Abstract
Introduction
The high prevalence of insulin resistance in women with polycystic ovary syndrome (PCOS) is considered to be one of the major pathophysiological changes in PCOS that leads to anovulatory infertility. We hypothesise that electroacupuncture pretreatment improves insulin sensitivity and leads to a higher ovulation rate and greater chances of live birth after the induction of ovulation. The effect of electroacupuncture pretreatment followed by ovulation induction in women with anovulatory PCOS has not been investigated before, and we present here a randomised controlled trial to test this hypothesis by comparing electroacupuncture pretreatment followed by letrozole versus letrozole alone in anovulatory women with PCOS.
Methods/analysis
This is a multicentre, randomised,and controlled trial. A total of 384 patients will be enrolled in this study and will be randomly allocated by a central randomisation system to the treatment group or the control group in a 1:1 ratio. The treatment group will undergo 16 weeks of electroacupuncture pretreatment followed by 4 cycles of letrozole, and the control group will only undergo 4 cycles of letrozole. The primary outcome will be the live birth rate. All statistical analyses will be performed using the SPSS program V.21.0 (SPSS, Chicago, Illinois, USA), and a p value <0.05 will be considered statistically significant.
Ethics/dissemination
This study has been approved by the ethics committees of each participating centre. Written consent will be obtained from each patient and her husband before any study procedure is performed. Adverse events will be categorised, and the percentage of patients experiencing adverse events or serious adverse events during the treatment period will be documented. The results of this trial will be disseminated in peer-reviewed journals and presented at international meetings.
Trial registration number
Keywords: acupuncture pretreatment, live birth rate, letrozole, polycystic ovary syndrome
Strengths and limitations of this study.
This is the first randomised controlled trial comparing electroacupuncture pretreatment followed by letrozole versus letrozole alone on live birth in anovulatory infertile women with polycystic ovary syndrome (PCOS).
This is the first trial that seeks to improve insulin sensitivity in women with PCOS by using electroacupuncture as the pretreatment.
Complications during pregnancy will also be recorded to verify the prospective effects of acupuncture on women with PCOS in this study.
Placebo or sham acupuncture will not be offered in the control group because it might not be truly inert and might still have some physiological effects.
The researchers, acupuncturists and the participants cannot be blinded to treatment allocation.
Background
Polycystic ovary syndrome (PCOS) is a common endocrine and metabolic disorder affecting 6–10% of women of reproductive age,1 and it accounts for 70–80% of anovulatory infertility.2 PCOS is characterised by amenorrhoea, hirsutism, acne, obesity, polycystic ovaries and infertility, and it can have serious effects on the general health and quality of life. Insulin resistance (IR), hyperinsulinaemia and dyslipidaemia worsen with ageing, and the risk of miscarriage is three times greater in women with PCOS compared with healthy women.3 Women with PCOS are also at an increased risk of pregnancy complications such as impaired glucose tolerance, gestational diabetes mellitus, pregnancy-induced hypertension and pre-eclampsia, and small for gestational age (SGA) children.3 Thus, the metabolic abnormalities of PCOS can affect the woman's health as well as that of her children.3
Ovulation induction in women with PCOS suffering from anovulatory infertility includes the use of antioestrogen, insulin sensitisers, aromatase inhibitors, gonadotropin and surgical treatment.4 Clomiphene citrate has been the first-line medication, but a recent large multicentre randomised study5 demonstrated that letrozole, an aromatase inhibitor, results in a higher ovulation rate and a higher live birth rate than clomiphene citrate.
Despite the fact that pharmacological methods of ovulation induction are often effective, a large number of patients still have difficulty conceiving and they have a high risk of miscarriage that might be related to reduced endometrial receptivity.6 The high prevalence of IR (up to 60%) in women with PCOS is considered to be one of the major pathophysiological changes in PCOS7 8 and studies indicate that IR and hyperinsulinaemia affect endometrial receptivity in many ways, leading to difficulty in conceiving and an increase in miscarriage.6
Acupuncture is becoming popular in research and clinical practice, and recent studies indicate that acupuncture can increase the ovulation rate,9–11 improve endocrine profile by decreasing circulating sex steroids, increase menstrual frequency12 13 and decrease weight.14 15 It has been shown that acupuncture can regulate endogenous systems, including the sympathetic nervous system, the endocrine system and the neuroendocrine system.16 17 Acupuncture appears to improve endometrial receptivity in rats18 19 by decreasing the impedance of the uterine artery blood flow and improving the blood flow to the uterus,20 which might enhance implantation and increase the chances of successful pregnancy and live birth.
Furthermore, electroacupuncture has been shown to improve insulin sensitivity in experimental trials. Electroacupuncture can significantly enhance the decrease in blood glucose levels after insulin injection, suggesting an improvement in insulin sensitivity.21 The result indicates that electroacupuncture improves glucose tolerance both in wild-type and transgenic mice fed a high-fat diet, and that the effect is related to the stimulation parameters used and is probably due to the reduction of free fatty acid.21 In rat PCOS models, repeated acupuncture has also been demonstrated to normalise insulin sensitivity.22 23 Another study showed that electroacupuncture can normalise insulin sensitivity, and ameliorate IR and hyperinsulinemia in rats with PCOS, probably by regulating the function of pancreatic islets β-cells and by reducing oxidative stress and free androgen.24 During stimulation, electroacupuncture improves insulin sensitivity in a dihydrotestosterone-induced rat PCOS model more than does acupuncture with manual stimulation. However, poststimulation, both electrical stimulation and manual stimulation show equal effects, and this might be due to the activation of sensory afferents by both methods.25
Until now, there are still no studies investigating whether low-frequency electroacupuncture pretreatment followed by ovulation induction will increase the live birth rate. On the basis of previous studies, we hypothesise that acupuncture pretreatment improves insulin sensitivity and leads to a higher ovulation rate and higher live birth rate. This study is intended to test this hypothesis.
Objective
The objective of this multicentre randomised controlled trial (RCT) is to evaluate whether 16 weeks of acupuncture pretreatment followed by letrozole compared with letrozole alone leads to a higher live birth rate in Chinese women with PCOS and anovulatory infertility.
Methods/design
Study design
This is a multicentre, randomised, and controlled trial comparing the live birth rate after acupuncture pretreatment followed by letrozole versus letrozole alone for ovulation induction in Chinese women with PCOS and anovulatory infertility. Reporting of the study results will follow the STRICTA26 and CONSORT guidelines.27
This study is referred to as the Clinical Trial of Acupuncture Pre-treatment on PCOS (PCOSAPct). It has been registered at clinicaltrial.gov (NCT02491320) and with the Chinese Clinical Trials Registry (ChiCTR-ICR-15006640).
Participants
A total of 384 women with PCOS and anovulatory infertility will be recruited from three hospitals in mainland China: the Department of Traditional Chinese Medicine in the First Affiliated Hospital of Guangzhou Medical University, the Department of Gynecology in Xuzhou Maternity and Child Health Hospital, and the Department of Traditional Chinese Medicine in Hexian Memorial Affiliated Hospital of Southern Medical University. Each centre will screen participants until the target population of 128 participants/site is achieved. After detailed explanation, counselling and signing the informed consent form, the eligible participants will be randomly allocated to either the electroacupuncture pretreatment with letrozole group or the letrozole-only group.
Inclusion criteria
Women aged between 20 and 40 years.
Confirmed diagnosis of PCOS according to the Rotterdam criteria: oligomenorrhoea (an intermenstrual interval>35 days or <8 cycles in the past year) or amenorrhoea (an intermenstrual interval>90 days) together with polycystic ovarian morphology, that is, the presence of ≥12 antral follicles (≤9 mm) and/or ovarian volume >10 mL on transvaginal scanning, and/or clinical or biochemical hyperandrogenism. Clinical hyperandrogenism in China is defined as a Ferriman-Gallwey (FG) score ≥5,28 and biochemical hyperandrogenism is defined as total testosterone (T) >2.6 nmol/L and free testosterone ≥6.0 pg/mL.29
A husband whose sperm concentration meets the WHO standards (2010) of ≥15×106/mL and a total motility of ≥40% or a total motile sperm count of ≥9 million.
At least one patent tube shown by hysterosalpingogram or diagnostic laparoscopy within 3 years if the patient does not have a history of abortion or pelvic operation. If the patient has a history of pregnancy and no history of pelvic operation within the past 5 years, she is not required to undergo a tubal patency test.
Exclusion criteria
- Exclusion of other endocrine disorders:
- Patients with hyperprolactinaemia (defined as 2 prolactin (PRL) levels of ≥25 ng/mL at least 1 week apart or as determined by local normative values). The goal of eliminating patients with documented hyperprolactinaemia is to decrease the heterogeneity of the PCOS population. These patients might be candidates for ovulation induction with alternate regimens (dopamine agonists). A normal level within the past year or being on treatment is adequate for entry.
- Patients with follicle-stimulating hormone (FSH) levels >15 mIU/mL. A normal level within the past year is adequate for entry.
- –Patients with uncorrected thyroid disease (defined as thyroid-stimulating hormone (TSH) <0.2 or >5.5 mIU/mL). A normal level within the past year is adequate for entry.
- Patients diagnosed with type I or type II diabetes who are poorly controlled (defined as a glycated haemoglobin (HbA1c) level >7.0%) or patients receiving antidiabetic medications such as insulin, thiazolidinediones, acarbose or sulfonylureas that are likely to confound the effects of electroacupuncture. Patients currently receiving metformin XR (extended release) for a diagnosis of type I or type II diabetes or for PCOS are also specifically excluded.
- Patients with suspected Cushing's syndrome.
Use of hormonal or other medication, including Chinese herbal prescriptions, in the past 2 months that might affect the outcome of the study treatment.
Acupuncture in the past 2 months.
Pregnancy within the past 6 weeks.
Abortion or having given birth in the past 6 weeks.
Breast feeding within the past 6 months.
Not willing to give written consent to the study.
Patients enrolled simultaneously in other investigative studies that require medications, prohibit the use of the study medications, limit intercourse or otherwise prevent compliance with the study protocol.
Patients who anticipate taking longer than a 1 month break from treatments during the study protocol.
- Additional exclusion criteria:
- Patients with a suspected adrenal or ovarian tumour that is secreting androgens.
- Couples with previous sterilisation procedures (vasectomy, tubal ligation) that have been reversed. The prior procedure might affect the study outcomes, and patients with both a reversed sterilisation procedure and PCOS are rare enough that exclusion should not adversely affect recruitment.
- Participants who have undergone a bariatric surgery procedure in the recent past (<12 months) and are in a period of acute weight loss or who have been advised against pregnancy by their bariatric surgeon.
- Patients with untreated or poorly controlled hypertension defined as a systolic blood pressure of 160 mm Hg or a diastolic blood pressure of 100 mm Hg obtained on two occasions at least 60 min apart.
- Patients with known congenital adrenal hyperplasia.
- Patients on oral contraceptives, depot progestins or hormonal implants (including Implanon). A 2-month washout period will be required prior to screening for patients on these agents. Longer washouts might be necessary for certain depot contraceptive forms or implants, especially when the implants are still in place. A 2-month washout will be required for patients on oral cyclic progestins.
- Patients with liver (LR) disease defined as aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >2 times normal or total bilirubin >2.5 mg/dL or patients with renal disease defined as blood urea nitrogen (BUN) >30 mg/dL or serum creatinine >1.4 mg/dL.
- Patients with significant anaemia (haemoglobin <10 g/dL).
- Patients with a history of deep venous thrombosis, pulmonary embolus or cerebrovascular accident.
- Patients with known heart disease that is likely to be exacerbated by pregnancy.
- Patients with a history of, or suspected, cervical carcinoma, endometrial carcinoma or breast carcinoma. A normal Pap smear or thinprep cytologic test (TCT) result will be required for women 21 years and older.
- Patients with a current history of alcohol abuse. Alcohol abuse is defined as >14 drinks/week or binge drinking.
- Patients taking other medications known to affect reproductive function or metabolism. These medications include oral contraceptives, gonadotropin-releasing hormone (GnRH) agonists and antagonists, antiandrogens, gonadotropins, antiobesity drugs, Chinese herbal formulas, antidiabetic drugs such as metformin and thiazolidinediones, somatostatin, diazoxide, ACE inhibitors and calcium channel blockers. The washout period for all of these medications will be 2 months.
Randomisation and allocation concealment
A central randomisation system will be used to allocate patients. The random number sequence will be generated by computer software and the allocation sequence will be made by Chinese Clinical Trial Registry with the web-based Research Management (ResMan) database. When a new participant is enrolled, the allocation information will be recorded with a special code in ResMan. There is no way to speculate on the allocation status of the participant before getting this information.
The patients in the treatment group will receive acupuncture treatment before ovulation induction, but the control group will not receive acupuncture treatment. Since the intervention in this study consists of the distribution of acupuncture, the researchers, acupuncturists and participants cannot be blinded to treatment allocation. To prevent contamination, participants in the treatment group will not be allowed to contact the control participants.
Interventions
Eligible patients will be randomised into one of the two arms.
Treatment group—a 16-week acupuncture pretreatment followed by letrozole. Electroacupuncture treatment will start on days 3–5 after a spontaneous period or after a withdrawal bleeding following progestin (100 mg twice a day for 5 days, Zhejiang Xianju Pharmaceutical Co, Taizhou). All participants will be requested to use contraception during the 16-week pretreatment with electroacupuncture according to a fixed protocol (see below). They will receive electroacupuncture treatment three times a week. Each treatment session will last for 30 min, with a maximum of 48 treatment sessions over 16 weeks. After 16 weeks of acupuncture treatment, letrozole will start on days 2–3 after a spontaneous period or after a withdrawal bleeding after progestin administration. The participants will be treated for up to four cycles, and they will be instructed to have intercourse on a regular basis during the cycles.
Control group—letrozole only. Letrozole will be started on days 3–5 after a spontaneous period or a withdrawal bleeding following progestin. Participants will be treated for up to four cycles, and they will be instructed to have intercourse on a regular basis during the cycles.
Acupuncture protocol
The rationale for the acupuncture protocol is based on traditional Chinese and Western medical theories, and the study protocol follows the CONSORT27 and STRICTA26 recommendations with detailed descriptions of the treatment, including the number of needles used, how the needles will be stimulated (manual, electrical), the frequency of the sessions and the length of treatment period. We will use a fixed acupuncture protocol. Acupuncture treatment will start on days 3–5 after a spontaneous period or after a withdrawal bleeding following progestin. All participants will receive acupuncture treatment for three sessions a week. Each treatment session will last for 30 min, and there will be a maximum of 48 treatment sessions over the 16 weeks. The acupuncture protocol of this RCT follows the protocol inClinicalTrial.gov NCT01457209 and NCT02026323.
Acupuncture treatment will be given by a Traditional Chinese Medicine doctor with a master's degree and at least 1 year of clinical experience in acupuncture. Before the start of the study, we will conduct a one-day study-specific education, including theoretical sessions with a traditional Chinese and Western medical approach to acupuncture physiology, practical sessions with demonstrations of acupuncture treatment and lectures on research methodology with a focus on RCTs.
Disposable, single-use, sterilised needles made of stainless steel, 0.25 mm×30 mm and 0.30 mm×40/50 mm (Huanqiu, Suzhou Acupuncture Goods Co, Suzhou, China), will be inserted to a depth of 15–35 mm in segmental acupuncture points. Two sets of acupuncture points will be alternated every other treatment (table 1).30 The first set will consist of conception vessel (CV) 3, CV12 and stomach (ST) 29 bilaterally and in the muscles above the knee, ST34 and ST33 bilaterally and below the knee, and spleen (SP) 6 and ST36. Needles will also be placed bilaterally in the extrasegmental large intestine (LI) 4 acupuncture point that does not innervate the ovaries. In total, 14 needles will be placed, and all will be stimulated manually by rotating the needle to evoke needle sensation (DeQi) once inserted. The following points will be connected to an electrical stimulator (Export Abteilung, Schwa-Medico GmbH, Wetzlarer Str. 41-43; 35630 Ehringshausen): CV3 to CV12, ST29 bilateral, and ST34 to ST33 bilateral. Electroacupuncture stimulation will be given at a frequency of 2 Hz with a 0.3 ms pulse length and intensity adjusted to produce local muscle contractions without pain or discomfort. Needles not connected to the electrical stimulator will be manually stimulated to evoke needle sensation every 10 min for a total of four stimulations. The second set consists of 14 needles placed in segmental abdominal points ST27 bilaterally and CV6 connected to CV10 (electrical stimulation), leg points SP10 connected to a non-acupuncture point located 6 cun proximal to the patella’s medial border (electrical stimulation) and SP6, and LR3 bilaterally (manual stimulation). Extrasegmental points are pericardium (PC) 6 bilaterally (manual stimulation).
Table 1.
Acupuncture protocol30
| Point | Stimulation | Location | Muscle | Muscle innervation |
|---|---|---|---|---|
| Set 1 | ||||
| Zhongji (CV3) | EA | 4 cun caudal to the umbilicus | Fibrous tissue, linea alba | L1 |
| Zhongwan (CV12) | EA | On the midline, 4 cun superior to the umbilicus | Fibrous tissue, linea alba | Th7–8 |
| Guilai (bilateral) (ST29) | EA | 1 cun cranial to the pubic bone and 2 cun lateral to the midline | M. rectus abdominis | Th6–12 |
| Liangqiu (bilateral) (ST34) | EA | 2 cun above the superior lateral border of the patella on the line connecting the anterior superior iliac spine found with the knee flexed | M. quadriceps femoris | Femoral nerve |
| Yinshi (bilateral) (ST33) | EA | 3 cun above the superior lateral border of the patella on the line connecting the anterior superior iliac spine found with the knee flexed | M. quadriceps femoris | Femoral nerve |
| Sanyinjiao (bilateral) (SP6) | DeQi 4 times | 3 cun proximal to the medial malleolus | Mm. flexor digitorum longus, tibialis posterior | L4–5, S1–2 |
| Zusanli (bilateral) (ST36) | DeQi 4 times | On the anterior lateral side of the leg, 3 cun below Dubi (ST35), one finger width (middle finger) from the anterior crest of the tibia | M. tibialis anterior | L4–5, S1 |
| Hegu (bilateral) (LI4) | DeQi 4 times | On the highest point at the musculi interosseus dorsalis | Mm. interosseus dorsalis I, lumbricalis II, adductor pollicis | C8, Th1 |
| Set 2 | ||||
| Daju (bilateral) (ST27) | EA | 3 cun cranial to the pubic bone and 2 cun lateral to the midline | M. rectus abdominis | Th6–12 |
| Qihai (CV6) | EA | 1.5 cun caudal to the umbilicus | Fibrous tissue, linea alba | Th11 |
| Xiawan (CV10) | EA | 2 cun cranial to the umbilicus | Fibrous tissue, linea alba | Th8 |
| Extrameridian point (bilateral) | EA | 6 cun above the patella in line with SP10 | M. quadriceps femoris | L2–4 |
| Xuehai (bilateral) (SP10) | EA | With the knee flexed, on the medial side of the thigh 2 cun above the superior medial corner of the patella on the prominence of the medial head of the quadriceps muscle of the thigh | M. quadriceps femoris | L2–4 |
| Sanyinjiao (bilateral) (SP6) | DeQi 4 times | 3 cun proximal to the medial malleolus | Mm. flexor digitorum longus, tibialis posterior | L4–5, S1–2 |
| Taichong (bilateral) (LR3) | DeQi 4 times | Between metatarsal I and II, just distal to the caput | M. interosseus dorsalis I | S2–3 |
| Neiguan (bilateral) (PC6) | DeQi 4 times | 2 cun proximal to the processus styloideus radii, between the tendons of the palmaris longus and the flexor carpi radialis | M. flexor digitorum superficialis | C8, Th1 |
The two sets will be alternated for every other treatment.
C, cervical vertebra; CV, conception vessel; EA, electroacupuncture; L, lumbar vertebra; LI, large intestine; LR, liver; M., musculi; Mm., musculus; PC, pericardium; S, sacral vertebra; SP, spleen; ST, stomach; Th, thoracic vertebra.
Needle insertion technique
Needle insertion should be gentle. The depth of the needle insertion will vary from patient to patient depending on their body mass index (BMI), but they should be placed deep enough to reach muscle/fibrous tissue. When the needles are inserted, they will be gently rotated until DeQi (needle sensation reflecting activation of sensory afferents) is obtained. The needles connected to the electric stimulator will be stimulated at 2 Hz and 300 revolutions per minute.
Letrozole
Patients in the treatment group following 16 weeks of acupuncture pretreatment and those in the control group will receive letrozole (Femara, Novartis Pharmaceuticals, Basel, Switzerland) starting from 2.5 mg (1 pill) daily from days 3–5 after a spontaneous period or after a withdrawal bleeding following progestin and will continue for 5 days. If there is an adequate response with ovulation (ie, the serum progesterone level on the 3rd week of the cycle is higher than 3 ng/mL) or if the increase in progesterone is delayed by 1 week, this dose will be maintained. In patients with no response, the letrozole dose will be increased to 5 mg (2 pills) a day for 5 days. If there is still no response, the dose will be increased to 7.5 mg per day for 5 days in the next cycle. The maximum daily dose of letrozole will be 7.5 mg (3 pills) daily. Participants will be treated for up to four cycles. If the patient becomes pregnant, the treatment will be stopped.
Study-specific visits and procedures
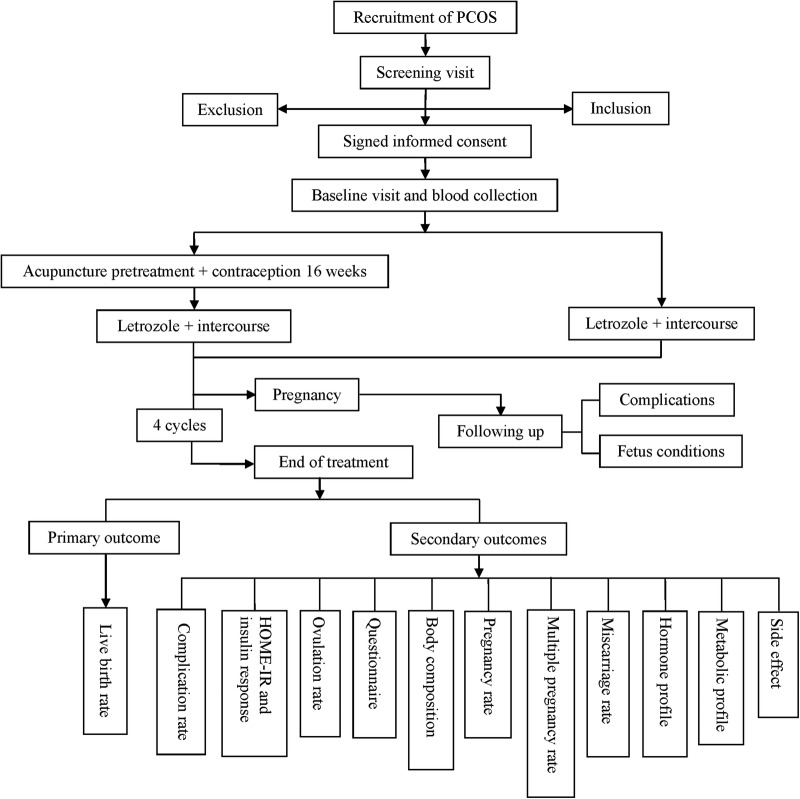
The trial phase will involve treatment with electroacupuncture pretreatment for 16 weeks followed by four cycles of letrozole or four cycles of letrozole alone (figure 1). Patients will attend up to seven visits, including the screening visit, baseline visit, treatment visit, end of pretreatment visit, pregnancy visit, end of treatment visit and follow-up visit. Adverse events and concomitant medications will be recorded during every visit. Face-to-face adherence reminder sessions will take place at the initial product dispensing and at each study visit thereafter. The overview of the study visits is shown in table 2.
Figure 1.
The study flow chart Main Document. HOMA-IR, homeostatic model assessment of insulin resistance; PCOS, polycystic ovary syndrome.
Table 2.
Overview of study visits
| Screening visit | Baseline visit | Treatment visit | End of pretreatment visit | Pregnancy visit | End of treatment visit | Follow-up visit | |
|---|---|---|---|---|---|---|---|
| Physical examination | √ | √ | |||||
| Menstrual cycle/intercourse diary | √ | √ | √ | √ | √ | ||
| Fasting blood samples for metabolic and safety profile | √ | √ | |||||
| Fasting blood samples for sex hormone steroids | √ | √ | |||||
| Preconception counselling | √ | ||||||
| Transvaginal ultrasound | √ | √ | √ | √ | |||
| Questionnaire | √ | √ | |||||
| OGTT and insulin release test | √ | √ | √ | ||||
| Pregnancy test | √ | √ | √ | √ | |||
| Hysterosalpingogram | √ | ||||||
| Semen analysis | √ | ||||||
| TCT | √ | ||||||
| Serum progesterone | √ | ||||||
| Pregnancy and neonatal records | √ | √ | |||||
| Fasting phlebotomy and faeces | √ | √ | |||||
| Query for adverse event and concomitant medications | √ | √ | √ | √ | √ | √ | √ |
Physical examination: weight, height, waist circumference, hip circumference, FG/acne.
Fasting blood samples for metabolic and safety profile: FGLU, FINS, C peptide, HbA1c, TC, TG, HDL-C, LDL-C, CBC, renal and liver profile.
Fasting blood samples for sex hormone steroids: FSH, LH, SHBG, T, free testosterone, E2, TSH and PRL.
Transvaginal ultrasound: endometrial thickness, ovarian volume, antral follicle count, and size of ovarian cysts or developing follicles.
Preconception counselling: TORCH and HIV screening.
Fasting phlebotomy and faeces: serum for the central core laboratory and faeces for microbiological detection.
CBC, complete blood count; E2, estradiol; FG, Ferriman-Gallwey; FGLU, fasting blood glucose; FINS, fasting insulin; FSH, follicle-stimulating hormone; HbA1c, glycated haemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LH, luteinising hormone; OGTT, oral glucose tolerance test; PRL, prolactin; SHBG, sex hormone-binding globulin, T, total testosterone; TC, total cholesterol; TCT, thinprep cytologic test; TG, triglycerides; TORCH, Toxoplasmosis, Rubella, Cytomegalovirus, and Herpes virus; TSH, thyroid-stimulating hormone.
Screening visit
Participants who are interested in participating will first be screened by one of the principal investigators (PIs) to verify that they meet the basic inclusion criteria (ie, age, oligomenorrhoea, partner availability, etc). Those who qualify will be scheduled for a screening visit.
Obtain signed informed consent
Every participant who agrees to participate in this study must sign the informed consent form (see online supplementary appendix 1). Participants will be mailed a study packet with a copy of the informed consent document and other relevant study materials for them to review prior to the visit. The study will be explained in detail, and all questions will be answered prior to signing the written informed consent to participate in the study. The husband will also be required to sign a consent form at the time of the screening visit. It is important to obtain his consent for participation in the study due to the requirements of intercourse and semen analysis in the inclusion criteria and the information collected on the quality-of-life surveys. Additional blood and faeces samples will be obtained from the patients to be stored for DNA analysis and microbiological detection.
bmjopen-2015-010955supp_appendix.pdf (163.6KB, pdf)
Complete physical examination
A complete physical examination, including height, weight, hip and waist measurements, will be performed. Height and weight will be recorded to the nearest 0.1 cm and 0.1 kg, respectively. Waist and hip circumference will be recorded to the nearest 1 cm. Hirsutism will be assessed by FG and acne by standard acne lesion counts; also, a pelvic examination with Pap smear or TCT will be performed.
Transvaginal ultrasound of the uterus and ovaries
Uterine dimensions, endometrial thickness and echo type, other uterine abnormalities, and the presence and size of leiomyoma will be obtained through transvaginal ultrasound.
Ovarian size in three dimensions, the size of the largest ovarian follicle/cyst and the size of every follicle with a mean diameter >10 mm, and total antral follicle count (small follicles with a mean diameter <10 mm) of each ovary will be obtained through transvaginal ultrasound.
Pregnancy test
A urine pregnancy test will be performed to exclude pregnancy.
Laboratory examination
Serum levels of sex hormone steroids, including blood FSH, luteinising hormone (LH), estradiol (E2), PRL, T, free testosterone, sex hormone-binding globulin (SHBG) and TSH, will be evaluated on the second day of the spontaneous period or a withdrawal bleeding.
Fasting blood samples will be taken to determine the metabolic and safety profile, including fasting blood glucose (FGLU), fasting insulin (FINS), C peptide, HbA1c, triglycerides (TG), total cholesterol (TC), apolipoprotein A 1 (ApoA1), apolipoprotein B (ApoB), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and complete blood count (CBC) as well as renal and LR profiles.
The oral glucose tolerance test (OGTT) and insulin release test with 75 g glucose will be performed in all participants after an overnight fast. Blood samples will be obtained to measure plasma glucose and serum insulin at 0, 60 and 120 min during the OGTT and insulin release test.
Complete questionnaires
Quality of life will be assessed using the health-related quality-of-life questionnaire for PCOS (PCOSQ).31
Exclusion of other infertility factors of the couple
The semen quality of the husband and the tubal patency of the patient, including hysterosalpingogram, hysterosalpingocontrast sonography (HyCoSy) or diagnostic laparoscopy with chromotubation, will be assessed.
Preconception counselling
TORCH screening (Toxoplasmosis, Rubella, Cytomegalovirus and Herpes virus), HIV screening and folic acid prescription will be completed.
Progestin withdrawal
Progestin will be prescribed to induce withdrawal bleeding with instructions to begin medication once eligibility is determined.
Baseline visit
▸ Distribute the home pregnancy test strip and folate to each participant.
▸ Distribute intercourse and menstrual journal logs to each participant.
Take blood sample for repository and DNA analysis
A 20 mL blood sample will be collected from each participant; 10 mL will be centrifuged for serum separation, and the other 10 mL will be stored for DNA analysis.
Take faeces sample for microbiological detection
Treatment visit
The patients in the treatment group will be required to use contraception during the first 16 weeks, and all participants will be instructed to have intercourse on a regular basis during the letrozole treatment for ovulation induction. The optimal frequency of intercourse will be once every 2 or 3 days. A urine pregnancy test will be performed after ovulation, and a serum progesterone level test will be performed in the local laboratory during the third week of the ovulation cycle. An elevated level of progesterone >3 ng/mL is considered to be a response.32 If the patient does not have ovulation, the serum progesterone level test will be checked every week until ovulation.
The transvaginal ultrasound examination will take place in the anticipated luteal phase of the cycle, and it will initially be scheduled for 2 weeks after the initiation of medication with a window of 4 days on either side of this day33 (ie, days 12–20, assuming a day 3 medication start, or days 14–22, assuming a day 5 start). The ultrasound includes the endometrial thickness and echogenicity as well as the number, size and echogenic characteristics of follicles/cysts in the ovary (including an antral follicle count).
We will determine whether the patients have anovulation response based on the serum progesterone level and transvaginal ultrasound. There will be three possible scenarios: ovulation, ovulation delay and non-ovulation. (1) Ovulation: a serum progesterone level in the third week >3 ng/mL and ultrasound showing presumptive evidence of ovulation. A urine pregnancy test will be performed to exclude pregnancy, the letrozole treatment will be started on days 3–5 of menstruation, and the dose of letrozole will be maintained. (2) Ovulation delay: a serum progesterone level <3 ng/mL, but ultrasound showing that there is a evidence of follicular development (ie, a follicle with a mean diameter ≥10 mm). In this case, we will wait for one more week, and check the serum progesterone level again. A urine pregnancy test will be performed to exclude pregnancy, letrozole treatment will be started on days 3–5 of menstruation, and the dose of letrozole will be maintained. (3) Non-ovulation: a serum progesterone level <3 ng/mL and ultrasound showing no developing follicles with a mean diameter ≥10 mm. These patients will receive letrozole on days 3–5 of their next cycle and will be instructed to increase letrozole by one tablet per day for 5 days until the maximum daily dose of 7.5 mg (3 pills) daily.
Every menstruation should be recorded, including the date, volume and duration of menstruation.
End of pretreatment visit
Repeat the OGTT and insulin release test.
Collect menstrual journal logs.
Pregnancy visit (only with conception)
Complete the adverse event questionnaire.
Take blood samples for measuring serum quantitative human chorionic gonadotropin (hCG) levels until ultrasound shows the gestational sacs (the threshold level of hCG is 2000–4000 IU/mL).33
Perform transvaginal ultrasound to determine the number of gestational sacs and the location, dimensions, presence and size of fetal structures and to document the visualisation of fetal heart motion and any pregnancy-related abnormalities.
End of treatment visit
Perform a physical examination, including vital signs, height, weight, hip and waist measurements, and repeat the hirsutism and acne assessments after the end of treatment or pregnancy.
Repeat the measurements of serum levels of sex hormone steroids and the metabolic profile.
Repeat the OGTT and insulin release test.
Collect 20 mL of blood and store 10 mL after serum separation with the other 10 mL for DNA analysis.
Repeat the PCOSQ.
Collect the menstrual and intercourse journal logs.
Record adverse events and concomitant medications.
Arrange follow-up for participants who have conceived and obtain release of records for pregnancy and neonatal records.
Follow-up visit
For those women who have an ongoing pregnancy, arrangements will be made to follow the outcome of the pregnancy at the end of the first trimester and also after delivery or termination of gestation. All pregnancies (including multiples) will be followed to monitor weight, glucose tolerance, blood pressure and fetal growth and to determine the abortion rate, complication rate and pregnancy outcomes. The glucose tolerance will be measured by OGTT in all pregnancies at 24–28 weeks of pregnancy. Patients will be informed to notify the study personnel of the outcome of the pregnancy, and we will obtain release of record forms from treating physicians to obtain copies of relevant medical records. Delivery records of both the mother and newborn will be requested to determine the birth weight, length of gestation, and any prenatal complications of the mother or neonatal complications of the infant. Phone contacts will be initiated if the patient has not contacted the study personnel within 6 weeks of the original estimated date of childbirth.
We will collect pregnancy outcome data, and we will track the outcomes of all participants who have a positive serum pregnancy screen during the course of this study. We will record biochemical pregnancies (defined as positive serum pregnancy screens without ultrasonically detected pregnancies), ectopic pregnancies and all intrauterine pregnancy losses both before and after 20 weeks, including missed abortions, spontaneous abortions, elective abortions, fetal deaths and stillbirths.
Outcome measures
Primary outcome
Live birth rate defined as a delivery after ≥20 weeks gestation.
Secondary outcomes
Ovulation rate;
Ongoing pregnancy rate (around 8–10 weeks gestation);
Multiple pregnancy rate;
Miscarriage rate (loss of an intrauterine pregnancy before 20 completed weeks of gestation);
Complications of pregnancy, including SGA, low birth weight, preterm delivery, pre-eclampsia, antepartum haemorrhage, congenital anomaly and perinatal mortality;
Hormone profile: FSH, LH, T, and SHBG;
Metabolic profile: glucose and insulin concentrations, C peptide, HbA1c, TC, TG, HDL-C, and LDL-C;
The homeostatic model assessment of insulin resistance (HOMA-IR) index and the insulin response to glucose assessed by calculating the area under the curve during the OGTT and insulin release test performance for glucose (AUCglu) and insulin (AUCins) using the trapezoidal rule;34
Body composition: weight, BMI, waist-to-hip circumference, FG score and acne lesion counts;
PCOSQ Questionnaire;
Side effect profile.
Safety analysis
The major risks to the participant are side effects from letrozole and acupuncture and the risks of pregnancy. The most common adverse reactions (>20%) of letrozole are hot flashes, arthralgia, flushing, asthenia, oedema, arthralgia, headache, dizziness, hypercholesterolaemia, increased sweating, bone pain and musculoskeletal pain.35 The major risks of acupuncture are local skin irritation, discomfort and vasovagal reactions during the procedure.30 Adverse events will be categorised, and the percentage of patients experiencing adverse events and serious adverse events during the treatment period will be documented and reported to the Data and Safety Monitoring Board (DSMB). χ2 Tests will be performed to examine differences in the proportions of total events and categories of adverse events.
Interim analysis
We propose not to perform an interim analysis, and the final data analysis will be completed after all live births in the trial.
Statistics
Statistical tests
The Kolmogorov-Smirnov test will be used to test the normal distribution of continuous variables. Continuous variables will be shown as means±SDs if they are normally distributed or as medians with IQRs if they are not normally distributed. Statistical comparison will be carried out according to the intention to treat by Student's t-test, Mann-Whitney U-test and Wilcoxon signed ranks test for continuous variables and by χ2 tests for dichotomous variables where appropriate. A p value <0.05 will be considered statistically significant.
Comparisons of variables such as live birth rate, ovulation rate, ongoing pregnancy rate, multiple pregnancy rate and miscarriage rate will include relative risk (RR) and 95% CIs in addition to the χ2 test.
All statistical analyses of the data will be performed using the SPSS program V.21.0 (SPSS, Chicago, Illinois, USA).
Sample size estimation
We hypothesise that the live birth rate after letrozole for four cycles is 35% and the live birth rate after 16 weeks of acupuncture pretreatment followed by letrozole for four cycles is 55%. Thus, the difference between the two groups will be 20% (δ=0.2); the α error and β error are estimated as 0.05 and 0.20, respectively, and the power will be 80%. This will require a sample size of 96 participants in both groups. A total of 60% of the women with PCOS will be assumed to have IR (IR is defined by HOMA-IR, which is FINS concentration×fasting glucose concentration/22.5, and a value ≥2.14 is considered to be indicative of IR36), which will require 160 patients per group. The sample size is inflated from 160 to 192 per group to allow for a dropout rate of slightly over 20%, and this gives a total of 384 cases for the two groups.
Imputation procedure for missing data
We will report reasons for withdrawal for both randomisation groups and compare the reasons qualitatively. The effect that any missing data might have on the results will be assessed via sensitivity analysis of augmented data sets. Dropouts (essentially participants who withdraw consent for continued follow-up) will be included in the analysis by modern imputation methods for missing data.
Data management and quality control of the data
Case report forms (CRFs) will be developed for recording and depositing the individual patient data, and the web-based public clinical platform ResMan will be used to manage the data. Quality control of the data will be handled at three different levels. The investigators are required to ensure the accuracy of the data as the first level of control. The second level will include data monitoring and validation via ResMan on a regular basis by the Data Coordination Centre (DCC). The third level will be the site visits, during which data in ResMan will be compared with the source documents. Identified errors will be resolved between the DCC and the clinical sites.
Data monitoring and access
We will establish an independent DSMB. The responsibility of the DSMB will be to review and interpret the data generated from the study, and its primary objectives will be to ensure the safety of the study participants and the integrity of the research data. The DSMB will hold regular conference calls to review the protocol with respect to ethical and safety standards, to monitor the safety of the trials, to monitor the integrity of the data with respect to the original study design, and to provide advice on study conduct. It will also review the progress of the trial, adjudicate adverse events and decide on any premature closure of the study.
The DCC will oversee the intrastudy data sharing process with input from the DSMB. All PIs will be given access to the cleaned data sets. Project data sets will be housed on the ResMan database system for the study, and all data sets will be password protected. The PIs will have direct access to their own site's data sets as well as access to other sites' data by request. To ensure confidentiality, data dispersed to project team members will be blinded to any identifying participant information.
Auditing
An audit trail will be designed as another security measure. This will ensure that only authorised additions, deletions or alterations of information in the electronic record will have occurred and will allow a means to reconstruct significant details about study conduct and source data collection that are necessary to verify the quality and integrity of the data. Computer-generated, time-stamped audit trails will be implemented for tracking changes in the electronic source documentation.
Controls will be built to ensure that the date and time of the system are correct. Dates and times will include the year, month, day, hour and minute provided by international standard-setting agencies. The ability to change the date or time will be limited to authorised personnel, and such personnel will be notified if a system date or time discrepancy is detected.
Internal safeguards will be built into the computerised system. Data will be stored at the servers housed at the First Affiliated Hospital of Guangzhou Medical University with access overseen by professor TW. Records will be regularly backed up, and record logs will be maintained to prevent a catastrophic loss of data and to ensure the quality and integrity of the data.
Ethics and dissemination
All participants and their husbands will be asked to sign a consent form prior to joining the study, and they will be made fully aware that they are free to withdraw from the study at any time. The results of this trial will be disseminated in peer-reviewed journals and presented at international meetings.
Confidentiality and privacy
The investigators have always maintained a strict privacy policy. All correspondence to the department is held confidentially, and at no time will participants' personal and/or identifying information be shared outside of our organisation for any reason.
All study participants have the right to access their personal data and known study results, if and when needed, and they enjoy rights for the protection of the confidentiality of their personal data such as those regarding the collection, custody, retention, management, control and use (including analysis or comparison) of the data.
By signing and dating this consent form, all participants agree to and do give up, waive, disclaim, adjust or in any way abrogate any of the aforementioned rights. For any questions, they should consult our office (020-83062452) as to the proper monitoring or supervision of their personal data protection so that their full awareness and understanding of the significance of compliance with the law governing the privacy of their data is assured.
For questions about the study or reporting of adverse events, the participants should contact their physician.
Research group
The research group consists of the PIs and research physicians, the Steering Committee (SC), the Trial Management Committee, the DSMB, the DCC and the Publication Committee. The members will all have different roles and responsibilities to guarantee that the research is carried out scientifically and rigorously (table 3).
Table 3.
Composition and responsibilities for the research group
| Composition | Member name | Affiliation | Roles and responsibilities |
|---|---|---|---|
| PIs and research physicians | Hongxia Ma Ernest HY Ng Elisabet Stener-Victorin Juan Li Maohua Lai Hua Liu Zhenxing Hu Yongxia Zheng Kewei Quan Meifang Li |
1st Affiliated Hospital, Guangzhou Medical University The University of Hong Kong Karolinska Institutet 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University Xuzhou Maternity and Child Health Hospital Hexian Memorial Affiliated Hospital of Southern Medical University 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University |
Design and conduct of the study. Preparation of protocol and revisions. Preparation of the investigator's SOP and CRFs. Organising the SC meetings. In each participating centre, a site investigator will be identified and will be responsible for patient identification and recruitment, data collection and completion of CRFs as well as follow-up of study patients and adherence to the study protocol and SOP. |
| SC |
Ernest HY Ng Hongxia Ma Elisabet Stener-Victorin Juan Li Taixiang Wu Maohua Lai Hua Liu Zhenxing Hu Yongxia Zheng Kewei Quan Meifang Li |
Chair The University of Hong Kong PIs 1st Affiliated Hospital, Guangzhou Medical University Karolinska Institutet Co-investigators 1st Affiliated Hospital, Guangzhou Medical University Chinese Clinical Trial Registry Site investigators and research physicians 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University Xuzhou Maternity and Child Health Hospital Hexian Memorial Affiliated Hospital of Southern Medical University 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University |
Authorisation of the final study protocol. All PIs will be SC members. Reviewing the progress of the study and, if necessary, agreeing to changes to the protocol and/or the SOP to facilitate the smooth running of the study. |
| TMC (PIs, research physicians, administrators) | Ernest HY Ng Hongxia Ma Elisabet Stener-Victorin Taixiang Wu Juan Li Maohua Lai Hua Liu Zhenxing Hu Yongxia Zheng Kewei Quan Meifang Li |
The University of Hong Kong 1st Affiliated Hospital, Guangzhou Medical University Karolinska Institutet Chinese Clinical Trial Registry 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University Xuzhou Maternity and Child Health Hospital Hexian Memorial Affiliated Hospital of Southern Medical University 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University |
Study planning. Provide annual risk reports to the DSMB and the ethics committee. Reporting serious unexpected suspected adverse events to the DSMB. Responsible for the trial master file. Budget administration and contractual issues with individual centres. Advice for PIs. Auditing of 6-month feedback forms and deciding when site visits should occur. Assistance with independent ethics committee applications. Data verification. Organisation of the central serum sample collection. |
| DSMB | Hongying Kuang Mei Han Min Hu |
Heilongjiang University of Chinese Medicine Beijing University of Chinese Medicine Goteborg University |
Review and interpret the data generated from the study. Ensure the safety of the study participants. Hold regular conference calls. Review the protocol with respect to ethical and safety standards. Monitor the safety of the trials. Monitor the integrity of the data with respect to the original study design. Provide advice on study conduct. Review the progress of the trial. Adjudicate adverse events. Decide on any premature closure of the study. |
| DCC | Taixiang Wu | Chinese Clinical Trial Registry | Oversee the data collection and management (including quality assurance/compliance measures). Oversee the intrastudy data sharing process. Generate and disclose the randomisation scheme for the study. Design the password of the web-based ResMan database to protect the safety of the data. |
| Publication Committee | Ernest HY Ng Hongxia Ma Elisabet Stener-Victorin Taixiang Wu Juan Li Maohua Lai Zhenxing Hu |
The University of Hong Kong 1st Affiliated Hospital, Guangzhou Medical University Karolinska Institutet Chinese Clinical Trial Registry 1st Affiliated Hospital, Guangzhou Medical University 1st Affiliated Hospital, Guangzhou Medical University Xuzhou Maternity and Child Health Hospital |
Publication of study reports. Document the contributions to the study of each author. Draft and revise the manuscript. |
CRFs, case report forms; DCC, Data Coordination Centre; DSMB, Data and Safety Monitoring Board; PIs, principal investigators; ResMan, Research Management; SC, Steering Committee; SOP, standard operating procedure; TMC, Trial Management Committee.
Protocol version and amendments
The first version of the protocol was finished on 20 May 2014. After numerous discussions and amendments by the authors and experts in the research group, there are 10 versions of the protocol. Such amendments will be agreed on by the SC and DSMB and will be approved by the ethics committee prior to implementation. Administrative changes to the protocol are minor corrections and/or clarifications that have no effect on the way the study is to be conducted.
Dissemination policy
It is anticipated that there will be up to 10–20 authors per major manuscript. The authorship order for the participating sites will be based on participant recruitment, data accuracy and promptness of data reporting and will start at position 5 and go to position 15. Each site's PI will be responsible for documenting the contributions to the study of that site's authors.
The participants, all of whom belong to couples with infertility, will benefit from receiving treatment that might offer an increased chance for pregnancy. This study will help guide therapy for future couples with the same issues, and establishing the evidence-based nature of these practices is an important public health aim.
Discussion
To the best of our knowledge, no studies have investigated whether acupuncture pretreatment followed by ovulation induction will increase the live birth rate in women with PCOS. The present study is the first RCT to compare acupuncture pretreatment followed by letrozole with letrozole alone on live birth in anovulatory infertile women with PCOS. The hypothesis is that acupuncture pretreatment improves insulin sensitivity and leads to a higher ovulation rate and live birth rate, which is different from the available evidence suggesting that acupuncture influences ovulation by affecting the levels of various hormones. We are also interested in the prospective effects of acupuncture on women with PCOS, and complications of pregnancy such as impaired glucose tolerance, gestational diabetes mellitus, pregnancy-induced hypertension and pre-eclampsia, SGA, low birth weight, preterm delivery, pre-eclampsia, antepartum haemorrhage, congenital anomaly, perinatal mortality and multiple pregnancies will all be observed to verify the efficacy of the treatment in this study.
According to a recent systematic review,37 there are no studies that have been designed to investigate live birth—which is one of the important clinical outcomes—and there is no evidence of a difference in pregnancy rate between true and sham acupuncture. Based on this, live birth was chosen as the primary outcome in this study, and we chose letrozole alone as the control to replace placebo or sham acupuncture. In addition, low numbers of participants is one of the factors that has limited the quality of the studies performed until now. A high-quality study with an adequate sample size is urgently needed, and our estimates suggest that 384 patients recruited from three hospitals at the same time will ensure a sample size that is sufficient to test our hypothesis.
The study was designed in 2014, and the first participant was randomised on 18 August 2015. At the time of manuscript submission, we have recruited 90 patients and the recruitment is ongoing.
Footnotes
Contributors: JL and EHYN contributed equally to this work. EHYN and HM conceived and designed the study. JL, EHYN and ES-V drafted and critically revised the manuscript for important intellectual content. HM and JL sought funding and ethical approval. TW was responsible for randomisation and data management. All authors contributed to the further writing of the manuscript and approved the final manuscript.
Funding: This work was supported in part by the Natural Science Foundation of Guangdong Province, China (grant number 2015A030310508) and funded by the Science and Technology Planning Project of Guangdong Province, China (grant number 2014A020221060).
Disclaimer: The funding sources had no role in the design of this study and will not have any role during its execution, analysis, data interpretation or decision to submit the results.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: This study has been approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University, Xuzhou Maternity and Child Health Hospital, and Hexian Memorial Affiliated Hospital of Southern Medical University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ghazeeri G, Kutteh WH, Bryer-Ash M et al. Effect of rosiglitazone on spontaneous and clomiphene citrate-induced ovulation in women with polycystic ovary syndrome. Fertil Steril 2003;79:562–6. 10.1016/S0015-0282(02)04843-4 [DOI] [PubMed] [Google Scholar]
- 2.Qiao J. [The diagnosis and treatment for amenorrhea in polycystic ovary syndrome]. Zhongguo Shi Yong Fu Ke Yu Chan Ke Za Zhi 2008;24:902–4. [Google Scholar]
- 3.Katulski K, Czyzyk A, Podfigurna-Stopa A et al. Pregnancy complications in polycystic ovary syndrome patients. Gynecol Endocrinol 2015;31:87–91. 10.3109/09513590.2014.974535 [DOI] [PubMed] [Google Scholar]
- 4.Lin SQ, He FF, Chen ZJ et al. [Clinical progress of gynecological endocrinology]. Edition 1. Beijing: Tsinghua Tongfang CD-ROM electronic publishing house 2013:106–9.
- 5.Legro RS, Brzyski RG, Diamond MP et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med 2014;371:119–29. 10.1056/NEJMoa1313517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang CY, Ding CF. [Research progress on insulin resistance and endometrial receptivity of polycystic ovary syndrome]. Zhejiang Zhong Xi Yi Jie He Za Zhi 2012;22:155–8. [Google Scholar]
- 7.DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 2005;83:1454–60. [DOI] [PubMed] [Google Scholar]
- 8.Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 2004;59:141–54. 10.1097/01.OGX.0000109523.25076.E2 [DOI] [PubMed] [Google Scholar]
- 9.Stener-Victorin E, Waldenstrom U, Tagnfors U et al. Effects of electro-acupuncture on anovulation in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand 2000;79:180–8. 10.1080/j.1600-0412.2000.079003180.x [DOI] [PubMed] [Google Scholar]
- 10.Johansson J, Redman L, Veldhuis PP et al. Acupuncture for ovulation induction in polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2013;304:934–43. 10.1152/ajpendo.00039.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastore LM, Williams CD, Jenkins J et al. True and sham acupuncture produced similar frequency of ovulation and improved LH to FSH ratios in women with polycystic ovary syndrome. J Clin Endocrinol Metab 2011;96:3143–50. 10.1210/jc.2011-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai MH, Ma HX, Yao H et al. [Effect of abdominal acupuncture therapy on the endocrine and metabolism in obesity-type polycystic ovarian syndrome patients]. Zhen Ci Yan Jiu 2010;35:298–302. [PubMed] [Google Scholar]
- 13.Jedel E, Labrie F, Odén A et al. Impact of electro-acupuncture and physical exercise on hyperandrogenism and oligo/amenorrhea in women with polycystic ovary syndrome: a randomized controlled trial. Am J Physiol Endocrinol Metab 2011;300:37–45. 10.1152/ajpendo.00495.2010 [DOI] [PubMed] [Google Scholar]
- 14.Zheng YH, Wang XH, Lai MH et al. Effectiveness of abdominal acupuncture for patients with obesity-type polycystic ovary syndrome: a randomized controlled trial. J Altern Complement Med 2013;19:740–5. 10.1089/acm.2012.0429 [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Qu HQ, Fang HL. [Effect of electroacupuncture combined with auricular point tapping and pressing on serum insulin and testosterone in the patients of obese women with polycystic ovary syndrome]. Zhongguo Zhen Jiu 2009;29:441–3. [PubMed] [Google Scholar]
- 16.Stener-Victorin E, Wu X. Effects and mechanisms of acupuncture in the reproductive system. Auton Neurosci 2010;157:46–51. 10.1016/j.autneu.2010.03.006 [DOI] [PubMed] [Google Scholar]
- 17.Raja-Khan N, Stener-Victorin E, Wu X et al. The physiological basis of complementary and alternative medicines for polycystic ovary syndrome. Am J Physiol Endocrinol Metab 2011;301:E1–10. 10.1152/ajpendo.00667.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang WY, Huang GY, Liu J et al. Acupuncture-induced improvement of endometrial receptivity of rats with polycystic ovary syndrome treated by clomiphene for ovarian stimulation. World J Acupunct Moxibustion 2009;19:30–7. [Google Scholar]
- 19.Zhang WY, Huang GY, Liu J et al. [Influences of acupuncture on infertility of rats with polycystic ovarian syndrome]. Zhongguo Zhong Xi Yi Jie He Za Zhi 2009;29:997–1000. [PubMed] [Google Scholar]
- 20.Stener-Victorin E, Waldenstrom U, Andersson SA et al. Reduction of blood flow impedance in the uterine arteries of infertile women with electro-acupuncture. Hum Reprod 1996;11:1314–17. 10.1093/oxfordjournals.humrep.a019378 [DOI] [PubMed] [Google Scholar]
- 21.Yin J, Kuang J, Chandalia M et al. Hypoglycemic effects and mechanisms of electroacupuncture on insulin resistance. Am J Physiol Regul Integr Comp Physiol 2014;307:R332–9. 10.1152/ajpregu.00465.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson J, Yi F, Shao R et al. Intense acupuncture normalizes insulin sensitivity, increases muscle GLUT4 content, and improves lipid profile in a rat model of polycystic ovary syndrome. Am J Physiol Endocrinol Metab 2010;299:E551–9. 10.1152/ajpendo.00323.2010 [DOI] [PubMed] [Google Scholar]
- 23.Manneras L, Jonsdottir IH, Holmang A et al. Low-frequency electro-acupuncture and physical exercise improve metabolic disturbances and modulate gene expression in adipose tissue in rats with dihydrotestosterone-induced polycystic ovary syndrome. Endocrinology 2008;149:3559–68. 10.1210/en.2008-0053 [DOI] [PubMed] [Google Scholar]
- 24.Zheng YH, Ding T, Ye DF et al. Effect of low-frequency electroacupuncture intervention on oxidative stress and glucose metabolism in rats with polycystic ovary syndrome. Zhen Ci Yan Jiu 2015;40:125–30. [PubMed] [Google Scholar]
- 25.Benrick A, Maligueo M, Johansson J et al. Enhanced insulin sensitivity and acute regulation of metabolic genes and signaling pathways after a single electrical or manual acupuncture session in female insulin-resistant rats. Acta Diabetol 2014;51:963–72. 10.1007/s00592-014-0645-4 [DOI] [PubMed] [Google Scholar]
- 26.MacPherson H, Altman DG, Hammerschlag R et al. Revised STandards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA): extending the CONSORT statement. PLoS Med 2010;7:e1000261 10.1371/journal.pmed.1000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moher D, Hopewell S, Schulz KF et al. CONSORT 2010 Explanation and Elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao X, Ni R, Li L et al. Defining hirsutism in Chinese women: a cross-sectional study. Fertil Steril 2011;96:792–6. 10.1016/j.fertnstert.2011.06.040 [DOI] [PubMed] [Google Scholar]
- 29.Ni RM, Mo Y, Chen X et al. Low prevalence of the metabolic syndrome but high occurrence of various metabolic disorders in Chinese women with polycystic ovary syndrome. Eur J Endocrinol 2009;161:411–18. 10.1530/EJE-09-0298 [DOI] [PubMed] [Google Scholar]
- 30.Yanhua Z, Stener-Victorin E, Ng HE et al. How does acupuncture affect insulin sensitivity in women with polycystic ovary syndrome and insulin resistance? Study protocol of a prospective pilot study. BMJ Open 2015;5:e007757 10.1136/bmjopen-2015-007757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cronin L, Guyatt G, Griffith L et al. Development of a health-related quality-of-life questionnaire (PCOSQ) for women with polycystic ovary syndrome (PCOS). J Clin Endocrinol Metab 1998;83:1976–87. 10.1210/jcem.83.6.4990 [DOI] [PubMed] [Google Scholar]
- 32.Guermandi E, Vegetti W, Bianchi MM et al. Reliability of ovulation tests in infertile women. Obstet Gynecol 2001;97:92–6. [DOI] [PubMed] [Google Scholar]
- 33.Legro RS, Kunselman AR, Brzyski RG et al. The pregnancy in polycystic ovary syndrome II (PPCOS II) trial: rationale and design of a double-blind randomized trial of clomiphene citrate and letrozole for the treatment of infertility in women with polycystic ovary syndrome. Contemp Clin Trials 2012;33:470–81. 10.1016/j.cct.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahn SE, Prigeon RL, McCulloch DK et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–72. [DOI] [PubMed] [Google Scholar]
- 35.Drug insert of Femara. Initial U.S. approval in 1997. Revised in 2014. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation.
- 36.Chen X, Yang D, Li L et al. Abnormal glucose tolerance in Chinese women with polycystic ovary syndrome. Hum Reprod 2006;21:2027–32. 10.1093/humrep/del142 [DOI] [PubMed] [Google Scholar]
- 37.Lim CE, Ng RW, Xu K et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rve 2016;(5):CD007689 10.1002/14651858.CD007689.pub3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2015-010955supp_appendix.pdf (163.6KB, pdf)