Abstract
Introduction
Our aim was to evaluate a community-based exercise (CBE) intervention with the goal of reducing disability and enhancing health for community-dwelling people living with HIV (PLWH).
Methods and analysis
We will use a mixed-methods implementation science study design, including a prospective longitudinal interrupted time series study, to evaluate a CBE intervention with PLWH in Toronto, Canada. We will recruit PLWH who consider themselves medically stable and safe to participate in exercise. In the baseline phase (0–8 months), participants will be monitored bimonthly. In the intervention phase (8–14 months), participants will take part in a 24-week CBE intervention that includes aerobic, resistance, balance and flexibility exercise at the YMCA 3 times per week, with weekly supervision by a fitness instructor, and monthly educational sessions. In the follow-up phase (14–22 months), participants will be encouraged to continue to engage in unsupervised exercise 3 times per week. Quantitative assessment: We will assess cardiopulmonary fitness, strength, weight, body composition and flexibility outcomes followed by the administration of self-reported questionnaires to assess disability and contextual factor outcomes (coping, mastery, stigma, social support) bimonthly. We will use time series regression analysis to determine the level and trend of outcomes across each phase in relation to the intervention. Qualitative assessment: We will conduct a series of face-to-face interviews with a subsample of participants and recreation providers at initiation, midpoint and completion of the 24-week CBE intervention. We will explore experiences and anticipated benefits with exercise, perceived impact of CBE for PLWH and the strengths and challenges of implementing a CBE intervention. Interviews will be audio recorded and analysed thematically.
Ethics and dissemination
Protocol approved by the University of Toronto HIV/AIDS Research Ethics Board. Knowledge translation will occur with stakeholders in the form of presentations and publications in open access peer-reviewed journals.
Trial registration number
NCT02794415; Pre-results.
Keywords: REHABILITATION MEDICINE, Exercise, community-based exercise, physical activity
Strengths and limitations of this study.
Strengths include our evaluation of the process and outcomes of a community-based exercise (CBE) intervention for adults living with HIV using an Implementation Science approach with the RE-AIM (Reach-Evaluation-Adoption-Implementation-Maintenance) Framework. Partnerships with the YMCA and HIV community will facilitate the successful translation of CBE into the community.
The CBE intervention, which is based on self-management and health promotion approaches to engaging people living with HIV in exercise, is less costly in contrast to highly structured, prescriptive protocols where individuals may have less input into their type of exercise activity.
The interrupted time series design, which involves assessing outcomes at baseline (pretesting phase), during (intervention phase) and after the CBE intervention (post-testing phase), will enable us to evaluate the short-term and long-term translation and sustainability of CBE with adults living with HIV.
Results will lead to the first known HIV-specific CBE intervention in Canada evaluated for effectiveness and successful translation with the HIV community.
Potential challenges include recruitment and retention of participants across the 22-month study and potential burden of assessments. Ongoing collaboration with community partners will be critical throughout.
Introduction
As people living with HIV (PLWH) in resource-rich countries age, they are experiencing the health-related consequences of HIV.1–7 In addition to morbidity resulting from HIV and associated treatments, many PLWH experience multimorbidity from cardiovascular disease, diabetes,8 bone and joint disorders,9 10 neurocognitive disorders11 and non-AIDS-defining cancers.12–14 These health-related consequences may be characterised as disability, including the physical, cognitive, mental and emotional symptoms and impairments, difficulties carrying out daily activities, uncertainty or worrying about future health and challenges to social inclusion for PLWH.15
As the burden of disability increases, so does the role for rehabilitation in the context of HIV.16 Rehabilitation is broadly defined as any service or health intervention that may address or prevent disability.16 Rehabilitation, such as physical therapy and occupational therapy, can mitigate disability associated with HIV and multimorbidity, such as fatigue, pain, cognitive impairments, body composition changes and employment challenges. Rehabilitation thus has the potential to improve health and quality of life for PLWH. However, access to rehabilitation services can be limited for those without private insurance or the ability to pay out of pocket for services.17 With few rehabilitation professionals adequately serving PLWH,18 individuals may use self-management strategies to deal with their daily health challenges.19
Exercise is a rehabilitation intervention and self-management strategy that can address disability and improve or sustain the health of PLWH.20 Exercise is defined as any physical activity involving bodily movement produced by skeletal muscles that requires energy expenditure including (but not limited to) aerobic, resistance, flexibility and neuromotor activity that go beyond activities of daily living to improve and maintain physical fitness and health.21 22 Although systematic reviews indicate that exercise is safe and can lead to benefits in cardiopulmonary fitness, strength, weight and body composition, and psychological status for PLWH,23–27 the included studies focused on highly supervised interventions by physical therapists and exercise physiologists that can be costly and unsustainable for PLWH. Few PLWH engage in regular exercise or achieve recommended levels of physical activity.28–31 Hence, it is essential to consider exercise interventions that are accessible and practical for PLWH to sustain over the long term.
Community-based exercise (CBE) is one approach for enhancing the health of PLWH. CBE involves a group of individuals exercising under the supervision of a health or fitness instructor with the goal of promoting regular exercise in the community.32–34 CBE can foster social interaction, support and encouragement to exercise,32–34 and promote emotional, cognitive and behavioural self-management strategies to help PLWH independently manage health challenges associated with chronic conditions.33 35 However, the impact of CBE when translated into the HIV community setting and its sustainability over the long term is unknown.
Authors of a qualitative study identified factors to consider in the development and implementation of a CBE programme for people who experience the complexity of HIV and multimorbidity.36 Building on this work, we developed and piloted a 4-month CBE intervention with 28 PLWH at the Toronto YMCA. Exercise prescription included a combination of aerobic, resistive, flexibility and balance exercise three times a week, supervision of exercise once weekly and monthly group education sessions.37 Fifteen (54%) participants attended ≥40% of the weekly supervised sessions. Reasons for not attending the exercise sessions included challenges with scheduling, health status and lack of motivation. The CBE intervention was positively received by participants who completed the study.37 These results directly informed the refinement of the CBE intervention, recruitment strategy and data collection methods for this study protocol.
Our aim is to evaluate a CBE intervention for PLWH within the community with the goal of reducing disability and enhancing health (cardiopulmonary, strength, weight and body composition, flexibility) and intrinsic and extrinsic factor outcomes (social support, stigma, mastery, coping) for PLWH.
We will use the RE-AIM Framework to evaluate the CBE intervention. Derived from the field of Implementation Science, the RE-AIM Framework emphasises the importance of considering multiple aspects of an intervention beyond clinical efficacy.38 The Framework includes criteria to evaluate the impact and translation of an intervention at individual and organisational levels in order to promote uptake, transferability and ultimately enhance the public health impact of health promotion interventions.39 Specific study objectives are: (1) to determine the extent to which adults with HIV participate in a CBE intervention (proportion of eligible individuals who consent, initiate and complete the intervention); (2) to assess the effect of a CBE intervention on (i) disability and health outcomes (physical (cardiopulmonary fitness, strength, weight, body composition and anthropometrics, flexibility), mental–emotional, cognitive health, daily function, social inclusion) and (ii) intrinsic and extrinsic factor outcomes (social support, stigma, mastery, coping) for PLWH; (3) to assess engagement in CBE for adults with HIV over time (adherence, level of physical activity); and (4) to evaluate the process (strengths and challenges; feasibility; accessibility, long-term sustainability) of implementing a CBE intervention within the community from the perspective of recreation providers (fitness instructors; managers) and PLWH.
Methods
We will conduct a prospective longitudinal study using mixed methods to evaluate a CBE intervention with PLWH.
Study design
We will use an interrupted time series (ITS) design in combination with qualitative interviews to assess outcomes at baseline (pretesting phase), during the CBE intervention (intervention phase) and after the CBE intervention (post-testing phase) to evaluate the short-term and long-term effect of CBE. In an ITS study design, ‘data are collected at multiple instances over time before and after an intervention (interruption) to detect whether the intervention has an effect significantly greater than the underlying secular trend’.40 We did not choose a randomised control trial given that the benefits of exercise are well known; rather what remains unclear is the long-term durability effect of exercise over time, and the optimal implementation of a CBE intervention for PLWH. Thus, a single-group ITS study design will allow us to evaluate how community-based interventions are translated into, and influenced by, a real-world setting.41 42 As per the RE-AIM Framework, we are particularly interested in assessing the long-term impact of exercise, the ability to integrate CBE into the community and the ability of PLWH to integrate exercise into their daily lives over time.43 Using qualitative methods in combination with ITS to evaluate effectiveness will enable us to determine the impact of the intervention as perceived by PLWH and provide insight into the personal and environmental factors that may influence the benefits of exercise and its implementation into the HIV community.44 See online supplementary file 1 for the WHO Trial Registration Data Set.
Supplemental-File_1.pdf (89.4KB, pdf)
Community partnerships
This study represents a community–academic–clinical partnership. Collaborating partners include community-based organisations, a specialty hospital (Casey House) and the Central Toronto YMCA. The study will be guided by a Community Advisory Committee comprised of PLWH, representatives from community-based organisations, recreation fitness providers and policy stakeholders.
Participants and recruitment
We will include adults living with HIV (18 years and older) who consider themselves medically stable and safe to participate in exercise as determined by the self-administered Physical Activity Readiness Questionnaire (PAR-Q+).45 To evaluate the process of CBE implementation, we will include recreation providers (managers and fitness instructors) from community organisations who engage in the CBE implementation (objective #4).
We will recruit adults living with HIV in Toronto through a combination of community-based organisations, realize (formally known as the Canadian Working Group on HIV and Rehabilitation (CWGHR)), a specialty hospital (Casey House) and the YMCA. Recruitment will occur via posters, brochures and recruitment cards placed at collaborating organisations and/or circulated to their members via email. Additional participants may be recruited via word of mouth. We will tailor our recruitment to ensure diversity of our study population with regards to gender, ethnocultural background, age and length of time since HIV diagnosis. To evaluate the process of CBE implementation, we will additionally recruit fitness instructors and managers involved in the CBE intervention.
Interested individuals will be asked to contact the research team by email or telephone to set up an ‘Initial CBE Study Screening Meeting’ with the Study Coordinator to discuss details of the study. During this meeting, individuals will complete the PAR-Q+45 and then review the eligibility criteria, including level of commitment, details of the study and the information letter and consent form.
CBE intervention
This 22-month study consists of an 8-month baseline phase, 6-month (24-week) exercise intervention at the Central Toronto YMCA and an 8-month postintervention phase.
Baseline (0–8 months)
Participants will be monitored bimonthly during the baseline (preintervention phase) for 32 weeks. This will serve as the ‘control’ phase for comparing the level and trend of outcomes during and postintervention in the ITS study design.
Intervention—exercise and self-management education (8–14 months)
The HIV CBE intervention is a 24-week exercise programme at the Central Toronto YMCA. The intervention is derived from high-quality research evidence on the effectiveness of exercise with PLWH, recommendations from the American College of Sports Medicine,21 qualitative consultation with community (PLWH and fitness instructors),36 pilot work37 and existing HIV rehabilitation services in the UK,46 collectively adapted for the Canadian context. We chose a 6-month CBE duration because it aligns with transtheoretical model evidence on the stages of behaviour change whereby the ‘action’ of practicing a new behaviour (exercise) lasts up to 24 weeks followed by the ‘maintenance’ stage during which commitment to sustaining the new behaviour (self-monitored exercise) is solidified.47 48 This intervention is based on a chronic illness and self-management approach to engaging PLWH in exercise.49 The intervention will include: framing exercise in the broader context of health through educational sessions; providing participants with the opportunity to choose activities of interest to enhance engagement and adherence to exercise; problem-solving support where participants will have access to staff to assist with goal setting and overcoming barriers to exercise; and communication among participants, fitness instructors and study staff to ensure participants receive feedback on their progress and goal attainment.50
Prior to the intervention, fitness instructors will take part in realize's (formally CWGHR's) online interprofessional learning course Rehabilitation in the Context of HIV, and an education session on HIV, rehabilitation and exercise for PLWH.50 Participants will meet one-on-one with a fitness instructor to assess their goals and establish an individualised exercise programme that will include a combination of aerobic, resistive, neuromotor and flexibility training.
Aerobic exercise will include physical activity 3 days/week (frequency) at 60–70% heart rate maximum (intensity) for at least 30 min (time) with a variety of types of activity based on participant choice (type). Resistance exercise will include strength training for each major muscle group ∼8–10 exercises (type) 3 days/week (frequency) at 60–70% 1 repetition maximum (intensity) with 10–12 repetitions each (time). Flexibility exercise will include stretching major muscle–tendon groups in a static stretch for 10–30 s each with two repetitions. Neuromotor exercise will include a range of activities focused on motor skills (eg, balance, agility, coordination and gait) for ∼20–30 min.21 To achieve these exercise elements, participants will engage in a combination of individual exercise (eg, weight circuit training, endurance treadmill walking/jogging, stationary bike, swimming) and group-based exercise in the form of a class (eg, circuit training, spinning, yoga, aerobics, dancing). The combination of group and individual exercise will ensure that the intervention will be tailored to the individual, while promoting efficacy and social support that come from group activity.51 Note that, in this study, we adopt a ‘guided adaptation’ approach whereby the intervention may be adapted based on the ongoing assessment of the needs and ability of participants by the fitness instructor.
Participants will attend exercise sessions for ∼1.5 hours, three times per week for 24 weeks. Sessions will be supervised weekly by a fitness instructor who will monitor participant progress, adjust exercise intensity, and complete a coaching log after each weekly session. Participants will also attend monthly group educational sessions co-led by a health or fitness professional and/or PLWH focused on topics related to self-management and healthy living with HIV.46 A variety of topics will be covered in six sessions, including stress management and relaxation, pain management, nutrition, fatigue management, smoking cessation, goal setting, confidence to self-manage, community services and supports and rehabilitation in the context of HIV.46 Throughout the intervention, participants will be asked to complete a weekly online exercise log to document their physical activity. The exercise log will be administered in the form of a secure web-based questionnaire.
Postintervention—self-monitored exercise (14–22 months)
Participants will be encouraged to continue unsupervised exercise three times per week. As per usual practice at the YMCA, a fitness instructor will be available to monitor participants monthly. Participants will be asked to continue completing the weekly online exercise log throughout to document the frequency, intensity, time and type of activity, thus allowing us to assess long-term engagement in exercise. Participants will also be provided with a wireless activity tracker (Fitbit) to self-monitor steps, distance and calories burned during both exercise phases (8–22 months).52 Participants will be asked to download their Fitbit data (steps, distance and calories) weekly to an individualised Fitbit account to measure physical activity of participants in the intervention and postintervention phases.
Research procedure
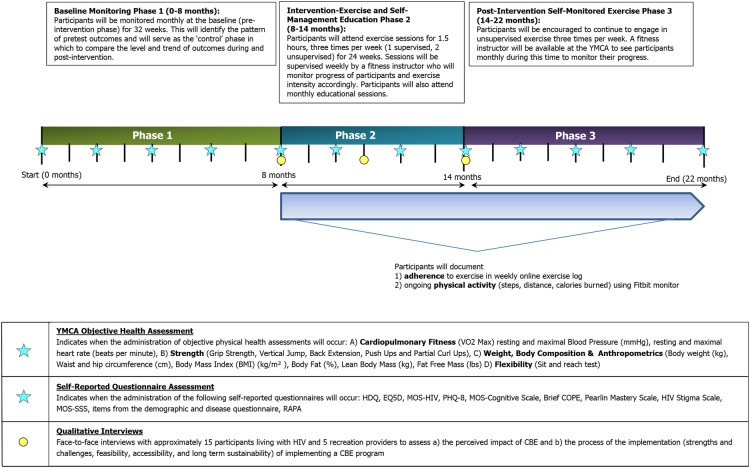
Participants will be followed up over the course of the intervention and assessments administered at 12 time points: baseline (0, 2, 4 and 6 months), intervention (8, 10, 12 and 14 months) and postintervention (16, 18, 20 and 22 months). Objective and self-reported questionnaire assessments will be completed within 1 week of each other. See figure 1 for an overview of the study timeline and assessments. We will evaluate the CBE intervention with PLWH in the community using the RE-AIM Framework.
Figure 1.
CBE study timeline of intervention and assessments (22 months). CBE, community-based exercise; EQ5D, European Quality of Life—5 Dimensions; HDQ, HIV Disability Questionnaire; MOS-HIV, Medical Outcomes Study HIV Questionnaire; MOS-SSS, Medical Outcomes Study Social Support Scale; PAR-Q, Physical Activity Readiness Questionnaire; PHQ, Patient Health Questionnaire; PLWH, people living with HIV; RAPA, Rapid Assessment of Physical Activity Questionnaire.
Objective 1 (Reach)—To determine the extent to which PLWH participate in the CBE intervention: We will measure the number of individuals who (1) are eligible but do not consent, (2) consent but do not initiate the CBE intervention, (3) initiate but do not complete the CBE intervention, (4) complete the CBE intervention but not self-monitored exercise and (5) complete the CBE intervention and self-monitored exercise. We will track the number of participants who withdraw, the week they withdraw and reasons for withdrawal. We will similarly monitor any participants lost to follow-up.
Objective 2 (Effectiveness)—To assess the effectiveness of the CBE intervention: We will use a combination of an ITS analysis and qualitative interviews. ITS analyses assess the effectiveness of an intervention by comparing the level (intercept) and the pattern of pretest responses (baseline), with a pattern of post-test responses (postintervention). An effect is demonstrated when there is a change in the level or slope (trend) of the interruption or post-treatment responses when compared with the pretest responses. We will assess disability and health outcomes bimonthly at baseline (0–8 months), during intervention (8–14 months) and postintervention (14–22 months) for a total of 12 time points (figure 1). We will use a questionnaire to ask participants whether they experienced any life events or illnesses since the last assessment that may have had an impact on their ability to engage in exercise. We will also conduct a series of face-to-face interviews with a subsample of 15 PLWH participants at initiation (8 months), midpoint (11 months) and completion (14 months) of the 24-week CBE intervention.
We will use the Episodic Disability Framework to inform the dimensions of health and disability against which to assess the impact of CBE, including dimensions of disability (physical, cognitive, mental and emotional symptoms and impairments, difficulties carrying out day-to-day activities, challenges to social inclusion and uncertainty about future health) and extrinsic and intrinsic contextual factors (social support, stigma, living strategies and personal attributes) that may exacerbate or alleviate dimensions of disability.15 53 Using this Framework to inform our quantitative and qualitative assessment of CBE will promote consideration of not only health and disability outcomes but also the influence that contextual factors may have on the effect of, and engagement in, exercise.
Part A—objective physical health assessment
Fitness staff at the YMCA will conduct the following objective assessments bimonthly for a total of 12 time points (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20 and 22 months).
Physical health symptoms and impairments
The Central Toronto YMCA Performance Centre provides comprehensive physical fitness assessment services that include (1) cardiopulmonary fitness (maximum oxygen consumption (VO2max), resting and maximal heart rate and blood pressure); (2) strength for upper and lower body (grip strength, vertical jump, back extension, pushups, partial curl ups); (3) weight, body composition and anthropometrics (weight, body mass index, body fat per cent, lean body mass, waist and hip circumference, fat free mass); and (4) flexibility (lower back, hamstrings).54 55 Fitness staff at the YMCA with certified fitness training experience will conduct the objective assessments. If a participant does not wish to or is unable to do the VO2max test, coaches will conduct either the Treadmill Walking Test or the One Mile Walk Test (suboptimal VO2max tests) as an alternative. The above assessment will take ∼1.5 hours.
VO2max will be considered the primary outcome for this study as it is a critical indicator for cardiovascular disease prevention, a common comorbidity among PLWH.56 57 (1) We hypothesise that there will be a significant change (p<0.05) in the level and trend of VO2max between the baseline and intervention phases suggesting an intervention effect. (2) We hypothesise that there will be a significant change in the trend of VO2max (decreased slope) between the intervention and postintervention phase suggesting a levelling off of the intervention effect in the postintervention (or self-monitoring phase).
Collectively the above outcomes were similarly assessed in the Cochrane Collaboration systematic reviews, but the impact of a less supervised longer duration intervention on these outcomes for PLWH is unknown. Assessing these outcomes will also enable us to interpret findings from our study in relation to the more heavily supervised exercise interventions included in the systematic reviews.23 24 We will not assess CD4 count or viral load, as they do not change with exercise.23 24
Part B—self-reported questionnaire assessment
We will administer self-report questionnaires bimonthly for a total of 12 time points (0, 2, 4, 6, 8, 10, 12, 14, 16, 18, 20 and 22 months) to assess disability and the impacts of contextual factors as indicated in the Episodic Disability Framework.15 53 We estimate that the questionnaire assessments will take ∼1.5 hours to complete at each time point.
Disability
HIV Disability Questionnaire (HDQ): The HDQ is a 69-item self-administered instrument developed to measure disability experienced by PLWH across six domains: physical, cognitive and mental–emotional health symptoms, difficulties with day-to-day activities, challenges to social inclusion and uncertainty.57 58 The HDQ has demonstrated sensibility, validity and reliability among PLWH;60–62 however, responsiveness and interpretability have yet to be assessed. Assessing these properties of the HDQ is a secondary aim of this research and beyond the focus of this protocol. We will also administer a generic disability questionnaire, the EuroQol-5D (EQ5D) five-level response option version, to evaluate the impact of the CBE intervention.63 The EQ5D is a health status questionnaire comprised of five domains (mobility, self-care, usual activities, pain and anxiety/depression). The EQ5D has demonstrated responsiveness and has been used to assess quality of life among PLWH receiving physical therapy.64–67
Quality of Life
Medical Outcomes Study HIV (MOS-HIV) Questionnaire: The MOS-HIV consists of 35 questions that assess the 10 dimensions of health: general health perceptions, pain, physical functioning, role functioning, social functioning, mental health, energy/fatigue, cognitive function, health distress and quality of life. This questionnaire has been used extensively and is reliable and valid for PLWH.66 67
Mental health symptoms and impairments
Patient Health Questionnaire (PHQ-8): The PHQ-8 is an 8-item measure of depression severity. Items are rated using a Likert-type scale from 0 to 3, with a total score range of 0–24.69 A score of 10 or greater is considered major depression and 20 or more is severe major depression. We chose to use the PHQ-8 because the ninth item in the PHQ-9 assesses suicidal or self-injurious ideation and is commonly omitted in studies where individuals administering the questionnaire are not trained mental health providers, which is the case in this study. The PHQ-8 has been used among PLWH and is comparable to the originally developed PHQ-9.70 71
Cognitive health symptoms and impairments
MOS Cognitive Functioning Scale includes four items with a 6-point ordinal scale that measures challenges across reasoning and problem solving, memory, attention, and concentration and thinking.68 The scale has been validated for use with PLWH.72
Intrinsic contextual factors
Living strategies will be measured using the following two instruments: The Brief COPE is a 28-item instrument, comprised of 14 scales with two items each. The scales are as follows: Active Coping, Planning, Positive Reframing, Acceptance, Humour, Religion, Using Emotional Support, Using Instrumental Support, Self-Distraction, Denial, Venting, Substance Use, Behavioural Disengagement and Self-Blame.73 74 The Brief COPE has been used when measuring function and quality of life with PLWH.75 The Pearlin Mastery Scale is a self-administered questionnaire which consists of seven items that assess sense of personal control over important life forces or outcomes.76 The items have been developed to reflect whether individuals feel they have control over the decisions they make, what happens to them and whether they can deal with their problems appropriately and effectively. Each item in the scale is measured using a Likert scale with four response categories, and summary scores are generated indicating level of mastery (limited, moderate, great). This scale has demonstrated construct validity and good reliability for caregivers of PLWH, and individuals with Alzheimer's disease and dementia.77–79
Extrinsic contextual factors
Stigma and social support are extrinsic factors that may influence the health and disability of PLWH.53 Inclusion of scales to measure these constructs is integral to obtaining a comprehensive understanding of how CBE may affect perceived stigma and social support and subsequently the health of PLWH. The HIV Stigma Scale is a self-administered questionnaire comprised of 40 items that capture the complexity and multidimensionality of HIV-related stigma across four subscales: personalised stigma, disclosure, negative self-image and concern with public attitudes.80 This scale possesses construct validity and reliability when used with PLWH.81 82
The MOS Social Support Scale (MOS-SSS) is a self-administered questionnaire designed to measure five dimensions of social support among patients with chronic illness: emotional/informational support, tangible support, positive social interaction and affectionate support.83 The scale consists of 20 items that are measured using a scale with five response categories ranging ‘none of the time’ to ‘all of the time’. Higher overall scores are indicative of higher levels of social support. The MOS-SSS possesses construct validity and reliability when used with PWLH.83
Personal attributes (demographic and disease characteristics)
We will administer a demographic and disease questionnaire to capture characteristics including but not limited to age, gender, length of time since HIV diagnosis, ethnocultural background, antiretroviral use and adherence, smoking history, geographical status and number and type of comorbidities. Items on medication adherence, smoking history and comorbidities will be readministered at each time point. We will readminister items from this questionnaire bimonthly (comorbidities, medication adherence, smoking history) and also ask whether there are any events that may influence participants' ability to exercise since the last assessment.
Part C—qualitative interviews
We will conduct a series of face-to-face interviews with a subsample of 15 PLWH participants at initiation (8 months), midpoint (11 months) and completion (14 months) of the 24-week CBE intervention. Participants will be purposively sampled to achieve diversity in baseline physical activity levels. Using a semi-structured interview guide, we will explore their (1) current experience with exercise, (2) anticipated benefits of exercise (initiation) and (3) perceived impact of CBE on their health and disability over time (midpoint and post). In addition, we will specifically explore the influence of extrinsic factors (social support, stigma) and intrinsic factors (personal attributes, coping strategies) on the impact and level of engagement in exercise. All interviews will be audio recorded and later transcribed verbatim.
Intake and phase completion/withdrawal questionnaires
At initiation of the study (month 0) we will administer a CBE Study Intake Questionnaire which will ask participants for their contact information, how they found out about the study and previous exercise experience (new exerciser vs experienced exerciser). At the completion of each phase, we will administer the Goal Attainment Scale (GAS)85 and a CBE Study Phase Completion Questionnaire. If a participant withdraws from the study prior to study completion, we will ask him/her to complete a Withdrawal/Study Exit Questionnaire, GAS and revised version of the demographic questionnaire in order to capture the reasons for withdrawal and participant characteristics upon exit from the study.
Objective 3 (Maintenance)—To assess engagement in CBE over time: We will measure maintenance by assessing physical activity and adherence to the CBE intervention over 14 months. Physical activity: We will measure physical activity by administering the Rapid Assessment of Physical Activity (RAPA) questionnaire at each of the 12 time points and objectively using the Fitbit, a wireless activity monitor that will be used to track participant steps, distance and calories burned.52 The RAPA is a 9-item questionnaire developed to examine the frequency and intensity a participant spends in vigorous or moderate activity, including strength and flexibility within the last week.86 While developed originally for assessing activity among adults older than 50 years of age, based on our review of the items, the instrument appears to possess face validity for assessing physical activity for adults living with HIV. The Fitbit has been validated for use in healthy populations.87 Adherence: We will document adherence and long-term engagement in exercise by asking participants to complete a weekly online log to track frequency, intensity, time and type of exercise throughout the intervention (8–14 months) and postintervention (14–22 months). The questionnaire will be administered using an online secure web-based platform. We are currently piloting an online version of the weekly exercise log for study implementation.
Objective 4 (Adoption and implementation)—To evaluate the process of implementing the CBE intervention: We will conduct face-to-face interviews with a sample of five recreation providers (managers, fitness instructors) and subsample of 15 participants at initiation, midpoint and completion of the 6-month CBE intervention. We will explore anticipated concerns regarding engagement in exercise prior to the intervention (initiation, 8 months), insights on the strengths and challenges of the CBE implementation and uptake (midpoint, 11 months) and reflections (post, 14 months) on the overall process of implementing the intervention with fidelity in a community setting immediately postintervention. Using a semi-structured interview guide, we will specifically explore the (1) strengths and challenges of implementing the CBE intervention, (2) accessibility and feasibility of the intervention and (3) potential for long-term sustainability of the programme. The interview guide will be revised over the course of the three qualitative time points of the study. All interviews will be audio recorded and later transcribed verbatim. Throughout we will document strengths, challenges and lessons learnt on feasibility and implementation process using a CBE Study Process Log with members of the team and Community Advisory Committee.
Analysis
We will describe participant characteristics using descriptive statistics.
Objective 1 (Reach): We will report descriptive statistics for individuals who express interest to participate in the CBE intervention. For proportion, we will obtain an estimate based on the total number of eligible PLWH from the recruitment sites. For representativeness, we will assess the characteristics of participants who engage in the programme in relation to the broader HIV community comparing the sample to epidemiological data available in Toronto, Canada.88 We will report the descriptive statistics for individuals who are eligible and agree to participate, participants who withdraw from the programme and the reasons for withdrawal over time. We will also document the duration of time between participants' consent to participate to initiation of the study to document any delays that may occur due to capacity at the YMCA. We will assess differences in characteristics of those who complete versus withdraw from the intervention using independent t-tests and Mann-Whitney U tests.
Objective 2 (Effectiveness): Disability and health status: We will calculate descriptive statistics as frequencies and proportions for categorical variables and means and SDs (normally distributed data) and medians and IQRs (non-normally distributed data) for continuous variables across all time points. We will assess the distribution of domain and total scores pre-CBE and post-CBE intervention as well as change scores. We will calculate the proportion of items at the floor or ceiling of the scales in order to determine floor or ceiling effects, respectively.
Quantitative analysis (ITS): We will compare patterns of responses to the health and disability outcomes for the sample population at baseline (0–8 months), intervention (8–14 months) and postintervention (14–22 months) phases using time series regression analysis.89 (1) We will graph the mean and SD scores for all health and disability outcomes taken bimonthly to determine the structure or trend of the data. (2) We will test for the presence of autocorrelation (extent to which the data are dependent on each other) using the Durbin-Watson statistic.90 (3) If present, then we will adjust for autocorrelation. We will use the Prais-Winston estimator, which uses the generalised least-squares method91 to determine regression estimates that express the level (value of outcome at the beginning of a segment; intercept) and trend (rate of change of outcome; slope) of the outcomes across each phase in the series in relation to the intervention. We will adjust for seasonality of exercise using dummy variables to define seasons. We will specifically determine the slope and 95% CI for each phase and significance in the change in slope. We will evaluate each comparison separately (baseline to intervention; intervention to postintervention) to determine the effect of CBE.42 92 Regression coefficients corresponding to two standardised effect sizes will be obtained for each comparison: a change in level (also called ‘step change’) and a change in trend before and after the intervention. A change in level is defined as the difference between the observed level at the first intervention time point and that predicted by the preintervention time trend, and a change in trend is defined as the difference between postintervention and preintervention slopes.40 Statistical analysis will be conducted using SAS statistical software (SAS Computer Software 9.3 [program], 2011).
Objective 3 (Maintenance): Physical activity: We will calculate median (IQR) change scores of the RAPA questionnaire across all time points. We will also calculate median and IQR steps, distance and calories burned as measured by the Fitbit at each time point. We will define maintenance as the ability to sustain similar RAPA scores (highly correlated ≥0.7) postintervention. Adherence: Using the weekly exercise log, we will measure adherence to exercise over the intervention and self-monitoring phases. We will calculate the proportion of exercise sessions attended. Adherence will be defined as engaging in 75% or more of the three weekly exercise sessions throughout.93 In order to align with definitions of adherence derived specifically with PLWH, we will additionally determine how many participants engage in ≥40% of the exercise sessions.94 We will also calculate the proportion of participants who achieved the Canadian Physical Activity Guidelines (CPAG) of moderate to vigorous activity of at least 150 min a week for each of the study weeks, and the proportion of weeks in which the majority of participants achieved the CPAG guidelines.95 We will calculate the median number of days per week participants achieved 20–30 min of physical activity, and the proportion of participants who achieved a minimum of 3 days per week of at least 20 min of physical activity. We will additionally test for associations between adherence and health and disability outcomes.
Objective 2 (Effectiveness) and Objective 4 (Implementation and adoption): Qualitative data analysis: We will use content analysis methods to explore perceptions of PLWH participants on the impact of the CBE intervention (objective 2) and perceptions of PLWH participants and recreation providers on the process of implementing the CBE intervention and its long-term sustainability (objective 4).96 We will analyse the transcripts cross-sectionally (at each time point) as well as longitudinally to identify changes in participant perceptions over time.97 All transcripts will be analysed using line-by-line coding and codes clustered into broader categories.98 When all interview data are analysed, we will formulate a summary of perceived impact and the influence of extrinsic and intrinsic contextual factors on exercise behaviour (objective 2) and strengths and challenges associated with the CBE intervention and recommendations for long-term implementation in the community (objective 4). We will use NVivo software to facilitate analysis (NVivo qualitative data analysis software. Version 7 [program]: QSR International Pty. Ltd, 2006). We will review and refine findings with the Community Advisory Committee. The quantitative and qualitative data pertaining to effectiveness (objective 2) will be analysed separately but concurrently and will be combined at the point of interpretation with the Advisory Committee.
Objective 4 (Assessing the CBE implementation): The research team and Community Advisory Committee will meet throughout all phases of the project (start-up, recruitment, intervention and data collection, analysis and knowledge translation) to assess the process (strengths, challenges and recommendations for revision) related to the recruitment strategy, eligibility screening process, CBE implementation, retention in the study and outcome assessment. During teleconference and face-to-face team meetings, these discussions will be formally documented.
Sample size estimation and justification
Quantitative assessment (ITS): In ITS, sample size refers to the number of observational time points rather than number of participants. For a model of 12 time points, to detect level and trend change, assuming an effect size of 1, equal preintervention and postintervention time periods, statistical significance p<0.05 and an autoregression error time series model with lag 1, and autocorrelation estimate of 0.3, we would expect a power of 0.80.99 100 We will aim to recruit ∼120 PLWH with the goal for 75 participants to complete the study, the number of recommended observations in ITS to achieve acceptable variability of the estimates at each time point.100 This is due to the observed ∼60% retention rate observed in the pilot study (4 months)37 and was determined as feasible for the YMCA to accommodate administering the fitness assessments and providing weekly coaching during the intervention phase. The retention rate in the pilot study is higher than observed in other HIV-related exercise programmes;46 nevertheless, our sample size is limited to the capacity of participants the YMCA is able to accommodate for the fitness assessments and coaching. This is a feasible sample to recruit representing <10% of the total number of clients served at Toronto PWA (n∼1000), Casey House (n∼180) and community organisations such as realize. Given the increased length of the study compared to the pilot, we may recruit an additional wave of participants if required.
Qualitative interviews: We will recruit a subsample of 15 PLWH who engaged in the CBE intervention and 5 fitness instructors and managers involved in the intervention to participate in 3 interviews. Previous work with PLWH and providers suggests that this number of participants will enable us to reach saturation as it relates to the strengths and challenges of implementing the CBE intervention.61
Ethics
This protocol was approved by the HIV/AIDS Research Ethics Board (REB) at the University of Toronto (Protocol Reference #32910). We will inform potential participants of all study procedures, risks and benefits, and the time commitment involved in participation (see online supplementary file 2 for an example consent form). We will submit any proposed amendment(s) to the protocol, for review by the REB. We will report any unanticipated or adverse events that occur, and the study protocol will undergo annual review by the REB.
Supplemental-File_2.pdf (102.9KB, pdf)
Token of appreciation: We will offer each PLWH participant a 14-month open access YMCA membership as a token of appreciation for their participation in the study. The membership will be provided in two waves. The first membership will be for 6 months (intervention phase) and the second membership will be for 8 months (postintervention phase). Participants will be able to keep the Fitbit wireless activity monitor provided at the initiation of the intervention. Participants and recreation providers who complete the interview sessions will receive a $25 e-gift card at the end of each interview.
Potential risks and benefits: It is possible that participants will experience injury with exercise and the physical assessments. If this does occur, fitness staff from the YMCA will follow appropriate emergency procedures (in accordance with the YMCA general safety procedures and guidelines). It is also possible that participants may find some of the questions on the questionnaires to be personal or sensitive in nature. If participants experience any form of physical injury or emotional distress, investigators will refer participants to their physician, qualified counsellor or local community support group, if available. Finally, given the individual and group nature of the CBE intervention, and the monthly self-management information sessions, it is possible that participants may feel a loss of privacy related to participating in the YMCA programmes on site. During the consent process, we will indicate to participants that all individuals involved in this study, including other participants, researchers, knowledge users, and the fitness instructors and assessment staff at the YMCA, will know that participants in this study are HIV positive.
Confidentiality and data management: All information will remain confidential and available only to study investigators, research staff and the University of Toronto REB that reviewed this protocol, and other regulatory authorities for the purpose of monitoring this study, unless required by law. Participants will be matched with a fitness instructor at the YMCA who will have access to participants' contact information collected at intake in order to be able to directly liaise with their respective participants about scheduling their fitness sessions.
Data collected at the YMCA will be uploaded to a password-protected and encrypted file share system,102 and subsequently transferred to the University of Toronto for storage. Any hard copy documents, including questionnaires and consent forms, will be stored in a locked filing cabinet in the laboratory of the principal investigator (PI) at the University of Toronto. The document linking names of participants to their assigned numeric code will be kept in a password-protected file on the UofT server accessible to the coordinator and PI.
As per the REB approval, participant level data will not be publicly accessible. Members of the public who wish to access the full protocol and statistical code may contact the corresponding author with their request.
Dissemination
Results will be translated among PLWH, researchers, future and current health providers, government stakeholders, community-based agencies and policymakers. Integrated knowledge translation (KT) with PLWH will be led by the Community Advisory Committee using a series of ‘HIV CBE Community Meetings' and facilitated by collaborator organisations. Knowledge translation will occur through publications in open access peer-reviewed scientific journals, podium and poster presentations at scientific meetings, lectures in health professional and research trainee curricula, presentations at community-based organisations and newsletters. Further translation may occur through collaborations with realize and YMCA centres across Canada.
Discussion
Results will lead to the first known HIV-specific CBE intervention in Canada evaluated for effectiveness and successful translation with the HIV community. This research involves a partnership between the YMCA and HIV community that will facilitate the successful translation of CBE into the community while building capacity about HIV in the broader fitness sector.
Strengths of our approach include an intervention based on a self-management and health promotion approaches to engaging PLWH in exercise that is less costly in contrast to earlier interventions, where participants followed highly structured, prescriptive protocols with less input into the type of exercise activity.23 24 The 6-month CBE intervention and 8-month follow-up will surpass 3-month interventions commonly assessed in existing evidence, allowing us to evaluate the long-term impact of CBE and retention of benefits. Potential challenges will include recruitment and retention of participants across the 22-month study and potential burden of quantitative and qualitative assessments. Ongoing collaboration with community partners, including the YMCA, will be critical throughout. Overall, if deemed successful, this intervention may be transferable to other geographic regions where partnerships between community-based HIV organisations and fitness centres could enhance exercise and health promotion for PLWH.
Acknowledgments
The authors acknowledge the YMCA staff, including Mehdi Zobeiry, Katie Lowe, Maria Rapalini, Ivan Ilic, Emily Sas, Liam Dick, Dexter Wilson, Helen Trent, Katharine Stanbridge, Christine Hsu, Cristina Granados and Letizia Lepore, who were integral to the pilot work that informed the protocol development. The authors acknowledge the members of the Community Advisory Committee who were involved in this work, including Ken King (Community Member), Chris Godi and Murray Jose-Boerbridge (Toronto PWA Foundation), and James Murray (Ontario Ministry of Health and Long-Term Care). The authors acknowledge Sean Rourke who assessed neurocognitive outcomes with the pilot CBE intervention, beyond the scope of this manuscript. The authors thank Melanie Bisnauth for her assistance with figure 1.
Footnotes
Contributors: KKO led the conceptualisation of the study objectives, and drafted the protocol and is the lead investigator on the study. AMD, PS, AMB, AN and AT are members of the research team, were involved in the pilot work, conceptualisation of the study design and development of the protocol. KM is a knowledge user and member of the Community Advisory Committee. She is involved in the recruitment of participants and provision of training for the YMCA fitness instructors through the realize (formally CWGHR) online interprofessional learning module. SCC is a collaborator and member of the Community Advisory Committee. She is involved in recruitment of participants. MZ is the principal knowledge user in this study in collaboration with the YMCA (see acknowledgements) involved in developing the protocol as it relates to the physical assessments, fitness instruction and CBE intervention at the YMCA. All authors have read and approved the final protocol manuscript.
Funding: This study is supported by the Canadian Institutes of Health Research (CIHR) HIV/AIDS Community-Based Research (CBR) Program (Funding Reference Number #CBR-139685; 160 Elgin Street, Ottawa, Ontario, Canada K1A 0W9). KKO is supported by a New Investigator Award from the Canadian Institutes of Health Research (CIHR). AMB is supported by the Foundation Baxter and Alma Ricard Chair in Inner City Health at St. Michael's Hospital and the University of Toronto. AT is supported by a personnel award from the Heart and Stroke Foundation, Ontario Provincial Office (CS I 7468).
Disclaimer: The CIHR had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data or decision to submit results.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data collected during and/or analysed during the study are not publicly available in accordance with our study protocol that was approved by the University of Toronto HIV/AIDS Research Ethics Board. Data may be available on reasonable request by contacting the corresponding author.
References
- 1.Rusch M, Nixon S, Schilder A et al. . Impairments, activity limitations and participation restrictions: prevalence and associations among persons living with HIV/AIDS in British Columbia. Health Qual Life Outcomes 2004;2:46 10.1186/1477-7525-2-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rusch M, Nixon S, Schilder A et al. . Prevalence of activity limitation among persons living with HIV/AIDS in British Columbia. Can J Public Health 2004;95:437–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaidhane AM, Zahiruddin QS, Waghmare L et al. . Assessing self-care component of activities and participation domain of the international classification of functioning, disability and health (ICF) among people living with HIV/AIDS. AIDS Care 2008;20:1098–104. 10.1080/09540120701808820 [DOI] [PubMed] [Google Scholar]
- 4.Palella FJ Jr, Baker RK, Moorman AC et al. . Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 2006;43:27–34. 10.1097/01.qai.0000233310.90484.16 [DOI] [PubMed] [Google Scholar]
- 5.Willard S, Holzemer WL, Wantland DJ et al. . Does “asymptomatic” mean without symptoms for those living with HIV infection? AIDS Care 2009;21:322–8. 10.1080/09540120802183511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guaraldi G, Orlando G, Zona S et al. . Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53:1120–6. 10.1093/cid/cir627 [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Penney AT, Iudicello JE, Riggs PK et al. . Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS 2013;27:5–16. 10.1089/apc.2012.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vance DE, Mugavero M, Willig J et al. . Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care 2011;22:17–25. 10.1016/j.jana.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 9.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 2006;20:2165–74. 10.1097/QAD.0b013e32801022eb [DOI] [PubMed] [Google Scholar]
- 10.Mallon PW. Aging with HIV: osteoporosis and fractures. Curr Opin HIV AIDS 2014;9:428–35. 10.1097/COH.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 11.Heaton RK, Clifford DB, Franklin DR Jr et al. . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2011;75:2087–96. 10.1212/WNL.0b013e318200d727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiels MS, Cole SR, Kirk GD et al. . A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 2009;52:611–22. 10.1097/QAI.0b013e3181b327ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasse B, Ledergerber B, Furrer H et al. . Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011;53:1130–9. 10.1093/cid/cir626 [DOI] [PubMed] [Google Scholar]
- 14.Kendall CE, Wong J, Taljaard M et al. . A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health 2014;14:161 10.1186/1471-2458-14-161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien KK, Bayoumi AM, Strike C et al. . Exploring disability from the perspective of adults living with HIV/AIDS: development of a conceptual framework. Health Qual Life Outcomes 2008;6:76 10.1186/1477-7525-6-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worthington C, Myers T, O'Brien K et al. . Rehabilitation in HIV/AIDS: development of an expanded conceptual framework. AIDS Patient Care STDS 2005;19:258–71. 10.1089/apc.2005.19.258 [DOI] [PubMed] [Google Scholar]
- 17.Canadian Working Group on HIV and Rehabilitation, Wellesley Institute. Equitable access to rehabilitation: realizing potential, promising practices, and policy directions, 2012. http://www.wellesleyinstitute.com/wp-content/uploads/2012/06/Equitable-Access-to-Rehabilitation-Discussion-Paper1.pdf
- 18.Worthington C, Myers T, O'Brien K et al. . Rehabilitation professionals and human immunodeficiency virus care: results of a national Canadian survey. Arch Phys Med Rehabil 2008;89:105–13. 10.1016/j.apmr.2007.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: an integrative framework. AIDS Care 2009;21:1321–34. 10.1080/09540120902803158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botros D, Somarriba G, Neri D et al. . Interventions to address chronic disease and HIV: strategies to promote exercise and nutrition among HIV-infected individuals. Curr HIV/AIDS Rep 2012;9:351–63. 10.1007/s11904-012-0135-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garber CE, Blissmer B, Deschenes MR et al. . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334–59. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Health topics: physical activity 2013. http://www.who.int/topics/physical_activity/en/
- 23.O'Brien KK, Tynan AM, Nixon SA et al. . Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis 2016;16:182 10.1186/s12879-016-1478-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien K, Tynan AM, Nixon S et al. . Effects of progressive resistive exercise in adults living with HIV/AIDS: systematic review and meta-analysis of randomized trials. AIDS Care 2008;20:631–53. 10.1080/09540120701661708 [DOI] [PubMed] [Google Scholar]
- 25.Gomes Neto M, Conceicao CS, Oliveira Carvalho V et al. . Effects of combined aerobic and resistance exercise on exercise capacity, muscle strength and quality of life in HIV-infected patients: a systematic review and meta-analysis. PLoS ONE 2015;10:e0138066 10.1371/journal.pone.0138066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes Neto M, Ogalha C, Andrade AM et al. . A systematic review of effects of concurrent strength and endurance training on the health-related quality of life and cardiopulmonary status in patients with HIV/AIDS. Biomed Res Int 2013;2013:319524 10.1155/2013/319524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomes-Neto M, Conceicao CS, Oliveira Carvalho V et al. . A systematic review of the effects of different types of therapeutic exercise on physiologic and functional measurements in patients with HIV/AIDS. Clinics (Sao Paulo) 2013;68:1157–67. 10.6061/clinics/2013(08)16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fillipas S, Bowtell-Harris CA, Oldmeadow LB et al. . Physical activity uptake in patients with HIV: who does how much? Int J STD AIDS 2008;19:514–18. 10.1258/ijsa.2007.007237 [DOI] [PubMed] [Google Scholar]
- 29.Clingerman EM. Participation in physical activity by persons living with HIV disease. J Assoc Nurses AIDS Care 2003;14:59–70. 10.1177/1055329003255284 [DOI] [PubMed] [Google Scholar]
- 30.Clingerman E. Physical activity, social support, and health-related quality of life among persons with HIV disease. J Community Health Nurs 2004;21:179–97. 10.1207/s15327655jchn2103_5 [DOI] [PubMed] [Google Scholar]
- 31.Schuelter-Trevisol F, Wolff FH, Alencastro PR et al. . Physical activity: do patients infected with HIV practice? How much? A systematic review. Curr HIV Res 2012;10:487–97. 10.2174/157016212802429794 [DOI] [PubMed] [Google Scholar]
- 32.Winward C. Supporting community-based exercise in long-term neurological conditions: experience from the Long-term Individual Fitness Enablement (LIFE) project. Clin Rehabil 2011;25:579–87. 10.1177/0269215510392075 [DOI] [PubMed] [Google Scholar]
- 33.Salbach NM, Howe JA, Brunton K et al. . Partnering to increase access to community exercise programs for people with stroke, acquired brain injury and multiple sclerosis. J Phys Act Health 2014;11:838–45. 10.1123/jpah.2012-0183 [DOI] [PubMed] [Google Scholar]
- 34.Stuart M, Benvenuti F, Macko R et al. . Community-based adaptive physical activity program for chronic stroke: feasibility, safety, and efficacy of the Empoli model. Neurorehabil Neural Repair 2009;23:726–34. 10.1177/1545968309332734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee LL, Arthur A, Avis M. Using self-efficacy theory to develop interventions that help older people overcome psychological barriers to physical activity: a discussion paper. Int J Nurs Stud 2008;45:1690–9. 10.1016/j.ijnurstu.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 36.Li A, McCabe T, Silverstein E et al. . Community-based exercise program for adults with HIV: Factors to consider from the perspectives of adults with HIV rehabilitation professionals and recreation providers. Journal of the International Association of Providers of AIDS Care (JIAPAC). Accepted August 2016. In Press. [Google Scholar]
- 37.O'Brien KK, Nayar A, Bayoumi AM et al. . Implementing a community-based exercise intervention to improve the health of adults living with HIV: a pilot study. 25th Annual Canadian Conference on HIV/AIDS Research Realizing Our Potential: Local to Global and Back CAHR Abstracts, 2016. [Google Scholar]
- 38.Glasgow RE, Eckstein ET, Elzarrad MK. Implementation science perspectives and opportunities for HIV/AIDS research: integrating science, practice, and policy. J Acquir Immune Defic Syndr 2013;63(Suppl 1):S26–31. 10.1097/QAI.0b013e3182920286 [DOI] [PubMed] [Google Scholar]
- 39.Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–7. 10.2105/AJPH.89.9.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramsay CR, Matowe L, Grilli R et al. . Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. Int J Technol Assess Health Care 2003;19:613–23. 10.1017/S0266462303000576 [DOI] [PubMed] [Google Scholar]
- 41.Biglan A, Ary D, Wagenaar AC. The value of interrupted time-series experiments for community intervention research. Prev Sci 2000;1:31–49. 10.1023/A:1010024016308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michielutte R, Shelton B, Paskett ED et al. . Use of an interrupted time-series design to evaluate a cancer screening program. Health Educ Res 2000;15:615–23. 10.1093/her/15.5.615 [DOI] [PubMed] [Google Scholar]
- 43.Hanbury A, Farley K, Thompson C et al. . Immediate versus sustained effects: interrupted time series analysis of a tailored intervention. Implement Sci 2013;8:130 10.1186/1748-5908-8-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curran VR, Mugford JG, Law RM et al. . Influence of an interprofessional HIV/AIDS education program on role perception, attitudes and teamwork skills of undergraduate health sciences students. Educ Health (Abingdon) 2005;18:32–44. 10.1080/13576280500042606 [DOI] [PubMed] [Google Scholar]
- 45.Warburton DER, Jamnik VK, Bredin SSD et al. . The Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and Electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fitness J Canada 2011;4:3–23. [Google Scholar]
- 46.Brown D, Claffey A, Harding R. Evaluation of a physiotherapy-led group rehabilitation intervention for adults living with HIV: referrals, adherence and outcomes. AIDS Care 2016;28:1495–1505. 10.1097/QAD.0000000000001250 [DOI] [PubMed] [Google Scholar]
- 47.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot 1997;12:38–48. 10.4278/0890-1171-12.1.38 [DOI] [PubMed] [Google Scholar]
- 48.Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390–5. 10.1037/0022-006X.51.3.390 [DOI] [PubMed] [Google Scholar]
- 49.Elzarrad MK, Eckstein ET, Glasgow RE. Applying chronic illness care, implementation science, and self-management support to HIV. Am J Prev Med 2013;44(Suppl 2):S99–107. 10.1016/j.amepre.2012.09.046 [DOI] [PubMed] [Google Scholar]
- 50.Canadian Working Group on HIV and Rehabilitation. E-module for evidence-informed HIV rehabilitation (e-module). Canadian Working Group on HIV and Rehabilitation (CWGHR), 2016.
- 51.Glasgow RE, McKay HG, Piette JD et al. . The RE-AIM framework for evaluating interventions: what can it tell us about approaches to chronic illness management? Patient Educ Couns 2001;44:119–27. 10.1016/S0738-3991(00)00186-5 [DOI] [PubMed] [Google Scholar]
- 52. Fitbit Inc. Fitbit® official site 2014. http://www.fitbit.com/ca/zip.
- 53.O'Brien KK, Davis AM, Strike C et al. . Putting episodic disability into context: a qualitative study exploring factors that influence disability experienced by adults living with HIV/AIDS. J Int AIDS Soc 2009;12:5 10.1186/1758-2652-2-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wells KF, Dillon EK. The sit and reach. A test of back and leg flexibility. Res Q 1952;23:115–18. [Google Scholar]
- 55.Mayorga-Vega D, Merino-Marban R, Viciana J. Criterion-related validity of sit-and-reach tests for estimating hamstring and lumbar extensibility: a meta-analysis. J Sports Sci Med 2014;13:1–14. [PMC free article] [PubMed] [Google Scholar]
- 56.Hand GA, Jaggers JR, Lyerly GW et al. . Physical activity in cardiovascular disease prevention in patients with HIV/AIDS. Curr Cardiovasc Risk Rep 2009;3:288–95. 10.1007/s12170-009-0044-5 [DOI] [Google Scholar]
- 57.Esser S, Gelbrich G, Brockmeyer N et al. . Prevalence of cardiovascular diseases in HIV-infected outpatients: results from a prospective, multicenter cohort study. Clin Res Cardiol 2013;102:203–13. 10.1007/s00392-012-0519-0 [DOI] [PubMed] [Google Scholar]
- 58.O'Brien KK, Bayoumi AM, King K et al. . Community engagement in health status instrument development: experience with the HIV disability questionnaire. Prog Community Health Partnersh 2014;8:549–59. 10.1353/cpr.2014.0071 [DOI] [PubMed] [Google Scholar]
- 59.O'Brien KK, Bayoumi AM, Stratford P et al. . Which dimensions of disability does the HIV Disability Questionnaire (HDQ) measure? A factor analysis. Disabil Rehabil 2015;37:1193–201. 10.3109/09638288.2014.949358 [DOI] [PubMed] [Google Scholar]
- 60.O'Brien KK, Solomon P, Bayoumi AM. Measuring disability experienced by adults living with HIV: assessing construct validity of the HIV Disability Questionnaire using confirmatory factor analysis. BMJ Open 2014;4:e005456 10.1136/bmjopen-2014-005456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Brien KK, Bayoumi AM, Bereket T et al. . Sensibility assessment of the HIV Disability Questionnaire. Disabil Rehabil 2013;35:566–77. 10.3109/09638288.2012.702848 [DOI] [PubMed] [Google Scholar]
- 62.O'Brien KK, Solomon P, Bergin C et al. . Reliability and validity of a new HIV-specific questionnaire with adults living with HIV in Canada and Ireland: the HIV Disability Questionnaire (HDQ). Health Qual Life Outcomes 2015;13:124 10.1186/s12955-015-0310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brooks R, Rabin R, Charro F. The measurement and valuation of health status using EQ-5D: a European perspective. Dordrecht, The Netherlands: Kluwer Academic Publishers, 2003. [Google Scholar]
- 64.Brown D, Nelson M, Bower M et al. . Presence of complex comorbidity and functional disability when ageing with HIV; review of referrals to specialist HIV outpatient physiotherapy in the UK. HIV Medicine Abstracts of the 22nd Annual Conference of the British HIV Association (BHIVA); British HIV Association (BHIVA), 2016;17:14–71. [Google Scholar]
- 65.Mathews WC, May S. EuroQol (EQ-5D) measure of quality of life predicts mortality, emergency department utilization, and hospital discharge rates in HIV-infected adults under care. Health Qual Life Outcomes 2007;5:5 10.1186/1477-7525-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu AW, Hanson KA, Harding G et al. . Responsiveness of the MOS-HIV and EQ-5D in HIV-infected adults receiving antiretroviral therapies. Health Qual Life Outcomes 2013;11:42 10.1186/1477-7525-11-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brown D, Nelson M, Bower M et al. . People living with HIV present with worse health status than elderly people at risk of hospitalisation, when referred to specialist HIV outpatient physiotherapy in the UK. HIV Medicine Abstracts of the 22nd Annual Conference of the British HIV Association (BHIVA). British HIV Association (BHIVA), 2016;17:14–71. [Google Scholar]
- 68.Wu AW, Revicki DA, Jacobson D et al. . Evidence for reliability, validity and usefulness of the Medical Outcomes Study HIV Health Survey (MOS-HIV). Qual Life Res 1997;6:481–93. 10.1023/A:1018451930750 [DOI] [PubMed] [Google Scholar]
- 69.Kroenke K, Strine TW, Spitzer RL et al. . The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–73. 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- 70.Do AN, Rosenberg ES, Sullivan PS et al. . Excess burden of depression among HIV-infected persons receiving medical care in the United States: data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS ONE 2014;9:e92842 10.1371/journal.pone.0092842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wells TS, Horton JL, LeardMann CA et al. . A comparison of the PRIME-MD PHQ-9 and PHQ-8 in a large military prospective study, the Millennium Cohort Study. J Affect Disord 2013;148:77–83. 10.1016/j.jad.2012.11.052 [DOI] [PubMed] [Google Scholar]
- 72.Revicki DA, Chan K, Gevirtz F. Discriminant validity of the Medical Outcomes Study cognitive function scale in HIV disease patients. Qual Life Res 1998;7:551–9. 10.1023/A:1008866122441 [DOI] [PubMed] [Google Scholar]
- 73.Carver CS. You want to measure coping but your protocol's too long: consider the brief COPE. Int J Behav Med 1997;4:92–100. 10.1207/s15327558ijbm0401_6 [DOI] [PubMed] [Google Scholar]
- 74.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. J Pers Soc Psychol 1989;56:267–83. 10.1037/0022-3514.56.2.267 [DOI] [PubMed] [Google Scholar]
- 75.Vosvick M, Koopman C, Gore-Felton C et al. . Relationship of functional quality of life to strategies for coping with the stress of living with HIV/AIDS. Psychosomatics 2003;44:51–8. 10.1176/appi.psy.44.1.51 [DOI] [PubMed] [Google Scholar]
- 76.Pearlin LI. Pearlin mastery scale. J Health Soc Behav 1978;19:2–21. 10.2307/2136319 [DOI] [PubMed] [Google Scholar]
- 77.Skaff MM, Pearlin LI, Mullan JT. Transitions in the caregiving career: effects on sense of mastery. Psychol Aging 1996;11:247–57. 10.1037/0882-7974.11.2.247 [DOI] [PubMed] [Google Scholar]
- 78.Adams KB, Smyth KA, McClendon MJ. Psychosocial resources as moderators of the impact of spousal dementia caregiving on depression. J Appl Gerontol 2005;24:475–89. 10.1177/0733464805278812 [DOI] [Google Scholar]
- 79.Turner HA, Pearlin LI, Mullan JT. Sources and determinants of social support for caregivers of persons with AIDS. J Health Soc Behav 1998;39:137–51. 10.2307/2676396 [DOI] [PubMed] [Google Scholar]
- 80.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health 2001;24:518–29. 10.1002/nur.10011 [DOI] [PubMed] [Google Scholar]
- 81.Wright K, Naar-King S, Lam P et al. . Stigma scale revised: reliability and validity of a brief measure of stigma for HIV+ youth. J Adolesc Health 2007;40:96–8. 10.1016/j.jadohealth.2006.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bunn JY, Solomon SE, Miller C et al. . Measurement of stigma in people with HIV: a reexamination of the HIV Stigma Scale. AIDS Educ Prev 2007;19:198–208. 10.1521/aeap.2007.19.3.198 [DOI] [PubMed] [Google Scholar]
- 83.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–14. 10.1016/0277-9536(91)90150-B [DOI] [PubMed] [Google Scholar]
- 84.Yu Y, Yang JP, Shiu CS et al. . Psychometric testing of the Chinese version of the Medical Outcomes Study Social Support Survey among people living with HIV/AIDS in China. Appl Nurs Res 2015;28:328–33. 10.1016/j.apnr.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turner-Stokes L. Goal attainment scaling (GAS) in rehabilitation: a practical guide. Clin Rehabil 2009;23:362–70. 10.1177/0269215508101742 [DOI] [PubMed] [Google Scholar]
- 86.Topolski TD, LoGerfo J, Patrick DL et al. . The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 87.Takacs J, Pollock CL, Guenther JR et al. . Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport 2014;17:496–500. 10.1016/j.jsams.2013.10.241 [DOI] [PubMed] [Google Scholar]
- 88.Public Health Agency of Canada (2015). Summary: estimates of HIV, incidence, prevalence and proportion undiagnosed in Canada, 2014. http://healthycanadians.gc.ca/publications/diseases-conditions-maladies-affections/hiv-aids-estimates-2014-vih-sida-estimations/alt/hiv-aids-estimates-2014-vih-sida-estimations-eng.pdf
- 89.Abeysinghe T, Balasooriya U, Tsui A. Small-sample forecasting regression or ARIMA models? J Quant Econ 2003;1:103–13. [Google Scholar]
- 90.Durbin J. Testing for serial correlation in least-squares regressions when some of the regressors are lagged dependent variables. Econometrica 1970;38:410–21. 10.2307/1909547 [DOI] [Google Scholar]
- 91.Prais SJ, Winston CB. Trend estimatorsand serial correlation. Cowles Commission Discussion Paper No. 383. Chicago, 1954;383. [Google Scholar]
- 92.Zhang F, Wagner AK, Soumerai SB et al. . Methods for estimating confidence intervals in interrupted time series analyses of health interventions. J Clin Epidemiol 2009;62:143–8. 10.1016/j.jclinepi.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hicks GE, Benvenuti F, Fiaschi V et al. . Adherence to a community-based exercise program is a strong predictor of improved back pain status in older adults: an observational study. Clin J Pain 2012;28:195–203. 10.1097/AJP.0b013e318226c411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Petroczi A, Hawkins K, Jones G et al. . HIV patient characteristics that affect adherence to exercise programmes: an observational study. Open AIDS J 2010;4:148–55. 10.2174/1874613601004010148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Canadian Society for Exercise Physiology. Canadian physical activity, and sedentary behaviour guidelines, 2012. http://www.csep.ca/en/guidelines/
- 96.Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 97.Molloy D, Woodfield K, Bacon J. Longitudinal qualitative research approaches in evaluation studies. London: Her Majesty's Stationary Office, 2002. [Google Scholar]
- 98.Strauss A, Corbin J. Basics of qualitative research: techniques and procedures for developing grounded theory. Thousand Oaks: (CA: ): Sage Publications, 1998. [Google Scholar]
- 99.Zhang F, Wagner AK, Ross-Degnan D. Simulation-based power calculation for designing interrupted time series analyses of health policy interventions. J Clin Epidemiol 2011;64:1252–61. 10.1016/j.jclinepi.2011.02.007 [DOI] [PubMed] [Google Scholar]
- 100.McLeod AI, Vingilis ER. Power computations in time series analyses for traffic safety interventions. Accid Anal Prev 2008;40:1244–8. 10.1016/j.aap.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wagner AK, Soumerai SB, Zhang F et al. . Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther 2002;27:299–309. 10.1046/j.1365-2710.2002.00430.x [DOI] [PubMed] [Google Scholar]
- 102.Citrix. ShareFile, ©2016 Citrix Systems, Inc., 2016. http://www.sharefile.com/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental-File_1.pdf (89.4KB, pdf)
Supplemental-File_2.pdf (102.9KB, pdf)