Abstract
Purpose
To evaluate the risk of locoregional recurrence (LRR) associated with locoregional treatment of women with primary breast cancer tumors negative for estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 (triple-negative breast cancer [TNBC]).
Patients and Methods
Patients diagnosed with TNBC were identified from a cancer registry in a single institution (n=768). LRR-free survival was estimated using Kaplan-Meier analysis. The Cox proportional hazards regression model was used to determine risk of LRR on the basis of locoregional management: breast-conserving therapy (BCT; ie, lumpectomy and adjuvant radiation therapy [RT]) and modified radical mastectomy (MRM) in the TNBC population and T1-2N0 subgroup.
Results
At a median follow-up of 7.2 years, 77 patients (10%) with TNBC developed LRR. Five-year LRR-free survival was 94%, 85%, and 87% in the BCT, MRM, and MRM + RT groups, respectively (P < .001). In multivariate analysis, MRM (compared with BCT), lymphovascular invasion and lymph node positivity were associated with increased LRR. Conversely, adjuvant chemotherapy was associated with decreased risk of LRR. For patients with T1-2N0 tumors, 5-year LRR-free survival was 96% and 90% in the BCT and MRM groups, respectively (P =.027), and MRM was the only independent prognostic factor associated with increased LRR compared with BCT (hazard ratio, 2.53; 95% CI, 1.12 to 5.75; P= .0264).
Conclusion
Women with T1-2N0 TNBC treated with MRM without RT have a significant increased risk of LRR compared with those treated with BCT. Prospective studies are warranted to investigate the benefit of adjuvant RT after MRM in TNBC.
INTRODUCTION
Breast cancer is a heterogeneous disease that encompasses several distinct molecular profiles with different clinical behaviors and responses to therapy. Currently, patients with breast cancer are managed using clinical and histologic parameters, such as tumor size, lymph node (LN) status, and grade in conjunction with standardized immunohistochemical assessment of hormone receptors (ie, estrogen receptor [ER], progesterone receptor [PR]) and human epidermal growth factor receptor 2 (HER2) testing. Locoregional management of breast cancer has been implemented based on results of randomized controlled trials comparing breast-conserving therapy (BCT; ie, lumpectomy and adjuvant radiation therapy [RT]) and modified radical mastectomy (MRM).1–3 In those studies, locoregional outcome was not investigated with respect to molecular and/or biologic heterogeneity of breast cancer. Indeed, over the past decade, genomic and molecular profiling have paved the way to a paradigm shift toward new molecular classification with at least four major molecular subtypes4,5 associated with differences in survival and response to treatment.4–6 To approximate these molecular subtypes, most studies have focused on biologic subtyping using ER, PR, and HER2 as biomarkers.7,8 In particular, triple-negative breast cancers (TNBCs), which account for approximately 10% to 17% of all patients with breast cancer,9,10 present poorly differentiated tumors lacking expression of ER, PR, and HER2 on immunohistochemical analysis; they are characterized by a high proliferation rate11 and increased aggressiveness compared with other subtypes.9,12 Because endocrine and HER2-targeted therapies cannot be offered, conventional cytotoxic chemotherapy followed by adjuvant RT is the standard of care for patients with TNBC. The paucity of therapeutic options emphasizes the urgent need to optimize the current locoregional management of patients with TNBC and reduce their risk of locoregional recurrence (LRR).
Several retrospective studies7,8,13–17 have used biologic subtype to assess risk of LRR in large populations of patients with breast cancer, which proportionally included small cohorts of patients with TNBC. Those studies showed an increased risk of LRR in patients with TNBC as compared with those with other biologic subtypes. However, they did not analyze risk of LRR based on initial locoregional management (ie, BCT v MRM) in patients with TNBC.
Our study investigates risk of LRR associated with locoregional treatment (ie, BCT v MRM) in a large population-based cohort of patients with TNBC treated in a single institution. To our knowledge, our study is the first to highlight the increased risk of LRR in patients with T1-2N0 TNBC treated with MRM without RT compared with those treated with BCT.
PATIENTS AND METHODS
Study Population
Patients with newly diagnosed TNBC between January 1998 and December 2008 in a single cancer center were included in this study. We identified this population of patients with TNBC tumors from the Alberta Cancer Registry and assessed risk of LRR associated with locoregional treatment. Immunohistochemical staining for ER, PR, and HER2 was performed centrally and prospectively on tissue sections using standard methods.18,19 Patients with in situ disease and metastatic breast cancer at presentation were excluded. Of 1,189 patients identified, 421 were excluded from final analysis as follows: breast cancer diagnosis before January 1998 (n=184), no adjuvant treatment (n= 80), diagnosed with multiple primary malignancies (n=86), or neoadjuvant chemotherapy (n=71). Data collected included standard prognostic factors such as tumor size; LN, ER, PR, and HER2 status; modified Scarff-Bloom-Richardson tumor grade; lymphovascular invasion (LVI); type and date of surgery; adjuvant treatment received; time and site of first LRR and subsequent metastatic progression; last follow-up; and death.
Patient Management and Follow-Up
All patient cases were reviewed by a multidisciplinary group, and patients were offered guideline-based staging, surgery, adjuvant chemotherapy, and RT as per published recommendations.20–23 The Cross Cancer Institute is the only center in northern Alberta delivering RT. All patients with breast cancer in this study were diagnosed and/or reviewed by pathologists (members of regional breast pathology team). Adjuvant chemotherapy was offered to all LN-positive and high-risk LN-negative patients. Adjuvant RT delivered to the breast (50 Gy in 25 fractions or 42.5 Gy in 16 fractions) was offered to all patients after segmental resection. RT boost to the tumor bed (administered as 10 Gy in five fractions) was left to the discretion of the attending radiation oncologist. Regional LN irradiation was offered to patients with four or more positive LNs. After mastectomy, patients were offered chest wall and regional LN RT (50 Gy in 25 fractions) if they had one or more positive LNs or locally advanced disease (ie, greater than T3 tumor). Follow-up was provided as per Canadian guidelines. Local relapse was defined as recurrence within the breast/chest wall, and regional relapse as recurrence in LNs, including ipsilateral supraclavicular fossa, axilla, or internal mammary LNs.
Primary End Points and Statistical Analysis
The primary end point of this study was LRR-free survival. LRR refers to any progression in the breast, skin, or muscles of the chest wall and/or LNs. Time to LRR was measured from date of surgery to date of clinical relapse. The secondary end point was overall survival (OS). Statistical analysis was carried out using SAS version 9.1 (SAS Institute, Cary, NC). The following variables were analyzed: tumor size and grade, LN status, LVI, adjuvant RT, adjuvant chemotherapy, locoregional treatment (BCT v MRM or MRM + RT). The differences in clinicopathologic features and adjuvant treatment between the three groups (BCT, MRM, and MRM + RT) were examined using χ2 tests. LRR-free survival and OS curves were estimated using the Kaplan-Meier method, and survival differences were assessed using the log-rank test. The Cox proportional hazards regression model was used for univariate and multivariate analyses of LRR-free survival and OS in the TNBC population and T1-2N0 subgroup. Univariate Cox regression analysis was performed for each prognostic variable, and those variables with P ≤ .10 in univariate analysis were included in the multivariate Cox model analysis. Multivariate Cox regression analysis included locoregional treatment as the primary prognostic variable, with tumor size, grade, LN status, LVI, and adjuvant chemotherapy added as covariables in the model, and used backward elimination with factor removal set at P > .05. Multivariate analysis provided significance levels with hazard ratios (HRs) and 95% CIs for the clinicopathologic and treatment covariates to identify significant predictive factors associated with LRR and OS. In addition, T1-2N0 patients were matched based on tumor size (T1 and T2), and multivariate Cox regression analysis was conducted on this matched pair data set with adjuvant chemotherapy and LVI included in the model as covariates. All reported P values are two-sided, and differences were considered statistically significant when P < .05.
RESULTS
Patients and Treatment Characteristics
In this cohort of women with TNBC (n=768), median age was 56 years, and median follow-up time for LRR was 7.2 years. As previously reported,24 this population presented a high percentage of young women, with 40% of patients younger than 50 years of age at diagnosis. The most striking pathologic features are the association between TNBC and small tumor size (T1=52%), grade 3 tumor (83%), and low incidence of LN involvement (N0=64%). In this study, to investigate risk of LRR in TNBC, 768 patients with TNBC were stratified by locoregional treatment (Table 1). Patients underwent BCT (319 of 768 patients; 42%), MRM (287 of 768; 37%), or MRM + RT (162 of 768; 21%). In the BCT group, all patients received RT: 262 (82%) received RT to the breast/chest, and 57 (18%) received locoregional RT (breast/chest and LNs). In the MRM + RT group, 10 patients (6%) received chest wall RT alone, and 152 (94%) received locoregional RT. Eighty-five percent of patients received adjuvant chemotherapy (P < .001; Table 1).
Table 1.
Distribution of Clinical and Treatment Characteristics Among Patients With TNBC
| Characteristic | BCT (n=319) |
MRM (n=287) |
MRM + RT (n=162) |
P | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Age at diagnosis, years | |||||||
| Median | 52 | 56 | 51 | ||||
| Range | 26-85 | 27-90 | 27-89 | ||||
| Tumor size, cm | <.001 | ||||||
| T1 (< 2) | 207 | 65.0 | 140 | 49.0 | 54 | 33.0 | |
| T2 (2-5) | 107 | 33.0 | 134 | 47.0 | 87 | 54.0 | |
| T3 (> 5) | 5 | 2.0 | 13 | 4.0 | 21 | 13.0 | |
| Tumor grade | .9572 | ||||||
| 1-2 | 50 | 16.0 | 57 | 20.0 | 21 | 13.0 | |
| 3 | 269 | 84.0 | 230 | 80.0 | 141 | 87.0 | |
| LN status | <.001 | ||||||
| N0 | 236 | 74.0 | 242 | 84.3 | 17 | 11.0 | |
| N1 (one to three positive) | 63 | 19.8 | 34 | 11.9 | 80 | 49.0 | |
| N2 (> three positive) | 20 | 6.3 | 11 | 3.8 | 65 | 40.0 | |
| Extracapsular extension | .0035 | ||||||
| No | 297 | 93.0 | 267 | 93.0 | 89 | 55.0 | |
| Yes | 22 | 7.0 | 20 | 7.0 | 73 | 45.0 | |
| LVI | <.001 | ||||||
| Negative | 226 | 71.0 | 206 | 72.0 | 55 | 34.0 | |
| Positive | 93 | 29.0 | 81 | 28.0 | 107 | 66.0 | |
| Adjuvant RT | |||||||
| No | 0 | 0.0 | 287 | 100.0 | 0 | 0.0 | < .001 |
| Yes | 319 | 100.0 | 0 | 0.0 | 162 | 100.0 | |
| Breast/chest wall alone | 262 | 82.0 | 0 | 0.0 | 10 | 6.0 | < .001 |
| Locoregional | 57 | 18.0 | 0 | 0.0 | 152 | 94.0 | |
| Adjuvant chemotherapy | <.001 | ||||||
| No | 83 | 26.0 | 111 | 39.0 | 24 | 15.0 | |
| Yes | 236 | 74.0 | 176 | 61.0 | 138 | 85.0 | |
Abbreviations: BCT, breast-conserving therapy; LN, lymph node; LVI, lymphovascular invasion; MRM, modified radical mastectomy; RT, radiation therapy; TNBC, triple-negative breast cancer.
Locoregional and Distant Relapse in Patients With TNBC
Of 768 patients, 155 (20%) developed disease progression, 77 (10%) experienced LRR, 103 (13%) developed distant metastasis (DM), and 123 (LRR, 54; DM, 69) died as a result of disease progression. Seventy-seven (10%) developed LRR as a first event, including 50 (65%) breast/chest wall relapses and 41 (53%) isolated LN relapses. Of these 77 patients, 25 (32%) were diagnosed with LRR and simultaneous DM. Five-year LRR-free survival rate was 89%, and 5-year OS rate was 81%.
Univariate analysis of LRR-free survival and OS (Figs 1A and 1B, respectively) revealed significant differences among the three groups. Five-year LRR-free survival was 94%, 85%, and 87% in the BCT, MRM, and MRM + RT groups, respectively (P < .001; Fig 1A). Five-year OS was 87%, 82%, and 68% in the BCT, MRM, and MRM + RT groups, respectively (P < .001; Fig 1B). In univariate analysis, the rate of LRR relapse was significantly higher in the MRM (HR, 2.61; 95% CI, 1.5 to 4.55; P < .001) and MRM + RT (HR, 2.38; 95% CI, 1.25 to 4.53; P < .001) groups compared with the BCT group (Table 2). The rate of DM was not significantly different in the MRM compared with the BCT group but was significantly higher in the MRM + RT group (HR, 3.49; 95% CI, 2.19 to 5.55; P < .001; Table 2).
Fig 1.
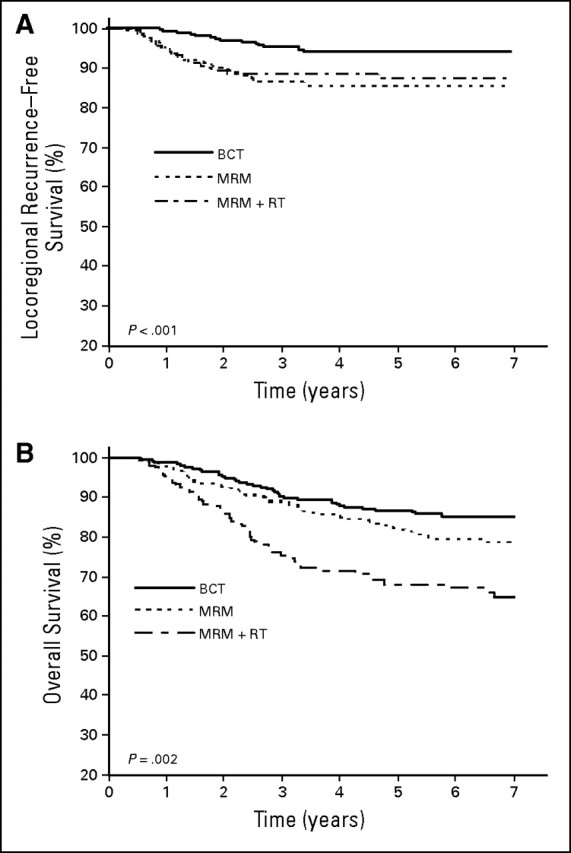
(A) Locoregional recurrence–free and (B) overall survival stratified by locoregional treatment (breast-conserving therapy [BCT], modified radical mastectomy [MRM], MRM + radiation therapy [RT]).
Table 2.
Locoregional and Distant Relapse Stratified by Locoregional Management of Patients With TNBC
| Locoregional Treatment | BCT (n=319)* |
MRM (n=287) |
MRM + RT (n=162) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | HR | No. | HR | 95% CI | P | No. | HR | 95% CI | P | |
| Relapse | ||||||||||
| LRR | 18 | 1 | 40 | 2.61 | 1.5 to 4.55 | < .001 | 19 | 2.38 | 1.25 to 4.53 | .0086 |
| DM | 30 | 1 | 28 | 1.12 | 0.6 to 1.87 | 0.67 | 45 | 3.49 | 2.19 to 5.55 | < .001 |
| LRR + DM | 45 | 1 | 55 | 1.45 | 0.9 to 2.15 | .064 | 55 | 2.80 | 1.89 to 4.16 | < .001 |
| LRR | ||||||||||
| Breast/chest wall | 9 | 1 | 25 | 3.26 | 1.5 to 6.98 | .0024 | 16 | 4.0 | 1.77 to 9.05 | < .001 |
| LN | 10 | 1 | 24 | 2.82 | 1.3 to 5.89 | .0059 | 7 | 1.58 | 0.60 to 4.14 | .35 |
| DM | ||||||||||
| Organ (liver, lung, brain) | 26 | 1 | 20 | 0.92 | 0.51 to 1.65 | 0.78 | 31 | 2.77 | 1.64 to 4.68 | < .001 |
| Bone | 8 | 1 | 9 | 1.40 | 0.54 to 3.65 | 0.49 | 11 | 3.46 | 1.37 to 8.74 | .008 |
| Skin/other | 5 | 1 | 10 | 2.36 | 0.81 to 6.91 | 0.11 | 18 | 8.24 | 3.06 to 22.20 | < .001 |
Abbreviations: BCT, breast-conserving therapy; DM, distant metastasis; HR, hazard ratio; LN, lymph node; LRR, locoregional recurrence; MRM, modified radical mastectomy; RT, radiation therapy; TNBC, triple-negative breast cancer.
Reference group.
Prognostic Factors Associated With LRR
We investigated the prognostic factors associated with increased risk of LRR in the whole cohort of patients with TNBC. Multivariate Cox regression analysis included locoregional treatment as the primary prognostic variable, with tumor size, grade, LN status, LVI, and adjuvant chemotherapy added as covariables in the model. Compared with BCT, MRM (without RT) was an independent predictor of LRR in patients with TNBC (HR, 3.44; 95% CI, 2.04 to 5.80; P < .001; Table 3). However, there was no significant difference between MRM + RT and BCT (HR, 0.72; 95% CI, 0.36 to 1.43; P =.34; Table 3), suggesting that BCT or MRM + RT provides adequate locoregional control to patients with TNBC compared with MRM without RT.
Table 3.
Multivariate Analysis of Predictors for LRR and OS of Patients With TNBC
| Variable | LRR |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Tumor size | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.14 | 0.70 to 1.85 | .6078 | 1.8 | 1.25 to 2.60 | < .001 |
| T3 | 1.42 | 0.63 to 3.21 | .4018 | 2.16 | 1.19 to 3.90 | .0109 |
| Tumor grade | ||||||
| 1-2 | 1 | 1 | ||||
| 3 | 1.07 | 0.57 to 2.00 | .83 | 1.06 | 0.67 to 1.67 | .811 |
| LN status | ||||||
| N0 | 1 | 1 | ||||
| N1 (one to three positive) | 3.54 | 1.95 to 6.40 | < .001 | 2.64 | 1.69 to 4.14 | < .001 |
| N2-N3 (> three positive) | 8.67 | 4.33 to 17.38 | < .001 | 6.48 | 3.83 to 10.94 | < .001 |
| LVI | ||||||
| Negative | 1 | 1 | ||||
| Positive | 2.08 | 1.23 to 3.51 | .0062 | 1.86 | 1.27 to 2.71 | < .001 |
| Treatment | ||||||
| Locoregional therapy | ||||||
| BCT | 1 | 1 | ||||
| MRM | 3.44 | 2.04 to 5.80 | < .001 | 1.31 | 0.81 to 1.92 | .3267 |
| MRM + RT | 0.72 | 0.36 to 1.43 | .34 | 0.87 | 0.55 to 1.40 | .5744 |
| Adjuvant chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.39 | 0.24 to 0.66 | < .001 | 0.29 | 0.20 to 0.42 | < .001 |
Abbreviations: BCT, breast-conserving therapy; DM, distant metastasis; HR, hazard ratio; LN, lymph node; LRR, locoregional recurrence; MRM, modified radical mastectomy; OS, overall survival; RT, radiation therapy; TNBC, triple-negative breast cancer.
In addition, we found that LN positivity (HR, 3.54; 95% CI, 1.95 to 6.40; P < .001 and HR, 8.67; 95% CI, 4.33 to 17.38; P < .001 for N1 and N2/3, respectively) and presence of LVI (HR, 2.08; 95% CI, 1.23 to 3.51; P =.0062) were associated with increased risk of LRR. Adjuvant chemotherapy was associated with decreased risk of LRR (HR, 0.39; 95% CI, 0.24 to 0.66; P < .001; Table 3). Margins were not included in this analysis because all patients had clear margins (guidelines: > 3 mm for in situ and invasive component) before starting their adjuvant treatment. Tumor size (T2 and T3 v T1), LN positivity (N1 and N2/3 v N0), and LVI were independent prognostic variables associated with poor OS. Adjuvant chemotherapy was significantly associated with improvement of OS (HR, 0.29; 95% CI, 0.20 to 0.42; P < .001; Table 3), as previously reported.25 However, locoregional management was not an independent prognostic factor affecting OS.
LRR in T1-2N0 Treated With MRM Without RT Compared With BCT
We investigated risk of LRR in patients with T1-2N0 tumors treated with MRM without adjuvant RT compared with those receiving BCT. As per National Comprehensive Cancer Network guidelines, patients with T1-2N0 TNBC tumors treated with MRM were not offered adjuvant RT.26 In our population, 468 patients with T1-2N0 tumors were treated with BCT (n=233) or MRM without RT (n=235). These two groups displayed similar clinicopathologic features (Table 4). Five-year LRR-free survival for T1-2N0 was 96% and 90% in the BCT and MRM groups, respectively (P =.022). There was no significant difference in OS between these groups (Appendix Fig A1, online only). In univariate analysis, the rate of LRR was significantly higher in the MRM compared with the BCT group (HR, 2.52; 95% CI, 1.11 to 5.72; P =.027; Fig 2A; Appendix Table A1, online only). The rate of DM was not significantly different between BCT and MRM groups (Appendix Table A1). In multivariate Cox regression analysis, MRM without RT was the only independent prognostic factor associated with increased LRR in patients with TNBC compared with BCT (HR, 2.53; 95% CI, 1.12 to 5.75; P =.0264; Appendix Table A2, online only). However, adjuvant chemotherapy was not associated with decreased risk of LRR (HR, 0.51; 95% CI, 0.24 to 1.06; P =.07; Appendix Table A2). Locoregional management (MRM v BCT) was not an independent prognostic factor for OS. However, use of adjuvant chemotherapy was significantly associated with improvement of OS (HR, 0.34; 95% CI, 0.19 to 0.60; P < .001; Appendix Table A2).
Table 4.
Patient and Treatment Characteristics in TNBC T1-2N0 Tumors Stratified by BCT and MRM
| Characteristic | BCT |
MRM |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Total patients | 233 | 49.8 | 235 | 50.2 | |
| Age at diagnosis, years | .20 | ||||
| Median | 53 | 55 | |||
| Range | 26-83 | 27-89 | |||
| Tumor grade | .72 | ||||
| 1-2 | 42 | 18.0 | 47 | 20.0 | |
| 3 | 191 | 82.0 | 188 | 80.0 | |
| LVI | .73 | ||||
| Negative | 186 | 80.0 | 183 | 78.0 | |
| Positive | 47 | 20.0 | 52 | 22.0 | |
| Adjuvant chemotherapy | .90 | ||||
| No | 152 | 65.0 | 155 | 66.0 | |
| Yes | 81 | 35.0 | 80 | 34.0 | |
Abbreviations: BCT, breast-conserving therapy; MRM, modified radical mastectomy; LVI, lymph vascular invasion; TNBC, triple-negative breast cancer.
Fig 2.
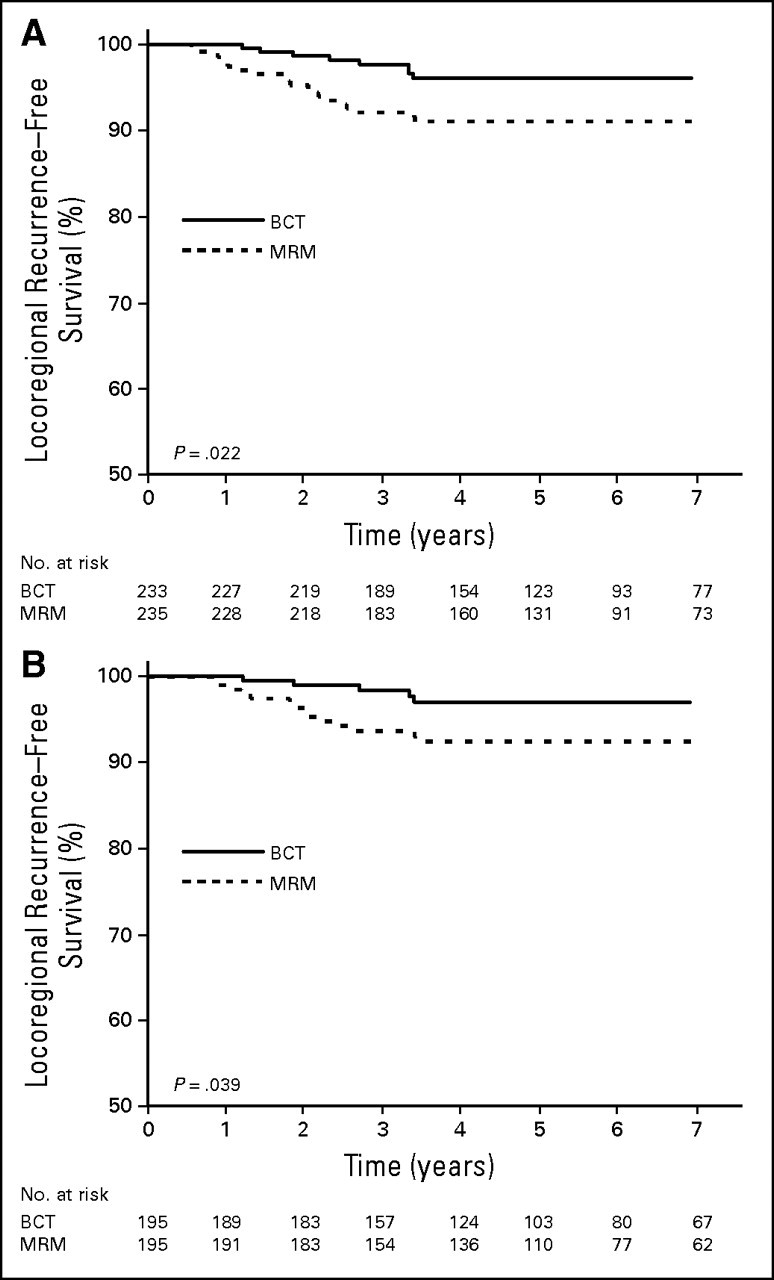
Locoregional recurrence–free survival in triple-negative breast cancer T1-2N0 treated with breast-conserving therapy (BCT) and modified radical mastectomy (MRM) for (A) unmatched and (B) matched data sets.
Next, we created a matched-pair data set in the T1-2N0 group. Patients without LN involvement (N0) were matched based on tumor size (T1 and T2). Of the 468 patients with T1-2N0 tumors, 195 matched pairs were stratified either by BCT or MRM. A multivariate Cox regression analysis was conducted on this matched pair data set. Locoregional treatment (BCT v MRM) was the primary prognostic variable. Adjuvant chemotherapy and LVI were included in the Cox model as covariates. This multivariate analysis confirmed that MRM was the only independent prognostic factor associated with increased risk of LRR (HR, 2.82; 95% CI, 1.04 to 7.82; P =.04; Fig 2B).
DISCUSSION
Risk of LRR with respect to locoregional management (BCT v MRM with and without RT) has been scarcely investigated in TNBC. Current guidelines recommend adjuvant RT after MRM based on tumor size and LN status, without specific consideration of biologic subtype.26 Although some studies have suggested that T1-2N0 patients may benefit from adjuvant RT after MRM,27,28 this treatment is offered only to patients with LN-positive or T3N0 tumors.26 In our study, MRM without RT was the only independent prognostic factor associated with increased risk of LRR in patients with T1-2N0 TNBC compared with BCT.
In accordance with this study, Kaplan et al29 reported that although patients with TNBC with T1N0 tumors are currently treated as a low-risk category with respect to LRR, they have greater risk of distant relapse than hormone receptor–positive/HER2-negative patients and should rather be treated with adjuvant chemotherapy. Interestingly, growing evidence from the current study and others30 shows that TNBC subtype is associated with lower incidence of axillary LN involvement. Furthermore, recent studies have shown that tumor size is an unreliable predictor of LN metastasis.31 The uncoupling between tumor size and LN status and increased risk of LRR for patients with TNBC treated with MRM compared with BCT suggest that these prognostic factors should not be considered as the only determinants of locoregional treatment decisions after MRM. Taken together, our findings emphasize that the current guidelines should take into account the intrinsic risk associated with this biologic subtype. Hence, the benefit of adjuvant RT after MRM in T1-2N0 TNBC should be further investigated in prospective studies.
Despite an absolute reduction of LRR risk by 6% in T1-2N0 TNBC treated with BCT compared with MRM, there was no significant difference in OS between the BCT and MRM groups. These results are not surprising, because follow-up for the current cohort was short to evaluate the impact of LRR reduction on OS. Indeed, the Early Breast Cancer Trialists’ Collaborative Group3 reported that reduction of 20% in risk of LRR at 5 years was associated with a 5.2% improvement in survival at 15 years.
Compared with other biologic subtypes, TNBC tumors exhibit high proliferative potential and are presumed to be clinically radioresistant.8 In the Danish breast cancer study,8 outcome in 152 patients with TNBC who underwent MRM was associated with increased risk of LRR. For this high-risk LN-positive cohort, the authors reported significant reduction of LRR after RT in patients with TNBC treated with MRM compared with patients who did not receive RT after MRM. However, the extent of LRR reduction was significantly smaller in TNBC compared with hormone receptor–positive/HER2-negative subtype. These results highlight the highly proliferative and aggressive behavior of TNBC subtype, which is not necessarily a radioresistant phenotype.
Although proliferation is a key determinant for LRR in breast cancer, this biologic feature has not been well documented in TNBC cell lines or tumors exposed to RT. Ionizing radiation reduced significantly the rate of cell proliferation in TNBC cell lines, despite their higher cell proliferation rate compared with hormone receptor–positive/HER2-negative cell lines (unpublished data). These findings warrant further investigation to understand the biologic response (ie, proliferation, invasion, and metastasis) of TNBC tumors to RT in vivo, which may account for the potential benefit of RT in reducing LRR.
The strengths of our study include the comprehensive nature of the registry database with patient characteristics, treatment, and complete ascertainment of patient status at regular follow-up intervals. As reported in our previous studies,18,19 hormone receptor and HER2 testing, treatment, and follow-up of patients are all centralized in our institution within the auspices of a province-wide cancer care system. Because of the size of this population, we could specifically address the issue of LRR risk associated with locoregional management (BCT v MRM) in a relatively less common biologic subtype of breast cancer. On the other hand, we acknowledge the limitations of the present study, including its retrospective nature. Type of adjuvant chemotherapy was at the discretion of the oncologist and in general aligned with National Comprehensive Cancer Network guidelines, which reflects the population-based nature of our study.
In sum, our study suggests that patients with T1-2N0 TNBC treated with MRM without RT have worse outcome with significant increased risk of LRR compared with those treated with BCT. These findings should have direct implications for locoregional RT after MRM. Although contributing to the evolving concept of biologic subtype and associated risk of LRR, our study requires further validation from prospective clinical trials addressing the issue of locoregional management and risk of LRR specifically in TNBC, which may lead to tailoring of locoregional treatment based on risk of LRR in TNBC.
Supplementary Material
Acknowledgment
We thank the members of the Alberta Cancer Registry for the identification of this population of patients with breast cancer and the technicians from the Department of Laboratory Medicine and Pathology for their support in immunohistochemistry.
Appendix
Table A1.
Locoregional and Distant Relapse in TNBC T1-2N0 Tumors Stratified by Locoregional Treatment
| Locoregional Treatment | BCT (n=233)* |
MRM (n=235) |
||||
|---|---|---|---|---|---|---|
| No. | HR | No. | HR | 95% CI | P | |
| LRR | 8 | 1 | 20 | 2.52 | 1.11 to 5.72 | .027 |
| Breast/chest wall | 3 | 1 | 14 | 4.7 | 1.35 to 16.35 | .015 |
| LN | 6 | 1 | 9 | 1.51 | 0.54 to 4.25 | .433 |
| DM | 15 | 1 | 17 | 1.17 | 0.58 to 2.35 | .655 |
| Organ (lung, liver, brain) | 12 | 1 | 10 | 0.87 | 0.38 to 2.03 | .75 |
| Bone | 5 | 1 | 6 | 1.33 | 0.40 to 4.43 | .63 |
| Skin/other | 5 | 1 | 8 | 1.60 | 0.52 to 4.88 | .41 |
Abbreviations: BCT, breast-conserving therapy; DM, distant metastasis; HR, hazard ratio; LN, lymph node; LRR, locoregional recurrence; MRM, modified radical mastectomy; TNBC, triple-negative breast cancer.
Reference group.
Table A2.
Multivariate Analysis of Predictors for LRR and OS in T1-2N0 Group Treated With MRM Compared With BCT
| Variable | LRR |
OS |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Tumor grade | ||||||
| 1-2 | 1 | 1 | ||||
| 3 | 1.13 | 0.43 to 2.96 | .79 | 0.82 | 0.42 to 1.6 | .56 |
| LVI | ||||||
| Negative | 1 | 1 | ||||
| Positive | 1.17 | 0.48 to 2.87 | .73 | 1.9 | 1.16 to 4.08 | .0155 |
| Locoregional therapy | ||||||
| BCT | 1 | 1 | ||||
| MRM | 2.53 | 1.12 to 5.75 | .0264 | 1.13 | 0.63 to 2.02 | .68 |
| Adjuvant chemotherapy | ||||||
| No | 1 | 1 | ||||
| Yes | 0.51 | 0.24 to 1.06 | .07 | 0.34 | 0.19 to 0.60 | < .001 |
Abbreviations: BCT, breast-conserving therapy; HR, hazard ratio; LRR, locoregional recurrence; LVI, lymphovascular invasion; MRM, modified radical mastectomy; OS, overall survival; RT, radiation therapy.
Fig A1.
Overall survival in triple-negative breast cancer with T1-2N0 tumors treated with breast-conserving therapy (BCT) and modified radical mastectomy (MRM).
Footnotes
See accompanying editorial on page 2841
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTSOF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Bassam S. Abdulkarim, Siham Sabri
Administrative support: Bassam S. Abdulkarim
Collection and assembly of data: Bassam S. Abdulkarim, Julie Cuartero, John Hanson
Data analysis and interpretation: Bassam S. Abdulkarim, Julie Cuartero, John Hanson, Jean Deschênes, David Lesniak, Siham Sabri
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 4.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PL, Taghian AG, Katz MS, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 8.Kyndi M, Sørensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: The Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 9.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 10.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: Primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura R, Arima N. Is triple negative a prognostic factor in breast cancer? Breast Cancer. 2008;15:303–308. doi: 10.1007/s12282-008-0042-3. [DOI] [PubMed] [Google Scholar]
- 12.Bauer KR, Brown M, Cress RD, et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: A population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 13.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 14.Freedman GM, Anderson PR, Li T, et al. Locoregional recurrence of triple-negative breast cancer after breast-conserving surgery and radiation. Cancer. 2009;115:946–951. doi: 10.1002/cncr.24094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voduc KD, Cheang MC, Tyldesley S, et al. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 16.Gabos Z, Thoms J, Ghosh S, et al. The association between biological subtype and locoregional recurrence in newly diagnosed breast cancer. Breast Cancer Res Treat. 2010;124:187–194. doi: 10.1007/s10549-010-1135-1. [DOI] [PubMed] [Google Scholar]
- 17.Albert JM, Gonzalez-Angulo AM, Guray M, et al. Estrogen/progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,bN0 breast cancer. Int J Radiat Oncol Biol Phys. 77:1296–1302. doi: 10.1016/j.ijrobp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Lesniak D, Xu Y, Deschênes J, et al. Beta1-integrin circumvents the antiproliferative effects of trastuzumab in human epidermal growth factor receptor-2-positive breast cancer. Cancer Res. 2009;69:8620–8628. doi: 10.1158/0008-5472.CAN-09-1591. [DOI] [PubMed] [Google Scholar]
- 19.Gabos Z, Sinha R, Hanson J, et al. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006;24:5658–5663. doi: 10.1200/JCO.2006.07.0250. [DOI] [PubMed] [Google Scholar]
- 20.Levine M. Clinical practice guidelines for the care and treatment of breast cancer: Adjuvant systemic therapy for node-negative breast cancer (summary of the 2001 update) CMAJ. 2001;164:644–646. [PMC free article] [PubMed] [Google Scholar]
- 21.Truong PT, Olivotto IA, Whelan TJ, et al. Clinical practice guidelines for the care and treatment of breast cancer: 16. Locoregional post-mastectomy radiotherapy. CMAJ. 2004;170:1263–1273. doi: 10.1503/cmaj.1031000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whelan T, Olivotto I, Levine M. Clinical practice guidelines for the care and treatment of breast cancer: Breast radiotherapy after breast-conserving surgery (summary of the 2003 update) CMAJ. 2003;168:437–439. [PMC free article] [PubMed] [Google Scholar]
- 23.Shenkier T, Weir L, Levine M, et al. Clinical practice guidelines for the care and treatment of breast cancer: 15. Treatment for women with stage III or locally advanced breast cancer. CMAJ. 2004;170:983–994. doi: 10.1503/cmaj.1030944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reis-Filho JS, Tutt AN. Triple negative tumours: A critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 25.Colleoni M, Cole BF, Viale G, et al. Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple-negative, node-negative breast cancer: Results from two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Clin Oncol. 2010;28:2966–2973. doi: 10.1200/JCO.2009.25.9549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carlson RW, Allred DC, Anderson BO, et al. Breast cancer: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2009;7:122–192. doi: 10.6004/jnccn.2009.0012. [DOI] [PubMed] [Google Scholar]
- 27.Jagsi R, Raad RA, Goldberg S, et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: Implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62:1035–1039. doi: 10.1016/j.ijrobp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Truong PT, Lesperance M, Culhaci A, et al. Patient subsets with T1-T2, node-negative breast cancer at high locoregional recurrence risk after mastectomy. Int J Radiat Oncol Biol Phys. 2005;62:175–182. doi: 10.1016/j.ijrobp.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan HG, Malmgren JA, Atwood M. T1N0 triple negative breast cancer: Risk of recurrence and adjuvant chemotherapy. Breast J. 2009;15:454–460. doi: 10.1111/j.1524-4741.2009.00789.x. [DOI] [PubMed] [Google Scholar]
- 30.Crabb SJ, Cheang MC, Leung S, et al. Basal breast cancer molecular subtype predicts for lower incidence of axillary lymph node metastases in primary breast cancer. Clin Breast Cancer. 2008;8:249–256. doi: 10.3816/CBC.2008.n.028. [DOI] [PubMed] [Google Scholar]
- 31.Foulkes WD, Grainge MJ, Rakha EA, et al. Tumor size is an unreliable predictor of prognosis in basal-like breast cancers and does not correlate closely with lymph node status. Breast Cancer Res Treat. 2009;117:199–204. doi: 10.1007/s10549-008-0102-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.