relapsed ≤ 6 months after first CR (CR1) achieved remission (two CRs and two CRps) after reinduction with 350 mg/m2 AEG35156 and chemotherapy (Table 2). Of these four patients, one achieved CR1 with decitabine and two did not receive consolidation chemotherapy after achieving CR1 after a conventional induction regimen with cytarabine and daunorubicin. Among patients refractory to ≥ two induction regimens who received 350 mg/m2 of AEG35156 in combination with chemotherapy, the response rate was poor, as only one (8%) of 13 patients achieved CR. Among the patients who received 350 g/m2 of AEG35156 and had a short CR1 or who were refractory to ≥ 2 induction regimens, 67% had higher than 90% knockdown of XIAP mRNA. Moreover, target knockdown did not differ between patients who did and did not achieve CR or CRp after AEG35156 in combination with chemotherapy. Of note, samples for XIAP mRNA analysis were available from six patients treated at the highest planned dose of 350 mg/m2. Of these six patients, three were refractory to a single induction regimen and all achieved CR/CRp. All three of these patients had evidence of target knockdown with maximal target knockdowns of 100%, 99%, and 42%. The three other patients on whom pharmacodynamics samples were available were more heavily pretreated and did not achieve CR/CRp. However, these three patients also had target knockdown with maximal target knockdowns of 90%, 90%, and 68%. Thus, in highly refractory AML patients, inhibition of XIAP was not sufficient to sensitize them to chemotherapy.
Table 2.
Rate of CR/CRp Response After AEG35156 in Combination With Idarubicin and High-Dose Cytarabine Reinduction Chemotherapy
| Parameter |
AEG35156 (dosage by mg/m2) |
|||||
|---|---|---|---|---|---|---|
| 12-250 (n = 24) |
350 (n = 31) |
|||||
| No. | % | 95% CI | No. | % | 95% CI | |
| Relapse ≤ 6 months* | 0/14 | 0 | 0 to 23 | 4/8 | 50 | 16 to 84 |
| 1 refractory† | 1/1 | 100 | 10/11 | 91 | 59 to 100 | |
| > 2 refractory‡ | 0/9 | 0 | 0 to 34 | 1/13 | 8 | 0 to 23 |
| Overall response rate | 4 | 0 to 24 | 47 | 29 to 65 | ||
Abbreviations: CR, complete response; CRp, CR with incomplete platelet count recovery.
Relapse ≤ 6 months after first complete response.
Refractory to a single induction chemotherapy regimen.
Refractory to ≥ 2 induction chemotherapy regimens.
Safety
AEG35156 in combination with reinduction chemotherapy was generally well tolerated. Grade 3 and 4 toxicities possibly related to AEG35156 included two cases of peripheral neuropathy that were noted in the phase II portion of the study where patients received repeated courses of 350 mg/m2 AEG35156 after achieving CR after the study induction regimen. A 52-year-old man developed a grade 4 sensory peripheral neuropathy 2 months after the last dose of AEG35156. At the time the peripheral neuropathy developed, the patient was in CR and had received six doses of AEG35156. The peripheral neuropathy prevented the patient from walking. However, it gradually resolved over 3 months. A 66-year-old female developed grade 3 mixed sensory and motor peripheral neuropathy while in CR after receiving AEG35156. The peripheral neuropathy developed during consolidation after eight doses of AEG35156. The patient's AML relapsed and she died from progressive disease without resolution of the peripheral neuropathy. As a result of these two cases, the protocol was amended to administer AEG35156 on days 1 through 3 and day 8 with no subsequent cycles. After the change in the protocol, eight patients were enrolled on the study with no further cases of peripheral neuropathy nor any discernable effects on efficacy.
Among patients receiving 12 to 250 mg/m2 AEG35156 with reinduction chemotherapy, two (8%) of 24 patients died before day 35 from infection (n = 1) and renal failure (n = 1). At the highest dose of AEG35156 350 mg/m2 with chemotherapy, five (16%) of 32 patients died before day 35 from infection. Complement activation was not noted in any patient.
DISCUSSION
We report a phase I/II clinical trial of escalating doses of AEG35156 XIAP antisense in combination with high-dose cytarabine and idarubicin reinduction chemotherapy in patients with relapsed and refractory AML. In this study, AEG35156 knocked down its target, XIAP, and target knockdown correlated with the dose of XIAP antisense. At the highest planned dose, all patients had higher than 30% target knockdown and a median knockdown of 90%. Target mRNA knockdown in blasts was assessed by Q-RT-PCR, as the there were insufficient samples to assess protein loss. However, XIAP has a short half-life, so knockdown of the mRNA likely translates into protein knockdown. In cell culture models 50% to 80% knockdown of XIAP mRNA with AEG35156 and similar sequence AEG35146 was associated with cell death and sensitized cells to TRAIL.9,10 In lung cancer xenograft studies, higher than 60% XIAP mRNA knockdown with AEG35146 delayed tumor growth in mice.9 However, the effects of lesser amounts of target knockdown were not evaluated in either cell culture or animal models. High levels and frequency of knockdown of target knockdown were observed in patients whose disease had rapidly relapsed or those refractory to ≥ 2 induction regimens. However, knockdown of XIAP was not sufficient to result in clinical responses in these patients. Thus, in these patients, resistance to chemotherapy likely involves mechanisms beyond XIAP.
In the subgroup of patients who were refractory to a single induction chemotherapy regimen, AEG35156 consistently knocked down its target and the drug in combination with chemotherapy produced a CR/CRp rate of 91%. These 12 patients who were refractory to an initial induction regimen included one patient who received 165 mg/m2 of AEG35156 and 11 patients who received 350 mg/m2. Interestingly, the patient who received 165 mg/m2 of AEG35156 had 80% target knockdown and patients who received 350 mg/m2 had a median knockdown of 90%. Thus, the knockdown of XIAP with AEG35156 may explain the remarkable response rate to reinduction chemotherapy.
Of the 12 patients enrolled in this study who were refractory to a single induction regimen, four were initially treated with a noncytarabine-based induction. Therefore, it is difficult to predict their likely response to reinduction as they did not have a conventional induction regimen. However, the 91% response rate observed in these 12 patients is higher than response rates to primary induction chemotherapy that have been previously reported.17,18
The observed high response rates may have reflected entry of patients with relatively low percentages of blasts in the marrow at the time of reinduction (eg, 5% to 10%). In particular, these patients may have achieved CR or CRp with reinduction chemotherapy alone without the addition of AEG35156. To address this issue, readers are referred to our analysis of covariates predicting response to reinduction chemotherapy with high-dose cytarabine regimens without AEG35156 in patients refractory to first-line induction with standard dose cytarabine and an anthracycline.19 Of note, the entry criteria for that study were similar to the ones used here. In this study, increased age, poor-risk cytogenetics, increased percentage of blasts in the marrow at the time of reinduction and secondary AML were independent adverse predictors of response to a second course of chemotherapy with high-dose cytarabine in patients refractory to a single induction regimen containing cytarabine (100 to 200 mg/m2 × 7 days) in combination with daunorubicin (45 to 60 mg/m2 × 3days).19 The overall response rate to reinduction chemotherapy with high-dose cytarabine was 53%. Thus, while, the number of patients in our study was small, the results suggest that the use of AEG35156 may improve response rates.
In this study, AEG35156 was well tolerated up to the highest planned dose of 350 mg/m2. Notable adverse effects, involved two cases of peripheral neuropathy after the administration of multiple AEG35156 doses in patients achieving CR. The cause of the neuropathy is uncertain, but was likely related to the administration of study drug in association with cytarabine and idarubicin since no cases of peripheral neuropathy were observed in the AEG3516 single agent phase I studies.12,13 The mechanism by which AEG35156 enhanced chemotherapy induced peripheral neuropathy is unclear. However, since full recovery was noted within 3 months in one of the two patients who had prolonged survival, it is unlikely that the neuropathy was due to neuronal cell death. Rather, we speculate that it may represent a reversible demyelination after glial cell cotoxicity.
In summary, the addition of AEG35156 to reinduction chemotherapy was well tolerated and possibly effective in first salvage. A high rate of target knockdown was observed, potentially supporting the clinical efficacy of the drug. Therefore, a randomized phase II study in this patient population would be useful to confirm this observation. Specifically, such a study would include adult patients with AML who experienced treatment failure with a standard dose cytarabine-based first-line induction regimen. If the trend to improved response rate with the addition of AEG35156 to reinduction chemotherapy is maintained in this phase II study, then a randomized phase III study would likely be needed to prove the efficacy of AEG35156 in this setting.
Acknowledgment
We thank Ann Kasaboski for administrative assistance and Gabriele Cherton-Horvat, Charles Lefebvre, Martine St-Jean, and Matthew Martin for technical assistance.
Appendix
Eligibility criteria.
Patients age older than 18 years with Eastern Cooperative Oncology Group performance status 0 to 2 and with relapsed or primary refractory acute myeloid leukemia (AML) except acute promyelocytic leukemia were eligible if they relapsed 6 months or fewer after their initial complete response (CR) or if they were refractory to at least one induction chemotherapy regimen. The diagnosis of relapsed or refractory AML required higher than 10% blasts in the marrow or blood or 5% to 10% blasts in the blood or marrow unrelated to recovery of normal hematopoesis plus at least one cytopenia. Circulating blasts had to be lower than 50,000/μL and not expected to rise above 50,000/μL in the first 5 days of treatment. Patients were ineligible if they had an ejection fraction lower than 50%, active CNS AML, or abnormal organ function. The study was approved by the research ethics boards of all participating institutions, and all patients provided written informed consent before study treatment.
Dose escalation and trial design.
The standard 3 + 3 escalation rule was used for the dose escalation portion of the trial. Dose-limiting toxicity defined as grade 3 or 4 toxicity possibly related to AEG35156 including ≥ 3-fold increase in complement split product Bb or C5a at the end of infusion or 2 hours after infusion relative to the preAEG35156 dose baseline value. Complement activation was defined as a grade 3/4 toxicity to give a more robust definition of safety as prior studies in primates with first generation antisense oligonucleotides identified complement activation as a potential toxicity (Henry SP, Giclas PC, Leeds J, et al: J Pharmacol Exp Ther 281:810-816, 1997; Galbraith WM, Hobson WC, Giclas PC, et al: Antisense Res Dev 4:201-206, 1994). However, no evidence of complement activation was seen in primates treated with AEG35156 (data not shown) or antisense oligonucleotides with a similar chemical design (Chen HX, Marshall JL, Ness E, et al: Clin Cancer Res 6:1259-1266, 2000). In the dose escalation portion of the trial the 110 mg/m2 cohort was expanded to five patients as there were two induction deaths before day 25 that were judged probably unrelated to AEG35156. Expansion of the cohort to four patients at 250 mg/m2 was planned to give a more robust indication of safety of this dose level.
A Simon two-stage minimax design was used in the phase II portion of the trial which investigated the highest planned dose of 350 mg/m2. The design set 20% as the null CR/CRp rate (corresponding to the historical rate) and a CR/CRp rate of at least 40% as desirable. Setting the false-positive and false-negative rates at 10% the study would stop if there were less than four CR or CRp in the first 19 patients and if the trial proceeded to a second stage the treatment would be rejected if there were less than 11 of 36 CR or CRp overall.
The trial was sponsored by Aegera Therapeutics and designed by the principle investigators in collaboration with the sponsor. Data collection and management and were performed by the sponsor. Statistical analysis was performed by the principle investigators and the sponsor. The first draft of the report was written by the lead author, and subsequent drafts were revised and edited by all the authors. All authors vouch for the accuracy and completeness of the data.
Pharmacodynamics.
Peripheral blood samples were collected on days 1, 2, and 3 before the start of the AEG35156 infusion and on day 4 before the start of the idarubicin and cytarabine. Mononuclear cells were separated by Ficoll-Hypaque density gradient centrifugation (Sigma-Aldrich, St Louis, MO). Leukemic blasts were enriched from the mononuclear cells by depleting CD19+ and CD3+ expressing cells with Miltenyi Microbeads and MACS Columns (Miltenyi, Auburn, CA).
Footnotes
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Christine Jacob, Aegera Therapeutics (C); Stephen J. Morris, Aegera Therapeutics (C); Jacques Jolivet, Aegera Therapeutics (C) Consultant or Advisory Role: Aaron D. Schimmer, Aegera Therapeutics (C); Elihu H. Estey, Aegera Therapeutics (C); Gautam Borthakur, Aegera Therapeutics (C); Michael Andreeff, Aegera Therapeutics (C) Stock Ownership: None Honoraria: None Research Funding: Aaron D. Schimmer, Aegera Therapeutics; David W. Hedley, Aegera Therapeutics; Michael Andreeff, Aegera Therapeutics Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Aaron D. Schimmer, Elihu H. Estey, Bing Z. Carter, David W. Hedley, Stephen J. Morris, Eric LaCasse, Jacques Jolivet, Michael Andreeff
Administrative support: Christine Jacob
Provision of study materials or patients: Aaron D. Schimmer, Elihu H. Estey, Gautam Borthakur, Gary J. Schiller, Martin S. Tallman, Jessica K. Altman, Judith E. Karp, Jeannine Kassis, Michael Andreeff
Collection and assembly of data: Aaron D. Schimmer, Elihu H. Estey, Bing Z. Carter, David W. Hedley, Joseph Brandwein, Wei Xu, Duncan H. Mak, Christine Jacob, Stephen J. Morris, Eric LaCasse, Jacques Jolivet, Michael Andreeff
Data analysis and interpretation: Aaron D. Schimmer, Elihu H. Estey, Gautam Borthakur, Bing Z. Carter, Martin S. Tallman, Jessica K. Altman, Judith E. Karp, David W. Hedley, Joseph Brandwein, Wei Xu, Stephen J. Morris, Jacques Jolivet, Michael Andreeff
Manuscript writing: Aaron D. Schimmer, Elihu H. Estey, Gary J. Schiller, Martin S. Tallman, Jessica K. Altman, Judith E. Karp, Jeannine Kassis, Joseph Brandwein, Wei Xu, Jacques Jolivet
Final approval of manuscript: Aaron D. Schimmer, Elihu H. Estey, Gautam Borthakur, Bing Z. Carter, Gary J. Schiller, Martin S. Tallman, Jessica K. Altman, Judith E. Karp, Jeannine Kassis, David W. Hedley, Joseph Brandwein, Wei Xu, Duncan H. Mak, Christine Jacob, Stephen J. Morris, Eric LaCasse, Jacques Jolivet, Michael Andreeff
REFERENCES
- 1.Deveraux QL, Takahashi R, Salvesen GS, et al. X-linked IAP is a direct inhibitor of cell death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 2.Sun C, Cal M, Gunasekera A, et al. NMR structure and mutagenesis of the inhibitor-of-apoptosis protein XIAP. Nature. 1999;401:818–821. doi: 10.1038/44617. [DOI] [PubMed] [Google Scholar]
- 3.Riedl SJ, Renatus M, Schwarzenbacher R, et al. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791–800. doi: 10.1016/s0092-8674(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 4.Shiozaki EN, Chai J, Rigotti DJ, et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- 5.Schimmer AD, Welsh K, Pinilla C, et al. Small-molecule antagonists of apoptosis suppressor XIAP exhibit broad antitumor activity. Cancer Cell. 2004;5:25–35. doi: 10.1016/s1535-6108(03)00332-5. [DOI] [PubMed] [Google Scholar]
- 6.Carter BZ, Gronda M, Wang Z, et al. Small-molecule XIAP inhibitors derepress downstream effector caspases and induce apoptosis of acute myeloid leukemia cells. Blood. 2005;105:4043–4050. doi: 10.1182/blood-2004-08-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter BZ, Milella M, Tsao T, et al. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17:2081–2089. doi: 10.1038/sj.leu.2403113. [DOI] [PubMed] [Google Scholar]
- 8.Lacasse EC, Kandimalla ER, Winocour P, et al. Application of XIAP antisense to cancer and other proliferative disorders: Development of AEG35156/ GEM(R)640. Ann N Y Acad Sci. 2005;1058:215–234. doi: 10.1196/annals.1359.032. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y, Cherton-Horvat G, Dragowska V, et al. Antisense oligonucleotides targeting XIAP induce apoptosis and enhance chemotherapeutic activity against human lung cancer cells in vitro and in vivo. Clin Cancer Res. 2003;9:2826–2836. [PubMed] [Google Scholar]
- 10.Cummings J, Ward TH, LaCasse E, et al. Validation of pharmacodynamic assays to evaluate the clinical efficacy of an antisense compound (AEG 35156) targeted to the X-linked inhibitor of apoptosis protein XIAP. Br J Cancer. 2005;92:532–538. doi: 10.1038/sj.bjc.6602363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shaw TJ, Lacasse EC, Durkin JP, et al. Downregulation of XIAP expression in ovarian cancer cells induces cell death in vitro and in vivo. Int J Cancer. 2008;122:1430–1434. doi: 10.1002/ijc.23278. [DOI] [PubMed] [Google Scholar]
- 12.Dean E, Jodrell D, Connolly K, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol. 2009;27:1660–1666. doi: 10.1200/JCO.2008.19.5677. [DOI] [PubMed] [Google Scholar]
- 13.Dean E, Ward T, Denney O, et al. A phase I trial of AEG35156 (XIAP antisense) administered as a 2-hour intravenous infusion in patients with advanced tumours. J Clin Oncol. 2008;26(suppl):163s. abstr 3541. [Google Scholar]
- 14.Carter BZ, Mak DH, Morris MJ, et al. Pharmacodynamic study of phase 1/2 trial of the XIAP antisense oligonucleotide (AEG35156) in combination with chemotherapy in patients with relapsed/refractory AML. Blood. 2008;112 abstr 1943. [Google Scholar]
- 15.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 16.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Buchner T, Hiddemann W, Berdel WE, et al. 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by sequential HAM in adult patients at all ages with de novo acute myeloid leukemia (AML): A randomized trial of the German AML Cooperative Group. J Clin Oncol. 2003;21:4496–4504. doi: 10.1200/JCO.2003.02.133. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JE, Kopecky KJ, Willman CL, et al. Outcome after induction chemotherapy for older patients with acute myeloid leukemia is not improved with mitoxantrone and etoposide compared to cytarabine and daunorubicin: A Southwest Oncology Group study. Blood. 2002;100:3869–3876. doi: 10.1182/blood-2001-12-0354. [DOI] [PubMed] [Google Scholar]
- 19.Brandwein JM, Gupta V, Schuh AC, et al. Predictors of response to reinduction chemotherapy for patients with acute myeloid leukemia who do not achieve complete remission with frontline induction chemotherapy. Am J Hematol. 2008;83:54–58. doi: 10.1002/ajh.21034. [DOI] [PubMed] [Google Scholar]