Abstract
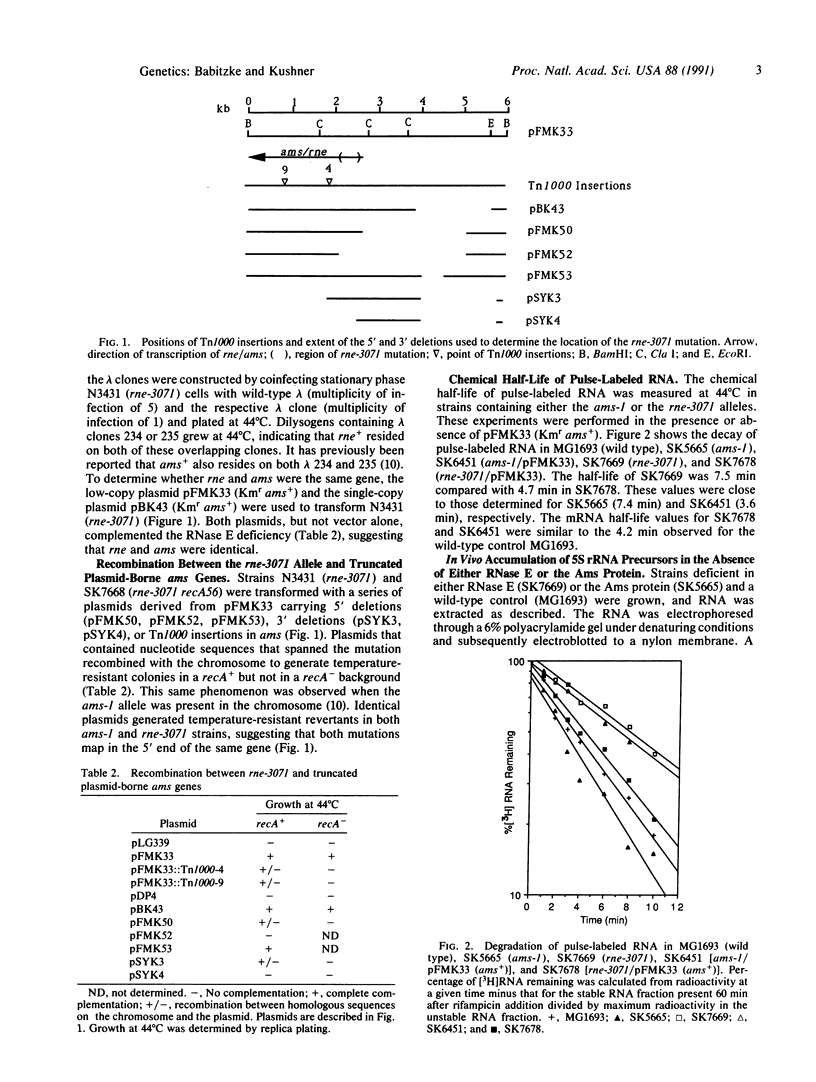
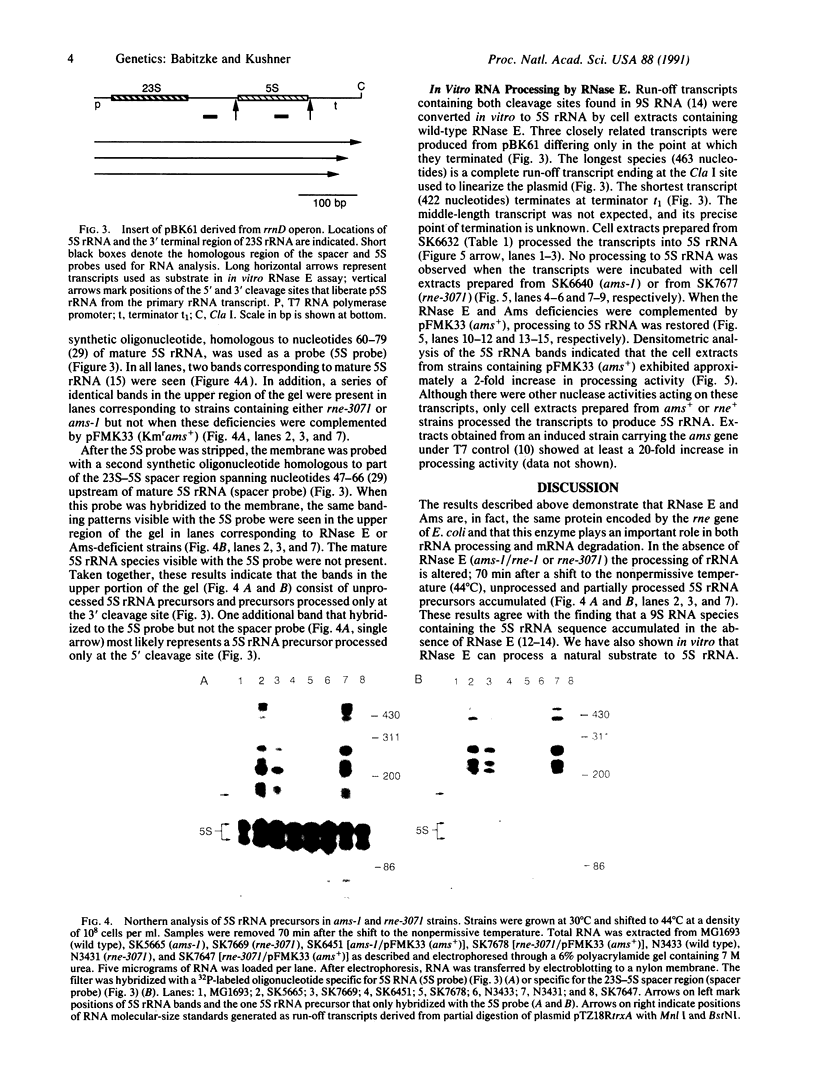
The in vitro and in vivo analysis of the ribonuclease E-deficient (rne-) and the altered mRNA stability protein-deficient (ams-) strains of Escherichia coli has demonstrated that they carry mutations in the same structural gene. Strains encoding either thermolabile RNase E (rne-3071) or Ams protein (ams-1) are defective in both rRNA processing and mRNA turnover. Immediately after a shift to the nonpermissive temperature, the chemical decay rate of bulk mRNA is slowed 2- to 3-fold, and within 70 min, precursors to 5S rRNA begin to accumulate. In addition, all of the phenotypes associated with either the rne-3071 or the ams-1 alleles were complemented by a recombinant plasmid carrying ams+. When taken together with previous genetic studies, these results suggest that the role of ribonuclease E in mRNA turnover involves endonucleolytic cleavages at the proposed ACAG(A/U)AUUUG consensus sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D. Isolation, genetic mapping and some characterization of a mutation in Escherichia coli that affects the processing of ribonuleic acid. Genetics. 1978 Dec;90(4):659–671. doi: 10.1093/genetics/90.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Lassar A. B. A conditional lethal mutant of Escherichia coli which affects the processing of ribosomal RNA. J Biol Chem. 1978 Mar 10;253(5):1738–1742. [PubMed] [Google Scholar]
- Arraiano C. M., Yancey S. D., Kushner S. R. Stabilization of discrete mRNA breakdown products in ams pnp rnb multiple mutants of Escherichia coli K-12. J Bacteriol. 1988 Oct;170(10):4625–4633. doi: 10.1128/jb.170.10.4625-4633.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belasco J. G., Higgins C. F. Mechanisms of mRNA decay in bacteria: a perspective. Gene. 1988 Dec 10;72(1-2):15–23. doi: 10.1016/0378-1119(88)90123-0. [DOI] [PubMed] [Google Scholar]
- Claverie-Martin F., Diaz-Torres M. R., Yancey S. D., Kushner S. R. Cloning of the altered mRNA stability (ams) gene of Escherichia coli K-12. J Bacteriol. 1989 Oct;171(10):5479–5486. doi: 10.1128/jb.171.10.5479-5486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Jan;83(1):120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G. L., Holmes W. M. The distal end of the ribosomal RNA operon rrnD of Escherichia coli contains a tRNA1thr gene, two 5s rRNA genes and a transcription terminator. Nucleic Acids Res. 1980 Sep 11;8(17):3793–3807. doi: 10.1093/nar/8.17.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra C. C., Prasher D., Kushner S. R. Physical and biochemical analysis of the cloned recB and recC genes of Escherichia coli K-12. J Bacteriol. 1984 Jan;157(1):21–27. doi: 10.1128/jb.157.1.21-27.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford R. M., Holmes W. M. Fusion of the tandem Escherichia coli rrnA promoters to a transcription termination signal from the end of rrnD. J Mol Biol. 1983 Aug 15;168(3):557–561. doi: 10.1016/s0022-2836(83)80301-5. [DOI] [PubMed] [Google Scholar]
- Ghora B. K., Apirion D. Structural analysis and in vitro processing to p5 rRNA of a 9S RNA molecule isolated from an rne mutant of E. coli. Cell. 1978 Nov;15(3):1055–1066. doi: 10.1016/0092-8674(78)90289-1. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Krzyzek R., Rogers P. Arginine control of transcription of argECBH messenger ribonucleic acid in Escherichia coli. J Bacteriol. 1972 Jun;110(3):945–954. doi: 10.1128/jb.110.3.945-954.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra T. K., Apirion D. RNase E, an RNA processing enzyme from Escherichia coli. J Biol Chem. 1979 Nov 10;254(21):11154–11159. [PubMed] [Google Scholar]
- Mudd E. A., Carpousis A. J., Krisch H. M. Escherichia coli RNase E has a role in the decay of bacteriophage T4 mRNA. Genes Dev. 1990 May;4(5):873–881. doi: 10.1101/gad.4.5.873. [DOI] [PubMed] [Google Scholar]
- Mudd E. A., Prentki P., Belin D., Krisch H. M. Processing of unstable bacteriophage T4 gene 32 mRNAs into a stable species requires Escherichia coli ribonuclease E. EMBO J. 1988 Nov;7(11):3601–3607. doi: 10.1002/j.1460-2075.1988.tb03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Kuwano M. A conditional lethal mutation in an Escherichia coli strain with a longer chemical lifetime of messenger RNA. J Mol Biol. 1979 Apr 15;129(3):343–357. doi: 10.1016/0022-2836(79)90500-x. [DOI] [PubMed] [Google Scholar]
- Ono M., Kuwano M. Chromosomal location of a gene for chemical longevity of messenger ribonculeic acid in a temperature-sensitive mutant of Escherichia coli. J Bacteriol. 1980 Apr;142(1):325–326. doi: 10.1128/jb.142.1.325-326.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Apirion D. Characterization of DNA from the rne gene of Escherichia coli: uniqueness of the rne DNA. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1361–1367. doi: 10.1016/s0006-291x(82)80148-4. [DOI] [PubMed] [Google Scholar]
- SPAHR P. F. PURIFICATION AND PROPERTIES OF RIBONUCLEASE II FROM ESCHERICHIA COLI. J Biol Chem. 1964 Nov;239:3716–3726. [PubMed] [Google Scholar]
- Singer M., Baker T. A., Schnitzler G., Deischel S. M., Goel M., Dove W., Jaacks K. J., Grossman A. D., Erickson J. W., Gross C. A. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol Rev. 1989 Mar;53(1):1–24. doi: 10.1128/mr.53.1.1-24.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeberényi J., Roy M. K., Apirion D. 7 S RNA: a single site substrate for the RNA processing enzyme ribonuclease E of Escherichia coli. Biochim Biophys Acta. 1983 Aug 2;740(3):282–290. doi: 10.1016/0167-4781(83)90137-9. [DOI] [PubMed] [Google Scholar]
- Tomcsányi T., Apirion D. Processing enzyme ribonuclease E specifically cleaves RNA I. An inhibitor of primer formation in plasmid DNA synthesis. J Mol Biol. 1985 Oct 20;185(4):713–720. doi: 10.1016/0022-2836(85)90056-7. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Mount D. W. Genetic analysis of recombination-deficient mutants of Escherichia coli K-12 carrying rec mutations cotransducible with thyA. J Bacteriol. 1969 Nov;100(2):923–934. doi: 10.1128/jb.100.2.923-934.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. G., Rogers P. Expression of arg genes of Escherichia coli during arginine limitation dependent upon stringent control of translation. J Bacteriol. 1987 Apr;169(4):1644–1650. doi: 10.1128/jb.169.4.1644-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]






