Abstract
Objectives
At least 30% of young people with chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) also have symptoms of depression. This systematic review aimed to establish which treatment approaches for depression are effective and whether comorbid depression mediates outcome.
Setting
A systematic review was undertaken. The search terms were entered into MEDLINE, EMBASE, PsycInfo and the Cochrane library.
Participants
Inclusion and exclusion criteria were applied to identify relevant papers. Inclusion criteria were children age <18, with CFS/ME, defined using CDC, NICE or Oxford criteria, and having completed a valid assessment for depression.
Results
9 studies were identified which met the inclusion criteria, but none specifically tested treatments for paediatric CFS/ME with depression and none stratified outcome for those who were depressed compared with those who were not depressed. There is no consistent treatment approach for children with CFS/ME and comorbid depression, although cognitive–behavioural therapy for CFS/ME and a multicomponent inpatient programme for CFS/ME have shown some promise in reducing depressive symptoms. An antiviral medication in a small scale, retrospective, uncontrolled study suggested possible benefit.
Conclusions
It is not possible to determine what treatment approaches are effective for depression in paediatric CFS/ME, nor to determine the impact of depression on the outcome of CFS/ME treatment. Young people with significant depression tend to have been excluded from previous treatment studies.
Keywords: chronic fatigue syndrome, CFS/ME, paediatric
Strengths and limitations of this study.
A systematic approach was taken, which aimed to identify the highest quality evidence available.
Two reviewers independently completed the screening and data extraction process, and a third reviewer arbitrated when there was a lack of consensus.
Articles in foreign languages were included so these results have wide applicability.
Grey literature and unpublished material were not included.
A formal quality assessment of the observational studies was not undertaken.
Background
Chronic fatigue syndrome (CFS)/myalgic encephalomyelitis (ME) in young people are common and disabling. It has a significant impact on a young person's functioning, including school attendance1 with approximately half of young people with CFS/ME being bedbound at some stage of their illness, and missing, on average, 1 year of school.2 The estimated prevalence of paediatric CFS/ME is between 1% and 2.4% depending on the methodology and diagnostic criteria used.3 4 There are a number of recognised and widely accepted diagnostic criteria for CFS/ME, including the Centers for Disease Control and Prevention (CDC) criteria, also known as the Fukuda definition,5 the Oxford6 criteria and the criteria defined by the National Institute for Health and Care Excellence7 (table 1). In summary, to meet a diagnosis of CFS/ME, these criteria require the presence of recurrent or persistent fatigue which is debilitating, has lasted for at least 37 or 6 months5 in duration but is not lifelong, is not explained by ongoing exertion, is not alleviated by rest, is not explained by other conditions (including depression) and has a substantial impact on activity.8
Table 1.
Diagnostic criteria for CFS/ME
| Oxford criteria6 | CDC criteria5 | Canadian criteria35 | NICE criteria7 | |
|---|---|---|---|---|
| Principal symptom | Fatigue | Fatigue | Fatigue | Fatigue |
| Other symptoms | Myalgia, mood, sleep disturbance | At least four of: sore throat, tender lymph nodes, muscle pain, joint pain, headaches, unrefreshing sleep, postexertional malaise, impaired memory or concentration | Postexertional malaise and/or postexertional fatigue, unrefreshing sleep or sleep disturbance, pain. Cognitive dysfunction | Malaise, headaches, sleep disturbances, difficulties with concentration and muscle pain and/or joint pain, painful lymph nodes, sore throat, dizziness and/or nausea, and palpitations with no identifiable heart problem |
| Onset | Definite onset but not life long | Of new or definite onset (not lifelong) | Not stated | New, persistent and/or recurrent |
| Duration | Minimum of 6 months, for ≥50% of the time | ≥6 months. Persistent or relapsing | ≥3 months in a child or young person. Persistent or reoccurring | ≥3 months in a child or young person |
| Impact on functioning | Severe, disabling. Impacts on physical and mental functioning | Results in a substantial reduction in occupational, educational, social or personal functioning | Results in substantial reduction in previous levels of educational, social and personal functioning | Substantial reduction in activity levels |
| Exclusions | Medical conditions known to result in ongoing fatigue. Current diagnosis of schizophrenia, manic depressive illness, substance abuse, eating disorder or organic brain disease | Fatigue is not substantially alleviated by rest, and is not the result of ongoing exertion. Fatigue is clinically evaluated and unexplained | Fatigue is clinically evaluated and unexplained. Current psychiatric conditions that may explain the presence of chronic fatigue, including schizophrenia or psychotic disorders, bipolar disorder, alcohol or substance abuse, anorexia nervosa or bulimia nervosa and depressive disorders |
Fatigue not explained by other conditions. The diagnosis of CFS/ME should be reconsidered if none of the following key features are present: postexertional fatigue or malaise, cognitive difficulties, sleep disturbance and chronic pain |
| Subtypes | Two syndromes: Chronic fatigue syndrome (CFS) Postinfectious fatigue syndrome (PIFS) |
None specified | None specified | None specified |
Although the diagnosis of CFS/ME cannot be made if significant depression is the cause of the fatigue, a diagnosis can be made in the presence of comorbid depression which does not fully explain the disabling fatigue. At least 30% of children with CFS/ME have symptoms indicative of depression9 10 which is significantly higher than the prevalence of depression in the healthy population of between 0.92%11 and 5%.12 Rates of depression in children with CFS/ME are also higher than the rates of depression in children with other chronic illnesses, for example, cystic fibrosis.13
The impact of having a comorbid mood disorder for young people with CFS/ME is unclear. Depression during adolescence in the normal population increases the risk of subsequent depression, interpersonal difficulties and suicide in adulthood.14 While the long-term impact of depression in paediatric CFS/ME specifically is not known, cross-sectional studies have shown that young people with CFS/ME and comorbid depression are also more functionally disabled with worse fatigue and more pain compared with those without depression.10 Comorbid depression in adults with CFS/ME has been associated with a worse prognosis in some clinical trials,15–19 but not in others.20–22 It is plausible that comorbid paediatric depression in CFS/ME may impact on treatment outcome, but this has yet to be investigated.
Despite the recognised comorbidity and clear guidance that children/young people with CFS/ME should be screened and treated for depression,7 23 little is known about the efficacy and effectiveness of treatments for paediatric CFS/ME with comorbid depression, and the impact of the comorbid depression on recovery in CFS/ME. This review aims to synthesise the existing evidence regarding treatments for paediatric CFS/ME and comorbid depression by addressing the following questions:
What does the existing quantitative and qualitative literature tell us about treatment approaches for depression in children with CFS/ME?
What is the outcome for children with CFS/ME who are depressed compared with children who are not depressed?
Does the outcome for children with CFS/ME and comorbid depression vary between studies? Do particular treatment approaches have different outcomes?
Methods
This review was registered on Prospero in February 2015, registration numberCRD42015016813. The protocol is available from http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42015016813.
Data sources and search strategy
The search strategy was designed to identify studies examining depression in children or young people (<18) with CFS/ME. MEDLINE, EMBASE, PsycInfo and the Cochrane library were searched using search terms developed in collaboration with an information specialist, designed to include the concepts ‘paediatric’, ‘CFS/ME’ and ‘depression’ (details of search terms used available via http://dx.doi.org/10.15125/BATH-00127 and as an online supplementary file). Citation lists of included articles were hand-searched. Searches were carried out in February 2015.
bmjopen-2016-012271supp.pdf (247.7KB, pdf)
Study selection (inclusion and exclusion criteria)
Titles and abstracts of all articles were assessed for inclusion by two reviewers (MEL and EAS) and conflicts discussed and resolved. Articles for possible inclusion were reviewed in full and independently assessed for inclusion by two reviewers (MEL and EAS), with all conflicts discussed and reviewed by a third reviewer (EC).
Inclusion criteria were children age <18, with CFS/ME, defined using CDC criteria,5 NICE7 or Oxford criteria6 and having completed a valid assessment for depression. This review was limited to studies with young people (age <18 years) from 1991 onwards as this is when the Oxford diagnostic criteria were published.
Studies of children who were fatigued due to other causes and studies where chronic fatigue was not defined using one of the above criteria were excluded. Studies of predominantly adult samples, where the data were not separable for the <18 years, were also excluded.
Observational studies and clinical trials (randomised or quasi-randomised) of children with CFS/ME where symptoms of depression were measured were included, as were qualitative studies that reported on the treatment of depression in children with CFS/ME. The outcome data of interest was a change in depression and/or fatigue on psychometrically validated assessments or validated diagnostic interviews, and studies presenting such data, whether there was an intervention or not, were included.
Studies published in foreign languages were considered for inclusion. Where English language abstracts were not available, or where the titles and abstracts indicated that the study potentially met the inclusion criteria and merited full-text review, the papers were translated by native speakers to determine whether they met the inclusion criteria. None of these papers met the full inclusion criteria.
Data extraction and appraisal
Data extraction was completed independently by two reviewers (MEL and EAS) using a purpose-designed data extraction form, designed to capture the following aspects: details of the setting of the study, how children were recruited for the study, date of the study, participant characteristics (including age and gender), the study design, the CFS/ME definition used, how depression was assessed, definition of response and the treatment/intervention offered and outcomes.
Data synthesis
If sufficient data were available, a meta-analysis would have been conducted. The interventions were heterogeneous and the data were across a range of outcome measures which precluded this; therefore, a narrative review was carried out.
Quality assessment
An assessment of study methodological quality was undertaken for the included randomised controlled trials (RCTs) using the Cochrane risk of bias assessment tool.24–26
Results
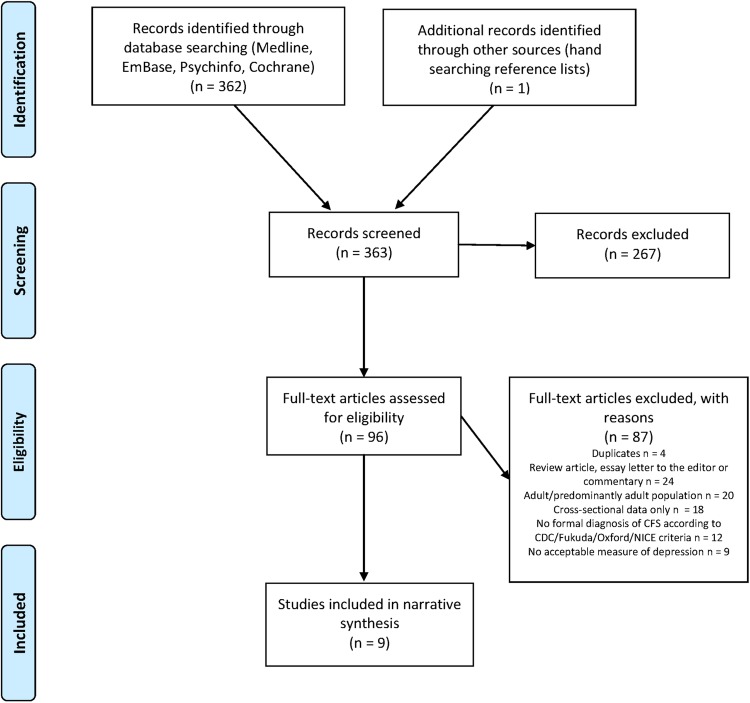
The systematic review identified 362 papers, with 1 further paper identified by hand searching reference lists of included articles (figure 1). Of these 363 papers, 96 were included at full review, with the remainder excluded at the screening stage. Of the 363 papers, 8 were foreign language articles (N=8; 2 French, 1 Catalan, 1 Russian, 2 Japanese, 1 Dutch, 1 Greek). Nine studies met the inclusion criteria for the review.
Figure 1.
Flow chart for systematic review based on PRISMA: Preferred Reporting Items for Systematic reviews and Meta-Analyses.49
Characteristics of included studies
Quality assessment
Of the nine studies that met the inclusion criteria, there was one RCT.27 The Cochrane risk of bias assessment tool was applied to this study (table 2). In summary, there was a low risk of bias in some domains, but a high risk of bias, or an uncertain risk of bias in others.
Table 2.
Risk of bias assessment for Gordon et al27 trial
| Domain | Description | Review author's judgement regarding risk of bias |
|---|---|---|
| Random sequence generation | Drawing a piece of paper out of an envelope | Low risk |
| Allocation concealment | Unclear | Uncertain risk of bias |
| Blinding of participants and personnel | Not possible | High risk |
| Blinding of outcome assessment | Blinded assessor completed baseline and follow-up assessments | Low risk |
| Incomplete outcome data | Missing data points substituted with the last known measure for each outcome. Intention to treat analysis using MANOVA | Low risk |
| Selective reporting | Insufficient information | Uncertain risk of bias |
| Other sources of bias | Unclear | Uncertain risk of bias |
Study design
In summary, one included study was an RCT, which compared two variations of the physical exercise intervention of a multicomponent treatment programme in an inpatient setting. The remaining eight studies were observational. Of these, six studies described outcome after outpatient (three investigating CBT and one antiviral medication) or inpatient (two studies investigating a multicomponent programme) treatment. Two more studies were epidemiological studies. Further details of the study designs are described below and in tables 3 (methodology) and 4 (findings). All studies, including the RCT, had relatively small sample sizes, with the smallest including 4 patients with CFS/ME, and the largest including 63 patients, of whom 52 were followed up.
Table 3.
Summary of methodology and study design of included studies
| Authors (year) | Design | Number of participants | CFS/ME diagnostic criteria applied | Mean age—years (SD) | Measure of CFS/ME | Measure of depression | Intervention | Was the treatment specifically targeted at or adapted for depression? | Was the outcome of treatment stratified by depressed vs non-depressed? | Length of follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Chalder et al (2002) | Observational (outpatient) | 23 | Sharpe et al (1991)* | (Range 11–18, median 15) | CFS | HADS | CBT-based rehabilitation programme. Up to 15 hourly sessions | No | No | 6 months |
| Denborough et al (2003) | Observational (inpatient) | 39 (19 at 6 months follow-up) | Fukuda et al (1994) | 16.2 | Chronic Fatigue Illness Disability Scale FSS |
BDI- | 4-week inpatient programme, focused on graded exercise using hydrotherapy and physiotherapy. | No | No | 6 months |
| Gordon and Lubitz (2009) | Observational (inpatient) | 16 | Fukuda et al (1994) | 16 (1.28) | FSS | BDI | 4-week inpatient programme, including graded exercise therapy, psychological/psychiatric support, attendance at school, recreation and leisure intervention | No | No | Post-treatment |
| Gordon et al (2010) | RCT (inpatient) | 22 | Fukuda et al (1994) | 16.2 (0.8) | Exercise tolerance Muscle strength FSS |
BDI | 4-week inpatient programme, including graded exercise therapy, psychological/psychiatric support, attendance at school. Aerobic training compared with resistance training | No | No | Post-treatment |
| Henderson (2014) | Observational (outpatient, retrospective case series) | 15 (14 at follow-up) | Fukuda et al (1994) | 15.46 (3.11) | Self-reported improvement FSS Multidimensional Fatigue Symptom Inventory-Short Form |
CDI | Valacyclovir (antiviral) medication, initially 500 mg two times a day, increasing after 2–3 weeks. Duration of treatment ranged from 3 to 60 months (mean 27.9 months) | No | Yes | Varied—post-treatment |
| Kawatani et al (2011) | Observational (outpatient) | 19 | Jason et al (2006) | 13.6 (0.7) | CFS | Zung self-rating depression scale | CBT (average of 5 sessions over 6 months) and pharmacotherapy | No | No | 6 months |
| Lloyd et al (2012) | Observational (outpatient) | 63 (52 at follow-up) | Sharpe et al (1991)* | (Median 15) | CFS | Birleson Depression Scale | CBT via telephone based guided self-help—6 fortnightly sessions, 30mins duration | No | No | 6 months |
| Rimes et al (2007) | Observational (prospective, community) | 1 case of CFS at time 1; 4 cases CFS at identified at time 2 | Fukuda et al (1994) | (Range 11–15) | Diagnostic interview using CDC criteria | Development and Well-being Assessment (interview) | None | No | No | 4–6 months |
| van de Putte et al (2007) | Observational (prospective, community) | 40 at baseline (36 at follow-up) | Fukuda et al (1994) | 16.0 (1.5) | Subjective fatigue subscale of CIS-20 | CDI at baseline only | None | No | No | 18 months |
*In the studies using the Oxford criteria,6 it is unclear if the criteria for CFS or postinfectious fatigue syndrome (PIFS) were applied.
BDI, Beck Depression Inventory; CDI, Children's Depression Inventory; CFS, Chalder Fatigue Scale; CIS-20, Checklist of Individual Strength; FSS, Fatigue Severity Scale; HADS, Hospital Anxiety and Depression Scale.
Table 4.
Summary of outcomes for depressive symptoms and other relevant findings for included studies
| Authors (year) | Measure of depression | Pretreatment | Post-treatment (unless otherwise stated) | Statistical analysis of change in depressive symptomatology | Summary of other relevant findings |
|---|---|---|---|---|---|
| Chalder et al (2002) | HADS | Mean 8.4 (IQR 5.7–11) | 6-month follow-up Mean 3 (IQR 3–5) |
Wilcoxon signed-ranks test −3.33 (two-tailed significance 0.00) | All 20 treatment completers returned to school at 6 months follow-up, with 95% attending full time. Depression significantly improved, as did social adjustment |
| Denborough et al (2003) | BDI | Mean score 21 | Mean score 15 | Improvement p<0.001 Maintained at 6-month follow-up (p<0.038) |
On discharge, the mean depression score significantly better than on admission. Also significant improvement in Chronic Fatigue Illness Disability score and significant decrease in FSS score (maintained at 6-month follow-up). Achenbach/Youth Self-Report scores improved significantly by discharge, but returned to above admission levels at 6 months |
| Gordon and Lubitz (2009) | BDI | Mean 19.88 SD 8.62 | Mean 11.44 SD 10.98 | Paired t-test p value 0.001 sig 0.008 | Significant improvement in BDI scores, Fatigue Severity scores |
| Gordon et al (2010) | BDI | Resistance Arm Pretreatment 20.9±11.3 |
Resistance arm Post-treatment 14.2±10.0 |
Resistance arm Difference −6.7±8.5 p=0.03 |
Significant improvement in BDI scores in both arms |
| Aerobic arm Pretreatment 16.4±4.3 |
Aerobic arm Post-treatment 12.2±6.7 |
Aerobic arm Difference −4.2±4.8 p=0.002 |
|||
| Henderson (2014) | CDI | Mean score 14±2.83 (4 patients with mood disorder, 16.8±1.92) (11 patients without mood disorder 12.73±2.00) | Not stated | Not reported | All patients reported at least 80% self-rated improvement. Significant reduction in FSS, MSFI (all subscales) |
| Kawatani et al (2011) | Zung self-rating depression scale | 53.3±6.7 | Not stated | Not reported | No significant change between baseline fatigue scores and fatigue scores 6 m follow-up. Significant improvement in performance status scores (self-reported impact on functioning) |
| Lloyd et al (2012) | Birleson Depression Scale | Baseline mean 13.38 (SD 4.76) Pretreatment mean 12.91 (SD 5.57) |
Post-treatment mean 10.98 (SD 5.35) 3-month follow-up mean 10.47 (SD 5.87) 6-month follow-up mean 9.22 (SD 5.36) |
Multilevel modelling and Wald tests Treatment effect estimate at 6 months −3.69 (CI −5.17 to −2.21) Significance (two-tailed) <0.001, effect size 0.78 |
Significant change in fatigue and school attendance, with improvements in depression, impairment and adjustment at 6 months |
| Rimes et al (2007) | 3 of 4 had at least 1 psychiatric diagnosis at baseline | 4 participants developed CFS/ME at follow-up (4–6 months) | Not reported | Of the 4 participants who developed CFS/ME over the follow-up period, 3 of 4 had at least 1 psychiatric diagnosis at baseline, 3 had reported being ‘much more tired and worn out than usual over the last month’ at time 1, 2 participants had frequent headaches at time 1, 1 also had sleep problems and postexertional malaise at time 1 | |
| Van de Putte et al (2007) | CDI | Mean score at baseline 11.7 SD 6.1 | Not stated | Not reported | 47% of adolescents ‘fully recovered’ (below score ie, mean plus 2 SD of subjective fatigue distribution in health adolescents). |
BDI, Beck Depression Inventory; CDI, Children's Depression Inventory; CFS, Chalder Fatigue Scale; CIS-20, Checklist of Individual Strength; FSS, Fatigue Severity Scale; HADS, Hospital Anxiety and Depression Scale.
Three studies evaluated the outcomes of CBT for CFS/ME. Chalder et al28 administered a CBT-based outpatient rehabilitation programme focused on remediating CFS/ME of up to 15 hourly face-to-face sessions. Lloyd et al29 offered a less intensive intervention of CBT-based guided self-help, focused on remediating CFS/ME and also emotional symptoms where appropriate, via six telephone sessions, of 30 min in duration, at fortnightly intervals. Kawatani et al30 offered, on average, five sessions of CBT over a 6-month period, combined with pharmacotherapy (6 of the 19 patients were prescribed SSRIs with or without other medications, 5 patients received an antihypotensive medication, midodrine hydrochloride, 4 received other medications and 4 received no medication).
Three studies, including an RCT, evaluated the outcomes of a 4-week inpatient programme, focused on graded exercise using hydrotherapy and physiotherapy, school attendance and psychiatric input as required. The RCT specifically compared two variations of physiotherapy, aerobic exercise and resistance training, within this programme. These studies were all small in sample size, and due to the multidisciplinary and multicomponent nature of the intervention (which included psychological therapy where indicated with no further details specified about this), it is not possible to draw any conclusions about what the key components of the approach may have been. The studies by Gordon and Lubitz31 and Gordon et al27 are also limited by a lack of follow-up data postdischarge.
One study was a retrospective case series of an antiviral medication (outpatient) with a small sample size (N=15) and variable length of intervention (ranging from 3 to 60 months). This study is further limited by the lack of a comparator or control condition, and the uncontrolled nature of this study, which does not report on other interventions (eg, psychological input) which the patient may have had concurrently. There are also considerable missing data, particularly in regard to depression outcomes, which could bias the study findings.
Two studies did not offer any active intervention. Rimes et al32 conducted a prospective study of a random sample of British adolescents (n=842) from the general population who were assessed at baseline and ∼4–6 months later as part of the Office for National Statistics study of mental health in children. van de Putte et al33 undertook a longitudinal study to explore alexithymia (the inability to identify and/or describe one's emotions) in paediatric CFS/ME. The research aimed to establish whether alexithymia was a prognostic factor for recovery from CFS/ME. As part of this study, they sought to establish the number of participants who had ‘recovered’ from CFS/ME at 18 months (n=40), where recovery is defined as scoring within 2 SDs of the average fatigue score within a population of healthy adolescents on the Subjective Fatigue Subscale of the Checklist Individual Strength (CIS-20). CFS/ME patients were compared with healthy controls (n=36) at baseline. No differences in recovery were evident between those adolescents with alexithymia compared with those who were not alexithymic.
Only one study stratified their findings by depressed versus non-depressed participants,34 and no studies stratified their results by the severity of depression (mild/moderate/severe).
Of the included studies, no studies were specifically aimed at treating depression in the context of paediatric CFS/ME. Thus, it appears that approaches commonly used for CFS/ME are used for this subgroup of patients without any particular adaptations. At the current time, evidence of efficacy or effectiveness for specific treatments tailored to the subgroup of patients with depression in the context of paediatric CFS/ME does not exist.
The effectiveness of interventions in reducing depression symptoms
Cognitive–behavioural therapy
The outcomes of CBT for CFS/ME varied across the three studies included. Chalder et al28 found that, in those 20 patients who completed treatment, depression, fatigue, functioning and social adjustment significantly improved following their relatively intensive CBT programme. Lloyd et al29 showed that a less intensive CBT intervention resulted in a significant change in fatigue and school attendance, with improvements in depression, impairment and adjustment in the 52 patients retained in the study at 6-month follow-up. However, Kawatani et al30 assessed depressive symptomatology in 19 patients at baseline, and, on average, most of them were in the mildly depressed range. In this group, they did not find a significant improvement in fatigue at 6-month follow-up; mood was not reassessed. This study applied the Jason et al35 diagnostic criteria, which defines depressive disorders in the exclusionary criteria, meaning that all those who were clinically depressed would have been excluded from the study.
Multicomponent inpatient programme
All three studies27 31 36 evidence an improvement in mood post-treatment (which applied to both arms of the RCT), as well as a decrease in fatigue symptoms and an improvement in functioning. Denborough et al36 showed that improvements in mood and fatigue are maintained at 6-month follow-up, although parental-rated and self-reported internalising problems had escalated to above preadmission levels.
Antiviral medication
Henderson34 presents separate data on the post-treatment scores of one subscale (emotional subscale) of the Multidimensional Fatigue Symptom Inventory-Short Form (MSFI-SF) for three patients who were diagnosed with mood disorder when compared with seven participants who were not diagnosed with mood disorder (data on this variable are missing for the remaining five patients in the study) at assessment for treatment with Valacyclovir. The length of treatment was variable (mean 27.9 months, range 3–60 months). Patients with a mood disorder had a mean pretreatment score on the emotional subscale of the MSFI of 13.00±6.16 and a mean post-treatment score of 6.67±2.89, when compared with patients without a mood disorder who had a pretreatment score of 8.40±2.76 and a mean post-treatment score of 1.5±1.6. Thus, depression symptoms decreased in depressed and non-depressed patients over the course of treatment. The remaining outcome data (self-rated improvement, sleep, school performance, Fatigue Severity Scale (FSS) scores, Fatigue Symptom Inventory (FSI), and the fatigue, physical, mental and vigour subscales of the MSFI) indicate significant improvements but are not stratified for the patients with and without mood disorder.
Epidemiological studies. Rimes et al32 found that four cases of CFS (according to CDC criteria) had developed at 6-month follow-up in their population sample, three of whom had had at least one psychiatric diagnosis at baseline. Furthermore, three cases had reported being more worn out than usual at time 1. There was also one case of CFS diagnosed at baseline, although further information about follow-up assessments is not separately available for this participant. van de Putte et al33 found that 47% of the 36 CFS/ME participants in their longitudinal study had ‘recovered’ from CFS/ME at 18 months. At baseline, the CFS participants were found to have significantly higher depression scores on the Children's depression inventory (CDI) compared with healthy controls, although the mean CDI score in the CFS group still fell below the clinical cut-off for depressive disorder. The outcomes in terms of recovery are not stratified by depression severity.
Discussion
Despite the high prevalence of depression in young people with CFS/ME, there is little evidence about the effectiveness of treatment for this population, and no specifically adapted treatments have been trialled in this population. In the studies included in this review, the mean depression scores of the participants tended to be below clinical cut-offs for depression pretreatment. Thus, the samples in treatment studies, in which potential participants with significant depression tend to be excluded, do not appear to be representative of those in clinical cohort studies, which have found a much higher prevalence of depression in paediatric CFS/ME.10 In these skewed samples, treatments aimed at remediating CFS/ME, including CBT and a multicomponent inpatient programme can result in improvements in mood.
The strengths of this review are that a systematic approach was taken, which sought to identify the highest quality evidence available. Furthermore, two reviewers independently completed the screening and data extraction procedures, with a third reviewer arbitrating when there were differences of opinion. Articles in foreign languages were included so these results are not limited to UK patients. However, a formal review of the quality of the observational studies was not undertaken. This would have been undertaken had those studies provided significant evidence to inform the review questions.
There is a lack of evidence to inform clinicians on how best to help young people with CFS/ME who are also low in mood, which is particularly surprising, given that psychological models of the perpetuation of chronic fatigue and disability include depression as part of the maintenance cycle.37 Cognitive–behavioural therapy, a multicomponent inpatient programme and antiviral medication are the interventions used in the included studies. CBT and the multicomponent inpatient programme are broadly consistent with the evidence-based guidelines from the National Institute for Health and Care Excellence for the management and treatment of CFS/ME7 and the management and treatment of depression.38 The participants appear to have benefited from these interventions, including in terms of mood, although the data do not allow comparisons to be made about whether those who were low in mood benefited less, as much, or possibly more than those who were not low in mood. Larger sample sizes, with more separable data about those participants who are depressed versus those who are not depressed at baseline would enable further conclusions to be drawn about the effectiveness of these treatment approaches for those with comorbid depression.
The lack of evidence about what treatment approach is most effective with young people who have CFS/ME and depression is further compounded by contradictions within treatment approaches and the recommendations for the disorders separately. Certain recommendations made by NICE for the management of CFS7 contradict the recommendations for the management of depression.38 For example, exercising for up to three sessions, 45 min to an hour in duration of moderate exercise, per week for 10–12 weeks39 may be effective for depression but could exacerbate symptoms in patients with CFS/ME. Interventions for depression such as behavioural activation would be limited by the activity management approach advocated for managing the CFS/ME.7 Furthermore, standard cognitive therapy is cognitively demanding and may be beyond the capacity of a young person who is cognitively limited in attentional capacity and memory by fatigue.40
Similarly, the existing guidance and literature does not provide any clear direction about using medication. There is no known pharmacological treatment for CFS/ME, although medications can be used for symptom management.7 In the current review, one study used antiviral medication with possible benefit, although this study was small scale, uncontrolled, retrospective and had a variable length of follow-up, and therefore, it is difficult to draw any firm conclusions about the findings. There is variable evidence about the efficacy of antidepressant medications (SSRIs) in young people with a primary diagnosis of depression; while antidepressants can result in improvements to young people's mood,41 42 there are indications that SSRIs can increase suicidality.43 The NICE38 recommendation is that medication should only be offered in combination with a specific psychological therapy unless the latter is declined. Furthermore, there is currently no evidence about whether antidepressant medication is beneficial for young people with CFS and depression; in the current review, one paper did include SSRIs in combination with CBT, but only for a proportion of their sample, and outcome data were not separately presented for those with depression.30 Additionally, there is mixed evidence about the utility of antidepressant medication in adult CFS populations with some studies finding no effect of fluoxetine in comparison to placebo44 45 and others showing significant improvements in CFS/ME and depressive symptomatology in response to s-citalopram.46
It is notable that a number of the large-scale studies of recovery in paediatric CFS/ME such as the Dutch trials of FITNET47 48 do not appear to have specifically included low mood or depression as an outcome variable, although a broader well-being measure was included in this study and a specific measure of depression was used at the baseline assessment to exclude the most severely depressed young people. Furthermore, in these trials, and a number of other trials of treatments for paediatric CFS/ME, participants with psychiatric diagnoses are excluded. Therefore, uncertainty remains about how best to treat young people who have diagnosable depression in the context of paediatric CFS/ME.
Conclusion
This review has highlighted the relative lack of evidence regarding effective treatment for paediatric CFS/ME and comorbid depression. Given the levels of comorbidity,10 impact on functioning and potentially, impact on response to treatment, developing and trialling potentially efficacious and effective treatments for children and young people with CFS/ME and depression is a priority for future research.
Footnotes
Contributors: MEL and EC conceptualised and designed the study. MEL and EAS carried out the data search and analysis. MEL drafted the manuscript. EC contributed to the analysis process by when there was uncertainty about decisions about whether studies met the inclusion criteria. EC reviewed and revised the manuscript. All authors reviewed and approved the manuscript prior to submission.
Funding: This study was funded by donated Funds from the Royal National Hospital for Rheumatic Diseases (RNHRD) grant number RNP00267331143E. EC is funded by the NIHR (Senior Research Fellowship, SRF-2013-06-013).
Disclaimer: This report is independent research. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Competing interests: EC is an unpaid medical advisor to the Association for young people with ME and the Sussex & Kent ME/CFS Society.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: This systematic review draws on published research only. No new data were collected or generated in undertaking this study.
References
- 1.Crawley E, Sterne JA. Association between school absence and physical function in paediatric chronic fatigue syndrome/myalgic encephalopathy. Arch Dis Child 2009;94:752–6. 10.1136/adc.2008.143537 [DOI] [PubMed] [Google Scholar]
- 2.Garralda ME, Rangel L. Impairment and coping in children and adolescents with chronic fatigue syndrome: a comparative study with other paediatric disorders. J Child Psychol Psychiatry 2004;45:543–52. 10.1111/j.1469-7610.2004.00244.x [DOI] [PubMed] [Google Scholar]
- 3.Garralda ME, Chalder T. Practitioner review: chronic fatigue syndrome in childhood. J Child Psychol Psychiatry 2005;46:1143–51. 10.1111/j.1469-7610.2005.01424.x [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie C, Wray A. Chronic fatigue syndrome in children and young people. Paediatr Child Health 2013;23:35–9. 10.1016/j.paed.2012.10.001 [DOI] [Google Scholar]
- 5.Fukuda K, Straus SE, Hickie I et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med 1994;121:953–9. 10.7326/0003-4819-121-12-199412150-00009 [DOI] [PubMed] [Google Scholar]
- 6.Sharpe MC, Archard LC, Banatvala JE et al. A report—chronic fatigue syndrome: guidelines for research. J R Soc Med 1991;84:118–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.NICE. Chronic fatigue syndrome/myalgic encephalomyelitis (or encephalopathy): diagnosis and management of CFS/ME in adults and children. Excellence NIfHaC, 2007. [Google Scholar]
- 8.Reeves WC, Wagner D, Nisenbaum R et al. Chronic fatigue syndrome—a clinically empirical approach to its definition and study. BMC Med 2005;3:19 10.1186/1741-7015-3-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight S, Harvey A, Towns S et al. How is paediatric chronic fatigue syndrome/myalgic encephalomyelitis diagnosed and managed by paediatricians? An Australian Paediatric Research Network Study. J Paediatr Child Health 2014;50:1000–7. 10.1111/jpc.12677 [DOI] [PubMed] [Google Scholar]
- 10.Bould H, Collin SM, Lewis G et al. Depression in paediatric chronic fatigue syndrome. Arch Dis Child 2013;98:425–8. 10.1136/archdischild-2012-303396 [DOI] [PubMed] [Google Scholar]
- 11.Ford T, Goodman R, Meltzer H. The British child and adolescent mental health survey 1999: the prevalence of DSM-IV disorders. J Am Acad Child Adolesc Psychiatry 2003;42:1203–11. 10.1097/00004583-200310000-00011 [DOI] [PubMed] [Google Scholar]
- 12.Zhou X, Michael KD, Liu Y et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry 2014;14:340 10.1186/s12888-014-0340-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walford GA, Nelson WM, McCluskey DR. Fatigue, depression, and social adjustment in chronic fatigue syndrome. Arch Dis Child 1993;68:384–8. 10.1136/adc.68.3.384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fombonne E, Wostear G, Cooper V et al. The Maudsley long-term follow-up of child and adolescent depression. 1. Psychiatric outcomes in adulthood. Br J Psychiatry 2001;179:210–17. 10.1192/bjp.179.3.210 [DOI] [PubMed] [Google Scholar]
- 15.Wearden AJ, Dunn G, Dowrick C et al. Depressive symptoms and pragmatic rehabilitation for chronic fatigue syndrome. Br J Psychiatry 2012;201:227–32. 10.1192/bjp.bp.111.107474 [DOI] [PubMed] [Google Scholar]
- 16.White PD, Naish VA. Graded exercise therapy for chronic fatigue syndrome. Physiotherapy 2001;87:285–8. 10.1016/S0031-9406(05)60762-6 [DOI] [Google Scholar]
- 17.Kempke S, Goossens L, Luyten P et al. Predictors of outcome in a multi-component treatment program for chronic fatigue syndrome. J Affect Disord 2010;126:174–9. 10.1016/j.jad.2010.01.073 [DOI] [PubMed] [Google Scholar]
- 18.Flo E, Chalder T. Prevalence and predictors of recovery from chronic fatigue syndrome in a routine clinical practice. Behav Res Ther 2014;63:1–8. 10.1016/j.brat.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 19.Darbishire L, Seed P, Risdale L. Predictors of outcome following treatment for chronic fatigue. Br J Psychiatry 2005;186:350–1. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda Y, Matsui T, Kataoka K et al. A two-year follow-up study of chronic fatigue syndrome comorbid with psychiatric disorders. Psychiatry Clin Neurosci 2009;63:365–73. 10.1111/j.1440-1819.2009.01954.x [DOI] [PubMed] [Google Scholar]
- 21.Schreurs KMG, Veehof MM, Passade L et al. Cognitive behavioural treatment for chronic fatigue syndrome in a rehabilitation setting: effectiveness and predictors of outcome. Behav Res Ther 2011;49:908–13. 10.1016/j.brat.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 22.Prins J, Bleijenberg G, Rouweler EK et al. Effect of psychiatric disorders on outcome of cognitive-behavioural therapy for chronic fatigue syndrome. Br J Psychiatry 2005;187:184–5. [DOI] [PubMed] [Google Scholar]
- 23.RCPCH. Evidence Based Guideline for the Management of CFS/ME (Chronic Fatigue Syndrome/Myalgic Encephalopathy) in Children and Young People. Royal College of Paediatrics and Child Health, 2004. [Google Scholar]
- 24.Higgins JPT, Altman DG, Gøtzsche PC et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. Cochrane handbook for systematic reviews of interventions version 50, 2008.
- 26.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Wiley Online Library, 2008. [Google Scholar]
- 27.Gordon BA, Knapman LM, Lubitz L. Graduated exercise training and progressive resistance training in adolescents with chronic fatigue syndrome: a randomized controlled pilot study. Clin Rehabil 2010;24:1072–9. 10.1177/0269215510371429 [DOI] [PubMed] [Google Scholar]
- 28.Chalder T, Tong J, Deary V. Family cognitive behaviour therapy for chronic fatigue syndrome: an uncontrolled study. Arch Dis Child 2002;86:95–7. 10.1136/adc.86.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd S, Chalder T, Sallis HM et al. Telephone-based guided self-help for adolescents with chronic fatigue syndrome: a non-randomised cohort study. Behav Res Ther 2012;50:304–12. 10.1016/j.brat.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 30.Kawatani J, Mizuno K, Shiraishi S et al. Cognitive dysfunction and mental fatigue in childhood chronic fatigue syndrome—a 6-month follow-up study. Brain Dev 2011;33:832–41. 10.1016/j.braindev.2010.12.009 [DOI] [PubMed] [Google Scholar]
- 31.Gordon BA, Lubitz L. Promising outcomes of an adolescent chronic fatigue syndrome inpatient programme. J Paediatr Child Health 2009;45:286–90. 10.1111/j.1440-1754.2009.01493.x [DOI] [PubMed] [Google Scholar]
- 32.Rimes KA, Goodman R, Hotopf M et al. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: a prospective community study. Pediatrics 2007;119:e603–9. 10.1542/peds.2006-2231 [DOI] [PubMed] [Google Scholar]
- 33.van de Putte EM, Engelbert RH, Kuis W et al. Alexithymia in adolescents with chronic fatigue syndrome. J Psychosom Res 2007;63:377–80. 10.1016/j.jpsychores.2007.07.009 [DOI] [PubMed] [Google Scholar]
- 34.Henderson TA. Valacyclovir treatment of chronic fatigue in adolescents. Adv Mind Body Med 2014;28:4–14. [PubMed] [Google Scholar]
- 35.Jason LA, Bell DS, Rowe K et al. A pediatric case definition for myalgic encephalomyelitis and chronic fatigue syndrome. J Chron Fatigue Syndr 2006;13:1–44. 10.1300/J092v13n02_01 [DOI] [Google Scholar]
- 36.Denborough P, Kinsella S, Stevens J et al. Evaluation of a multidisciplinary inpatient rehabilitation programme for adolescents with chronic fatigue syndrome. Australas Psychiatry 2003;11:319–24. 10.1046/j.1440-1665.2003.00559.x [DOI] [Google Scholar]
- 37.Browne T, Chalder T. Chronic fatigue syndrome. Psychiatry 2006;5:48–51. 10.1383/psyt.2006.5.2.48 [DOI] [Google Scholar]
- 38.NICE. Depression in children and young people: psychological interventions for mild depression and pharmacological interventions for moderate to severe depression (update) 2015.
- 39.NICE. Depression in children and young people: identification and management in primary, community and secondary care. Excellence NIoHaC; 2005. [PubMed] [Google Scholar]
- 40.Haig-Ferguson A, Tucker P, Eaton N et al. Memory and attention problems in children with chronic fatigue syndrome or myalgic encephalopathy. Arch Dis Child 2009;94:757–62. 10.1136/adc.2008.143032 [DOI] [PubMed] [Google Scholar]
- 41.March J, Silva S, Petrycki S et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA 2004;292:807–20. 10.1001/jama.292.7.807 [DOI] [PubMed] [Google Scholar]
- 42.Emslie GJ, Mayes T, Porta G et al. Treatment of resistant depression in adolescents (TORDIA): week 24 outcomes. Am J Psychiatry 2010;167:782–91. 10.1176/appi.ajp.2010.09040552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry 2006;63:332–9. 10.1001/archpsyc.63.3.332 [DOI] [PubMed] [Google Scholar]
- 44.Vercoulen JHMM, Hoofs MPE, Bleijenberg G et al. Randomised, double-blind, placebo-controlled study of fluoxetine in chronic fatigue syndrome. Lancet 1996;347:858–61. 10.1016/S0140-6736(96)91345-8 [DOI] [PubMed] [Google Scholar]
- 45.Wearden AJ, Morriss RK, Mullis R et al. Randomised, double-blind, placebo-controlled treatment trial of fluoxetine and graded exercise for chronic fatigue syndrome. Br J Psychiatry 1998;172:485–90. 10.1192/bjp.172.6.485 [DOI] [PubMed] [Google Scholar]
- 46.Amsterdam JD, Shults J, Rutherford N. Open-label study of s-citalopram therapy of chronic fatigue syndrome and co-morbid major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008;32:100–6. 10.1016/j.pnpbp.2007.07.019 [DOI] [PubMed] [Google Scholar]
- 47.Nijhof SL, Bleijenberg G, Uiterwaal CS et al. Fatigue in teenagers on the interNET—the FITNET Trial. A randomized clinical trial of web-based cognitive behavioural therapy for adolescents with chronic fatigue syndrome: study protocol. [ISRCTN59878666]. BMC Neurol 2011;11:23 10.1186/1471-2377-11-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nijhof SL, Priesterbach LP, Bleijenberg G et al. Functional improvement is accompanied by reduced pain in adolescent chronic fatigue syndrome. Pain Med 2013;14:1435–8. 10.1111/pme.12181 [DOI] [PubMed] [Google Scholar]
- 49.Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-012271supp.pdf (247.7KB, pdf)